Abstract
Sensorineural hearing loss is a normal consequence of aging and results from a variety of extrinsic challenges such as excessive noise exposure and certain therapeutic drugs, including the aminoglycoside antibiotics. The proximal cause of hearing loss is often death of inner ear hair cells. The signaling pathways necessary for hair cell death are not fully understood and may be specific for each type of insult. In the lateral line, the closely related aminoglycoside antibiotics neomycin and gentamicin appear to kill hair cells by activating a partially overlapping suite of cell death pathways. The lateral line is a system of hair cell-containing sense organs found on the head and body of aquatic vertebrates. In the present study, we use a combination of pharmacologic and genetic manipulations to assess the contributions of p53, Bax, and Bcl2 in the death of zebrafish lateral line hair cells. Bax inhibition significantly protects hair cells from neomycin but not from gentamicin toxicity. Conversely, transgenic overexpression of Bcl2 attenuates hair cell death due to gentamicin but not neomycin, suggesting a complex interplay of pro-death and pro-survival proteins in drug-treated hair cells. p53 inhibition protects hair cells from damage due to either aminoglycoside, with more robust protection seen against gentamicin. Further experiments evaluating p53 suggest that inhibition of mitochondrial-specific p53 activity confers significant hair cell protection from either aminoglycoside. These results suggest a role for mitochondrial p53 activity in promoting hair cell death due to aminoglycosides, likely upstream of Bax and Bcl2.
Keywords: aminoglycoside, ototoxicity, neuromast, hearing loss, Danio rerio
Introduction
Aminoglycosides are a class of potent antibiotics with broad spectrum activity against gram-negative bacteria. Aminoglycoside use in developed countries is widespread in cystic fibrosis patients and premature infants; worldwide, these drugs are more widely used due to their low cost and high efficacy against a variety of severe or recalcitrant bacterial infections, including drug-resistant tuberculosis (Rizzi and Hirose 2007; Durante-Mangoni et al. 2009). Aminoglycoside ototoxicity, resulting in hearing loss and balance disorders, is a serious clinical issue for up to 20 % of patients who received these life-sustaining antibiotics (reviewed in Rizzi and Hirose 2007; Xie et al. 2011). Despite early recognition of the ototoxic side effects of aminoglycoside treatment, the cellular pathways underlying aminoglycoside-induced sensorineural hearing loss and balance disorders are not fully understood (Cheng et al. 2005; Schacht and Hawkins 2006; Warchol 2010).
Aminoglycoside ototoxicity likely involves activation of several cell death pathways, as morphological features of both apoptotic-like and non-apoptotic modes of cell death are visible in a single sensory organ (Li et al. 1995; Forge and Li 2000; Owens et al. 2007; Taylor et al. 2008). Caspase inhibition protects hair cells from neomycin toxicity in the mouse utricle in vitro and from gentamicin otoxicity in chick in vivo, suggesting that activation of classical apoptotic-like pathways may lead to hair cell death in some tissues or species (Cheng et al. 2002; Cunningham et al. 2002; Matsui et al. 2003; Kaiser et al. 2008). In vivo experiments in rats and zebrafish (Danio rerio), however, reveal evidence for caspase-independent hair cell death (Jiang et al. 2006; Coffin et al. 2013), suggesting that alternative cell death pathways may be involved.
Previous reports by our group and others suggest that different aminoglycosides appear to activate distinct sets of cell death pathways (Coffin et al. 2009, 2013; Owens et al. 2009; Mazurek et al. 2012; Vlasits et al. 2012). In the lateral line of larval zebrafish, neomycin induces rapid hair cell death in a dose-dependent manner, while gentamicin activates both rapid and slower cell death responses (Coffin et al. 2009; Owens et al. 2009). Studies with cell death inhibitors suggest that the rapid phase of death induced by neomycin or gentamicin represents a set of shared cell signaling pathways, while the slower phase activated only by gentamicin represents a distinct set of pathways (Vlasits et al. 2012; Coffin et al. 2013). These data suggest that comparing mechanisms of hair cell death activated by different aminoglycosides in a single system is warranted in order to develop informed interventions for patients receiving antibiotic treatment.
The zebrafish lateral line system has been established as a useful preparation for studies of hair cell death (e.g., Williams and Holder 2000; Harris et al. 2003; Ton and Parng 2005; Coffin et al. 2010). Lateral line hair cells cluster in sensory organs called neuromasts that are externally located on the head and body of the animal (Metcalf et al. 1985; Coombs et al. 1989; Raible and Kruse 2000). This sensory system is used to detect near-field water movement and mediates a number of important behaviors including predator avoidance, prey capture, and orientation to flow (Dijkgraaf 1963; Montgomery and MacDonald 1987; Montgomery et al. 1997; Coombs et al. 2001; New et al. 2001; Suli et al. 2012). Lateral line hair cells and mammalian inner ear hair cells exhibit similar responses to ototoxic drugs, making the lateral line a suitable preparation for ototoxicity studies (Harris et al. 2003; Ou et al. 2007; Owens et al. 2007; Hirose et al. 2011). In larval zebrafish, hair cells in the lateral line are relatively mature by 5 days post-fertilization (dpf) and they respond robustly to aminoglycoside exposure (Murakami et al. 2003; Santos et al. 2006). Given the small size, ease of hair cell labeling and assessment, and the large number of larvae that can be quickly generated by this fecund species, the zebrafish lateral line system provides a robust platform for quantitative assessment of ototoxin-induced hair cell death.
The present study examines the roles of the mitochondrial-associated cell death proteins Bax and Bcl2 and the tumor suppressor protein p53 in neomycin- and gentamicin-induced hair cell toxicity. The roles for these proteins were initially identified in a screen of a custom cell death inhibitor library for modulators of aminoglycoside-induced hair cell death (Coffin et al. 2013). Bax is a member of the Bcl2 family that acts by forming channels in the mitochondrial outer membrane, facilitating cytoplasmic translocation of mitochondrial proteins such as cytochrome c and activation of downstream cell death signaling (Putcha et al. 1999; Wei et al. 2001; Scorrano and Korsemeyer 2003). Through this route, Bax is closely associated with caspase activation and classical apoptosis (reviewed in van Delft and Huang 2006; Tait and Green 2010). However, Bax is also necessary for mitochondrial changes and activation of downstream cell death pathways in a caspase-independent manner (Middleton et al. 2000; Besirli et al. 2003; Cheung et al. 2005). Bcl2 family members have been linked to aminoglycoside ototoxicity in rodent models and noise-induced hearing loss (Cunningham et al. 2004; Vicente-Torres and Schacht 2006; Yamashita et al. 2008; Pfannenstiel et al. 2009).
p53 is best recognized as a transcription factor with hundreds of downstream targets, playing a central role in DNA repair, cell cycle regulation, and cell death (reviewed in Pietsch et al. 2008; Levine and Oren 2009). However, transcription-independent p53 activity is also important in many cell death processes by its association with cytoplasmic or mitochondrial proteins, including members of the Bcl2 family (Moll et al. 2005; Chipuk and Green 2006; Green and Kroemer 2009). p53 is implicated in cisplatin ototoxicity (Zhang et al. 2003), but a potential role in aminoglycoside-induced hair cell death has not been explored. The current study uses a combination of genetic and pharmacologic modulation of p53, Bcl2, and Bax to demonstrate a differential contribution of these three molecules in hair cell death following neomycin or gentamicin exposure in the zebrafish lateral line.
Methods
Animals
Larval zebrafish (*AB strain) were acquired through paired or group mating and raised at 28.5 °C in Petri dishes containing embryo medium (see Westerfield 2000). Animals were raised in either the University of Washington or Washington State University Vancouver zebrafish facilities. As hair cells in zebrafish younger than 5 dpf are relatively resistant to aminoglycoside toxicity (Murakami et al. 2003; Santos et al. 2006), all experiments used 5–6 dpf larvae. All procedures were approved by the University of Washington and Washington State University Animal Care and Use Committees.
Drug treatments
Neomycin and gentamicin solutions (10 and 50 mg/ml, respectively) were acquired from Sigma-Aldrich (St. Louis, MO, USA) and diluted in E2 embryo medium (EM) containing 1 mM MgSO4, 120 μM KH2PO4, 74 μM Na2HPO4, 1 mM CaCl2, 500 μM KCl, 15 mM NaCl, and 500 μM NaHCO3 in dH2O (Westerfield 2000). All drug treatments were performed by adding the compound(s) to EM and allowing the fish to swim freely during treatment. Fish were treated with one of two aminoglycoside exposure paradigms, defined here as either “acute” or “continuous.” Acute exposure consisted of a 30-min incubation in 0–400 μM aminoglycoside followed by four rinses in fresh EM and a 60-min recovery period in EM with no drug present. Continuous exposure was performed by incubating the larvae in 0–400 μM aminoglycoside for 6 h. These exposure times were selected based on the time course of hair cell death described previously (Owens et al. 2009). Experiments where treatment times deviated from this two paradigm system (e.g., washout experiments) are indicated in the text.
We used the following compounds to modulate aminoglycoside-induced hair cell death: the Bax channel blocker (±)-1-(3,6-dibromocarbazol-9-yl)-3-piperazin-1-yl-propan-2-ol, bis TFA (Bombrun et al. 2003), the p53 inhibitors pifithrin-α (PFTα) (Komarov et al. 1999; Endo et al. 2006) and pifithrin-μ (PFTμ) (Strom et al. 2006), and the Mdm2 inhibitor nutlin-3a (Vassilev et al. 2004). All inhibitors were purchased from EMD Millipore (Darmstadt, Germany) and dissolved in DMSO. Fish were treated with inhibitor for 1 h, then cotreated with inhibitor and aminoglycoside using either the acute or continuous exposure paradigm described above. Control animals were treated with 0.1–1 % DMSO (to match the DMSO concentration in the inhibitor treatment) in combination with the appropriate aminoglycoside. Inhibitors were initially used within a 0–100-μM range in order to determine the concentration that optimally affected hair cell death without causing hair cell or fish morbidity. Once an optimal concentration was determined for each inhibitor, it was used in subsequent experiments.
