Abstract
Age-related hearing loss (presbyacusis) has a complex etiology. Results from animal models detailing the effects of specific cochlear injuries on audiometric profiles may be used to understand the mechanisms underlying hearing loss in older humans and predict cochlear pathologies associated with certain audiometric configurations (“audiometric phenotypes”). Patterns of hearing loss associated with cochlear pathology in animal models were used to define schematic boundaries of human audiograms. Pathologies included evidence for metabolic, sensory, and a mixed metabolic + sensory phenotype; an older normal phenotype without threshold elevation was also defined. Audiograms from a large sample of older adults were then searched by a human expert for “exemplars” (best examples) of these phenotypes, without knowledge of the human subject demographic information. Mean thresholds and slopes of higher frequency thresholds of the audiograms assigned to the four phenotypes were consistent with the predefined schematic boundaries and differed significantly from each other. Significant differences in age, gender, and noise exposure history provided external validity for the four phenotypes. Three supervised machine learning classifiers were then used to assess reliability of the exemplar training set to estimate the probability that newly obtained audiograms exhibited one of the four phenotypes. These procedures classified the exemplars with a high degree of accuracy; classifications of the remaining cases were consistent with the exemplars with respect to average thresholds and demographic information. These results suggest that animal models of age-related hearing loss can be used to predict human cochlear pathology by classifying audiograms into phenotypic classifications that reflect probable etiologies for hearing loss in older humans.
Keywords: metabolic presbyacusis, sensory presbyacusis, endocochlear potential, animal models, audiogram classification, supervised machine learning classifiers
Introduction
Hearing loss is one of the most commonly reported chronic impairments in older persons, affecting approximately 30 % of the US population older than 65 years of age (Leske 1981; Lin et al. 2011). As the average age of the population increases, the number of people affected by hearing loss also increases. The complex genetic and environmental factors affecting hearing over the lifespan contribute to a large variation in audiometric profiles and other measures of auditory function. Whereas pathological findings from humans are limited to postmortem morphologic data, experimental procedures in animals can be designed to disrupt specific cochlear systems or minimize environmental exposures, while measuring subsequent changes in hearing across frequency. Thus, the effects on hearing and auditory function resulting from exposure to excessive noise or ototoxic drugs, or from aging in the absence of environmental exposures, can be better understood in terms of cochlear pathologies and their corresponding audiometric patterns in animal models. Moreover, an accurate audiogram classification system based on information from cochlear pathology from well-characterized animal models has the potential to provide a better understanding of underlying mechanisms of age-related hearing loss in humans and lead to improved diagnosis and treatment strategies.
There have been numerous attempts over the past 70 years to relate the underlying causes of age-related hearing loss to behavioral or electrophysiologic measures of auditory function, including analyses of audiograms (e.g., Hinchcliffe 1959; Glorig and Nixon 1962). Similarly, distortion-product otoacoustic emission and auditory brainstem response input–output functions have been used to differentiate noise induced from age-related hearing loss (Mills 2003, 2006; Mills and Schmiedt 2004). There is a long history of work that has correlated histopathological abnormalities of the cochlea observed in human temporal bones with specific audiometric patterns (e.g., Crowe et al. 1934; Schuknecht 1964; Suga and Lindsay 1976; Halpin and Rauch 2009; Nadol 2010). For several reasons, including long delays between threshold measures and temporal bone donations, results in the early work were often inconsistent and contradictory (Nelson and Hinojosa 2006). Nevertheless, four categories of presbyacusis were commonly accepted for many years, based on extensive human data from classic studies of human temporal bones (Schuknecht 1974): (1) sensory, characterized by atrophy and degeneration of sensory hair cells and supporting cells; (2) neural, typified by loss of spiral ganglion neurons; (3) metabolic, characterized by atrophy and degeneration of the lateral wall of the cochlea, especially the stria vascularis; and (4) mechanical, where the inner ear is hypothesized to change its conductive characteristics. After additional research on human temporal bones, Schuknecht and Gacek (1993) made significant revisions to their classification scheme, paraphrased as follows: “(1) sensory cell losses are the least important type of loss in the aged ear; (2) neuronal losses are constant and predictable expressions of aging; and (3) atrophy of the stria vascularis is the predominant lesion of the aging ear.” Thus, although hair cell loss is reported in some animal models of presbyacusis (Dayal and Bhattacharyya 1986; Sha et al. 2008), the Schuknecht and Gacek (1993) perspective downplays the significance of age-related loss of sensory cells, emphasizes the importance of age-related degeneration of the stria vascularis and auditory nerve, and brings consistency between results from human temporal bones and from experiments with aging animals.
Classification of hearing loss types in older persons based on audiometric configuration alone is challenging because heredity, age, noise history, injury, disease, medication, diet, and other factors can work individually and jointly to alter the audiogram of an older adult. Studies characterizing audiometric configuration can be divided into two approaches (Ciletti and Flamme 2008). One approach is to use statistical methods such as cluster analysis to group individuals based on the statistical patterns of the individual pure-tone thresholds (e.g., Yuen and McPherson 2002; Allen and Eddins 2010). This approach can classify audiometric profiles into groups, but the groupings are based entirely on the distribution and statistical properties of the threshold data and not on pathophysiologic considerations. As a result, this unsupervised approach has limited power to explain the etiologies or mechanisms underlying hearing loss. A second approach is to classify audiograms into a limited number of configurations based on shape, severity, and etiology, given that certain audiometric profiles suggest specific underlying cochlear pathologies.
Hearing loss of cochlear origin is a common etiology in older humans, with aging and excessive noise exposure being the most notable contributors. Effects of aging and noise exposure are almost always confounded and difficult to separate in human studies. Animal models have been used to characterize noise and aging effects on hearing thresholds by raising animals in quiet or controlled noise environments (e.g., Mills et al. 1990; Schmiedt et al. 1990, 2002; Tarnowski et al. 1991). In many of these animal studies, morphological pathologies were matched directly to hearing thresholds. Thus, the audiograms of quiet-aged and noise-exposed gerbils, many of which were tested through their life span of 30–36 months, provide information with which to classify audiograms according to well-established effects of aging and noise or drug exposures.
Consistent with the reclassification of Schuknecht and Gacek (1993), a striking result of the gerbil studies in particular is that age-related hearing loss is typically not a sensory, but a metabolic and neural disorder. Age-related hearing loss in the absence of noise exposure is hypothesized to be due, in part, to the decrease in the endocochlear potential (EP) present in the scala media fluid (endolymph). The EP is maintained by cells within the lateral wall and the stria vascularis, which may be highly susceptible to aging because of their high metabolic activities (Gruber et al. 2008). As a result, the normal potential of ∼90 mV can decrease with increasing age to ∼60 mV or lower throughout the gerbil cochlea (Schmiedt 1996).
With the EP acting as the power supply to the outer hair cells (OHCs), which comprise the cochlear amplifier (Davis 1983; Russell 1983), the potential directly affects the gain (sensitivity) of the amplifier as reflected in the pattern of threshold shifts (Sewell 1984; Ruggero and Rich 1991; Schmiedt et al. 2002; Schmiedt 2010). In the cochlear base, the relationship of cochlear sensitivity in the cat and gerbil to EP change is ∼1 dB per 1 mV decline in EP, whereas in the apex, the amplifier is less sensitive to changes in EP (Sewell 1984; Schmiedt 1996, 2010; Schmiedt et al. 2002). Results from animal models (Cooper and Rhode 1997; Robles and Ruggero 2001) also provide estimates of the maximum gain of the cochlear amplifier that are less in the apex (about 20 dB) than in the base (about 50–70 dB). As a result, a decrease in the EP of ∼30 mV throughout the cochlea does not result in a constant threshold shift across frequency, but rather results in a relatively flat loss at lower frequencies of ∼20 dB coupled with a gradually sloping loss at higher frequencies. Thus, the loss of EP with increasing age defines the audiometric profile of metabolic presbyacusis, as seen in the animal model of the quiet-aged gerbil (Mills et al. 1990, 1996, 2006; Hellstrom and Schmiedt 1990, 1991, 1996; Schmiedt et al. 1990, 2002; Tarnowski et al. 1991; Schulte and Schmiedt 1992; Schmiedt 1996, 2010; Gratton et al. 1997), and is hypothesized to affect the audiogram of older humans in similar fashion.
