Abstract
The study of human developmental microcephaly is providing important insights into brain development. It has become clear that developmental microcephalies are associated with abnormalities in cellular production, and that the pathophysiology of microcephaly provides remarkable insights into how the brain generates the proper number of neurons that determine brain size. Most of the genetic causes of ‘primary’ developmental microcephaly (i.e., not associated with other syndromic features) are associated with centrosomal abnormalities. In addition to other functions, centrosomal proteins control the mitotic spindle, which is essential for normal cell proliferation during mitosis. However, the brain is often uniquely affected when microcephaly genes are mutated implying special centrosomal related functions in neuronal production. Although models explaining how this could occur have some compelling data, they are not without controversy. Interestingly, some of the microcephaly genes show evidence that they were targets of evolutionary selection in primates and human ancestors, suggesting potential evolutionary roles in controlling neuronal number and brain volume across species. Mutations in DNA repair pathway genes also lead to microcephaly. Double stranded DNA breaks appear to be a prominent type of damage that needs to be repaired during brain development, yet why defects in DNA repair affect the brain preferentially and if DNA repair relates to centrosome function, are not clearly understood.
Abnormal brain development resulting in intellectual disability is frequently associated with microcephaly (small head). In most cases, microcephaly is equivalent to microencephaly (small brain) and we will be using the terms interchangeably. In a study of children at 7 years old, of those with a head circumference 2 to 3 standard deviations below the mean, 10% had an intelligence quotient (I.Q.) <70 (two standard deviations below the mean) while only 14% had an I.Q. >100, (where I.Q. of 100 is mean). With head circumferences <3 standard deviations below the mean, 51% had an I.Q. <70 while none were above average 1.
Microcephaly can be developmental resulting from abnormalities of proper development or degenerative with normal development and subsequent loss of cells. Microcephaly vera (true microcephaly), sometimes called primary microcephaly, is a group of autosomal recessive diseases of brain development that results in intellectual disability but not other neurological abnormalities. These patients were thought to have no significant brain malformations other than a small brain (Figure 1), but now it is clear that the phenotypes are not completely uniform, and that there is a continuum between patients that have microcephaly with normal gyral pattern, and microcephaly associated with other malformation 2–4. Other forms of microcephalies that are very consistently associated with abnormal brain structure, for instance microlissencephaly (small, smooth brain), are interpreted as reflecting a gene’s requirement for both producing the proper number of neurons and subsequent stages of neuronal development. To understand the causes of microcephaly, one must understand the basic processes of brain growth and neuronal proliferation, where many advances have been made in the field over the past two decades.
Figure 1.
MRI images from two 12 month old children, the top with developmental microcephaly and bottom with a head circumference in normal range. The images are T1 weighted sequence in mid-sagittal and axial planes. Note the dramatic reduction in brain volume with the relative preservation in structure and size of facial features. The images are scaled to the same size (bar is 5 cm).
Nearly all neurons in the cerebral cortex have completed proliferation by mid-gestation and almost none are generated after birth 5, although glial genesis and brain volume continues to grow until adulthood (Figure 2A). This is because the vast majority of cerebral volume is made up of neuropil (glial processes, axons, dendrites, etc.). Therefore, brain volume can increase without changing the number of neurons as the child develops, during which the brain acquires and prunes connections. The dramatic increase in brain size in a child during their first year of life is thought therefore to reflect predominantly an increase in neuronal processes, and an increase in the number and size of glial cells that invest them (Figure 2B). Head circumference, a reasonable relative proxy for brain size, continues to increase with age, though the head grows as much in the first year of life as it does over the next 17 years. This ongoing head growth, due presumably to increases in cellular processes and glial cells, relates to why children with developmental microcephaly appear to worsen as they age. The microcephalic head grows more slowly than normal and does not reflect loss of brain volume (Figure 2A). This is because the volume of neuropil is dependent upon the number of neurons present 6. Therefore, severe developmental microcephaly is nearly always caused by deficiencies in the number of neurons.
Figure 2.
(A) Examples of head circumference growth curves for Boys aged 0 (Birth) to 18 years (in months). The mean with 2 standard deviations above (+2 SD) and below (−2 SD) the mean to illustrate normal growth patterns. Note the very rapid expansion in head circumference during the first year of life. The purple line with Xs shows, a child with a developmental microcephaly that starts below the normal growth curves. The head growth remain below the normal curves and follow it’s own trajectory with a shallower slope due to diminished brain growth potential. Clinically, these children often gain milestones more slowly compared to other children and the developmental potential eventually plateaus. However, there is no developmental milestone loss unless there are additional complications, neurological or otherwise. An example of a child with a degenerative condition leading to microcephaly is shown in light blue with Xs. Note that the child starts within the normal range then starts to cross percentile curves during the mid to late first year of life. Clinically, the child may gain early developmental milestones such as smiling, rolling over and sitting without support but then loses them as the neurodegenerative process proceeded. (B) Pre and post-natal brain growth from ~13 weeks post-fertilization to 36 months after being born at full term (prenatal ages in green and post-natal ages in black). Also shown are qualitative illustrations of the approximate timing of cerebral cortical development of neurons 171, 172 (yellow), astrocytes (purple) 173, myelination 174 (blue) and synapses175 (green). Developmental processes are demonstrating the relative temporal peak of each process with arbitrary units for the Y-axis while no attempt is made to show the relative contribution to brain volume. The exact extent of each process is not represented as each phenomenon (except for neurogenesis) continues at some level into adolescence. Comparisons between processes is very difficult because were different methods used for each type of study. In addition, some studies measure the density of phenomena in a growing volume further complicating extrapolation. The growth curves are a mathematic composite of fetal 176 and childhood177 data and are intended for illustrative purposes not clinical use.
We will be discussing the development of glutamatergic (excitatory) neurons of the cerebral cortex because they make up the majority of neurons in the cerebral cortex. For information regarding the development of the inhibitory GABAergic neuron and the first-born neurons of the cerebral cortex the reader is referred to other excellent recent reviews 7–11. To understand the pathogenesis of the human diseases, we will discuss studies from various animal models, particularly mice due to the ability to manipulate their genetics, with the assumption that mammals with larger brains show many of the same processes. However, we will note when there are limitations in these models to determine the pathogenesis in humans due to differences in development.
Like the rest of the central nervous system, the cerebral cortex starts as pseudostratified epithelium, with cells initially proliferating symmetrically with every cell division, producing two ‘daughter’ progenitors, and hence increasing the number of cells exponentially 12. The nuclei move up and down within the pseudostratified epithelium replicating their DNA at the pial surface and mitosis occurring at the ventricular surface 13 (Figure 3A). In mice, the founding pool of precursor cells in the ventricular zone at E11 (embryonic day 11, i.e., 11 days after conception) is an important determinant of eventual brain size. Targeted deletion of genes required for apoptosis greatly enlarges the cerebral cortex due to excessive precursor cells at the start of neurogenesis (the time in which neurons are generated), not from lack of apoptosis later in development 14–17. Therefore, there is control in the eventual number of mature neurons even prior to neurogenesis. However, little else is understood concerning the regulation the precursor pool at this stage of development.
Figure 3.
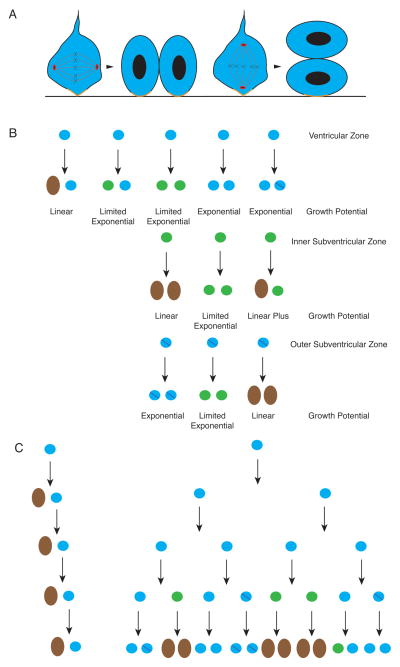
(A) Illustration of pseudostratified epithelium of the developing cerebral cortex prior to neurogenesis. It has the superficial appearance of layers due to nuclei being scattered from the ventricular to pial surface. However, cells are apparently uniform in character and all retain contacts with both the ventricular and pial surfaces. Within each cell, nuclei move upward toward the pial surface where they synthesize DNA and downward to the pial surface where they divide in mitosis. This pattern of nuclei movement is retained within the ventricular zone later in development described in B. Prior to neurogenesis, nearly all cells are uncommitted progenitors and the proliferative pool exponentially. (B) A diagram of the layering found during neurogenesis to emphasize the proliferative pools. Within the ventricular zone (represented by plain blue circles), uncommitted progenitors divide at the apical surface (dividing cells marked with red dot (indicative of the mitotic marker phospho-histone H3). Some of the progeny will become committed but retain limited proliferative capacity, start to express the marker TBR2, and move the inner subventricular zone, marked in green and also referred to intermediate precursors. They also divide (red dots, phospho-histone H3) but remain in the subventricular zone and do not have a connection with the apical surface. Cells within the outer subventricular zone (blue circles with purple slash) have many of the same markers of the ventricular zone, are uncommitted, have extensive proliferative capacity, but do not have connection with the apical surface, unlike the ventricular zone cells. The intermediate zone is cell sparse and the cortical plate consists of differentiated neurons that will become the cerebral cortex.
