Abstract
The neocortex, the evolutionarily newest part of the cerebral cortex, controls nearly all aspects of behavior, including perception, language and decision-making. It contains an immense number of neurons that can be broadly divided into two groups, excitatory neurons and inhibitory interneurons. These neurons are predominantly produced through extensive progenitor cell divisions during the embryonic stages. Moreover, they are not randomly dispersed, but spatially organized into horizontal layers that are essential for neocortex function. The formation of this laminar structure requires exquisite control of neuronal migration from their birthplace to their final destination. Extensive research over the past decade has greatly advanced our understanding of the production and migration of both excitatory neurons and inhibitory interneurons in the developing neocortex. In this review, we aim to give an overview on the molecular and cellular processes of neocortical neurogenesis and neuronal migration.
Introduction
A prominent trait of the neocortex is its complex yet well-organized cellular architecture, the formation of which relies on the production and positioning of its diverse neuronal populations. There are two major groups of neurons in the neocortex — glutamatergic excitatory neurons and γ-aminobutyric acid (GABA)-ergic inhibitory interneurons, which are responsible for generating excitation and inhibition, respectively. Excitatory neurons constitute the vast majority (~70–80%) of neocortical circuit neurons and are responsible for generating the output. On the other hand, inhibitory interneurons provide a rich variety of inhibitions that shape the output of functional circuits. Proper neocortex function critically depends on the production and positioning of a correct number of excitatory and inhibitory neurons, which largely occur during the embryonic stages.
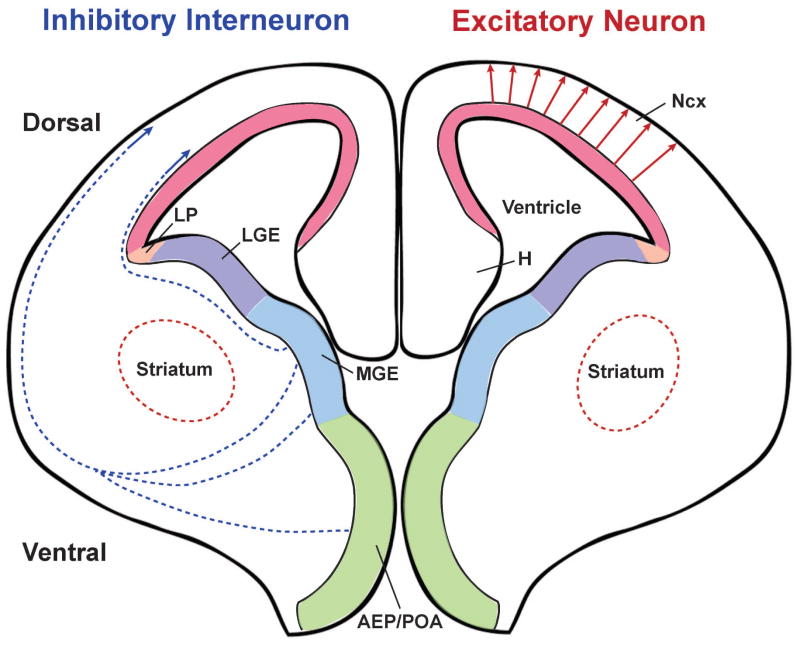
With the advent of improved fate-mapping tools, including genetically engineered mice, excitatory and inhibitory neurons in the neocortex were found to arise from different developmental lineages. In rodents, the progenitor cells of these two neuronal populations are fully segregated in space (Figure 1). Excitatory neurons are generated in the proliferative zone of the dorsal telencephalon and then migrate radially to constitute the future neocortex. In contrast, inhibitory interneurons are produced in the proliferative zone of the ventral telencephalon and migrate tangentially to reach the neocortex, where they co-assemble with excitatory neurons into functional circuits. This spatial segregation in the excitatory and inhibitory neuron progenitors no doubt further complicates the coordinated production and positioning of these neurons.
Figure 1. Distinct origins of excitatory neurons and inhibitory interneurons in the developing mouse neocortex.
Excitatory neurons are generated in the proliferative zone of the dorsal telencephalon and then migrate radially to the cortical plate (red arrows). In contrast, inhibitory interneurons are produced in the proliferative zone of the ventral telencephalon, especially the MGE and AEP/POA, and migrate tangentially to reach the neocortex following two major routes (blue dashed arrows). The colored regions indicate the proliferative zones across the embyronic brain expressing different transcription factors that are essential for proper neurogenesis of distinct neuronal populations. AEP: anterior entopeduncular area; H: hippocampus; LGE: lateral ganglionic eminence; LP: lateral pallium; MGE: medial ganglionic eminence; Ncx: neocortex; POA: preoptic area.
The production of neocortical excitatory neurons has been extensively studied and various types of neural progenitor cells have been revealed in the proliferative zone of the dorsal telencephalon (Figure 2). In addition, the radial migration of excitatory neurons has been characterized in great detail. Relatively speaking, our knowledge of the neurogenesis and migration of neocortical interneurons remains limited, in part due to their origin outside the developing neocortex and to their extraordinary diversity.
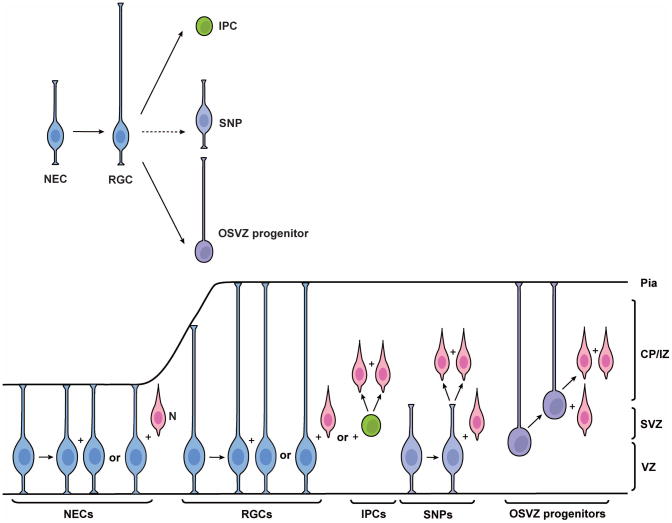
Figure 2. Diverse populations of excitatory neuron progenitor cells in the mouse neocortex.
During early brain development, neuroepithelial cells (NECs) are the major neural progenitors in the neocortex. NECs divide symmetrically to generate additional NECs, some of which give rise to the first group of neurons (N) through asymmetric division. As the developing brain epithelium thickens, NECs elongate and transit to radial glial cells (RGCs). RGCs can also divide symmetrically to expand the progenitor pool or asymmetrically to generate neurons either directly or indirectly through intermediate progenitor cells (IPCs), which generate neurons directly through symmetric division in the subventricular zone (SVZ). It remains unclear how short neural progenitors (SNPs) are generated. It is possible that they are generated from RGCs or they may be a distinct population originated directly from NECs. Most SNPs produce postmitotic neurons directly in the ventricular zone (VZ). Outer subventricular zone (OSVZ) progenitors likely originate from RGCs through oblique division, which leads to loss of the apical process and ascension of the nucleus towards the cortical plate/intermediate zone (CP/IZ). The minor population of OSVZ progenitors can also generate neurons through asymmetric division outside of the VZ.
I. Diversity of excitatory neuron progenitor cells
In the developing vertebrate nervous system, the neural tube is the embryo’s precursor to the central nervous system (CNS) and the neuroepithelial cells that constitute the neural tube are the precursors of all future neurons, including those in the neocortex 1.
Neuroepithelial cells (NECs)
In the early stages of embryonic development, most neuroepithelial progenitor cells undergo symmetric division – two neuroepithelial cells are produced at each division, thereby expanding the population of founder cells that will ultimately produce the CNS including the neocortex. Retrovirally-mediated lineage tracing demonstrated that many neuroepithelial cells are multi-potent progenitor cells 2. They are capable of generating the first group of neurons in the neocortex and radial glial cells (RGCs), the major neural progenitor cells responsible for producing neurons and glia. In the mouse neocortex, the transition of NECs to RGCs appears to occur around embryonic day 9.5–10.5 (E9.5-E10.5). This transition is characterized by the initiation of the expression of astroglial markers such as astrocyte-specific glutamatetransporter, SLC1A3, formerly known as GLAST and fatty acid binding protein 7 (FABP7) 3,4, and an alteration in tight junctions including loss of Occludin 5,6.
