Abstract
Objective
A cluster-randomized trial was performed to evaluate an educational intervention to improve parental attitudes and vaccine uptake in vaccine-hesitant parents.
Methods
Two primary care sites were randomized to provide families with either usual care, or an intervention (video and written information) for vaccine-hesitant parents. Eligible parents included those presenting for their child’s 2 week well visit with performance on the Parent Attitudes about Childhood Vaccines (PACV) survey suggesting vaccine hesitancy (score ≥25). Enrollees completed PACV surveys at the 2 month well visit and vaccination status at 12 weeks of age was assessed. The primary outcome was the difference in PACV scores obtained at enrollment and 2 months between the two groups. The proportion of on-time vaccination was also compared at 12 weeks.
Results
454 parents were approached and 369 (81.3%) participated; 132 had PACV scores ≥ 25 and were enrolled [67 in the control group (mean PACV score = 37) and 55 in the intervention group (mean PACV score = 40)]. Two month PACV surveys were completed by 108 (~90%) of enrollees. Parents in the intervention group had a significant decrease in PACV score at two months compared to control (median difference = 6.7, p=0.049); this remained significant after adjustment for baseline PACV score, race/ethnicity, and income (p=0.044). There was no difference in the on-time receipt of vaccines between groups at 12 weeks.
Conclusions
A brief educational intervention for vaccine-hesitant parents was associated with a modest but significant increase in measured parental attitudes towards vaccines.
Keywords: immunizations, parental attitudes, parental beliefs, vaccines
Introduction
Increasing numbers of parents are choosing to delay or refuse one or more of the recommended childhood vaccines.1–4 When children are unvaccinated, the protection afforded by herd immunity wanes.5 In fact, resurgence of diseases such as measles in the United States and Europe has been recently documented6–10 and parental refusal of pertussis, varicella and pneumococcal vaccinations has been associated with increased risks of these infections in children.11–13 The trend of increasing parental concern regarding childhood vaccines may be due to conflicting information parents receive about the safety and risks of vaccines. Several groups including the Centers for Disease Control and Prevention and the American Academy of Pediatrics (AAP) have developed online tools for providers and parents to find accurate information regarding childhood vaccines.14,15 However, other sources widely available on the internet question the safety and effectiveness of vaccines. It is unclear if parents are able to determine which publicly available information is trustworthy and based in science; therefore it is increasingly important that parents are directed to accurate information so that they can make an informed decision regarding childhood vaccinations.
Earlier studies have shown that most vaccine-hesitant parents prefer to receive information about childhood vaccines from their healthcare providers prior to the first vaccine visit.16,17 However, given time restrictions on clinic visits, it is logistically challenging for providers to comprehensively answer all questions.18 The goal of our pilot study was to develop an effective educational tool to address the most common concerns about childhood vaccines for vaccine-hesitant parents that could be implemented in the clinic. We hypothesized that providing vaccine information to vaccine-hesitant parents at the time of the two week office visit would improve parental attitudes regarding childhood vaccines in this population.
Patients and Methods
A two-arm cluster-randomized trial was conducted with subjects from two private pediatric practices in middle Tennessee (Table 1). The two sites were randomly assigned as either the intervention or the control site by coin flip and maintained the same designation for the duration of the study. All parents of infants at two week well child visits were asked by their healthcare provider or nurse/nursing assistant if they were willing to speak with a study team member. If they agreed, the study was introduced to the parents by the study team member in a private examination room prior to the provider examination or immediately after completion of the visit.
Table 1.
