Abstract
The cortex in spina bifida myelomeningocele (SBM) is atypically organized, but it is not known how specific features of atypical cortical organization promote or disrupt cognitive and motor function. Relations of deviant cortical thickness and gyrification with IQ and fine motor dexterity were investigated in 64 individuals with SBM and 26 typically developing (TD) individuals, aged 8–28 years. Cortical thickness and 3D local gyrification index (LGI) were quantified from 33 cortical regions per hemisphere using FreeSurfer. Results replicated previous findings, showing regions of higher and lower cortical thickness and LGI in SBM relative to the TD comparison individuals. Cortical thickness and LGI were negatively associated in most cortical regions, though less consistently in the TD group. Whereas cortical thickness and LGI tended to be negatively associated with IQ and fine motor outcomes in regions that were thicker or more gyrified in SBM, associations tended to be positive in regions that were thinner or less gyrified in SBM. The more deviant the levels of cortical thickness and LGI—whether higher or lower relative to the TD group—the more impaired the IQ and fine motor outcomes, suggesting that these cortical atypicalities in SBM are functionally maladaptive, rather than adaptive.
Keywords: atypical cortical organization, cortical thickness, gyrification, neurobehavioral function, spina bifida
Introduction
The classical view of neurodevelopmental disorders attributed adverse cognitive and motor function to a generalized reduction in brain volumes and/or microcephaly. Consistent with this view, reduced total or regional brain volumes have been reported in anatomical MRI studies of 22q11.2 deletion syndrome (Eliez et al. 2000), Angelman syndrome (Tan et al. 2011), Down syndrome (Weis et al. 1991), Fragile X syndrome (Kates et al. 2002), and Rett syndrome (Subramaniam et al. 1997). At the same time, anatomical MRI studies also show larger brain volumes in a number of disorders associated with poor functional outcomes. Cohen syndrome, an autosomal-recessive mental retardation, is associated with an enlarged midsagittal corpus callosum (Kivitie-Kallio et al. 1998). Individuals with Turner syndrome have higher volumes of the superior and middle temporal lobe, which are associated with poorer performance on language tasks (Rae et al. 2004). Congenital amusia is associated with extra volume in the right inferior frontal gyrus and auditory cortex (Hyde et al. 2007). Individuals with autism show more radiate white matter in the primary motor cortex, an anomaly associated with motor impairment (Mostofsky et al. 2007).
A range of studies demonstrate that regional brain size atypicality occurs at each end of the spectrum, with individual brain regions being variously smaller and larger relative to normative expectations. These studies, covering a variety of disorders, raise important questions about atypical cortical organization in neurodevelopmental disorders. One question is which brain regions develop as “too large” or “too small”? Another is whether different measures of brain size are congruent with each other (e.g. are measures of cortical thickness deviant in the same direction as measures of gyrification?). An important question is whether regional brain size deviance affects function and, if so, whether it does so at one or both ends of the deviance spectrum (e.g. is either a too large or too small brain region associated with poorer outcomes?). Aside from group differences, it is important to explore whether function varies with regional size variations within individual brains. Questions about cortical volume may be explored using measures of cortical thickness and gyrification, 2 distinct properties of cortical gray matter that contribute to overall cortical volume.
Cortical Development
Typical development of the cortex is characterized by an inverted U-shaped developmental trajectory of mean cortical thickness, with a period of initial increase peaking around middle childhood, followed by a decrease through early adulthood (Giedd et al. 1999; Giedd 2004; Gogtay et al. 2004; Shaw et al. 2008; Raznahan et al. 2011). This cortical thinning is followed by a relative stabilization of cortical thickness in adulthood (Sowell et al. 2003; Shaw et al. 2008). Gyrification describes the process by which the cortical surface folds to create gyral and sulcal regions. The development of gyrification begins prenatally; most cortical convolutions are formed during the third trimester of pregnancy (Armstrong et al. 1995), with continuing small changes in gyrification throughout the lifespan (Mangin et al. 2010; White et al. 2010). Emerging evidence suggests an inverted U-shaped developmental trajectory similar to that of cortical thickness, involving a moderate increase in global gyrification during early childhood followed by a gradual decrease in gyrification beginning at age 6 or earlier and continuing through adulthood (Magnotta et al. 1999; White et al. 2010; Raznahan et al. 2011).