Hair cell assessment
Hair cell survival was either assessed by vital dye labeling in living larvae or by immunocytochemistry in fixed fish. For vital dye labeling, fish were incubated for 15 min in 0.005 % 2-(4-(dimethylamino)styryl)-N-ethylpyridinium iodide (DASPEI) (Life Technologies, Grand Island, NY, USA), a mitochondrial dye that is specific for lateral line hair cells using our incubation protocol (Harris et al. 2003; Owens et al. 2007). Excess DASPEI was removed with two rinses in fresh EM and the fish were then anesthetized with 0.001 % MS-222 (Sigma-Aldrich). Fish were viewed with a Leica MZFLIII fluorescent stereomicroscope and ×50 magnification. We quantified hair cell survival using a scoring system based on the relative fluorescent intensity of DASPEI labeling for each of 10 head neuromasts per fish (Harris et al. 2003). A neuromast with intense labeling was scored “2,” one with dim labeling was scored “1,” and one with no labeling was scored “0,” yielding a score of 0–20 for each fish. We assessed the same 10 neuromasts for each fish, which have highly stereotyped positioning in 5–6 dpf zebrafish (Metcalf et al. 1985; Raible and Kruse 2000). DASPEI assessment was performed for 7–14 fish per treatment combination.
Immunocytochemical analysis of hair cells was performed using the optimal concentration of each inhibitor (as determined by DASPEI assessment) with a single concentration of either neomycin (acute exposure) or gentamicin (acute or continuous exposure). Fish were euthanized by cold-water bath and fixed in 4 % paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4) for either 1 h at room temperature or overnight at 4 °C. Fish were then rinsed in PBS, blocked in PBS supplemented with 0.1 % Triton-X and 5 % normal goat serum (both from Sigma-Aldrich), and incubated at 4 °C overnight in antiparvalbumin antibody (mouse monoclonal antibody from EMD Millipore). Primary antibody was diluted 1:500 in PBS with 0.1 % Triton-X and 1 % goat serum. Fish were then rinsed in PBS + 0.1 % Triton-X and incubated in Alexa 488 or 568 goat antimouse secondary antibody (Life Technologies). Following additional rinses in PBS + 0.1 % Triton-X and PBS, fish were stored in 1:1 PBS/glycerol at 4 °C.
Fish with labeled hair cells were mounted on bridged coverslips and viewed with a Zeiss Axioplan 2ie epifluorescent microscope with a ×40 objective. Hair cell counts were performed in seven neuromasts per fish (SO1, SO2, IO1, IO2, IO3, OP1, and M2; see Raible and Kruse 2000 for neuromast nomenclature), and counts were summed to arrive at one value per fish. Hair cell counts were performed for 8–10 fish per treatment.
Genetic manipulation of cell death pathways
The p53zdf1 line contains a point mutation that results in an amino acid change (M214K) in the DNA binding domain of p53 and a loss of transcriptional activity, as measured by a p53 transactivation assay (Berghmans et al. 2005). Homozygous p53zdf1 fish were obtained through the Zebrafish International Resource Center and bred for these experiments. In order to confirm identity, fish were genotyped by PCR using the protocol described in Berghmans et al. (2005). Briefly, genomic DNA was extracted from tail fin clips of breeders or entire larvae (posttreatment) and PCR-amplified using the following primer pair: forward ACA TGA AAT TGC CAG AGT ATG TGT C; reverse TCG GAT AGC CTA GTG CGA GC. PCR products were digested with MboII, which specifically cleaves a restriction site in p53zdf1 mutants. DNA from a subset of fish was also sequenced to confirm genotyping results. Dose–response experiments with p53zdf1 fish were conducted as described above for neomycin or gentamicin treatment. The p53zdf1 fish are maintained as a homozygous line, so wild-type siblings were not available as controls. As this mutation occurs on an AB background, age-matched wild-type *AB fish, which are genetically similar to AB fish, were used as controls. Similar sensitivity to aminoglycoside-induced hair cell death has been demonstrated in multiple fish strains, providing confidence that small genetic differences between wild-type lines will not confound our results (e.g., Williams and Holder 2000; Harris et al. 2003).
In order to determine the effect of overexpressing the cell survival protein Bcl2 on hair cell toxicity, we created a transgenic line using the Tol2 system and Life Technologies’ Gateway cloning architecture. The zebrafish Bcl2 coding sequence, fused to the 3′ end of EGFP, was kindly provided by Dr. A. T. Look (Langenau et al. 2005). This fusion gene was PCR-amplified with primers containing the appropriate attB sites for cloning into the Gateway middle entry vector (see Kwan et al. 2007). PCR was performed with Phusion DNA polymerase (New England BioLabs, Ipswich, MA, USA) using forward primer GGGGACAAGTTTGTACAAAAAAGCAGGCTGCGCCACCATGGTGAGCAAGGGCGAGG and reverse primer GGGGACCACTTTGTACAAGAAAGCTGGGTTCACTTCTGAGCAAAAAAGGCTCC. The resulting PCR product was cloned into pME-MCS. The final vector was constructed by Gateway cloning the zebrafish myo6b promoter (Kindt et al. 2012; kindly provided by the Drs. Kindt and Nicolson), pME-EGFP-Bcl2, and a 3′ polyadenylation signal into the destination vector pDestTol2CG2, which contains the cmlc2:EGFP transgenesis marker. This construct, along with transposase mRNA, was injected into *AB zebrafish embryos at the one-cell stage. Transgene-expressing offspring were raised to adulthood and crossed to generate a stable line. Animals used in the present experiments are from the F2 generation. Tg(myo6b:EGFP-bcl2) larvae were treated with aminoglycosides as described above to assess the effect of Bcl2 overexpression on hair cell survival. As the GFP interferes with assessment of DASPEI fluorescence intensity, all experiments in transgenic animals were conducted using counts of antiparvalbumin-labeled hair cells.
Data analysis
Data were analyzed with GraphPad Prism (V. 5.0) using either one- or two-way ANOVA as appropriate followed by Bonferroni-corrected post hoc analysis. For graphical presentation, data were normalized to untreated controls such that 100 % represents hair cell survival in control animals. All data are presented as mean ± 1 SD.
Results
Bax inhibition protects hair cells from neomycin toxicity
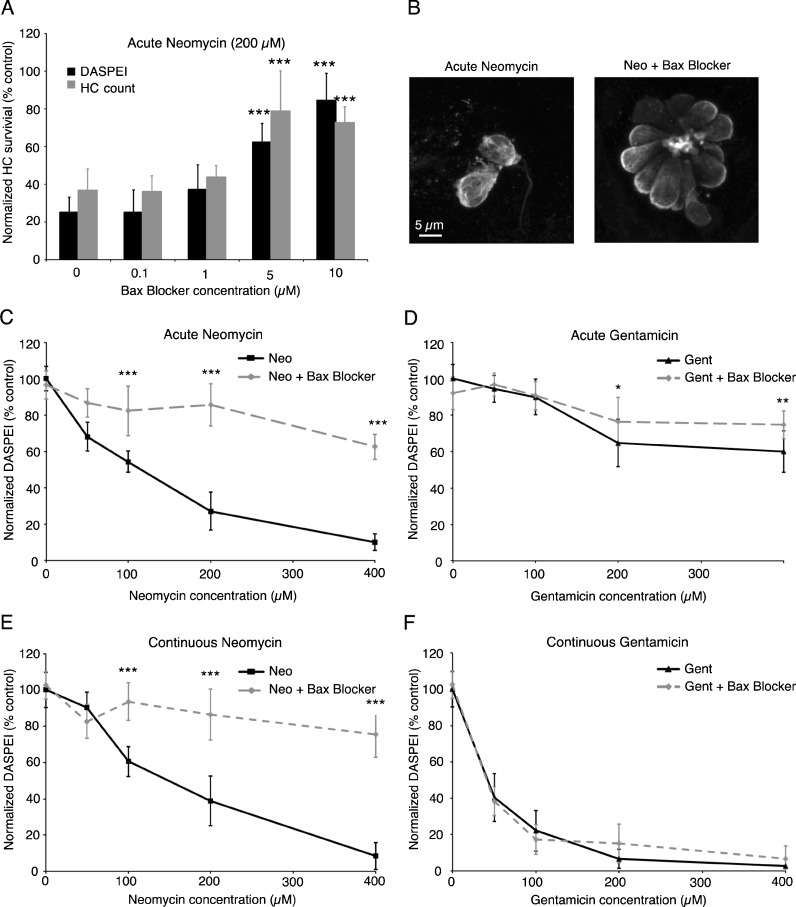
Our previous screen of a cell death inhibitor library suggested that a Bax channel blocker conferred protection from aminoglycoside toxicity (Coffin et al. 2013). Here, we fully characterize this protective effect. As seen in Figure 1, Bax inhibition robustly protects hair cells from neomycin toxicity. Protection is apparent using DASPEI scoring or with direct counts of antiparvalbumin-labeled hair cells, as seen by the confocal images in Figure 1B. Bax inhibition protects hair cells from damage with acute or continuous neomycin with no apparent differences due to exposure time. In contrast, Bax inhibition appears to only provide modest protection from acute gentamicin damage and no hair cell protection during continuous gentamicin exposure. These results support our hypothesis that neomycin and acute gentamicin exposure may activate related suites of cell death pathways but that additional pathways are activated during continuous gentamicin treatment. Therefore, the remainder of this paper compares acute neomycin damage with both acute and continuous gentamicin treatments. We do not show additional experiments with continuous neomycin because our previous experiments suggest that there are no differences in cell death pathway activation between acute and continuous neomycin exposures (Fig. 1, Owens et al. 2009; Coffin et al. 2013).
FIG. 1.