In mice, cells dividing within the ventricular zone during neurogenesis are dynamic in their proliferative characteristics. These factors include cell cycle length, which along with the period of neurogenesis determine the number of cell divisions that occur. Once neurogenesis begins, the control of the fate of dividing neuronal precursors appears to be critical in defining the ultimate size of the cortex. In the simplest models, during neurogenesis as precursors divide, there are 3 possibilities for the fate of progeny: two additional precursors, one precursor and one differentiated neuron or two differentiated neurons. If a cell proliferates symmetrically, producing two precursors every cell division, after 5 cell divisions one cell has produced 32 precursor cells. If a cell proliferates asymmetrically, producing one precursor and one differentiated neuron every cell division, after 5 cell divisions one cell has produced 6 cells (5 neurons and 1 precursor). There are actually additional possible fates, such as neuronal committed with limited proliferation potential 18, but the concept is similar. Therefore, control over proliferative versus differentiative fate is critical. In mice, there are progressively longer cell cycle times and the ratio of cells committed to proliferation versus differentiation appears to be precisely controlled (reviewed in 19, 20). These authors found they could account for the number of neurons produced in a mouse by accounting for progenitor pool size, the number of cell cycles and the ratio of cells proliferating to differentiating neurons (quiescent/proliferative or q/p). Critically, they found that the q/p ratio changed radically over the period of mouse development 20. According to their modeling data, the q/p ratios are important because if too many neurons differentiate too early, there will be fewer neurons at the end of neurogenesis with microcephaly as a result.
It is now clear that there are several types of progenitor cells in the cerebral cortex with different proliferative capacities and complicated lineage relationships that have not been completely determined. For example, a significant fraction of cells committed to a neuronal lineage migrate away from the ventricular surface to the subventricular layer and begin to change their expression profile, becoming TBR2+ 21–25 (Figure 3B). These cells go through limited, probably symmetrical, proliferation to produce differentiated neurons. Moreover, recent evidence suggests that mammals with gyri (folds) in the cerebral cortex and larger brains have an additional source of dividing. Progenitors that are presumably derived from the ventricular zone form a layer above the committed TBR2+ cells in the subventricular zone forming an outer subventricular proliferative zone 18, 26, 27 (Figure 3B). These cells appear to have extensive proliferative capacity and may be an important source of neurons in larger brained mammals. They appear to be able to undergo multiple cell symmetric divisions producing more precursors prior to differentiation. These outer subventricular cells are very much reduced in number in mouse brain compared to brains of gyrencephalic mammals 28, and do not form a distinct outer subventricular layer as they do in larger brains. Initial evidence hints that these outer subventricular zone cells may ultimately be seen to play a critical role in cerebral cortical proliferative capacity in mammals with gyri, but the extent of their role remains to be definitively determined.
Microcephaly genes
There were originally seven genetic loci for microcephaly vera, all of which have now been connected to single genes: MCPH1 29, ASPM 30, CDK5RAP2 31, CENPJ 31, STIL 32, WDR62 2–4, and CEP152 33. A few additional loci recently have been identified including CEP63 34 and a new locus potentially identified but no gene mutation found 35. Remarkably, all of these microcephaly genes encode proteins associated with the centrosome or centrosomal-related activities. Centrosomes play multiple critical roles in cellular function including during mitosis where they are associated with microtubules and involved in formation of the mitotic spindle. However, they also are involved in coordinating microtubules in other cellular processes such as migration (for review of neuronal aspects 36, 37) and primary cilia formation.
Centrosomes have a complicated structure and life cycle in dividing cells 38 (Figure 4). Interestingly, microcephaly proteins appear to play various roles in the centrosome and centriole life cycle 39. After mitosis, the core centrioles remain loosely tethered together and CDK5RAP2 appears to be important in this activity 40. STIL appears to interact machinery to form the core centriole 41, 42. In addition, STIL may play a role in the control of entry into mitosis as well 43 CEP152 is needed for centrosome duplication and appears to interact with CEP63 34, 44–46. After the centrioles are duplicated, they are elongated and CENPJ is involved in this activity 47–49. MCPH1 has been implicated in both centrosomal activity and DNA repair. However, as discussed below both sets of literature can partially reconciled if MCPH1 helps link DNA repair signaling with centrosomal activity. ASPM appears to play a role in mitotic spindle orientation during mitosis 50, 51. So while there is a unifying theme that microcephaly vera genes play some role in centrosomal activity, they do not appear to play an identical role in centrosomal function. In the future, studies could show a unified function in these genes, however. Therefore, the nature of the deficit for each mutation and how it results in microcephaly must be determined.
Figure 4.
A cartoon schematic of centriole biogenesis in dividing cells (see the excellent review 38 for further details). The potential roles of microcephaly related genes are noted. After M phase, in G1 two centrioles (large green boxes) are attached via a linker that contains CDK5RAP2. At the G1-S transition and only once during a cell cycle, a new centriole (small green box) is formed adjacent to parental centriole (larger green box). CEP152 is required for this process and it interacts with CEP63. Centriole duplication potentially involves STIL via its interaction with SAS6. The new centriole remains attached to the older centriole (small orange line). The new centriole elongates during S-phase and CENPJ is potentially involved this activity. MCPH1 may play a role in cell-cycle checkpoint control at the G2/M transition. ASPM may play an important role in mitotic spindle orientation during M phase. The alterations of spindle orientation play an important role in cell fate decisions invertebrates and may play a role in mammalian cortical development. Each cell inherits one centrosome, but the centrioles have different levels of maturity as it takes 1 ½ cell cycles for a centriole to fully mature as it accumulates distal appendages (black lines) and other maturation markers (more mature has asterisks). The differences in maturity between centrioles have potentially important biological consequences.
There is evidence of general centrosomal dysfunction associated with Cdk5rap2 with some increase in apoptosis in the developing cerebral cortex 52. In addition, it was also found that there was evidence of alteration of the q/p fraction that is consistent with fewer neurons produced 52. Similar defects have also been found in Aspm 50 and Nde1 53. Finally, a microcephaly locus whose gene mutation remains unidentified appears to have chromosomal segregation abnormalities implying a potential centrosomal function as well 35. Interestingly, chromosome copy number abnormalities have been reported in the normal developing rodent brain 54–56. This could indicate that chromosome segregation dysfunction is common even in the absence of mutations in centrosomal genes, but the role and extent of this phenomenon remains to be determined. Interestingly, many genes that are associated with lissencephaly (smooth brain) result from neuronal migration abnormalities and with centrosomes or microtubules as well including PAFAH1B1 (AKA LIS1) 57, DCX 58–60, RELN 61, TUBA1A 62, NDE1 63, 64. This is not surprising since centrosomal function is not limited to cell division, but also plays a critical role in neuronal migration as well 36, 37. Critically, there is experimental evidence that some of the genes associated with lissencephaly can also alter neurogenesis, supporting a similar mechanism as the microcephaly genes 53, 65.
Mutations in some genes cause a combination of both microcephaly and somatic growth deficiencies, exemplified by Seckel syndrome. Interestingly, a centrosomal gene has been associated with Seckel, PCNT 66 that when mutated causes abnormalities in the activation of the DNA repair gene, ATR, also mutated in Seckel syndrome 67. In addition, CEP152 has been associated with both microcephaly and Seckel syndrome as well 33, 68. However, microcephaly is often found without significant somatic growth defects.
Potential mechanisms of microcephaly genes
Why microcephaly is usually associated with mutations in centrosomal proteins is not certain, but there are several intriguing possibilities. It could be simply a result of mitotic dysfunction leading to deficiencies in cellular proliferation. However, as stated above, developmental microcephaly generally does not have profound somatic growth abnormalities. Alternatively, neurons could be more sensitive to cell cycle dysfunction resulting in apoptosis. Excessive cell death was seen in mouse models for CDK5RAP2 and MCPH1 but it was not obvious that the levels of cell death could account for the microcephaly alone 43, 52. Therefore, microcephaly associated centrosomal dysfunction appears to have a brain specific pathophysiological mechanisms.
One potential mechanism to regulate cell proliferation and differentiation is the differential inheritance of signaling molecules that alter progenitor fate. Differential segregation of the evolutionarily conserved Par3 (Bazooka), Par6 and aPKC complex plays a critical in Drosophila cell fate determination 69–71. The ventricular zone of the developing mammalian cerebral cortex appears to be an excellent candidate for sharing such a mechanism as well. There is a dramatic asymmetric localization of factors at the apical surface, the apical membrane complex, including PARD3 (homologue of Par3), PARD6A (homologue of Par6) and PRKCA (homologue of aPKC) while CTNNB1 (beta-catenin) is adjacent but slightly more basilar 69. There is significant evidence that this complex plays an important role in cortical development as well. Alterations in the pattern of expression of Pard3 72, 73, Pard6a 72, Ctnnb1 74 and Mpp5 (AKA Pals1) 75 (another component of the apical membrane complex), all alter proliferative patterns of cortical progenitors. However, how the apical membrane complex controls the fate decisions in not certain in mammals.
