Abstract
While much is known about the metrics and kinematics of gaze shifts to visual targets in cats, little is known about gaze shifts to auditory targets. Here, cats were trained to localize auditory and visual targets via gaze shifts. Five properties of gaze shifts to sounds were observed. First, gaze shifts were accomplished primarily by large head movements. Unlike primates, the head movement in cats often preceded eye movement though the relative timing of eye in head and head latencies depended upon the target modality and gaze shift magnitude. Second, gaze shift latencies to auditory targets tended to be shorter than equivalent shifts to visual targets for some conditions. Third, the main sequences relating gaze amplitude to maximum gaze velocity for auditory and visual targets were comparable. However, head movements to auditory and visual targets were less consistent than gaze shifts and tended to undershoot the targets by 30 % for both modalities. Fourth, at the end of gaze movement, the proportion of the gaze shift accomplished by the eye-in-head movement was greater to visual than auditory targets. On the other hand, at the end of head movement, the proportion of the gaze shift accomplished by the head was greater to auditory than visual targets. Finally, gaze shifts to long-duration auditory targets were accurate and precise and were similar to accuracy of gaze shifts to long-duration visual targets. Because the metrics of gaze shifts to visual and auditory targets are nearly equivalent, as well as their accuracy, we conclude that both sensorimotor tasks use primarily the same neural substrates for the execution of movement.
Keywords: sound localization, saccade, gaze shift, cat
Introduction
Studies of sound localization have traditionally taken a two-pronged approach that uses behavioral experiments in human subjects to determine localization accuracy and precision (Rayleigh 1907; Stevens and Newman 1936; Mills 1958; Populin 2008) and physiological recordings in animals to examine how neural circuits encode these cues (Rose et al. 1966; Goldberg and Brown 1969; Boudreau and Tsuchitani 1968; Yin and Chan 1990). With a view towards integrating behavior and physiology into a single model, we have recently established a behavioral preparation in which cats are trained to look at sound sources. Similar to human psychophysical studies, we have now described the accuracy and precision of the localization of auditory targets by cats under a variety of sound stimulus conditions (Populin and Yin 1998; Tollin et al. 2005) and have shown that the cat experiences several sound localization illusions similarly to humans—the precedence effect (Tollin and Yin 2003; Tollin et al. 2004; Dent and Yin 2005; Dent et al. 2009) and the Franssen effect (Dent et al. 2004). In our studies, we have relied on the position of the cat’s gaze as the indicator of the apparent location where the animal believes the sound is located in space. While there is the tendency to attribute any errors in localization to deficits in sound localization perception, i.e. on the sensory side, at least some component of the error may be on the motor side, especially in animal studies where the instructions to the subject are necessarily indirect.
There are three main aims of this study. The first aim was to try to disambiguate errors on the sensory side from the motor side by comparing the metrics of gaze shifts to visual and acoustic targets placed at identical spatial locations in the same animal using the same motor response metric. If the kinematic parameters, such as the amplitude–velocity relationships (main sequence; Bahill et al. 1975) and the relative contribution of the head and eye in head to the saccades, are very similar in saccades to the two different modalities, then these data would support the hypothesis that there is a common neural substrate for the motor component of the saccades to targets of the two modalities. Moreover, if the gaze shift response error is larger for auditory targets than for visual ones, the difference could be attributed to a difference in the perception of auditory versus visual space. That the properties of saccades to acoustic targets might be different than those of the visual targets was demonstrated in the cat for cases where the head was restrained (Populin and Yin 1999); for example, saccades to auditory targets showed a slow velocity ramp in the direction of the target before the saccade was executed. In the present study, we extend our investigation of metrics to head-unrestrained gaze shifts.
The importance of using the same behavioral response metric to test hypotheses regarding sound localization can be appreciated by the wide variations in estimates of localization error in human psychophysical studies where the instructions to the human subjects were verbal but different behavioral response metrics were used. For example, with broadband noise targets in the frontal field (±45° or so) at zero elevation, Oldfield and Parker (1984) reported absolute errors of 6–8° in azimuth for subjects pointing to the targets with their hands, Makous and Middlebrooks (1990) found errors of 2–6° when subjects faced the target and pointed with their noses, and Wightman and Kistler (1989) found errors averaging 18° when subjects verbally estimated the target in degrees from the point straight ahead. Thus, estimation of errors in localization can differ by almost an order of magnitude depending upon the response method. Following from this observation, a second aim of these experiments was to compare two different metrics for estimating sound localization, position of the gaze, and position of the head. Of course, any differences between gaze and head pointing in the cat cannot be easily compared with differences in human studies since we cannot instruct the cat on how to respond. However, these differences are relevant to many animal studies that have used head pointing rather than gaze to deduce sound localization ability (May and Huang 1996; Huang and May 1996; Slee and Young 2010; Nodal et al. 2008; 2010). One of the important uses of behavioral estimates of localization accuracy and precision is to constrain models and hypotheses of the neural coding of sound source location. Thus, behavioral estimates that differ by an order of magnitude or more are not very useful in this pursuit. For this reason, it is critical to identify the behavioral response metric that best captures the perceptual capabilities of the animal being studied.
The third main aim was to examine the mechanisms by which cats move their eyes and heads to auditory and visual targets. While the overall goal of this research is to examine the mechanisms of sound localization in the cat via their gaze shifts to sound sources, this is complicated by the operation of the vestibulo-ocular reflex (VOR) which reflexively moves the eyes to compensate for head movements in order to keep the image stable on the retina. Since the purpose of a gaze shift, by definition, is to ultimately change the point of view of the retina, the VOR’s natural operation is counter-productive to the purpose of the gaze shift and therefore must be taken into account. The neuronal control/coordination of combined head and eye gaze shifts are usually subsumed in two general models. In one, the “linear summation” model (Morasso et al. 1973; Laurutis and Robinson 1986), the commands to the eyes for a saccade and from the vestibular organ for the VOR are assumed to summate linearly. In this case, the VOR is hypothesized to remain at full gain throughout the gaze shift despite the fact that the VOR signal is opposite to the gaze shift. The advantage of this model is that a single saccadic command can accurately drive the gaze shift, irrespective of the relative contribution of the head to the gaze shift. Support for the linear summation hypothesis comes from synchronous movement of the head and eyes in cats (Blakemore and Donaghy 1980; Guitton et al. 1984) and monkeys (Morasso et al. 1973), the stereotyped gaze shifts, and the reports of the effects of perturbations of the head in mid-saccade suggesting that the VOR gain is not suppressed during the saccade, at least for saccades to targets within the oculomotor range (but see below). However, there are compelling arguments against linear summation that have largely come from measurements of the VOR gain during gaze shifts.
Alternatively, the other popular model posits that the head and eyes receive separate neural drives which allow the VOR to be turned off or attenuated during the gaze shift. The usual way in which the VOR gain has been assessed comes from experiments in which the head movement is unexpectedly perturbed during the gaze shift. If the VOR is fully functioning during a gaze shift, a compensatory eye movement with a velocity equal to the VOR gain would be expected to be superimposed on the ensuing eye velocity. Evidence against linear summation has been reported in cats (Fuller et al. 1983), monkeys (Tomlinson and Bahra 1986a; Tomlinson 1990), and humans (Laurutis and Robinson 1986; Pelisson and Prablanc 1986; Guitton and Volle 1987; Pelisson et al. 1988; Lefevre et al. 1992; Tabak et al. 1996) with several reports that the gain of the VOR varies with the size of the gaze shift (Tomlinson and Bahra 1986a; Tabak et al. 1996; Cullen et al. 2004). Furthermore, recordings from the so-called position-vestibular-pause neurons that are thought to constitute the middle neuron of the three neuron arc of the VOR are well known to pause during gaze shifts (Scudder and Fuchs 1992a, b; Roy and Cullen 1998; Fuchs et al. 2005), consistent with the idea that the VOR is attenuated.
Possible explanations for these mixed results lie in reports of high inter-subject and inter-task variability in the VOR gain during gaze shifts (Guitton and Volle 1987; Cullen et al. 2004) as well as variability in the relative timing of the head and eye movement, the gaze shift amplitude, and the requirements of the task (e.g., whether the target is predictable or not). In primates, the earliest studies suggested that the eye movement began and finished before the onset of head movement (Bizzi et al. 1972; Zangmeister and Stark 1982a, b) in which case the VOR functioning at unity would be most beneficial to maintain the gaze stable in space. In cats, it is more common to find the head movement leading the eye movement (Guitton et al. 1984, 1990) though there are also reports that the eyes always move first (Blakemore and Donaghy 1980). Furthermore, if the VOR is attenuated or turned off during the gaze shift, then the timing of VOR suppression relative to the onset and offset of the head and eye movements must also be considered. Our data presented here in the cat support the idea that the level of attenuation of the VOR depends on the specific time during the saccade that it is being considered and whether the cat is stabilizing or changing direction of gaze.
Methods and materials
Many of our methods and materials have been described earlier (Populin and Yin 1998; Tollin et al. 2005; Tollin et al. 2013).
Subjects and surgery
In three adult female domestic cats, we implanted a stainless steel post on the head and fine wire coils (AS631 or AS632, Cooner Wire Co., Chatsworth, CA) around the globe of each eye under aseptic surgical conditions. During each experiment, only one eye was monitored at a time; the second coil was implanted as a back-up in case of coil breakage or malfunction. A fine wire coil (AS633) oriented in the coronal plane was also embedded in dental cement on the head to monitor head position. Anesthesia was induced with an intramuscular injection of ketamine (20 mg/kg) and maintained throughout the surgery by inhalation of isofluorane (1–2 % in O2) via a tracheal cannula. Postoperative analgesia was provided by ketoprofen (2.0 mg/kg) once a day for 3 days and penicillin was given for 7 days as an antibiotic. All surgical and experimental procedures complied with the guidelines of the University of Wisconsin Animal Care and Use Committee and the National Institutes of Health.
Experimental apparatus and stimuli
All experiments were conducted in a dimly illuminated (or dark) sound-attenuating chamber (2.2 × 2.5 × 2.5 m, IAC, Bronx, NY). All walls and major pieces of equipment were covered with sound-absorbing acoustic foam (10.2 cm, Sonex, Ilbruck, Minneapolis, MN) to minimize acoustic reflections. The dual-phase magnetic search coil (CNC Engineering, Seattle, WA) technique (Fuchs and Robinson 1966) was used to measure the positions of the eyes and head in space and the analog outputs of the coil systems were saved to disk with a sampling rate of 500 Hz.
Both visual and acoustic stimuli were presented from 1 of the 13 different locations distributed along two arcs (62-cm radius), on the horizontal and vertical meridians, ±45° and 0°, ±32° and 0°, ±18° and 0°, ±9° and 0°, 0° and +18°, 0° and +9°, 0° and −14°, 0° and −23°, and 0° and 0°, that were outside the field of the magnetic search coil. Visual stimuli were not available at (+32° and 0°) for cats 17 and 18. Acoustic stimuli consisted of a broadband noise (∼1.5 to 25 kHz) with durations of 15, 25, 40, 100, 164, and 1,000 ms. We used a variety of different auditory target durations to examine the effect of duration on accuracy, as well as to study differences between open-loop and closed-loop situations. Visual stimuli consisted of a red light-emitting diode (LED; λm = 635 nm) located at the center of each speaker cone. The overall amplitudes of the acoustic stimuli with different durations were adjusted to maintain approximately the same power spectrum. The overall level of each acoustic stimulus was varied from trial to trial by ±6 dB in 2-dB steps. The durations of visual stimuli were 1,000 or 25 ms.
Eye coil calibration
The eye coils were calibrated with a behavioral procedure that relied on the natural instinct of the cat to look at a small light source that suddenly appears in the visual field (Populin and Yin 1998). The output of the coil system was recorded when the cat’s eye assumed a stationary position at the end of eye movements evoked by visual stimuli presented from known positions. The vertical and horizontal components of these final eye positions were separately fit with first-order linear functions relating the output of the coil system to the target angle. The coefficients of the least mean square regression (slope and intercept) were then used by the data collection software to convert the voltage output of the coil system to degrees of visual angle. We used a spherical coordinate system specified by angles in azimuth and elevation from the straight-ahead position with positive angles corresponding to rightward in azimuth and upward in elevation. Within the spatial range of this experiment (±50o), the voltage output of the coil system and the location of the target were typically well fit by the first-order linear function. In all cases, the correlation coefficients for fits exceeded 0.96. Control experiments with a dummy coil also showed highly linear fits (r2 = 0.995) with no sign of nonlinearity out to ±60° if the coil was placed in the center of the magnetic field, or ±6 in. from the center in any direction. Six inches is the maximum the cat can move its head within the magnetic field during the behavioral experiments.
Head coil calibration
A small laser pointer was mounted on the cat’s head post, the pitch and yaw of which could be precisely adjusted independent of the cat’s head position. The laser could be gated on and off by the “reward” signal that the computer sent to the peristaltic pump. In this way, when the cat made a “correct” saccade yielding a food reward, the laser pointer would illuminate briefly (∼2 s) a point on the black translucent cloth that hid the speakers and LEDs. While the cat worked on the visual fixation task, we monitored where in space (i.e., azimuth and elevation) the laser pointed via closed-circuit infrared camera or directly when the cat would continue to work with an experimenter in the booth. For trials when the LED was straight ahead, which was defined as 0° and 0°, and the cat visually fixated it, small adjustments were made in the pitch and yaw of the laser pointer until, on average, the laser pointed directly at that LED when the cat fixated it. This procedure gave us the position that the cat held its head as measured by the head coil while looking straight ahead. We then calibrated the head coil by manually moving the cat’s head so that the laser pointed to each of the LEDs in turn along the vertical and horizontal meridians, similar to the method described for the eye coil calibration above. Control experiments with a dummy coil indicated that (1) the calibration was relatively pitch-invariant (which was expected based on our search coil apparatus) and (2) the presence of the experimenter in the recording chamber did not alter the calibration.
Psychophysical procedure and training
All data presented in this paper were collected in the saccade psychophysical task. Here, the cat was initially required to fixate an LED, usually the one straight ahead, and maintain fixation for a variable period of time (600–1,000 ms). Then an acoustic or visual target was presented from another location (within ±45°) and simultaneously the fixation LED was extinguished, which was the signal to the cat to make a gaze saccade to the perceived location of the target. It had to maintain fixation on the target within a prescribed window at that location for 600–1,000 ms in order to receive a food reward. During data analysis, all trials that proceeded to the point of the target coming on were analyzed, including trials with no reward. No requirements were imposed on head position.
Analysis of gaze, eye in head, and head latency
Onset times of movement from target presentation (latencies) of eye-in-head, gaze, and head movement were measured at the “end of fixation,” i.e., the time at which the eye or head position was statistically not zero. It is the time from target presentation until the velocity exceeded two standard deviations from resting velocity as described in Populin and Yin (1998). For the eye in head, latency was measured at the “end of fixation” for movement in the direction of the target, and therefore did not include the time of compensatory rotation in the opposite direction of the head due to the VOR. A negative head latency minus eye latency indicated that the head movement preceded eye-in-head movement.