Demographics between groups/baseline scores (Total enrolled = 122)
| Control (N=67) | Intervention (N=55) | Significance of difference between sites |
|
|---|---|---|---|
| Screening score (median) | 36.63 (IQR: 29.97–53.28) |
39.96 (IQR: 33.3–53.28) |
p = 0.254 a |
| Parental gender | 80.6% Female | 81.8% Female | p = 0.8651 b |
| Parental age (mean) | 32.1 years | 32.0 years | p = 0.9581 b |
| Ethnicity | 73.13 % White | 85.45 % White | p = 0.105 c |
| 14.93 % Black | 1.82 % Black | ||
| 8.96 % Other | 9.09 % Other | ||
| 2.99 % no response | 3.64% no response | ||
| Annual Income | 20.9 % < $49,999 | 3.64 % < $49,999 | p = 0.001 c |
| 16.42 % $50,000–$74,999 | 21.82 % $50,000–$74,999 | ||
| 22.39 % $75,000+ | 52.73 % $75,000+ | ||
| 40.86 % prefer not to answer or no response | 21.82 % prefer not to answer or no response | ||
| Highest level of Education | 1.5% high school or less | 5.5 % high school or less | p = 0.285 c |
| 7.5 % some college | 18 % some college | ||
| 59.7% college graduate | 43.6 % college graduate | ||
| 25.4 % post college graduate | 27.3 % post college graduate | ||
| 6.0 % prefer not to answer or no response | 5.5 % prefer not to answer | ||
| Number of children | 47.8% first child | 45.5% first child | p = 0.645 c |
| 52.2% at least one other child | 54.5% at least one other child | ||
| Prenatal care onset | 94 % first trimester | 93 % first trimester | p = 0.138 c |
| Insurance | 88.1 % private | 89.1% private | p = 0.766 c |
| 9.0 % Medicaid/Medicare | 5.5 % Medicaid/Medicare | ||
| 0 % self pay | 1.8 % self pay | ||
| 3% no response | 3.6 % no response | ||
(Wilcoxon rank sum)
(ttest)
(Fisher’s exact)
Inclusion criteria required the parent to be at least 18 years old with a full term (gestational age ≥ 36 weeks) infant less than 30 days old and parental proficiency in the English language (assessed by one of three study team members at time of informed consent).
Eligible and consented parents completed the Parent Attitudes about Childhood Vaccines (PACV) survey to stratify them according to their acceptance of childhood immunizations, or level of “vaccine hesitancy”. The PACV is a 15 question survey developed and validated by Opel, et al. to identify vaccine-hesitant parents.19 Scored from 0 to 100, higher scores on the PACV correspond to increasing vaccine hesitancy. Parents who scored 25 or more on the PACV survey were enrolled. While the original PACV validation study suggested a cut-point of 50 or greater was associated with underimmunization, that study was validated by mailing the instrument to parents of children aged 19 to 35 months in Washington state. We were administering the PACV in a new geographical area to parents of newborn infants, and a priori chose a lower PACV score for study enrollment to ensure that our study captured all potential vaccine-hesitant parents. Parents were asked to complete demographic information regarding the following variables: parental age, number of children in home, household income, race/ethnicity, trimester at onset of prenatal care, insurance status (private, public, self-pay), and level of education. Parents were also asked to identify where they obtained trusted vaccine information (physician, friend, family, news/media, and/or the internet). Parents enrolled at the intervention site were provided with the educational intervention, while parents at the control site received routine care.
The educational intervention included three components: 1) an 8 minute video developed by the Vanderbilt Vaccine Research Program, 2) an educational handout on common vaccine concerns developed by the Vaccine Education Center at the Children’s Hospital of Philadelphia,20 and 3) a handout with written instructions on how to find accurate medical information on the internet. The interventional video included the most commonly described concerns of vaccine-hesitant parents, including: fears about the effect on the child’s immunity, concerns that the vaccine preventable diseases were not serious, poor understanding of the risk of disease, poor understanding of research on vaccine safety testing prior to licensure, and the risk of chronic diseases (autism) resulting from vaccines. 21–25 The video also included three parental accounts of children contracting vaccine preventable illnesses provided by the Parents of Kids with Infectious Diseases organization.26 The video script was developed by experts in vaccine safety and behavioral health and piloted with vaccine-hesitant parents prior to finalizing the content for filming. The content was constructed using the Health Belief Model (HBM) theory of behavioral change.27 Constructs of HBM including “perceived susceptibility” to getting a disease, “perceived severity” of the disease, “perceived barriers” to vaccination, and “perceived benefits” of vaccination guided the inclusion or deletion of content. For example, a reference to a 2008 outbreak of a vaccine preventable disease was determined to map onto the “perceived susceptibility” construct and was included in the video. Providers at both sites were given no instructions or interventions. The video is now available on the Vanderbilt Vaccine Research Program website (www.vvrp.info/).
