Abstract
Recent investigations into the neural basis of elite sporting performance have focused on whether cortical activity might characterize individual differences in ability. However, very little is understood about how changes in brain structure might contribute to individual differences in expert motor control. We compared the behavior and brain structure of healthy controls with a group of karate black belts, an expert group who are able to perform rapid, complex movements that require years of training. Using 3D motion tracking, we investigated whether the ability to control ballistic arm movements was associated with differences in white matter microstructure. We found that karate experts are better able than novices to coordinate the timing of inter-segmental joint velocities. Diffusion tensor imaging revealed significant differences between the groups in the microstructure of white matter in the superior cerebellar peduncles (SCPs) and primary motor cortex—brain regions that are critical to the voluntary control of movement. Motor coordination, the amount of experience, and the age at which training began were all associated with individual differences in white matter integrity in the cerebellum within the karate groups. These findings suggest a role for the white matter pathways of the SCPs in motor expertise.
Keywords: cerebellum, diffusion tensor imaging, expertise, individual differences, motor control
Introduction
Professional athletes consistently perform with a level of skill that novices are unable to replicate, and that can only be obtained through thousands of hours of practice over many years (Ericsson et al. 1993). Although there has been a great deal of research exploring expert behavior (Ericsson 2006), the neural mechanisms that characterize elite sporting performance remain poorly understood (Yarrow et al. 2009). Recent investigations into the brain basis of expertise have revealed discipline-specific functional specializations, such as asymmetries in the cortical motor maps of elite racquet players between their playing and non-playing hand (Pearce et al. 2000), increased motor-evoked potentials in basketball players when asked to observe other players (Aglioti et al. 2008), and encoding of motor skills in the functional organization of the primary motor cortex and corticomuscular system of musicians (Gentner et al. 2010).
Using neuroimaging studies, a number of groups have also examined whether musical expertise is associated with changes in brain structure, and have reported volumetric differences in gray matter (Sluming et al. 2002; Gaser and Schlaug 2003) and differences in the microstructure of white matter in the brains of musicians compared with novices (Schmithorst and Wilke 2002; Bengtsson et al. 2005; Imfeld et al. 2009; Abdul-Kareem et al. 2011). These findings are consistently associated with the level of ability the individuals have attained, and may provide some explanation as to why novices can execute qualitatively similar movements to experts, but are incapable of achieving a comparable degree of control with any consistency.
It is thought that through the process of learning a skill, the associated patterns of brain activity also adapt as performance improves (Raichle et al. 1994; Sakai et al. 1998; Kelly et al. 2006). Recent investigations have demonstrated that not only brain activity, but also gray and white matter structures alter as a consequence of motor learning (Draganski et al. 2004; Scholz et al. 2009). Therefore, the structural brain changes observed in expert groups might reflect the effects of long-term learning (Maguire et al. 2000; Bengtsson et al. 2005). As expert groups demonstrate optimal behavior on specific tasks, differences in white matter structure relative to novices might reflect a “fine-tuning” of the connectivity between specific brain regions and hence provide further explanation as to their function. Although a small number of studies have documented functional differences between controls and athletes, there has been no report of a professional sporting group where measures of elite performance have been associated with structural differences in the white matter of the brain.
We chose to investigate karate experts as their ability to generate extremely high impact forces is not replicable by novices, and the mechanism by which they achieve this feat is still not fully understood. Early studies found that although karate experts were able to generate higher impact forces than controls, isometric muscle force and velocity measurements of individual joints were not significantly different to controls (Vos and Binkhorst 1966). Recent studies report that karate experts demonstrated higher peak acceleration in ballistic elbow extensions, but this was not related to activity in the biceps or triceps as measured by electromyography (Zehr et al. 1997). Further research demonstrated that these individuals are also better able to maintain body stability by reducing the amount of backward displacement during punching to produce higher impact forces (Cesari and Bertucco 2008). These studies provide some insight into why karate experts are able to generate high impact forces, but the mechanism by which they achieve these higher accelerations and impact forces remains unclear.
The aim of the present study was first to identify a behavioral measure that distinguished karate experts and novices on a simple punching task, and then examine the relationship between expert performance and the structure of white matter in the brain. Karate punches are interesting because they represent a rapid, ballistic movement, where performance is not significantly determined by muscular strength (Voigt and Klausen 1990). Therefore, our hypothesis was that a non-musculoskeletal factor such as the timing and coordination of arm movements might be predictive of optimal performance. Specifically, we were interested in whether the relative timing of different joint velocities might differ in expert individuals.
Although there is evidence to suggest that even rapid movements can be updated online through sensory feedback (Desmurget and Grafton 2000), it is thought that the control of ballistic movements is primarily governed by a predictive motor plan, known as a forward model (Miall and Wolpert 1996). Therefore, areas of the motor system that are involved in the coordination and timing of joint movements, or maintaining an intact representation of a sequence of actions may play an important role in determining the accuracy with which an individual can make fast arm movements. Lesion studies have demonstrated that the cerebellum is critical to the smooth control of movements, and it is also thought to contribute significantly to the generation of forward models (Thach 1998; Kawato et al. 2003). Cortical regions implicated in the control of voluntary movement include the supplementary motor complex, which comprises supplementary and pre-supplementary motor areas (SMA; preSMA) and has also been implicated in sequence learning (Nachev et al. 2008), and primary motor cortex (M1) that has been closely associated with motor skill acquisition (Li et al. 2001; Muellbacher et al. 2002). Since a karate punch could be considered to be a short-range reaching task, it is reasonable to assume that parietal cortex (PC) might also play a role in such actions as this area is associated with the visuo-motor coordination and estimation of internal states (Wolpert et al. 1998; Desmurget et al. 1999). We were interested in examining whether the white matter structure adjacent to these areas was associated with task performance, or differed between experts and controls. A positive finding would indicate that the contribution of given region might play an important role in expert motor control.
Recent neuroimaging studies into the effect that training on a visuo-motor coordination task has on brain structure report significant changes to the structure of both gray and white matter after only a matter of weeks. These alterations also resemble the progression of activation changes found in functional magnetic resonance imaging (fMRI) studies of motor skill acquisition (Duerden and Laverdure-Dupont 2008; Ilg et al. 2008). However, it should be noted that brain changes that are the result of many years of training might not necessarily follow the same pattern as short-term adaptations.
To investigate the effect that long-term learning has on the microstructure of white matter in the brain, we developed a novel behavioral task to capture the key features of a karate punch. We then used diffusion tensor imaging (DTI) to infer properties of the underlying microstructure in the white matter of the brain (Basser et al. 1994). DTI measures the passive self-diffusion of water molecules and can be used to calculate the level of fractional anisotropy (FA) in a voxel. In white matter, this value is thought to be influenced by a number of factors including the level of myelination, axonal packing density, and properties of the cell membrane (Beaulieu 2002). Differences in the level of FA adjacent to a particular brain area may also reflect changes in the functional connectivity of a white matter pathway (Boorman et al. 2007).