Whereas in flies and worms, asymmetrical cell fate is regulated by the asymmetrical inheritance of apical proteins, this is less clear in the cerebral cortex. It was first noted in ferrets that dividing precursors within the ventricular zone usually divide within a plane that bisects the cell, leaving two cells with apparently equal distribution of the apical membrane complex and a smaller percentage dividing in a skewed plane leaving unequal distribution 12. In this context, symmetric cell divisions has the additional connotation of precursors dividing in a plane that is perpendicular to the ventricular surface so the apical membrane complex is inherited equally (symmetrically) (Figure 5). This occurs at the same time most dividing cells produced two progenitors and the minority had asymmetric cell division resulting in one precursor and one neuron. This observation was confirmed in mouse 76–78. However, others have found that the differences in cleavage plane are not as great as other have reported 79, 80. Interestingly, some studies have suggested that altering cleavage plane orientation alters where neurons proliferate but not their proliferative fate 81. Other studies have suggested that that alteration of cleavage plane generates more neurons by shunting the neuronally committed progeny into the subventricular zone for additional rounds of proliferation prior to differentiation 82.
Figure 5.
(A) Illustration of different planes of cell division in the ventricular zone with an emphasis on the retention of the apical membrane complex (orange). When a dividing cell has centrosomes aligned so that the cleavage plane is perpendicular to the ventricular surface, the apical membrane complex is shared equally between progeny. When the centrosomes are aligned so that the cleavage plane is parellel to the ventricular surface, one cell inherits the apical membrane complex while the other does not. Since the apical membrane complex takes up a very small area of the ventricular surface, it has been hypothesized that slight deviations from a perpendicular cleavage plane may be enough to cause unequal distribution in progeny. (B) Illustration of potential progeny derived from different proliferative zones along with the growth potential. For instance, only linear growth occurs when a ventricular zone cell (blue circle) divides to produce one neuron (brown oval) and one uncommitted precursor. However, when two uncommitted precursors (in either ventricular zone (blue circle) or outer subventricular zone (blue circle with purple slash)) cell growth can be exponential. When the progeny include inner subventricular zone cells (green circle), there can be extensive expansion compared to producing neurons directly. However, true exponential growth is not possible because these neuronally committed cells divide a limited number of times before differentiating into neurons. The outer subventricular zone cells appear to be comparable to the proliferative capacity of the ventricular zone, however, it is not fully clear what cells it can produce or how these decisions are controlled. Presumably, fate decisions in this group can significantly alter the final number of neurons in the brain. (C) An example of the number of cells produced in 4 divisions from one ventricular zone cell with linear growth versus combined exponential, limited exponential and linear growth demonstrating how neuronal number could be controlled though control of cell fate. Note, neurons (brown) no longer divide but move out of the ventricular zone and migrate to the cortical plate.
Although the apical complex may regulate some aspects of cell fate for apical progenitors, the apical membrane complex is not even present within the subventricular zone, as these cells are not epithelial and do not have a connection with the ventricular surface. Although intermediate progenitors within the inner subventricular zone --such as TBR2 positive cells—appear to show limited cell production capacity 22–24, the outer subventricular zone cells appear to have extensive proliferative capacity and also do not have an apical membrane complex, since they do not have an apical process in connection with the ventricular surface 18, 27, 79. Because most studies of microcephaly genes have been carried out in mice, which have a minute outer subventricular zone, these studies have provided limited understanding of the role that the outer subventricular zone could be playing in microcephaly in humans. Animal models with gyri, such as ferret, may be required to determine the role of microcephaly genes in this cell type 28. Every proliferative neuronal precursor has various potential fates (Figure 5B) and the choices made will greatly influence the final number of neurons produced (Figure 5C). Centrioles duplicated in dividing cells are born at different times and have different levels of maturity 38, 83, 84 (Figure 4). One daughter cell will inherit the older, ‘maternal’ centriole while the other the less mature, ‘daughter’ centriole. Interestingly, a recent study has implicated a newly discovered microcephaly protein, CEP63, as localizing preferentially to the mother centriole but the implications of this finding remain to be determined34. One group has been found that cells in the developing cerebral cortex that inherit the mother centriole tend to remain proliferating, whereas those with the daughter centriole more frequently differentiate into neurons 85. However, it is unknown what accounts for these phenomena mechanistically. Intriguingly, cells that inherit the mother centriole develops a primary cilia faster, than cells that inherit the daughter centriole 86. While the mechanism for these phenomena is also unclear, it could be related to addition of CEP164 to the mother centriole at the G2/M transition that plays a role in primary cilia formation 87.
An intriguing hypothesis for ASPM suggests that it might influence WNT signaling 88, 89. Buchman et al. found that they could rescue the loss of ASPM with over expression of CTNNB1 (beta-catenin) that is downstream of WNT 90, 91. However, it was previously found that over expression of CTNNB1 resulted in excessive proliferation 74. Therefore, it is difficult to distinguish between ASPM interacting with WNT resulting in decrease in CTNNB1 or rescue by of decreased proliferation by CTNNB1 by a different mechanism. Interestingly, cilia function has been tied to both neurological disease and WNT signaling 92, 93. If the microcephaly related abnormalities in centrosomal activity could be tied to abnormal primary cilia formation or function, then it is possible that abnormal signaling through the primary cilia accounts for some microcephaly phenotype. However, it should be noted that there are many clinical differences between the microcephaly centrosomal diseases and the human diseases associated with ciliopathies94.
Microcephaly genes and brain evolution
The potential mechanisms of the evolutionary expansion in brain size in humans compared to other primates have been of interest for many decades. Recent work has indicated that the human is similar to other primates in terms of the number of neurons to volume ratio, with the implication that differences in brain volumes result from differences in neuronal number 6. As discussed above, there are several potential strategies that could allow humans to have more neurons than other primates: such as starting with more precursors at the start of neurogenesis via more proliferation or reduced apoptosis of the precursor pool, additional rounds of proliferation during neurogenesis, or alterations in the proliferative/differentiative (q/p) ratio during neurogenesis. Since mutations in microcephaly genes alter this process in pathological states, they were looked to as candidates to play a role in controlling cell number in evolution as well. One method to identify genes that play a role in evolution is to compare the relative number of nucleotide changes in protein coding regions that change amino acids (non-synonymous) to those that do not (synonymous) across species 95. The synonymous changes control for the frequency of nucleotide changes in a particular gene and the genetic distance between species while the non-synonymous changes potentially reflect adaptation that may have been selected for in evolution. Interestingly, numerous genes associated with microcephaly may have had positive selection in primates and/or humans including MCPH1, ASPM, CDK5RAP2, CENPJ and CEP63 34, 96–102 (reviewed in 103, 104). The changes in microcephaly genes that appear to be selected for in evolution could produce larger brains by producing more neurons secondary to altering q/p fraction or by a different mechanism such as shunting cells into other proliferative pools such as the inner or outer subventricular zones (Figure 5 B and C). Microcephaly genes are not unique in the potential primate evolutionary selection, as genes with many different biochemical functions also may have shown increased potential positive selection in primates and/or humans 105–110. However, this selection in recent human evolutionary lineages does not seem to be present in all genes expressed in the human brain 111, 112. In addition, microcephaly genes could even play a role in normal variation of head size and neurological diseases not associated with microcephaly 113, 114.
DNA damage repair and microcephaly
DNA damage responses and repair pathways along with the maintenance of genomic integrity are related to microcephaly genes in multiple aspects. Defects in the maintenance of genomic integrity can range from the alteration of a single nucleotide to errors in chromosomes segregation during mitosis. It is useful conceptually to separate different aspects of these cellular functions although it is sometimes difficult to do experimentally. DNA, while a superficially simple structure of 4 bases pairing appropriately, connected by deoxyribose sugars and phosphodiester bonds in very long strands forming anti-parallel helices, can be damaged in many ways and the requirement for DNA’s integrity is clear. Not surprisingly, different repair mechanisms exist for different types of damage. First, DNA damage such as double strand DNA breaks must be recognized followed by DNA damage response activation (Figure 6). This often includes arrest of the cell cycle presumably to allow completion of DNA repair so that damage is not propagated either through DNA replication or mitosis 115. In addition, if the amount of DNA damage is severe enough, programmed cell death pathways are activated. Finally, the appropriate enzymatic proteins for a particular repair pathway are recruited and coordinated to repair the damage. DNA damage response pathways are frequently disrupted in cancer probably because it encourages accumulation of mutations and prevents cell cycle arrest or programmed cell death when DNA damage does occur. In addition, disruptions in cell cycle control encourage both uncontrolled proliferation as well as reducing DNA repair by not allowing time for repair processes. On the other hand, aberrant sorting of chromosomes due to defects in the mitotic machinery has different mechanisms even though also common in cancer 116. However, recent work may indicate that DNA repair and aberrant chromosome segregation maybe more closely linked than previously thought 117.