Intervention subjects were given the two informational handouts with a brief explanation of what each handout is addressing and viewed the 8 minute educational video in the private examination room using a portable computer. Parents who were unable to view the video in the examination room were emailed the video to view at home.
The intervention group completed the PACV again immediately after watching the educational video to ascertain any immediate change in attitude following the video. Both groups completed follow up PACV surveys at the 2 month well child visit to measure change in parental attitude. The medical records of all enrolled subjects were reviewed after the infants reached 12 weeks of age. Subjects receiving all the recommended 2 month vaccinations between 6 weeks and 12 weeks of chronological age were considered on-time. If any of the vaccines recommended at the 2 month well visit had not been administered by 12 weeks of age, the enrolled subject was considered delayed. The recommended 2 month vaccines included; diphtheria, tetanus, and acellular pertussis, inactivated polio, hepatitis B, rotavirus, 13 valent pneumococcal conjugate, and haemophilus influenzae type b vaccines. Study data were collected and managed using REDCap electronic data capture tools.28
Data analysis
All analyses were conducted using Stata version 12. We compared demographic variables and baseline screening scores between the intervention and control groups using Wilcoxon rank-sum, t-test or Fisher’s exact test as appropriate. (Table 1) For our primary outcome, we analyzed the difference in PACV scores from the 2 week visit and the 2 month visit between the two study arms using nonparametric methods for non-normal distribution. We used the Wilcoxon rank-sum test to compare crude differences in the sum of the score ranks between groups. For multivariate analysis, we evaluated the few extreme values in PACV score differences and confirmed they were not data entry error, and therefore had no compelling reason to exclude them from analysis. Therefore, robust regression was chosen for multivariate analysis in order to include all data points and appropriately weigh outliers. We adjusted for baseline PACV score (continuous variable), income (categorical variable), and race/ethnicity (categorical variable) using robust regression. These variables were selected a priori for adjustment as the most meaningful covariates in vaccine-hesitant populations. 29,21,30,31
Although our study was not powered to detect a difference in on-time receipt of all recommended 2 month childhood vaccines between the two groups, we analyzed our results to assess for a potential trend as our secondary outcome. We used Pearson’s chi-squared test to compare the crude proportion of enrollees who did not receive all recommended 2 month vaccines by 12 weeks of age between the intervention and control groups. We then performed multivariate analysis to assess this outcome with logistic regression adjusting for baseline score, income and race/ethnicity.
Our sample size was calculated based on our primary study outcome using Dupont PS software. Using a standard deviation of 16 points on the 100 point scale, a sample size of 41 subjects per arm provided 80% power to detect a 10 point difference on the 100 point PACV scale between groups with a type 1 error of 0.05. Assuming a 5% loss to follow-up, we planned to enroll at least 43 subjects per arm.
The Vanderbilt Institutional Review Board reviewed and approved our study. Informed consent was obtained prior to screening for enrollment for all subjects. Subjects were provided with 70 free disposable diapers for participation in our study.
