Abstract
For most orthopedists, total knee arthroplasty (TKA) has been regarded as the most effective surgery for patients with severe knee diseases. Though seldom occur, postoperative infection certainly remains one of the most devastating and dreaded complications of TKA. Thus, careful and early diagnosis is needed. After diagnosis, categorize the infection type and choose a rightful and sequential step of treatment are recommended.
Keywords: Knee, Arthroplasty, Infection
Introduction
Total knee arthroplasty (TKA) is the most cost-effective and safe procedure for restoring function to the arthritic knee joint1-3). However, this life-improving procedure also has some complications and postoperative infection is the most dreaded one. Postoperative infection is an important cause of implant failure and revision arthroplasty. Treatment of infection would require long hospitalization and result in high incidences of morbidity and mortality, especially in the elderly. Infection can also cause a great burden to patients' economic and psychological status.
Schurman4) reported the infection rate as 9.1% in 1981. Improvement of surgical skill, shortening of operation time, and the use of prophylactic antibiotics have helped reduce the rate to 1%-2%5-7). In spite of the efforts to prevent infection, orthopedic surgeons still frequently encounter this complication after TKA. The purpose of this article is to improve the knowledge of postoperative infection with a review of the recently published articles.
Risk Factors for Infection
Epidemiological studies have reported risk factors for infection after TKA. According to Petty et al.8) the risk factors for early infection are patient factors and operation technique. Patient factors include poor nutritional state, diabetes mellitus (DM), old age, obesity (body mass index>30) and prolonged hospital admission period. They also claimed previous operation history and traumatic events can also be applied risk factors8). Chun et al.9) reported TKA can be a fine solution to osteoarthritis with DM. However, without great care after treatment, it will lead to an infection. Papavasiliou et al.10) also insisted that steroid injection before operation can lead to infection after operation. Some kinds of inflammatory knee joint diseases can be a risk factor. Rheumatic arthritis (RA) is a serious factor for increasing the incidence of infection after TKA11). Poss et al.7) also reported RA is the cause of delayed wound healing and poor immune system response. Thus, RA patients have 2.5 times higher rate of postoperative infection than osteoarthritis patients. Of chronic infection, Petty et al.8) claimed periodontal abscess, infection on foot, urological infection and other periprosthetic septic arthritis or osteomyelitis were related to infection.
Cause of Infection
Bacteriological diagnosis is the most important step before planning the treatment of infection following TKA. The effective samples are joint fluid obtained by aspiration, tissue culture specimens taken during operation. Cho et al.12) reported the infection strains are staphylococcus (52.9%), Escherichia coli (5.9%) and anaerobes (2.8%). On the subtypes of staphylococcus, methicillin-resistant staphylococcus aureus (67%) is most common, followed by methicillin-resistant coagulase-negative staphylococcus (22%) in Korea. Stefansdottir et al.13) reported that aerobic Gram-positive bacteria were the sole causative pathogen in 75.1% of the infections and polymicrobial infections accounted for 6.3% and Gram-negative bacteria, 6.1%. Staphylococcus aureus (67.7%) was the most common pathogen, followed by streptococci (19.2%). Song et al.14) reported a case of infection caused by candida albicans. Thus, fungus or mycobacterium tuberculosis could also be one of the causative microorganisms that bring about infection after TKA.
Classification
Of the various factors that should be taken into consideration to treat periprosthetic joint infection, duration of symptoms has been suggested as the most important factor that affects treatment outcomes. Thus, classification of infection is performed according to symptom duration: positive intraoperative culture, early postoperative, acute hematogenous, and chronic infection15). Positive intraoperative culture can be defined as more than two positive intraoperative culture findings. Early postoperative infection can be diagnosed when patients present with infection symptoms within four weeks after the initial arthroplasty. Acute hematogenous infection is characterized by an acute presentation of symptoms in a previously well-functioning joint after arthroplasty. Chronic infection can be diagnosed by the presence of more than one month of insidious clinical course after initial arthroplasty or if treatment is delayed more than 4 weeks after symptom onset in acute hematogenous infection (Table 1)16).
Table 1.
Classification of Periprosthetic Infection

Reprinted from Tingle et al.16) with permission from SLACK.