Radial glial cells (RGCs)
RGCs are a widespread non-neuronal cell type in the developing CNS of all vertebrates examined so far. They were first identified as a transient population that serves as a scaffold for neuronal migration 7–9; however, their fundamental role as neural progenitor cells has been well-demonstrated in the past decade 10–12. RGCs are defined by their characteristic radial bipolar morphology and their astroglial properties. These long bipolar cells expand across the entire thickness of the developing neocortex with a long basal radial process pointing to the pial surface, a short apical ventricular endfoot reaching the ventricular zone (VZ) surface, and the soma located in the VZ 13,14. During the early stages of neurogenesis, RGCs largely divide symmetrically to produce two radial glial progenitor cells after each division 15,16. At later stages, they predominantly undergo asymmetric cell division to self-renew and, at the same time, to generate a differentiating daughter cell that is either a neuron or an intermediate progenitor cell 12,17, or an outer subventricular zone (OSVZ) progenitor cell 18. At the end of neurogenesis, RGCs are shown to switch to gliogenesis producing astrocytes and oligodendrocytes 19.
Intermediate progenitor cells (IPCs)
Besides RGCs, another type of neuronal progenitor cell – the basal or intermediate progenitor cells (IPCs) – emerges at the onset of peak neurogenesis. They accumulate in the subventricular zone (SVZ), a second proliferative region that lies above the VZ. These IPCs are produced by RGCs in the VZ through asymmetric cell division and migrate radially to the SVZ, where they divide symmetrically to generate two postmitotic neurons 17,20,21. IPCs can also divide symmetrically to produce a pair of progenitors and each could subsequently generate a pair of neurons 17,21. The generation of neocortical neurons through IPCs would therefore significantly increase the number of neurons produced by individual RGCs. Hence, it has been postulated that the abundance of IPCs may contribute to the evolutionary expansion of the neocortex 22. Notably, IPCs are molecularly distinguishable from RGCs by their expression of the transcription factor genes Eomes (formerly known as Tbr2)23, Cux1/Cux2 24,25, and Satb2 26.
Short neural precursors (SNPs)
Studies using three-dimensional reconstruction of individually labeled progenitor cells from the VZ surface showed that the vast majority of VZ progenitor cells in the mouse brain (88% at E12.5; 66% at E16.5) have long radial processes extending towards the basal pial surface with a growth cone at their tips 27. Nevertheless, a subpopulation of VZ dividing cells that either possess a short basal process or lack the basal process altogether (‘club-shaped’) has also been revealed as morphologically, ultrastructurally and molecularly distinct from RGCs and they are termed as short neural precursors (SNPs) 28. Follow-up studies have suggested that SNPs and RGCs cohabit the VZ but display different cell cycle kinetics. They give rise to distinct neuronal lineages because of a differential reliance on IPC amplification 29. While RGCs often give rise to neuronal progeny indirectly through IPCs within the SVZ, most SNPs produce postmitotic neurons directly within the VZ 29. These studies suggest that progenitor cell variety could be a very important factor in neocortical development.
Outer SVZ progenitor cells
Recently, another distinct type of self-renewing progenitor cell – the outer subventricular zone (OSVZ) progenitors – was discovered in developing human and ferret neocortices 30–33. Although they originate from and behave similar to RGCs, OSVZ progenitor cells retain only the basal process and lack the apical process necessary for anchoring to the VZ surface. They were initially recognized as a special population of neural progenitor cells as an evolutionary consequence of neocortex expansion. Strikingly, a very small population of OSVZ progenitor cells, similar to those observed in developing human and ferret neocortices, has of late been observed in the developing mouse neocortex 18,34. They are likely generated by rare oblique RGC divisions 18.
II. Mechanisms of asymmetric progenitor cell division
It is generally accepted that asymmetric division of progenitor cells is responsible for producing nearly all of the neurons in the developing neocortex either directly or indirectly. However, the molecular and cellular basis of asymmetric progenitor cell division in the developing neocortex remains largely unknown. A number of molecular signaling pathways have been implicated in the regulation of progenitor cell division and neuronal differentiation in the neocortex. For example, NOTCH-, EGFR- and FGF-signaling have all been implicated in the maintenance of RGCs and are hence crucial for the expansion of the neural progenitor pool 35–41. NOTCH signaling in particular has been extensively studied in recent years, given its central role in controlling asymmetric division of neuroblasts in the Drosophila nervous system. In the developing neocortex, NOTCH signaling has also been thought to regulate self-renewal versus differentiation of RGCs 42, although the precise role of NOTCH signaling in regulating RGC asymmetric division remains unclear 43–48.
A number of models have been proposed to explain the cellular basis of RGC asymmetric division (Figure 3):
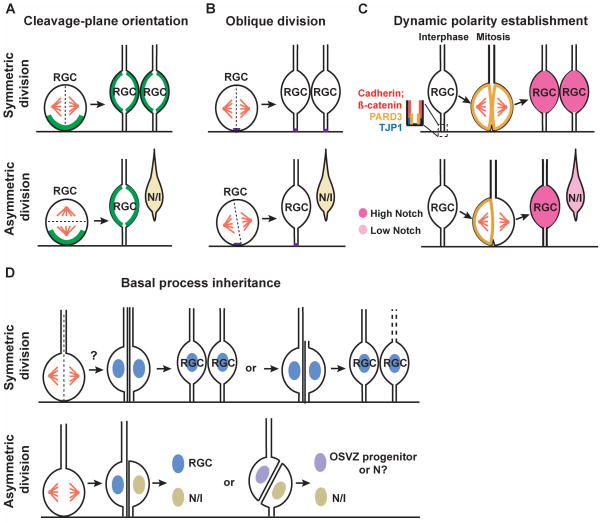
Figure 3. Models of symmetric and asymmetric divisions of radial glial progenitor cells in the mouse neocortex.
(A) Cleavage-plane orientation model. When the cleavage-plane is perpendicular to the VZ surface, the division of RGCs results in symmetric inheritance of critical fate determinants enriched at the VZ surface (represented by a green line) and consequently the same daughter cell fate specification (i.e. symmetric division). When the cleavage-plane is parallel to the VZ surface, the division results in asymmetric inheritance of critical fate determinants enriched at the VZ surface and consequently distinct daughter cell fate specification - one remains as RGC whereas the other becomes a postmitotic neuron (N) or an intermediate progenitor (I) (i.e. asymmetric division). (B) Oblique division model. Dividing progenitor cells possess a small patch of membrane close to the VZ surface (represented by a short purple line), the inheritance of which is critical for fate specification. Bisection of this small membrane patch leads to symmetric division, whereas a slight tilt in the vertical cleavage plane (i.e. oblique division) bypasses it and results in asymmetric division. (C) Dynamic polarity establishment model. The distribution of the evolutionarily conserved cell polarity protein PARD3 is dynamic depending on cell cycle progression, which becomes dispersed during mitosis. Even distribution of PARD3 in dividing RGCs leads to symmetric division, whereas polarized distribution of PARD3 results in its asymmetric inheritance by the two daughter cells, followed by differential activation of NOTCH signaling and subsequent distinct fate specification. (D) Basal process inheritance model. Inheritance of the basal process has been linked to fate specification though it is still controversial. The basal process can be bisected and inherited by both daughter cells during the symmetric division, although it has also been postulated that the basal process may be inherited by only one daughter RGC while the other has to elaborate a new basal process. For the asymmetric division, earlier studies suggested that the daughter cell that inherits the basal process becomes a differentiating neuron whereas the other daughter grows a new process and remains in the VZ as a progenitor. Recent work indicated that the daughter cell inheriting the basal process remains a progenitor (either a RGC or OSVZ progenitor) while the other differentiates (either a neuron or an intermediate progenitor).