Cortical Thickness and Gyrification in Developmental Disorders
Cortical thickness and gyrification have been investigated in a wide variety of conditions involving aberrant brain development, including developmental, genetic, neurological, and psychiatric disorders. Regions of significantly thicker cortex have been reported in fetal alcohol spectrum disorder (FASD; Sowell et al. 2008) and Williams syndrome (Thompson et al. 2005); whereas regions of thinner cortex have been reported in adolescents born preterm (Nagy et al. 2010), attention-deficit/hyperactivity disorder (ADHD; Makris et al. 2007; Narr et al. 2009; Batty et al. 2010), childhood- and adolescent-onset schizophrenia (White et al. 2003), and temporal lobe epilepsy (Lin et al. 2007; Mueller et al. 2009). Concomitantly thicker and thinner cortical regions have been reported in 22q11.2 deletion syndrome (Bearden et al. 2007, 2009; Schaer et al. 2009), autism spectrum disorder (Hyde et al. 2010; Jiao et al. 2010), and Turner syndrome (Raznahan et al. 2010).
Regions of higher gyrification have been reported in 22q11.2 deletion syndrome (Bearden et al. 2009), autism spectrum disorder (Awate et al. 2008), children born preterm (Kesler et al. 2006), and Williams syndrome (Thompson et al. 2005; Gaser et al. 2006); whereas regions of lower gyrification have been reported in ADHD (Wolosin et al. 2009), dyslexia (Casanova et al. 2004), generic intellectual disabilities (Zhang et al. 2010), obsessive-compulsive disorder (Wobrock et al. 2010), and Turner syndrome (Raznahan et al. 2010). Regions of both higher and lower gyrification have been reported in schizophrenia (White and Hilgetag 2011), and temporal lobe epilepsy (Lin et al. 2007; Voets et al. 2011).
Despite the number of studies characterizing cortical thickness and gyrification, it remains unclear whether there is a consistent relation between the 2 cortical properties in either typical or aberrant brain development. Most studies examining the statistical correlation between cortical thickness and gyrification report no consistent relation in either aberrant conditions or typically developing (TD) comparison groups (Thompson et al. 2005; Lin et al. 2007; Bearden et al. 2009). Further, the functional consequences of variations in cortical thickness and gyrification are poorly understood. The direction of associations between cortical thickness and IQ appear to vary with age during normal childhood and adulthood (Sowell et al. 2004; Shaw et al. 2006; Narr et al. 2007; Karama et al. 2009). Only 1 study has reported a positive correlation between gyrification and IQ in a healthy young adult sample (Im et al. 2006). In aberrant development, positive correlations between cortical thickness and cognitive outcome have been reported for 22q11.2 deletion syndrome (Schaer et al. 2009), schizophrenia (Hartberg et al. 2010), FASD (Sowell et al. 2008), dyslexia (Casanova et al. 2004), bipolar disorder (McIntosh et al. 2009), and adolescents at risk for schizophrenia (Stanfield et al. 2008); whereas negative associations have been reported for children born preterm (Kesler et al. 2006) and adolescents and adults with temporal lobe epilepsy (Oyegbile et al. 2004).
Spina Bifida Myelomeningocele
Individuals with spina bifida myelomeningocele (SBM) provide an opportunity to explore whether there are upper and lower limits to cortical thickness and gyrification for optimal cognitive and motor function within individuals and between brain regions. As the most common severely disabling congenital birth defect affecting the central nervous system, SBM involves failure of neural tube development and an atypically organized brain (Barkovich 2005). The Chiari II malformation affects the morphology of the cerebellum, and there is regional hypogenesis and/or hypoplasia of the corpus callosum (Barkovich 2005; Hannay et al. 2009). Quantitative MRI studies show reductions in regional gray and white matter with significantly higher volume of CSF (Fletcher et al. 2005) and compromised integrity of several long white matter pathways (Fletcher et al. 2005; Vachha et al. 2006; Hasan, Eluvathingal et al. 2008; Hasan, Sankar et al. 2008). Of particular interest, emerging MRI studies show bidirectional anomalies of cortical thickness and gyrification in children and young adults with SBM relative to TD comparison individuals (Juranek et al. 2008; Juranek and Salman 2010). Individuals with SBM have thicker cortex in frontal, superior parietal, and occipital regions, and thinner cortex in inferior parietal and temporal regions. Regions of higher gyrification include lateral frontal, inferior parietal, and posterior temporal regions, and regions of lower gyrification include the inferior frontal lobe and the medial surface of the parietal and temporal lobes.