Diagnosis
The diagnosis of infection should meet at least three of the five criteria that are presented below: 1) abnormal serology (erythrocyte sedimentation rate [ESR]>30 mm/hour and C-reactive protein [CRP]>1 mg/dL), 2) strong clinical and radiological suspicion of periprosthetic infection, 3) positive joint aspiration culture, 4) evidence of purulence during the subsequent surgical intervention, and 5) positive intraoperative culture17).
1. Physical Examination
The first step of diagnosis is careful physical examination and history taking. The starting point can be guessed by figuring out the period. Infection should be suspected if pain, swelling or erythema unrelated to operation remains. Morrey et al.18) reported pain associated with decreased range of motion may indicate infection. Pain relevant to infection should be considered, if it remains for a long time after operation. Teller et al.19) reported if the fever after operation is more than 37.5℃, sensitivity and specificity to infection are 9% and 96%, respectively.
2. Serology
ESR and CRP are representative serologic values for diagnosing infection. However, the values are not absolute indications of infection because CRP can be at its highest point after 48 to 72 hours from operation and it will slowly recover to its normal state in 3 weeks. However, it can take more than 3 weeks depending on patient's age or condition. Thus, Chun20) insisted the values of serologic test were not absolutely dependable and it should be compared with other examination results. According to Shih et al.21), ESR sensitivity was 82% and specificity was 85% and CRP sensitivity was 86% and specificity was 92%. There are other serologic tests that can be used to determine infection state. Glehr et al.22) reported that procalcitonin and interleukin (IL)-6 were helpful in detecting periprosthetic joint infection. Procalcitonin cutoff level of 0.35 ng/mL revealed a sensitivity of 80% and specificity of 37%. IL-6 cutoff level of 2.55 pg/mL had a sensitivity of 92% and specificity of 59%.
3. Aspiration
Aspiration is a standard and essential test for infection. Sensitivity could be increased with multiple trials. Leone and Hanssen23) insisted the best approach for aspiration is the lateral side of the upper pole of the patella. In cases of pyogenic arthritis without total knee replacement, the values are white blood count (WBC) of ≥50,000/mm3 and neutrophil of ≥80%24). If cytologic findings of aspirated fluid from a replaced knee joint are WBC of ≥5,000/mm3 and neutrophil of ≥65%, deep infection can be diagnosed. In 2007, Traumpz et al.25) reported that the possibility of infection exists if aspiration shows WBC is ≥1,700/mm3 or neutrophil count is more than 65%. Dinneen et al.26) also reported that the cutoff value for suspected periprosthetic joint infection is between 1,100/mm3 and 1,700/mm3. Mason et al.27) reported if aspiration values show WBC of ≥2,500/mm3 and neutrophil of ≥60%, the presence of infection is highly likely. On the other hand, Spangehl et al.28) insisted that guidelines for infection should be WBC of >5,000/mm3 and neutrophil of >80%. According to the guidelines announced by American Academy of Orthopaedic Surgeon (AAOS) in 2010, chronic infection should be diagnosed if WBC is >1,700 cell/µL and neutrophil is >65%29).
4. Tissue Culture and Frozen Biopsy
Intraoperative tissue culture and frozen biopsy can help diagnose infection. Feldman et al.30) conducted a retrospective study on 33 cases of total hip or knee replacement arthroplasty. In the study, infection could be confirmed if more than 5 multiple nucleated neutrophils were observed in high power field. Della Valle et al.31) defined infection as observation of 10 neutrophils in 5 different tissues. However, Cho et al.32) reported that successful reimplantation for infected TKA could be achieved despite detection of 5 to 20 neutrophils in frozen biopsy. They insisted that clinical and operative findings are as important as serological and biopsy findings. Booth and Lotke33) reported sensitivity and specificity could be increased by conducting both histological and biological tests together.
Tissue culture is the absolute standard for the diagnosis of periprosthetic infection. Intraoperative culture must be done before injection of antibiotics during surgery. Cultures must be obtained from various tissues such as synovium, synovial fluid, intramedullary tissue, granular tissue and bone. Chun20) reported that if tissue culture is negative and other clinical examinations suggest infection, infection treatment should be initiated. If tissue culture is positive and other examinations are negative, it indicates a false positive for tissue culture.