Figure 6.
Schematic of DNA damage response to double strand breaks. After a double strand break occurs ATM is activated via interaction with the MRN (MRE11A-RAD50-NBN (NBS1) complex. After ATM is activated it establishes a very broad cascade of second messengers (including the MRN complex) to perform a varieties of cellular functions. Progression of the cell cycle is arrested. This is thought to help prevent the propagation of the DNA damage and allow time for repair. Arrest of the cell cycle involves interaction with the cyclin dependent kinase machinery and potentially the centrosome as well. In addition, apoptotic response pathways related to TP53 (p53) are activated permitting the cell to initiate programmed cell death if the level of DNA damage is severe, but much remains to be determined as to how these decisions are made 178. Finally, the proteins that will perform the DNA repair are attracted to the site of damage break. Abnormalities in genes involved in the non-homologous end-joining pathway leads to microcephaly in humans including LIG4, NHEJ and PNKP and an analogous phenotype in mice Lig4 160, 161, Xrcc4 159, Xrcc6 (Ku70)/Xrcc5 (Ku80)163, or Prkdc (DNA-PKcs) 164.
MCPH1 has been implicated in both centrosomal and DNA repair activity and provides an excellent illustration of the difficulties of distinguishing between different aspects of genomic integrity maintenance. Microcephaly from mutations in MCPH1 is associated with premature condensation of chromosomes in G2 118, 119. MCPH1 defective cells have impaired G2 arrest after DNA damage from ionizing radiation, MCPH1 localizes to sites of double strand breaks, and the gene is mutated in certain types of cancer potentially implicating DNA damage repair as a central function120–124. The first published mouse models of Mcph1 mutations did not have defects in neuronal development 125, 126. However, a more recent mouse model has microcephaly due to premature differentiation of neurons 127. This was thought to be secondary to premature entry into mitosis and chromosomal alignment difficulties via disrupted cell cycle control of the CHEK1-CDC25C (AKA CHK1–CDC25) pathway. Therefore, MCPH1 neurological pathophysiology is likely from abnormal cell cycle control associated with centrosome function and not due to inability to DNA repair directly. MCPH1 may play an important role linking DNA damage response to centrosomal function and/or an additional role in DNA repair. This supports the conclusion that proliferative versus differentiated fate control may play the critical role in microcephaly in MCPH1 patients. In addition, CEP152 patient fibroblasts develop aneuploidy in culture indicating there are abnormalities in the control of chromosome segregation 68. Therefore, CEP152 likely plays an important role in genome integrity but not necessarily DNA repair and it is unclear if this gene plays any role in cancer.
Abnormalities in DNA repair do result in several different neurological phenotypes including developmental microcephaly as well as cancer and immunodeficiency. The broad reasons why abnormalities in DNA repair lead to cancer and immunodeficiencies are reasonably well understood even though many details remain to be determined 128–131. However, why the brain is so sensitive to abnormalities in DNA repair remains a mystery. The human neurological diseases associated with abnormalities in DNA repair can be broken down into three categories, microcephaly 67, 132–140, white and gray matter degeneration 141 or ataxia associated degenerative conditions 142–147 (reviewed in 148, 149).
Some DNA repair associated microcephaly disorder genes are involved in the early steps of DNA repair signaling activation, including NBN (AKA NBS1), RAD50, MRE11A and ATR 67, 132–134, 138, 140. MRE11A, RAD50 and NDN form the MRN complex that helps regulate ATM while ATR is a paralog of ATM 150, 151 (Figure 6). Proteins from the MRN complex also involved more directly in the repair of DNA and other signaling functions 152, 153. Interestingly, mutations in ATM cause the ataxia-associated degenerative disease, ataxia-telangiectasia, not a developmental microcephaly. To make matters even more complex, MRE11 mutations can cause either an ataxia-telangiectasia like degenerative disease or a developmental microcephaly 140, 143. Perhaps, the difficulty of interpreting the neurological phenotypes in relation to specific DNA repair pathways should not be surprising since both ATM and ATR play central roles in DNA repair signaling pathways and can phosphorylate over 700 proteins in response to DNA damage 154. Even though mutations in NBN and ATR both cause microcephaly, ATR also cause somatic growth restriction67, 132–134. Recent work suggests that the microcephaly associated with NBN and ATR mutations may have different pathogenetic mechanisms 155. Better insight into specific pathways of DNA repair can be gained focusing on proteins downstream of these early signaling molecules.
Mutations in LIG4 or NHEJ1 result in developmental microcephaly and both genes play a critical role in non-homologous end joining (NHEJ) in which double strand breaks are repaired by trimming DNA ends, bringing the ends together and ligating the strands 135–137, 156. NHEJ can be used in any part of the cell cycle but generally introduces some sequence changes to the DNA while completing repairs. A different double strand break pathway, homologous recombination, is only available late S-phase and in G2 157, 158. This because homologous recombination uses a duplicated strand as a template to create an exact replacement of the broken strand. Interestingly, mice in which NHEJ genes have been knocked out demonstrate significant and sometimes massive cell death in differentiated neurons around mid-gestation that is analogous to the human microcephaly phenotype 159–164. This means apoptosis during neurogenesis is likely an important mechanism of microcephaly in humans in these diseases although other mechanisms remain a possibility as well. The apoptosis is most likely secondary to cell death programs activated by the lack of repair of double strand breaks mediated though ATM and/or TP53 (formerly known as p53) 162, 165–167. Homologous recombination appears to be required for murine brain development as determined via targeted deletion of Xrcc2 or Brca2 167, 168. Interestingly, it appears that homologous recombination is required for precursors during proliferation and NHEJ is needed after differentiation 167. This may be because homologous recombination can compensate for the loss of NHEJ until the cells differentiate, permanently entering G1 where homologous recombination is no longer available.
Analogous to the association between abnormalities in NHEJ and microcephaly, the combined gray-white neurodegeneration typified by Cockayne syndrome is associated with abnormalities in nucleotide excision repair 141. The DNA repair diseases associated with ataxia do not have a clear, single DNA repair pathway defect pathway identified yet even though there is significant overlap of their neurological phenotypes. There is considerable neurological phenotypic overlap within a group of DNA repair diseases, but very little phenotypic overlap between the DNA repair diseases cause that microcephaly, Cockayne, or ataxia. This suggests the likely correlation between DNA repair deficiency pathway and neurological phenotype; non-homologous end joining pathway way for developmental microcephaly, nucleotide excision repair for Cockayne like diseases and a yet to be determined pathway for ataxia diseases. PNKP can play a role in multiple repair pathways and mutations cause both microcephaly and seizures 139, 169, 170. PNKP’s role in NHEJ likely explains the microcephaly phenotype 139, 170. However, seizures are not a very common feature of other microcephaly vera disorders or DNA repair associated microcephaly implying that the seizures may be secondary to defects in additional DNA repair pathways. Interestingly, mice with brain specific defects in the base excision repair pathway appear to have defects in interneurons and seizure like activity and PNKP can be used in this pathway as well 139, 169, 171.
Diseases that lead to developmental microcephaly have given significant insight into the genes required for normal brain development. However, pathophysiological mechanisms of microcephaly reviewed here generate many new questions. Why do mutations centrosomal genes that are used in all dividing cells generally result in a brain specific phenotype? Is there a unifying mechanism between the centrosomal genes? Fate decisions during neurogenesis are clearly important for determining cell final cell number. However, in which cell types are these decision made? There is tantalizing data suggesting that that centrosomal genes are playing a role in these decisions and may have over the course of evolution. How they may be helping control cell fates remains to be definitively determined. In addition, the DNA repair pathways that lead to microcephaly are used in all cells, but the brain is sensitive to their perturbation while most other tissues develop normally (with the exception of lymphocytes). The reasons why the brain is sensitive to loss of DNA repair genes remain a mystery. It is not clear if neuroblasts have more DNA breaks than other cells during their development and if so, what that source might be. Alternatively, neurons may be more sensitive to the presence of DNA damage found in all dividing cells and activate programmed cell death pathways when repair pathways are disrupted. Distinguishing between these hypotheses is difficult experimentally. However, it does appear that defects in specific DNA repair pathways lead to specific phenotypes whether that is NHEJ in microcephaly, nucleotide excision repair in Cockayne and an as of yet to be determined pathway in ataxia.
Acknowledgments
The authors are supported by grants from the US National Institute of Neurological Disorders and Stroke, E.C.G. (5K08NS059673-02) and C.A.W. (NSR01-35129). C.A.W. is also supported by the Fogarty International Center (R21 NS061772), the Manton Center for Orphan Disease Research, the Simons Foundation and by Dubai Harvard Foundation for Medical Research. C.A.W. is an Investigator of the Howard Hughes Medical Institute.