Of critical importance for characterizing the pathophysiology underlying human audiometric configurations, results from other animal models have shown that injuries due to excessive noise or ototoxic drug exposure are largely confined to the OHCs, typically in the cochlear base. Without OHC function (or in the absence of OHCs), the gain of the cochlear amplifier is dramatically reduced by 50–70 dB in the base of the cochlea, resulting in a notch or a fairly well-defined threshold shift of 50–70 dB at higher frequencies (Dallos and Harris 1978; Liberman and Kiang 1978; Ryan et al. 1979; Schmiedt 1984, 2010; Schmiedt et al. 1990; Cooper and Rhode 1997; Robles and Ruggero 2001). Studies of the effects of ototoxic drugs are perhaps the most definitive with regard to the effects of pure OHC loss on thresholds. Typically, OHC loss due to ototoxic drug exposure can be very sharply defined morphologically, yielding steeply sloping threshold shifts at higher frequencies to a plateau of ∼50–70 dB (e.g., Dallos and Harris 1978; Ryan et al. 1979). Thus, audiogram configurations arising from OHC losses from defined noise and drug exposures are quite different from those arising from chronic EP declines associated with metabolic presbyacusis.
The goal of the current study was to determine if results from animal models could be used to analyze audiograms from human subjects and predict probable etiologies of cochlear pathology in older adults that segregate with demographic and hearing history variables. We examined the extent to which unique and consistent patterns of “audiometric phenotypes” can be identified from pure-tone audiograms in a large sample of older adults. To provide support for this approach, the audiometric classifications were related to predicted distributions of age, gender, and noise exposure histories for certain cochlear pathologies. Thus, the aims were to (1) examine the consistency of proposed audiometric phenotypes, (2) correlate these classifications with subjects’ demographic information, and (3) develop automated classifiers that could be applied to a new and larger sample of older adults that reflected the varied distribution of audiograms in this population.
Methods
Subjects and measurement procedures
The Medical University of South Carolina (MUSC) database consists of measures of auditory function and medical/biological data from human subjects enrolled in an ongoing longitudinal study of age-related hearing loss, which began in 1987. Subjects in this study are in good general health and have no evidence of conductive hearing loss, active otologic disease, or significant cognitive declines (as screened by the Mini-Mental State Exam; Folstein et al. 1975). Audiometric measures consist of hearing for pure tones (at 0.25, 0.5, 1.0, 2.0, 3.0, 4.0, 6.0, and 8.0 kHz), thresholds at extended high frequencies (Matthews et al. 1997), ability to understand speech in quiet and in noise (Dubno et al. 1995, 1997), otoacoustic emissions, upward and downward spread of masking, middle ear function, and auditory brainstem responses. Subjects provide written and oral responses to questionnaires related to medical history, use of prescription and over-the-counter drugs (Lee et al. 1998), occupational and non-occupational noise history, hearing aid history, self-assessed hearing handicap, tinnitus, smoking, and handedness. All subjects receive an otologic examination. For a large proportion of subjects, DNA is extracted from blood to identify and characterize genes that are under- or over-expressed with age. For these subjects, a genetics counselor obtains a family pedigree for hearing loss. Following enrollment, subjects are scheduled approximately once per month for a total of three to six laboratory visits to complete the test battery; an audiogram is obtained at nearly every visit. After completion, subjects are scheduled annually to obtain an audiogram and speech recognition measures, and to update medical history and contact information, and then every 2–3 years to repeat the entire test battery. To date, more than 1,300 subjects have participated in this ongoing study; of these, longitudinal data covering at least a 3-year period are available from nearly 500 subjects.
The current analysis included 1,728 audiograms from the MUSC database (865 subjects, thresholds from two ears were missing), collected from subjects ranging in age from 50.4 to 97.5 years, with a mean age of 69.9 years; the vast majority of subjects (94.4 %) were 60 years and older. Consistent with the distribution of gender in most studies of aging, 44.4 % of cases were male and 55.6 % were female. To improve the accuracy of the audiograms and reduce small irregularities due to the 5-dB step size used in the measurement of pure-tone thresholds, an average audiogram for each ear of each subject was computed from all available audiograms obtained during the subject’s first year of participation (number of laboratory visits = 1–8, mean = 2.9).
Pure-tone thresholds were measured with either a Madsen OB822 or OB922 clinical audiometer calibrated to appropriate American National Standards Institute (ANSI) standards (American National Standards Institute 1969, 1989, 1996, 2004, 2010) and equipped with TDH-39 headphones mounted in MX-41/AR cushions, using standard measurement procedures recommended by the American Speech-Language-Hearing Association (2005). If no response was obtained at the audiometers’ maximum outputs, the following values were assigned: 115 dB HL for 0.25–6.0 kHz and 105 dB HL for 8.0 kHz. Additional details of subject selection and outcome measures are available in publications reporting results from the MUSC longitudinal study of age-related hearing loss (e.g., Dubno et al. 1995, 1997, 2008; Lee et al. 2005).
To assess noise exposure history, a seven-item noise history questionnaire on occupational and non-occupational noise exposure was used as an index of noise exposure. Subjects answered yes or no to questions related to noisy work environments (including the military) and exposure to noise from guns, music, power tools, and farm machinery. Of the 865 subjects, 731 had noise history information with 49.4 % of these subjects reporting a positive noise history, related primarily to occupational noise exposure. There were substantial differences according to gender; that is 82.4 % of males but only 22.2 % of females reported a positive noise history. These results, along with demographic (age and gender) information, provided the means to validate the classifications, as discussed later.
Defining the exemplars
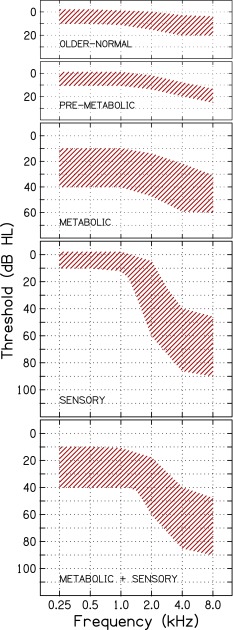
To determine if consistent patterns of “audiometric phenotypes” could be identified from pure-tone audiograms of older adults, schematic boundaries of audiograms were first defined based on five hypothesized conditions of cochlear pathology obtained from animal results. Boundaries were defined based on knowledge of previously published audiograms from a variety of animal models used to study effects of exposures to excessive noise or ototoxic drugs, or from animals raised in quiet, as described earlier, which were hypothesized to affect audiometric shapes. Mapping age-related changes in audiometric configurations of the gerbil to human audiograms was accomplished by a simple shift in the frequency axis. The transition between the constant loss at lower frequencies and the gradually sloping loss at higher frequencies is a predominant feature of metabolic presbyacusis and chronic EP loss, which also differentiates metabolic from sensory loss. This breakpoint frequency is ∼4.2 kHz in gerbil and ∼1.3 kHz in older humans with no significant noise history. This mapping is more fully described and schematized in Schmiedt et al. (2002; see Fig. 7, bottom panel).
FIG. 7.
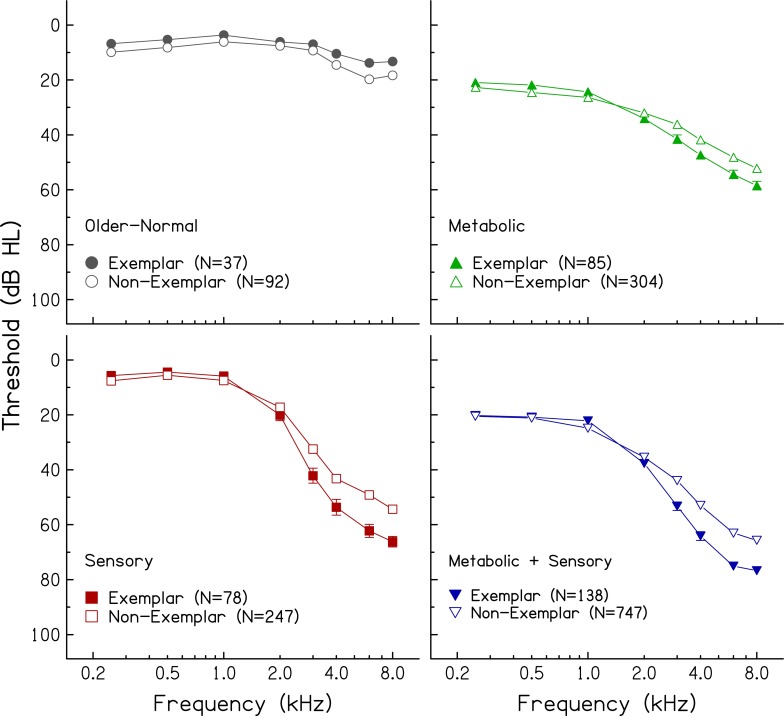
Mean audiograms and standard errors of exemplars (filled symbols) and non-exemplars (open symbols) in four audiometric phenotypes.