5. Other Examinations
For early diagnosis, bone scan can be more useful than simple radiography. To increase the accuracy of diagnosis, a technetium 99 m labeled WBC scan can be used. Palestro et al.34) reported that increased accuracy of diagnosis of infected knee prostheses could be obtained by using both an isotope as indium-111 and methyl diphosphste-sulfur colloid.
Sonication is a recently introduced method to increase the accuracy of diagnosis. Achermann et al.35) evaluated the value of multiplex polymerase chain reaction (PCR) for detection of microbial DNA in sonication fluid from removed implant. The removed implant was sonicated, and the resulting sonication fluid was cultured and subjected to multiplex PCR. The pathogen was identified by the multiplex PCR in 78.5% of the cases.
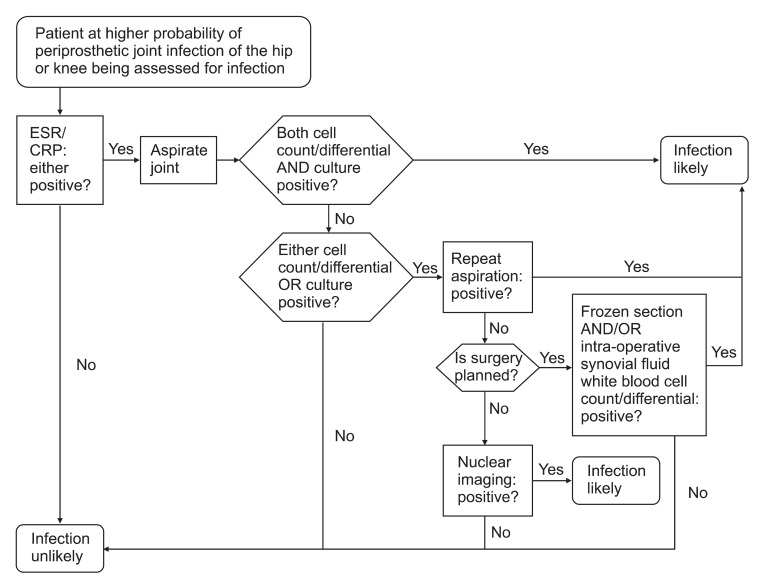
The Algorithm for Diagnosis
In 2010, AAOS released an algorithm for diagnosis of periprosthetic joint infections of the hip and knee29)(Fig. 1).
Fig. 1.
An algorithm for diagnosis of periprosthetic joint infections of the hip and knee. ESR: erythrocyte sedimentation rate, CRP: C-reactive protein. Reprinted from American Academy of Orthopaedic Surgeons29).
Treatment
Although there are numerous treatments for periposthetic joint infection, the single goal is providing painless and functional joint without infection. Thus, the selection for proper treatment option must be based on carefully established criteria. The treatment options include antibiotic suppression alone, debridement with antibiotics, single-stage prosthetic exchange, second-stage arthroplasty, arthrodesis, and amputation. Currently, second stage revision arthroplasty is the gold standard for the treatment of infected TKA. However, if infection remains after all these treatments, amputation can be considered. Bone cement with antibiotics increases the concentration of antibiotics at the local region to prevent infection. Chiu et al.36) reported a decreased infection rate in 44 patients with a history of DM after primary TKA.
Indications for conservative treatment without implant removal are very limited: 1) early infection is present after TKA, 2) stability of implants is firm and, 3) cultured bacteria are weak compared to other cases37).
1. Antibiotic Suppression
Antibiotic treatment alone cannot eradicate deep infection. Thus, it cannot be used on young or active patients. It can be used as a suppressive treatment only in a few conditions: the microorganism has low virulence, the microorganism is susceptible to oral antibiotics, the implant is well-fixed and/or the patients have a medical condition that cannot endure an operative procedure.
2. Debridement with Antibiotics
Irrigation and debridement without prosthesis removal, in addition to antibiotic treatment, can be used in patients with acute symptoms. In case of the well-fixed prosthesis with acute infection, irrigation with debridement can be a very useful treatment option. The reported success rate of irrigation and debridement varies from 16% to 80%38-40). However, if infection affects multiple joints, prosthesis loosening observed and the duration of infection is longer than 30 days, the prosthesis must be removed.