Previous studies have demonstrated that karate experts are able to generate higher punching forces, velocities, impulse and accelerations than controls. As karate training focuses on learning specific movements to generate higher impact forces rather than relying on muscle mass, we hypothesized that karate experts would demonstrate a significantly higher degree of limb and upper body coordination than controls. Specifically, the relative timing of limb movements would determine the force generated at impact. Based on the findings of previous studies of expertise, we predicted that individual differences in expert behavior would be associated with significantly higher indices of white matter integrity adjacent to motor brain regions, primarily: cerebellum, primary motor cortex, and corticospinal tract. In addition, we hypothesized that the variability of FA in these regions would be positively correlated with different levels of elite performance. We also selected additional regions of interest (ROIs) to examine whether the effect of long-term learning had a more widespread impact on white matter microstructure adjacent to regions often activated in motor learning tasks: preSMA, SMA, and PC.
Materials and Methods
Written informed consent was obtained from a group of 12 male karate experts of at least the level of first Dan Blackbelt with a mean = 13.8 years (standard error, SE = 1.7) training, and from 12 age-matched male controls. The controls exercised regularly, at least once a week, but had no previous experience of karate training or martial arts, and were not experts in any other discipline. All participants were right-handed as determined by the Edinburgh Handedness Inventory (Oldfield 1971). The mean score was 80.4 (SE = 5.6) for controls and 86.6 (SE = 5.9) for experts. The groups were matched for age (experts, mean = 32.7, SE = 2.2; controls, mean = 30.3, SE = 1.2) and body-mass index (BMI; experts, mean = 24.4, SE = 0.98; controls, mean = 24.3, SE = 0.91). The study was approved by the Hammersmith local research ethics committee and all participants gave written informed consent to participant in the study.
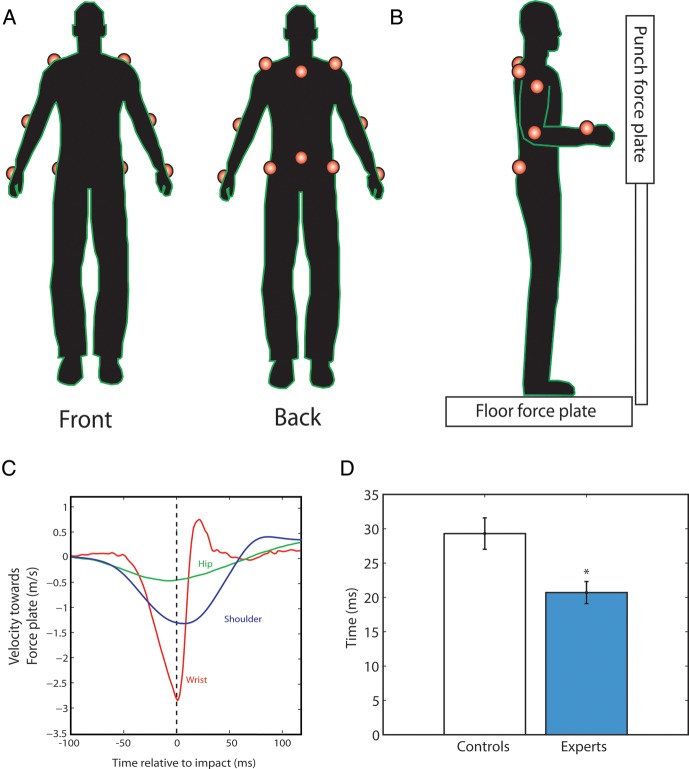
To record the movement of the participants during the experiment, infrared markers were attached in 12 locations on the torso and arms (Fig. 1). These were sampled at 400 Hz using 3 Codamotion mpx30 cameras (Charnwood Dynamics, Leicestershire, UK). Participants stood in bare feet on the center of a force plate (Kistler type 9281B; Kistler Instrumente AG, CH-8408 Winterthur, Switzerland), with their feet 10 cm apart. In some of the experiments, participants were also required to punch a vertical force plate (Kistler type 9286A; Kistler Instrumente AG), padded with an 18-mm thick layer of high-density foam in order to prevent injuries. The data from the force plates were collected at a 1-kHz sampling rate. The experimental apparatus was designed in order to minimize technique-dependent performance differences between the 2 groups. Our aim was to design a task that captured key features of a karate punch, but which both experts and controls could perform with minimal learning. It became apparent during piloting that the task had to be sufficiently constrained so that it could be easily performed by the controls, and also to protect the equipment from the karate experts.
Figure 1.
Behavioral experimental apparatus and results. (A) To record the movement of the participants during the experiment, infrared markers were placed on the radius and ulna bones of the wrist, on the elbow and the shoulder of each arm, (B) on either side of the hips (the iliac crests), and along the midline at the level of lumbar vertebra L5 and cervical vertebra C7. The participants stood on a floor force plate and, in the punching tasks, struck a vertical force plate with their right hand. (C) Mean velocity profiles for the expert group, immediately before and after punching on the 5 cm task. (D) The temporal difference between the peak velocities of the wrist and shoulder of the right hand when punching from a distance of 5 cm was significantly smaller in the karate expert group (blue) and the controls (white).
In the first task, participants were instructed to punch the vertical force plate as hard as they could with their right hand from a distance of 5 cm. At the beginning of each trial, the right forearm of the subjects was held at right angles to the upper arm. A string was attached to the force plate and connected to the back of the right hand; withdrawal of the fist away from the plate caused the string to detach and signal that the punch was invalid. Each punch began at a distance of 5 cm between the knuckles and the force plate. The signal to punch was given by an electronic beep. Participants were instructed that this was not a reaction time task; the emphasis was on punching as hard as they could. Subjects performed 3 practice punches, and then 20 valid punches in total. In a control condition, the task was repeated but began with the fist just touching the foam on the force plate. This task was effectively a push, rather than a punch. In the third task, participants were asked to stand on the floor force plate with their feet together and stand motionless for 80 s. As a measure of postural stability, the average velocity traveled by the infrared marker located at the base of the spine (due to body sway) was calculated during 2 conditions: eyes open and eyes closed.
MRI Data Acquisition
DTI data were acquired using a 1.5-T Siemens Magnetom Vision system at Charing Cross Hospital, London. Four sets of whole-brain diffusion-weighted volumes were acquired (12 directions; b = 1000 s mm−2; 48 slices; voxel size 2 × 2 × 3 mm3; repetition time (TR) = 8.6 s; echo time (TE) = 94 ms), plus 4 volumes without diffusion weighting (b = 0 s mm−2). A T1-weighted anatomical image was also acquired using a MP-RAGE sequence (TR = 1160 ms; TE = 4.38 ms; flip angle = 15; voxel size 1 × 1 × 1 mm3).