As illustrated in Figure 1 and specified in the first four columns of Table 1, the five audiometric phenotypes were: older-normal, pre-metabolic, metabolic, sensory, and a mixed metabolic + sensory phenotype. Audiograms classified as “older-normal” have thresholds of ≤10 dB HL from 0.25 to 1.0 kHz and 0 to 20 dB HL at higher frequencies. Audiograms classified as “pre-metabolic” have thresholds of ≤10 dB HL from 0.25 to 1.0 kHz and 10–25 dB HL at higher frequencies. Audiograms classified as “metabolic” (mild to severe) have relatively flat hearing loss in the lower frequencies ranging from 10 to 40 dB HL and gradually sloping hearing loss from 30 to 60 dB HL in the higher frequencies, with slopes ranging from 10 to 20 dB/oct. Audiograms classified as “sensory” have normal thresholds in the lower frequencies (≤10 dB HL) with a steeply sloping increase in thresholds in the higher frequencies to a notch or plateau between 40 and 70 dB HL, with slopes >20 dB/oct. Audiograms classified as “metabolic + sensory” have characteristics of metabolic presbyacusis in the lower frequencies (relatively flat loss ranging from 10 to 40 dB HL) and characteristics of sensory loss in the higher frequencies (steeply sloping loss with slopes >20 dB/oct).
FIG. 1.
Schematic boundaries of audiograms corresponding to five phenotypes of age-related hearing loss, based on five hypothesized conditions of cochlear pathology (adapted from Schmiedt 2010).
TABLE 1.
Characteristics of five audiometric phenotypes of age-related hearing loss
| Phenotype | Low frequency (0.25–1.0 kHz) | High frequency (2.0–8.0 kHz) | Age | Gender | Noise History | ||
|---|---|---|---|---|---|---|---|
| Range (dB HL) | Slope (dB/oct) | Range (dB HL) | Slope (dB/oct) | ||||
| Older-normal | ≤10 | −5 to 5 | 0 to 20 | −5 to 5 | Younger | Female>Male | No>Yes |
| Pre-metabolic | ≤10 | −5 to 5 | ≤25 | 0 to 10 | Younger | Female>Male | No>Yes |
| Metabolic | 10 to 40 | −5 to 5 | 30 to 60 | 10 to 20 | Older | Female>Male | No>Yes |
| Sensory | ≤10 | >5 | >40 | ≥20 | Younger | Male>Female | Yes>No |
| Metabolic + Sensory | 10 to 40 | −5 to 5 | >40 | ≥20 | Older | Male>Female | Yes>No |
Next, 1,728 audiograms from older human subjects stored in the MUSC database were searched for “exemplars” (best examples) of these five phenotypes, without knowledge of demographic information. The selection of the exemplars was performed by a single human expert (RAS) with >35 years of experience with animal models of age-related hearing loss, including models of metabolic presbyacusis and noise-induced and ototoxic-drug-induced hearing loss (Schmiedt and Zwislocki 1980; Schmiedt et al. 1980, 2002; Schmiedt 1984, 2010). RAS initially identified 374 audiograms as exemplars. Intra-rater reliability was performed to determine the extent to which these exemplars were consistently selected for each phenotype (alpha = 0.995). Because inter-rater reliability with a second human expert (JRD) was lower (alpha = 0.701), RAS and JRD discussed inconsistencies to arrive at a final group of exemplars. Following these reviews, 25 cases (6.7 % of the original set of exemplars, 1.4 % of all searched audiograms) were excluded after raters agreed they were not exemplars.
Statistical reliability and classification of exemplars
One challenge in defining and classifying audiograms from human subjects is the nonlinearity of the audiometric configuration. Supervised learning methods that take advantage of nonlinearities for feature identification were used to establish reliability and generalizability of findings for the audiometric phenotypes. Three supervised nonlinear classification methods for classifying audiometric phenotype were used to determine the extent to which: (1) classifier models can be trained to identify empirically based exemplar cases with a high degree of accuracy (>80 % consistency with the human expert) and (2) classification estimates for each phenotype correspond to predicted demographics (age, gender, noise exposure history) in non-exemplar cases (i.e., those for which classification by the human expert was difficult, by definition). Importantly, we determined the extent to which the exemplar groups exhibited the same patterns of demographic and noise exposure histories as the non-exemplar cases that were defined using the machine learning methods, thereby providing validation. Phenotypes of the exemplar audiograms were predicted using Quadratic Discriminant Analysis, Support Vector Machines, and Random Forests, to provide evidence for the consistency of classification across different classifier algorithms. For each method, a tenfold cross-validation was performed to establish the reliability of a classifier on the trained exemplar dataset (Kohavi 1995). That is, 90 % of the exemplar cases were selected to train each classifier, which was then tested on the remaining 10 % of cases. This procedure was repeated 10 times, each time with a new set of audiograms as trainers. The classification probabilities were then combined from the 10 classification runs and the highest probability was used to classify cases.
Quadratic Discriminant Analysis (QDA)
QDA was used to predict phenotype classification of the exemplars using pure-tone thresholds (0.25–8.0 kHz). The simplest case of a discriminant analysis is the determination of a dichotomous group membership (normal hearing vs. hearing impaired), based on a single variable, such as pure-tone average. In this case, the discriminant function is a simple linear regression. When several measured variables and subjects are classified into one of several groups, a set of linear discriminant functions is constructed based on a multivariate normal distribution. In this study, a nonlinear QDA was used because the covariances across frequencies are not equivalent and the exemplar phenotypes (thresholds as a function of frequency) are nonlinear. Thus, QDA was selected to capture nonlinearities in the audiogram and relations among thresholds that differentiated the phenotypes.
The exemplars provided the training set for the construction of discriminant functions and contained a sufficient sample size to obtain estimates for the 176 parameters (8 frequency means and 36 variance–covariance parameters across the five phenotypes). After the tenfold cross-validation reliability of QDA was confirmed, the non-exemplar cases were classified based on the quadratic discriminant functions constructed from the exemplars. Using the QDA classifier on the non-exemplar cases provided a means for estimating the likelihood that these difficult-to-classify cases have a particular etiology. Subsequent comparisons of thresholds between the exemplar and non-exemplar cases, and demographic (age, gender, and noise history) distributions across the phenotypes, were used to determine the validity of the non-exemplar classifications.
Support Vector Machines (SVM)
Multiclass SVM was used to identify patterns or structure in the audiometric thresholds that reflect the exemplar classification. SVM identifies and maximizes support vectors or points that define the width between sets of cases with different distributions of data between variables. These vectors define a nonlinear hyperplane that separates cases into different groups. Weka (2.6.1; Hall et al. 2009) and the libSVM wrapper (Chang and Lin 2001) were used to perform SVM on Z score-transformed audiometric data from the exemplars. Support vector classification and a radial basis function kernel were used in the tenfold cross-validation analysis. SVM has been widely used for predicting neurological disorders, as an example see reviews in Haller et al. 2011; Orrù et al. 2012.
Random Forests (RF)
Multiclass RF was used to build an exemplar classifier using Weke (2.6.1; Hall et al. 2009) by growing decision trees from groups of ears. The RF algorithm subsamples data and classifies cases based on variables that best separate the groups and builds a model for classification across many decision trees. Classification accuracy is then estimated for each unsampled case for a decision tree and averaged across trees. More specifically, for each treey, a random number (m) of ears was selected as a bootstrap sample to train treey. The random sample of ears for treey typically contains about two thirds of all the ears because one third of ears are selected twice. The one third of ears that were not randomly selected to grow treey were included in treey’s out of box (ooby) ears that were used to test the classification error rate for treey. The classification error rate for each tree was then determined using the ooby remaining ears and expressed as the percentage of correct classification across 100 trees in each forest. Consistent with previous RF observations, the classification accuracy/generalization error (root mean square classification error) reached asymptote (0.2) with increasing numbers of trees beyond 100 (Breiman 2001). Tenfold cross validation was performed 10 times with different starting values to obtain average and stable estimates of classification accuracy. RF also is a widely used classifier because of its strength in classifying cases without overfitting the data, thereby making the classifier likely to generalize to new datasets (see Touw et al. 2012 for additional information).
Results
Audiograms from 349 of the 1,728 ears (20.2 %) were identified as exemplars of one of the five phenotypes in that they exhibited characteristics seen in the audiograms of animal models of metabolic and/or sensory pathology, as reviewed earlier. The remaining audiograms (N = 1,379) did not fit precisely into any of the five phenotypes and were referred to as non-exemplars.
Only 11 of the 1,728 audiograms were identified as exemplars of the “pre-metabolic” phenotype (3 % of exemplars). The small sample size was, therefore, not amenable to the supervised learning aim of this study. The 11 audiograms originally classified as pre-metabolic were added to the non-exemplars and only four phenotypes were used in the exemplar modeling process (N = 338). After the classification rules were established, 5 of the 11 audiograms originally identified as pre-metabolic were classified by QDA as older-normal and six were classified as metabolic. Additional information about the pre-metabolic phenotype is included in the “Discussion” section.