Cleavage-plane orientation model
In Drosophila, delaminating neuroblasts from the epithelium retain the apical-basal polarity, which orients the mitotic spindle perpendicular to the epithelial layer. This precise orientation of the mitotic spindle ensures asymmetric segregation of the critical fate determinants such as NUMB and Prospero-related family, resulting in distinct fate specification 49,50. In the developing neocortex, RGCs in the VZ display interkinetic nuclear oscillation as they progress through the cell cycle. While their nuclei are located at the top of the ventricular zone during S phase, mitosis occurs only upon arrival of the nuclei at the VZ surface. It has been postulated that analogous to the Drosophila nervous system, critical fate determinants in these cells are enriched at the VZ surface 51,52. Therefore, when the cleavage plane is parallel to the VZ surface, this horizontal division results in asymmetric inheritance of critical fate determinants enriched at the VZ surface and consequently distinct daughter cell fate specification (i.e. asymmetric division). On the other hand, when the cleavage plane is perpendicular to the VZ surface, this vertical division results in symmetric inheritance of critical fate determinants and consequently the same daughter cell fate specification (i.e. symmetric division) (Figure 3A).
While this cleavage plane orientation hypothesis has wielded a dominant influence on the understanding of progenitor cell asymmetric division and neurogenesis in the developing neocortex, it is being challenged by recent experimental data. Based on this hypothesis, asymmetric, neurogenic division should assume the horizontal cleavage plane, while symmetric, proliferative division should assume the vertical cleavage plane. However, recent studies including time-lapse imaging analysis of RGC divisions clearly demonstrated that the vast majority of asymmetric, neurogenic divisions assume the vertical cleavage plane 53–55, arguing that vertical divisions can be asymmetric and neurogenic.
Oblique division model
To resolve the aforementioned discrepancy that vertical divisions can be either symmetric or asymmetric, a new model – oblique division model – has been put forward 15. This model suggests that dividing progenitor cells possess a small patch of membrane (1–2% of the total plasma membrane) close to the VZ surface and that the inheritance of this small patch of membrane is critical for fate specification 56. When a vertical cleavage plane bisects this small membrane patch, the division is symmetric. On the other hand, given that this membrane patch is so small, a slight tilt in the vertical cleavage plane (i.e. oblique division) would bypass it and result in asymmetric division (Figure 3B) 56. The nature of this small membrane patch in individual dividing progenitor cells remains elusive, even though it is largely considered to be the apical domain of the progenitor cells. The first and only molecular marker reported to mark this domain is PROMININ 1 (CD133), a membrane glycoprotein specifically associated with plasma membrane protrusions such as microvilli 57,58. Although PROMININ 1 is usually localized to the apical surface in epithelial cells and is a well-characterized stem cell marker, it is not a typical apical membrane marker. None of the well-characterized apical protein complexes, such as the partition-defective (PAR) protein complex and the PATJ-PALS1-CRBS complex, have been reported to label this domain in interphase and dividing progenitor cells. In fact, it is technically challenging to explicitly reveal the apical/basal polarity of individual dividing progenitor cells at the VZ surface 59, even though the polarity of interphase progenitor cells in the VZ has been well-established 60. Recent studies suggested that the oblique division of radial glial cells is critical for the production of OSVZ progenitor cells 18 or IPCs 54 in the mouse neocortex. This difference in the daughter cell fate specification further emphasizes the importance to understand the upstream components that control the orientation of the mitotic spindle and the downstream factors inherited by the daughter cells that regulate the fate specification.
Dynamic polarity model
Recently, it has been shown that the distribution of PARD3, an evolutionarily conserved polarity protein that marks the apical domain, in RGCs is dynamic depending on cell cycle progression 59. While it is selectively localized at the TJP1 (formerly known as ZO-1)-expressing lateral membrane domain in the ventricular endfeet during interphase, PARD3 becomes dispersed during mitosis. The dynamic distribution of PARD3 in individual dividing RGCs can result in polarized distribution of PARD3 followed by its asymmetric inheritance by the two daughter cells, which results in differential activation of NOTCH signaling and subsequent distinct cell fate specification. These data suggest the daughter cell that inherits a greater amount of PARD3 develops high NOTCH signaling activity and remains an RGC, whereas the one that inherits less PARD3 has low NOTCH signaling activity and differentiates into a neuron or IPC (Figure 3C) 59. Interestingly, while asymmetric inheritance of PARD3 occurs in dividing progenitor cells with different cleavage plane orientations (mostly vertical/oblique, and to a lesser extent horizontal), PARD3 polarity is always perpendicular to the cleavage plane orientation, raising the possibility that the dynamic establishment of polarity in dividing progenitor cells influences the orientation of the cleavage plane and subsequent daughter cell fate specification.PARD3 has also been implicated in progenitor cell asymmetric division in the zebrafish neural tube 61. However, in this model PAR3 inheritance appears to correlate with a neuronal fate. This difference calls for a closer examination of the behavior and function of endogenous PARD3 across species.
Basal process inheritance model
Being intricately associated with cleavage-plane orientation and apical membrane inheritance, inheritance of the basal process has also been linked to fate specification, despite much controversial data (Figure 3D). For symmetric divisions that expand the progenitor pool at the onset of neurogenesis, the basal process of NECs can be bisected and inherited by both daughter cells 62. On the other hand, it has been postulated that in symmetric divisions at later stages, the basal process is inherited by only one of the daughter cells while the other daughter cell has to elaborate a new basal process 17,20. For asymmetric divisions during later neurogenesis, it has been indicated that the neuronal daughter cell inherits the basal process and migrates towards the cortical plate (CP, the future neocortex) through somal translocation, whereas the other daughter cell could grow a new basal radial process and remain in the VZ as a progenitor 63,64. In contrast, time-lapse imaging studies by Noctor and colleagues revealed that the daughter cell inheriting the basal process remains an RGC 12,17. Furthermore, by inducing oblique division in RGCs to split the apical and basal processes to the two daughters, recent studies by Matsuzaki group indicated that the daughter cell retaining the basal process mostly self-renews outside the VZ, similar to the OSVZ progenitors reported in primates and ferrets, while the apical daughter cell that inherits the apical membrane at the VZ surface differentiates 18,55. Only when a daughter cell inherits both the basal process and the apical membrane does it become an RGC. It remains unclear where these discrepancies stem from and future work that identifies the signals associated with the basal process may help clarify these issues.
Cell cycle length model
An increase in cell cycle length, specifically G1 phase, is naturally associated with the progression of neurogenesis 65. A recent model, referred to as “cell cycle length hypothesis”, has been proposed, largely on the basis of the observation that lengthening the neuroepithelial cell cycle alone is sufficient to promote the transition from symmetric proliferative to asymmetric neurogenic divisions 66. This hypothesis emphasizes that unequal inheritance of a cell fate determinant by daughter cells may or may not lead to distinct fate specification. Instead, the length of cell cycle determines the time window in which the cell fate determinant is allowed to act 1,66, thereby resulting in fate specification. This concept has been further supported by more recent observations showing that progenitors undergoing neurogenic divisions are characterized by a significantly longer cell cycle than progenitors undergoing proliferative divisions 67.
Asymmetric centrosome inheritance model
The asymmetric inheritance of the mother versus daughter centrosome has been suggested to play a critical role in RGC asymmetric division. Centrosome duplication during mitosis generates a pair of centrosomes, one retaining the original mature mother centriole (i.e. the mother centrosome) and the other possessing the original less mature daughter centriole (i.e. the daughter centrosome) 68. During asymmetric division of RGCs, the mother centrosome is preferentially inherited by the renewing progenitor cell, while the daughter centrosome is inherited by the differentiating daughter cell 68. Interestingly, the asymmetric inheritance of the mother versus daughter centrosome has also been described in asymmetrically dividing Drosophila male germline stem cells 69 and neuroblasts 70,71. In germline stem cells, the mother centrosome is preferentially inherited by renewing stem cells. On the other hand, in neuroblasts the mother centrosome is preferentially inherited by differentiating daughter cells; however, during the process the mother centrosome loses most of its pericentriolar material (PCM), while the daughter centrosome gains PCM. This intriguing dynamics of PCM points to the importance of the property, rather than the absolute birth date, of the centrosome in regulating asymmetric division of progenitor/stem cells.