Bax inhibition protects hair cells from neomycin-induced hair cell death. (A) The Bax channel blocker confers dose-dependent protection from 200 μM neomycin, with 5 μM Bax blocker providing significant protection (one-way ANOVA, DASPEI scoring: F(4, 53) = 55.02, P < 0.001; HC count: F(4, 32) = 12.89, P < 0.001) without any overt toxicity to hair cells or fish health. Quantification of hair cell survival using DASPEI scoring (black bars) closely matches direct counts of labeled hair cells (gray bars). (B) Confocal images of antiparvalbumin-labeled hair cells demonstrate that protected hair cells have normal morphology. The scale bar in the left panel applies to both images. (C–F) 5 μM Bax channel blocker robustly protects hair cells from neomycin damage using either acute (C) or continuous (E) exposure paradigms. Limited protection is observed from (D) acute gentamicin while no protection was noted with (F) continuous gentamicin exposure. Two-way ANOVA analyses are as follows: acute neomycin F(1, 09) = 247.5, P < 0.001; acute gentamicin F(1, 116) = 6.29, P = 0.01; continuous neomycin F(1, 111) = 227.3, P < 0.001; continuous gentamicin F(1, 112) = 1.08, P = 0.30. Asterisks indicate significant differences from neomycin-only controls (A) or significant pairwise differences (C–F) using Bonferroni-corrected post hoc testing (*P < 0.05, **P < 0.01, ***P < 0.001). N = 10–14 animals per treatment, data are presented as mean ± 1 SD.
p53 inhibition protects hair cells from neomycin- or gentamicin-induced damage
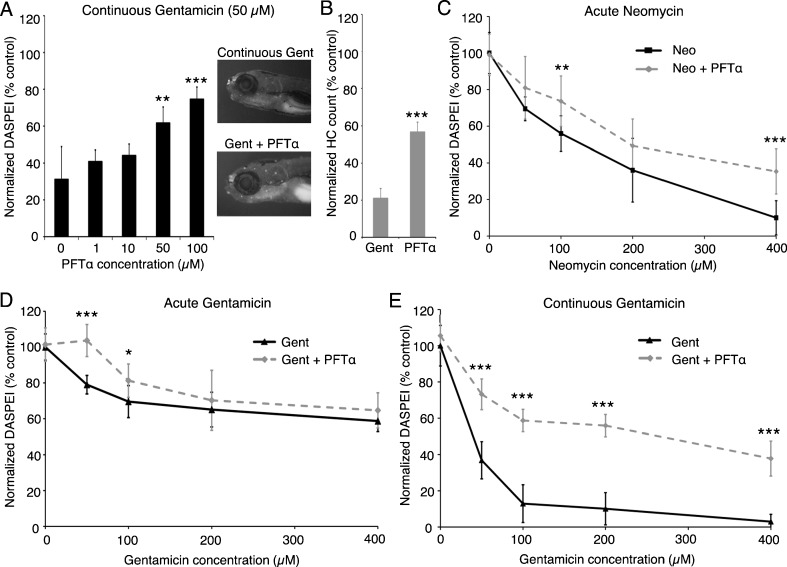
Bax can participate in many cell death signaling pathways, including those driven by p53 (Chipuk et al. 2004; Geng et al. 2010). p53 can transcriptionally regulate Bax, and p53 can also interact directly with Bax protein and other Bcl2 family members in the cytosol or mitochondria (Mihara et al. 2003; Moll et al. 2005; Chipuk and Green 2006). In a previous screen of a cell death inhibitor library, we demonstrated that p53 inhibition protects hair cells from gentamicin damage (Coffin et al. 2013). Here we show that the p53 inhibitor pifithrin-α (PFTα) confers dose-dependent protection from gentamicin toxicity (Fig. 2A). Although maximum protection was observed with 100 μM PFTα, this inhibitor concentration caused larval morbidity, so we performed our remaining experiments with 50 μM PFTα, a dose that was well-tolerated by the animals. Dose–response testing showed that p53 inhibition significantly protected hair cells from neomycin or gentamicin damage (Fig. 2). However, PFTα confers only modest protection against acute neomycin or gentamicin toxicity, while more substantial protection is observed following continuous gentamicin exposure. These findings are further supported by direct counts of antiparvalbumin-labeled hair cells (Fig. 2B).
FIG. 2.
The p53 inhibitor pifithrin-α (PFTα) protects hair cells from neomycin or gentamicin damage. A PFTα protects hair cells from continuous 50 μM gentamicin exposure (one-way ANOVA, F(4, 45) = 26.66, P < 0.001); 100 μM PFTα provides maximum protection but is detrimental to fish health, so 50 μM PFTα was used for the remaining experiments. The images in A show DASPEI-labeled larvae treated with 50 μM continuous gentamicin (top) or gentamicin + PFTα (bottom). B Protection conferred by PFTα was validated with counts of antiparvalbumin-labeled hair cells. Fish treated continuously with 50 μM gentamicin and 50 μM PFTα had significantly more hair cells than fish treated with gentamicin only (two-tailed t test, P < 0.001). C–E Dose–response curves using 50 μM PFTα and variable concentrations of neomycin (C) and gentamicin (D, E). PFTα confers modest protection from acute neomycin (two-way ANOVA, F(1, 110) = 4.99, P < 0.001) and acute gentamicin (two-way ANOVA, F(1, 113) = 6.98, P < 0.001) and robust protection from continuous gentamicin exposure (two-way ANOVA, F(1, 107) = 22.55, P < 0.001). Asterisks indicate significant pairwise differences using Bonferroni-corrected post hoc testing (**P < 0.01, ***P < 0.001). The lack of significant pairwise differences in some comparisons (e.g., C 200 μM neomycin) stems from our selection of the conservation Bonferroni correction for post hoc analysis. N = 7–13 animals per treatment, data are presented as mean ± 1 SD.
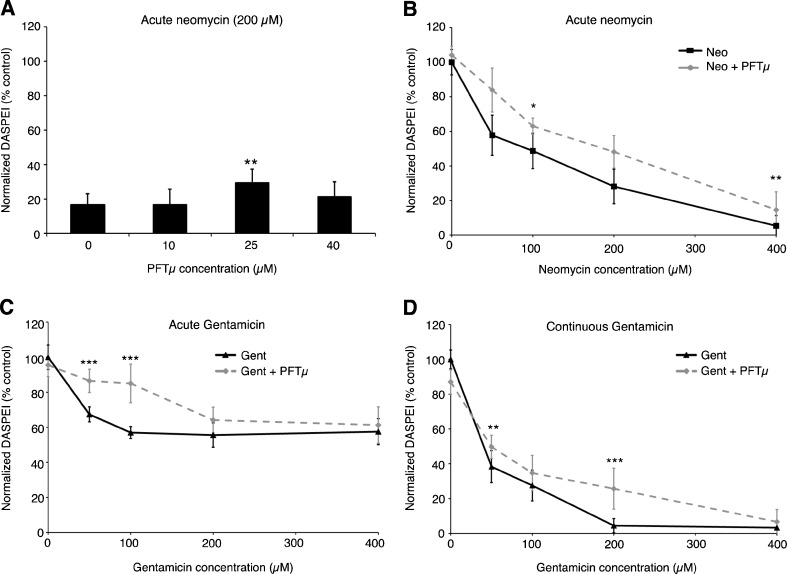
p53 is a multifunctional molecule, with noncanonical cytoplasmic and mitochondrial activity in addition to its well-described transcriptional activity (Vaseva and Moll 2009; Vousden and Prives 2009). Aminoglycosides are reported to induce changes in mitochondrial morphology and function in hair cells, suggesting a central importance for mitochondria in hair cell death signaling (Owens et al. 2007; Jensen-Smith et al. 2012). We therefore examined the necessity for mitochondrial p53 activity using the specific inhibitor PFTμ (Strom et al. 2006); 25 μM PFTμ provided statistically significant but modest protection from acute neomycin or acute gentamicin exposure (Fig. 3), with the magnitude of protection virtually identical to that seen with PFTα. Higher concentrations of PFTμ were toxic to hair cells (data not shown). These results suggest that mitochondrial p53 activity is a contributor to, but not essential for, the acute phase of aminoglycoside damage. In contrast to the substantial protection seen with PFTα during exposure to continuous gentamicin, PFTμ provided only modest protection from continuous gentamicin exposure, suggesting an additional requirement for p53 during continuous gentamicin damage that is independent of p53 mitochondrial activity.
FIG. 3.
The mitochondrial-specific p53 inhibitor PFTμ protects hair cells from neomycin or gentamicin toxicity. A Moderate concentrations of PFTμ provide protection from 200 μM neomycin (two-way ANOVA, F(3, 41) = 14.96, P < 0.001), although higher concentrations of PFTμ are toxic to hair cells; 25 μM PFTμ provides incomplete but significant protection from A acute neomycin (two-way ANOVA, F(1, 102) = 15.57, P < 0.001), B acute gentamicin (two-way ANOVA, F(1, 109) = 83.45, P < 0.001), and C continuous gentamicin toxicity (two-way ANOVA, F(1, 102) = 16.38, P < 0.001). Asterisks indicate significant pairwise differences using Bonferroni-corrected post hoc testing (*P < 0.05, **P < 0.01, ***P < 0.001). N = 10–13 animals per treatment, data are presented as mean ± 1 SD.
p53 inhibition protects hair cells from gentamicin toxicity post-exposure
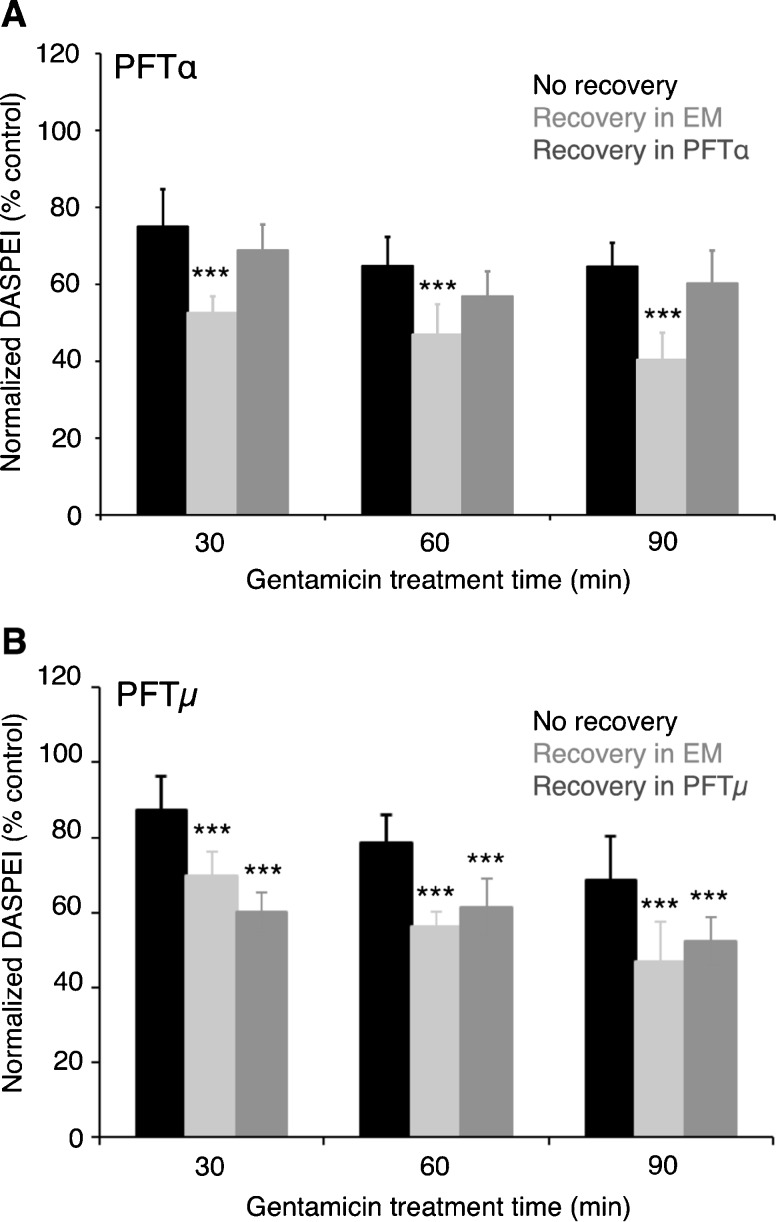
We next conducted a washout experiment to examine the role of p53 during the prolonged phase of gentamicin toxicity. In the zebrafish lateral line system, hair cell death due to gentamicin exposure continues after gentamicin removal, allowing us to assess the specific role for p53 in the slower phase of hair cell death (Owens et al. 2009). As shown in Figure 4, PFTα protected hair cells from gentamicin toxicity when the inhibitor was added after gentamicin removal, consistent with our hypothesis that p53 contributes to the slow phase of gentamicin-induced hair cell death. In contrast, the mitochondrial-specific p53 inhibitor PFTμ did not attenuate the slow phase of gentamicin-induced damage, providing further evidence that p53 mitochondrial activity is involved in the acute phase of damage. No protection was seen if a similar washout experiment was performed with PFTα and neomycin, consistent with previous evidence that neomycin only induces hair cell death by activating acute damage pathways (Owens et al. 2009; data not shown).