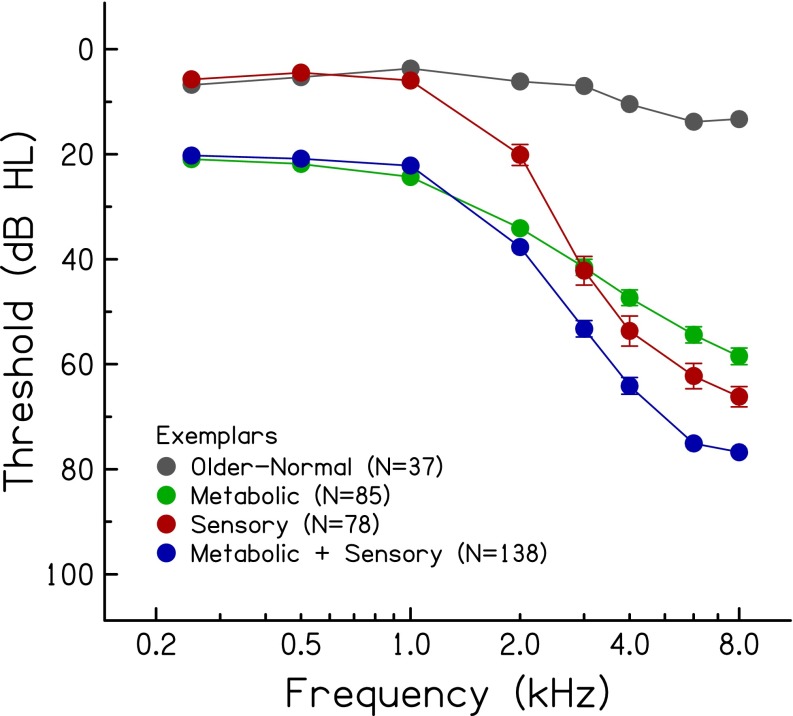
Of the 338 exemplar audiograms, 11 % were classified as older-normal, 25 % were classified as metabolic, 23 % were classified as sensory, and 41 % were classified as a mixed metabolic + sensory phenotype. The highest percentage for the mixed phenotype is consistent with the notion that age-related hearing loss in older humans reflects the accumulated effects of various exposures throughout the lifespan. The mean audiograms of the exemplars representing the four phenotypes are shown in Figure 2. With relatively large sample sizes, the standard errors are small, which obscures substantial overlap of thresholds of individual audiograms between phenotypes. For example, thresholds at higher frequencies (3.0–8.0 kHz) are generally similar for the metabolic and sensory phenotypes. These phenotypes are differentiated primarily by thresholds in the lower frequencies (better thresholds for sensory than metabolic) and by the slope of the hearing loss between 1.0 and 4.0 kHz (steeper slope for sensory than metabolic). Lower frequency thresholds are similar for the phenotypes with metabolic characteristics (metabolic and metabolic + sensory), but slopes for higher frequency thresholds are steeper for the mixed phenotype due to the sensory component. The sensory and mixed metabolic + sensory phenotypes have similar higher frequency slopes, but lower frequency thresholds are poorer for the mixed phenotype (due to the metabolic component). These results are consistent with the predefined schematic boundaries, as shown in Figure 1 and Table 1.
FIG. 2.
Mean thresholds (±1 standard error) of 338 exemplars in four audiometric phenotypes.
Classification rates of the exemplars
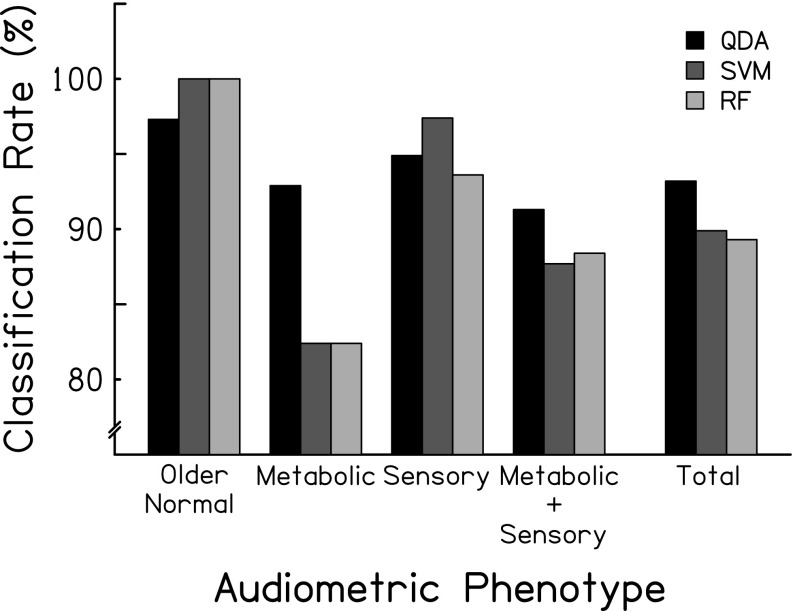
Table 2 and Figure 3 summarize the classification accuracies/errors for each supervised learning method across each of the four phenotypes. The three classification methods were consistent with respect to overall classification accuracy, primarily due to high rates of correct classification for exemplar audiograms in the older-normal and sensory phenotypes. For example, there was a 2.7 % difference in accuracy between QDA and SVM or RF for the older-normal phenotype. Classification errors for each method were also similar, although the QDA classifier exhibited greater accuracy for metabolic cases than SVM or RF (∼10 % difference in accuracy). Each method was more likely to misclassify metabolic and metabolic + sensory exemplars given the lower classification rates for these phenotypes as compared to older-normal and sensory phenotypes. Because QDA is a widely understood method compared to SVM and RF, and because it provided results comparable to or better than the other methods, subsequent classification results are described for the QDA classifier.
TABLE 2.
Error matrix for the classification of the 338 exemplars of four audiometric phenotypes determined by a human expert (rows) and by Quadratic Discriminant Analysis (QDA), Support Vector Machines (SVM), and Random Forests (RF) (columns). Overall accuracy of the three methods was 93.2, 89.9, and 89.3 %, respectively
| Older-normal | Metabolic | Sensory | Metabolic + Sensory | Percent correct | |
|---|---|---|---|---|---|
| QDA | |||||
| Older-normal | 36 | 0 | 1 | 0 | 97.3 |
| Metabolic | 0 | 79 | 0 | 6 | 92.9 |
| Sensory | 0 | 1 | 74 | 3 | 94.9 |
| Metabolic + Sensory | 0 | 9 | 3 | 126 | 91.3 |
| SVM | |||||
| Older-normal | 37 | 0 | 0 | 0 | 100.0 |
| Metabolic | 0 | 70 | 1 | 14 | 82.4 |
| Sensory | 0 | 2 | 76 | 0 | 97.4 |
| Metabolic + Sensory | 0 | 15 | 2 | 121 | 87.7 |
| RF | |||||
| Older-normal | 37 | 0 | 0 | 0 | 100.0 |
| Metabolic | 0 | 71 | 2 | 12 | 83.5 |
| Sensory | 1 | 1 | 73 | 3 | 93.6 |
| Metabolic + Sensory | 0 | 14 | 1 | 123 | 89.1 |
FIG. 3.
Classification rates for exemplars for four audiometric phenotypes and the overall classification rate.