III. Migration of excitatory neurons
After exiting the cell cycle, new-born excitatory neurons need to migrate out of the proliferative zone and into the CP, where they further differentiate and form distinct layers. This stereotypic laminar organization of the neocortex is crucial for its function. Birth-dating studies have shown that layers II–VI of the neocortex are generated in an ‘inside-out’ fashion, such that neurons generated early reside in the deep layers, whereas later-born neurons migrate past the existing neurons to occupy more superficial layers (Figure 4) 72,73.
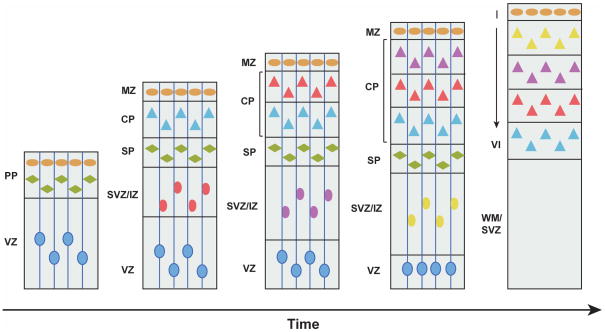
Figure 4. Inside-out layer formation of excitatory neurons in the neocortex.
The PP is formed by the production of the first wave of post-mitotic neurons that migrate from the VZ to the pial surface. Then, a second wave of new-born neurons (blue triangles) migrate through the SVZ/IZ and split the PP into the more superficial MZ (neurons in the MZ are represented by orange ovals) and the more deeply located SP (neurons in the SP are represented by green diamonds), creating the CP – the future neocortex. Neurons generated subsequently (represented by triangles with different colors, pink, purple and yellow by birth order) expand the CP in an inside-out fashion, as later-born neurons pass the existing neurons to occupy more superficial layers. The VZ progressively shrinks as the neural progenitor cells decrease during later embryonic development. During the postnatal development towards adulthood, the SP degenerates and leaves behind a six-layered neocortex (panel on the right). CP: cortical plate; IZ: intermediate zone; MZ: marginal zone; PP: preplate; SP: subplate; SVZ: subventricular zone; VZ: ventricular zone; WM: white matter.
New-born excitatory neurons that migrate radially into the CP are thought to do so through two distinct modes: somal translocation or locomotion. Early in neocortical development, the principal mode of neuronal migration appears to be somal translocation 64,74,75, in which neurons typically have a long radial process and maneuver their soma towards the leading edge of the radial process that is attached to the pial surface 74. How neurons acquire these long radial processes remains controversial. One possibility is that a newly generated neuron quickly elaborates a new radial ascending process before migration 74. Alternatively, the daughter neuron inherits the original radial process through asymmetric, neurogenic division 64. At later stages of neocortical development, new-born neurons predominantly adopt a second migratory mode where they make use of the radial processes of RGCs as a scaffold to reach their final positions, and this is known as radial glia-guided migration or locomotion 17,76,77. Time-lapse imaging studies in brain slice cultures have revealed many details of the locomotion of new-born neurons. These neurons do not migrate directly to the CP but instead exhibit four distinct phases of migration as characterized by Noctor, Kriegstein and colleagues 17. During phase one, new-born neurons assume a bipolar shape and ascend rapidly from the VZ to the SVZ. The second phase consists of migratory arrest for 24 hours or more with a multipolar morphology, followed by a third phase of retrograde migration toward the ventricle with a switch back to bipolar morphology. This retrograde migration is intriguing and raises the possibility that migrating neurons may receive certain signals from the ventricle, although future experiments are needed to confirm that it does occur in native conditions. After coming into contact with the ventricle, the daughter cells enter the fourth phase of migration, when they reverse polarity and migrate toward the CP 17. Notably, once their leading process reaches the marginal zone (MZ), locomoting cells can detach from the radial glial fibers and switch to “terminal translocation” mode 74. It has been further suggested that this change in the migratory mode occurs just beneath the primitive cortical zone (PCZ) and is critical for the disabled 1 (Dab1)-dependent inside-out lamination of the neocortex 78. Excitatory neuron migration in the neocortex may not be strictly radial. Lineage studies using retroviral labeling showed that clones of neurons derived from individual progenitors could disperse tangentially 79–81. However, the mechanisms responsible for this tangential dispersion remain unclear. Tangential dispersion may occur between symmetrically proliferating progenitor cells in the VZ/SVZ 17,81,82, and/or postmitotic neurons in the SVZ/IZ 83,84. Notably, multipolar cells in the SVZ/IZ, often observed at the second phase of radial migration, do not seem to closely associate with the radial fiber and have been shown to occasionally ‘jump’ tangentially from one radial glial fiber to another 17,85. Serum response factor (SRF), a transcription factor regulated by changes in the ratio of polymerized to unpolymerized actin, has been recently shown to direct excitatory neurons to choose a radial migration pathway along radial glia over a tangential migration mode 86. Despite the discovery of other migratory modes, radial glia-guided locomotion remains a central mechanism for establishing the architecture of the neocortex.
Many molecules have been implicated in the regulation of excitatory neuron radial migration in the developing neocortex. Of these, REELIN, an extracellular matrix protein secreted by Cajal-Retzius cells in the marginal zone, appears to be a key player. When mutated spontaneously in Reeler mice, the birth date-dependent inside-out fashion of the positioning of excitatory neurons is reversed, such that the layers are generated in an ‘outside-in’ manner 87–93. Subsequently, other mutants in REELIN signaling such as Scrambler which lacks a functional Dab1 gene have been shown to recapitulate the Reeler phenotype 94–96. Interestingly, in all mutants with abnormal neocortical lamination, the development of the preplate is normal. This observation is consistent with the idea that early-born neurons adopt a different mode of migration – soma translocation – which is unaffected by the cascade of signaling mechanisms that control the radial glia-guided locomotion of later-born neurons occupying the CP. Genetic analysis of mouse mutants and human neurologic disorders associated with disrupted cortical laminar organization has identified other molecules including cyclin-dependent kinase 5 (Cdk5) 97, Cdk5r1 98, transcription factor Pax6 99,100, neurotrophin 5 (Ntf5) 101, integrins 102–104, and doublecortin 105 as critical regulators of neuronal migration, and thereby layer formation in the developing neocortex.
IV. Interneuron neurogenesis and migration
In contrast to excitatory neurons generated directly in the proliferative zone of the neocortex, most, if not all, neocortical interneurons arise from the ventral telencephalon, predominantly the medial ganglionic eminence (MGE) 106,107, caudal ganglionic eminences (CGE) 108,109, and the preoptic area (POA) 110 (Figure 1). Even though the germinal zones of pyramidal neurons and interneurons are spatially segregated, they all harbor RGCs in their VZ 10,111,112. However, the RGCs responsible for producing excitatory neurons and inhibitory interneurons are distinct. RGCs in the VZ of the neocortex express Pax6 and Emx1, two transcription factor genes essential for specifying neocortex features, without which the neocortex is converted into structures normally formed by the ventral telencephalon 113. Conversely, RGCs in the ventral telencephalon express different sets of transcription factor genes such as Gsx1/Gsx2 and Olig2 4. Notably, expression analysis has shown that neocortical RGCs are highly heterogeneous and can be divided into multiple antigenically distinct subpopulations that evolve in a stereotypical manner as development proceeds 3. It is possible that FABP7-expressing RGCs in the ventral telencephalon are also heterogeneous, which might be related to the great diversity of neocortical interneurons.
A recent study using retroviral labeling and live imaging analysis clearly demonstrated that RGCs in the VZ of the MGE undergo asymmetric cell division to produce neocortical interneurons 114. In addition, there are abundant IPCs in the SVZ that divide symmetrically to produce neocortical interneurons (Figure 5). In fact, the SVZ in the ventral telencephalon is substantially larger than that in the neocortex, indicating that symmetrically dividing IPCs represent an important source of neocortical interneurons 4,115.
Figure 5. Clonal production and organization of inhibitory interneurons in the mouse neocortex.