3. Single-Stage Prosthetic Exchange (One-Stage Arthroplasty)
Single-stage prosthetic exchange is a strenuous treatment option since it removes prosthesis with resection and reimplants all components in a single operation. Single-stage prosthetic exchange has advantages such as limiting the number of surgeries and recovery time that leads to decreased expenses. The antibiotics act as a local therapy when mixed in cement for fixation of prosthesis. Freeman et al.41) insisted one-stage revision can be performed if the patient shows mild symptoms of infection without fistula, culture results of gram positive bacteria are identified and systemic toxic reaction is not observed. Jenny et al.42) also reported one-stage reimplantation can be successful in chronically infected TKA, showing no infection recurrence at 3 year follow-up. The reported success rate of direct exchange varies widely from 73% to 100%43-45). However, single-stage prosthesis has limited indication. It can be performed on patients whose medical condition cannot endure multiple operations and for infection by a single pathogen that is non-resistant and non-virulent.
4. Second-Stage Arthroplasty
Second-stage arthroplasty consists of first stage debridement and delayed antibiotic treatment. After 4 to 6 weeks of follow-up, second-stage arthroplasty is performed if serological examination results are within normal range46). After first stage debridement, a static spacer or an articulating spacer made from antibiotic mixed cement can be placed in the joint space. Inserting an articular space can solve the complication caused by prolonged immobilization of the joint with use of a static spacer. By inserting an articulating spacer, soft tissue contracture can be prevented and early range of motion can be obtained. It can also help use a suitable approach and increase the range of motion after revision arthroplasty33,47). Hoffmann et al.48) reported that the average range of motion was 101° in 26 cases. Lee and Choi49) reported that a temporary articulation system composed of an autoclaved femoral component, a resterilized polyethylene insert and antibiotic-impregnated bone cement can be used instead of an articulating spacer. They insisted that their method was effective for eradication of infection as well as for the recovery of soft tissue health and knee function. Second-stage revision arthroplasty using a static or a mobile spacer can be performed after an infection is controlled. Brunnekreef et al.50) reported that the use a mobile spacer promoted better and faster recovery of knee function after operation. They also claimed that using a mobile spacer requires shorter operation time.
5. Arthrodesis
Before improvement of medical care or antibiotics and operational skills, arthrodesis was the standard treatment for infected arthroplasty. Instead of gaining benefits, such as decreased pain, eliminated infection and stable knee function, patients suffered limitation of social activities due to eliminated knee motion. Thus, this treatment option seems less favorable to most patients and surgeons in present days. However, arthrodesis can be applied to patients with high functional demands, single joint diseases, young age, disruption of extensor mechanism, poor soft tissue that requires wide soft tissue reconstruction, systemic immunocompromise and microorganisms that require highly toxic antibiotic treatment or are resistant to formal antibiotics. Arthrodesis cannot be applied to bilateral knee infection, ipsilateral ankle or hip diseases, or contralateral extremity amputation state. Successful rate of arthrodesis depends on the type of prior knee implant, amount of bone deficiency and arthrodesis techniques. The fixation techniques for arthrodesis are external fixation and internal fixation with intramedullary nail or plate fixation. Intramedullary nailing is the most commonly used techniques with higher rates of union. However, it also has higher risk of recurrent infection.
6. Amputation
Amputation is the last method after failure of other methods. This treatment has been conducted in only 5% of the infected arthoplasty patients. It is performed in cases of life-threatening systemic sepsis or persistent infection with massive bone loss. Amputation must be performed with the level that maximizes the function and completes eradication of infection. The local muscle transposition of the gastrocnemius during the procedure should be done for dead space management and to envelope the amputation stamp.