The general objective of the present study was to investigate the relations between deviant levels of cortical thickness and gyrification with cognitive and motor function in individuals with SBM. Measures of IQ and fine motor dexterity were selected because they have been shown to be variably impaired in SBM and significantly related to quantitative neuroimaging area and volume brain measures (Fletcher et al. 2005). We proposed the following hypotheses:
Individuals with SBM will have anomalous cortical thickness and gyrification at both ends of the size continuum, relative to the TD group. In agreement with earlier reports from our laboratory (Juranek et al. 2008; Juranek and Salman 2010), we predicted that the SBM group would demonstrate: (a) significantly thicker cortex in frontal, superior parietal, and occipital regions; (b) significantly thinner cortex in inferior parietal and temporal regions; (c) significantly higher gyrification in lateral frontal, inferior parietal, and posterior temporal regions; and (d) significantly lower gyrification in the inferior frontal lobe and the medial surface of the parietal and temporal lobes.
Cortical thickness and gyrification will be significantly associated in individuals with SBM. Although little is known about the relation between cortical thickness and gyrification in either typical or aberrant development, we predicted that the 2 properties would be significantly associated in SBM, given possible common underlying mechanisms involving hydrocephalus and aberrant brain development contributing to their anomalous values.
In individuals with SBM, anomalous cortical thickness and gyrification will be associated with compromised cognitive and motor outcomes. Given reports of cognitive and motor impairments in other neurodevelopmental disorders associated with both too-large and too-small brains, we predicted that lower IQ and fine motor dexterity performance would be associated with anomalies at both ends of the cortical thickness and gyrification spectra.
Materials and Methods
Participants
Participants included 64 individuals with SBM and 26 TD comparison individuals recruited from clinics associated with Texas Children's Hospital and Shriners Hospital for Children in Houston, as well as from parent groups for individuals with SBM and community contacts. Individuals were excluded from participation if they had neurological disorders unrelated to SBM, severe psychiatric disorders that precluded adequate cooperation (autism, psychosis), uncontrolled seizure disorder, uncorrected vision or hearing impairment, or inability to control the upper limbs. Participants and their families gave informed consent in accordance with institutional review guidelines. Participants ranged in age from 8 to 28 years.
Table 1 contains participant demographic information and IQ and fine motor scores. The SBM and TD groups did not differ in age, t(35.28) = 0.59, p = 0.561, gender, χ2(1) = 0.001, p = 0.992, ethnicity, χ2(4) = 8.42, p = 0.077, or handedness, p = 0.136, Fisher's exact test. The group with SBM had significantly lower SES as estimated by Hollingshead's 4-factor index of social status (1975), t(88) = 2.21, p = 0.030. After controlling for SES, individuals with SBM also showed lower IQ, F(2,87) = 36.35, p < 0.001, and fine motor dexterity, F(2,87) = 70.13, p < 0.001, performances characteristic of the disorder.
Table 1.
Demographic information and IQ and fine motor scores for SBM and TD groups
| SBM (n= 64) | TD (n= 26) | |
|---|---|---|
| Years of age at MRI (M [SD]) | 13.85 (4.2) | 14.62 (6.1) |
| SES (M [SD])* | 33.77 (12.9) | 40.12 (10.9) |
| Gender (% male) | 0.58 | 0.58 |
| Handedness (% RHD) | 0.92 | 0.77 |
| Ethnicity | ||
| % African American | 0.09 | 0.11 |
| % Asian | 0 | 0.08 |
| % Caucasian | 0.36 | 0.23 |
| % Hispanic | 0.55 | 0.54 |
| % Other | 0 | 0.04 |
| IQ (LSM [SE])** | 84.73 (1.49) | 101.76 (2.36) |
| Fine Motor (LSM [SE])** | −2.83 (0.14) | −0.57(0.23) |
Note. LSM, least-squares mean; M, mean; RHD, right-hand dominant; SBM, spina bifida myelomeningocele; SD, standard deviation; SE, standard error; SES, socioeconomic status.