FIG. 4.
The general p53 inhibitor PFTα protected hair cells from the slow phase of damage after gentamicin treatment. Fish were treated with 100 μM gentamicin for 30, 60, or 90 min and then either assessed immediately, or the gentamicin was washed out and the fish were allowed to recover for either 4.5, 5, or 5.5 h for a total experimental time of 6 h. Recovery took place in either embryo medium, 50 μM PFTα (A), or PFTμ (B). A There is an overall effect of recovery on hair cell survival (two-way ANOVA, F(2, 86) = 70.08, P < 0.001). Some hair cell loss was evident immediately after treatment but additional loss occurred during the recovery period (comparing black and gray bars). Addition of PFTα after gentamicin exposure significantly protected hair cells, with effects again visible at all time points (comparing dark and light gray bars, two-way ANOVA, F(1, 58) = 76.54, P < 0.001). B Again, there is an overall effect of recovery on hair cell survival (F(2, 95) = 76.87, P < 0.001). In this case, however, addition of the mitochondrial p53 inhibitor PFTμ did not protect hair cells from damage due to gentamicin washout (comparing dark and light gray bars, two-way ANOVA, F(1, 63) = 0.03, P = 0.87). Asterisks indicate treatments that are significantly different from the “recovery in EM” group (**P < 0.01, ***P < 0.001). N = 9–13 fish per treatment, data are presented as mean + 1 SD.
Mdm2 inhibition sensitizes hair cells to gentamicin
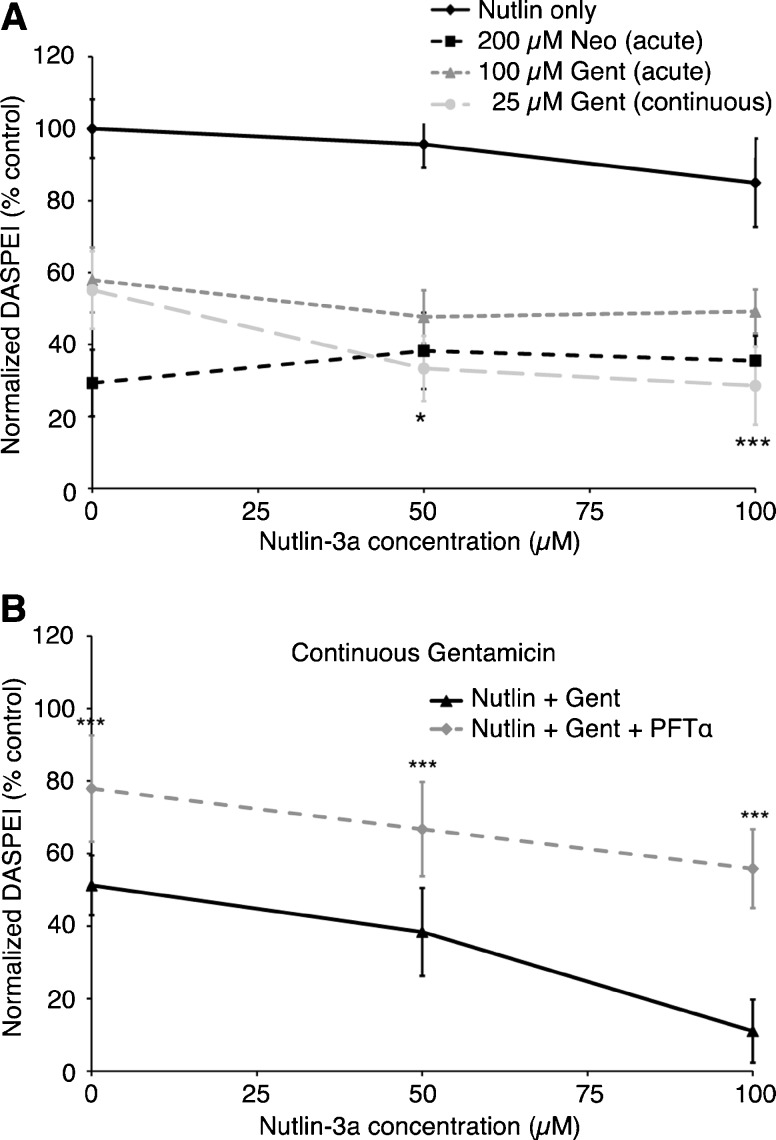
We next asked if stabilizing p53 promoted hair cell death by treating fish with nutlin-3a, which inhibits the endogenous p53 antagonist Mdm2 and prevents p53 degradation (Vassilev et al. 2004). Figure 5A shows that nutlin-3a treatment sensitized hair cells to toxicity resulting from continuous exposure to a moderate gentamicin concentration, but not to acute neomycin or gentamicin toxicity. To rule out that this sensitization was simply the result of longer exposure to an inhibitor, we also examined the effect of nutlin-3a treatment in a continuous neomycin exposure paradigm. Nutlin-3a did not alter the effect of 6 h of neomycin exposure (data not shown), suggesting that Mdm2 inhibition specifically promotes gentamicin-induced hair cell death during the slow phase of damage.
FIG. 5.
Inhibition of the p53 antagonist mdm2 facilitates gentamicin-induced hair cell death. A The Mdm2 inhibitor nutlin-3a sensitizes hair cells to damage caused by a 6-h continuous gentamicin exposure (one-way ANOVA, F(2, 29) = 12.90, P < 0.001), but not to damage from acute neomycin or gentamicin treatment (one-way ANOVAs, neomycin: F(2, 28) = 2.71, P = 0.08; gentamicin: F(2, 32) = 3.03, P = 0.06). Nutlin-3a alone was not toxic to hair cells during the 7-h treatment period (one-way ANOVA, F(2, 30) = 0.49, P = 0.61). B Nutlin-3a attenuates the protection provided by PFTα. Fish were cotreated with nutlin-3a and 25 μM gentamicin with or without PFTα. Hair cell survival was greater in all PFTα groups relative to those without PFTα (two-way ANOVA, F(1, 50) = 105.8, P < 0.001), but survival decreased with increasing concentrations of nutlin-3a in the presence of PFTα (one-way ANOVA, F(2, 23) = 4.81, P = 0.02). Asterisks indicate significant differences between treatment pairs with vs. without PFTα (***P < 0.001). N = 5–11 animals per treatment, data are presented as mean ± 1 SD.
If p53 contributes to hair cell death due to gentamicin exposure, it stands to reason that we can pharmacologically titrate the relative concentration of p53 and manipulate hair cell survival. We tested this hypothesis by cotreating fish with 50 μM PFTα and variable concentrations of nutlin-3a (Fig. 5B). While PFTα conferred significant protection against continuous gentamicin exposure, this protection was attenuated by exposure to increasing concentrations of nutlin-3a. In all cases, however, hair cell survival was greater when gentamicin-exposed fish were cotreated with nutlin-3a and PFTα as compared to fish treated with nutlin-3a alone. These data suggest that the relative level of p53 is an important regulator of death or survival in gentamicin-treated hair cells.