Demographics of the exemplars
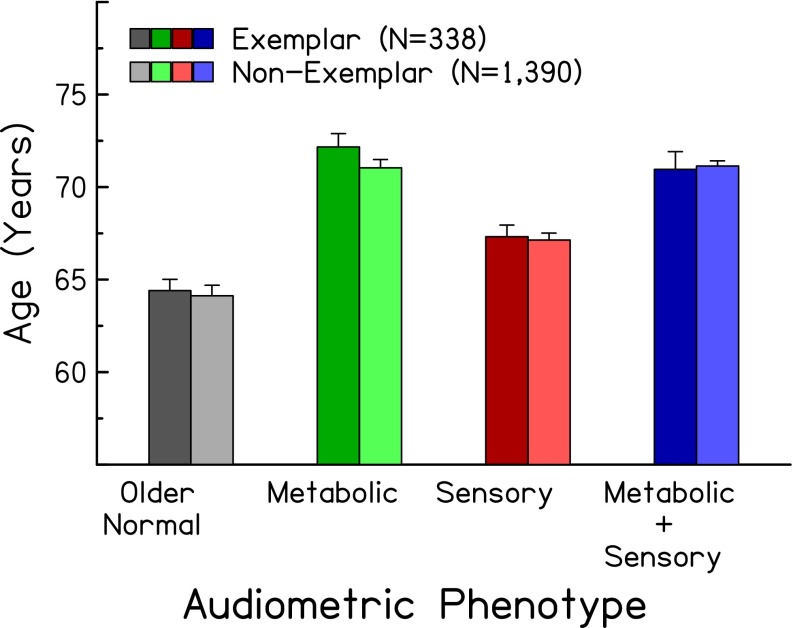
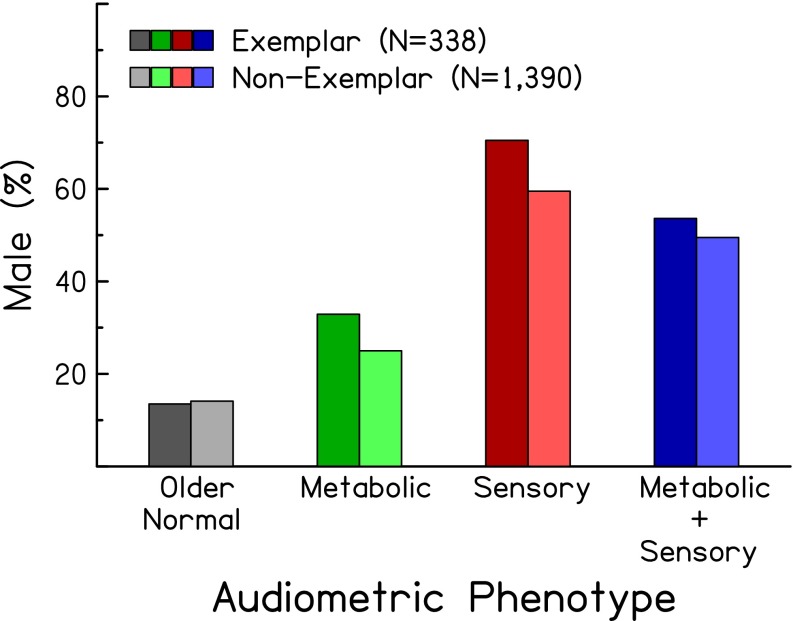
Because the expert identification and automated classification were performed using audiometric data only, subjects’ demographic information can be used to indirectly verify the consistency of the identifications and classifications. Figures 4 and 5 show the mean age and percentage of male subjects, respectively, of exemplars in the four audiometric phenotypes (left, darker bars). As predicted (see right-hand columns of Table 1), subjects classified in the older-normal and sensory phenotypes were younger than those in the metabolic phenotypes (F(1,334) = 52.75, p < 0.0001). Also as predicted, subjects classified in the sensory phenotype were predominately male (Chi-square (df = 1) = 13.13, p < 0.0001). Figure 6 shows the distribution of exemplar audiograms with respect to noise exposure history (left, darker bars). These results show that subjects classified in the sensory phenotype were more likely to have positive noise histories (exposure to gun noise: Chi-square (df = 1) = 14.63, p < 0.0001; power tool use: Chi-square (df = 1) = 24.37, p < 0.00001; incidence of sudden loud noise exposure: Chi-square (df = 1) = 5.29, p = 0.0215) than those classified in the metabolic phenotype.
FIG. 4.
Mean (±1 standard error) ages of exemplars (left, darker bars) and non-exemplars (right, lighter bars) for four audiometric phenotypes.
FIG. 5.
Percentage of male subjects for exemplars (left, darker bars) and non-exemplars (right, lighter bars) for four audiometric phenotypes.
FIG. 6.
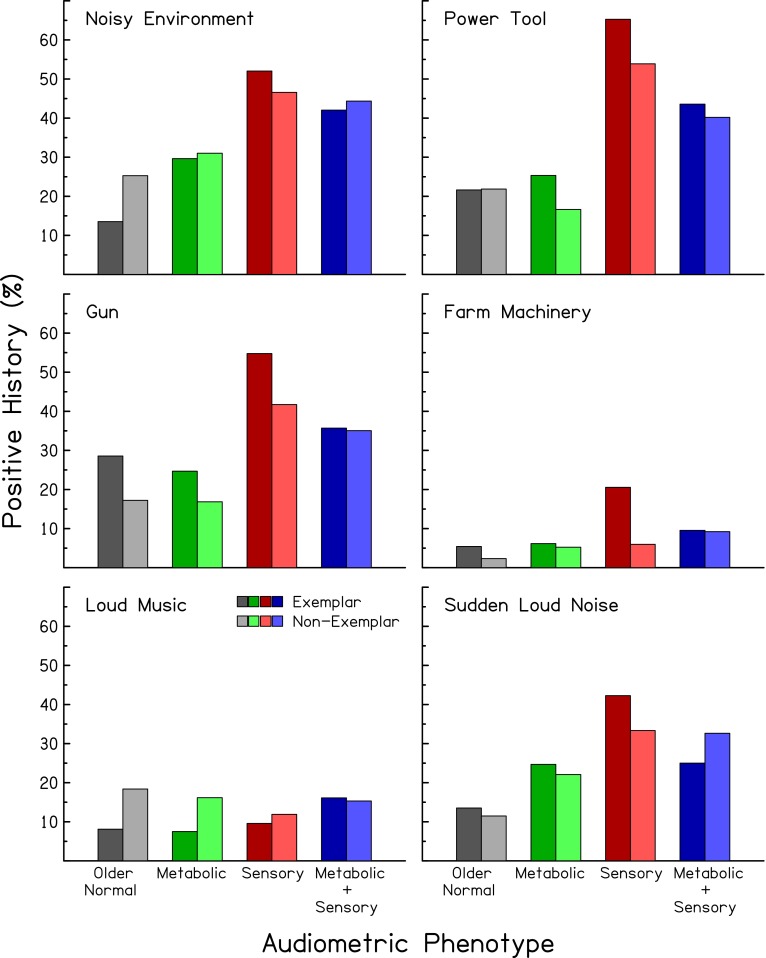
Percentage of subjects reporting positive noise histories for exemplars (left, darker bars) and non-exemplars (right, lighter bars) for four audiometric phenotypes. Each panel reports noise histories for different types of noise exposures.
To explore further the apparent differences in lower frequency thresholds for the metabolic and sensory phenotypes, differences in age and thresholds at higher frequencies for subjects in these two phenotypes were controlled in a repeated measures ANOVA. The results confirmed that thresholds at 0.25–2.0 kHz averaged 16.5 dB better for sensory than metabolic phenotypes (F(1,203) = 276.0, p < 0.001). One hypothesis for this difference is that, for sensory losses, transduction currents are reduced by OHC loss (Salt and Konishi 1979), which has the effect of reducing the load on the EP generator, yielding a higher EP throughout the cochlea. Moreover, there is evidence that EP is most effectively generated in the cochlear base (Wu and Hoshino 1999), which coincides with the location of the OHC lesions in the sensory phenotype. This hypothesis further suggests that sensory loss may mask a metabolic loss until strial degeneration overwhelms this unloading effect. Given that subjects classified in the sensory phenotype were primarily male, these results are not consistent with previous reports of better lower frequency thresholds for females than males (e.g., Gates et al. 1993; Jerger et al. 1993)
Classification of non-exemplars
We examined the extent to which demographic patterns observed for the exemplar phenotypes were present when phenotype classification of the non-exemplar cases was defined by QDA. Using the classification parameters obtained from the exemplars, non-exemplar cases were classified into one of the four phenotypes based on the highest probability estimate. Figures 4, 5, and 6 show comparisons of ages, gender, and noise histories of the exemplars and non-exemplars (darker vs. lighter bars in each figure). Note that the significant gender (Chi-square (df = 1) = 67.38, p < 0.0001), age (F(1,334) = 41.89, p < 0.0001), and noise history associations (gun noise: Chi-square (df = 1) = 39.91, p < 0.0001; power tools: Chi-square (df = 1) = 80.60, p < 0.0001; sudden noise: Chi-square (df = 1) = 8.32, p = 0.00393) with phenotypes in the exemplar cases were also present in the non-exemplar cases that were defined with QDA. The average audiograms of the four phenotypes for the non-exemplar cases are shown in Figure 7. The older-normal phenotype for the non-exemplar cases exhibited slightly elevated (worse) thresholds than those of the exemplars, whereas mean high-frequency thresholds of the metabolic, sensory, and metabolic + sensory phenotypes for the non-exemplars were ∼10 dB lower (better) than those of the exemplars (4.0 kHz: F(1,1720) = 16.96, p < 0.0001; 6.0 kHz: F(1,1720) = 22.48, p < 0.0001; 8.0 kHz: F(1,1720) = 21.73, p < 0.0001). It is likely that these threshold differences relate to the fact that all audiograms were classified into one of the four phenotypes based on the highest probability estimate, and that a small percentage of audiograms would have been more appropriately classified into an “unknown etiology” category.