Analysis of gaze, head, and eye main sequence
A commonly used metric for experiments studying the metrics of eye and/or head movements is the main sequence (Bahill et al. 1975; Guitton et al. 1990; Freedman and Sparks 1997) which, for gaze, is the relationship of maximum gaze velocity versus gaze amplitude. Corresponding plots for the main sequence of eye in head and head were also calculated for both auditory and visual targets. If there were two saccades in one trial, only the first saccade was included in the analysis of main sequence. Amplitude data were grouped into 5° bins and expressed as mean maximum velocity ± 1 standard deviation (SD).
Analysis of eye-in-head and head amplitudes as a function of gaze shift
Each trial had both a gaze and a head movement. Orbit position within the head was computed by subtracting head position from gaze position (referred to as “eye-in-head” movement, shown in Figures 1C and 2C) (gaze − head = eye in head). Eye-in-head saccade amplitude was defined as the maximum displacement of the eye in the head in the direction of the target. Head amplitude was defined as the amplitude of head displacement at the end of head movement.
FIG. 1.
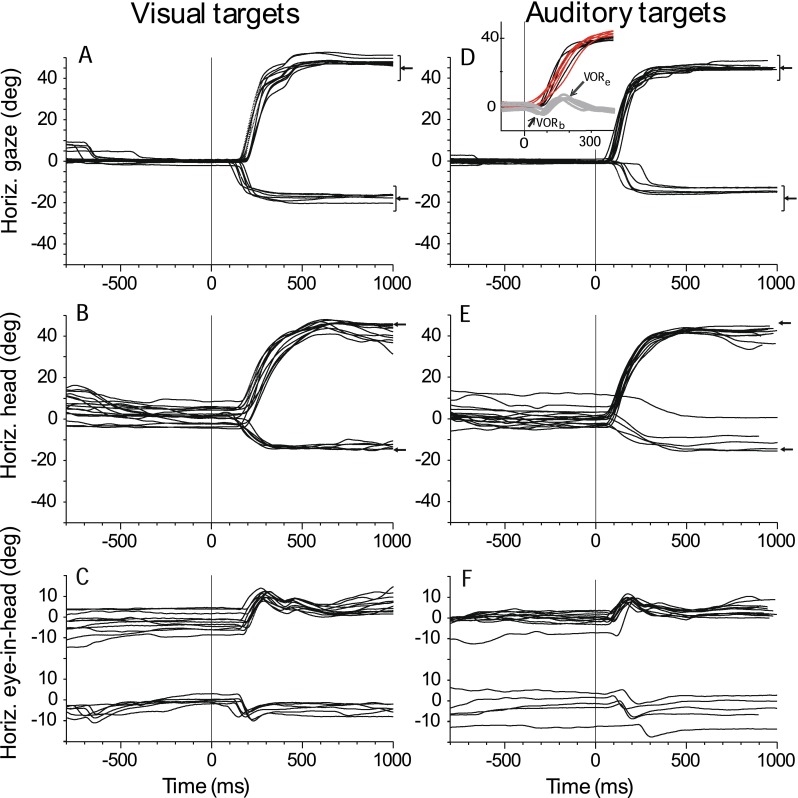
Typical visual (A) and auditory (D) horizontal gaze shifts, visual (B) and auditory (E) head shifts, and visual (C) and auditory (F) eye-in-head movement of cat 18 to long-duration (1,000 ms) targets. The horizontal component of all successful and failed trials from the primary position (0 ° and 0 °) to two targets located along the horizontal ((+45 ° and 0 °) and (−18 ° and 0 °)) axis are plotted as a function of time and synchronized to stimulus onset time (time = 0 ms). Left-pointing arrows in (A) and (D) illustrate the positions of the targets, and the brackets illustrate the sizes of the acceptance window surrounding each target during the session from which these data were taken. Inset in (D) shows an enlargement of the portions of the trials exhibiting the VOR with the gaze (black trace), head (red), and eye-in-head (gray) movements plotted on the same axis. The two components of the VOR at the beginning (VORb) and end (VORe) of the eye movement are indicated by the arrows.
FIG. 2.
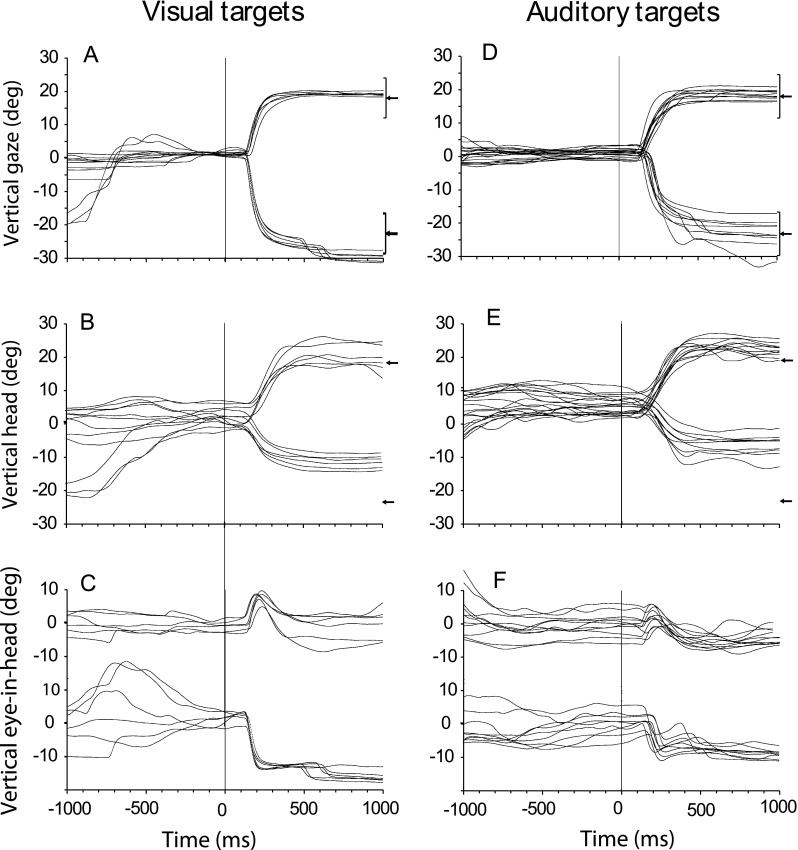
Same as Figure 1 except for two targets in elevation ((0° and +18°) and (0° and −23°)).
Analysis of final gaze and head position
The primary dependent variable in this experiment was the final gaze or head position at the completion of the saccadic shift to the apparent location of the target. Examples of saccadic gaze and head shifts are shown in Figures 1 and 2. We used the method outlined in Populin and Yin (1998) (Fig. 3) to compute separately the final horizontal and vertical gaze or head location. Briefly, we determined the beginning and ending of gaze and head movements by determining the time at which the magnitude of the velocity trace continuously and significantly departed and returned, respectively, to the baseline of steady fixation (i.e., a nominal velocity of zero). The final gaze or head position was the position at the time of the ‘return to fixation’ at the end of the saccade. On some trials, corrective movements were made. Provided the correction occurred within 200 ms of the end of the initial saccade, the final gaze or head position was determined from the return to fixation of the corrective saccade. For the analysis comparing accuracy to noise targets of different durations, we calculated accuracy of localization both incorporating the second saccade (if present) and also utilizing only the first saccade, and compared the results. We did not consider any trials with gaze shifts made greater than 500 ms after the onset of the target or after the reward.
FIG. 3.
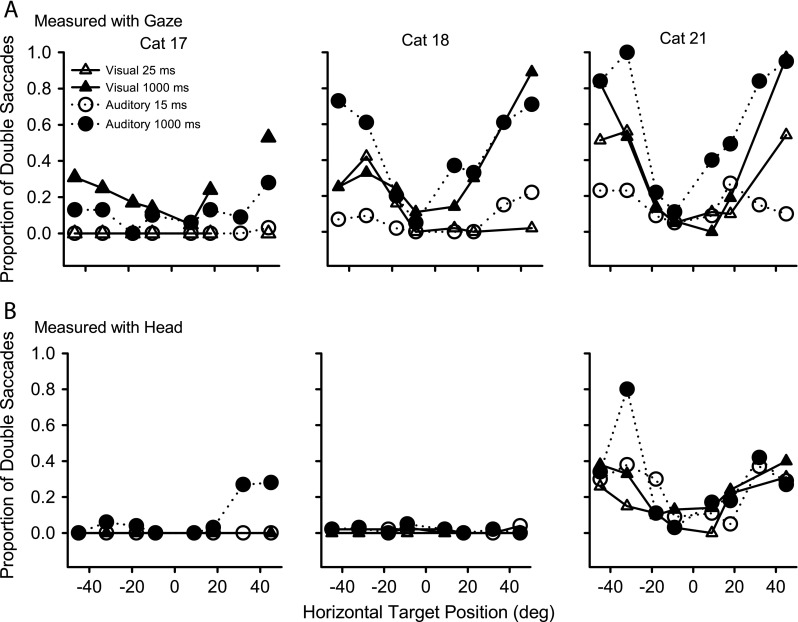
The proportion of horizontal gaze (A) and head (B) movements in the three cats (columns) that were performed using double saccades, plotted against the horizontal location of the target, for long-duration (closed symbols) and transient (open symbols) visual (triangles) and auditory (circles) stimuli.
Data analysis
We define motor error as the difference between the target-in-space position and the initial gaze/head position at the time of target onset: it is the magnitude of the gaze/head shift needed to acquire the target position given the initial gaze/head position. In our experiments, since the cats were always required to fixate the LED at 0° and 0° before the saccade, the gaze motor error is usually nearly equivalent to the position of the target in space. However, because there is no fixation requirement imposed on head position, head motor error may be quite different from the position of the target in space. The gaze/head shift was defined as the angular magnitude and direction of the gaze or head movement. In order to compare data across conditions (e.g., between final gaze and head position), the initial motor error and the final gaze/head shift were computed for each trial, separately for horizontal and vertical components. To obtain a quantitative measure of the localization performance across all target locations, a linear function was fit to the plot of gaze/head shift vs. motor error, separately for the horizontal and vertical components of the target locations (Tollin et al. 2005). This procedure was performed for both the gaze and the head for both visual and auditory stimuli with various durations. The coefficients of the fits are indicators of localization performance. The slope of the response-target localization function, which we shall refer to as a ‘gain’, indicates the accuracy with which the cats localized the targets. A gain of 1.0 indicates that, on average across all trials and all target positions, the cats located the targets to their actual positions, while gains of <1.0 indicate that the localization responses undershot the target. Standard statistical bootstrapping techniques (Efron and Tibshirani 1986) were used to obtain an estimate of the 95 % confidence intervals of the gain. Using the empirical dataset, we also computed the standard deviation of the residuals of the fitted function, which represents the distribution of behavioral responses about the mean gain, again separately for the horizontal and vertical components. This latter value gives a numerical estimate of the precision (or consistency) of the localization responses, which we call δ.
Results
These experiments were designed to examine the metrics of the gaze, head and eye in head in cats to visual and acoustic targets using gaze saccades with their heads unrestrained. The results and statistical analyses are based on the localization performance of three adult female cats.
Gaze, head, and eye-in-head movement to visual and auditory targets
Figure 1A shows typical horizontal saccadic gaze shifts as a function of time from cat 18 to long-duration (1,000 ms) visual and auditory stimuli from the center of gaze to two locations, +45° to the right and −18° to the left, on the horizontal plane. The targets were turned on and the initial fixation LED at 0° and 0° was simultaneously turned off at time = 0. These results support and extend information presented in our earlier report (Tollin et al. 2005), by incorporating horizontal targets beyond the cat’s oculomotor range, increasing from ±18° to ±45° azimuth, and by the analysis of the relative contributions of head and eye movement to gaze. Visual inspection of the gaze traces in the left and right columns of Figure 1 show that gaze shifts to long-duration auditory targets were comparable in general trajectory and in accuracy and precision to those made to long-duration visual targets. The substantial undershooting of the target in saccades with the head restrained (Populin and Yin 1998) was essentially eliminated when the head was also free to move (Tollin et al. 2005). The gaze shifts shown in Figures 1A were accomplished by a combination of head (Fig. 1B) and eye-in-head movements (Fig. 1C) but the similarity of the head and gaze movements shows that most of the gaze shift consists of the head movement component. Figure 2 shows vertical saccades to two long-duration targets, +18° and –23°, on the vertical meridian in the same format as Figure 1. The gaze, head and eye-in-head movements to vertical targets are generally similar to those seen for horizontal targets. One difference is that downward head movements were smaller (Fig. 2B) which resulted in larger downward eye-in-head movements (Fig. 2C). As expected, the initial head position shows more variability than initial gaze position for the horizontal (Fig. 1B) and vertical (Fig. 2B) targets ,since there is no behavioral contingency on head position like there is for gaze position. It is apparent that even with the gaze fixed at (0°, 0°), initial head position at the time of target presentation frequently deviated from 0° to about ±10°. Head position traces to both visual and auditory targets were also more variable than gaze traces, often with undershooting to horizontal, and undershooting or overshooting to vertically placed targets.
It is apparent from the horizontal and vertical eye-in-head movements (Figs. 1C and 2C) that the VOR can be active at both the beginning and end of the gaze shifts. For the horizontal auditory targets (Fig. 1, right column), the head began to move before the eye, resulting in small eye-in-head movements in the opposite direction of the target due to the VOR at the beginning of the gaze shift (referred to here as VORb in the inset to Fig. 1D). Similarly, because of the lower inertia and greater velocity of the eye, in most cases the eye attained the target earlier than the head. At that point, the eye remained fixed on the perceived target position as the head continued to move toward the target, resulting in a VOR at the end of the gaze shift (VORe). As the head moved toward the target, the eye moved in the opposite direction within the head, so that gaze could remain fixed on target (Figs. 1F and 2F). To better illustrate the temporal relationships between the gaze, head and eye-in-head movements, Figure 1D (inset) superimposes all three movement traces.