Results
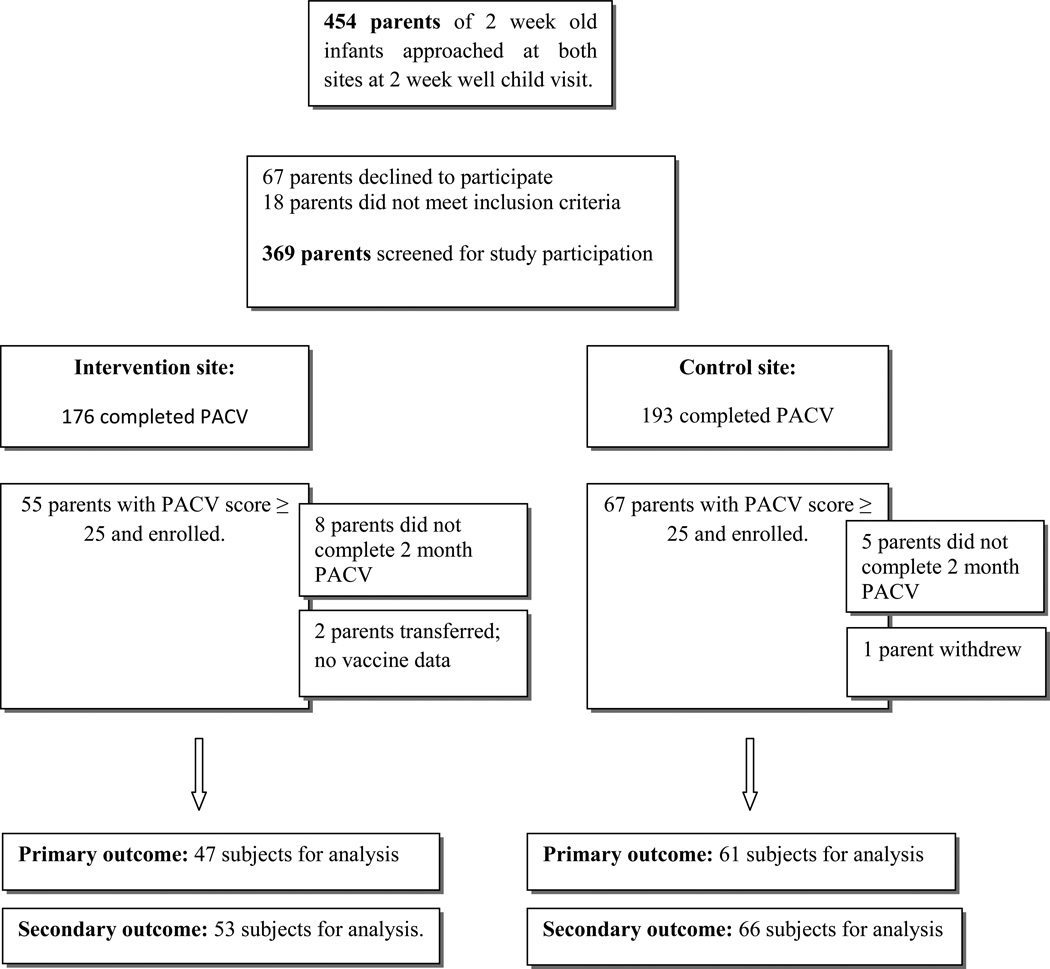
From May 2011 through September 2012, 454 parents of 2 week old infants were approached. Of these, 369 (81.3%) completed the PACV survey (67 parents declined to participate and 18 parents did not meet inclusion criteria). A total of 122 participants had PACV scores of ≥ 25 and were enrolled; 67 at the control site and 55 at the intervention site. (Figure 1) Table 1 displays the demographics of enrolled subjects in each group. There was no significant difference in median baseline scores between groups [control group = 36.63 (IQR: 29.97–53.28); intervention group = 39.96 (IQR: 33.30–53.28)]. There were no significant differences between groups regarding parental age, gender, education, number of children, prenatal care onset, race/ethnicity and insurance status. There was a significant difference between groups in reported household income.
Figure 1.
Enrollment Flowchart
For our primary outcome, 108 of the total 122 enrollees (88.5%) completed the 2 month PACV survey. (Figure 1) Although both groups improved in PACV score, we found a crude statistically significant improvement in PACV score among enrollees at the intervention site compared with the control site [median difference of 6.7 points less vaccine-hesitant PACV score compared to control (IQR: −13.3 to 33.3); p = 0.049]. The mean difference between intervention and control sites was also significantly different after adjusting for baseline PACV score, household income and race/ethnicity using robust regression [mean difference of 5.2 points less vaccine-hesitant (95% CI: 0.13–10.34); p = 0.044].
Within the intervention group, the median PACV score did not change from when it was assessed post intervention at the 2 week visit [median PACV score = 30 (IQR 6.7–63.3)] and when it was repeated at the two month visit [median PACV score = 30 (IQR 20–66.6)]. Among participants with complete PACV surveys at 2 months, the difference between the mean PACV screening score at 2 weeks [41.3 (95% CI: 37.4–45.3)] and 2 months [34.5 (95% CI: 29.7–39.2)] for the control group was 6.8 points, while the difference between the mean PACV screening score at 2 weeks [43.7 (95% CI: 39.7–47.7)] and 2 months [32.9 (95% CI: 27.3–38.6)] for the intervention group was 10.8 points.