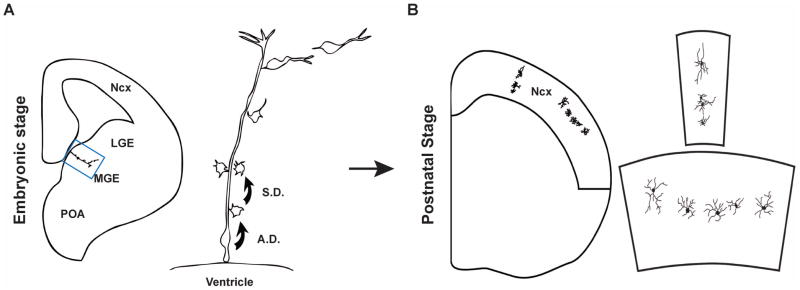
(A) At embryonic stage, RGCs in the VZ of the MGE divide asymmetrically to self-renew and to simultaneously produce differentiating interneurons or IPCs that divide symmetrically in the SVZ to produce differentiating interneurons. The progeny of the same RGC initially migrate along the mother RGC and form radially aligned clonal clusters (the clone in the MGE is shown at higher magnification in the right panel). The new-born cells progressively move away from the VZ, differentiate, and migrate tangentially towards the neocortex. (B) After arriving at their destination in the neocortex, inhibitory interneuron clones do not randomly disperse, but form spatially organized clusters which are either vertically or horizontally oriented (one example in each case is shown at higher magnification in the right panel). A.D., asymmetric division; S.D., symmetric division. Adapted from Fig. S14 114.
Neocortical interneurons are remarkably diverse and can be subdivided into distinct subgroups based on neurochemical marker expression, morphology, firing pattern and synaptic connectivity. An open question related to neocortical interneuron neurogenesis is how this bewilderingly diverse group of interneurons is generated. Genetic and transplantation studies have demonstrated that distinct regions of the GEs generate distinct interneuron subtypes 109,116–118. In addition, distinct temporal origins of physiologically defined interneuron subtypes have also been reported 109,116–118. However, the basis of the production of distinct groups of neocortical interneurons at individual progenitor cell level is largely unknown. It appears that individual RGCs in the VZ of the MGE are capable of producing different subtypes of neocortical interneurons 114. It will be interesting in the future to determine the lineage trees of different subtypes of neocortical interneurons.
In contrast to excitatory neurons, neocortical interneurons produced at the ventral telencephalon need to embark on a lengthy tangential migration to reach the dorsal neocortex, and then migrate radially to arrive at their final proper laminar positions 106,108,119–128. On the other hand, new-born interneurons in the MGE and POA, similar to excitatory neurons in the neocortex, initially migrate radially along the mother radial glial progenitor cell before starting their tangential migration 114. The physiological significance of this initial radial migration is unclear. It may allow proper neuronal differentiation of the new-born cells and subsequent proper tangential migration. Previous studies have identified a diverse set of temporally and spatially distinct tangential migratory routes associated with different progenitor zones within the ventral telencephalon 123,129. Early during development (E11.5), neocortical interneurons arise primarily from the MGE and the anterior entopeduncular area (AEP)/POA, and follow a superficial route outside the developing striatum 123. At mid-embryonic stages (E12.5-E14.5), the MGE appears to be the principal source of neocortical interneurons, which migrate either deeply or superficially, relative to the developing striatum. During this period, the LGE and CGE also start to produce interneurons that migrate to the neocortex 128. At later stages (E15.5), the CGE reaches its peak of neurogenesis and becomes the more prominent/substantive source of cortical interneurons, which prefer a deeper migratory route 128,129.
Once an interneuron fate has been specified and a tangential migratory pathway is undertaken, there are at least three different types of factors that can regulate this process: 1) motogenic factors that stimulate the movement of interneurons, such as hepatocyte growth factor (also known as scatter factor) 130, brain-derived neurotrophic factor (BDNF) and neurotrophin 5 (NTF5) 101,131; 2) extracellular substrates that serve as scaffolds for migration, e.g. migrating interneurons in the lower intermediate zone may use corticofugal axons as the substrate for their migration 83,132,133; 3) guidance factors that direct different migratory streams through appropriate routes towards their targets, such as chemorepulsive SLIT/ROBO 134–136 and semaphorin/neuropilin signaling 137, and chemoattractive CXCL12/CXCR4 signaling 138–141. Notably, this long-distance tangential migration of individual interneurons is believed to be mostly random 142, although it is unclear how their random walking behavior could lead to an organized distribution of interneurons in the neocortex for functional circuit assembly, such as birth date-dependent laminar distribution of MGE-derived interneurons. Interestingly, a recent study demonstrated that clonally related interneurons do not randomly disperse but form spatially organized clusters in the neocortex 114, indicating a lineage-dependent organization of interneurons in the neocortex (Figure 5).
After entering the neocortex, interneurons continue to disperse tangentially via highly stereotyped routes in the MZ, the subplate, and the lower intermediate zone/the SVZ 106. Eventually, interneurons switch from tangential to radial migration to adopt their final laminar position in the neocortex 124,142,143. Proper cell-autonomous development of neocortical interneurons has been indicated as a critical factor in their radial positioning, as defective laminar distribution is often observed in mutant mice with abnormal interneuronal differentiation 144–147. Moreover, neocortical interneurons are intrinsically programmed to modulate expression of the K+/Cl− co-transporter SLC12A5 (formerly known as KCC2) in order to sense the ambient extracellular levels of GABA and glutamate as a way to determine when to stop migration in the neocortex 148,149.
Furthermore, many interneurons and excitatory neurons that are born at a similar time end up occupying the same neocortical layer 108,150–152, indicating that the mechanisms controlling the layer acquisition of these two neuronal types are highly coordinated. However, little is known about how this coordination is achieved. Recently, it has been shown that RGCs may serve as a scaffold for both further tangential and radial migration of interneurons within the developing neocortex 124,153. When the radial glia scaffold is disrupted, neither excitatory neurons nor inhibitory interneurons migrate properly into the cortical plate 154,155. Also, gap junctions, which play an important role in the radial migration of excitatory neurons 156–158, have been recently suggested to regulate the switch from tangential to radial migration as interneurons enter the neocortex 159. In addition, interneurons invade the neocortex only after their excitatory neuron partners, possibly reflecting a need for signals from appropriately located excitatory neurons 140. Consistent with this idea, interneurons are found to distribute abnormally in the cortex of the Reeler mice, in which pyramidal neuron lamination is nearly inverted 160,161. Further evidence of a significant role of excitatory neurons in influencing interneuron migration and positioning came from a recent study using Fezf2−/− mice, which demonstrated that distinct subtypes of excitatory neurons could uniquely and differentially determine the laminar distribution of neocortical interneurons 162. Thus, it is possible that the pairing of synchronously-born excitatory neurons and interneurons relies on the expression of complementary molecules, which may have evolved to ensure the coordinated positioning of these two distinct neuronal populations.
Concluding remarks
During the past decade, there has been remarkable progress in our understanding of the production and migration of neocortical excitatory and inhibitory neurons at the mechanistic and cell biological level. However, many key questions remain inadequately addressed. For example, how is symmetric versus asymmetric division of progenitor cells controlled? How is the rich diversity of neuronal types generated? How is the migration of different types of neurons regulated to generate the stereotypic laminar architecture? The greatest challenge presumably lies in the feasibility of systematically addressing these questions, as the regulation of these cellular processes likely involves a comprehensive set of molecular pathways that function in a coherent manner. With the development of new experimental approaches, it is safe to predict that our understanding of neurogenesis and neuronal migration in the developing neocortex will be enriched in the near future.
Acknowledgments
We apologize to the authors whose work we could not cite owing to space limitations. We thank Stewart. A. Anderson, M. Elizabeth Ross, Kirsten Hively and Yvette Chin for critical reading of the manuscript. Our research is supported by grants from the National Institute of Health (R01DA024681, R21NS072483, R21MH083624 and P01NS048120), the McKnight Foundation and the March of Dimes Foundation.