Disrupted p53 DNA binding activity does not protect hair cells from aminoglycoside damage
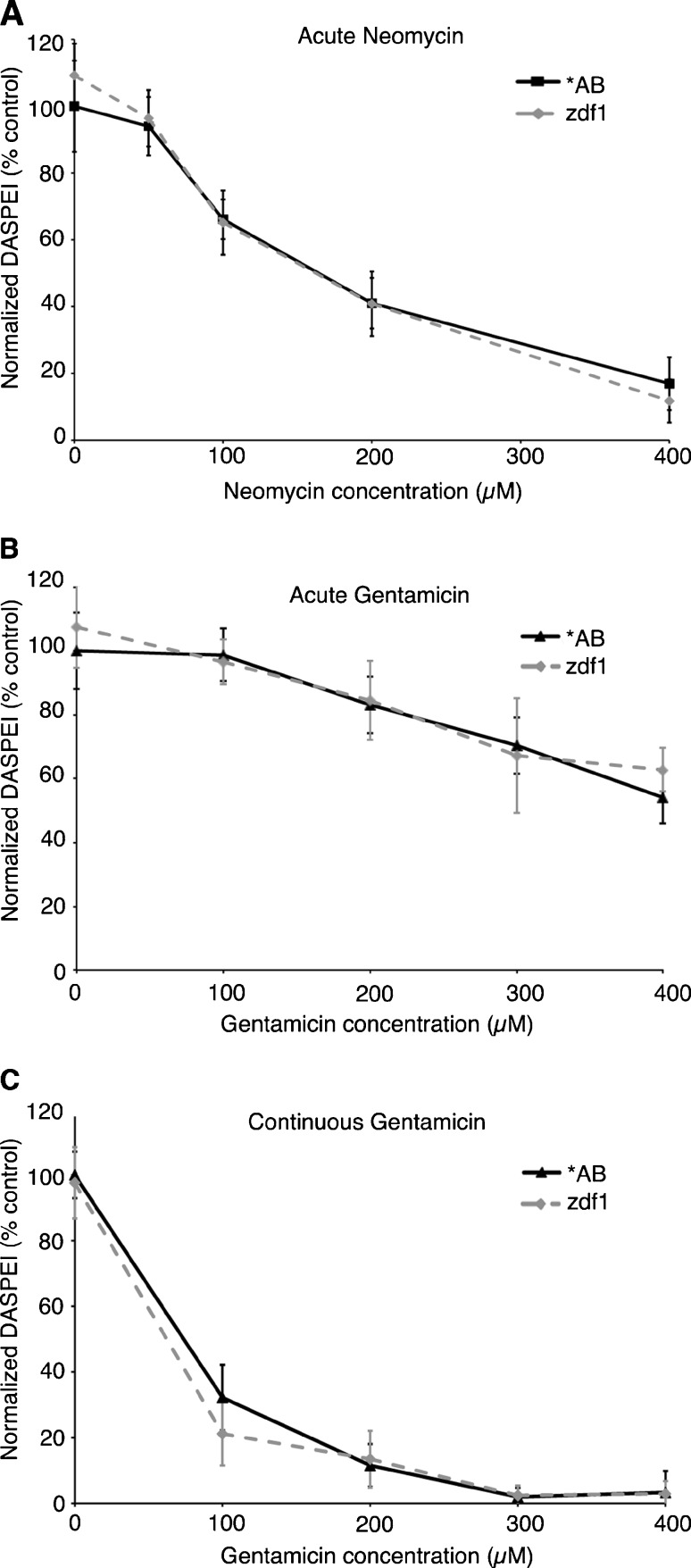
The p53zdf1 allele bears a point mutation in the DNA binding domain of p53, eliminating its transcriptional activity (Berghmans et al. 2005). The effects of this mutation on transcription-independent p53 activity are unknown, but it is likely that some p53 functionality remains. We found that hair cells in p53zdf1 homozygotes were not resistant to either neomycin or gentamicin damage using either acute or continuous exposure paradigms (Fig. 6). Furthermore, nutlin-3a treatment facilitated hair cell loss in the p53zdf1 line to the same degree as in wild-type fish (data not shown). These results suggest that p53 transcriptional activity is not required for aminoglycoside toxicity in the lateral line system.
FIG. 6.
p53 transcriptional activity is not required for aminoglycoside-induced hair cell death. Hair cells in the zdf1 mutant line are not protected from A acute neomycin, B acute gentamicin, or C continuous gentamicin-induced damage, as compared to *AB wild-type fish. Analysis was conduced by two-way ANOVA, acute neomycin F(1, 107) = 0.40, P = 0.53; acute gentamicin F(1, 100) = 1.33, P = 0.25; continuous gentamicin F(1, 105) = 2.64, P = 0.11. N = 7–13 animals per group, data are presented as mean ± 1 SD.
Bcl2 overexpression protects hair cells from gentamicin toxicity
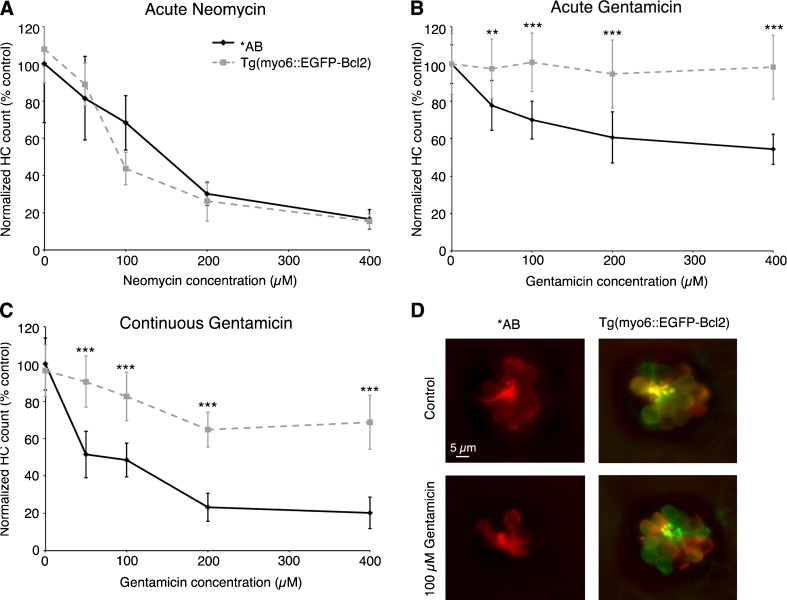
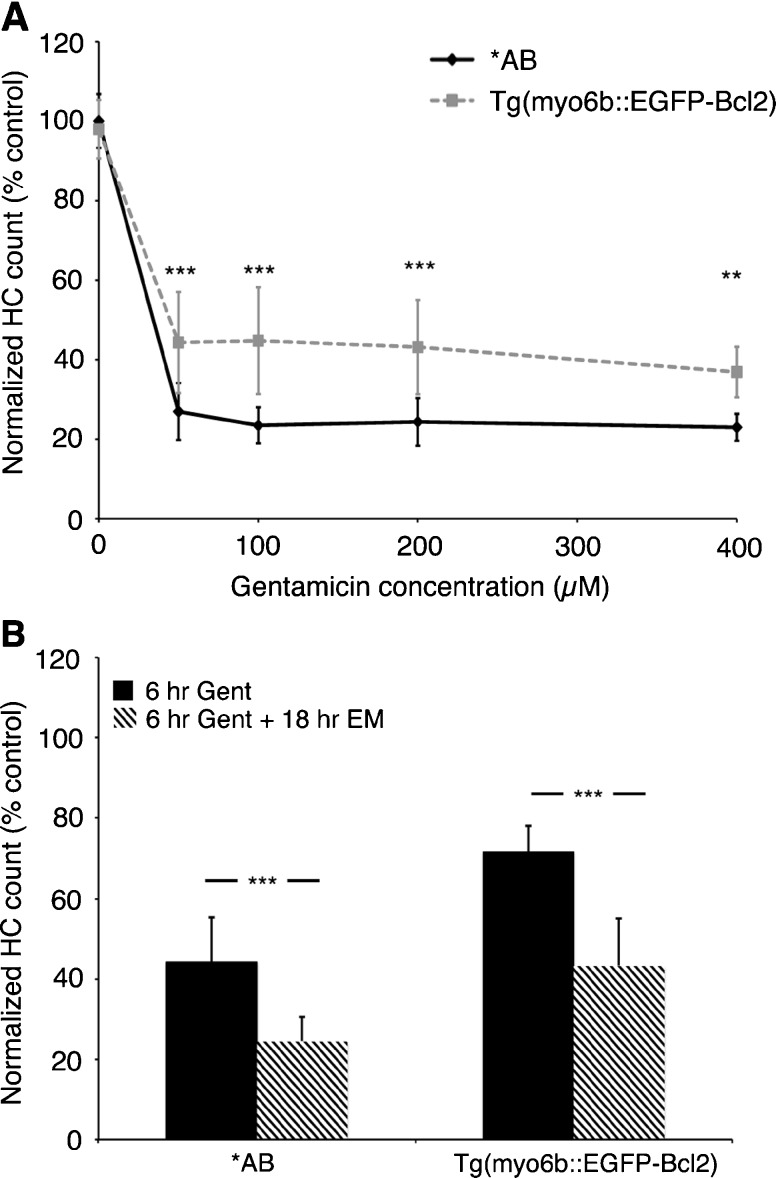
p53 is thought to compete with Bax for Bcl2 family pro-survival proteins (Jiang and Milner 2003; Chipuk and Green 2006). PFTμ is hypothesized to inhibit p53 mitochondrial activity by disrupting binding of p53 to Bcl2 or Bcl-xL, which then allows them to bind and inhibit Bax and thereby promote cell survival (Strom et al. 2006; Morita et al. 2010). We therefore asked if overexpressing Bcl2 would protect hair cells from aminoglycoside damage. Bcl2 overexpression robustly protected hair cells from either acute or continuous gentamicin damage but not from acute neomycin toxicity, as shown in Figure 7. We also examined Bcl2 overexpressing fish 1 day after gentamicin treatment to assess whether Bcl2 overexpression prevented hair cell death, as opposed to delaying it. As seen in Figure 8, hair cells in transgenic larvae were still significantly protected from gentamicin toxicity. However, the protection conferred by Bcl2 overexpression was attenuated by 18 h posttreatment when compared to fish assessed immediately after gentamicin exposure (Fig. 8B). This result suggests that some hair cell loss occurs in the presence of excess Bcl2, perhaps via activation of distinct cell death pathways. PFTα treatment during this 18-h recovery period did not prevent the loss of hair cells that occurred in either wild-type or transgenic animals (data not shown). These data indicate that the moderate hair cell death observed in transgenic fish after continuous gentamicin exposure is not dependent on p53.
FIG. 7.
Excess Bcl2 protects hair cells from gentamicin damage. Overexpression of hair cell-specific Bcl2 does not protect hair cells from A acute neomycin (two-way ANOVA, F(1, 100) = 0.65, P = 0.42). Significant protection is seen from B acute gentamicin toxicity (two-way ANOVA, F(1, 73) = 113.5, P < 0.001) or C continuous gentamicin exposure (two-way ANOVA, F(1, 90) = 181.60, P < 0.001). Data represent counts of antiparvalbumin-labeled hair cells from seven neuromasts per fish, N = 7–19 fish per group, data are presented as mean ± 1 SD. Asterisks represent significant pairwise differences using Bonferroni-corrected post hoc testing (**P < 0.01, ***P < 0.001). D Confocal images (z-series projections) of *AB (left column) and Tg(myo6b:EGFP-bcl2) (right column) neuromasts labeled with antiparvalbumin (red) and anti-GFP (green). Images are shown of control neuromasts (top row) and those treated with 100 μM gentamicin for 6 h (bottom row), demonstrating robust hair cell survival in gentamicin-treated transgenic hair cells. The scale bar in the upper left panel is 5 μM and applies to all panels.