References
- 1.Dolk H. The predictive value of microcephaly during the first year of life for mental retardation at seven years. Dev Med Child Neurol. 1991;33:974–983. doi: 10.1111/j.1469-8749.1991.tb14813.x. [DOI] [PubMed] [Google Scholar]
- 2.Bilguvar K, Ozturk AK, Louvi A, Kwan KY, Choi M, Tatli B, Yalnizoglu D, Tuysuz B, Caglayan AO, Gokben S, et al. Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature. 2010;467:207–210. doi: 10.1038/nature09327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu TW, Mochida GH, Tischfield DJ, Sgaier SK, Flores-Sarnat L, Sergi CM, Topcu M, McDonald MT, Barry BJ, Felie JM, et al. Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat Genet. 2010;42:1015–1020. doi: 10.1038/ng.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholas AK, Khurshid M, Desir J, Carvalho OP, Cox JJ, Thornton G, Kausar R, Ansar M, Ahmad W, Verloes A, et al. WDR62 is associated with the spindle pole and is mutated in human microcephaly. Nat Genet. 2010;42:1010–1014. doi: 10.1038/ng.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci U S A. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 8.Di Cristo G. Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin Genet. 2007;72:1–8. doi: 10.1111/j.1399-0004.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- 9.Vitalis T, Rossier J. New insights into cortical interneurons development and classification: contribution of developmental studies. Dev Neurobiol. 2011;71:34–44. doi: 10.1002/dneu.20810. [DOI] [PubMed] [Google Scholar]
- 10.Siegenthaler JA, Pleasure SJ. We have got you ‘covered’: how the meninges control brain development. Curr Opin Genet Dev. 2010;21:249–255. doi: 10.1016/j.gde.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer G. Building a human cortex: the evolutionary differentiation of Cajal-Retzius cells and the cortical hem. J Anat. 2010;217:334–343. doi: 10.1111/j.1469-7580.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 13.Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 14.Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 15.Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 16.Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM, Mak TW. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- 18.Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caviness VS, Takahashi T, Nowakowski RS. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–383. doi: 10.1016/0166-2236(95)93933-o. [DOI] [PubMed] [Google Scholar]
- 20.Caviness VS, Goto T, Tarui T, Takahashi T, Bhide PG, Nowakowski RS. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb Cortex. 2003;13:592–598. doi: 10.1093/cercor/13.6.592. [DOI] [PubMed] [Google Scholar]
- 21.Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- 23.Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 24.Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- 25.Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- 26.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 27.Reillo I, de Juan Romero C, Garcia-Cabezas MA, Borrell V. A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex. 2011;21:1674–1694. doi: 10.1093/cercor/bhq238. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Tsai JW, LaMonica B, Kriegstein AR. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci. 2011;14:555–561. doi: 10.1038/nn.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson AP, Eastwood H, Bell SM, Adu J, Toomes C, Carr IM, Roberts E, Hampshire DJ, Crow YJ, Mighell AJ, et al. Identification of microcephalin, a protein implicated in determining the size of the human brain. Am J Hum Genet. 2002;71:136–142. doi: 10.1086/341283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bond J, Roberts E, Mochida GH, Hampshire DJ, Scott S, Askham JM, Springell K, Mahadevan M, Crow YJ, Markham AF, et al. ASPM is a major determinant of cerebral cortical size. Nat Genet. 2002;32:316–320. doi: 10.1038/ng995. [DOI] [PubMed] [Google Scholar]
- 31.Bond J, Roberts E, Springell K, Lizarraga SB, Scott S, Higgins J, Hampshire DJ, Morrison EE, Leal GF, Silva EO, et al. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet. 2005;37:353–355. doi: 10.1038/ng1539. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Girimaji SC, Duvvari MR, Blanton SH. Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. Am J Hum Genet. 2009;84:286–290. doi: 10.1016/j.ajhg.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guernsey DL, Jiang H, Hussin J, Arnold M, Bouyakdan K, Perry S, Babineau-Sturk T, Beis J, Dumas N, Evans SC, et al. Mutations in centrosomal protein CEP152 in primary microcephaly families linked to MCPH4. Am J Hum Genet. 2010;87:40–51. doi: 10.1016/j.ajhg.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sir JH, Barr AR, Nicholas AK, Carvalho OP, Khurshid M, Sossick A, Reichelt S, D’Santos C, Woods CG, Gergely F. A primary microcephaly protein complex forms a ring around parental centrioles. Nat Genet. 2011 doi: 10.1038/ng.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchal JA, Ghani M, Schindler D, Gavvovidis I, Winkler T, Esquitino V, Sternberg N, Busche A, Krawitz P, Hecht J, et al. Misregulation of mitotic chromosome segregation in a new type of autosomal recessive primary microcephaly. Cell Cycle. 2011;10:2967–2977. doi: 10.4161/cc.10.17.16871. [DOI] [PubMed] [Google Scholar]
- 36.Kerjan G, Gleeson JG. Genetic mechanisms underlying abnormal neuronal migration in classical lissencephaly. Trends Genet. 2007;23:623–630. doi: 10.1016/j.tig.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Manzini MC, Walsh CA. What disorders of cortical development tell us about the cortex: one plus one does not always make two. Curr Opin Genet Dev. 2011;21:333–339. doi: 10.1016/j.gde.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nigg EA, Stearns T. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol. 2011;13:1154–1160. doi: 10.1038/ncb2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thornton GK, Woods CG. Primary microcephaly: do all roads lead to Rome? Trends Genet. 2009;25:501–510. doi: 10.1016/j.tig.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graser S, Stierhof YD, Nigg EA. Cep68 and Cep215 (Cdk5rap2) are required for centrosome cohesion. J Cell Sci. 2007;120:4321–4331. doi: 10.1242/jcs.020248. [DOI] [PubMed] [Google Scholar]
- 41.Azimzadeh J, Marshall WF. Building the centriole. Curr Biol. 2010;20:R816–825. doi: 10.1016/j.cub.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevens NR, Dobbelaere J, Brunk K, Franz A, Raff JW. Drosophila Ana2 is a conserved centriole duplication factor. J Cell Biol. 2010;188:313–323. doi: 10.1083/jcb.200910016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castiel A, Danieli MM, David A, Moshkovitz S, Aplan PD, Kirsch IR, Brandeis M, Kramer A, Izraeli S. The Stil protein regulates centrosome integrity and mitosis through suppression of Chfr. J Cell Sci. 2011;124:532–539. doi: 10.1242/jcs.079731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cizmecioglu O, Arnold M, Bahtz R, Settele F, Ehret L, Haselmann-Weiss U, Antony C, Hoffmann I. Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome. J Cell Biol. 2010;191:731–739. doi: 10.1083/jcb.201007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dzhindzhev NS, Yu QD, Weiskopf K, Tzolovsky G, Cunha-Ferreira I, Riparbelli M, Rodrigues-Martins A, Bettencourt-Dias M, Callaini G, Glover DM. Asterless is a scaffold for the onset of centriole assembly. Nature. 2010;467:714–718. doi: 10.1038/nature09445. [DOI] [PubMed] [Google Scholar]
- 46.Hatch EM, Kulukian A, Holland AJ, Cleveland DW, Stearns T. Cep152 interacts with Plk4 and is required for centriole duplication. J Cell Biol. 2010;191:721–729. doi: 10.1083/jcb.201006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohlmaier G, Loncarek J, Meng X, McEwen BF, Mogensen MM, Spektor A, Dynlacht BD, Khodjakov A, Gonczy P. Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr Biol. 2009;19:1012–1018. doi: 10.1016/j.cub.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt TI, Kleylein-Sohn J, Westendorf J, Le Clech M, Lavoie SB, Stierhof YD, Nigg EA. Control of centriole length by CPAP and CP110. Curr Biol. 2009;19:1005–1011. doi: 10.1016/j.cub.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Tang CJ, Fu RH, Wu KS, Hsu WB, Tang TK. CPAP is a cell-cycle regulated protein that controls centriole length. Nat Cell Biol. 2009;11:825–831. doi: 10.1038/ncb1889. [DOI] [PubMed] [Google Scholar]