Voxel-wise statistical analysis of the diffusion-weighted data using a region of interest approach was carried out using tract-based spatial statistics, part of FSL (Smith et al. 2004, 2006). FA images were created by fitting a tensor model to the raw diffusion data using FMRIB's diffusion toolbox, and then brain-extracted using Brain Extraction Tool. All subjects' FA data were then aligned into a common space using the nonlinear registration tool FNIRT. A mean FA image was created and thinned to generate a mean FA skeleton representing the centers of all tracts common to the group. Each subject's aligned FA data were then projected onto this skeleton, which was used as the basis for voxel-wise statistical analysis. At this stage, we also generated images of the radial diffusivity (DR) within each voxel. This was calculated by averaging the values for the secondary and tertiary diffusion directions.
First, we tested for differences in the FA of white matter between the 2 groups. ROIs were chosen based on regions previously associated with motor control and comprised: preSMA, SMA, primary motor cortex (M1), parietal cortex (PC), internal capsule (IC), inferior and superior cerebellar peduncles (ICPs; SCPs). To generate our white matter ROIs, the overlap between the FMRIB58 white matter skeleton with the John Hopkins University ICBM-91 white matter and the Juelich histological atlases included in the FSL software package was used (Eickhoff et al. 2005; Mori et al. 2005). To determine the location of the cortical ROIs, coordinates were taken from the literature based on activation studies. Three slices were taken anteriorly and posteriorly from the center, and the overlap between the white matter skeleton and the probabilistic histological atlas was then used as the ROI. The complete overlap with the white matter skeleton was used for the cerebellar ROIs. For the IC ROI, slices taken superiorly and inferiorly from the center, determined by the JHU ICBM-91 atlas. This approach ensured that the ROIs were of comparable size in order to make meaningful comparisons between regions, varying slightly due to anatomical differences (mean = 320 voxels, SE = 35; Fig. 2). The size of the ROIs was not normalized as the statistical method employed adjusts for additional comparisons due to any small differences in volume.
Figure 2.
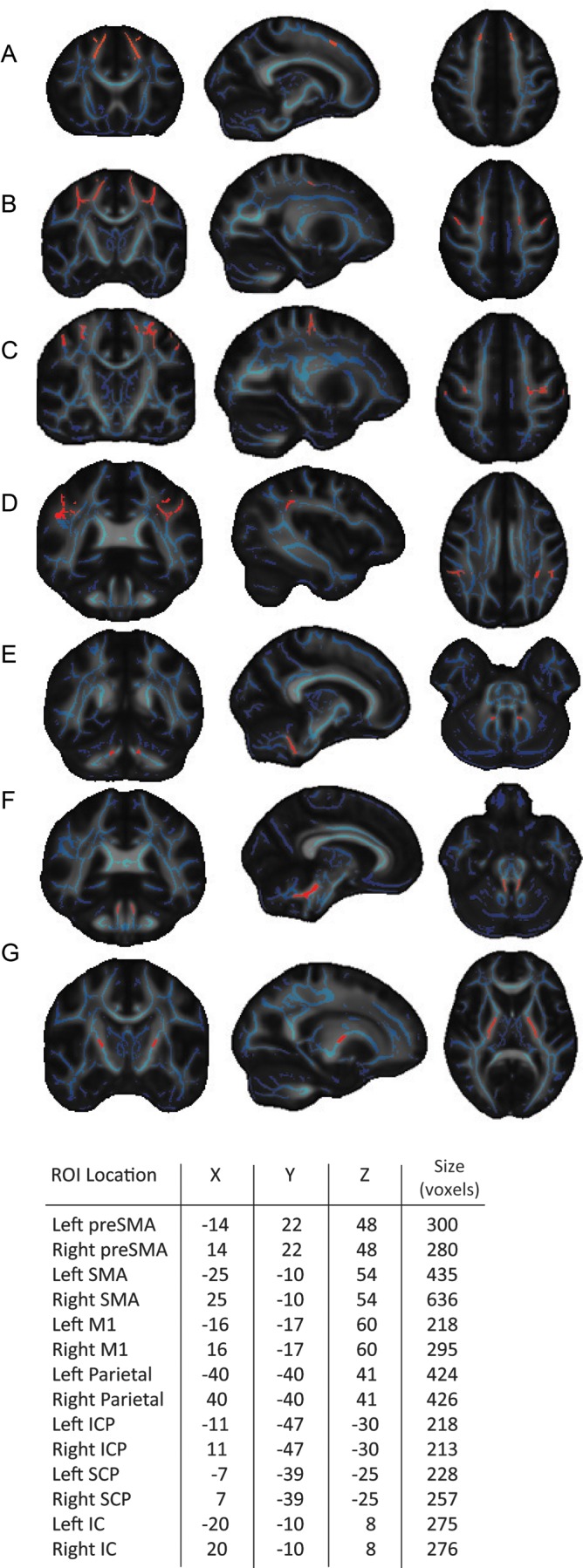
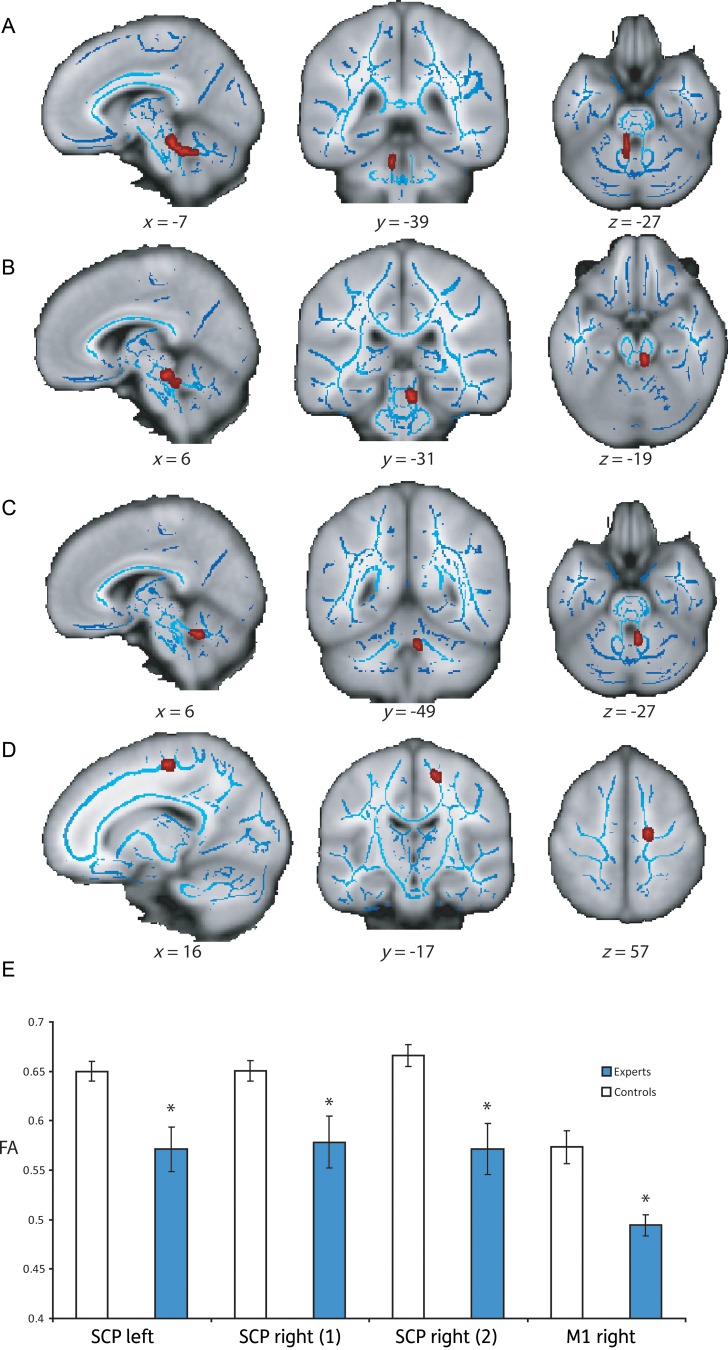
Regions of interest (ROIs) employed in the white matter comparison analysis. Coordinates for the center of mass and the size of ROIs (red) used in the comparison analysis overlaid on mean FA maps (grayscale) and group FA skeleton (blue), Talairach coordinates (mm). (A–D) Cortical ROIs: pre-supplementary motor area (preSMA), supplementary motor area (SMA), primary motor cortex (M1), and parietal cortex (PC). (E–G) Subcortical ROIs: inferior cerebellar peduncle (ICP), superior cerebellar peduncle (SCP), and internal capsule (IC).