Discussion
The results of this study suggest that phenotypes of age-related hearing loss can be classified from human audiograms when using results from animal models of age-related hearing loss. Nevertheless, the audiometric phenotypes characterized in this study are highly consistent with expected demographic and noise history patterns that segregate with patterns of hearing loss. Moreover, these associations were confirmed in a second sample of cases that were phenotyped using an automated classification of non-exemplar audiograms. The development and testing of an automated classifier makes it possible to apply the methods used in this study to new samples to further replicate and validate our results, with the long-term goal of evaluating genetic and biological mechanisms of age-related hearing loss in humans. Until that occurs, the phenotypic classifications, and their etiologic foundations based on animal studies of metabolic and sensory loss, should be considered putative in nature.
The challenge in developing classifications for the pathophysiology of age-related hearing loss is that qualitatively distinct but overlapping nonlinear patterns of hearing loss are observed in older adults. This multidimensional problem was addressed in the current study with empirically defined rules based on the contribution of the EP to specific patterns of neural threshold shifts in animal models, coupled with nonlinear supervised classifiers. Findings from quiet-aged and furosemide-exposed gerbils demonstrating specific contributions of EP loss to neural thresholds across frequency (Schmiedt et al. 2002) was a key feature for classifying audiograms and characterizing evidence for metabolic presbyacusis. The relatively limited influence of noise exposure history on lower frequency thresholds provides one phenotypic difference that was leveraged in the current study. While the classification of metabolic presbyacusis in contrast to metabolic + sensory loss becomes problematic when the magnitude of hearing loss increases across frequency, overall magnitude of hearing loss across frequency was important for differentiating older-normal or metabolic + sensory cases from metabolic or sensory cases.
Noise and ototoxic drug exposures in animal studies typically produce OHC loss in the cochlear base and relatively steep slopes of higher frequency thresholds. In humans, it is generally accepted that sensory loss is more common in older males than females, primarily due to differences in occupational and non-occupational noise exposures. The gender ratios and noise exposure histories in Figures 5 and 6 show that subjects classified in the older-normal and metabolic phenotypes were primarily females with negative noise histories, and that subjects classified in the sensory and metabolic + sensory phenotypes were primarily males with positive noise histories. These consistencies between animal models and human data are encouraging signs that the four phenotypes reflect probable etiologies for hearing loss in older human subjects.
The initial framework of this study was that a pre-metabolic phenotype would be prominent in our sample. A pre-metabolic phenotype was initially included to represent audiograms that exhibited early stages of metabolic (EP) loss and a transition between the older-normal and metabolic phenotypes. However, only a small percentage of exemplars were classified into this phenotype, which may be indicative of pre-metabolic decline that begins in middle age. Although subject ages in the current analysis ranged from ∼50 to 98 years, the vast majority of subjects (94.4 %) were ≥60 years. In support of this premise, the average age of the 11 pre-metabolic exemplars was 65.1 years. In addition, a transition from older-normal to metabolic phenotypes that occurs fairly rapidly (a few months to a few years) would yield a relatively small number of pre-metabolic exemplars. Longitudinal studies of additional subjects in their 40s, 50s, and 60s will be necessary to establish the stability of the pre-metabolic phenotype.
The expert human classifier identified 144 of the 865 subjects as having audiograms from the left and right ears that were the same phenotype. Similarly, QDA classified 132 of these 144 subjects (91.6 %) with left and right ears as having the same phenotype. Conversely, 624 of the 865 subjects had left and right ears identified as non-exemplars, but only 60.6 % had their left and right ears classified in the same phenotype. Thus, subjects with similar patterns of hearing loss in left and right ears were more likely to have their audiograms chosen as exemplars by the human expert. This was expected, given that the presumed mechanisms underlying strial and sensory pathology are systemic and would likely affect both ears in similar ways (with the exception of primarily unilateral noise exposures; Wilson 2011). In this study, audiogram classification by QDA for one ear was not informed by data from the other ear. In future studies, probability estimates from both ears may be combined to increase the consistency of classification between ears.
The use of left and right ear data as unique data points enhanced the power of the current study, but should be considered in the context of exemplar classification reliability and validity. Subjects with both ears classified as exemplars were more likely to have consistent audiometric phenotypes matching their age and noise history. This is an important consideration because discriminant functions from QDA, for example, are essentially nonlinear regression functions, which may require independent samples. Thus, including thresholds from left and right ears in the classification process may violate the assumption of independent samples. To address this concern, separate analyses were performed that included only one audiogram per subject and revealed similar classification rates to the original dataset (not shown). From a statistical standpoint, including thresholds from both ears may benefit the modeling process if, in a weighted regression, data with smaller variances are weighted more heavily than data with larger variances because data with smaller variances are more reliable.
The supervised classifiers were used to obtain automated classifications of phenotypes of age-related hearing loss that exhibited a high degree of classification accuracy with the human classifications. While all three classifiers performed similarly, QDA was particularly successful at classifying cases and was selected for the non-exemplar analyses because it is conceptually the most basic classifier of the three and because QDA coefficients can be made available to be used by other groups to classify audiograms of older adults. Importantly, the QDA classifier appeared to transfer to classification of the non-exemplar cases based on the similar average audiometric profiles of common phenotypes from exemplar and non-exemplar cases.
In the current analysis, the classification accuracies and demographic group comparisons were based on classification into the highest probability of group membership. The degree of similarity between a particular audiogram and a schematic pattern (or the likelihood that an audiogram belongs to a particular phenotype) can be expressed with discriminant analysis as a probability using Mahalanobis distance and multinormal distributions (Morrison 1976). High probability classification values indicated that a good match was found between the audiogram and the phenotype’s schematic pattern. For example, Figure 8 shows that exemplar cases received high classification probabilities. As expected, the classifiers exhibited less confidence in the classification of the non-exemplar cases, and a small percentage of cases may reflect unknown etiologies. Probability values may be particularly useful in the context of characterizing and communicating the likelihood for a particular phenotype in patients where the efficacy of interventions is expected to vary depending of the mechanism of hearing loss. Thus, probability values may provide a metric for evaluating interventions with the perspective that one treatment or intervention plan may not benefit all patients to the same degree (Halpin and Rauch 2009; Fig. 2).
FIG. 8.
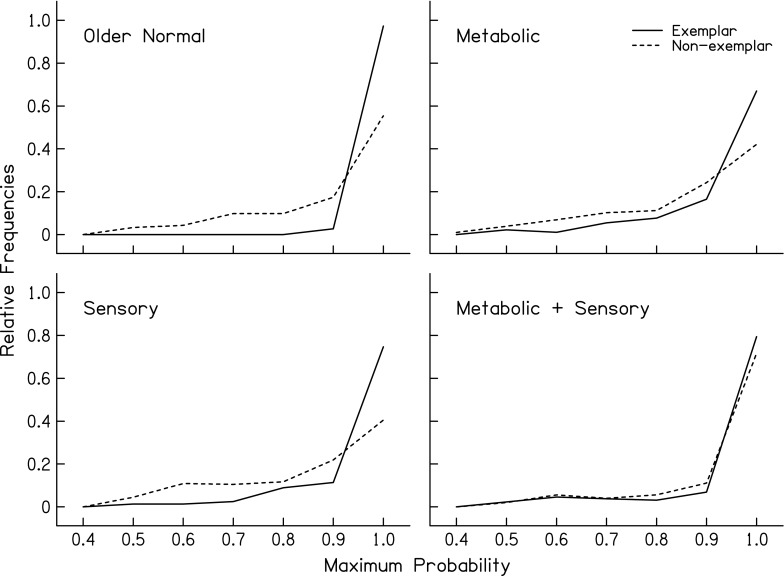
Distributions of the maximum probabilities from quadratic discriminant analysis (QDA) for exemplar and non-exemplar audiograms for four audiometric phenotypes.
Schmiedt et al. (2002) demonstrated that metabolic presbyacusis can be modeled by lateral wall and strial dysfunction that results in a reduced EP. Results from this study further demonstrated that age-related hearing loss can be observed in patterns of audiograms of human subjects that are similar to those observed in gerbil animal models. This is consistent with an assumption that a reduction in the EP may also underlie the characteristic audiograms of older humans. Metabolic hearing loss in animals is not a sensory loss, per se, but results in elevated pure-tone thresholds because cochlear amplifier gain is reduced, especially at higher frequencies. Although OHCs may remain viable, they are not functioning as well as with the EP at its normal, higher state. As a result, the consequences of metabolic loss on auditory function may be quite different than the consequences of pure sensory loss. Future studies will examine predictions from animal models that nonlinearities (such as basilar membrane compression) and otoacoustic emissions are still present in metabolic cases, while relatively diminished in sensory cases.