Double gaze saccades are often used to acquire peripherally located targets
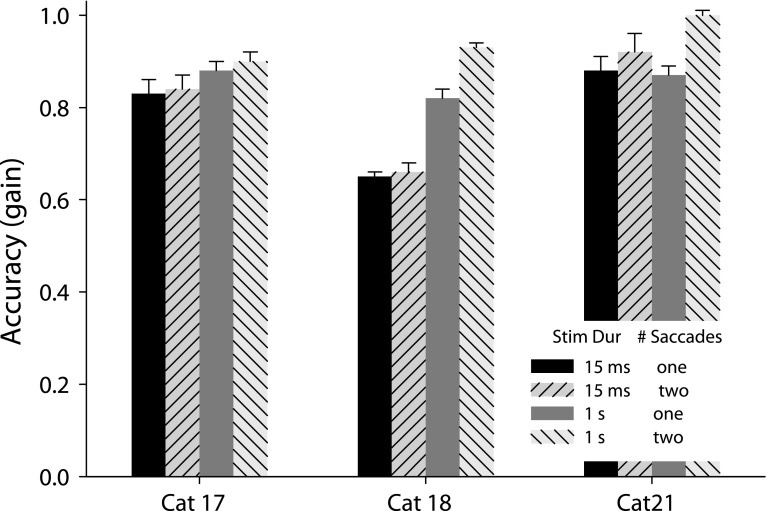
A possible reason for the improvement of localization accuracy with stimulus duration (see Tollin et al. 2005) was that longer stimuli allowed for corrective saccades to be made following the initial saccades. In Figures 1 and 2 it is apparent that many of the gaze and head movements have an associated secondary, or corrective, movement. To be considered a corrective movement in our study, it had to be executed within 200 ms after the end of the initial saccade. Figure 3A shows the proportion of trials that were performed using double saccades, plotted against the azimuth of the target, for long-duration (filled symbols) and transient (open symbols) targets to visual (triangles) and auditory (circles) stimuli. Although the three cats differed from each other in their tendencies to use double saccades, some common patterns were evident. All three cats made fewer double saccades for transient than sustained targets, and more double saccades for peripheral than proximal targets. Cat 17 made more double saccades to visual than auditory targets, whereas the other two cats made more double saccades to auditory targets. The percentage of long-duration visual targets for cats 18 and 21 that elicited double saccades was comparable to the percentage for cat 17, about 10 % for proximal targets, and from 25 % to 95 % for targets out to 45°.
Double head movements to auditory or visual targets are unusual
Figure 3B shows that for two (cats 17 and 18) of our three cats, the head typically reached its final position with one saccade, even for the most peripheral targets. This can also be seen by comparing the raw gaze and head traces of Figures 1 and 2. The other cat (cat 21), who had a substantial number of double head movements also had the greatest percentage of double gaze saccades and the most variability in initial head position during fixation (data not shown). Interestingly, she showed the highest accuracy among all the subjects for localizing long-duration auditory targets (See below.)
Latencies of eye and head movements
Since gaze movement can be initiated by either a head or an eye-in-head movement, a comparison of head and gaze movement latency is needed to determine the overall eye-in-head latency. The distribution of gaze latencies seen in our experiments provides an opportunity to examine whether the head and eye movements are synchronized for visual and acoustic targets. Furthermore a comparison of head and gaze latencies can shed light on whether the VOR is attenuated during the head/gaze shift.
Gaze shift
Figure 4 shows the latency of gaze saccades as a function of gaze motor error for targets in azimuth for all of our subjects for both visual and acoustic targets. Considerable variability was observed both within and across subjects. Cat 21 had the largest variation of latencies, showing nearly uniform dispersion throughout the range, for each target in azimuth. She also showed the most similarity between transient and long-duration and visual and auditory targets. Latencies were distributed from about 20 to 300 ms with some saccades having latencies near 500 ms. Saccades to auditory targets had slightly shorter latencies than those to visual targets. Latencies to proximal targets were no different than latencies to peripheral targets.
FIG. 4.
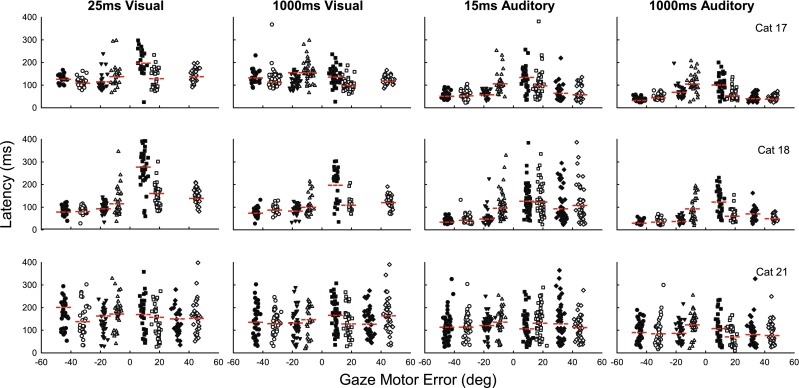
The latency of gaze saccades for the three cats (rows) as a function of motor error for visual (left two columns) and auditory (right two columns) targets in azimuth. Each symbol represents an individual trial. Different shapes and shading of symbols represent saccades to different amplitude targets.
The other two cats showed a greater tendency for shorter saccade latencies to auditory targets, compared with visual targets. The difference between the two modalities was most evident at the shortest latencies, whereas the spread of latencies was comparable. These two cats showed considerably more spread in the latency distribution for proximal than peripheral targets with correspondingly shorter latencies to more peripheral targets, although on many trials, latencies were comparably short for targets at all azimuths. Curiously, both cats 17 and 18 had longer latencies to transient auditory and visual stimuli to the right than to the left.
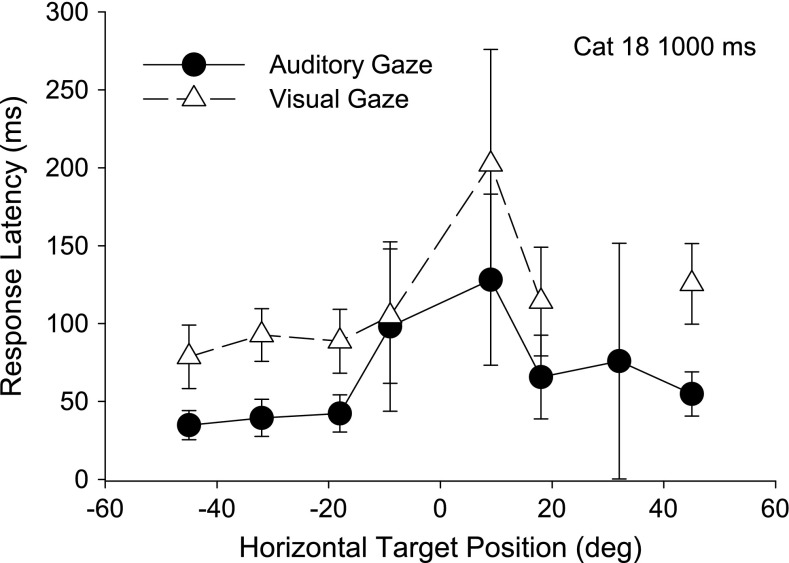
Figure 5 shows the average results of Figure 4 for cat 18, as an example of the few typical generalities noted between cats. Gaze saccades to horizontal auditory targets had significantly shorter latencies than those to visual targets for all three cats (t ranging from −20.93 to −3.15, p < .002) at all but the −9° target for cats 18 and 21 (t = .56, p = .58; t = −1.36, p = .12, respectively). Saccades to the more peripheral targets at 18°, 32°, and 45° tended to have shorter latencies than those to the proximal 9 ° targets, although this difference was not significant.
FIG. 5.
Representative mean gaze latency for one cat (18) with standard deviation bars as a function of azimuth for the same long-duration (1,000 ms) auditory (black, filled circles) and visual (gray, triangles) trials in Figure 4.
Head shift
Qualitatively the head latency data looks strikingly similar to the gaze data for each cat, with similar distinct differences between the cats (not shown). Head movements to auditory targets had slightly shorter latencies than visual, somewhat shorter latencies to long-duration targets than transient ones, as well as a tendency for shorter latencies to more peripheral targets than to proximal targets. As with her gaze latencies, cat 18 has shorter head latencies with transient auditory saccades to the left than to the right.
Synchronization of Gaze and Head Latency
To compare the synchronization of eye and head latencies, we computed the difference between gaze and head latency (Fig. 6A) as well as the difference between eye-in-head and head latency (Fig. 6B). For long-duration (1000 ms) stimuli, for most peripheral targets from ±18° to ±45° the mean of the head and gaze started within 20 ms of each other, for both visual and auditory saccades. For the proximal auditory targets at ±9°, the head tended to lead the gaze by about 30 to 70 ms for all three cats. For visual targets at ±9°, the head led the gaze by about 30 to 50 ms for cat 17 and cat 18; for cat 21 visual targets, head and gaze start at about the same time (Fig. 6A). The mean head latency is always shorter than the mean eye-in-head latency to both auditory and visual targets (Fig. 6B), and the difference between head and eye-in-head latency is greater for proximal than distal targets. Differences between auditory and visual latencies for the comparisons shown in Figure 6A and B were generally not significant due to the high variability in the latencies (Fig. 4).
FIG. 6.
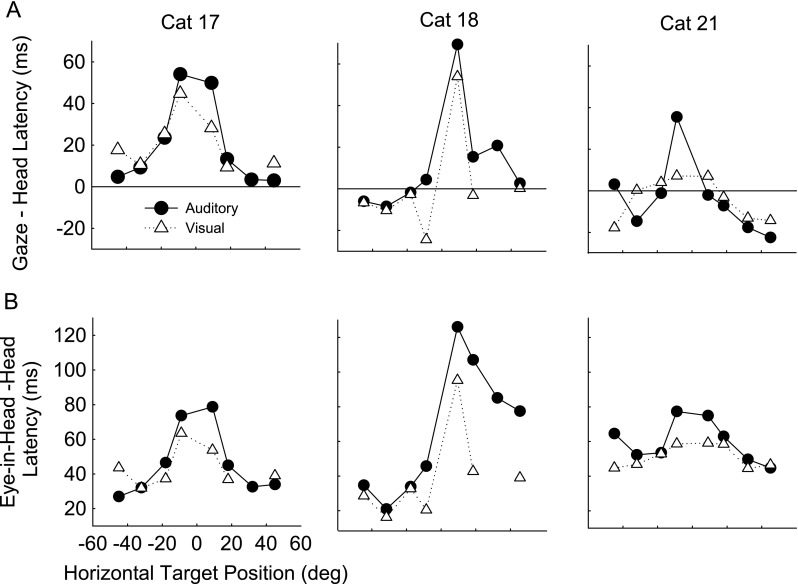
A Gaze minus head latency for the three cats (columns) plotted against target position for auditory (black, filled circles) and visual (gray, triangles) trials to long-duration (1,000 ms) horizontal targets. B Eye-in-head minus head latency plotted against target position. Each symbol represents the mean of 20–49 trials (average 36). SD were large and in all cases, differences were not significant but show only trends; for example, cat 17, the cat with the lowest SD, had gaze minus head latencies to visual targets with SD ranging from 10 to 49 ms while cat 21 had the largest SDs ranging from 48 to 103 ms for gaze minus head latencies to auditory targets.
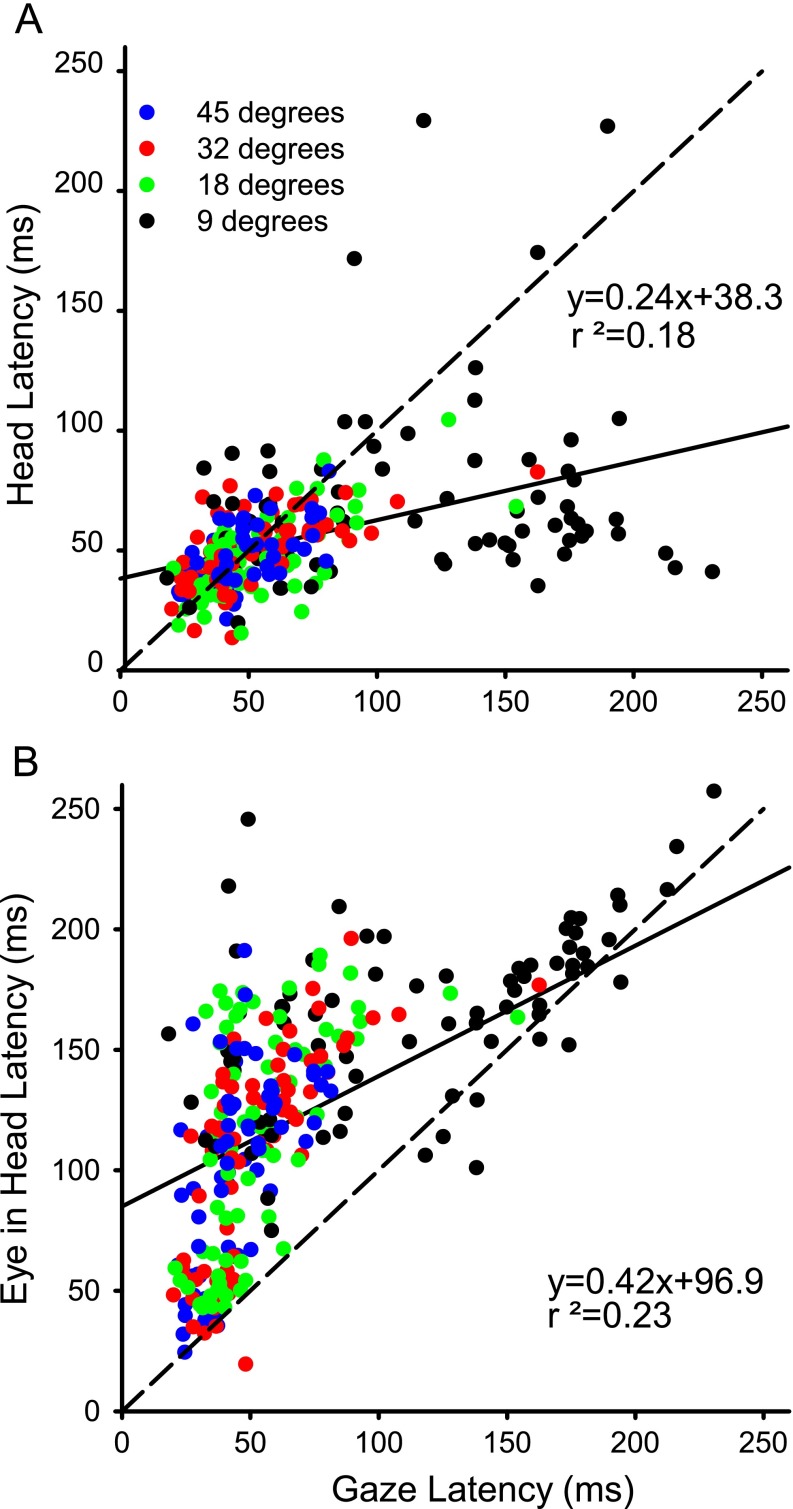
One of the unusual features of the latency data shown in Figure 4 was the wide distribution of latencies at many of the azimuth locations in all three cats, especially for proximal targets. Figure 7A plots the head latency as a function of gaze latency for long-duration auditory targets for cat 18. Clearly, the data do not cluster along the line of unity and the linear regression has a low r2 value (0.18). The lack of a strong correlation suggests that the commands to move the gaze and the head are largely independent. Moreover, the majority (53 %, 151/283) of the data points are below the line of unity which reflects the propensity for the head to move first in the cat; this is especially prominent for the most proximal target (9°), where 53/74 (72 %) are below the line. Similar results were seen for short duration targets and for visual targets in all three cats. For the three cats, for four types of targets (auditory and visual for long and short duration) the mean r2 value under these 12 conditions was 0.24 (n = 286–623).