- 50.Fish JL, Kosodo Y, Enard W, Paabo S, Huttner WB. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc Natl Acad Sci U S A. 2006;103:10438–10443. doi: 10.1073/pnas.0604066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Voet M, Berends CW, Perreault A, Nguyen-Ngoc T, Gonczy P, Vidal M, Boxem M, van den Heuvel S. NuMA-related LIN-5, ASPM-1, calmodulin and dynein promote meiotic spindle rotation independently of cortical LIN-5/GPR/Galpha. Nat Cell Biol. 2009;11:269–277. doi: 10.1038/ncb1834. [DOI] [PubMed] [Google Scholar]
- 52.Lizarraga SB, Margossian SP, Harris MH, Campagna DR, Han AP, Blevins S, Mudbhary R, Barker JE, Walsh CA, Fleming MD. Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors. Development. 2010;137:1907–1917. doi: 10.1242/dev.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng Y, Walsh CA. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron. 2004;44:279–293. doi: 10.1016/j.neuron.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 54.Rehen SK, McConnell MJ, Kaushal D, Kingsbury MA, Yang AH, Chun J. Chromosomal variation in neurons of the developing and adult mammalian nervous system. Proc Natl Acad Sci USA. 2001;98:13361–13366. doi: 10.1073/pnas.231487398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaushal D, Contos JJ, Treuner K, Yang AH, Kingsbury MA, Rehen SK, McConnell MJ, Okabe M, Barlow C, Chun J. Alteration of gene expression by chromosome loss in the postnatal mouse brain. J Neurosci. 2003;23:5599–5606. doi: 10.1523/JNEUROSCI.23-13-05599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang AH, Kaushal D, Rehen SK, Kriedt K, Kingsbury MA, McConnell MJ, Chun J. Chromosome segregation defects contribute to aneuploidy in normal neural progenitor cells. J Neurosci. 2003;23:10454–10462. doi: 10.1523/JNEUROSCI.23-32-10454.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dobyns WB, Reiner O, Carrozzo R, Ledbetter DH. Lissencephaly. A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. JAMA. 1993;270:2838–2842. doi: 10.1001/jama.270.23.2838. [DOI] [PubMed] [Google Scholar]
- 58.des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y, et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- 59.Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 60.Sossey-Alaoui K, Hartung AJ, Guerrini R, Manchester DK, Posar A, Puche-Mira A, Andermann E, Dobyns WB, Srivastava AK. Human doublecortin (DCX) and the homologous gene in mouse encode a putative Ca2+-dependent signaling protein which is mutated in human X-linked neuronal migration defects. Hum Mol Genet. 1998;7:1327–1332. doi: 10.1093/hmg/7.8.1327. [DOI] [PubMed] [Google Scholar]
- 61.Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND, Walsh CA. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2000;26:93–96. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- 62.Keays DA, Tian G, Poirier K, Huang GJ, Siebold C, Cleak J, Oliver PL, Fray M, Harvey RJ, Molnar Z, et al. Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell. 2007;128:45–57. doi: 10.1016/j.cell.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alkuraya FS, Cai X, Emery C, Mochida GH, Al-Dosari MS, Felie JM, Hill RS, Barry BJ, Partlow JN, Gascon GG, et al. Human mutations in NDE1 cause extreme microcephaly with lissencephaly [corrected] Am J Hum Genet. 2011;88:536–547. doi: 10.1016/j.ajhg.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bakircioglu M, Carvalho OP, Khurshid M, Cox JJ, Tuysuz B, Barak T, Yilmaz S, Caglayan O, Dincer A, Nicholas AK, et al. The essential role of centrosomal NDE1 in human cerebral cortex neurogenesis. Am J Hum Genet. 2011;88:523–535. doi: 10.1016/j.ajhg.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yingling J, Youn YH, Darling D, Toyo-Oka K, Pramparo T, Hirotsune S, Wynshaw-Boris A. Neuroepithelial stem cell proliferation requires LIS1 for precise spindle orientation and symmetric division. Cell. 2008;132:474–486. doi: 10.1016/j.cell.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griffith E, Walker S, Martin CA, Vagnarelli P, Stiff T, Vernay B, Al Sanna N, Saggar A, Hamel B, Earnshaw WC, et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet. 2008;40:232–236. doi: 10.1038/ng.2007.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’driscoll M, Ruiz-Perez V, Woods C, Jeggo P, Goodship J. A splicing mutation affecting expression of ataxia–telangiectasia and Rad3–related protein (ATR) results in Seckel syndrome. Nat Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 68.Kalay E, Yigit G, Aslan Y, Brown KE, Pohl E, Bicknell LS, Kayserili H, Li Y, Tuysuz B, Nurnberg G, et al. CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nat Genet. 2011;43:23–26. doi: 10.1038/ng.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roegiers F, Jan YN. Asymmetric cell division. Curr Opin Cell Biol. 2004;16:195–205. doi: 10.1016/j.ceb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 70.Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- 71.Morin X, Bellaiche Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev Cell. 2011;21:102–119. doi: 10.1016/j.devcel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 72.Costa MR, Wen G, Lepier A, Schroeder T, Gotz M. Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development. 2008;135:11–22. doi: 10.1242/dev.009951. [DOI] [PubMed] [Google Scholar]
- 73.Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 75.Kim S, Lehtinen MK, Sessa A, Zappaterra MW, Cho SH, Gonzalez D, Boggan B, Austin CA, Wijnholds J, Gambello MJ, et al. The apical complex couples cell fate and cell survival to cerebral cortical development. Neuron. 2010;66:69–84. doi: 10.1016/j.neuron.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhong W, Feder JN, Jiang MM, Jan LY, Jan YN. Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron. 1996;17:43–53. doi: 10.1016/s0896-6273(00)80279-2. [DOI] [PubMed] [Google Scholar]
- 77.Haydar TF, Ang E, Jr, Rakic P. Mitotic spindle rotation and mode of cell division in the developing telencephalon. Proc Natl Acad Sci U S A. 2003;100:2890–2895. doi: 10.1073/pnas.0437969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wakamatsu Y, Maynard TM, Jones SU, Weston JA. NUMB localizes in the basal cortex of mitotic avian neuroepithelial cells and modulates neuronal differentiation by binding to NOTCH-1. Neuron. 1999;23:71–81. doi: 10.1016/s0896-6273(00)80754-0. [DOI] [PubMed] [Google Scholar]
- 79.Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508:28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morin X, Jaouen F, Durbec P. Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat Neurosci. 2007;10:1440–1448. doi: 10.1038/nn1984. [DOI] [PubMed] [Google Scholar]
- 82.Postiglione MP, Juschke C, Xie Y, Haas GA, Charalambous C, Knoblich JA. Mouse Inscuteable Induces Apical-Basal Spindle Orientation to Facilitate Intermediate Progenitor Generation in the Developing Neocortex. Neuron. 2011;72:268–284. doi: 10.1016/j.neuron.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J Cell Biol. 2000;149:317–330. doi: 10.1083/jcb.149.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14:25–34. doi: 10.1016/s0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- 85.Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461:947–955. doi: 10.1038/nature08435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anderson CT, Stearns T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr Biol. 2009;19:1498–1502. doi: 10.1016/j.cub.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Graser S, Stierhof YD, Lavoie SB, Gassner OS, Lamla S, Le Clech M, Nigg EA. Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol. 2007;179:321–330. doi: 10.1083/jcb.200707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Major MB, Roberts BS, Berndt JD, Marine S, Anastas J, Chung N, Ferrer M, Yi X, Stoick-Cooper CL, von Haller PD, et al. New regulators of Wnt/beta-catenin signaling revealed by integrative molecular screening. Sci Signal. 2008;1:ra12. doi: 10.1126/scisignal.2000037. [DOI] [PubMed] [Google Scholar]
- 89.Buchman JJ, Durak O, Tsai LH. ASPM regulates Wnt signaling pathway activity in the developing brain. Genes Dev. 2011;25:1909–1914. doi: 10.1101/gad.16830211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 91.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 92.Lee JE, Gleeson JG. Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol. 2011;24:98–105. doi: 10.1097/WCO.0b013e3283444d05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldman N, Yang Z. A codon-based model of nucleotide substitution for protein-coding DNA sequences. Mol Biol Evol. 1994;11:725–736. doi: 10.1093/oxfordjournals.molbev.a040153. [DOI] [PubMed] [Google Scholar]
- 96.Zhang J. Evolution of the human ASPM gene, a major determinant of brain size. Genetics. 2003;165:2063–2070. doi: 10.1093/genetics/165.4.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Evans PD, Anderson JR, Vallender EJ, Gilbert SL, Malcom CM, Dorus S, Lahn BT. Adaptive evolution of ASPM, a major determinant of cerebral cortical size in humans. Hum Mol Genet. 2004;13:489–494. doi: 10.1093/hmg/ddh055. [DOI] [PubMed] [Google Scholar]