The parietal ROIs were chosen based on the overlap between the left and right histological probability areas for the first and second anterior intraparietal sulcus hlPl maps and the white matter skeleton. The coordinates were taken from a study that used fMRI to identify brain activation during object manipulation (Binkofski et al. 1999). For the SMA ROI, the overlap between the white matter skeleton and the BA6 histological map was used in conjunction with peak activation coordinates from a study examining movement preparation in SMA (Lee et al. 1999). For the M1 ROI, the overlap between anterior and posterior BA4 histological maps and the white matter skeleton was used. The arm area of the primary motor cortex ROI was targeted using the coordinates taken from 2 functional activation studies that investigated the somatotopic representation of M1 (Lotze et al. 2000; Luft et al. 2002). The location of the ROI for the preSMA was based on coordinates taken from functional activations reported in a previous study (Nachev et al. 2005) and the anterior portion of the BA6 histological region. In addition a vertical line to the intercommissural line taken at the anterior commissure was used as an anatomical landmark.
A voxel-wise comparison analysis was performed to compare the level of FA and DR in each ROI between the expert and control groups (Experts > Controls; Controls > Experts). Results were considered significant for P < 0.05, corrected for multiple comparisons within each ROI using threshold-free cluster enhancement, an approach that avoids the choice of an arbitrary threshold for initial cluster formation (Smith and Nichols 2009). Regions where differences were detected between the 2 groups were used as a mask to perform a voxel-wise analysis to examine whether there was a correlation between the levels of FA and behavioral measures within the expert group. To determine the specificity of the areas where differences were found, we also conducted voxel-wise correlation analysis with the behavioral measures in the other ROIs, corrected for multiple comparisons. For illustrative purposes, the FSL program TBSS_fill was used to highlight the significant clusters. This creates a darker expanded outline around the cluster images (Fig. 3).
Figure 3.
Group differences in FA in the SCPs and M1. (A–D) The location of the clusters of voxels that had significantly different higher FA in the control group. The clusters (highlighted in red) are superimposed on a structural T1 image (gray), and the skeletonized FA image (blue), Talairach coordinates (mm). (D) Bar chart showing the mean level of FA in each cluster for the expert (blue) and control groups (white).
Results
Behavioral Analysis
In the first task, participants punched a force plate with their right hand from a distance of 5 cm with maximum force. The mean peak force was calculated for each subject, and then normalized using the BMI. Although the expert group was matched with physically active controls, this step ensured that comparisons could be made between the groups by controlling for body size and type. Comparisons of the behavioral performance between the groups were made using 2-tailed unpaired t-tests. The karate group generated a significantly higher mean peak force than the controls (0.077 kN kg−1 m2, SE = 0.0077; 0.051 kN kg−1 m2, SE = 0.007; t = 2.5, P < 0.05), and a significantly shorter rise time, that is, the time taken to reach peak force (4.72 ms, SE = 0.13; 6.21 ms, SE = 0.37; t = −3.8, P < 0.001). The relative velocities of the major joints (wrist, shoulder, and hip) involved in this right-handed punching movement were then examined. The progression of joint motions began with the hip velocity peaking first, followed by the wrist and shoulder velocities peaking close to impact (Fig. 1). The experts demonstrated significantly higher peak wrist velocities (mean = 2.87 ms−1, SE = 0.094; mean = 2.42 ms−1, SE = 0.11; t = 3.1, P < 0.01) and shoulder velocities (1.22 ms−1, SE = 0.0.78; 0.83 ms−1, SE = 0.11; t = 2.7, P < 0.01) than the controls, but showed no significant differences in terms of the mean peak velocity of the right hip prior to impact (t = 1.7, P > 0.09). The experts also showed significant higher peak acceleration of the wrist prior to impact than the controls (0.050 ms−2, SE = 0.002; 0.044 ms−2, SE = 0.0017; t = 2.1, P < 0.05). We also investigated the evidence of learning during the task, but found no significant differences between initial punch force and final punch force in the expert group (t = 1.87, P > 0.09; paired-samples t-test), or the control group (t = 1.14, P > 0.28).
As previous studies have reported superior postural control in martial artists (Perrot et al. 1998; Perrin et al. 2002), we examined whether the ground reaction forces during punching were predictive of ability on this task. In both the groups, the pattern of movement prior to punching involved a small reduction in ground force followed by a larger increase immediately before impact. This pattern was due to the participants initially crouching slightly followed immediately by an extension of the legs resulting in a negative-positive deflection in the ground reaction force. The expert group demonstrated a significantly higher initial reduction in ground reaction force prior to punching than controls (0.0031 kN kg−1 m2, SE = 0.51; 0.0012 kN kg−1 m2, SE = 0.36; t = 2.9, P < 0.01). However, there was no significant difference between the subsequent increase in ground force prior to punching (0.0081 kN kg−1 m2, SE = 1.7; 0.0046 kN kg−1 m2, SE = 0.97; t = 1.87, P > 0.07). We then analyzed whether the ground force minimum was predictive of task performance within the expert group. We found no significant correlations between ground force minimum and peak punch force from 5 cm (r = −0.10, P > 0.74), rise time (r = 0.12, P > 0.7), or peak acceleration (r = 0.12, P > 0.71). Neither was there a significant difference between the timing of the ground force minimum in the experts compared with the controls (152 ms, SE = 17.8; 135 ms, SE = 27.8; t = 0.65, P > 0.5). Based on these data, it appears that the ground reaction forces do not significantly predict performance on this task.