FIG. 7.
A Representative head latency as a function of gaze latency for cat 18 to long-duration (1,000 ms) auditory sources. B Eye-in-head latency for cat 18 as a function of gaze latency. Each symbol represents a single trial. Dashed line is line of unity while solid line is the linear regression.
Figure 7B plots eye-in-head latency as a function of gaze latency for the same data as in Figure 7A. While more of the points are clustered around the line of unity, a prominent feature of these data is that 261/283 (92 %) of the points are above the line, meaning that the gaze latency was almost always shorter than eye latency. As expected from the data of cat18 shown in Figure 4, the data points with long gaze latencies tend to be to proximal targets and for these points the difference between the onset of head and gaze latency is large (the cluster of points in the lower right quadrant of Fig. 7A) while the same trials in Figure 7B tend to be near the 45° line in the upper right quadrant of Figure 7B. Similar results were seen in all three cats under all conditions.
In order for the gaze latency to be regularly shorter than the eye-in-head latency (Fig. 7B), the gaze must be changing due to a head movement that precedes the eye movement. On the other hand since the percentage of trials with head latency shorter than gaze latency is only 53 %, this suggests that for many trials the onset of gaze must be due to the head moving with the VOR turned off or attenuated so that the head and gaze latency are similar. In Figure 7A, the many points at short latencies that cluster around the line of unity must reflect trials in which the head and gaze tended to move together while the eye-in-head followed later (Fig. 7B).
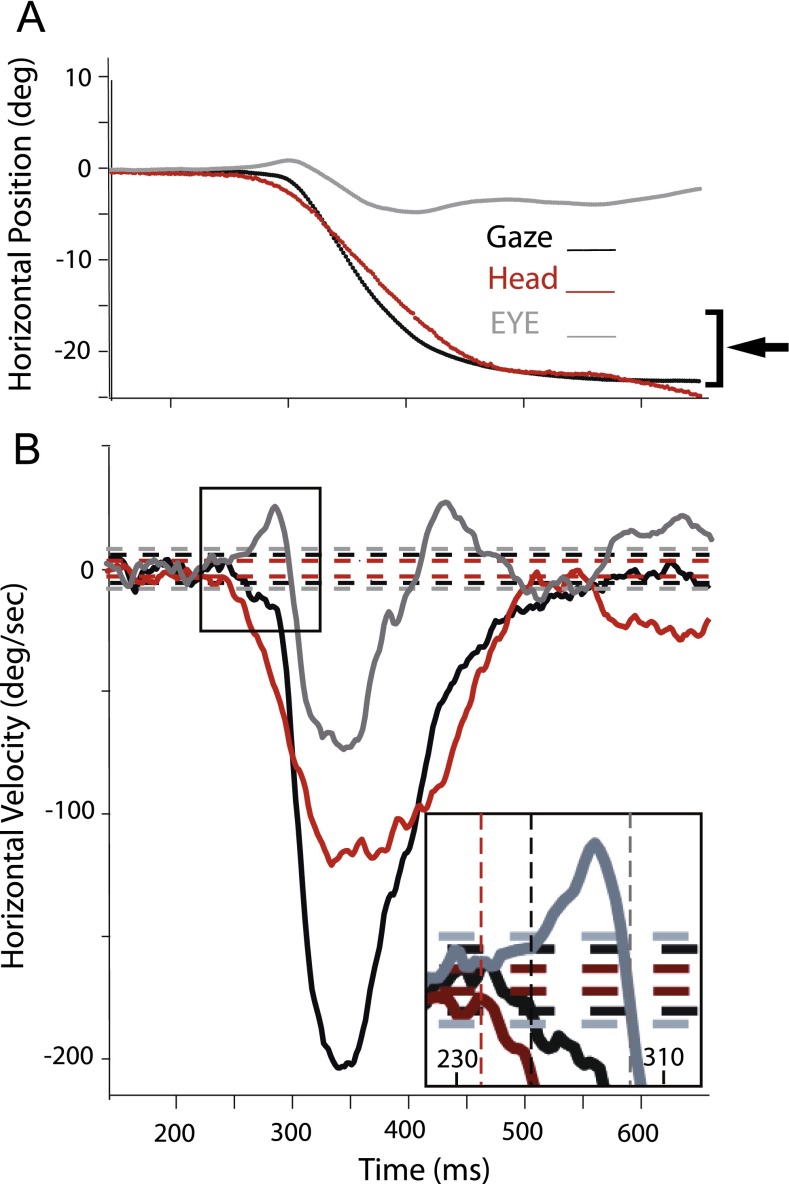
A typical example of the positions and velocities of gaze, head and eye-in-head positions to a horizontal target is illustrated in Figure 8 and shows that when the head moves first, the eye-in-head momentarily moves in the opposite direction due to the action of the VORb (Fig. 1 and inset of Fig. 8, bottom panel). For the example shown in Figure 8, the VORb gain must be < 1 since the gaze is not stable immediately following the head movement but begins to drift in the direction of the head. Thus, the fact that for over 90 % of trials the gaze latency is shorter than eye-in-head latency (Fig. 7B) suggests that the VOR is turned off or attenuated before the eye saccade is executed so that the eye is no longer stabilized in space by the VOR and drifts in the direction of the moving head towards the target. This gaze movement tends to have a slower velocity ramp than the subsequent gaze movement that appears to be coincident with the onset of the eye-in-head movement. Table 1 shows the percent of the trials that the head led the gaze, and the mean difference between the latency of onset of the gaze and head movement in each of the three cats for auditory and visual trials. Table 2 shows similar data for head leading eye in head and the difference in latency between eye in head and head.
FIG. 8.
Typical (A) position and (B) velocity traces to a long-duration noise target located at −18 ° and 0 °. Horizontal lines in (B) indicate ±2 SD from the mean resting velocity for the head (red), gaze (black), and eye-in-head (gray) movements. Vertical dashed lines in the inset of (B) indicate the beginning of movement as defined in methods for the head (red), gaze (black), and eye-in-head (gray) movements.
TABLE 1.
Mean difference ± SD and percent time head leads gaze
| Cat | Auditory (A) or visual (V) | Gaze—head (ms) latency | Head leads |
|---|---|---|---|
| 17 | A | 19.94 ± 33.07 | 74 % (178/241) |
| 18 | A | 13.24 ± 46.72 | 53 % (151/283) |
| 21 | A | −4.07 ± 56.75 | 46 % (144/315) |
| Mean | A | 9.7 | 58 % |
| 17 | V | 20.94 ± 33.3 | 73 % (165/227) |
| 18 | V | 0.32 ± 58.31 | 44 % (98/223) |
| 21 | V | −5.02 ± 76.67 | 43 % (136/315) |
| Mean | V | 5.02 | 53 % |
TABLE 2.
Mean difference ± SD and percent time head leads eye
| Cat | Auditory (A) or visual (V) | Eye—head (ms) latency | Head leads |
|---|---|---|---|
| 17 | A | 48.00 ± 31.74 | 98 % (236/241) |
| 18 | A | 67.01 ± 51.07 | 92 % (261/283) |
| 21 | A | 59.46 ± 53.80 | 89 % (280/315) |
| Mean | A | 58.2 | 93 % |
| 17 | V | 45.85 ± 34.79 | 93 % (212/227) |
| 18 | V | 38.41 ± 60.00 | 90 % (198/223) |
| 21 | V | 51.31 ± 66.64 | 82 % (259/315) |
| Mean | V | 45.12 | 88 % |
To summarize the results of latency measurements, gaze shifts to auditory targets had consistently shorter latencies than to visual targets; for both modalities the head movement latencies were usually shorter than both gaze and eye-in-head movement latencies. For over 90 % of trials, the gaze latency was shorter than eye-in-head latency showing that the VOR is shut off or attenuated during the head movement. Latencies were shorter for more peripheral targets than central ones, particularly in the case of auditory saccades.
Main sequence
For comparisons with other studies, we studied whether the gaze, head, and eye-in-head saccades were well described by their main sequence (Bahill et al. 1975). Figure 9 plots, for long-duration stimuli for cat 18, the main sequence for gaze, head, and eye in head and shows a linear relationship between amplitude and maximum velocity, one criterion for describing a movement as a “saccade.” Head main sequence amplitude/peak velocity functions showed only about 50 % of the maximum velocities of gaze or eye-in-head main sequences. Figure 10 shows for another cat (cat 21) saccade duration plotted against amplitude for long-duration targets for both auditory and visual targets for gaze, head, and eye-in-head saccades. Functions describing movements to horizontal targets show a linear relationship between amplitude and saccade duration, although not as orderly as the amplitude/peak velocity main sequence functions. Eye-in-head movements, but not gaze or head movements, to vertical targets show a weak linear relationship between amplitude and duration. In general, there was very little difference in the slopes for auditory compared with visual main sequence (see Table 3 for amplitude/peak velocity summary.)
FIG. 9.
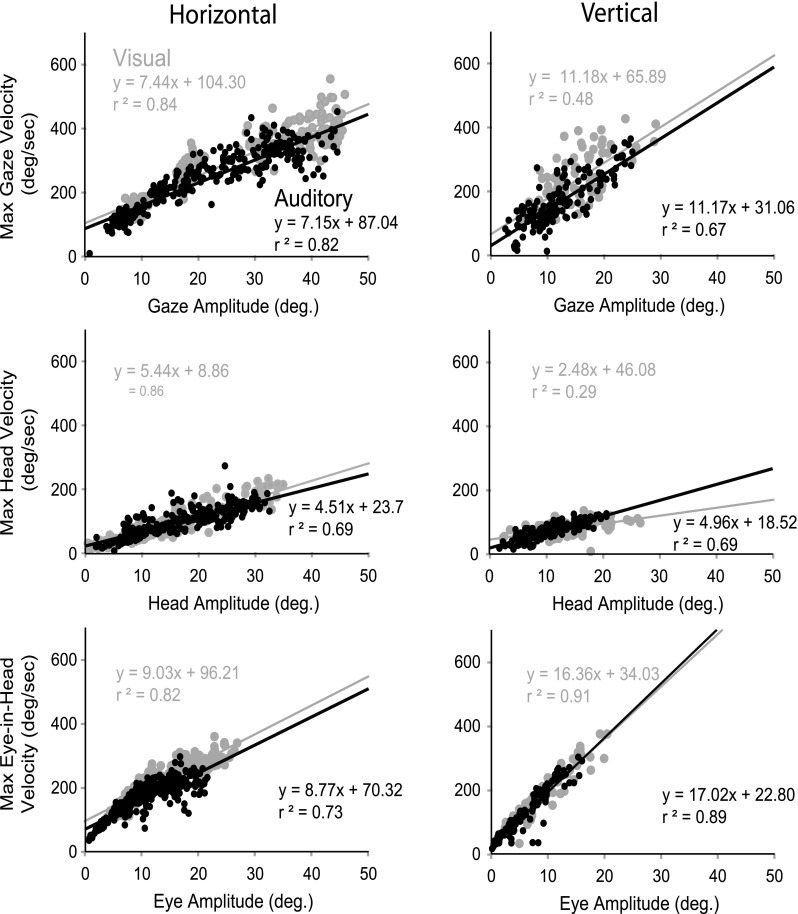
Representative plot of main sequence for one cat (18) relating peak velocity and amplitude for gaze (A), head (B), and eye in head (C), for horizontal (left) and vertical (right) long-duration auditory (black) and visual (gray) targets. Black and gray lines indicate linear regressions for auditory and visual sources, respectively.
FIG. 10.
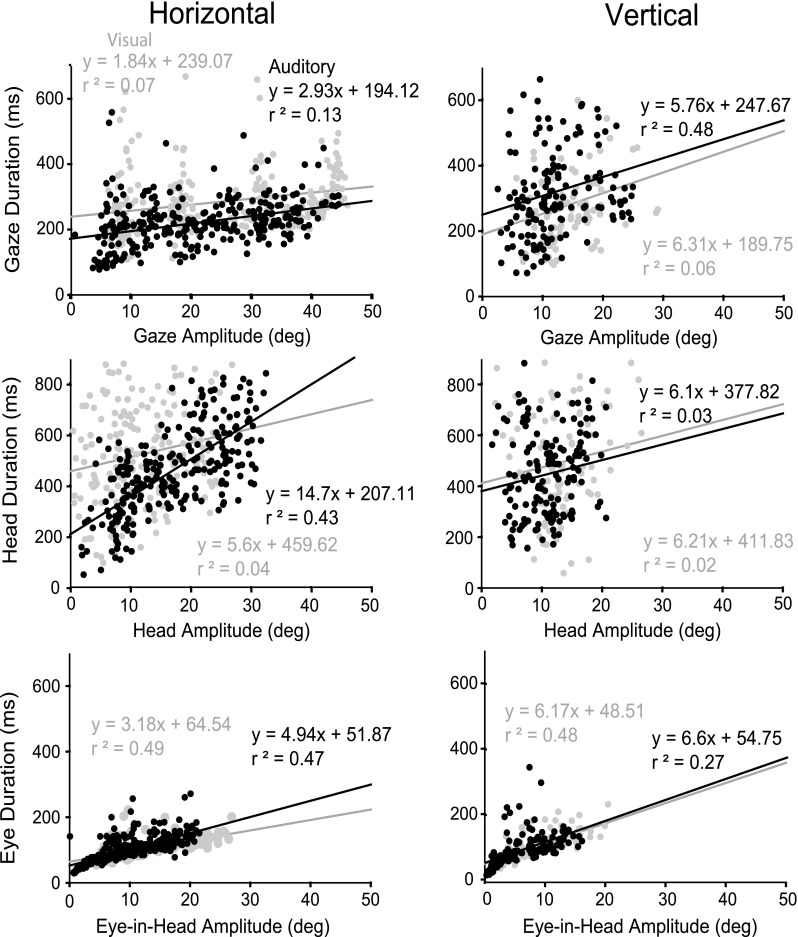
Representative duration of movement for one cat (21) plotted against amplitude for gaze (A), head (B), and eye in head (C), for horizontal (left) and vertical (right) long-duration auditory (black) and visual (gray) targets. Black and gray lines indicate linear regressions for auditory and visual sources, respectively.
TABLE 3.