Thirteen parents in the intervention group (24%) viewed the educational video at home after the clinic visit and completed the 2 month PACV survey. There was no difference in the PACV survey score difference from 2 weeks to 2 months between parents who viewed the video at home or in the clinic [mean PACV score difference for parents viewing at home = 9.99 (95% CI: 0.99 – 18.99); mean PACV score difference for parents viewing in clinic = 11.07 (95% CI: 7.5–14.6); p=0.78 (Student’s t-test)].
For our secondary outcome, we had complete 2 month vaccine data for 119 enrollees (97.5%) and found no significant difference in on-time completion of all recommended 2 month vaccines by 12 weeks between the intervention and control groups (p=0.864). Approximately 80% of enrolled infants in each group received all recommended 2 month vaccines, while approximately 8–10% of infants in each group received none of the recommended vaccines. (Table 2)
Table 2.
On-time receipt of all recommended two month vaccines by site
| Control (N=66) |
Intervention (N=53) |
|
|---|---|---|
| Any two month vaccine not received by 12 weeks | 12 (18.2%) | 9 (17.0%) |
| OR | ref |
0.92 (95% CI: 0.36–2.38) |
| aOR | ref |
0.71 (95% CI: 0.24–2.05) |
| No vaccines by 12 weeks | 5 (7.6%) | 5 (9.4%) |
| OR | ref |
1.27 (95% CI: 0.35–4.64) |
| aOR | ref |
1.28 (95% CI: 0.32–5.14) |
OR: Odds Ratio
aOR: Adjustment for screening PACV score, household income, and ethnicity
Most parents (86.9%) identified the healthcare provider as their source for trustworthy vaccine information. Parents also identified the internet (39.3%), friends (26.2%), family (25.4%) and news or media (13.9%) as sources for trustworthy vaccine information. We conducted a post hoc analysis to assess whether identifying the internet as a source of trusted vaccine information was associated with not being on-time with all recommended 2 month vaccines and found no association [p=0.977 (Pearson’s chi-squared)].
Discussion
Efforts to provide accurate childhood vaccine information in an efficient and convenient manner are needed.24 A recent study in Oregon found a near four-fold increase (from 2.5% to 9.5% between 2006 and 2009) in the proportion of parents utilizing alternative immunization schedules.2 As the rate of vaccine hesitancy increases, providers are challenged to address the many complex issues related to vaccine safety in the time-limited well child visit.18 In 2011, Kempe, et al reported that 53% of physicians spend 10–19 minutes, and 8% spend greater than or equal to 20 minutes discussing vaccines with parents of infants who have significant concerns. Pediatricians were also significantly more likely to report their job as less satisfying because of parental vaccine concerns.18 Our study demonstrated that a brief educational intervention provided in the clinical setting improved parental attitudes about childhood vaccines when compared with a control group and suggests that further studies should be conducted to evaluate whether clinic based educational programs can offset some or all of the time spent by healthcare providers on vaccine topics.
A multifaceted approach is needed to provide accurate information to parents who are concerned about the inherent safety and necessity of vaccines.32 Local population-based strategies are currently being developed and tested in Washington,33 direct provider-to-patient communication strategies are being evaluated,34 and groups are assessing the effectiveness of social media interfaces to address questions from vaccine-hesitant parents. We believe that our intervention provides another critical arm of this multifaceted approach in that it addresses several aspects identified by providers and patients as important or challenging in this effort. These include: 1) it can be implemented without additional provider time, 2) the tool can address concerns prior to the first visit requiring vaccines, and 3) the tool can be used in the clinic under the supervision of the healthcare provider, who parents report as the most trustworthy information source.