The higher peak accelerations and shorter rise times observed suggest “why” higher forces were measured in the expert group, but not how they were achieved. To investigate this question, we examined the “relative timing” of peak velocities of the right wrist, shoulder, and hip joints for each individual—a measure of motor coordination. The mean interval between peak wrist and shoulder velocities of the experts was significantly “shorter” than the controls (19.1 ms, SE = 1.6; 27.7 ms, SE = 2.3; t = 3.1, P < 0.01), but there was no significant difference between the timing of wrist–hip (t = 0.80, P > 0.43) or shoulder–hip (t = 1.58, P > 0.13) peak velocities between the groups. The size of the wrist–shoulder temporal interval was significantly negatively correlated with peak force and acceleration of the wrist (r = −0.45, P < 0.05; r = −0.42, P < 0.05), and significantly positively correlated with rise time (r = 0.65; P < 0.001). We also examined within-subject variability in the relative timing of wrist–shoulder peak velocities. This revealed no significant difference between the 2 groups (t = 1.84, P > 0.08), indicating that the experts and controls exhibited a similar level of “precision” in their movements. The significantly shorter interval between peak wrist and shoulder velocities suggests that the experts were more closely coordinated and accurate in their movements than controls. Thus, smaller temporal separations between peak velocities of the wrist and shoulder were associated with higher acceleration of the wrist, impact force, and increased transfer of momentum.
Control Tasks
In a control condition, the participants performed the same punching movement, but began with their fist lightly touching the foam on the force plate. No significant differences were found in mean peak force generated by the experts and the controls (mean = 2.69 kN kg−1 m2, SE = 0.38; mean = 2.2 kN kg−1 m2, SE = 0.30; t = 0.69, P > 0.5), but the experts were significantly quicker to reach peak velocity, that is shorter rise time (35.8 ms, SE = 5.13; 54.5 ms, SE = 7.4; t = 2.1, P < 0.05). This suggests that the higher forces generated by the karate experts in the first task were not due to differences in muscular strength between the 2 groups. The mean within-subject variability in punch force (standard deviation = 168.9 N) was low compared with the group variation (1210 N). This indicates that the control task was sufficiently sensitive to detect differences between individuals, while maintaining a high degree of reliability for repeated measurements.
A further control task was employed to establish whether karate expertise would generalize to the control of other movements. In this task, the karate experts were not significantly different to the controls in the eyes-open condition (mean = 3.49 mm−s, &&SE = 0.31; mean = 4.61 mm−s, SE = 0.36) or the eyes-closed condition (mean = 7.02 mm−s, SE = 0.91; mean = 6.05 mm−s, SE = 0.45). There was a significant main effect of visual condition with all participants more stable in the eyes-open condition (F = 22.28, P < 0.001), although no significant group effect (t = 0.17, P > 0.87) or interaction was observed (t = 1.58, P > 0.13).
These results suggest that the karate experts' motor expertise is specific to movements of the limbs and upper body, which is consistent with the training that these individuals have undertaken. In addition, their skills do not extend to superior balance control—an area where some martial artists have been found to demonstrate superior ability (Perrot et al. 1998; Perrin et al. 2002).
White Matter Analysis
To investigate whether there were any significant FA differences between the experts and controls in the chosen ROIs, a comparison analysis was performed (Experts > Controls; Controls > Experts). This revealed 4 clusters of white matter (1 in the left SCP, 2 in the right SCP, 1 in the right primary motor cortex), where the level of FA was significantly lower in the karate expert group than in the controls (left SCP: tmax= 3.8, P < 0.01, size = 137 voxels [x = −7, y = −39, z = −27 mm]; right SCP1: tmax= 3.8, P < 0.01, size = 98 voxels [x = 6, y = −31, z = −19]; right SCP2: tmax= 3.9, P < 0.001, size = 41 voxels [x = 6, y = −49, z = −27 mm]; right M1: tmax = 2.81, P < 0.05, size = 46 voxels [x = 16, y = −17, z = 57]; corrected for multiple comparisons; Fig. 3). No significant differences were found between the groups in any of the other cortical or subcortical ROIs. We also explored whether these differences were due to changes in the radial diffusivity by performing the same analysis (Experts > Controls; Controls > Experts). However, this revealed no significant differences in radial diffusivity within these regions.
To test whether the variation in FA measured in the SCPs was associated with expert motor performance, a voxel-wise correlation analysis of FA and motor coordination on the 5-cm punching task was performed within the white matter clusters where significant differences in FA were detected in the comparison analysis. The data were examined to investigate whether the variation of FA within the karate group was also related to task performance, and whether the amount of training each subject had received or the age that training had begun predicted the level of FA in the SCPs.
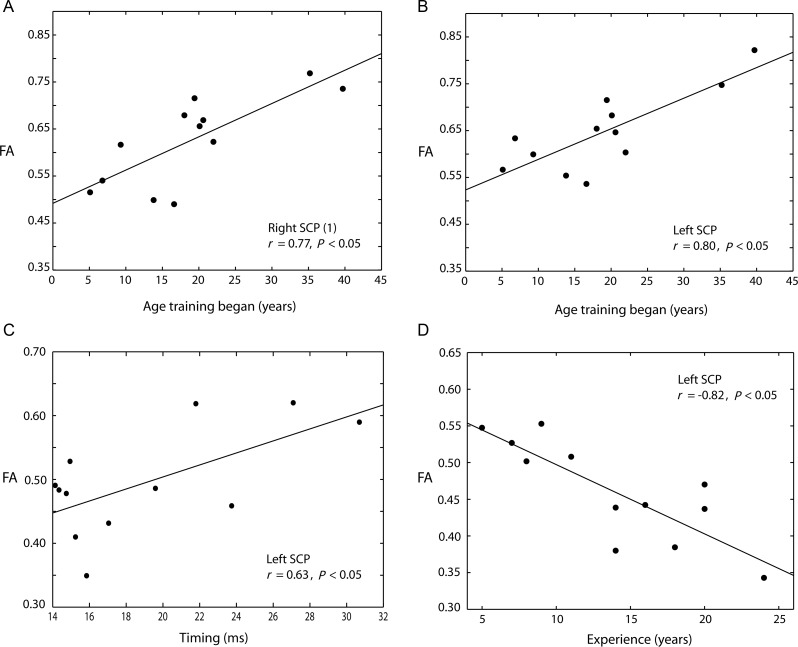
The coordination of shoulder and wrist peak velocities (measured as temporal alignment) was highly significantly correlated with FA in the left SCP cluster within the karate group (tmax= 2.9, r = 0.60, P < 0.05, size = 69 voxels [x = −7, y = −44, z = −28 mm]), indicating that the level of FA in the left SCP may be associated with different levels of expertise. There were no significant correlations between FA and timing in the right SCP or M1 after correcting for multiple comparisons. This suggests that higher FA in SCPs is associated with longer intervals between peak shoulder and wrist velocity on the 5-cm punching task, and hence poorer performance. This finding is in line with the results of the comparison analysis where the experts exhibited lower levels of FA than the controls. Conversely, superior motor expertise, associated with tightly synchronized movements, is associated with lower FA in the left SCP.