Slope of amplitude/peak velocity functions (in seconds), mean ± SD
| Cat | Visual | Auditory |
|---|---|---|
| Gaze | ||
| 17 | ||
| Elevation | 4.95 (3.34–6.53) | 3.23 (2.17–3.89) |
| Azimuth | 8.31 (7.61–8.93) | 11.15 (10.48–11.61) |
| 18 | ||
| Elevation | 11.18 (8.66–12.96) | 11.17 (9.7–12.2) |
| Azimuth | 7.44 (7.02–7.88) | 7.15 (6.6–7.47) |
| 21 | ||
| Elevation | 8.54 (6.78–10.09) | 9.41 (7.44–10.76) |
| Azimuth | 7.32 (6.72–7.84) | 5.56 (5.03–5.97) |
| Head | ||
| 17 | ||
| Elevation | 1.94 (.82–2.84) | 2.81 (1.81–3.62) |
| Azimuth | 5.76 (5.45–6.04) | 7.45 (7.05–7.83) |
| 18 | ||
| Elevation | 2.48 (1.88–3.3) | 4.96 (4.33–5.42) |
| Azimuth | 5.44 (5.09–5.69) | 4.51 (4.12–4.76) |
| 21 | ||
| Elevation | 5.32 (4.33 6.3) | 5.24 (4.14–6.16) |
| Azimuth | 5.04 (4.57–5.46) | 4.48 (4.07–4.78) |
| Eye | ||
| 17 | ||
| Elevation | 12.69 (10.78–14.17 | 16.00 (14.65–16.89) |
| Azimuth | 11.77 (10.94–12.6) | 15.68 (14.71–16.77) |
| 18 | ||
| Elevation | 16.35 (15.14–17.58) | 17.01 (16.03–18.00) |
| Azimuth | 9.03 (8.38–9.57) | 8.77 (7.89–9.34) |
| 21 | ||
| Elevation | 15.01 (12.85–16.93) | 11.60 (9.01–13.58) |
| Azimuth | 9.44 (8.34–10.3) | 7.29 (6.67–8.12) |
In order to perform a statistical comparison between auditory and visual peak velocity, trials were binned in 5° increments and expressed as mean peak velocity ±1 SD. No statistical differences (p > 0.05) were noted between visual and auditory maximum velocities as a function of saccade amplitude, for any gaze, head, or eye-in-head saccade except that the maximum vertical gaze saccades for cat 17 were greater to visual targets than to auditory targets (data not shown).
Eye-in-head amplitude and velocity as a function of target position
As target eccentricity increased, both amplitude and peak velocity of eye in head increased. The mean maximum eye-in-head amplitude for the three cats to visual targets was 24.8° in azimuth and 18.0° in elevation. For auditory targets, the mean maximum eye-in-head amplitude in azimuth was 21.9°, in elevation 15.7° (Table 4). Peak eye-in-head amplitude was statistically greater (p < 0.05) in visual compared with auditory saccades to the same amplitude horizontal targets to 26/34 targets for the three cats. Peak eye-in-head velocity was statistically greater (p < 0.05) in visual compared with auditory saccades to the same amplitude targets for 25/34 horizontal and vertical targets.
TABLE 4.
Mean maximum eye-in-head amplitude (in degrees)
| Cat | Visual | Auditory | ||
|---|---|---|---|---|
| Azimuth | Elevation | Azimuth | Elevation | |
| 17 | 25.5 | 16.34 | 22.56 | 14.76 |
| 18 | 26.97 | 20.53 | 21.24 | 16.05 |
| 21 | 21.87 | 17.02 | 21.94 | 16.22 |
| Mean | 24.78 | 17.96 | 21.91 | 15.68 |
Gaze and head accuracy and precision
Figure 11A shows the final horizontal and vertical gaze position for the 12 most extensively tested target locations with varying stimulus duration and modality for cat 18. The responses to long-duration visual and auditory trials were tightly clustered (good precision) and located near each target in azimuth and elevation (good accuracy). It is apparent from the scatter plots in Figure 11A that the visual responses were slightly more precise and accurate than the auditory for both long and short duration stimuli. To quantify these qualitative observations, the eight figures comprising Figure 11B show scatter plots of actual gaze displacement (i.e., the angular distance and direction that the eyes moved in space) as a function of gaze motor error (i.e., the angular distance between the initial position of the eyes and the actual target) for cat 18 for the same four stimulus conditions. Separate plots are presented for horizontal and for vertical components of the responses. The assumption that gaze and head shift changed linearly with target eccentricity can be evaluated by the first-order correlation coefficient r which was between 0.91 and 1.00 for azimuth and between 0.66 and 0.99 for elevation (mean r = 0.94 ± 0.06, data not shown). The correlation coefficients of the fitted functions for all conditions (gaze and head position, see later) and all cats are highly significant (p < 0.05). As in the raw data shown in Figures 1A, 2A, and 11A, the cats’ responses were nearly as accurate in elevation as in azimuth and were similar for auditory and visual targets of long duration. In all cases, the accuracy and precision are improved for the long-duration stimuli as compared with the short duration as measured by the gain and delta measures, respectively.
FIG. 11.
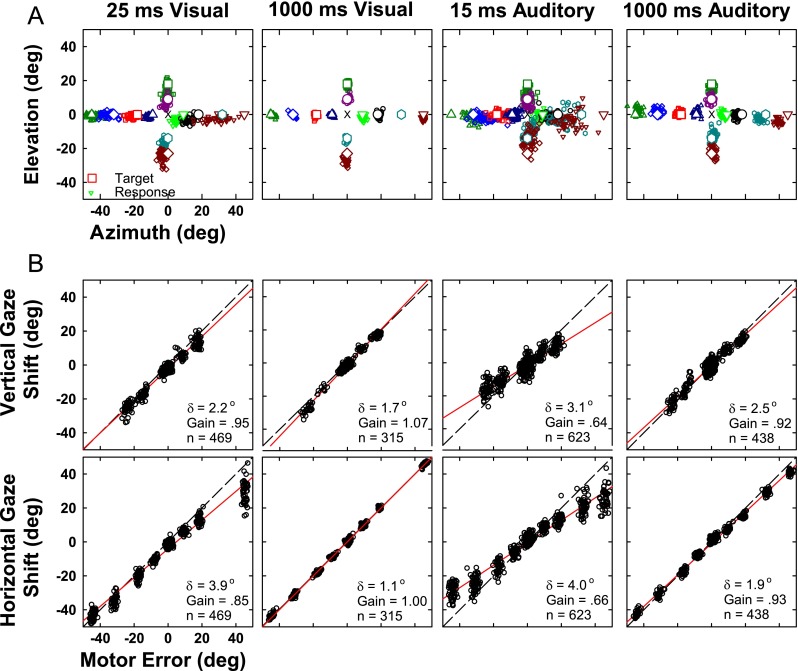
Localization of long-duration (1,000 ms) and transient (25 ms) visual and (15 ms) auditory targets. A Final two-dimensional gaze position (small symbols) for stimuli presented from 12 target locations (corresponding large open symbols at locations ±45 ° and 0 °, ±32 ° and 0 °, ±18 ° and 0 °, ±9 ° and 0 °, 0 ° and 18 °, 0 ° and 9 °, 0 ° and −14 °, and 0 ° and −23 °). Central fixation LED is shown as an error mark. B Accuracy of the vertical (saccade amplitude elevation, top) and horizontal (saccade amplitude azimuth, bottom) components of the saccades to the 12 targets. Each point corresponds to a single trial. The motor error (abscissa) is the horizontal or vertical component of the distance between the initial gaze position on each trial and the actual position of the target. The saccade amplitude (ordinate) is the corresponding horizontal or vertical component of the response to that target position from the initial gaze position. Solid red line indicates the linear regressions of saccade amplitude component and the motor error. Gain is the slope of the regression line and represents localization accuracy (gain = 1 corresponds to perfect localization accuracy). δ is the residual error after regression and is an indication of response precision or consistency. n is the number of trials. Dashed line, line of unity. Data are from cat 18.
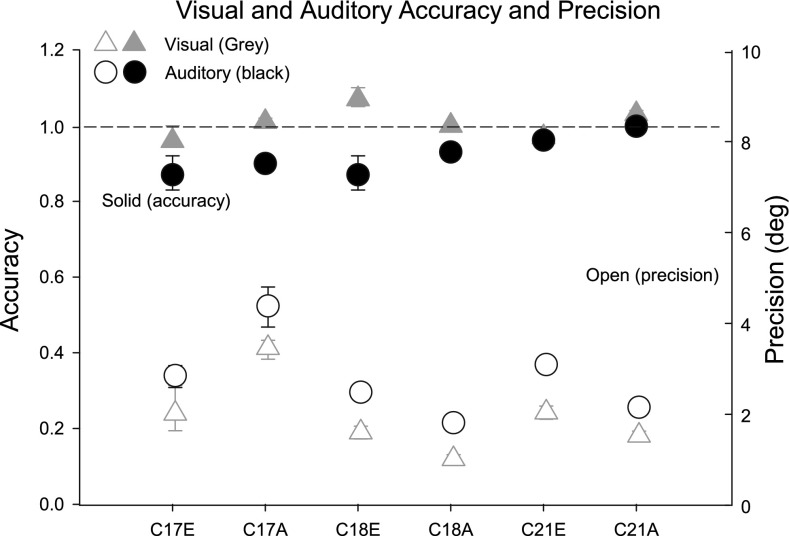
Accuracy and precision of gaze responses to long-duration visual and auditory stimuli for each of the three cats are displayed in Figure 12. Averaging responses of all three cats to long-duration targets in both azimuth and elevation, the responses were less precise (greater δ), for long-duration auditory (mean δ = 3.0°) than long-duration visual (mean δ = 2.0°) targets. The difference in precision between auditory and visual targets was significant (p < 0.05) for all three cats in azimuth, and two of three cats in elevation. For cats 18 and 21, responses to long-duration auditory and visual vertical targets were significantly less precise than responses to long-duration horizontal targets, as determined by bootstrapping techniques.
FIG. 12.
Plots of response accuracy (gain (filled symbols)) and precision (δ (open symbols)) with associated 95 % confidence intervals for the three cats to sources in elevation (E) and azimuth (A). The subjects are identified along the abscissa; for example “C17E” refers to data from cat 17 for sources in E. Auditory sources are indicated by black circles and visual targets by gray triangles.
In the same format as Figure 11A for final gaze position, Figure 13A shows the final horizontal and vertical head position for cat 18. Responses as measured by head position showed more scatter and considerably more undershooting than the same trials as measured by gaze position (Fig. 11). The undershooting is most evident in the gain values in Figure 13B which ranged from 0.41 to 0.72 (mean of 0.56) as compared with gain values in Figure 11B which ranged from 0.64 to 1.07 (mean of 0.88). Little difference was obvious between auditory and visual, or transient and the long-duration targets. Comparison of Figures 11 and 13 shows that localization accuracy as measured by head position produced gains only 60 to 70 % of those same trials measured by gaze position.
FIG. 13.
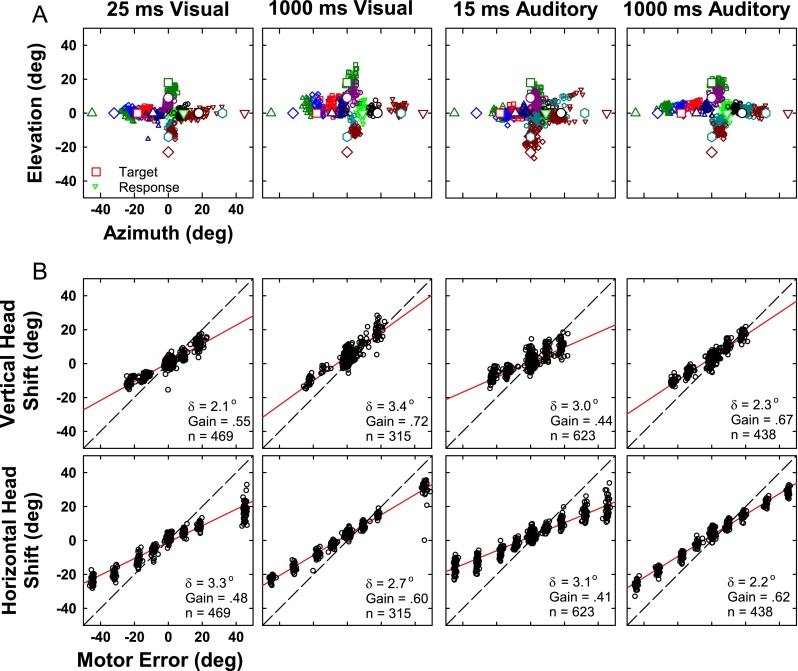
Same as Figure 10 except for head position.
Eye-in-head and head amplitudes as a function of gaze amplitude
For all three cats, the head contributed to gaze saccades of all amplitudes. Table 5 shows the slope and confidence intervals of head and eye-in-head amplitude as a function of gaze shift amplitude for visual and auditory trials to horizontal and vertical targets. The slope of these functions is indicative of the head contribution to gaze (at the end of head movement) and the eye-in-head contribution to gaze (at the peak eye-in-head movement), respectively. Note that the sum of head contribution and eye-in head contribution is not equal to 1.0 because these measurements are taken at different time points in the gaze shift. Overall on average the head contribution to gaze is about 78 % for targets in azimuth and elevation for both auditory and visual sources. For two of three cats in azimuth and in elevation, head contribution to gaze shift was greater in auditory than visual trials. For cat 18, at the end of head movement to upward vertical targets, the position of the eye is actually at a more negative position in the head, on average, than it was at the start of the saccade (Fig. 2C right). The head overshoots the target so much that in order to maintain gaze fixation on the target, the eye must counter rotate in the head to a position that is more negative than the starting position. For three of three cats in azimuth and two of three cats in elevation, eye-in-head contribution to gaze was greater in visual than auditory trials.
TABLE 5.
Head contribution to gaze shift (at end of head movement) gain (95 % confidence interval)
| Cat | Azimuth | Elevation | ||||
|---|---|---|---|---|---|---|
| Auditory | Visual | Significance | Auditory | Visual | Significance | |
| 17 | 0.76 (0.75–0.77) | 0.76 (0.74–0.77) | N | 1.23 (1.14–1.31) | 1.04 (0.99–1.1) | Y |
| 18 | 0.66 (0.65–0.67) | 0.60 (0.57–0.61) | Y | 0.71 (0.69–0.74) | 0.64 (0.60–0.68) | Y |
| 21 | 0.71 (0.69–0.72) | 0.68 (0.66–0.69) | Y | 0.81 (0.77–0.86) | 0.81 (0.76–0.85) | N |
For all three cats, there was a tendency for the cat to undershoot downward targets with the head for both visual and auditory targets as compared with upward movements, which is most evident in the scatter diagrams of Figure 13A, with few responses near the target at −23° down. Despite the small downward head movements to that target, localization by vertical gaze was accurate (Fig. 11A), which suggests a difference in the motor programming of downward localization in both modalities.