*p< 0.05.
**p < 0.001.
Individuals with SBM were born with myelomeningocele (verified by medical record review, including pathology and neurosurgical operative reports when available) and all were shunted for hydrocephalus. Additional clinical characteristics, including blinded radiological coding of anatomical features of SBM, are provided in Table 2. As expected, the majority of individuals with SBM had a Chiari II malformation of the hindbrain with an anomalous corpus callosum. There were 6 exceptions: 1 participant had a Chiari I malformation and 5 participants with no identified Chiari malformation on radiological review had aqueductal stenosis, a possible mechanism of their hydrocephalus. As Table 2 demonstrates, the sample is representative of the population of individuals with SBM and hydrocephalus with most participants having lower level spinal lesions, 0–4 shunt revisions, no history of seizures, and impaired ambulation.
Table 2.
Clinical characteristics of group with SBM
| Percent of SBM participants (n = 64a) | |
|---|---|
| Lesion level | |
| Above L1 | 0.17 |
| Below T12 | 0.83 |
| Chiari malformation | |
| None | 0.08 |
| I | 0.02 |
| II | 0.91 |
| Corpus callosum | |
| Normal | 0.02 |
| Hypoplastic | 0.66 |
| Dysgenetic | 0.33 |
| Shunt revisions | |
| 0 | 0.11 |
| 1 | 0.26 |
| 2–4 | 0.51 |
| 5–9 | 0.10 |
| >10 | 0.02 |
| Ambulatory status | |
| Normal | 0.03 |
| Independent | 0.31 |
| With support | 0.33 |
| Unable | 0.33 |
| Seizure disorder | |
| No | 0.85 |
| Past | 0.11 |
| Present | 0.04 |
Note. SBM, spina bifida myelomeningocele.
aParticipants missing data for shunt revisions: n= 3; ambulatory status: n= 3; seizure disorder: n= 11; participants missing data were not included in percentage calculations.
IQ and Fine Motor Dexterity Measures
All participants were administered the 4-subtest short form of the Stanford-Binet Intelligence Test, 4th Edition (Thorndike et al. 1986), from which a composite IQ score was generated. Test–retest reliability estimates of the 4-subtest composite range from r = 0.93 to r = 0.98 for age 8–23 years (Thorndike et al. 1986).
Fine motor dexterity was measured using the Purdue Pegboard Test (Tiffen 1968). Requiring participants to place small pins into a perforated board as quickly as possible in 30 s time intervals, the Purdue Pegboard Test is consistently but variably impaired in individuals with SBM, likely because of the Chiari II malformation and the effects of injury to the white matter from hydrocephalus (Fletcher et al. 2005). Test–retest reliability estimates for single-trial scores range from r = 0.63 to r = 0.82 (Tiffen 1968; Reddon et al. 1988). The z-scores for each hand were calculated by comparing each participant's performance with age-based norms (Lafayette Instruments 1999) and averaged to provide an estimate of overall fine motor dexterity. Five participants (4 SBM and 1 TD) did not complete the Purdue Pegboard Test because of time constraints (n = 2), family relocation (n = 1), or unknown reasons (n = 2). Group means for fine motor dexterity were imputed to prevent dropping of cases in mixed model and correlational analyses.
MRI Acquisition
High-resolution brain MR images were acquired on a Philips 3T scanner with SENSE (Sensitivity Encoding) technology (described in Juranek et al. 2008). Whole-brain coverage was obtained through a 3D T1-weighted sequence following conventional sagittal scout and coronal T2-weighted sequences. Acquisition parameters of the 3D turbo fast spin-echo sequence were as follows: repetition time/echo time = 6.5–6.7/3.04–3.14; flip angle = 8°; field of view = 240 × 240 mm; matrix = 256 × 256; slice thickness = 1.5 mm; in-plane pixel dimensions (x, y) = 0.94, 0.94 mm; number of excitations (NEX) = 2.