We then analyzed whether the amount of training, or the stage of development in which training began, was associated with individual differences in the white matter microstructure of the SCPs. The number of years of training each individual had undertaken (mean = 13.8, SE = 2.9) was significantly negatively correlated with FA in the left SCP (tmax= 3.7, r = −0.82, P < 0.05, size = 91 voxels [x = −7, y = −36, z = −24 mm]), but no significant correlation was found in the right SCP. There was also a significant positive correlation between the age at which training began (mean = 18.9, SE = 1.7) and FA in both left (tmax= 3.7, r = 0.77, P < 0.05, size = 34 voxels [x = −7, y = −37, z = −26 mm]) and right SCPs (tmax= 3.3, r = 0.80. P < 0.05, size = 61 voxels [x = 6, y = −38, z = −26 mm]).
Thus, the karate experts demonstrate a highly significant focal difference in the microstructure of white matter bilaterally in the cerebellum, which is negatively correlated with the degree of temporal motor coordination and the number of years experience in the left SCP. The age at which training began was also significantly positively correlated with FA in both left and right SCPs. These data suggest that the stage in development that karate instruction began and the duration of training are associated with differences in the microstructure of white matter in the SCPs and the ability to coordinate rapid multi-joint movements.
Discussion
When striking a target from close range, karate experts are able to consistently generate impact forces that novices find impossible to replicate (Vos and Binkhorst 1966; Cesari and Bertucco 2008). This may be due in part to a superior ability to coordinate the timing of limb movements. We find that when executing a short-range punch, karate experts coordinate the peak velocities of the shoulder and wrist such that the 2 segments move in unison with a higher degree of accuracy than non-experts. It appears that this synchronization has functional benefit because the magnitude of the temporal interval was negatively correlated with peak impact force and acceleration, and negatively associated with force rise time. Closer control of peak shoulder and wrist velocities is associated with higher acceleration and thus higher impact force. We argue that the ability of karate experts to minimize the temporal separation between the peak velocities of the distal and proximal upper-limb parts allows them to maximize the punch force and its rate of change. The mechanical advantage offered by this precise inter-joint timing may arise partly from an increase in forward momentum, produced by higher wrist velocity in space and greater effective mass (torso plus arm), and partly from a stiffer arm-torso structure, produced by high instantaneous active muscle stiffness through precisely timed contractions, as has been previously suggested (Vos and Binkhorst 1966).
We then employed DTI to examine the relationship between expert performance and structural connectivity in the brain. We compared indices of white matter structure adjacent to brain areas, which have previously been associated with motor control between expert and control groups. This analysis revealed 3 clusters of white matter in the SCPs and 1 in the right primary motor cortex that had lower FA in the karate experts than in the control group. This finding is surprising because higher FA has often been associated with superior performance (Klingberg et al. 2000; Begré et al. 2007; Johansen-Berg et al. 2007), and there is evidence to suggest that FA may also reflect the level of functional connectivity (Boorman et al. 2007). However, a number of studies have also reported “negative” associations between measures of white matter microstructural integrity and performance in healthy individuals (Baird et al. 2005), and lower structural indices in expert groups compared with controls (Schmithorst and Wilke 2002; Bengtsson et al. 2005; Imfeld et al. 2009). As the FA measurement is thought to be the result of numerous factors at the cellular level, such as myelin thickness and axonal packing density (Beaulieu 2002), the relationship between FA and large-scale network function is still not fully understood. In the context of expertise, differences in the integrity of white matter microstructure may reflect a subtle fine-tuning of white matter connectivity to optimize performance on a specific task. Therefore, lower regional FA—as found here—may not necessarily imply reduced “network” functionality.
We then investigated the relationship between individual performance and the variability of FA in SCPs and M1 within the expert group. FA in left SCP was significantly negatively correlated with timing control—indexed by the degree of temporal separation between shoulder and wrist velocities in the punching task—and the number of years of karate training (Fig. 4). This suggests that lower FA in these areas is associated with superior motor control on this task and might also reflect adaptations as a result of long-term learning. However, no significant association was found with these measures in right SCP or right M1. We also examined whether the age at which training began was related to the level of FA in these regions, and this revealed a significant positive correlation in the white matter of both left and right SCPs. This is in agreement with a previous study of expertise which reported that differences in white matter were associated with the stage of development that musical instruction began (Bengtsson et al. 2005).
Figure 4.
Expert group white matter analysis. (A and B) Positive peak correlations within the expert group between FA and the age at which karate training began in the right and left SCPs. (C) Positive peak correlation between timing and FA in the left SCP. (D) Negative peak correlation between number of years of training and FA in the left SCP.
The absence of significant differences between the groups in the microstructure of white matter adjacent to other brain areas associated with motor control may be due to the sample size, or it is possible that the effects of long-term motor learning in karate result in changes primarily to the major efferent pathways of the cerebellum and white matter adjacent to the primary motor cortex. As karate combines training in both left and right arm movements, we would predict correlations between white matter and performance in both hemispheres. However, the right hemisphere lateralization of the correlations would suggest specialization for left-sided limb movements. A possible explanation for these findings may be that extensive karate training results in reduced lateralization toward the dominant hand. Thus, as these individuals were all right-hand dominant, there may be more sensitivity to detect adaptations as a result of experiential learning in right hemisphere brain areas responsible for left-sided movements. An alternative explanation is that since the diffusion-weighted data were acquired using a magnetic field strength of 1.5 T rather than 3 T, and a limited number of diffusion directions, the lower signal to noise ratio could have impaired our ability to detect a significant association in right SCP.
A limitation of the TBSS approach and MRI parameters employed here is that the diffusion sequence used to acquire the data may not have had sufficient sensitivity to detect more subtle changes in white matter microstructure adjacent to cortical structures and cannot fully explain the physiological reasons for differences in the level of FA. It is possible that the finding of lower FA in the karate expert group may be explained by an increase in the proportion of crossing fibers in this region. However, this is a question which is difficult to answer when employing only the standard diffusion tensor model. Recent advances in DTI analysis have shown considerable potential to differentiate between changes in the diffusion signal (Tournier et al. 2011). Future studies may wish to employ such techniques in order to elucidate the structural basis for differences in the diffusion signal. The lack of significant differences between the groups in terms of radial diffusivity suggests that the FA differences may be due to a combination of microstructural changes affecting both radial and axial diffusivity, resulting in a significant overall change in the level of FA.
A possible physiological explanation for the findings reported here should be motivated by our understanding of the requirements of karate. Our findings suggest a role for SCP pathways in the control of rapid ballistic arm movements and are supported by previous studies which found that the cerebellum plays a critical role in our ability to produce complex and coordinated movements (Thach 1998; Imfeld et al. 2009). The SCPs are the primary efferent pathway conveying fibers from the cerebellar nuclei to both cortical and subcortical brain areas. This tract consists of projections from the interpositus nucleus via SCPs, which terminate in the red nucleus (RN) or ventral lateral (VL) thalamus, and a small number of fibers from fastigial nucleus which follow a similar path. The dentate nucleus contributes the majority of efferent fibers in SCPs and projects chiefly to cerebral cortex via VL thalamus. Patients with cerebellar lesions also demonstrate impairments when executing motor plans that require the coordination of multiple muscle groups such as throwing a ball (Timmann et al. 1999) or reaching (Day et al. 1998).