In the longer term, results can be confirmed with biological markers, such as genetic profiles to provide a framework for comparative analyses beyond affected groups (hearing impaired) vs. non-affected groups (normal hearing). Studies of otopathology from human temporal bones will also help refine and add to the classifications presented in the current study. In particular, an additional phenotype of neural presbyacusis is expected to be observed, but may not have a significant impact on the shape of the audiogram until there is a substantial loss of spiral ganglion architecture (perhaps consistent with the older-normal phenotype in the current classification scheme). Indeed, spiral ganglion cells can incur significant damage without changes in hearing thresholds (e.g., Kujawa and Liberman 2009). Thus, neural presbyacusis may segregate with older-normal, sensory, or mixed metabolic + sensory phenotype, or may have an additive and unique behavioral/biological phenotype, or may reveal itself only through results of suprathreshold auditory measures. With additional replication and validation, the long-term potential for classifiers of phenotypes of age-related hearing loss are (1) advancing our understanding of hearing loss in older adults and (2) informing clinical decisions.
Conclusions
Findings from animal models of age-related hearing loss were used to classify audiograms from older human subjects. Large numbers of audiograms from older human subjects were classified in a two-step process by a human expert familiar with animal models of metabolic and sensory pathology. Physiological findings in quiet-aged and furosemide-exposed gerbils provided the conceptual framework for this study. Exemplar audiometric phenotypes demonstrated demographic and noise exposure history patterns that are consistent with findings from animal studies. Supervised learning methods were used to develop classifiers for audiograms that were based on exemplar cases for hearing loss in older humans as derived from known threshold shifts in animals after documented metabolic or sensory losses. The same audiometric phenotypes were also identified with these classifiers that were applied to non-exemplar audiograms. Importantly, the phenotypes in the non-exemplar data exhibited demographic and noise history patterns that were observed in the exemplar data, thereby providing cross-validation of the phenotypes. Thus, human audiometric phenotypes appear consistent with predictions from animal findings associated with sensory and strial pathology. These results indicate that empirically derived models of hearing loss, such as human audiometric phenotypes, can be used to meaningfully classify audiometric data into groups that reflect probable etiologies of age-related hearing loss, which can be further validated in future studies of auditory function and biological markers.
Acknowledgments
This work was supported (in part) by grant P50 DC00422 from NIH/NIDCD, with a supplement from the American Recovery and Reinvestment Act. The project also received support from the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCRR Grant number UL1 RR029882. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR14516 from the National Center for Research Resources, National Institutes of Health. Assistance by Jayne B. Ahlstrom, John H. Mills, and Bradley A. Schulte is gratefully acknowledged.
Contributor Information
Judy R. Dubno, Phone: +843-792-7978, FAX: +843-0792-7736, Email: dubnojr@musc.edu
Mark A. Eckert, Email: eckert@musc.edu
Fu-Shing Lee, Email: leefs@musc.edu.
Lois J. Matthews, Email: matthelj@musc.edu
Richard A. Schmiedt, Email: schmiera@musc.edu
References
- Allen PD, Eddins DA. Presbycusis phenotypes form a heterogeneous continuum when ordered by degree and configuration of hearing loss. Hear Res. 2010;264:10–20. doi: 10.1016/j.heares.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American National Standards Institute (1969) Specification for audiometers. ANSI S3.6-1969, American National Standards Institute, New York
- American National Standards Institute (1989) Specification for audiometers. ANSI S3.6-1989, American National Standards Institute, New York
- American National Standards Institute (1996) Specification for audiometers. ANSI S3.6-1996, American National Standards Institute, New York
- American National Standards Institute (2004) Specification for audiometers. ANSI S3.6-2004, American National Standards Institute, New York
- American National Standards Institute (2010) Specification for audiometers. ANSI S3.6-2010, American National Standards Institute, New York
- Guidelines for manual pure-tone threshold audiometry. Rockville, MD: American Speech–Language–Hearing Association; 2005. [Google Scholar]
- Breiman L. Random forests. Mach Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- Chang CC, Lin CJ (2001). LIBSVM: a library for support vector machines. Software available http://www.csie.ntu.edu.tw/∼cjlin/papers/libsvm
- Ciletti L, Flamme GA. Prevalence of hearing impairment by gender and audiometric configuration: results from the National Health and Nutrition Examination Survey (1999–2004) and The Keokuk County Rural Health Study (1994–1998) J Am Acad Audiol. 2008;19:672–685. doi: 10.3766/jaaa.19.9.3. [DOI] [PubMed] [Google Scholar]
- Cooper N, Rhode W. Mechanical responses to two-tone distortion products in the apical and basal turns of the mammalian cochlea. J Neurophysiol. 1997;78:261–270. doi: 10.1152/jn.1997.78.1.261. [DOI] [PubMed] [Google Scholar]
- Crowe SJ, Guild SR, Polvogt LM. Observations on the pathology of high tone deafness. Bull Johns Hopkins Hosp. 1934;54:315–379. [Google Scholar]
- Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol. 1978;41:365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- Davis H. An active process in cochlear mechanics. Hear Res. 1983;9:79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- Dayal VS, Bhattacharyya TK. Comparative study of age-related cochlear hair cell loss. Ann Otol Rhinol Laryngol. 1986;95:510–513. doi: 10.1177/000348948609500513. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Lee FS, Klein AJ, Matthews LJ, Lam CF. Confidence limits for maximum word-recognition scores. J Speech Hear Res. 1995;38:490–502. doi: 10.1044/jshr.3802.490. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Lee FS, Matthews LJ, Mills JH. Age-related and gender-related changes in monaural speech recognition. J Speech Lang Hear Res. 1997;40:444–452. doi: 10.1044/jslhr.4002.444. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Lee FS, Matthews LJ, Ahlstrom JB, Horwitz AR, Mills JH. Longitudinal changes in speech recognition in older persons. J Acoust Soc Am. 2008;123:462–475. doi: 10.1121/1.2817362. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cobb JL, D'Agostino RB, Wolf PA. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Head Neck Surg. 1993;119:156–161. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- Glorig A, Nixon J. Hearing loss as a function of age. Laryngoscope. 1962;72:1596–1610. doi: 10.1288/00005537-196211000-00006. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Smythe BJ, Lam CF, Boettcher FA, Schmiedt RA (1997) Decline in the endocochlear potential corresponds to decreased Na,K-ATPase activity in the lateral wall of quiet-aged gerbils. Hear Res 108:9–16 [DOI] [PubMed]
- Gruber J, Schaffer S, Halliwell B (2008) The mitochondrial free radical theory of ageing—where do we stand? Front Biosci 13:6554–6579 [DOI] [PubMed]
- Hall M, Frank E, Holmes G, Pfahringer B, Reutemann P, Witten IH. The WEKA Data Mining Software: an update. SIGKDD Explorations. 2009;11(1):10–18. doi: 10.1145/1656274.1656278. [DOI] [Google Scholar]
- Haller S, Lovblad KO, Giannakopoulos P. Principles of classification analyses in mild cognitive impairment (MCI) and Alzheimer disease. J Alzheimers Dis. 2011;26(Suppl 3):389–394. doi: 10.3233/JAD-2011-0014. [DOI] [PubMed] [Google Scholar]
- Halpin C, Rauch SD. Hearing aids and cochlear damage: the case against fitting the pure tone audiogram. Otolaryngol Head Neck Surg. 2009;140:629–632. doi: 10.1016/j.otohns.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Hellstrom LI, Schmiedt RA. Compound action potential input/output functions in young and quiet-aged gerbils. Hear Res. 1990;50:163–174. doi: 10.1016/0378-5955(90)90042-N. [DOI] [PubMed] [Google Scholar]
- Hellstrom LI, Schmiedt RA. Rate-level functions of auditory-nerve fibers have similar slopes in young and old gerbils. Hear Res. 1991;53:217–221. doi: 10.1016/0378-5955(91)90055-E. [DOI] [PubMed] [Google Scholar]