Our study did not show a statistical improvement in our secondary outcome of vaccine uptake at 12 weeks. We limited our immunization time point to two month immunizations rather than incorporating more doses over a longer time period for two primary reasons: 1) our pilot study was not powered on this outcome, and 2) given the lack of statistical power, we believed any effect from our small study would be seen by examining the uptake of vaccines provided in close proximity to the intervention. Because the rates of on-time vaccination are high, even in populations identified as vaccine-hesitant, larger sample sizes are needed to detect a statistical difference in vaccine uptake for studies assessing immunization behavior. A larger trial powered to detect a difference in vaccine uptake between groups should be considered which could also ascertain whether the improvement in parental attitude corresponds to ultimate immunization behavior. Further, it may be that participants who did not complete the recommended vaccines for their infants held strong beliefs prior to enrollment that could not be swayed by additional information.
Although it is uncertain whether a 6.7 point difference in PACV survey scores between groups corresponds to a clinical impact of actual vaccine uptake, the PACV validation data suggests that decreasing PACV scores corresponds significantly to fewer days of underimmunization.35 The results of our intervention are strengthened by our finding that the improvement in PACV scores in the intervention arm immediately following the intervention was stable through the 2 month visit. Future studies could also evaluate the quantitative impact of implementing a similar intervention on the average time spent on well child visits with vaccine-hesitant parents.
Our study has several limitations. First, our control group could have been influenced to ask additional vaccine related questions due to exposure to the PACV survey, thus increasing their knowledge and potentially impacting their vaccination decisions (i.e., Hawthorne effect). However, we would expect this to reduce the effect of our intervention. Additionally, parents may have reported less vaccine-hesitant responses given that the surveys were conducted in person (social desirability bias). Further, we cannot account for differences in vaccine education that enrollees received from different providers at the two different sites. By providing no specific intervention or education to one study site, we hoped to minimize the bias of intervention site providers having gained more knowledge regarding the specific concerns of vaccine-hesitant parents and thereafter being more inclined to thoroughly address these concerns. We collected information on multiple demographic items to assess for any differences between groups and found that in general enrollees at both sites were similar except that there was a significant difference in the reported household income between the two sites. However, our results remained significant after adjusting for income in our regression analysis.
Because we enrolled parents with PACV scores of 25 or greater, and the previously validated PACV survey found parents who scored 50 or greater were significantly more underimmunized, inclusion of parents with lower PACV scores could have resulted in a dilution of the effect of our intervention.35 Our study population is not inclusive of the general U.S. population; therefore, future studies should be extended to broader socioeconomic populations. We did not anticipate that parents would choose to watch the educational video at home rather than in clinic, and therefore we did not have a method for ensuring the fidelity of our intervention in this instance. Our future efforts will offer this option to parents in addition to in-clinic educational opportunities and we will monitor interventional fidelity through the requirement of unique study identification numbers for electronic tool (video) access and a priori analytic process measures.
Although we intervened in private examination rooms, parents in the intervention arm could potentially have shared knowledge with enrollees in the control group. We therefore chose to use physically separate clinic sites for the intervention and control groups, rather than to randomize patients at the individual level. An optimal design for a future study might include several sites for a multi-site cluster randomization trial.
Conclusion
With minimal time investment by pediatricians, we found that an educational intervention, including an 8 minute video and written information tailored to the most common concerns of vaccine-hesitant parents and applied in the clinical setting, could improve attitudes regarding childhood vaccines among vaccine-hesitant parents. Further research is needed to identify optimal strategies for transmitting accurate vaccine information to concerned parents in an effective and efficient manner with the goal to modify parental attitudes about vaccines and enhance vaccine acceptance.
What's New.
Vaccine-hesitant parents are more likely to delay or refuse recommended childhood vaccinations. There are limited data on strategies to improve parental attitudes or vaccine uptake in this population. This study suggests that a brief educational intervention can be beneficial.
Acknowledgments
Funding: This project was supported through funding from the Vanderbilt Institute for Clinical and Translational Research grant support (UL1TR000011 from NCATS/NIH) and NRSA 5T32 HS 013833-09 (Agency AHRQ).
Abbreviations
- PACV
Parent Attitudes about Childhood Vaccines
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures:
SEW, MAS, RLR, PAO, KME: none.