Conclusions
TKA has been known as one of the most effective surgical procedures for arthritic patients. However, like all the other procedures, TKA has complications, and infection is the most disastrous one among them. Infection after TKA increases both morbidity and mortality in patients. Although prevention strategies, such as the administration of prophylactic antibiotics, have reduced the incidence of infection after primary arthroplasty, surgeons still encounter this complication frequently. As dreadful as it seems, there is a solution to this problem. Early diagnosis with sequential treatment can resolve the complication without leaving sequelae. To do so, we must remind that infection can happen randomly to any patients and surgeons should always be careful in every process of procedures.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Felson DT, Lawrence RC, Hochberg MC, McAlindon T, Dieppe PA, Minor MA, Blair SN, Berman BM, Fries JF, Weinberger M, Lorig KR, Jacobs JJ, Goldberg V. Osteoarthritis: new insights: part 2: treatment approaches. Ann Intern Med. 2000;133:726–737. doi: 10.7326/0003-4819-133-9-200011070-00015. [DOI] [PubMed] [Google Scholar]
- 2.Jones CA, Beaupre LA, Johnston DW, Suarez-Almazor ME. Total joint arthroplasties: current concepts of patient outcomes after surgery. Clin Geriatr Med. 2005;21:527–541. doi: 10.1016/j.cger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Lavernia CJ, Guzman JF, Gachupin-Garcia A. Cost effectiveness and quality of life in knee arthroplasty. Clin Orthop Relat Res. 1997;(345):134–139. [PubMed] [Google Scholar]
- 4.Schurman DJ. Functional outcome of GUEPAR hinge knee arthroplasty evaluated with ARAMIS. Clin Orthop Relat Res. 1981;(155):118–132. [PubMed] [Google Scholar]
- 5.Bae DK, Kim SS, Kwon OS. Effect of antibiotic-impregnated bone cement in total knee arthroplasty. J Korean Knee Soc. 1996;8:167–172. [Google Scholar]
- 6.Grogan TJ, Dorey F, Rollins J, Amstutz HC. Deep sepsis following total knee arthroplasty: ten-year experience at the University of California at Los Angeles Medical Center. J Bone Joint Surg Am. 1986;68:226–234. [PubMed] [Google Scholar]
- 7.Poss R, Thornhill TS, Ewald FC, Thomas WH, Batte NJ, Sledge CB. Factors influencing the incidence and outcome of infection following total joint arthroplasty. Clin Orthop Relat Res. 1984;(182):117–126. [PubMed] [Google Scholar]
- 8.Petty W, Bryan RS, Coventry MB, Peterson LF. Infection after total knee arthroplasty. Orthop Clin North Am. 1975;6:1005–1014. [PubMed] [Google Scholar]
- 9.Chun CH, Park JY, Kim JW, Kim JY. Total knee arthroplasty in diabetic patients. J Korean Knee Soc. 2005;17:105–111. [Google Scholar]
- 10.Papavasiliou AV, Isaac DL, Marimuthu R, Skyrme A, Armitage A. Infection in knee replacements after previous injection of intra-articular steroid. J Bone Joint Surg Br. 2006;88:321–323. doi: 10.1302/0301-620X.88B3.17136. [DOI] [PubMed] [Google Scholar]
- 11.Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72:878–883. [PubMed] [Google Scholar]
- 12.Cho WS, Jeong YG, Park JH, Shin HK, Kim KY, Seon MW. Treatment of infected TKRA. J Korean Orthop Assoc. 2001;36:561–567. [Google Scholar]
- 13.Stefansdottir A, Johansson D, Knutson K, Lidgren L, Robertsson O. Microbiology of the infected knee arthroplasty: report from the Swedish Knee Arthroplasty Register on 426 surgically revised cases. Scand J Infect Dis. 2009;41:831–840. doi: 10.3109/00365540903186207. [DOI] [PubMed] [Google Scholar]
- 14.Song MH, Kim BH, Ahn SJ, Yoo SH, Kim TH. Successful salvage of primary TKA infected with Candida albicans: a case report. J Korean Orthop Assoc. 2005;40:356–360. [Google Scholar]
- 15.Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am. 2003;85(Suppl 1):S75–S80. doi: 10.2106/00004623-200300001-00014. [DOI] [PubMed] [Google Scholar]