- Hellstrom LI, Schmiedt RA. Measures of tuning and suppression in single-fiber and whole-nerve responses in young and quiet-aged gerbils. J Acoust Soc Am. 1996;100:3275–3285. doi: 10.1121/1.417211. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe R. The threshold of hearing as a function of age. Acustica. 1959;9:303–308. [Google Scholar]
- Jerger J, Chmiel R, Stach B, Spretjnak M. Gender affects audiometric shape in presbyacusis. J Am Acad Audiol. 1993;4:42–49. [PubMed] [Google Scholar]
- Kohavi R (1995) A study of cross-validation and bootstrap for accuracy estimation and model selection. In: Proceedings of the Fourteenth International Joint Conference on Artificial Intelligence San Francisco, CA: Morgan Kaufmann, pp 1137–1143
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Matthews LJ, Mills JH, Dubno JR, Adkins WY. Gender-specific effects of medicinal drugs on hearing levels of older persons. Otolaryngol Head Neck Surg. 1998;118:221–227. doi: 10.1016/S0194-5998(98)80020-X. [DOI] [PubMed] [Google Scholar]
- Lee FS, Matthews LJ, Dubno JR, Mills JH. Longitudinal study of pure-tone thresholds in older persons. Ear Hear. 2005;26:1–11. doi: 10.1097/00003446-200502000-00001. [DOI] [PubMed] [Google Scholar]
- Leske MC. Prevalence estimates of communicative disorders in the US. ASHA. 1981;23:229–237. [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- Lin FR, Niparko JK, Luigi Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med. 2011;171:1851–1853. doi: 10.1001/archinternmed.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews LJ, Lee FS, Mills JH, Dubno JR. Extended high-frequency thresholds in older adults. J Speech Lang Hear Res. 1997;40:208–214. doi: 10.1044/jslhr.4001.208. [DOI] [PubMed] [Google Scholar]
- Mills DM. Differential responses to acoustic damage and furosemide in auditory brainstem and otoacoustic emission measures. J Acoust Soc Am. 2003;113:914–924. doi: 10.1121/1.1535942. [DOI] [PubMed] [Google Scholar]
- Mills DM. Determining the cause of hearing loss: differential diagnosis using a comparison of audiometric and otoacoustic emission responses. Ear Hear. 2006;27:508–525. doi: 10.1097/01.aud.0000233885.02706.ad. [DOI] [PubMed] [Google Scholar]
- Mills DM, Schmiedt RA. Metabolic presbycusis: differential changes in auditory brainstem and otoacoustic emission responses with chronic furosemide application in the gerbil. J Assoc Res Otolaryngol. 2004;5:1–10. doi: 10.1007/s10162-003-4004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JH, Schmiedt RA, Kulish LF. Age-related changes in auditory potentials of Mongolian gerbil. Hear Res. 1990;46:201–210. doi: 10.1016/0378-5955(90)90002-7. [DOI] [PubMed] [Google Scholar]
- Mills JH, Lee FS, Dubno JR, Boettcher FA. Interactions between age-related and noise-induced hearing loss. In: Axelsson A, Borchgrevink H, Hamernik R, Hellstrom P, Henderson D, Salvi R, editors. Scientific basis of noise-induced hearing loss. New York: Thieme Medical; 1996. pp. 193–212. [Google Scholar]
- Mills JH, Schmiedt RA, Schulte BA, Dubno JR. Age-related hearing loss: a loss of voltage, not hair cells. Semin Hear. 2006;27:228–236. doi: 10.1055/s-2006-954849. [DOI] [Google Scholar]
- Morrison DF. Multivariate statistical methods. 2. New York: McGraw-Hill; 1976. [Google Scholar]
- Nadol JB. Disorders of aging. In: Merchant SN, Nadol JB, editors. Schuknecht’s pathology of the ear. 3. Shelton CT: PHPH-USA; 2010. pp. 431–475. [Google Scholar]
- Nelson E, Hinojosa R. Presbycusis: a human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Laryngoscope. 2006;116:1–12. doi: 10.1097/01.mlg.0000236089.44566.62. [DOI] [PubMed] [Google Scholar]
- Orrù G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using support vector machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci Biobehav Rev. 2012;36:1140–1152. doi: 10.1016/j.neubiorev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev. 2001;81:1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC. Furosemide alters organ of corti mechanics: evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci. 1991;11:1057–1067. doi: 10.1523/JNEUROSCI.11-04-01057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ (1983) Origin of the receptor potential in inner hair cells of the mammalian cochlea—evidence for Davis’ theory. Nature 301:334–336 [DOI] [PubMed]
- Ryan A, Dallos P, McGee T. Psychophysical turning curves and auditory thresholds after hair cell damage in the chinchilla. J Acoust Soc Am. 1979;66:370–378. doi: 10.1121/1.383194. [DOI] [PubMed] [Google Scholar]
- Salt AN, Konishi T. Effects of noise on cochlear potentials and endolymph potassium concentration recorded with potassium-selective electrodes. Hear Res. 1979;1:343–363. doi: 10.1016/0378-5955(79)90005-4. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. Acoustic injury and the physiology of hearing: a review and tutorial. J Acoust Soc Am. 1984;76:1293–1317. doi: 10.1121/1.391446. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. Effects of aging on potassium homeostasis and the endocochlear potential in the gerbil. Hear Res. 1996;102:125–132. doi: 10.1016/S0378-5955(96)00154-2. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. The physiology of cochlear presbyacusis. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. The aging auditory system. New York: Springer; 2010. pp. 9–38. [Google Scholar]
- Schmiedt RA, Mills JH, Adams JC. Tuning and suppression in auditory nerve fibers of aged gerbils raised in quiet or noise. Hear Res. 1990;45:221–236. doi: 10.1016/0378-5955(90)90122-6. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Lang H, Okamura H, Schulte BA. Effects of furosemide applied chronically to the round window: a model of metabolic presbycusis. J Neurosci. 2002;22:9643–9650. doi: 10.1523/JNEUROSCI.22-21-09643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Zwislocki JJ. Effects of hair cell lesions on responses of cochlear nerve fibers. II. Single- and two-tone intensity functions in relation to tuning curves. J Neurophysiol. 1980;43:1390–1405. doi: 10.1152/jn.1980.43.5.1390. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Zwislocki JJ, Hamernik RP. Effects of hair cell lesions on responses of cochlear nerve fibers. I. Lesions, tuning curves, two-tone inhibition, and responses to trapezoidal-wave patterns. J Neurophysiol. 1980;43:1367–1389. doi: 10.1152/jn.1980.43.5.1367. [DOI] [PubMed] [Google Scholar]
- Schuknecht H. Further observations on the pathology of presbycusis. Arch Otolaryngol. 1964;80:369–382. doi: 10.1001/archotol.1964.00750040381003. [DOI] [PubMed] [Google Scholar]
- Schuknecht H. Pathology of the ear. Cambridge: Harvard University Press; 1974. [Google Scholar]
- Schuknecht H, Gacek M. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102(Suppl 158):1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Schulte BA, Schmiedt RA. Lateral wall Na, K-ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hear Res. 1992;61:35–46. doi: 10.1016/0378-5955(92)90034-K. [DOI] [PubMed] [Google Scholar]
- Sewell WF. The effects of furosemide on the endocochlear potential and auditory-nerve fiber tuning curves in cats. Hear Res. 1984;14:305–314. doi: 10.1016/0378-5955(84)90057-1. [DOI] [PubMed] [Google Scholar]
- Sha S-H, Kanicki A, Dootz G, Talaska AE, Halsey K, Dolan D, Altschuler R, Schacht J. Age-related auditory pathology in the CBA/J mouse. Hear Res. 2008;243:87–94. doi: 10.1016/j.heares.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga F, Lindsay J. Histopathological observations of presbyacusis. Ann Otol Rhinol Laryngol. 1976;85:169–184. doi: 10.1177/000348947608500201. [DOI] [PubMed] [Google Scholar]
- Tarnowski B, Schmiedt RA, Hellstrom LI, Lee FA, Adams JC. Age-related changes in cochleas of Mongolian gerbils. Hear Res. 1991;54:123–134. doi: 10.1016/0378-5955(91)90142-V. [DOI] [PubMed] [Google Scholar]
- Touw WG, Bayjanov JR, Overmars L, Backus L, Boekhorst J, Wels M, van Hijum SA. Data mining in the life sciences with random forest: a walk in the park or lost in the jungle? Brief Bioinform. 2012 doi: 10.1093/bib/bbs034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RH. Some observations on the nature of the audiometric 4000 Hz notch: data from 3430 veterans. J Am Acad Audiol. 2011;22:23–33. doi: 10.3766/jaaa.22.1.4. [DOI] [PubMed] [Google Scholar]
- Wu R, Hoshino T. Changes in off-lesion endocochlear potential following localized lesion in the lateral wall. Acta Otolaryngol. 1999;119:550–554. doi: 10.1080/00016489950180775. [DOI] [PubMed] [Google Scholar]
- Yuen KC, McPherson B. Audiometric configurations of hearing impaired children in Hong Kong: implications for amplification. Disabil Rehabil. 2002;24:904–913. doi: 10.1080/09638280210148602. [DOI] [PubMed] [Google Scholar]