Localization accuracy is better represented by final gaze than final head position
Plots like those shown for cat 18 in Figures 11 and 13 were constructed for each of the 3 cats and performance was quantified, separately for the azimuthal and elevational components of the responses, in terms of response gain and scatter about the mean as described in the “Methods and materials.” Figure 14 summarizes the performance separately for all three cats. The gains with the 95 % confidence intervals of localization as a function of stimulus duration are plotted for visual (left column), and auditory (right column) conditions for the azimuthal (bottom) and elevational (top) components of the gaze (closed symbols) and head (open symbols) movement responses. It is apparent for all cats with the exception of cat 17 in elevation for both visual and long-duration auditory stimuli, that for all conditions, the final gaze position gains were higher than the final head position gains. The gains of final head position were generally more similar within a cat than between cats. (cat 17, 0.70 to 0.76; cat 18, 0.57 to 0.66; and cat 21, 0.65 to 0.72) In other words, the degree to which final gaze was accomplished with a head movement was more a function of individual subjects than stimulus condition.
FIG. 14.
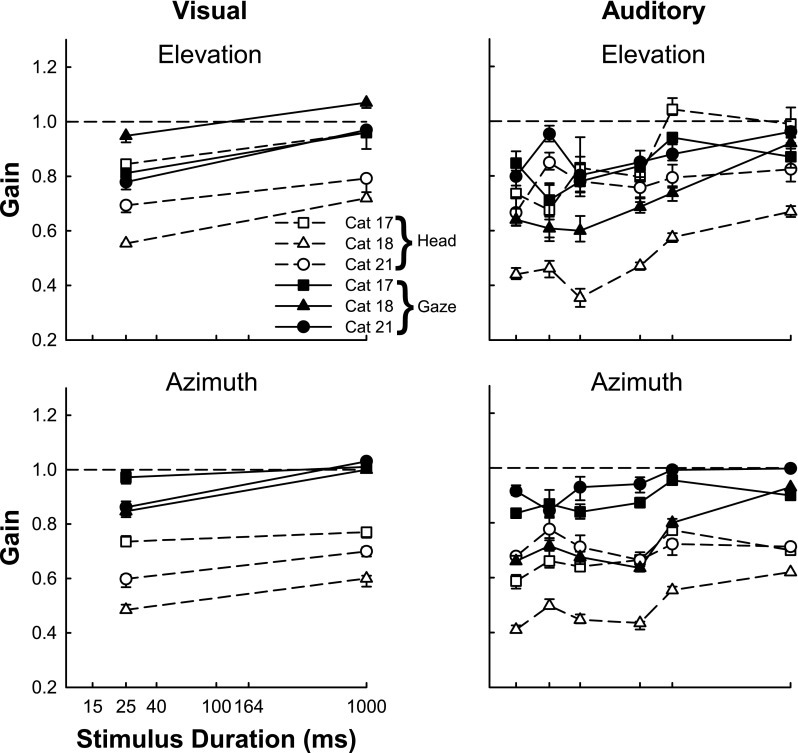
Localization accuracy improves for auditory and visual targets when measured with the gaze compared with the head and with increasing stimulus duration. Response accuracy (gain) and associated 95 % confidence intervals (see “Methods and materials”) for all three cats (filled and empty squares, cat 17; filled and empty triangles, cat 18 and; filled and empty circles, cat 21) are plotted as a function of stimulus duration for the gaze (solid line and filled symbols) and the head (dashed line and empty symbols).
Gaze localization affected by stimulus duration
As shown in Figure 11 for cat 18 and summarized in Figure 14 for all cats, accuracy and precision declined at the shortest stimulus durations, similarly for both visual and auditory targets, under all conditions. This is not surprising since at the shortest duration the eye movement latency is longer than the stimulus duration so there is an element of memory required to make the saccade to the target. Decrease in accuracy may also be attributed to an increased propensity to make corrective gaze shifts in the long-duration case where new evaluation of sensory information may contribute. The effect of sound duration on the spectral characteristics of the stimuli may contribute, as well as perceptual decision factors based on increased decision time to form the location judgment independent of motor output. To further explore this we analyzed the localization accuracy of short and long-duration stimuli, using the first saccade only, and compared these results to when corrective movements were included in the analysis. Figure 15 shows for three cats the accuracy of localization (error bars show 95 % confidence intervals) to short and long-duration noise targets in azimuth using data from both saccades (if present) compared with data from only the first saccade. For cat 21, addition of information from the second saccade improved accuracy for both short and long-duration stimuli. For the other two cats, accuracy generally improved for longer duration stimuli even when only the first saccade was considered. Accuracy improved further by including information gained from the second saccade, to varying degrees in the two cats.
FIG. 15.
Localization accuracy improves for longer duration stimuli even if only the first saccade to the stimulus is considered. Response accuracy (gain) and associated 95 % confidence intervals (averaged and displayed in one direction) for all three cats.
Discussion
Although the metrics and kinematics of gaze shifts to visual targets in cats has been well documented (Guitton et al. 1984, 1990), much less is known about the metrics of gaze shifts to auditory targets. Likewise, there has been extensive work describing eye-head coordination to visual targets in humans and monkeys (see Scudder et al. 2002; Freedman 2008 for review), while relatively less is known about these mechanisms to auditory targets (Whittington et al. 1981; Populin and Rajala 2011; (monkeys); Fuller 1992; Goossens and Van Opstal 1997; Zambarbieri et al. 1997; Corneil and Munoz 1999; Populin et al. 2002 (humans)). In this discussion we will first compare our study of visual saccades to those of others in cats. We will show that there are only subtle differences between the metrics of auditory and visual saccades in our cats, suggesting that any differences in accuracy and precision can be attributed to differences in localization perception. We will discuss the presence of the VOR in visual and auditory saccades. Finally, we will discuss our preference to use the position of gaze rather than position of head for determining where cats localize targets in space.
Comparison to other studies: metrics
Eye/head movements to visual targets in cats
Although their visual targets and behavioral tasks were different than ours (visual objects rather than LEDs), the properties of saccades to horizontal visual targets in our data agree well with other studies in cats (Guitton et al. 1984; 1990; Blakemore and Donaghy 1980). In those studies, the maximal eye-in-head amplitudes with head free saccades all fall in the range of 25° to 28° (Guitton et al. 1990; Blakemore and Donaghy 1980). Our three cats had mean maximal eye-in-head amplitude of 24.8° for horizontal saccades to visual targets. Despite a limited oculomotor range, cats can accomplish saccades to targets well outside this range with one smooth coordinated movement of the eye and head, although secondary corrective saccades are also common. In addition our results confirm these earlier studies that head movement accompanies saccades of all amplitudes, to targets both inside and outside of the oculomotor range as well as that main sequence functions show a linear relationship between gaze saccade amplitude and maximum velocity, one criterion for describing a movement as a “saccade”. The average slope for gaze saccades to horizontal visual targets for our cats was 7.7 s (Table 3). Slopes estimated from prior studies (e.g., Guitton et al. 1990, Fig. 4D) show comparable mean slopes of 7–16 s. Peak eye-in-head velocities in our study were as high as 325 s. In agreement, Guitton et al. (1984) and Blakemore and Donaghy (1980) measured velocities as high as 300 and 400 s, respectively.
Our results also confirm the observations of Guitton et al. (1984) and (1990) who found the head to lead the eye by an average of about 40 and 15 ms, respectively. We found that in visual trials, 88 % (Table 1) of the time the head latency was shorter than the eye-in-head latency with a mean difference of about 40 ms to distal targets and 60 ms to proximal targets. This disagrees with the results of Blakemore and Donaghy (1980) and Harris (1980) who reported the eye to lead the head by 20 to 50 ms or 30 ms, respectively. It is not clear why Blakemore and Donaghy (1980) and Harris (1980) found the head to usually lag the eye movement but it may have been due to an additional load placed on the head due to their use of a head holder linked to a potentiometer to measure head movement in their studies. Guitton et al. (1984) found that although the head movement usually occurred prior to eye-in-head movement (in the direction of the target), gaze and eye-in-head movements were typically coincident due to full compensation by the VOR. As the head started to move, the eye-in-head moved in an equal and opposite direction resulting in the gaze remaining stable in space until the eye-in-head started to move in the direction of the target.
Table 1 shows that for visual trials the head movement latency was shorter than the gaze latency while gaze was almost always (88 %) earlier than the eye-in-head latency. This implies that for most trials, the head moved first while the VOR was on, which kept the gaze stable during VORb (Fig. 1D). Since gaze latency is consistently shorter than eye latency, in some cases by over 100 ms, the VOR must be turned off or attenuated before the eye saccade is executed (Fig. 8). Clearly there is considerable variability in individual trials and differences in different subjects. The low correlation in gaze and head latency suggests that the commands to move the gaze/eye and head are not synchronized, which is in disagreement with Guitton et al. (1990) who showed similarities in the shapes of the eye movement and head velocity profiles for a few examples.
Eye/head movements to visual targets
Cats have a retinal specialization called the area centralis, functionally similar to the fovea of the primate, where the density of cones and spatial acuity is highest. In both species this specialization is the basis for redirection of the eye to bring a stimulus of interest to the center of the visual field when the light initially stimulates in the periphery of the visual field. This structure is similarly shaped in the two species, and is a different specialization than the horizontal streak seen in the cat and the rabbit Although localization of targets is similar in cats and primates, a striking difference between cats and primates is the limited oculomotor range of cats, which is most evident when the head is restrained (Populin and Yin 1999; Guitton and Volle 1987; Freedman and Sparks 1997; Tomlinson and Bahra 1986b) but is also reflected in the maximal eye-in-head amplitudes with head free which all fall in the range of 25° to 28° (Guitton et al. 1990; Blakemore and Donaghy 1980) as compared with about 55 ° in primates (Freedman and Sparks 1997; Guitton and Volle 1987; Tomlinson and Bahra 1986b; Phillips et al. 1995). Despite their limited oculomotor range, cats, as well as primates, can make single-step rapid gaze shifts to targets well outside their range, although multiple saccades are often used to localize peripheral targets (Guitton and Volle 1987; Freedman and Sparks 1997; Tomlinson and Bahra 1986b).
In cats, we found the range of gaze latencies to both visual and auditory targets to be quite variable (Fig. 4). Evidence of this same variability in primates is seen in the literature, with latencies in nonhuman primates ranging from 20 to 600 ms (Populin and Rajala 2011) and from about 155 to 386 ms in humans (Goossens and Van Opstal 1997). In our cats, the head nearly always moves before the eye. Reports in primates, however, indicate that the eye typically leads the head (Bizzi et al. 1972; Guitton and Volle 1987; Freedman and Sparks 1997; Tomlinson and Bahra 1986b; McCluskey and Cullen 2007; Tweed et al. 1995). Goossens and Van Opstal (1997) and Phillips et al. (1995) report that there is considerable variability in eye/head onset, though their average values show slight eye leading. However, that the eye typically leads the head may be due partially to the methodology of most monkey studies to partially restrain the head to limit head motion to the horizontal axis (Tomlinson and Bahra 1986a, b; Phillips et al. 1995; McCluskey and Cullen 2007), which may have increased the inertia of the head or resistance to movement, delaying movement onset. Goossens and Van Opstal (1997) used a totally head free preparation. Freedman and Sparks (1997) trained monkeys with totally unrestrained heads but they were using a delayed saccade task and excluded trials with slow head movements during the fixation period. On the other hand, there are many examples in the published literature showing head movement before the eye in saccades to visual targets in monkeys and humans (Laurutis and Robinson 1986, Fig. 1; Phillips et al. 1995, Fig. 13; Freedman 2005, Fig. 6; Guitton and Volle 1987, Fig. 17; Tweed et al. 1995, Fig. 3; Goossens and Van Opstal 1997, Fig. 7). Populin and Rajala 2011, using a completely head free preparation, found on average, the head to move before the eye in saccades to visual targets greater than 15°. Perhaps eye–head latency coordination is more similar in cats and primates than we have assumed.
Cats typically use a combined eye–head gaze shift to localize targets of all amplitudes, even those within their oculomotor range (Guitton et al. 1984, 1990; Blakemore and Donaghy 1980). In monkeys, it appears that the head contributes to a saccade only for targets of >20° or so (Freedman 2005; Freedman and Sparks 1997; Tomlinson and Bahra 1986b). That is, for targets of <20°, the head does not move until after the gaze has reached the target. Typically, because the eye reaches the target much faster than the head, once the gaze is on target the head will continue to move and the VOR will move the eyes in an equal and opposite direction. Perhaps the difference between head contribution to gaze in cats and primates to targets of <20° is partially due to differences in the size and inertia of their respective heads rather than a difference in motor control strategies.
Saccades to visual versus auditory targets
We found no significant differences between the main sequence, i.e., the slope of peak velocity vs. amplitude of movement, of auditory and visual saccades for gaze, head, or eye. In contrast to our findings, Goossens and Van Opstal (1997) reported that when the amplitude of the visual and auditory gaze shifts (and eye-in-head saccades) were the same (i.e., movement amplitude for visual and auditory saccades were matched) velocities to visual targets were greater than to auditory. However, we found eye-in-head velocities were significantly higher to visual than auditory targets at identical locations because of the slight undershoot in auditory saccades. This is in agreement with Zambarbieri et al. (1997) who found that saccades in human subjects to visual targets had slightly greater velocities than saccades to the same amplitude auditory targets.
Most studies comparing visual and auditory eye saccades have been done in the head fixed condition (Jay and Sparks 1990 (monkey and human); Zahn et al. 1978; Zambarbieri et al. 1982 (human); Populin and Yin 1999[cat]), which may still provide insight into the eye component of the gaze saccade. These studies aimed to discern whether auditory and visual eye saccades utilize the same substrates for initiation and execution of movement, and if so, whether these neural components behave similarly when stimulated by different modalities. Jay and Sparks (1990), Zahn et al. (1978), and Zambarbieri et al. (1982) agree that in primates for saccades of the same amplitude, saccades to visual targets have greater peak velocity than saccades to auditory targets. This may be related to differences in the strength of responses of the saccade-related burst in the SC to visual versus auditory stimuli (Wallace et al. 1996; Bell et al. 2001, 2004; Populin and Yin 2002). Jay and Sparks (1990) concluded that auditory and visual saccades share a common motor circuit at the level of the SC though the different sensory inputs have different effects on the rate of discharge or duration of the saccade-related burst. This may, in turn, produce differences in latency, velocity and accuracy of saccades to targets of different modalities. Populin and Yin (1999) found no difference in the peak velocity of visual and auditory saccades of the same amplitude in the head fixed cat. The fact that visual saccade velocities are only slightly and non-significantly greater that auditory saccade velocities in our cats, (similar results to Populin and Yin (1999)), may reflect a species difference.
In our study, head amplitude as a function of gaze amplitude was greater to auditory than visual targets (Table 5). That is, by the time the eye, gaze and head movements all terminated, the head had moved relatively further in auditory than visual trials. Similar results were found in the monkey working with the head unrestrained by Populin and Rajala (2011) who attributed this to head movements preceding eye movements in acoustic trials This suggests that in the case of auditory saccades there is additional motor input to neck muscles (perhaps from different neural structures) than in visual saccades. Eye-in-head amplitude as a function of gaze amplitude was greater to visual than auditory targets (Table 6). That is, at the time of peak eye-in-head amplitude, (long before the end of head movement), the eye-in-head amplitude has moved proportionally further in the head for visual than auditory gaze saccades of the same amplitude. This may be a function of less neural input to the muscles of the head in visual compared with auditory saccades, especially early in the saccade. Goossens and Van Opstal (1997) found similar differences between auditory and visual saccades in human subjects.