The majority of investigations into the structural brain basis of motor expertise have employed musicians as specialist groups (e.g. Gaser and Schlaug 2003; Bengtsson et al. 2005; Abdul-Kareem et al. 2011) and have largely reported significant increases in white matter microstructure adjacent to cortical and cerebellar structures. However, the nature of musicianship relies on controlling fine finger movements—actions that are specifically not a focus of karate training. Therefore, our finding of lower FA in areas often associated with skilled performance might reflect a shifting of specialization away from structures closely involved in the fine control of hand movements to those governing the limbs and trunk. A hypothetical anatomical correlate for such a bias could be a down-regulation of connections with primary motor cortex to benefit connectivity with the RN. This area has previously been implicated in the control of voluntary limb movements in humans and animals (Ghez and Kubota 1977; Martin and Ghez 1988; Nioche et al. 2009; Hicks and Onodera 2012), and increased involvement of the rubrospinal system may account for the decreased microstructural integrity found in SCPs and primary motor cortex. This possibility would imply that regions of higher FA might exist in this expert group in the connections of the rubrospinal system; however, this hypothesis would have to be investigated using superior resolution data than available here. The differences in brain structure observed in these karate experts may explain their ability to coordinate their movements with such accuracy, reflecting a structural specialization developed over many years of training. However, longitudinal studies would be required to confirm that this is a direct result of karate training.
This study examined the behavioral and brain basis of expert motor control in karate experts. It was found that these individuals are able to repeatedly coordinate certain actions with a level of skill that novices are unable to reproduce. We argue that these abilities may be due primarily to changes to white matter structure in the SCPs, allowing the synchronization of movements of the upper limbs and trunk with a high degree of accuracy. This is the first example of a link between human cerebellar white matter and motor control measures in an elite sporting group. This has implications for our understanding of the role of white matter connectivity in motor coordination, the relationship between measures of white matter microstructure and elite performance, and how brain changes may be related to the stage of development in which learning begins. As the relationship between behavior and measures of brain structure is only beginning to be understood, it is important to carefully consider such findings within the context of the existing literature and our understanding of the brain systems involved.
Funding
This research was supported by the Medical Research Council, Wellcome Trust and the National Institute for Health Research Clinical Biomedical Centre at University College London Hospitals/University College London. Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust, (Prof Husain is a Wellcome PI).
Notes
We thank Mr Daniel Voyce for the design and construction of the punching frame. Conflict of Interest: None declared.
References
- Abdul-Kareem IA, Stancak A, Parkes LM, Al-Ameen M, AlGhamdi J, Aldhafeeri FM, Embleton K, Morris D, Sluming V. Plasticity of the superior and middle cerebellar peduncles in musicians revealed by quantitative analysis of volume and number of streamlines based on diffusion tensor tractography. Cerebellum. 2011;10(3):611–623. doi: 10.1007/s12311-011-0274-1. doi:10.1007/s12311-011-0274-1. [DOI] [PubMed] [Google Scholar]
- Aglioti SM, Cesari P, Romani M, Urgesi C. Action anticipation and motor resonance in elite basketball players. Nat Neurosci. 2008;11(9):1109–1116. doi: 10.1038/nn.2182. doi:10.1038/nn.2182. [DOI] [PubMed] [Google Scholar]
- Baird AA, Colvin MK, VanHorn JD, Inati S, Gazzaniga MS. Functional connectivity: integrating behavioral, diffusion tensor imaging, and functional magnetic resonance imaging data sets. J Cogn Neurosci. 2005;17(4):687–693. doi: 10.1162/0898929053467569. doi:10.1162/0898929053467569. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. doi:10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. doi:10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Begré S, Frommer A, von Känel R, Kiefer C, Federspiel A. Relation of white matter anisotropy to visual memory in 17 healthy subjects. Brain Res. 2007;1168:60–66. doi: 10.1016/j.brainres.2007.06.096. doi:10.1016/j.brainres.2007.06.096. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8(9):1148–1150. doi: 10.1038/nn1516. doi:10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund HJ. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci. 1999;11(9):3276–3286. doi: 10.1046/j.1460-9568.1999.00753.x. doi:10.1046/j.1460-9568.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- Boorman ED, O'Shea J, Sebastian C, Rushworth MF, Johansen-Berg H. Individual differences in white-matter microstructure reflect variation in functional connectivity during choice. Curr Biol. 2007;17(16):1426–1431. doi: 10.1016/j.cub.2007.07.040. doi:10.1016/j.cub.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Cesari P, Bertucco M. Coupling between punch efficacy and body stability for elite karate. J Sci Med Sport. 2008;11(3):353–356. doi: 10.1016/j.jsams.2007.05.007. doi:10.1016/j.jsams.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Day BL, Thompson PD, Harding AE, Marsden CD. Influence of vision on upper limb reaching movements in patients with cerebellar ataxia. Brain. 1998;121:357. doi: 10.1093/brain/121.2.357. doi:10.1093/brain/121.2.357. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci. 1999;2(6):563–567. doi: 10.1038/9219. doi:10.1038/9219. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4(11):423–431. doi: 10.1016/s1364-6613(00)01537-0. doi:10.1016/S1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427(6972):311–312. doi: 10.1038/427311a. doi:10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Laverdure-Dupont D. Practice makes cortex. J Neurosci. 2008;28(35):8655. doi: 10.1523/JNEUROSCI.2650-08.2008. doi:10.1523/JNEUROSCI.2650-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. doi:10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ericsson KA. The Cambridge handbook of expertise and expert performance. Cambridge (UK): Cambridge University Press; 2006. [Google Scholar]
- Ericsson KA, Krampe RT, Tesch-Römer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363–406. doi:10.1037/0033-295X.100.3.363. [Google Scholar]
- Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23(27):9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner R, Gorges S, Weise D, Buttmann M, Classen J. Encoding of motor skill in the corticomuscular system of musicians. Curr Biol. 2010;20(20):1869–1874. doi: 10.1016/j.cub.2010.09.045. doi:10.1016/j.cub.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Ghez C, Kubota K. Activity of red nucleus neurons associated with a skilled forelimb movement in the cat. Brain Res. 1977;129(1977):383–388. doi: 10.1016/0006-8993(77)90533-9. doi:10.1016/0006-8993(77)90533-9. [DOI] [PubMed] [Google Scholar]