- 16.Tintle SM, Forsberg JA, Potter BK, Islinger RB, Andersen RC. Prosthesis retention, serial debridement, and antibiotic bead use for the treatment of infection following total joint arthroplasty. Orthopedics. 2009;32(2):87. [PubMed] [Google Scholar]
- 17.Parvizi J, Ghanem E, Menashe S, Barrack RL, Bauer TW. Periprosthetic infection: what are the diagnostic challenges? J Bone Joint Surg Am. 2006;88(Suppl 4):138–147. doi: 10.2106/JBJS.F.00609. [DOI] [PubMed] [Google Scholar]
- 18.Morrey BF, Westholm F, Schoifet S, Rand JA, Bryan RS. Long-term results of various treatment options for infected total knee arthroplasty. Clin Orthop Relat Res. 1989;(248):120–128. [PubMed] [Google Scholar]
- 19.Teller RE, Christie MJ, Martin W, Nance EP, Haas DW. Sequential indium-labeled leukocyte and bone scans to diagnose prosthetic joint infection. Clin Orthop Relat Res. 2000;(373):241–247. doi: 10.1097/00003086-200004000-00029. [DOI] [PubMed] [Google Scholar]
- 20.Chun CH. Diagnosis of infected total knee arthroplasty. J Korean Knee Soc. 2009;21:205–211. [Google Scholar]
- 21.Shih LY, Wu JJ, Yang DJ. Erythrocyte sedimentation rate and C-reactive protein values in patients with total hip arthroplasty. Clin Orthop Relat Res. 1987;(225):238–246. [PubMed] [Google Scholar]
- 22.Glehr M, Friesenbichler J, Hofmann G, Bernhardt GA, Zacherl M, Avian A, Windhager R, Leithner A. Novel biomarkers to detect infection in revision hip and knee arthroplasties. Clin Orthop Relat Res. 2013;471:2621–2628. doi: 10.1007/s11999-013-2998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leone JM, Hanssen AD. Management of infection at the site of a total knee arthroplasty. J Bone Joint Surg Am. 2005;87:2335–2348. doi: 10.2106/00004623-200510000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J, Della Valle CJ. AAOS clinical practice guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18:771–772. doi: 10.5435/00124635-201012000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117:556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Dinneen A, Guyot A, Clements J, Bradley N. Synovial fluid white cell and differential count in the diagnosis or exclusion of prosthetic joint infection. Bone Joint J. 2013;95:554–557. doi: 10.1302/0301-620X.95B4.30388. [DOI] [PubMed] [Google Scholar]
- 27.Mason JB, Fehring TK, Odum SM, Griffin WL, Nussman DS. The value of white blood cell counts before revision total knee arthroplasty. J Arthroplasty. 2003;18:1038–1043. doi: 10.1016/s0883-5403(03)00448-0. [DOI] [PubMed] [Google Scholar]
- 28.Spangehl MJ, Masri BA, O'Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 29.American Academy of Orthopaedic Surgeons (AAOS) The diagnosis of periprothetic joint infections of the hip and knee: guideline and evidence report. Rosemont: AAOS; 2010. [Google Scholar]
- 30.Feldman DS, Lonner JH, Desai P, Zuckerman JD. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am. 1995;77:1807–1813. doi: 10.2106/00004623-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Della Valle CJ, Bogner E, Desai P, Lonner JH, Adler E, Zuckerman JD, Di Cesare PE. Analysis of frozen sections of intraoperative specimens obtained at the time of reoperation after hip or knee resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 1999;81:684–689. doi: 10.2106/00004623-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Cho WS, Byun SE, Cho WJ, Yoon YS, Dhurve K. Polymorphonuclear cell count on frozen section is not an absolute index of reimplantation in infected total knee arthroplasty. J Arthroplasty. 2013 Apr 30; doi: 10.1016/j.arth.2013.03.016. [Epub]. http://dx.doi.org/10.1016/j.arth.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Booth RE, Jr, Lotke PA. The results of spacer block technique in revision of infected total knee arthroplasty. Clin Orthop Relat Res. 1989;(248):57–60. [PubMed] [Google Scholar]