References
- 1.Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 2.Williams BP, Price J. Evidence for multiple precursor cell types in the embryonic rat cerebral cortex. Neuron. 1995;14:1181–1188. doi: 10.1016/0896-6273(95)90265-1. [DOI] [PubMed] [Google Scholar]
- 3.Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- 4.Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 5.Aaku-Saraste E, Hellwig A, Huttner WB. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure--remodeling of the neuroepithelium prior to neurogenesis. Dev Biol. 1996;180:664–679. doi: 10.1006/dbio.1996.0336. [DOI] [PubMed] [Google Scholar]
- 6.Mollgoard K, Saunders NR. Complex tight junctions of epithelial and of endothelial cells in early foetal brain. J Neurocytol. 1975;4:453–468. doi: 10.1007/BF01261375. [DOI] [PubMed] [Google Scholar]
- 7.Levitt P, Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol. 1980;193:815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- 8.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 9.Hatten ME. New directions in neuronal migration. Science. 2002;297:1660–1663. doi: 10.1126/science.1074572. [DOI] [PubMed] [Google Scholar]
- 10.Campbell K, Gotz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–238. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- 11.Kriegstein AR, Gotz M. Radial glia diversity: a matter of cell fate. Glia. 2003;43:37–43. doi: 10.1002/glia.10250. [DOI] [PubMed] [Google Scholar]
- 12.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 13.Bentivoglio M, Mazzarello P. The history of radial glia. Brain Res Bull. 1999;49:305–315. doi: 10.1016/s0361-9230(99)00065-9. [DOI] [PubMed] [Google Scholar]
- 14.Cameron RS, Rakic P. Glial cell lineage in the cerebral cortex: a review and synthesis. Glia. 1991;4:124–137. doi: 10.1002/glia.440040204. [DOI] [PubMed] [Google Scholar]
- 15.Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol. 2005;17:648–657. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Haydar TF, Ang E, Jr, Rakic P. Mitotic spindle rotation and mode of cell division in the developing telencephalon. Proc Natl Acad Sci U S A. 2003;100:2890–2895. doi: 10.1073/pnas.0437969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 18.Shitamukai A, Konno D, Matsuzaki F. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J Neurosci. 2011;31:3683–3695. doi: 10.1523/JNEUROSCI.4773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- 21.Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Cerdeno V, Noctor SC, Kriegstein AR. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb Cortex. 2006;16 (Suppl 1):i152–161. doi: 10.1093/cercor/bhk017. [DOI] [PubMed] [Google Scholar]
- 23.Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, Cunningham J, Gotz M, Walsh CA. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II–IV of the cerebral cortex. J Comp Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- 25.Zimmer C, Tiveron MC, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex. 2004;14:1408–1420. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]
- 26.Britanova O, Akopov S, Lukyanov S, Gruss P, Tarabykin V. Novel transcription factor Satb2 interacts with matrix attachment region DNA elements in a tissue-specific manner and demonstrates cell-type-dependent expression in the developing mouse CNS. Eur J Neurosci. 2005;21:658–668. doi: 10.1111/j.1460-9568.2005.03897.x. [DOI] [PubMed] [Google Scholar]
- 27.Hartfuss E, Forster E, Bock HH, Hack MA, Leprince P, Luque JM, Herz J, Frotscher M, Gotz M. Reelin signaling directly affects radial glia morphology and biochemical maturation. Development. 2003;130:4597–4609. doi: 10.1242/dev.00654. [DOI] [PubMed] [Google Scholar]
- 28.Gal JS, Morozov YM, Ayoub AE, Chatterjee M, Rakic P, Haydar TF. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J Neurosci. 2006;26:1045–1056. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stancik EK, Navarro-Quiroga I, Sellke R, Haydar TF. Heterogeneity in ventricular zone neural precursors contributes to neuronal fate diversity in the postnatal neocortex. J Neurosci. 2010;30:7028–7036. doi: 10.1523/JNEUROSCI.6131-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, Huttner WB. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- 31.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 32.Kelava I, Reillo I, Murayama AY, Kalinka AT, Stenzel D, Tomancak P, Matsuzaki F, Lebrand C, Sasaki E, Schwamborn JC, Okano H, Huttner WB, Borrell V. Abundant Occurrence of Basal Radial Glia in the Subventricular Zone of Embryonic Neocortex of a Lissencephalic Primate, the Common Marmoset Callithrix jacchus. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Tsai JW, LaMonica B, Kriegstein AR. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci. 14:555–561. doi: 10.1038/nn.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anthony TE, Mason HA, Gridley T, Fishell G, Heintz N. Brain lipid-binding protein is a direct target of Notch signaling in radial glial cells. Genes Dev. 2005;19:1028–1033. doi: 10.1101/gad.1302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dang L, Yoon K, Wang M, Gaiano N. Notch3 signaling promotes radial glial/progenitor character in the mammalian telencephalon. Dev Neurosci. 2006;28:58–69. doi: 10.1159/000090753. [DOI] [PubMed] [Google Scholar]
- 37.Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- 38.Yoon K, Nery S, Rutlin ML, Radtke F, Fishell G, Gaiano N. Fibroblast growth factor receptor signaling promotes radial glial identity and interacts with Notch1 signaling in telencephalic progenitors. J Neurosci. 2004;24:9497–9506. doi: 10.1523/JNEUROSCI.0993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmid RS, McGrath B, Berechid BE, Boyles B, Marchionni M, Sestan N, Anton ES. Neuregulin 1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc Natl Acad Sci U S A. 2003;100:4251–4256. doi: 10.1073/pnas.0630496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patten BA, Peyrin JM, Weinmaster G, Corfas G. Sequential signaling through Notch1 and erbB receptors mediates radial glia differentiation. J Neurosci. 2003;23:6132–6140. doi: 10.1523/JNEUROSCI.23-14-06132.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patten BA, Sardi SP, Koirala S, Nakafuku M, Corfas G. Notch1 signaling regulates radial glia differentiation through multiple transcriptional mechanisms. J Neurosci. 2006;26:3102–3108. doi: 10.1523/JNEUROSCI.4829-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 43.Rasin MR, Gazula VR, Breunig JJ, Kwan KY, Johnson MB, Liu-Chen S, Li HS, Jan LY, Jan YN, Rakic P, Sestan N. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10:819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- 44.Li HS, Wang D, Shen Q, Schonemann MD, Gorski JA, Jones KR, Temple S, Jan LY, Jan YN. Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron. 2003;40:1105–1118. doi: 10.1016/s0896-6273(03)00755-4. [DOI] [PubMed] [Google Scholar]
- 45.Zhong W, Jiang MM, Schonemann MD, Meneses JJ, Pedersen RA, Jan LY, Jan YN. Mouse numb is an essential gene involved in cortical neurogenesis. Proc Natl Acad Sci U S A. 2000;97:6844–6849. doi: 10.1073/pnas.97.12.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Y, Atkins JB, Rompani SB, Bancescu DL, Petersen PH, Tang H, Zou K, Stewart SB, Zhong W. The mammalian Golgi regulates numb signaling in asymmetric cell division by releasing ACBD3 during mitosis. Cell. 2007;129:163–178. doi: 10.1016/j.cell.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 47.Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- 48.Shen Q, Zhong W, Jan YN, Temple S. Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development. 2002;129:4843–4853. doi: 10.1242/dev.129.20.4843. [DOI] [PubMed] [Google Scholar]
- 49.Spana EP, Kopczynski C, Goodman CS, Doe CQ. Asymmetric localization of numb autonomously determines sibling neuron identity in the Drosophila CNS. Development. 1995;121:3489–3494. doi: 10.1242/dev.121.11.3489. [DOI] [PubMed] [Google Scholar]
- 50.Choksi SP, Southall TD, Bossing T, Edoff K, de Wit E, Fischer BE, van Steensel B, Micklem G, Brand AH. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Developmental cell. 2006;11:775–789. doi: 10.1016/j.devcel.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 52.Zhong W, Feder JN, Jiang MM, Jan LY, Jan YN. Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron. 1996;17:43–53. doi: 10.1016/s0896-6273(00)80279-2. [DOI] [PubMed] [Google Scholar]