WS: Dr. Schaffner has been a member of Data Safety Monitoring Boards for Sanofi-Pasteur and Merck within the past year; this work is now concluded. He is also an occasional consultant for GSK, Dynavax and Pfizer.
Conflicts of interest: none
References
- 1.Omer SB, Richards JL, Ward M, Bednarczyk RA. Vaccination policies and rates of exemption from immunization, 2005-2011. N Engl J Med. 2012 Sep 20;367(12):1170–1171. doi: 10.1056/NEJMc1209037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robison SG, Groom H, Young C. Frequency of alternative immunization schedule use in a metropolitan area. Pediatrics. 2012 Jul;130(1):32–38. doi: 10.1542/peds.2011-3154. [DOI] [PubMed] [Google Scholar]
- 3.Gilkey MB, McRee AL, Brewer NT. Forgone vaccination during childhood and adolescence: Findings of a statewide survey of parents. Prev Med. 2013 Jan 4; doi: 10.1016/j.ypmed.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glanz JM, Newcomer SR, Narwaney KJ, et al. A Population-Based Cohort Study of Undervaccination in 8 Managed Care Organizations Across the United States. JAMA pediatrics. 2013 Jan 21;:1–8. doi: 10.1001/jamapediatrics.2013.502. [DOI] [PubMed] [Google Scholar]
- 5.Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med. 2009 May 7;360(19):1981–1988. doi: 10.1056/NEJMsa0806477. [DOI] [PubMed] [Google Scholar]
- 6.Chen SY, Anderson S, Kutty PK, et al. Health care-associated measles outbreak in the United States after an importation: challenges and economic impact. J Infect Dis. 2011 Jun 1;203(11):1517–1525. doi: 10.1093/infdis/jir115. [DOI] [PubMed] [Google Scholar]
- 7.Imported measles case associated with nonmedical vaccine exemption--Iowa, March 2004. MMWR Morb Mortal Wkly Rep. 2004 Mar 26;53(11):244–246. [PubMed] [Google Scholar]
- 8.Measles - United States, 2011. MMWR Morb Mortal Wkly Rep. 2012 Apr 20;61:253–257. [PubMed] [Google Scholar]
- 9.Sugerman DE, Barskey AE, Delea MG, et al. Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics. 2010 Apr;125(4):747–755. doi: 10.1542/peds.2009-1653. [DOI] [PubMed] [Google Scholar]
- 10.Leuridan E, Sabbe M, Van Damme P. Measles outbreak in Europe: Susceptibility of infants too young to be immunized. Vaccine. 2012 Sep 7;30(41):5905–5913. doi: 10.1016/j.vaccine.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Glanz JM, McClure DL, Magid DJ, et al. Parental refusal of pertussis vaccination is associated with an increased risk of pertussis infection in children. Pediatrics. 2009 Jun;123(6):1446–1451. doi: 10.1542/peds.2008-2150. [DOI] [PubMed] [Google Scholar]
- 12.Glanz JM, McClure DL, Magid DJ, Daley MF, France EK, Hambidge SJ. Parental refusal of varicella vaccination and the associated risk of varicella infection in children. Arch Pediatr Adolesc Med. 2010 Jan;164(1):66–70. doi: 10.1001/archpediatrics.2009.244. [DOI] [PubMed] [Google Scholar]
- 13.Glanz JM, McClure DL, O'Leary ST, et al. Parental decline of pneumococcal vaccination and risk of pneumococcal related disease in children. Vaccine. 2011 Jan 29;29(5):994–999. doi: 10.1016/j.vaccine.2010.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Academy of Pediatrics. Immunization/Families. [Accessed March 1, 2013]. http://www2.aap.org/immunization/families/families.html. [Google Scholar]
- 15.CDC. [Accessed March 1, 2013];Vaccine Safety. http://www.cdc.gov/vaccinesafety/index.html.