- 34.Palestro CJ, Swyer AJ, Kim CK, Goldsmith SJ. Infected knee prosthesis: diagnosis with In-111 leukocyte, Tc-99m sulfur colloid, and Tc-99m MDP imaging. Radiology. 1991;179:645–648. doi: 10.1148/radiology.179.3.2027967. [DOI] [PubMed] [Google Scholar]
- 35.Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol. 2010;48:1208–1214. doi: 10.1128/JCM.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu FY, Lin CF, Chen CM, Lo WH, Chaung TY. Cefuroxime-impregnated cement at primary total knee arthroplasty in diabetes mellitus: a prospective, randomised study. J Bone Joint Surg Br. 2001;83:691–695. doi: 10.1302/0301-620x.83b5.11737. [DOI] [PubMed] [Google Scholar]
- 37.Whiteside LA. Treatment of infected total knee arthroplasty. Clin Orthop Relat Res. 1994;(299):169–172. [PubMed] [Google Scholar]
- 38.Bradbury T, Fehring TK, Taunton M, Hanssen A, Azzam K, Parvizi J, Odum SM. The fate of acute methicillin-resistant Staphylococcus aureus periprosthetic knee infections treated by open debridement and retention of components. J Arthroplasty. 2009;24(6 Suppl):101–104. doi: 10.1016/j.arth.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 39.Hartman MB, Fehring TK, Jordan L, Norton HJ. Periprosthetic knee sepsis: the role of irrigation and debridement. Clin Orthop Relat Res. 1991;(273):113–118. [PubMed] [Google Scholar]
- 40.Silva M, Tharani R, Schmalzried TP. Results of direct exchange or debridement of the infected total knee arthroplasty. Clin Orthop Relat Res. 2002;(404):125–131. doi: 10.1097/00003086-200211000-00022. [DOI] [PubMed] [Google Scholar]
- 41.Freeman MA, Sudlow RA, Casewell MW, Radcliff SS. The management of infected total knee replacements. J Bone Joint Surg Br. 1985;67:764–768. doi: 10.1302/0301-620X.67B5.4055878. [DOI] [PubMed] [Google Scholar]
- 42.Jenny JY, Barbe B, Gaudias J, Boeri C, Argenson JN. High infection control rate and function after routine one-stage exchange for chronically infected TKA. Clin Orthop Relat Res. 2013;471:238–243. doi: 10.1007/s11999-012-2480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buechel FF, Femino FP, D'Alessio J. Primary exchange revision arthroplasty for infected total knee replacement: a long-term study. Am J Orthop (Belle Mead NJ) 2004;33:190–198. [PubMed] [Google Scholar]
- 44.Silva M, Tharani R, Schmalzried TP. Results of direct exchange or debridement of the infected total knee arthroplasty. Clin Orthop Relat Res. 2002;(404):125–131. doi: 10.1097/00003086-200211000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Jamsen E, Stogiannidis I, Malmivaara A, Pajamaki J, Puolakka T, Konttinen YT. Outcome of prosthesis exchange for infected knee arthroplasty: the effect of treatment approach. Acta Orthop. 2009;80:67–77. doi: 10.1080/17453670902805064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grogan TJ, Dorey F, Rollins J, Amstutz HC. Deep sepsis following total knee arthroplasty: ten-year experience at the University of California at Los Angeles Medical Center. J Bone Joint Surg Am. 1986;68:226–234. [PubMed] [Google Scholar]
- 47.Chun CH, Kim JW, Kweon SH, Choi BS. Comparison of articular spacer versus static spacer in the treatment of infected total knee arthroplasty. J Korean Knee Soc. 2007;19:57–62. [Google Scholar]
- 48.Hofmann AA, Kane KR, Tkach TK, Plaster RL, Camargo MP. Treatment of infected total knee arthroplasty using an articulating spacer. Clin Orthop Relat Res. 1995;(321):45–54. [PubMed] [Google Scholar]
- 49.Lee JK, Choi CH. Two-stage reimplantation in infected total knee arthroplasty using a re-sterilized tibial polyethylene insert and femoral component. J Arthroplasty. 2012;27:1701–1706. doi: 10.1016/j.arth.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Brunnekreef J, Hannink G, Malefijt Mde W. Recovery of knee mobility after a static or mobile spacer in total knee infection. Acta Orthop Belg. 2013;79:83–89. [PubMed] [Google Scholar]