MRI Processing
All scans were analyzed by a rater blind to IQ and fine motor dexterity scores, age, and gender (see Juranek et al. 2008). T1-weighted images were reviewed for image quality prior to performing morphometric analyses. Using FreeSurfer v4.0.5 software (www.surfer.nmr.mgh.harvard.edu) on a 64-bit Linux computer, a fully automated process was used to skull-strip and segment each brain into 3 classes of voxels: gray matter, white matter, and CSF (Dale and Sereno 1993; Dale et al. 1999).
Cortical thickness values were automatically quantified within FreeSurfer on a vertex-by-vertex basis by computing the shortest distance between the white matter boundary and the pial surface (Fischl and Dale 2000). A fully automated algorithm for calculating 3D local gyrification index (LGI), a measure of gyrification, was also performed within FreeSurfer. Based on the method of Schaer et al. (2008), LGI quantifies the amount of cortex buried within the sulcal folds relative to the amount of cortex visible on the outer surface of the brain such that higher values indicate greater gyrification. LGI at each vertex of the pial surface is automatically computed in FreeSurfer through: (1) creation of an outer smoothed surface tightly wrapping the pial surface; (2) successive estimations of circular regions of interest (ROI) on the outer smoothed surface and their corresponding ROI on the pial surface; and (3) computation of the ratio between corresponding ROIs on the pial and outer smoothed surfaces. Figure 1 provides an illustration of the pial and outer smoothed surfaces used for LGI computation in SBM and TD brains.
Figure 1.
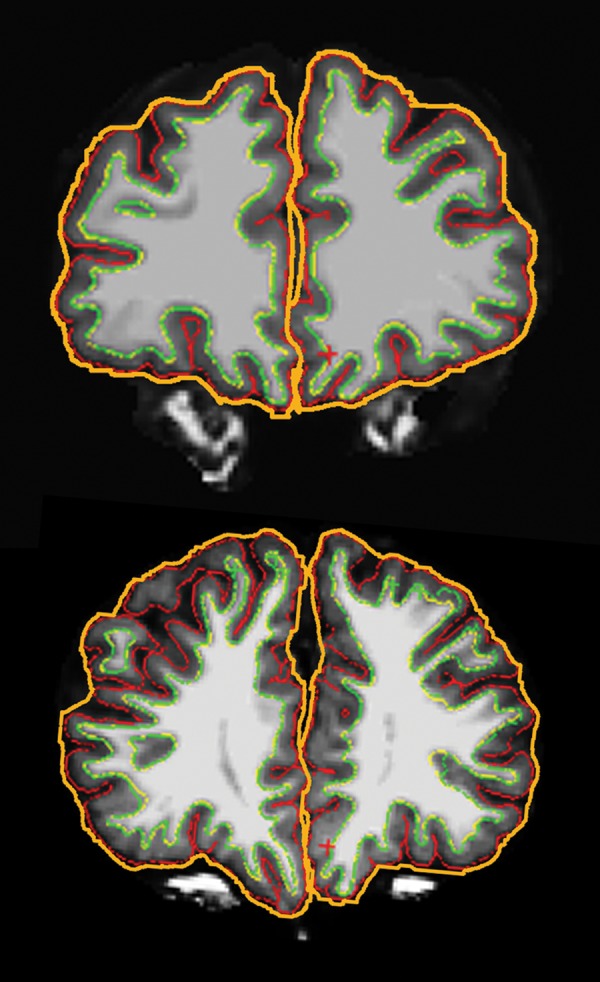
Gyrification patterns in a typically developing 14-year-old male (top) and a 15-year-old female with spina bifida myelomeningocele (bottom). Pial surface (red) and outer smoothed surface (orange) were used for 3D local gyrification index (LGI) computation in FreeSurfer. Coronal sections were taken at approximately the same anterior–posterior level, just anterior to the genu of the corpus callosum.