- Hicks TP, Onodera S. The mammalian red nucleus and its role in motor systems, including the emergence of bipedalism and language. Prog Neurobiol. 2012;96(2):165–175. doi: 10.1016/j.pneurobio.2011.12.002. doi:10.1016/j.pneurobio.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Ilg R, Wohlschläger AM, Gaser C, Liebau Y, Dauner R, Wöller A, Zimmer C, Zihl J, Mühlau M. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28(16):4210. doi: 10.1523/JNEUROSCI.5722-07.2008. doi:10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L. White matter plasticity in the corticospinal tract of musicians: a diffusion tensor imaging study. Neuroimage. 2009;46(3):600–607. doi: 10.1016/j.neuroimage.2009.02.025. doi:10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TEJ, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007;36:16–21. doi: 10.1016/j.neuroimage.2007.03.041. doi:10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato M, Kuroda T, Imamizu H, Nakano E, Miyauchi S, Yoshioka T. Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Prog Brain Res. 2003;142:171–188. doi: 10.1016/S0079-6123(03)42013-X. doi:10.1016/S0079-6123(03)42013-X. [DOI] [PubMed] [Google Scholar]
- Kelly C, Foxe JJ, Garavan H. Patterns of normal human brain plasticity after practice and their implications for neurorehabilitation. Arch Phys Med Rehabil. 2006;87:20–29. doi: 10.1016/j.apmr.2006.08.333. doi:10.1016/j.apmr.2006.08.333. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JDE, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25(2):493–500. doi: 10.1016/s0896-6273(00)80911-3. doi:10.1016/S0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Lee KM, Chang KH, Roh JK. Subregions within the supplementary motor area activated at different stages of movement preparation and execution. Neuroimage. 1999;9(1):117–123. doi: 10.1006/nimg.1998.0393. doi:10.1006/nimg.1998.0393. [DOI] [PubMed] [Google Scholar]
- Li CS, Padoa-Schioppa C, Bizzi E. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron. 2001;30(2):593–607. doi: 10.1016/s0896-6273(01)00301-4. doi:10.1016/S0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- Lotze M, Erb M, Flor H, Huelsmann E, Godde B, Grodd W. fMRI evaluation of somatotopic representation in human primary motor cortex. Neuroimage. 2000;11(5pt1):473–481. doi: 10.1006/nimg.2000.0556. doi:10.1006/nimg.2000.0556. [DOI] [PubMed] [Google Scholar]
- Luft AR, Smith GV, Forrester L, Whitall J, Macko RF, Hauser TK, Goldberg AP, Hanley DF. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum Brain Mapp. 2002;17(2):131–140. doi: 10.1002/hbm.10058. doi:10.1002/hbm.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RSJ, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Nat Acad Sci USA. 2000;97(8):4398–4403. doi: 10.1073/pnas.070039597. doi:10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Red nucleus and motor cortex: parallel motor systems for the initiation and control of skilled movement. Behav Brain Res. 1988;28(1–2):217–223. doi: 10.1016/0166-4328(88)90099-x. doi:10.1016/0166-4328(88)90099-X. [DOI] [PubMed] [Google Scholar]
- Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Netw. 1996;9(8):1265–1279. doi: 10.1016/s0893-6080(96)00035-4. doi:10.1016/S0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PC, Nagae-Poetscher LM. MRI atlas of human white matter. Amsterdam (The Netherlands): Elsevier; 2005. [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallet M. Early consolidation in human primary motor cortex. Nature. 2002;415(6872):640–644. doi: 10.1038/nature712. doi:10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9(11):856–869. doi: 10.1038/nrn2478. doi:10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Curr Biol. 2005;15(2):122–128. doi: 10.1016/j.cub.2005.01.006. doi:10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nioche C, Cabanis EA, Habas C. Functional connectivity of the human red nucleus in the brain resting state at 3t. Am J Neuroradiol. 2009;30(2):396–403. doi: 10.3174/ajnr.A1375. doi:10.3174/ajnr.A1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. Assessment and analysis of handedness - Edinburgh inventory. Neuropsycholia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pearce AJ, Thickbroom GW, Byrnes ML, Mastaglia FL. Functional organisation of the corticomotor projection to the hand in skilled racquet players. Exp Brain Res. 2000;130(2):238–243. doi: 10.1007/s002219900236. doi:10.1007/s002219900236. [DOI] [PubMed] [Google Scholar]
- Perrin P, Deviterne D, Hugel F, Perrot C. Judo, better than dance, develops sensorimotor adaptabilities involved in balance control. Gait Posture. 2002;15(2):187–194. doi: 10.1016/s0966-6362(01)00149-7. doi:10.1016/S0966-6362(01)00149-7. [DOI] [PubMed] [Google Scholar]
- Perrot C, Deviterne D, Perrin P. Influence of training on postural and motor control in a combative sport. J Hum Mov Stud. 1998;35(3):119–136. [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, Petersen SE. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex. 1994;4(1):8–26. doi: 10.1093/cercor/4.1.8. doi:10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Takino R, Sasaki Y, Pütz B. Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J Neurosci. 1998;18(5):1827–1840. doi: 10.1523/JNEUROSCI.18-05-01827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M. Differences in white matter architecture between musicians and non-musicians: a diffusion tensor imaging study. Neurosci Lett. 2002;321(1–2):57–60. doi: 10.1016/s0304-3940(02)00054-x. doi:10.1016/S0304-3940(02)00054-X. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. doi:10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluming V, Barrick T, Howard M, Cezayirli E, Mayes A, Roberts N. Voxel-based morphometry reveals increased gray matter density in Broca's area in male symphony orchestra musicians. Neuroimage. 2002;17(3):1613–1622. doi: 10.1006/nimg.2002.1288. doi:10.1006/nimg.2002.1288. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. doi:10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(S1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. doi:10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. doi:10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Thach WT. What is the role of the cerebellum in motor learning and cognition? Trends Cogn Sci. 1998;2(9):331–337. doi: 10.1016/s1364-6613(98)01223-6. doi:10.1016/S1364-6613(98)01223-6. [DOI] [PubMed] [Google Scholar]
- Timmann D, Watts S, Hore J. Failure of cerebellar patients to time finger opening precisely causes ball high-low inaccuracy in overarm throws. J Neurophysiol. 1999;82(1):103–114. doi: 10.1152/jn.1999.82.1.103. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;6:1532–1556. doi: 10.1002/mrm.22924. doi:10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt M, Klausen K. Changes in muscle strength and speed of an unloaded movement after various training programmes. Eur J Appl Physiolog Occup Physiol. 1990;60(5):370–376. doi: 10.1007/BF00713501. doi:10.1007/BF00713501. [DOI] [PubMed] [Google Scholar]
- Vos JA, Binkhorst RA. Velocity and force of some karate arm-movements. Nature. 1966;211(5044):89–90. doi: 10.1038/211089a0. doi:10.1038/211089a0. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2(9):338–347. doi: 10.1016/s1364-6613(98)01221-2. doi:10.1016/S1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- Yarrow K, Brown P, Krakauer JW. Inside the brain of an elite athlete: the neural processes that support high achievement in sports. Nat Rev Neurosci. 2009;10(8):585–596. doi: 10.1038/nrn2672. doi:10.1038/nrn2672. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Sale DG, Dowling JJ. Ballistic movement performance in karate athletes. Med Sci Sports Exerc. 1997;29(10):1366. doi: 10.1097/00005768-199710000-00014. [DOI] [PubMed] [Google Scholar]