FIG. 8.
Bcl2 overexpressing hair cells are protected from the slow phase of damage that occurs after gentamicin washout. A Significant protection is seen vs. wild-type fish when fish are treated with gentamicin for 6 h and allowed to recover in EM for 18 h prior to hair cell assessment (two-way ANOVA, F(1, 86) = 60.90, P < 0.001). Post hoc analysis shows significant protection at each gentamicin concentration (**P < 0.01, ***P < 0.001). B However, some additional loss of hair cells occurs in either wild-type or Bcl2 transgenic fish following 6 h of exposure to 200 μM gentamicin and 18 h of recovery as compared to immediately after gentamicin treatment (within-genotype two-tailed t test, P < 0.001). Data represent summed counts of antiparvalbumin-labeled hair cells in seven neuromasts per fish, N = 9–10 fish per group, data are presented as mean ± 1 SD.
As hair cell death due to neomycin exposure begins very quickly, we also asked if Bcl2 overexpressing hair cells was protected from neomycin damage if assessed early in neomycin exposure. There were no differences in the number of surviving hair cells in transgenic vs. wild-type fish after 15 min of neomycin treatment (data not shown), further evidence that Bcl2 promotes hair cell survival from continuous gentamicin toxicity but not acute neomycin damage.
Discussion
Our pharmacologic and genetic manipulations suggest differential involvement of Bax, Bcl2, and p53 proteins in aminoglycoside-induced hair cell death in the zebrafish lateral line. Bax channel inhibition robustly protected hair cells from acute neomycin exposure, but not from 6 h of gentamicin exposure, while Bcl2 overexpression showed the reverse pattern, conferring protection from acute or continuous gentamicin damage but not from neomycin toxicity. p53 inhibition attenuated hair cell death induced by either ototoxin, although protection from neomycin damage appears quite modest, and in this context, p53 appears to play a distinct mitochondrial role. Hair cell toxicity due to either neomycin or gentamicin appears to be independent of p53 transcriptional activity, suggesting multiple posttranslational roles for p53 in hair cell death processes. These results support our previous findings that there are distinct mechanisms underlying damage from acute or continuous exposure to neomycin and gentamicin (Owens et al. 2009; Coffin et al. 2013).
Two caveats might be considered in interpreting these data. First, the majority of the data we present are based on assessment of DASPEI-labeled hair cells. On the other hand, all pharmacologic or genetic manipulations were also assessed by direct counts of fixed, antibody-labeled hair cells in order to ensure that our data reflected hair cell survival and are not limited to changes in mitochondrial membrane potential. Second, it is difficult to directly compare drug dosages or the time course of hair cell death in the lateral line to studies in mammals because of dilution with systemic application as used in most mammalian studies. In terms of access of experimental drugs to hair cells, the lateral line more closely resembles an in vitro model than a mammalian inner ear tested in vivo. It is worth noting, however, that gentamicin exposure is reported to induce hair cell loss within 6 h in utricular cultures and within 18–24 h following intratympanic application in vivo (Forge and Li 2000; Suzuki et al. 2008). In addition, preliminary studies in guinea pigs demonstrate that cochlear perfusion of neomycin leads to rapid hair cell loss (60–90 min; Wang et al. 2009). These studies suggest that aminoglycosides may activate cell death pathways in mammalian hair cells quite rapidly, as in the lateral line system, when direct access is possible. On the other hand, it is yet to be determined if distinct cell death pathways are activated at different times in mammalian inner ear in vivo, as indicated by our zebrafish data.
p53 and aminoglycoside ototoxicity
The tumor suppressor protein p53 is a key regulator of many processes, including cell proliferation, senescence, and death (Pietsch et al. 2008; Vousden and Prives 2009). p53 is best known in its canonical role as a transcription factor, but p53 can function in cytoplasmic and mitochondrial roles in cell death independent of transcriptional activity (Caelles et al. 1994; Chipuk and Green 2006). The broadly acting p53 inhibitor PFTα significantly protected hair cells from neomycin or gentamicin damage, suggesting that p53 activity is important for aminoglycoside-induced hair cell death. Given that transcriptional and translational activity does not appear essential for aminoglycoside-induced hair cell death in the zebrafish lateral line system, a noncanonical p53 mechanism appears to be the necessary function (Coffin et al. 2013). PFTμ, a specific inhibitor of mitochondrial p53 activity, also protected hair cells from neomycin or gentamicin damage, albeit to a lesser extent than PFTα, while the Mdm2 inhibitor nutlin-3a sensitized hair cells to gentamicin toxicity and attenuated the protective effect of PFTα.
Collectively, these data suggest that p53 may have multiple modes of action in regulating aminoglycoside toxicity, one in the acute damage phase and a second, distinct role during the prolonged damage phase caused by continuous gentamicin exposure. A temporally biphasic role for p53 was previously demonstrated in γ-irradiated mice (Erster et al. 2004). However, this response was due to sequential activation of mitochondrial and transcriptional p53 activity, whereas our data suggest that both acute and continuous hair cell death mechanisms are independent of p53 transcriptional activity. There is a previous report of PFTα conferring protection against gentamicin damage in neonatal rat cochlear cultures (Zhang and Cramer 2006), but the role for p53 in aminoglycoside ototoxicity in mammals has not been explored in detail. A surprising result in our studies is that Mdm2 inhibition via nutlin-3a facilitates hair cell death due to gentamicin but not neomycin. Nutlin-3a can induce p53-mediated apoptosis in tumor cells in both a transcription-dependent and transcription-independent manner, suggesting that Mdm2 may modulate p53 activity in multiple ways (Chipuk et al. 2004; Kojima et al. 2006; Vaseva et al. 2009). It is possible that the action of nutlin-3a-mediated Mdm2 antagonism depends on the intracellular location and relative access of p53 to protein–protein interactions. Localization studies are needed to further understand these roles for p53.
We must also note that many of the pharmacologic inhibitors used in this study have known off-target effects, making it possible that p53 is not the central actor in these cell death processes, or that additional molecules are also inhibited by our treatments. For example, PFTα can inhibit zebrafish p73 in vivo and mammalian cyclin D1 in vitro, while PFTμ inhibits hsp70 and can interfere with autophagy (Davidson et al. 2008; Leu et al. 2009; Sohn et al. 2009; Pimkina and Murphy 2011). Hsp70 activation inhibits aminoglycoside-induced hair cell loss in mammalian models, making it unlikely that inhibiting hsp70 would exert a protective effect in zebrafish hair cells (Taleb et al. 2008, 2009). However, blocking autophagy does provide a modest protective effect from aminoglycoside damage in zebrafish (Coffin et al. 2013), so we cannot rule out this off-target effect of PFTμ in our present study, nor can we be certain that PFTα or PFTμ are not exerting an influence on additional targets. Further genetic studies are necessary to validate the involvement of p53 in lateral line hair cell death.
Bcl2 proteins and aminoglycoside ototoxicity
We have shown that treating hair cells with an inhibitor of the pro-cell death protein, Bax, significantly protects them from neomycin and acute gentamicin damage, but not from damage due to continuous gentamicin exposure. This finding is consistent with our previous hypothesis that neomycin and acute gentamicin activate similar cell death pathway(s) but that additional pathways are uniquely activated by continuous gentamicin exposure (Owens et al. 2009; Coffin et al. 2013). Recent evidence suggests that gentamicin and neomycin may activate distinct pathways in rat cochlear explants, indicating that differential pathway contributions may not be restricted to zebrafish (Mazurek et al. 2012).
In other cell types, cell death stimuli trigger Bax translocation to the mitochondrial outer membrane, where Bax forms channels that permeabilize mitochondria and allow mitochondrial proteins to leak into the cytosol (Antonsson 2001). Neomycin induces changes in mitochondrial membrane potential in the zebrafish lateral line system and Bax inhibition protects mitochondrial cytochrome c localization, consistent with Bax-induced mitochondrial membrane permeability (Owens et al. 2007; A. Coffin, unpublished data). Other pro-cell death Bcl2 proteins including Bak and Bad have been implicated in noise-induced hair cell damage, suggesting that Bcl2 proteins are required for responses to a variety of ototoxic stimuli (Vicente-Torres and Schacht 2006; Yamashita et al. 2008). It is possible that the Bax channel inhibitor used in the present study may bind additional targets with unrecognized effects, confounding our results. There is a high degree of functional conservation between mammalian and zebrafish Bcl2 family proteins, suggesting that the Bax channel blocker used here likely interacts with zebrafish Bax (Kratz et al. 2006; Jette et al. 2008). However, future experiments with genetic Bax manipulation are necessary, particularly since there are two Bax paralogs in zebrafish (Kratz et al. 2006).
In contrast to treatment with the Bax inhibitor, overexpressing Bcl2 protected lateral line hair cells from gentamicin damage but not from neomycin exposure. Bcl2 overexpression has been previously shown to confer protection against neomycin toxicity in cultured mature mouse utricles (Cunningham et al. 2004). These results suggest that Bcl2 family proteins may be involved in aminoglycoside toxicity. At the present time, it is unclear if the difference between the mouse utricle experiment and our present study is due to species differences or differences in hair cell responses in vivo vs. in vitro. Cunningham et al. (2004) used a neomycin treatment paradigm similar to our “continuous” exposure in the present study, although over a much longer treatment duration.
Why does Bcl2 overexpression not attenuate neomycin toxicity in the lateral line? While the ratio of Bcl2 to Bax is considered a rheostat of cell life or death, the Bcl2 family includes multiple pro-survival proteins, including Bcl2 and Bcl-xL, either of which can associate with Bax either directly or via BH3-only proteins (Korsmeyer et al. 1993; Finucane et al. 1999; Cheng et al. 2001; van Delft and Huang 2006). Similarly, both Bax and related pro-cell death family members such as Bak can induce mitochondrial membrane permeabilization and subsequent activation of downstream cell death pathways (Wei et al. 2001; van Delft and Huang 2006). It is therefore possible that a Bax association with an unidentified pro-survival protein is necessary for neomycin ototoxicity, while Bcl2 interacts with Bak or another pro-death protein in gentamicin-induced damage pathways. Additional BH3-only proteins may be important in neomycin-induced toxicity, perhaps by preventing binding of Bcl2 to Bax, which could explain the lack of protection seen in the Bcl2 overexpression fish. This conjecture requires further exploration of BH3-only proteins such as Bim and Noxa that modulate Bcl2 interactions with other protein targets (Antonsson 2001; Han et al. 2010).