TABLE 6.
Eye Contribution to Gaze Shift (at end of eye movement) Gain (95 % confidence interval)
| Cat | Azimuth | Elevation | ||||
|---|---|---|---|---|---|---|
| Auditory | Visual | Significance | Auditory | Visual | Significance | |
| 17 | 0.41 (0.40–0.42) | 0.43 (0.42–0.44) | Y | 0.30 (0.25–0.34) | 0.42 (0.38–0.45) | Y |
| 18 | 0.43 (0.42–0.43) | 0.48 (0.47–0.49) | Y | 0.36 (0.34–0.38) | 0.50 (0.47–0.51) | Y |
| 21 | 0.33 (0.32–0.34) | 0.38 (0.37–0.39) | Y | 0.32 (0.28–0.37) | 0.31 (0.27–0.34) | N |
In the monkey, Whittington et al. (1981) found the only difference between head free gaze saccades to visual and auditory targets was that the latency to auditory targets was 50 to 60 ms earlier than latencies to visual targets, which they attributed to the speed of the peripheral receptors. We found a similar difference in latency in two of our three cats. Zambarbieri et al. (1997) found that in human saccades to visual targets, latencies were on average shorter to proximal targets (210 ms) and longer to peripheral targets (275 ms at 70°); in saccades to auditory targets, latencies to proximal targets were longer (370 ms) than to peripheral targets (215 ms). We found in cats that saccade latencies on average were also longer to more proximal targets for both auditory and visual saccades, although there was considerable variability in some of our subjects (Fig. 4). Jay and Sparks (1990) found the same pattern in head fixed eye saccades and believe this is the result of motor rather than sensory processes, because latencies decrease with stimulus eccentricity, regardless of the position of the head.
Our latency measures (Figs. 4, 5, and 6) show that at least for some cats, localization of proximal targets is different than for peripheral targets for both visual and auditory stimuli. Furthermore, the plots of head and eye-in-head latency vs gaze latency show that for the proximal 9 ° targets where gaze latency was very long, the head latency was not. So for these trials, as the head initially began to turn, the VOR was still fully functional and kept the gaze stable. In other cases such as Figure 8, the VOR is attenuated so that the gaze latency is delayed with respect to head latency but still shorter than eye-in-head latency. This VORb is also evident from the raw movement traces (Figs. 1 and 8, inset). Shortly after the head began to turn, an eye saccade was executed to produce the shift in gaze and corresponding eye-in-head movement. Since the eye saccade is much shorter in duration than the head movement, the eye-in-head movement clearly shows the action of the VORe at the end of the gaze shift (Figs. 1D (inset) and 8).
Perhaps there are partially separate mechanisms for orienting (more automatic and reflexive) and localizing (more voluntary) as suggested by Thompson and Masterton (1978). More recently Nodal et al. (2008) found when studying ferrets that measures of acoustic orientation and approach-to-target were well correlated in individual trials in terms of latency and accuracy, suggesting that “… natural localization behavior (is) a sequence of sound-evoked responses, beginning with orientation and followed by locomotor response (Beitel and Kaas 1993).” Nodal et al. (2008) contend that at least part of the neural circuitry for translating sensory signals into motor commands is shared by the two behaviors. Perhaps in the case of our cats for both auditory and visual targets there is an initial orientation to peripheral targets (and sometimes proximal ones), after which more cognitive localization behavior blends into the ongoing task. Sometimes in the case of proximal targets there may be no need for initial orientation, but just the greater latency, cognitive localization. This may help explain why there is such a variety of latencies to auditory and visual targets within and between cats. We know that there are different pathways by which location information of an auditory target may reach the superior colliculus; from brainstem auditory structures (Oliver 1984a, b; Henkel and Spangler 1983; Shneiderman and Henkel 1987), from inferior colliculus (King et al. 1998; Harting and Van Lieshout 2000; Edwards et al. 1979; Anderson and Yoshida 1980), frontal eye fields (Harting et al. 1992; Russo and Bruce 1994), anterior ectosylvian sulcus (Clarey and Irvine 1986; Meredith and Clemo 1989), and the substantia nigra pars reticulata (Harting and Van Lieshout 1991). There is evidence that auditory information may reach brainstem motor neurons directly from frontal eye fields (Hanes and Wurtz 2001). The subject’s attention, level of motivation, and practice may all lead to differences in latency (Metzger et al. 2006). Cat 21, who had long latencies but was also very accurate, is perhaps exchanging speed for accuracy. Differences in latency may reflect parallel pathways by which sound location information reaches the motor centers.
Both Goossens and Van Opstal (1997) and Zambarbieri et al. (1997) found that the eye minus head latencies in human subjects were smaller in auditory than visual targets i.e., the head followed the leading eye more closely in auditory trials. We found in cats that to proximal targets, the head led the eye more to auditory than visual targets, although this difference was not significant. Zangemeister and Stark (1982a, b) studying variable interactions of head and eye latency in humans found that eye and especially head latencies were readily modified by experimental conditions such as instructions to the subject, frequency and predictability of the target, amplitude of movement, and fatigue. They attributed variability to higher level neurological processing.
One of our cats, cat 21, had many double head movements toward both visual and auditory targets. She also had the most variable initial head position and the most variability in gaze shift latency. Yet, she had the highest sound localization accuracy of the three cats. Her accuracy supports the contention of Vliegen et al. (2004) that the auditory system can process dynamically varying acoustic cues that result from self-initiated rapid head movements to construct a stable representation of the target. It could be that head movement may be a strategy used to improve the localization accuracy of long-duration, more eccentrically located targets (Thurlow and Runge 1967; Thurlow et al. 1967; Pollack and Rose 1967).
Accuracy and precision
In a previous study (Tollin et al. 2005), we compared the sound localization ability of cats with their heads restrained and unrestrained to targets within the oculomotor range of the head restrained cat (i.e., ±18°). In the present study we have expanded the range of targets in azimuth beyond the oculomotor range, out to ±45°. The measures of azimuthal localization accuracy, or gain, are generally equivalent (within 0.03) in our two studies for short and long-duration visual and auditory trials, except for the very shortest duration auditory targets (15–164 ms), where the localization accuracy for the most peripheral target (i.e., ±45°) decreased somewhat. The slight reduction in localization accuracy for the shortest duration sounds may be due to sound duration itself and/or the effect of sound duration on the spectral characteristics of the stimuli. The joint effect on localization accuracy and precision of sound duration and spectral content is beyond the scope of this paper.
May and Huang (1996) studied sound localization in cats, measured by head pointing. For 40 ms noise from horizontal targets out to 45°, mean gains were about 0.75, (13° undershoot for a 45° target). (Gain values were adapted from their data for direct comparison to our study.) Our cats had similar gains, as measured by head position, averaging 0.73 (12° undershoot) for 40 ms noise targets. These head gains were 0.23 to 0.34 (average, 0.27) less than the gains measured using eye position. Thompson and Masterton (1978) reported an error in accuracy near 0° (measured by the position of the head) when localizing sources of sound, though their measurement system (videotape recording) was only accurate to 15°. We also found the head to undershoot the target in comparison to the gaze position when localizing visual stimuli. On the other hand, Guitton et al. (1984) obtained a gain of 1 when plotting target eccentricity versus head amplitude. This very accurate result may have been a function of their practice of rewarding the cat for correct gaze shifts to visual targets, with food rewards offered at the location of the visual target, necessitating the cat to point their mouth to the target. We therefore conclude that although head pointing may be a viable means of measuring sound localization in humans, where training with specific head pointing instructions and cognitive feedback is possible (Perrott et al. 1987; Makous and Middlebrooks 1990; Carlile et al. 1997), it is less than optimal for measuring the cat’s perceived location of sound. This is not only because the head consistently undershoots the eye but also because there is such variability within and between cats as to how much discrepancy there is between the final gaze and head position. A caveat here is that we did not reward the animals based on their head movement but rather on their gaze. Had we rewarded head movement, perhaps head position would have been more accurate and/or precise. However, we like others studying humans (Zahn et al. 1978, 1979; Zambarbieri et al. 1982; Frens and Van Opstal 1995; Vliegen et al. 2004) and monkeys (Whittington et al. 1981; Jay and Sparks 1990), use the final position of the gaze as the best representation of where the cat perceives the location of the sound.
Acknowledgments
We thank R. Kochhar and J. Sekulski for designing and implementing the analysis software and the data collection software, respectively. We thank Dr. Yan Gai for helpful comments on an earlier version of the manuscript.
Grants
This work was supported by National Institutes of Deafness and other Communicative Disorders Grants DC-00116, DC-07177, and DC-02840 to T.C.T. Yin and an Individual National Research Service Award (DC-000376) to D. J. Tollin.
References
- Anderson ME, Yoshida M. Axonal branching patterns and location of nigrothalamic and nigrocollicular neurons in the cat. J Neurophysiol. 1980;43:883–895. doi: 10.1152/jn.1980.43.4.883. [DOI] [PubMed] [Google Scholar]
- Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math Biosci. 1975;24:191–204. doi: 10.1016/0025-5564(75)90075-9. [DOI] [Google Scholar]
- Beitel RE, Kaas JH. Effects of bilateral and unilateral ablation of auditory cortex in cats on the unconditioned head orienting response to acoustic stimuli. J Neurophysiol. 1993;70:351–369. doi: 10.1152/jn.1993.70.1.351. [DOI] [PubMed] [Google Scholar]
- Bell AH, Corneil BD, Meredith MA, Munoz DP. The influence of stimulus properties on multisensory processing in the awake primate superior colliculus. Can J Exp Psychol. 2001;55:123–132. doi: 10.1037/h0087359. [DOI] [PubMed] [Google Scholar]
- Bell AH, Fecteau JH, Munoz DP. Using auditory and visual stimuli to investigate the behavioral and neuronal consequences of reflexive covert orienting. J Neurophysiol. 2004;91:2172–2184. doi: 10.1152/jn.01080.2003. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Kalil RE, Morasso P. Two modes of active eye-head coordination in monkeys. Brain Res. 1972;40:45–48. doi: 10.1016/0006-8993(72)90104-7. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Donaghy M. Co-ordination of head and eyes in the gaze changing behaviour of cats. J Physiol. 1980;300:317–335. doi: 10.1113/jphysiol.1980.sp013164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J Neurophysiol. 1968;31:442–454. doi: 10.1152/jn.1968.31.3.442. [DOI] [PubMed] [Google Scholar]
- Carlile S, Leong P, Hyams S. The nature and distribution of errors in sound localization by human listeners. Hear Res. 1997;114:179–196. doi: 10.1016/S0378-5955(97)00161-5. [DOI] [PubMed] [Google Scholar]
- Clarey JC, Irvine DR. Auditory response properties of neurons in the anterior ectosylvian sulcus of the cat. Brain Res. 1986;386:12–19. doi: 10.1016/0006-8993(86)90136-8. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Munoz DP. Human eye-head gaze shifts in a distractor task. II. Reduced threshold for initiation of early head movements. J Neurophysiol. 1999;82:1406–1421. doi: 10.1152/jn.1999.82.3.1406. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Huterer M, Braidwood DA, Sylvestre PA. Time course of vestibuloocular reflex suppression during gaze shifts. J Neurophysiol. 2004;92:3408–3422. doi: 10.1152/jn.01156.2003. [DOI] [PubMed] [Google Scholar]
- Dent ML, Yin TC. Psychological and physiological studies of the precedence effect in cats. Acta Acoust U Acustica. 2005;91:463–470. [Google Scholar]
- Dent ML, Tollin DJ, Yin TC. Cats exhibit the Franssen effect illusion. J Acoust Soc Am. 2004;116:3070–3074. doi: 10.1121/1.1810136. [DOI] [PubMed] [Google Scholar]
- Dent ML, Tollin DJ, Yin TC. Influence of sound source location on the behavior and physiology of the precedence effect in cats. J Neurophysiol. 2009;102:724–734. doi: 10.1152/jn.00129.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SB, Ginsburgh CL, Henkel CK, Stein BE. Sources of subcortical projections to the superior colliculus in the cat. J Comp Neurol. 1979;184:309–329. doi: 10.1002/cne.901840207. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–77. doi: 10.1214/ss/1177013815. [DOI] [Google Scholar]
- Freedman EG. Head-eye interactions during vertical gaze shifts made by rhesus monkeys. Exp Brain Res. 2005;167:557–570. doi: 10.1007/s00221-005-0051-9. [DOI] [PubMed] [Google Scholar]
- Freedman EG. Coordination of the eyes and head during visual orienting. Exp Brain Res. 2008;190:369–387. doi: 10.1007/s00221-008-1504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. J Neurophysiol. 1997;77:2328–2348. doi: 10.1152/jn.1997.77.5.2328. [DOI] [PubMed] [Google Scholar]
- Frens MA, Van Opstal AJ. A quantitative study of auditory-evoked saccadic eye movements in two dimensions. Exp Brain Res. 1995;107:103–117. doi: 10.1007/BF00228022. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Ling L, Phillips JO. Behavior of the position vestibular pause (PVP) interneurons of the vestibuloocular reflex during head-free gaze shifts in the monkey. J Neurophysiol. 2005;94:4481–4490. doi: 10.1152/jn.00101.2005. [DOI] [PubMed] [Google Scholar]
- Fuller JH. Head movement propensity. Exp Brain Res. 1992;92:152–164. doi: 10.1007/BF00230391. [DOI] [PubMed] [Google Scholar]
- Fuller JH, Maldonado H, Schlag J. Vestibular-oculomotor interaction in cat eye–head movements. Brain Res. 1983;271:241–250. doi: 10.1016/0006-8993(83)90286-X. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol. 1969;32:613–636. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- Goossens HH, Van Opstal AJ. Human eye-head coordination in two dimensions under different sensorimotor conditions. Exp Brain Res. 1997;114:542–560. doi: 10.1007/PL00005663. [DOI] [PubMed] [Google Scholar]