- 53.Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508:28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Postiglione MP, Juschke C, Xie Y, Haas GA, Charalambous C, Knoblich JA. Mouse inscuteable induces apical-basal spindle orientation to facilitate intermediate progenitor generation in the developing neocortex. Neuron. 2011;72:269–284. doi: 10.1016/j.neuron.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- 56.Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corbeil D, Roper K, Hannah MJ, Hellwig A, Huttner WB. Selective localization of the polytopic membrane protein prominin in microvilli of epithelial cells -a combination of apical sorting and retention in plasma membrane protrusions. J Cell Sci. 1999;112 ( Pt 7):1023–1033. doi: 10.1242/jcs.112.7.1023. [DOI] [PubMed] [Google Scholar]
- 58.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chenn A, Zhang YA, Chang BT, McConnell SK. Intrinsic polarity of mammalian neuroepithelial cells. Mol Cell Neurosci. 1998;11:183–193. doi: 10.1006/mcne.1998.0680. [DOI] [PubMed] [Google Scholar]
- 61.Alexandre P, Reugels AM, Barker D, Blanc E, Clarke JD. Neurons derive from the more apical daughter in asymmetric divisions in the zebrafish neural tube. Nat Neurosci. 13:673–679. doi: 10.1038/nn.2547. [DOI] [PubMed] [Google Scholar]
- 62.Kosodo Y, Huttner WB. Basal process and cell divisions of neural progenitors in the developing brain. Dev Growth Differ. 2009;51:251–261. doi: 10.1111/j.1440-169X.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- 63.Tamamaki N, Nakamura K, Okamoto K, Kaneko T. Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci Res. 2001;41:51–60. doi: 10.1016/s0168-0102(01)00259-0. [DOI] [PubMed] [Google Scholar]
- 64.Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi T, Nowakowski RS, Caviness VS., Jr The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calegari F, Huttner WB. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J Cell Sci. 2003;116:4947–4955. doi: 10.1242/jcs.00825. [DOI] [PubMed] [Google Scholar]
- 67.Calegari F, Haubensak W, Haffner C, Huttner WB. Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J Neurosci. 2005;25:6533–6538. doi: 10.1523/JNEUROSCI.0778-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461:947–955. doi: 10.1038/nature08435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–521. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Januschke J, Llamazares S, Reina J, Gonzalez C. Drosophila neuroblasts retain the daughter centrosome. Nat Commun. 2011;2:243. doi: 10.1038/ncomms1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conduit PT, Raff JW. Cnn dynamics drive centrosome size asymmetry to ensure daughter centriole retention in Drosophila neuroblasts. Curr Biol. 2010;20:2187–2192. doi: 10.1016/j.cub.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 72.Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 73.Berry M, Rogers AW. The migration of neuroblasts in the developing cerebral cortex. J Anat. 1965;99:691–709. [PMC free article] [PubMed] [Google Scholar]
- 74.Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- 75.Morest DK. A study of neurogenesis in the forebrain of opossum pouch young. Z Anat Entwicklungsgesch. 1970;130:265–305. doi: 10.1007/BF00520999. [DOI] [PubMed] [Google Scholar]
- 76.Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- 77.Nadarajah B, Parnavelas JG. Modes of neuronal migration in the developing cerebral cortex. Nat Rev Neurosci. 2002;3:423–432. doi: 10.1038/nrn845. [DOI] [PubMed] [Google Scholar]
- 78.Sekine K, Honda T, Kawauchi T, Kubo K, Nakajima K. The outermost region of the developing cortical plate is crucial for both the switch of the radial migration mode and the Dab1-dependent “inside-out” lamination in the neocortex. J Neurosci. 31:9426–9439. doi: 10.1523/JNEUROSCI.0650-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walsh C, Cepko CL. Clonal dispersion in proliferative layers of developing cerebral cortex. Nature. 1993;362:632–635. doi: 10.1038/362632a0. [DOI] [PubMed] [Google Scholar]
- 80.Reid CB, Liang I, Walsh C. Systematic widespread clonal organization in cerebral cortex. Neuron. 1995;15:299–310. doi: 10.1016/0896-6273(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 81.Walsh C, Cepko CL. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science. 1992;255:434–440. doi: 10.1126/science.1734520. [DOI] [PubMed] [Google Scholar]
- 82.Fishell G, Mason CA, Hatten ME. Dispersion of neural progenitors within the germinal zones of the forebrain. Nature. 1993;362:636–638. doi: 10.1038/362636a0. [DOI] [PubMed] [Google Scholar]
- 83.O’Rourke NA, Sullivan DP, Kaznowski CE, Jacobs AA, McConnell SK. Tangential migration of neurons in the developing cerebral cortex. Development. 1995;121:2165–2176. doi: 10.1242/dev.121.7.2165. [DOI] [PubMed] [Google Scholar]
- 84.Britanova O, Alifragis P, Junek S, Jones K, Gruss P, Tarabykin V. A novel mode of tangential migration of cortical projection neurons. Dev Biol. 2006;298:299–311. doi: 10.1016/j.ydbio.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 85.Tabata H, Nakajima K. Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci. 2003;23:9996–10001. doi: 10.1523/JNEUROSCI.23-31-09996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pinheiro EM, Xie Z, Norovich AL, Vidaki M, Tsai LH, Gertler FB. Lpd depletion reveals that SRF specifies radial versus tangential migration of pyramidal neurons. Nat Cell Biol. 2011;13:989–995. doi: 10.1038/ncb2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caviness VS, Jr, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- 88.D’Arcangelo G. The role of the Reelin pathway in cortical development. Symp Soc Exp Biol. 2001:59–73. [PubMed] [Google Scholar]
- 89.D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 90.Drakew A, Frotscher M, Deller T, Ogawa M, Heimrich B. Developmental distribution of a reeler gene-related antigen in the rat hippocampal formation visualized by CR-50 immunocytochemistry. Neuroscience. 1998;82:1079–1086. doi: 10.1016/s0306-4522(97)00326-6. [DOI] [PubMed] [Google Scholar]
- 91.Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 92.Forster E, Tielsch A, Saum B, Weiss KH, Johanssen C, Graus-Porta D, Muller U, Frotscher M. Reelin, Disabled 1, and beta 1 integrins are required for the formation of the radial glial scaffold in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:13178–13183. doi: 10.1073/pnas.202035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Magdaleno S, Keshvara L, Curran T. Rescue of ataxia and preplate splitting by ectopic expression of Reelin in reeler mice. Neuron. 2002;33:573–586. doi: 10.1016/s0896-6273(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 94.Sweet HO, Bronson RT, Johnson KR, Cook SA, Davisson MT. Scrambler, a new neurological mutation of the mouse with abnormalities of neuronal migration. Mamm Genome. 1996;7:798–802. doi: 10.1007/s003359900240. [DOI] [PubMed] [Google Scholar]
- 95.Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- 96.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 97.Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- 99.Schmahl W, Knoedlseder M, Favor J, Davidson D. Defects of neuronal migration and the pathogenesis of cortical malformations are associated with Small eye (Sey) in the mouse, a point mutation at the Pax-6-locus. Acta Neuropathol. 1993;86:126–135. doi: 10.1007/BF00334879. [DOI] [PubMed] [Google Scholar]
- 100.Caric D, Gooday D, Hill RE, McConnell SK, Price DJ. Determination of the migratory capacity of embryonic cortical cells lacking the transcription factor Pax-6. Development. 1997;124:5087–5096. doi: 10.1242/dev.124.24.5087. [DOI] [PubMed] [Google Scholar]
- 101.Brunstrom JE, Gray-Swain MR, Osborne PA, Pearlman AL. Neuronal heterotopias in the developing cerebral cortex produced by neurotrophin-4. Neuron. 1997;18:505–517. doi: 10.1016/s0896-6273(00)81250-7. [DOI] [PubMed] [Google Scholar]
- 102.Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Z, Galileo DS. Retroviral transfer of antisense integrin alpha6 or alpha8 sequences results in laminar redistribution or clonal cell death in developing brain. J Neurosci. 1998;18:6928–6938. doi: 10.1523/JNEUROSCI.18-17-06928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- 105.Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 106.Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- 108.Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- 109.Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gelman DM, Martini FJ, Nobrega-Pereira S, Pierani A, Kessaris N, Marin O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mori T, Buffo A, Gotz M. The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr Top Dev Biol. 2005;69:67–99. doi: 10.1016/S0070-2153(05)69004-7. [DOI] [PubMed] [Google Scholar]