Neuronal cell death in response to DNA damage in vitro or prion expression in vivo can occur in a fast Bax-dependent or slow Bax-independent manner, paralleling our findings with neomycin- and gentamicin-induced hair cell death (Besirli et al. 2003; Li et al. 2007). Importantly, the slow form of Bax-independent death induced by DNA damage involved p53 activity (Besirli et al. 2003). p53 can also interact directly with pro-survival and pro-cell death Blc2 family members to activate mitochondrial-associated cell death pathways (Mihara et al. 2003; Chipuk et al. 2004; Moll et al. 2005; Han et al. 2010). Protein binding and colocalization experiments are necessary to further dissect the relative contributions of these proteins to aminoglycoside ototoxicity.
Acknowledgments
This research was funded by the National Institute on Deafness and Other Communication Disorders (NIDCD) grants DC004661, DC005987, DC009931, and DC011344. Additional support was provided by the Virginia Merrill Bloedel Hearing Research Center at the University of Washington and by Washington State University Vancouver. We thank David White for fish husbandry assistance, Kay Williamson and Lauren Hayashi for assistance with data collection, and three anonymous reviewers for comments that strengthened the manuscript.
Contributor Information
Allison B. Coffin, Phone: +1-360-5469748, FAX: +1-360-5469038, Email: Allison.coffin@wsu.edu
Edwin W. Rubel, Email: rubel@uw.edu
David W. Raible, Email: draible@uw.edu
References
- Antonsson B. Bax and other pro-apoptotic Bcl-2 family “killer-proteins” and their victim, the mitochondrion. Cell Tissue Res. 2001;306:347–361. doi: 10.1007/s00441-001-0472-0. [DOI] [PubMed] [Google Scholar]
- Berghmans S, Murphey RD, Wienholds E, Neuberg D, Kutok JL, Fletcher CD, Morris JP, Liu TX, Schulte-Merker S, Kanki JP, Plasterk R, Zon LI, Look AT. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci USA. 2005;102(2):407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besirli CG, Deckwerth TL, Crowder RJ, Freeman RS, Johnson EM., Jr Cytosine arabinoside rapidly activates Bax-dependent apoptosis and a delayed Bax-independent death pathway in sympathetic neurons. Cell Death Differ. 2003;10(9):1045–1058. doi: 10.1038/sj.cdd.4401259. [DOI] [PubMed] [Google Scholar]
- Bombrun A, Gerber P, Casi G, Terradillos O, Antonsson B, Halazy S. 3,6-Dibromocarbazole piperazine derivatives of 2-propanol as first inhibitors of cytochrome c release via bax channel modulation. J Med Chem. 2003;46(21):4365–4368. doi: 10.1021/jm034107j. [DOI] [PubMed] [Google Scholar]
- Caelles C, Heimberg A, Karin M. p53-Dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature. 1994;370:220–223. doi: 10.1038/370220a0. [DOI] [PubMed] [Google Scholar]
- Cheng E, H-Y A, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-XL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/S1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW (2002) Hair cell death activation in the avian basillar papilla: characterization of the in vitro model and caspase activation. J Assoc Res Otolaryngol 4(1):91–105 [DOI] [PMC free article] [PubMed]
- Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13(6):343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- Cheung ECC, Melanson-Drapeau L, Cregan SP, Vanderluit JL, Ferguson KL, McIntosh WC, Park DS, Bennett SAL, Slack RS. Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms. J Neurosci. 2005;25(6):1324–1334. doi: 10.1523/JNEUROSCI.4261-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk J, Green D. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13:994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuawana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Coffin AB, Reinhart KE, Owens KN, Raible DW, Rubel EW. Extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Hear Res. 2009;253(1–2):42–51. doi: 10.1016/j.heares.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Ou H, Owens KN, Santos F, Simon JA, Rubel EW, Raible DW. Chemical screening for hair cell loss and protection in the zebrafish lateral line. Zebrafish. 2010;7(1):3–11. doi: 10.1089/zeb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Williamson KL, Mamiya A, Raible DW, Rubel EW. Profiling drug-induced cell death pathways in the zebrafish lateral line. Apoptosis. 2013;18(4):393–408. doi: 10.1007/s10495-013-0816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs S, Görner P, Münz H. The mechanosensory lateral line: neurobiology and evolution. New York: Springer; 1989. [Google Scholar]
- Coombs S, Braun CB, Donovan B. The orienting response of Lake Michigan mottled sculpin is mediated by canal neuromasts. J Exp Biol. 2001;204:337–348. doi: 10.1242/jeb.204.2.337. [DOI] [PubMed] [Google Scholar]
- Cunningham LL, Cheng AG, Rubel EW. Caspase activation in hair cells of the mouse utricle exposed to neomycin. J Neurosci. 2002;22(19):8532–8540. doi: 10.1523/JNEUROSCI.22-19-08532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Matsui JI, Warchol ME, Rubel EW. Overexpression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. J Neurbiol. 2004;601(1):89–100. doi: 10.1002/neu.20006. [DOI] [PubMed] [Google Scholar]
- Davidson W, Ren Q, Kari G, Kashi O, Dicker AP, Rodeck U. Inhibition of p73 function by Pifithrin-alpha as revealed by studies in zebrafish embryos. Cell Cycle. 2008;7(9):1224–1230. doi: 10.4161/cc.7.9.5786. [DOI] [PubMed] [Google Scholar]
- Dijkgraaf S. The functioning and significance of the lateral line organs. Biol Rev. 1963;38:51–105. doi: 10.1111/j.1469-185X.1963.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Durante-Mangoni E, Grammatikos A, Util R, Falagas ME. Do we still need the aminoglycosides? Int J Antimicrob Agents. 2009;33(3):201–205. doi: 10.1016/j.ijantimicag.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Endo H, Kamada H, Nito C, Nishi T, Chan PH. Mitochondrial translocation of p53 mediates release of cytochrome c and hippocampal CA1 neuronal death after transient global cerebral ischemia in rats. J Neurosci. 2006;26(30):7974–7983. doi: 10.1523/JNEUROSCI.0897-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erster S, Mihara M, Kim RH, Petrenko O, Moll UM. In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol Cell Biol. 2004;42(15):6728–6741. doi: 10.1128/MCB.24.15.6728-6741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondrial is inhibitable by Bcl-xL. J Biol Chem. 1999;274(4):2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000;139(1–2):97–115. doi: 10.1016/S0378-5955(99)00177-X. [DOI] [PubMed] [Google Scholar]
- Geng Y, Walls KC, Ghosh AP, Akhtar RS, Klocke BJ, Roth KA. Cytoplasmic p53 and activated Bax regulate p53-dependent, transcription-independent neural precursor cell apoptosis. J Histochem Cytochem. 2010;58(3):265–275. doi: 10.1369/jhc.2009.954024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Kroemer G. Cytoplasmic functions of the tumor suppressor p53. Nature. 2009;458(7242):1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Goldstein LA, Hou W, Gastman BR, Rabinowich H. Regulation of mitochondrial apoptotic events by p53-mediated disruption of complexes between antiapoptotic Bcl-2 members and Bim. J Biol Chem. 2010;285(29):22473–22483. doi: 10.1074/jbc.M109.081042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2003;4(2):219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose Y, Simon JA, Ou H. Hair cell toxicity in anti-cancer drugs: evaluating an anti-cancer drug library for independent and synergistic toxic effects on hair cells using the zebrafish lateral line. J Assoc Res Otolaryngol. 2011;12(6):719–728. doi: 10.1007/s10162-011-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen-Smith HC, Hallworth R, Nichols MG. Gentamicin rapidly inhibits mitochondrial metabolism in high-frequency cochlear outer hair cells. PLoS One. 2012;7(6):e38471. doi: 10.1371/journal.pone.0038471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette CA, Flanagan AM, Ryan J, Pyati UJ, Carbonneau S, Stwerart RA, Langenau DM, Look AT, Letai A. BIM and other BCL-2 family proteins exhibit cross-species conservation of function between zebrafish and mammals. Cell Death Diff. 2008;15:1063–1072. doi: 10.1038/cdd.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Milner J. Bcl-2 constitutively suppresses p53-dependent apoptosis in colorectal cancer cells. Genes Dev. 2003;17:832–837. doi: 10.1101/gad.252603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Forge A, Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ. 2006;13(1):20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser CL, Chapman BJ, Guidi JL, Terry CE, Mangiardi DA, Cotanche DA. Comparison of activated caspase detection methods in the gentamicin-treated chick cochlea. Hear Res. 2008;240(1–2):1–11. doi: 10.1016/j.heares.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Finch G, Nicolson T. Kinocilia mediate mechanosensitivity in developing zebrafish hair cells. Dev Cell. 2012;23(2):329–341. doi: 10.1016/j.devcel.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Konopleva M, McQueen T, O’Brien S, Plunkett W, Andreeff M. Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood. 2006;108(3):993–1000. doi: 10.1182/blood-2005-12-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285(5434):1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4(6):327–332. [PubMed] [Google Scholar]
- Kratz E, Eimon PM, Mukhala K, Stern H, Zha J, Strasser A, Hart R, Ashkenazi A. Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ. 2006;13(10):1631–1640. doi: 10.1038/sj.cdd.4402016. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien C-B. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Langenau DM, Jette C, Berghmans S, Palomero T, Kanki JP, Kutok JL, Look AT. Suppression of apoptosis by bcl-2 overexpression in lymphoid cells of transgenic zebrafish. Blood. 2005;105(8):3278–3285. doi: 10.1182/blood-2004-08-3073. [DOI] [PubMed] [Google Scholar]
- Leu JI-J, Pimkina J, Frank A, Murphy ME, George DL. A small molecular inhibitor of inducible heat shock protein 70. Mol Cell. 2009;36:15–27. doi: 10.1016/j.molcel.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9(10):749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Nevill G, Forge A. Two modes of hair cell loss from the vestibular sensory epithelia of the guinea pig inner ear. J Comp Neurol. 1995;355(3):405–417. doi: 10.1002/cne.903550307. [DOI] [PubMed] [Google Scholar]