- 98.Kouprina N, Pavlicek A, Mochida GH, Solomon G, Gersch W, Yoon YH, Collura R, Ruvolo M, Barrett JC, Woods CG, et al. Accelerated evolution of the ASPM gene controlling brain size begins prior to human brain expansion. PLoS Biol. 2004;2:E126. doi: 10.1371/journal.pbio.0020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Evans PD, Anderson JR, Vallender EJ, Choi SS, Lahn BT. Reconstructing the evolutionary history of microcephalin, a gene controlling human brain size. Hum Mol Genet. 2004;13:1139–1145. doi: 10.1093/hmg/ddh126. [DOI] [PubMed] [Google Scholar]
- 100.Wang YQ, Su B. Molecular evolution of microcephalin, a gene determining human brain size. Hum Mol Genet. 2004;13:1131–1137. doi: 10.1093/hmg/ddh127. [DOI] [PubMed] [Google Scholar]
- 101.Evans PD, Vallender EJ, Lahn BT. Molecular evolution of the brain size regulator genes CDK5RAP2 and CENPJ. Gene. 2006;375:75–79. doi: 10.1016/j.gene.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 102.Ali F, Meier R. Positive selection in ASPM is correlated with cerebral cortex evolution across primates but not with whole-brain size. Mol Biol Evol. 2008;25:2247–2250. doi: 10.1093/molbev/msn184. [DOI] [PubMed] [Google Scholar]
- 103.Hill RS, Walsh CA. Molecular insights into human brain evolution. Nature. 2005;437:64–67. doi: 10.1038/nature04103. [DOI] [PubMed] [Google Scholar]
- 104.Vallender EJ, Mekel-Bobrov N, Lahn BT. Genetic basis of human brain evolution. Trends Neurosci. 2008;31:637–644. doi: 10.1016/j.tins.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Enard W, Przeworski M, Fisher SE, Lai CS, Wiebe V, Kitano T, Monaco AP, Paabo S. Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002;418:869–872. doi: 10.1038/nature01025. [DOI] [PubMed] [Google Scholar]
- 106.Burki F, Kaessmann H. Birth and adaptive evolution of a hominoid gene that supports high neurotransmitter flux. Nat Genet. 2004;36:1061–1063. doi: 10.1038/ng1431. [DOI] [PubMed] [Google Scholar]
- 107.Ferland RJ, Eyaid W, Collura RV, Tully LD, Hill RS, Al-Nouri D, Al-Rumayyan A, Topcu M, Gascon G, Bodell A, et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet. 2004;36:1008–1013. doi: 10.1038/ng1419. [DOI] [PubMed] [Google Scholar]
- 108.Wang YQ, Qian YP, Yang S, Shi H, Liao CH, Zheng HK, Wang J, Lin AA, Cavalli-Sforza LL, Underhill PA, et al. Accelerated evolution of the pituitary adenylate cyclase-activating polypeptide precursor gene during human origin. Genetics. 2005;170:801–806. doi: 10.1534/genetics.105.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dorus S, Anderson JR, Vallender EJ, Gilbert SL, Zhang L, Chemnick LG, Ryder OA, Li W, Lahn BT. Sonic Hedgehog, a key development gene, experienced intensified molecular evolution in primates. Hum Mol Genet. 2006;15:2031–2037. doi: 10.1093/hmg/ddl123. [DOI] [PubMed] [Google Scholar]
- 110.Vallender EJ, Lahn BT. A primate-specific acceleration in the evolution of the caspase-dependent apoptosis pathway. Hum Mol Genet. 2006;15:3034–3040. doi: 10.1093/hmg/ddl245. [DOI] [PubMed] [Google Scholar]
- 111.Shi P, Bakewell MA, Zhang J. Did brain-specific genes evolve faster in humans than in chimpanzees? Trends Genet. 2006;22:608–613. doi: 10.1016/j.tig.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Wang HY, Chien HC, Osada N, Hashimoto K, Sugano S, Gojobori T, Chou CK, Tsai SF, Wu CI, Shen CK. Rate of evolution in brain-expressed genes in humans and other primates. PLoS Biol. 2007;5:e13. doi: 10.1371/journal.pbio.0050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang JK, Li Y, Su B. A common SNP of MCPH1 is associated with cranial volume variation in Chinese population. Hum Mol Genet. 2008;17:1329–1335. doi: 10.1093/hmg/ddn021. [DOI] [PubMed] [Google Scholar]
- 114.Rimol LM, Agartz I, Djurovic S, Brown AA, Roddey JC, Kahler AK, Mattingsdal M, Athanasiu L, Joyner AH, Schork NJ, et al. Sex-dependent association of common variants of microcephaly genes with brain structure. Proc Natl Acad Sci U S A. 2010;107:384–388. doi: 10.1073/pnas.0908454107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Medema RH, Macurek L. Checkpoint control and cancer. Oncogene. 2010 doi: 10.1038/onc.2011.451. [DOI] [PubMed] [Google Scholar]
- 116.Thompson SL, Compton DA. Chromosomes and cancer cells. Chromosome Res. 2011;19:433–444. doi: 10.1007/s10577-010-9179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Janssen A, van der Burg M, Szuhai K, Kops GJ, Medema RH. Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science. 2011;333:1895–1898. doi: 10.1126/science.1210214. [DOI] [PubMed] [Google Scholar]
- 118.Neitzel H, Neumann LM, Schindler D, Wirges A, Tonnies H, Trimborn M, Krebsova A, Richter R, Sperling K. Premature chromosome condensation in humans associated with microcephaly and mental retardation: a novel autosomal recessive condition. Am J Hum Genet. 2002;70:1015–1022. doi: 10.1086/339518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trimborn M, Bell SM, Felix C, Rashid Y, Jafri H, Griffiths PD, Neumann LM, Krebs A, Reis A, Sperling K, et al. Mutations in microcephalin cause aberrant regulation of chromosome condensation. Am J Hum Genet. 2004;75:261–266. doi: 10.1086/422855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin SY, Rai R, Li K, Xu ZX, Elledge SJ. BRIT1/MCPH1 is a DNA damage responsive protein that regulates the Brca1-Chk1 pathway, implicating checkpoint dysfunction in microcephaly. Proc Natl Acad Sci USA. 2005;102:15105–15109. doi: 10.1073/pnas.0507722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alderton G, Galbiati L, Griffith E, Surinya K, Neitzel H, Jackson A, Jeggo P, O’driscoll M. Regulation of mitotic entry by microcephalin and its overlap with ATR signalling. Nat Cell Biol. 2006;8:725–733. doi: 10.1038/ncb1431. [DOI] [PubMed] [Google Scholar]
- 122.Rai R, Dai H, Multani AS, Li K, Chin K, Gray J, Lahad JP, Liang J, Mills GB, Meric-Bernstam F, et al. BRIT1 regulates early DNA damage response, chromosomal integrity, and cancer. Cancer Cell. 2006;10:145–157. doi: 10.1016/j.ccr.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wood JL, Singh N, Mer G, Chen J. MCPH1 functions in an H2AX-dependent but MDC1-independent pathway in response to DNA damage. J Biol Chem. 2007;282:35416–35423. doi: 10.1074/jbc.M705245200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wood JL, Liang Y, Li K, Chen J. Microcephalin/MCPH1 associates with the Condensin II complex to function in homologous recombination repair. J Biol Chem. 2008;283:29586–29592. doi: 10.1074/jbc.M804080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liang Y, Gao H, Lin SY, Peng G, Huang X, Zhang P, Goss JA, Brunicardi FC, Multani AS, Chang S, et al. BRIT1/MCPH1 is essential for mitotic and meiotic recombination DNA repair and maintaining genomic stability in mice. PLoS Genet. 2010;6:e1000826. doi: 10.1371/journal.pgen.1000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trimborn M, Ghani M, Walther DJ, Dopatka M, Dutrannoy V, Busche A, Meyer F, Nowak S, Nowak J, Zabel C, et al. Establishment of a mouse model with misregulated chromosome condensation due to defective Mcph1 function. PLoS One. 2010;5:e9242. doi: 10.1371/journal.pone.0009242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gruber R, Zhou Z, Sukchev M, Joerss T, Frappart PO, Wang ZQ. MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nat Cell Biol. 2011 doi: 10.1038/ncb2342. [DOI] [PubMed] [Google Scholar]
- 128.Kracker S, Gardes P, Mazerolles F, Durandy A. Immunoglobulin class switch recombination deficiencies. Clin Immunol. 2010;135:193–203. doi: 10.1016/j.clim.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 129.Preston BD, Albertson TM, Herr AJ. DNA replication fidelity and cancer. Semin Cancer Biol. 2010;20:281–293. doi: 10.1016/j.semcancer.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gostissa M, Alt FW, Chiarle R. Mechanisms that promote and suppress chromosomal translocations in lymphocytes. Annu Rev Immunol. 2011;29:319–350. doi: 10.1146/annurev-immunol-031210-101329. [DOI] [PubMed] [Google Scholar]
- 131.Luijsterburg MS, van Attikum H. Chromatin and the DNA damage response: The cancer connection. Mol Oncol. 2011;5:349–367. doi: 10.1016/j.molonc.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matsuura S, Tauchi H, Nakamura A, Kondo N, Sakamoto S, Endo S, Smeets D, Solder B, Belohradsky BH, Der Kaloustian VM, et al. Positional cloning of the gene for Nijmegen breakage syndrome. Nat Genet. 1998;19:179–181. doi: 10.1038/549. [DOI] [PubMed] [Google Scholar]