- 112.Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- 113.Muzio L, DiBenedetto B, Stoykova A, Boncinelli E, Gruss P, Mallamaci A. Conversion of cerebral cortex into basal ganglia in Emx2(-/-) Pax6(Sey/Sey) double-mutant mice. Nat Neurosci. 2002;5:737–745. doi: 10.1038/nn892. [DOI] [PubMed] [Google Scholar]
- 114.Brown KN, Chen S, Han Z, Lu CH, Tan X, Zhang XJ, Ding L, Lopez-Cruz A, Saur D, Anderson SA, Huang K, Shi SH. Clonal production and organization of inhibitory interneurons in the neocortex. Science. 2011;334:480–486. doi: 10.1126/science.1208884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sheth AN, Bhide PG. Concurrent cellular output from two proliferative populations in the early embryonic mouse corpus striatum. J Comp Neurol. 1997;383:220–230. [PubMed] [Google Scholar]
- 116.Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 117.Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Carlos JA, Lopez-Mascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci. 1996;16:6146–6156. doi: 10.1523/JNEUROSCI.16-19-06146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tan SS, Kalloniatis M, Sturm K, Tam PP, Reese BE, Faulkner-Jones B. Separate progenitors for radial and tangential cell dispersion during development of the cerebral neocortex. Neuron. 1998;21:295–304. doi: 10.1016/s0896-6273(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 121.Ware ML, Tavazoie SF, Reid CB, Walsh CA. Coexistence of widespread clones and large radial clones in early embryonic ferret cortex. Cereb Cortex. 1999;9:636–645. doi: 10.1093/cercor/9.6.636. [DOI] [PubMed] [Google Scholar]
- 122.Anderson S, Mione M, Yun K, Rubenstein JL. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9:646–654. doi: 10.1093/cercor/9.6.646. [DOI] [PubMed] [Google Scholar]
- 123.Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- 124.Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- 125.Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 126.Jimenez D, Lopez-Mascaraque LM, Valverde F, De Carlos JA. Tangential migration in neocortical development. Dev Biol. 2002;244:155–169. doi: 10.1006/dbio.2002.0586. [DOI] [PubMed] [Google Scholar]
- 127.Yozu M, Tabata H, Nakajima K. The caudal migratory stream: a novel migratory stream of interneurons derived from the caudal ganglionic eminence in the developing mouse forebrain. J Neurosci. 2005;25:7268–7277. doi: 10.1523/JNEUROSCI.2072-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 130.Powell EM, Mars WM, Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30:79–89. doi: 10.1016/s0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 131.Behar TN, Dugich-Djordjevic MM, Li YX, Ma W, Somogyi R, Wen X, Brown E, Scott C, McKay RD, Barker JL. Neurotrophins stimulate chemotaxis of embryonic cortical neurons. Eur J Neurosci. 1997;9:2561–2570. doi: 10.1111/j.1460-9568.1997.tb01685.x. [DOI] [PubMed] [Google Scholar]
- 132.Denaxa M, Chan CH, Schachner M, Parnavelas JG, Karagogeos D. The adhesion molecule TAG-1 mediates the migration of cortical interneurons from the ganglionic eminence along the corticofugal fiber system. Development. 2001;128:4635–4644. doi: 10.1242/dev.128.22.4635. [DOI] [PubMed] [Google Scholar]
- 133.Metin C, Godement P. The ganglionic eminence may be an intermediate target for corticofugal and thalamocortical axons. J Neurosci. 1996;16:3219–3235. doi: 10.1523/JNEUROSCI.16-10-03219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu Y, Li H, Zhou L, Wu JY, Rao Y. Cellular and molecular guidance of GABAergic neuronal migration from an extracortical origin to the neocortex. Neuron. 1999;23:473–485. doi: 10.1016/s0896-6273(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 135.Chen JH, Wen L, Dupuis S, Wu JY, Rao Y. The N-terminal leucine-rich regions in Slit are sufficient to repel olfactory bulb axons and subventricular zone neurons. J Neurosci. 2001;21:1548–1556. doi: 10.1523/JNEUROSCI.21-05-01548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, Rao Y. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marin O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- 138.Li G, Adesnik H, Li J, Long J, Nicoll RA, Rubenstein JL, Pleasure SJ. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci. 2008;28:1085–1098. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tanaka DH, Mikami S, Nagasawa T, Miyazaki J, Nakajima K, Murakami F. CXCR4 is required for proper regional and laminar distribution of cortical somatostatin-, calretinin-, and neuropeptide Y-expressing GABAergic interneurons. Cereb Cortex. 2010;20:2810–2817. doi: 10.1093/cercor/bhq027. [DOI] [PubMed] [Google Scholar]
- 140.Lopez-Bendito G, Sanchez-Alcaniz JA, Pla R, Borrell V, Pico E, Valdeolmillos M, Marin O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ang ES, Jr, Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci. 2003;23:5805–5815. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tanaka D, Nakaya Y, Yanagawa Y, Obata K, Murakami F. Multimodal tangential migration of neocortical GABAergic neurons independent of GPI-anchored proteins. Development. 2003;130:5803–5813. doi: 10.1242/dev.00825. [DOI] [PubMed] [Google Scholar]
- 144.Azim E, Jabaudon D, Fame RM, Macklis JD. SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci. 2009;12:1238–1247. doi: 10.1038/nn.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Batista-Brito R, Rossignol E, Hjerling-Leffler J, Denaxa M, Wegner M, Lefebvre V, Pachnis V, Fishell G. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron. 2009;63:466–481. doi: 10.1016/j.neuron.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2–1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miyoshi G, Fishell G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex. 2011;21:845–852. doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miller MW. Cogeneration of retrogradely labeled corticocortical projection and GABA-immunoreactive local circuit neurons in cerebral cortex. Brain Res. 1985;355:187–192. doi: 10.1016/0165-3806(85)90040-9. [DOI] [PubMed] [Google Scholar]
- 151.Fairen A, Cobas A, Fonseca M. Times of generation of glutamic acid decarboxylase immunoreactive neurons in mouse somatosensory cortex. J Comp Neurol. 1986;251:67–83. doi: 10.1002/cne.902510105. [DOI] [PubMed] [Google Scholar]
- 152.Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. J Neurosci. 2003;23:5113–5122. doi: 10.1523/JNEUROSCI.23-12-05113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yokota Y, Gashghaei HT, Han C, Watson H, Campbell KJ, Anton ES. Radial glial dependent and independent dynamics of interneuronal migration in the developing cerebral cortex. PLoS One. 2007;2:e794. doi: 10.1371/journal.pone.0000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Poluch S, Juliano SL. A normal radial glial scaffold is necessary for migration of interneurons during neocortical development. Glia. 2007;55:822–830. doi: 10.1002/glia.20488. [DOI] [PubMed] [Google Scholar]
- 155.Haubst N, Georges-Labouesse E, De Arcangelis A, Mayer U, Gotz M. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development. 2006;133:3245–3254. doi: 10.1242/dev.02486. [DOI] [PubMed] [Google Scholar]
- 156.Elias LA, Kriegstein AR. Gap junctions: multifaceted regulators of embryonic cortical development. Trends Neurosci. 2008;31:243–250. doi: 10.1016/j.tins.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 158.Fushiki S, Perez Velazquez JL, Zhang L, Bechberger JF, Carlen PL, Naus CC. Changes in neuronal migration in neocortex of connexin43 null mutant mice. J Neuropathol Exp Neurol. 2003;62:304–314. doi: 10.1093/jnen/62.3.304. [DOI] [PubMed] [Google Scholar]
- 159.Elias LA, Turmaine M, Parnavelas JG, Kriegstein AR. Connexin 43 mediates the tangential to radial migratory switch in ventrally derived cortical interneurons. J Neurosci. 2010;30:7072–7077. doi: 10.1523/JNEUROSCI.5728-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hevner RF, Daza RA, Englund C, Kohtz J, Fink A. Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration. Neuroscience. 2004;124:605–618. doi: 10.1016/j.neuroscience.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 161.Pla R, Borrell V, Flames N, Marin O. Layer acquisition by cortical GABAergic interneurons is independent of Reelin signaling. J Neurosci. 2006;26:6924–6934. doi: 10.1523/JNEUROSCI.0245-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lodato S, Rouaux C, Quast KB, Jantrachotechatchawan C, Studer M, Hensch TK, Arlotta P. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron. 2011;69:763–779. doi: 10.1016/j.neuron.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]