- 16.Dempsey AF, Schaffer S, Singer D, Butchart A, Davis M, Freed GL. Alternative vaccination schedule preferences among parents of young children. Pediatrics. 2011 Nov;128(5):848–856. doi: 10.1542/peds.2011-0400. [DOI] [PubMed] [Google Scholar]
- 17.Klein NP, Kissner J, Aguirre A, et al. Differential maternal responses to a newly developed vaccine information pamphlet. Vaccine. 2009 Dec 11;28(2):323–328. doi: 10.1016/j.vaccine.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 18.Kempe A, Daley MF, McCauley MM, et al. Prevalence of parental concerns about childhood vaccines: the experience of primary care physicians. Am J Prev Med. 2011 May;40(5):548–555. doi: 10.1016/j.amepre.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 19.Opel DJ, Mangione-Smith R, Taylor JA, et al. Development of a survey to identify vaccinehesitant parents: The parent attitudes about childhood vaccines survey. Hum Vaccin. 2011 Apr 1;7(4) doi: 10.4161/hv.7.4.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chidren's Hospital of Philadelphia. Vaccine Education Center. 2010. [Accessed October 21, 2010]. http://www.chop.edu/service/vaccine-education-center/home.html. [Google Scholar]
- 21.Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Parental vaccine safety concerns in 2009. Pediatrics. 2010 Apr;125(4):654–659. doi: 10.1542/peds.2009-1962. [DOI] [PubMed] [Google Scholar]
- 22.Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics. 2008 Oct;122(4):718–725. doi: 10.1542/peds.2007-0538. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee A, O'Keefe C. Current controversies in the USA regarding vaccine safety. Expert Rev Vaccines. 2010 May;9(5):497–502. doi: 10.1586/erv.10.36. [DOI] [PubMed] [Google Scholar]
- 24.Gust DA, Kennedy A, Weber D, Evans G, Kong Y, Salmon D. Parents questioning immunization: evaluation of an intervention. Am J Health Behav. 2009 May-Jun;33(3):287–298. doi: 10.5993/ajhb.33.3.7. [DOI] [PubMed] [Google Scholar]
- 25.Plotkin SA, Orenstein WA, Offit PA. In: Vaccines. 6th ed. Plotkin SA, Orenstein WA, Offit PA, editors. Saunders Elsevier; 2008. [Google Scholar]
- 26.Parents of Kids with Infectious Diseases. [Accessed 10/4/2012]. http://www.pkids.org/ [Google Scholar]
- 27.Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education. 4th ed. San Francisco: John Wiley and Sons, Inc; 2008. [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy AM, Gust DA. Measles outbreak associated with a church congregation: a study of immunization attitudes of congregation members. Public Health Rep. 2008 Mar-Apr;123(2):126–134. doi: 10.1177/003335490812300205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gust DA, Strine TW, Maurice E, et al. Underimmunization among children: effects of vaccine safety concerns on immunization status. Pediatrics. 2004 Jul;114(1):e16–e22. doi: 10.1542/peds.114.1.e16. [DOI] [PubMed] [Google Scholar]
- 31.Smith PJ, Humiston SG, Parnell T, Vannice KS, Salmon DA. The association between intentional delay of vaccine administration and timely childhood vaccination coverage. Public Health Rep. 2010 Jul-Aug;125(4):534–541. doi: 10.1177/003335491012500408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zacharyczuk C. Multifaceted approach advocated for vaccine-hesitant parents. Infectious Diseases in Children. 2011 [Google Scholar]
- 33.Opel DJ, Diekema DS, Lee NR, Marcuse EK. Social marketing as a strategy to increase immunization rates. Arch Pediatr Adolesc Med. 2009 May;163(5):432–437. doi: 10.1001/archpediatrics.2009.42. [DOI] [PubMed] [Google Scholar]
- 34.Opel DJ, Robinson JD, Heritage J, Korfiatis C, Taylor JA, Mangione-Smith R. Characterizing providers' immunization communication practices during health supervision visits with vaccine-hesitant parents: a pilot study. Vaccine. 2012 Feb 8;30(7):1269–1275. doi: 10.1016/j.vaccine.2011.12.129. [DOI] [PubMed] [Google Scholar]
- 35.Opel DJ, Taylor JA, Mangione-Smith R, et al. Validity and reliability of a survey to identify vaccine-hesitant parents. Vaccine. 2011 Sep 2;29(38):6598–6605. doi: 10.1016/j.vaccine.2011.06.115. [DOI] [PubMed] [Google Scholar]