- Guitton D, Volle M. Gaze control in humans: eye-head coordination during orienting movements to targets within and beyond the oculomotor range. J Neurophysiol. 1987;58:427–459. doi: 10.1152/jn.1987.58.3.427. [DOI] [PubMed] [Google Scholar]
- Guitton D, Douglas RM, Volle M. Eye-head coordination in cats. J Neurophysiol. 1984;52:1030–1050. doi: 10.1152/jn.1984.52.6.1030. [DOI] [PubMed] [Google Scholar]
- Guitton D, Munoz DP, Galiana HL. Gaze control in the cat: studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. J Neurophysiol. 1990;64:509–531. doi: 10.1152/jn.1990.64.2.509. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Wurtz RH. Interaction of the frontal eye field and superior colliculus for saccade generation. J Neurophysiol. 2001;85:804–815. doi: 10.1152/jn.2001.85.2.804. [DOI] [PubMed] [Google Scholar]
- Harris LR. The superior colliculus and movements of the head and eyes in cats. J Physiol. 1980;300:367–391. doi: 10.1113/jphysiol.1980.sp013167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting JK, Van Lieshout DP. Spatial relationships of axons arising from the substantia nigra, spinal trigeminal nucleus, and pedunculopontine tegmental nucleus within the intermediate gray of the cat superior colliculus. J Comp Neurol. 1991;305:543–558. doi: 10.1002/cne.903050403. [DOI] [PubMed] [Google Scholar]
- Harting JK, Van Lieshout D. Projections from the rostral pole of the inferior colliculus to the cat superior colliculus. Brain Res. 2000;881:281–295. doi: 10.1016/S0006-8993(00)02849-3. [DOI] [PubMed] [Google Scholar]
- Harting JK, Updyke BV, Van Lieshout DP. Corticotectal projections in the cat: anterograde transport studies of twenty-five cortical areas. J Comp Neurol. 1992;324:379–414. doi: 10.1002/cne.903240308. [DOI] [PubMed] [Google Scholar]
- Henkel CK, Spangler KM. Organization of the efferent projections of the medial superior olivary nucleus in the cat as revealed by HRP and autoradiographic tracing methods. J Comp Neurol. 1983;221:416–428. doi: 10.1002/cne.902210405. [DOI] [PubMed] [Google Scholar]
- Huang AY, May BJ. Sound orientation behavior in cats. II. Mid-frequency spectral cues for sound localization. J Acoust Soc Am. 1996;100:1070–1080. doi: 10.1121/1.416293. [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Localization of auditory and visual targets for the initiation of saccadic eye movements. In: Berkley M, Stebbins W, editors. Comparative perception. Vol. I: basic mechanisms. New York: Wiley; 1990. pp. 351–374. [Google Scholar]
- King AJ, Jiang ZD, Moore DR. Auditory brainstem projections to the ferret superior colliculus: anatomical contribution to the neural coding of sound azimuth. J Comp Neurol. 1998;390:342–365. doi: 10.1002/(SICI)1096-9861(19980119)390:3<342::AID-CNE4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Laurutis VP, Robinson DA. The vestibulo-ocular reflex during human saccadic eye movements. J Physiol. 1986;373:209–233. doi: 10.1113/jphysiol.1986.sp016043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre P, Bottemanne I, Roucoux A. Experimental study and modeling of vestibulo-ocular reflex modulation during large shifts of gaze in humans. Exp Brain Res. 1992;91:496–508. doi: 10.1007/BF00227846. [DOI] [PubMed] [Google Scholar]
- Makous JC, Middlebrooks JC. Two-dimensional sound localization by human listeners. J Acoust Soc Am. 1990;87:2188–2200. doi: 10.1121/1.399186. [DOI] [PubMed] [Google Scholar]
- May BJ, Huang AY. Sound orientation behavior in cats. I. Localization of broadband noise. J Acoust Soc Am. 1996;100:1059–1069. doi: 10.1121/1.416292. [DOI] [PubMed] [Google Scholar]
- McCluskey MK, Cullen KE. Eye, head, and body coordination during large gaze shifts in rhesus monkeys: movement kinematics and the influence of posture. J Neurophysiol. 2007;97:2976–2991. doi: 10.1152/jn.00822.2006. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Clemo HR. Auditory cortical projection from the anterior ectosylvian sulcus (Field AES) to the superior colliculus in the cat: an anatomical and electrophysiological study. J Comp Neurol. 1989;289:687–707. doi: 10.1002/cne.902890412. [DOI] [PubMed] [Google Scholar]
- Metzger RR, Greene NT, Porter KK, Groh JM. Effects of reward and behavioral context on neural activity in the primate inferior colliculus. J Neurosci Off J Soc Neurosci. 2006;26:7468–7476. doi: 10.1523/JNEUROSCI.5401-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AW. On the minimum audible angle. J Acoust Soc Amer. 1958;30:237–246. doi: 10.1121/1.1909553. [DOI] [Google Scholar]
- Morasso P, Bizzi E, Dichgans J. Adjustment of saccade characteristics during head movements. Exp Brain Res. 1973;16:492–500. doi: 10.1007/BF00234475. [DOI] [PubMed] [Google Scholar]
- Nodal FR, Bajo VM, Parsons CH, Schnupp JW, King AJ. Sound localization behavior in ferrets: comparison of acoustic orientation and approach-to-target responses. Neuroscience. 2008;154:397–408. doi: 10.1016/j.neuroscience.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodal FR, Kacelnik O, Bajo VM, Bizley JK, Moore DR, King AJ. Lesions of the auditory cortex impair azimuthal sound localization and its recalibration in ferrets. J Neurophysiol. 2010;103:1209–1225. doi: 10.1152/jn.00991.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield SR, Parker SP. Acuity of sound localisation: a topography of auditory space. I. Normal hearing conditions. Perception. 1984;13:581–600. doi: 10.1068/p130581. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Dorsal cochlear nucleus projections to the inferior colliculus in the cat: a light and electron microscopic study. J Comp Neurol. 1984;224:155–172. doi: 10.1002/cne.902240202. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Neuron types in the central nucleus of the inferior colliculus that project to the medial geniculate body. Neuroscience. 1984;11:409–424. doi: 10.1016/0306-4522(84)90033-2. [DOI] [PubMed] [Google Scholar]
- Pelisson D, Prablanc C. Vestibulo-ocular reflex (VOR) induced by passive head rotation and goal-directed saccadic eye movements do not simply add in man. Brain Res. 1986;380:397–400. doi: 10.1016/0006-8993(86)90244-1. [DOI] [PubMed] [Google Scholar]
- Pelisson D, Prablanc C, Urquizar C. Vestibuloocular reflex inhibition and gaze saccade control characteristics during eye-head orientation in humans. J Neurophysiol. 1988;59:997–1013. doi: 10.1152/jn.1988.59.3.997. [DOI] [PubMed] [Google Scholar]
- Perrott DR, Ambarsoom H, Tucker J. Changes in head position as a measure of auditory localization performance: auditory psychomotor coordination under monaural and binaural listening conditions. J Acoust Soc Am. 1987;82:1637–1645. doi: 10.1121/1.395155. [DOI] [PubMed] [Google Scholar]
- Phillips JO, Ling L, Fuchs AF, Siebold C, Plorde JJ. Rapid horizontal gaze movement in the monkey. J Neurophysiol. 1995;73:1632–1652. doi: 10.1152/jn.1995.73.4.1632. [DOI] [PubMed] [Google Scholar]
- Pollack I, Rose M (1967) Effect of head movement on the localization of sounds in the equatorial plane. Percept Psychophys 2(12):591–596
- Populin LC. Human sound localization: measurements in untrained, head-unrestrained subjects using gaze as a pointer. Exp Brain Res. 2008;190:11–30. doi: 10.1007/s00221-008-1445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Populin LC, Rajala AZ. Target modality determines eye-head coordination in nonhuman primates: implications for gaze control. J Neurophysiol. 2011;106:2000–2011. doi: 10.1152/jn.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Populin LC, Yin TCT. Behavioral studies of sound localization in the cat. J Neurosci. 1998;18:2147–2160. doi: 10.1523/JNEUROSCI.18-06-02147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Populin LC, Yin TC. Kinematics of eye movements of cats to broadband acoustic targets. J Neurophysiol. 1999;82:955–962. doi: 10.1152/jn.1999.82.2.955. [DOI] [PubMed] [Google Scholar]
- Populin LC, Yin TC. Bimodal interactions in the superior colliculus of the behaving cat. J Neurosci Off J Soc Neurosci. 2002;22:2826–2834. doi: 10.1523/JNEUROSCI.22-07-02826.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Populin LC, Tollin DJ, Weinstein JM. Human gaze shifts to acoustic and visual targets. Ann N Y Acad Sci. 2002;956:468–473. doi: 10.1111/j.1749-6632.2002.tb02857.x. [DOI] [PubMed] [Google Scholar]
- Rayleigh LJWS. On our perception of sound direction. Philos Mag. 1907;13:214–232. [Google Scholar]
- Rose JE, Gross NB, Geisler CD, Hind JE. Some neural mechanisms in the inferior colliculus of the cat which may be relevant to localization of a sound source. J Neurophysiol. 1966;29:288–314. doi: 10.1152/jn.1966.29.2.288. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. A neural correlate for vestibulo-ocular reflex suppression during voluntary eye-head gaze shifts. Nat Neurosci. 1998;1:404–410. doi: 10.1038/1619. [DOI] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ. Frontal eye field activity preceding aurally guided saccades. J Neurophysiol. 1994;71:1250–1253. doi: 10.1152/jn.1994.71.3.1250. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol. 1992;68:244–264. doi: 10.1152/jn.1992.68.1.244. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF. The error signal for modification of vestibuloocular reflex gain. Ann N Y Acad Sci. 1992;656:884–885. doi: 10.1111/j.1749-6632.1992.tb25283.x. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Kaneko CS, Fuchs AF. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res. 2002;142:439–462. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- Shneiderman A, Henkel CK. Banding of lateral superior olivary nucleus afferents in the inferior colliculus: a possible substrate for sensory integration. J Comp Neurol. 1987;266:519–534. doi: 10.1002/cne.902660406. [DOI] [PubMed] [Google Scholar]
- Slee SJ, Young ED. Sound localization cues in the marmoset monkey. Hear Res. 2010;260:96–108. doi: 10.1016/j.heares.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SS, Newman EB. The localization of actual sources of sound. Amer J Psychol. 1936;48:297–306. doi: 10.2307/1415748. [DOI] [Google Scholar]
- Tabak S, Smeets JB, Collewijn H. Modulation of the human vestibuloocular reflex during saccades: probing by high-frequency oscillation and torque pulses of the head. J Neurophysiol. 1996;76:3249–3263. doi: 10.1152/jn.1996.76.5.3249. [DOI] [PubMed] [Google Scholar]
- Thompson GC, Masterton RB. Brain stem auditory pathways involved in reflexive head orientation to sound. J Neurophysiol. 1978;41:1183–1202. doi: 10.1152/jn.1978.41.5.1183. [DOI] [PubMed] [Google Scholar]
- Thurlow WR, Runge PS. Effect of induced head movements on localization of direction of sounds. J Acoust Soc Am. 1967;42:480–488. doi: 10.1121/1.1910604. [DOI] [PubMed] [Google Scholar]
- Thurlow WR, Mangels JW, Runge PS. Head movements during sound localization. J Acoust Soc Am. 1967;42:489–493. doi: 10.1121/1.1910605. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Yin TC. Psychophysical investigation of an auditory spatial illusion in cats: the precedence effect. J Neurophysiol. 2003;90:2149–2162. doi: 10.1152/jn.00381.2003. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Populin LC, Yin TC. Neural correlates of the precedence effect in the inferior colliculus of behaving cats. J Neurophysiol. 2004;92:3286–3297. doi: 10.1152/jn.00606.2004. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Populin LC, Moore JM, Ruhland JL, Yin TC. Sound-localization performance in the cat: the effect of restraining the head. J Neurophysiol. 2005;93:1223–1234. doi: 10.1152/jn.00747.2004. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Ruhland JL, Yin TC. The role of spectral composition of sounds on the localization of sound sources by cats. J Neurophysiol. 2013;109:1658–1668. doi: 10.1152/jn.00358.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson RD. Combined eye-head gaze shifts in the primate. III. Contributions to the accuracy of gaze saccades. J Neurophysiol. 1990;64:1873–1891. doi: 10.1152/jn.1990.64.6.1873. [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, Bahra PS. Combined eye-head gaze shifts in the primate. II. Interactions between saccades and the vestibuloocular reflex. J Neurophysiol. 1986;56:1558–1570. doi: 10.1152/jn.1986.56.6.1558. [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, Bahra PS. Combined eye-head gaze shifts in the primate. I. Metrics. J Neurophysiol. 1986;56:1542–1557. doi: 10.1152/jn.1986.56.6.1542. [DOI] [PubMed] [Google Scholar]
- Tweed D, Glenn B, Vilis T. Eye-head coordination during large gaze shifts. J Neurophysiol. 1995;73:766–779. doi: 10.1152/jn.1995.73.2.766. [DOI] [PubMed] [Google Scholar]
- Vliegen J, Van Grootel TJ, Van Opstal AJ. Dynamic sound localization during rapid eye-head gaze shifts. J Neurosci Off J Soc Neurosci. 2004;24:9291–9302. doi: 10.1523/JNEUROSCI.2671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Wilkinson LK, Stein BE. Representation and integration of multiple sensory inputs in primate superior colliculus. J Neurophysiol. 1996;76:1246–1266. doi: 10.1152/jn.1996.76.2.1246. [DOI] [PubMed] [Google Scholar]
- Whittington DA, Hepp-Reymond MC, Flood W. Eye and head movements to auditory targets. Exp Brain Res. 1981;41:358–363. doi: 10.1007/BF00238893. [DOI] [PubMed] [Google Scholar]
- Wightman FL, Kistler DJ. Headphone simulation of free-field listening. II: Psychophysical validation. J Acoust Soc Am. 1989;85:868–878. doi: 10.1121/1.397558. [DOI] [PubMed] [Google Scholar]
- Yin TCT, Chan JCK. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol. 1990;64:465–488. doi: 10.1152/jn.1990.64.2.465. [DOI] [PubMed] [Google Scholar]
- Zahn JR, Abel LA, Dell’Osso LF. Audio-ocular response characteristics. Sens Processes. 1978;2:32–37. [PubMed] [Google Scholar]
- Zahn JR, Abel LA, Dell ’Osso LF, Daroff RB. The audioocular response: intersensory delay. Sens Processes. 1979;3:60–65. [PubMed] [Google Scholar]
- Zambarbieri D, Schmid R, Magenes G, Prablanc C. Saccadic responses evoked by presentation of visual and auditory targets. Exp Brain Res. 1982;47:417–427. doi: 10.1007/BF00239359. [DOI] [PubMed] [Google Scholar]
- Zambarbieri D, Schmid R, Versino M, Beltrami G. Eye–head coordination toward auditory and visual targets in humans. J Vestib Res. 1997;7:251–263. doi: 10.1016/S0957-4271(96)00181-4. [DOI] [PubMed] [Google Scholar]
- Zangemeister WH, Stark L. Types of gaze movement: variable interactions of eye and head movements. Exp Neurol. 1982;77:563–577. doi: 10.1016/0014-4886(82)90228-X. [DOI] [PubMed] [Google Scholar]
- Zangemeister WH, Stark L. Gaze latency: variable interactions of head and eye latency. Exp Neurol. 1982;75:389–406. doi: 10.1016/0014-4886(82)90169-8. [DOI] [PubMed] [Google Scholar]