- 133.Carney JP, Maser RS, Olivares H, Davis EM, Le Beau M, Yates JR, 3rd, Hays L, Morgan WF, Petrini JH. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. doi: 10.1016/s0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 134.Varon R, Vissinga C, Platzer M, Cerosaletti KM, Chrzanowska KH, Saar K, Beckmann G, Seemanova E, Cooper PR, Nowak NJ, et al. Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell. 1998;93:467–476. doi: 10.1016/s0092-8674(00)81174-5. [DOI] [PubMed] [Google Scholar]
- 135.O’Driscoll M, Cerosaletti KM, Girard PM, Dai Y, Stumm M, Kysela B, Hirsch B, Gennery A, Palmer SE, Seidel J, et al. DNA ligase IV mutations identified in patients exhibiting developmental delay and immunodeficiency. Mol Cell. 2001;8:1175–1185. doi: 10.1016/s1097-2765(01)00408-7. [DOI] [PubMed] [Google Scholar]
- 136.Buck D, Moshous D, De Chasseval R, Ma Y, Le Deist F, Cavazzana-Calvo M, Fischer A, Casanova J, Lieber M, De Villartay J. Severe combined immunodeficiency and microcephaly in siblings with hypomorphic mutations in DNA ligase IV. Eur J Immunol. 2006;36:224–235. doi: 10.1002/eji.200535401. [DOI] [PubMed] [Google Scholar]
- 137.Buck D, Malivert L, de Chasseval R, Barraud A, Fondaneche MC, Sanal O, Plebani A, Stephan JL, Hufnagel M, le Deist F, et al. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell. 2006;124:287–299. doi: 10.1016/j.cell.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 138.Waltes R, Kalb R, Gatei M, Kijas AW, Stumm M, Sobeck A, Wieland B, Varon R, Lerenthal Y, Lavin MF, et al. Human RAD50 deficiency in a Nijmegen breakage syndrome-like disorder. Am J Hum Genet. 2009;84:605–616. doi: 10.1016/j.ajhg.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shen J, Gilmore EC, Marshall CA, Haddadin M, Reynolds JJ, Eyaid W, Bodell A, Barry B, Gleason D, Allen K, et al. Mutations in PNKP cause microcephaly, seizures and defects in DNA repair. Nat Genet. 2010;42:245–249. doi: 10.1038/ng.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matsumoto Y, Miyamoto T, Sakamoto H, Izumi H, Nakazawa Y, Ogi T, Tahara H, Oku S, Hiramoto A, Shiiki T, et al. Two unrelated patients with MRE11A mutations and Nijmegen breakage syndrome-like severe microcephaly. DNA Repair (Amst) 2011;10:314–321. doi: 10.1016/j.dnarep.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 141.Lehmann A. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85:1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 142.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 143.Stewart GS, Maser RS, Stankovic T, Bressan DA, Kaplan MI, Jaspers NG, Raams A, Byrd PJ, Petrini JH, Taylor AM. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell. 1999;99:577–587. doi: 10.1016/s0092-8674(00)81547-0. [DOI] [PubMed] [Google Scholar]
- 144.Moreira MC, Barbot C, Tachi N, Kozuka N, Uchida E, Gibson T, Mendonça P, Costa M, Barros J, Yanagisawa T, et al. The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat Genet. 2001;29:189–193. doi: 10.1038/ng1001-189. [DOI] [PubMed] [Google Scholar]
- 145.Takashima H, Boerkoel C, John J, Saifi G, Salih M, Armstrong D, Mao Y, Quiocho F, Roa B, Nakagawa M, et al. Mutation of TDP1, encoding a topoisomerase I–dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat Genet. 2002;32:267–272. doi: 10.1038/ng987. [DOI] [PubMed] [Google Scholar]
- 146.Moreira MC, Klur S, Watanabe M, Nemeth AH, Le Ber I, Moniz JC, Tranchant C, Aubourg P, Tazir M, Schols L, et al. Senataxin, the ortholog of a yeast RNA helicase, is mutant in ataxia-ocular apraxia 2. Nat Genet. 2004;36:225–227. doi: 10.1038/ng1303. [DOI] [PubMed] [Google Scholar]
- 147.El-Khamisy SF, Saifi GM, Weinfeld M, Johansson F, Helleday T, Lupski JR, Caldecott KW. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature. 2005;434:108–113. doi: 10.1038/nature03314. [DOI] [PubMed] [Google Scholar]
- 148.Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 149.McKinnon PJ. DNA repair deficiency and neurological disease. Nat Rev Neurosci. 2009;10:100–112. doi: 10.1038/nrn2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- 151.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lamarche BJ, Orazio NI, Weitzman MD. The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett. 2010;584:3682–3695. doi: 10.1016/j.febslet.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stracker TH, Petrini JH. The MRE11 complex: starting from the ends. Nat Rev Mol Cell Biol. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 155.Zhou Z, Bruhn C, Wang ZQ. Differential function of NBS1 and ATR in neurogenesis. DNA Repair (Amst) 2011 doi: 10.1016/j.dnarep.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 156.Ben-Omran T, Cerosaletti K, Concannon P, Weitzman S, Nezarati M. A patient with mutations in DNA Ligase IV: Clinical features and overlap with Nijmegen breakage syndrome. Am J Med Genet. 2005;137A:283–287. doi: 10.1002/ajmg.a.30869. [DOI] [PubMed] [Google Scholar]
- 157.Weterings E, Chen DJ. The endless tale of non-homologous end-joining. Cell Res. 2008;18:114–124. doi: 10.1038/cr.2008.3. [DOI] [PubMed] [Google Scholar]
- 158.Hartlerode AJ, Scully R. Mechanisms of double-strand break repair in somatic mammalian cells. Biochem J. 2009;423:157–168. doi: 10.1042/BJ20090942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gao Y, Sun Y, Frank KM, Dikkes P, Fujiwara Y, Seidl KJ, Sekiguchi JM, Rathbun GA, Swat W, Wang J, et al. A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell. 1998;95:891–902. doi: 10.1016/s0092-8674(00)81714-6. [DOI] [PubMed] [Google Scholar]
- 160.Frank KM, Sekiguchi JM, Seidl KJ, Swat W, Rathbun GA, Cheng HL, Davidson L, Kangaloo L, Alt FW. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature. 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- 161.Barnes DE, Stamp G, Rosewell I, Denzel A, Lindahl T. Targeted disruption of the gene encoding DNA ligase IV leads to lethality in embryonic mice. Curr Biol. 1998;8:1395–1398. doi: 10.1016/s0960-9822(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 162.Frank KM, Sharpless NE, Gao Y, Sekiguchi JM, Ferguson DO, Zhu C, Manis JP, Horner J, DePinho RA, Alt FW. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol Cell. 2000;5:993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- 163.Gu Y, Sekiguchi J, Gao Y, Dikkes P, Frank K, Ferguson D, Hasty P, Chun J, Alt FW. Defective embryonic neurogenesis in Ku-deficient but not DNA-dependent protein kinase catalytic subunit-deficient mice. Proc Natl Acad Sci USA. 2000;97:2668–2673. doi: 10.1073/pnas.97.6.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vemuri MC, Schiller E, Naegele JR. Elevated DNA double strand breaks and apoptosis in the CNS of scid mutant mice. Cell Death Differ. 2001;8:245–255. doi: 10.1038/sj.cdd.4400806. [DOI] [PubMed] [Google Scholar]
- 165.Lee Y, Barnes DE, Lindahl T, McKinnon PJ. Defective neurogenesis resulting from DNA ligase IV deficiency requires Atm. Genes Dev. 2000;14:2576–2580. doi: 10.1101/gad.837100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sekiguchi J, Ferguson DO, Chen HT, Yang EM, Earle J, Frank K, Whitlow S, Gu Y, Xu Y, Nussenzweig A, et al. Genetic interactions between ATM and the nonhomologous end-joining factors in genomic stability and development. Proc Natl Acad Sci USA. 2001;98:3243–3248. doi: 10.1073/pnas.051632098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Orii KE, Lee Y, Kondo N, McKinnon PJ. Selective utilization of nonhomologous end-joining and homologous recombination DNA repair pathways during nervous system development. Proc Natl Acad Sci USA. 2006;103:10017–10022. doi: 10.1073/pnas.0602436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Frappart PO, Lee Y, Lamont J, McKinnon PJ. BRCA2 is required for neurogenesis and suppression of medulloblastoma. EMBO J. 2007;26:2732–2742. doi: 10.1038/sj.emboj.7601703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Whitehouse CJ, Taylor RM, Thistlethwaite A, Zhang H, Karimi-Busheri F, Lasko DD, Weinfeld M, Caldecott KW. XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell. 2001;104:107–117. doi: 10.1016/s0092-8674(01)00195-7. [DOI] [PubMed] [Google Scholar]
- 170.Koch CA, Agyei R, Galicia S, Metalnikov P, O’Donnell P, Starostine A, Weinfeld M, Durocher D. Xrcc4 physically links DNA end processing by polynucleotide kinase to DNA ligation by DNA ligase IV. EMBO J. 2004;23:3874–3885. doi: 10.1038/sj.emboj.7600375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee Y, Katyal S, Li Y, El-Khamisy SF, Russell HR, Caldecott KW, McKinnon PJ. The genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Nat Neurosci. 2009;12:973–980. doi: 10.1038/nn.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]