- Li A, Barmada SJ, Roth KA, Harris DA. N-terminally deleted forms of prior protein activate both Bax-dependent and Bax-independent neurotoxic pathways. J Neurosci. 2007;27(4):852–859. doi: 10.1523/JNEUROSCI.4244-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui JI, Haque A, Huss D, Messana EP, Alosi JA, Roberson DW, Cotanche DA, Dickman JD, Warchol ME. Caspase inhibitors promote vestibular hair cell survival and function after aminoglycoside treatment in vivo. J Neurosci. 2003;23(14):6111–6122. doi: 10.1523/JNEUROSCI.23-14-06111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek B, Lou X, Olze H, Haupt H, Szczepek AJ. In vitro protection of auditory hair cells by salicylate from gentamicin-induced but not neomycin-induced cell loss. Neurosci Lett. 2012;506:107–110. doi: 10.1016/j.neulet.2011.10.060. [DOI] [PubMed] [Google Scholar]
- Metcalf WK, Kimmel CB, Schabtach E. Anatomy of the posterior lateral line system in young larvae of the zebrafish. J Comp Neurol. 1985;233(3):377–389. doi: 10.1002/cne.902330307. [DOI] [PubMed] [Google Scholar]
- Middleton G, Cox SW, Korsmeyer S, Davies AM. Differences in bcl-2- and bax-independent function in regulating apoptosis in sensory neuron populations. Eur J Neurosci. 2000;12(3):819–827. doi: 10.1046/j.1460-9568.2000.00966.x. [DOI] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoscka P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11(3):577–590. doi: 10.1016/S1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Moll U, Wolff S, Speidel D, Deppert W. Transcription-independent proapoptotic functions of p53. Curr Opin Cell Biol. 2005;17:631–636. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Montgomery JC, MacDonald JA. Sensory tuning of lateral line receptors in Antarctic fish to the movements of planktonic prey. Science. 1987;235:195–196. doi: 10.1126/science.235.4785.195. [DOI] [PubMed] [Google Scholar]
- Montgomery JC, Baker CF, Carton AG. The lateral line can mediate rheotaxis in fish. Nature. 1997;389:960–963. doi: 10.1038/40135. [DOI] [Google Scholar]
- Morita A, Yamamoto S, Wang B, Tanaka K, Suzuki N, Aoki S, Ito A, Nanao T, Ohya S, Yoshino M, Zhu J, Enomoto A, Matsumoto Y, Funatsu O, Hosoi Y, Ikekita M. Sodium orthvanadate inhibits p53-mediated apoptosis. Cancer Res. 2010;79(10):2570265. doi: 10.1158/0008-5472.CAN-08-3771. [DOI] [PubMed] [Google Scholar]
- Murakami SL, Cunningham LL, Werner LA, Bauer E, Pujols R, Raible DW, Rubel EW. Developmental differences in susceptibility to neomycin-induced hair cell death in the lateral line neuromasts of zebrafish (Danio rerio) Hear Res. 2003;186(1–2):47–56. doi: 10.1016/S0378-5955(03)00259-4. [DOI] [PubMed] [Google Scholar]
- New JG, Fewkes LA, Khan SN. Strike feeding behavior in the muskellunge, Esox masquinongy: contributions of the lateral line and visual sensory systems. J Exp Biol. 2001;204:1207–1221. doi: 10.1242/jeb.204.6.1207. [DOI] [PubMed] [Google Scholar]
- Ou HC, Raible DW, Rubel EW. Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hear Res. 2007;233(1–2):46–53. doi: 10.1016/j.heares.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Cunningham DE, MacDonald G, Rubel EW, Raible DW, Pujol R. Ultrastructural analysis of aminoglycoside-induced hair cell death in the zebrafish lateral line reveals an early mitochondrial response. J Comp Neurol. 2007;502(4):522–543. doi: 10.1002/cne.21345. [DOI] [PubMed] [Google Scholar]
- Owens KN, Coffin AB, Hong LS, Bennett KO, Rubel EW, Raible DW. Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct cell death pathways. Hear Res. 2009;253(1–2):32–41. doi: 10.1016/j.heares.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannenstiel SC, Praetorius M, Plinkert PK, Brough DE, Staecker H. Bcl-2 gene therapy prevents aminoglycoside-induced degeneration of auditory and vestibular hair cells. Audiol Neurootol. 2009;14(4):254–266. doi: 10.1159/000192953. [DOI] [PubMed] [Google Scholar]
- Pietsch EC, Sykes SM, McMahon SB, Murphy ME. The p53 family and programmed cell death. Oncogene. 2008;27(50):6507–6521. doi: 10.1038/onc.2008.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimkina J, Murphy ME. Interaction of the ARF tumor suppressor with cytosolic HSP70 contributes to its autophagic function. Cancer Biol Ther. 2011;12(6):503–509. doi: 10.4161/cbt.12.6.15976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha GV, Deshmukh M, Johnson EM., Jr BAX translocation is a critical event in neuronal apoptosis: regulation by neuroprotectants, BCL-2, and caspases. J Neurosci. 1999;19(17):7476–7485. doi: 10.1523/JNEUROSCI.19-17-07476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421(2):189–198. doi: 10.1002/(SICI)1096-9861(20000529)421:2<189::AID-CNE5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Rizzi MD, Hirose K. Aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 2007;15(5):352–357. doi: 10.1097/MOO.0b013e3282ef772d. [DOI] [PubMed] [Google Scholar]
- Santos F, MacDonald G, Rubel EW, Raible DW. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio) Hear Res. 2006;213(1–2):25–33. doi: 10.1016/j.heares.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Schacht J, Hawkins JE. Sketches of otohistory. Part 11: ototoxicity: drug-induced hearing loss. Audiol Neurootol. 2006;11(1):1–6. doi: 10.1159/000088850. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Korsemeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304(3):437–444. doi: 10.1016/S0006-291X(03)00615-6. [DOI] [PubMed] [Google Scholar]
- Sohn D, Graupner V, Neise D, Essmann F, Schulze-Osthoff K, Jänicke RU. Pifithrin-α protects against DNA damage-induced apoptosis downstream of mitochondria independent of p53. Cell Death Differ. 2009;16:869–878. doi: 10.1038/cdd.2009.17. [DOI] [PubMed] [Google Scholar]
- Strom E, Sathe S, Komarov P, Chernova O, Pavlovska I, Shyshynova I, Bosykh D, Burdelya L, Macklis R, Skaliter R, Komarova E, Gudkov A. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat Chem Biol. 2006;2:474–479. doi: 10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- Suli A, Watson GM, Rubel EW, Raible DW. Rheotaxis in larval zebrafish is mediated by lateral line mechanosensory hair cells. PLoS One. 2012;7(2):e29727. doi: 10.1371/journal.pone.0029727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Ushio M, Yamasoba T. Time course of apoptotic cell death in guinea pig cochlea following intratympanic gentamicin application. Acta Otolaryngol. 2008;128(7):724–731. doi: 10.1080/00016480701714244. [DOI] [PubMed] [Google Scholar]
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11(9):621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- Taleb M, Brandon CS, Lee FS, Lomax MI, Dillmann WH, Cunningham LL. Hsp70 inhibits aminoglycoside-induced hair cell death and is necessary for the protective effect of heat shock. J Assoc Res Otolaryngol. 2008;9(3):277–289. doi: 10.1007/s10162-008-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb M, Brandon CS, Lee FS, Harris KC, Dillmann WH, Cunningham LL. Hsp70 inhibits aminoglycoside-induced hearing loss and cochlear hair cell death. Cell Stress Chaperones. 2009;14(4):427–437. doi: 10.1007/s12192-008-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RR, Nevill G, Forge A. Rapid hair cell loss: a mouse model for cochlear lesions. J Assoc Res Otolaryngol. 2008;9(1):44–64. doi: 10.1007/s10162-007-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton C, Parng C. The use of zebrafish for assessing ototoxic and otoprotective agents. Hear Res. 2005;208(1–2):79–88. doi: 10.1016/j.heares.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Van Delft MF, Huang DCS. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16:203–213. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- Vaseva A, Moll U. The mitochondrial p53 pathway. Biochim Biophys Acta. 2009;1787:414–420. doi: 10.1016/j.bbabio.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseva AV, Marchenko ND, Moll UM. The transcription-independent mitochondrial p53 program is a major contributor to nutlin-induced apoptosis in tumor cells. Cell Cycle. 2009;8(11):1711–1719. doi: 10.4161/cc.8.11.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L, Vu B, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu E. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Vicente-Torres MA, Schacht J. A BAD link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. J Neurosci Res. 2006;83(8):1564–1572. doi: 10.1002/jnr.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasits AL, Simon JA, Raible DW, Rubel EW, Owens KN. Screen of FDA-approved drug library reveals compounds that protect hair cells from aminoglycosides and cisplatin. Hear Res. 2012;294(1–2):153–165. doi: 10.1016/j.heares.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden K, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Wang J, Schmitt N, Rubel EW, Lenoir M, Raible DW, Puel J-L. Rapid hearing loss and hair cell degeneration following acute intracochlear perfusion of neomycin in guinea pig. Assoc Res Otolaryngol Midwinter Meet. 2009;32:54–55. [Google Scholar]
- Warchol ME. Cellular mechanisms of aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 2010;18(5):454–458. doi: 10.1097/MOO.0b013e32833e05ec. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong W-X, Cheng EH-Y, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for laboratory use of zebrafish (Danio rerio) 4. Eugene: University of Oregon Press; 2000. [Google Scholar]
- Williams JA, Holder N. Cell turnover in neuromasts of zebrafish larvae. Hear Res. 2000;143:171–181. doi: 10.1016/S0378-5955(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Xie J, Talaska AE, Schacht J. New developments in aminoglycoside therapy and ototoxicity. Hear Res. 2011;281(1–2):28–37. doi: 10.1016/j.heares.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita D, Minami SB, Kanzaki S, Ogawa K, Miller JM. Bcl-2 genes regulate noise-induced hearing loss. J Neurosci Res. 2008;86(4):920–928. doi: 10.1002/jnr.21533. [DOI] [PubMed] [Google Scholar]
- Zhang M, Cramer M (2006) Pifithrin-alpha protects gentamicin ototoxicity. Assoc Res Otolaryngol mid-winter meeting abstract # 121
- Zhang M, Liu W, Ding D, Salvi R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience. 2003;120(1):191–205. doi: 10.1016/S0306-4522(03)00286-0. [DOI] [PubMed] [Google Scholar]