Within each hemisphere, 33 cortical parcellation units of the cortex were automatically identified and labeled according to the Desikan atlas of gyral-based definitions included within the Freesurfer automatic cortical parcellation routine (Desikan et al. 2006). Boundary delineations for subcortical structures and cortical parcels were visually inspected using the FreeSurfer Tkmedit viewer and manually edited as needed before final quantitative analyses. Cortical thickness and LGI metrics were averaged across all vertices within each cortical parcellation unit for each participant, yielding 2 separate matrices of 33 average measurements per hemisphere per participant. For statistical analyses, cortical thickness and LGI values for each cortical parcellation unit in each hemisphere were extracted from FreeSurfer and analyzed in SAS v9.2.
Statistical Analysis
Following our previous approach (Juranek et al. 2008), group differences in cortical thickness and LGI were evaluated independently for each lobe (frontal, parietal, temporal, and occipital) and the cingulate cortex using a mixed model design with region (i.e. cortical parcellation unit; 10 in frontal, 6 in parietal, 9 in temporal, 4 in occipital, and 4 in cingulate) and hemisphere (left, right) as within subjects variables and group (SBM, TD) as a between subjects variable. Although groups were comparable in age and gender, analyses with and without age and gender as covariates were compared. Because the inclusion of age and gender did not significantly alter the results of group differences in cortical thickness and LGI, the covariates were trimmed from the models in order to preserve statistical power.
To examine possible relations between cortical thickness and LGI, Spearman rank-order partial correlations, controlling for age and gender, between the 2 cortical properties were examined in each hemisphere of all cortical regions in both the SBM and TD groups. Due to the exploratory nature of this analysis and the high number of correlations calculated, the magnitude of the associations was interpreted instead of p values.
Relations of deviant levels of cortical thickness and LGI with IQ and fine motor dexterity were examined using Spearman rank-order partial correlations, controlling for SES, in the SBM group only. For data reduction purposes, correlations were computed only in those regions demonstrating significant group differences (p<0.05) in cortical thickness or LGI. The inclusion of age and gender as covariates did not significantly alter the results of the partial correlations; therefore, they were trimmed from the analyses. We did not correct for multiple comparisons in order to reduce the probability of Type II error, and instead interpreted the magnitude of the associations and the pattern of results.
Results
Group Differences in Cortical Thickness
The mixed model analyses of cortical thickness yielded a significant region by hemisphere by group interaction for the temporal lobe, F(8,704) = 5.23, p<0.001. The 3-way interaction was not significant in the remaining lobes or the cingulate cortex (all p > 0.05). To follow-up the significant 3-way interaction for the temporal lobe, we examined the region by group interaction within each hemisphere. The region by group interaction was significant within both the left, F(8,704) = 4.15, p<0.001, and right, F(8,704) = 6.78, p<0.001, hemispheres. These results indicate that the pattern and magnitude of differences in cortical thickness between groups varied significantly between cortical regions and between hemispheres. As a result, follow-up analyses in the temporal lobe consisted of simple effect comparisons of thickness between groups at each cortical region within each hemisphere.
To follow-up the non significant 3-way interactions in the frontal, parietal, and occipital lobes and the cingulate cortex, we examined the region by group interaction collapsing across the left and right hemispheres. The region by group interaction collapsing across hemisphere was significant in each lobe and the cingulate cortex (frontal: F(9,792) = 8.13, p<0.001; parietal: F(5,440) = 22.82, p<0.001; occipital: F(3,264) = 4.47, p = 0.004; cingulate cortex: F(3,264) = 34.96, p < 0.001). These results indicate that the pattern and magnitude of differences in cortical thickness between groups varied significantly between cortical regions, but the pattern was comparable in the 2 hemispheres. As a result, follow-up analyses in the frontal, parietal, and occipital lobes and the cingulate cortex consisted of simple effect comparisons of thickness between groups at each cortical region collapsing across hemisphere.
Table 3 presents cortical thickness values and results of simple effect comparisons, showing results by hemisphere for the temporal lobe and collapsed across hemisphere for the other lobes and cingulate cortex. Figure 2 provides a visual representation of group differences in cortical thickness. Compared with the TD group, superior frontal and orbitofrontal regions of the frontal lobe, as well as the lateral and medial regions of the occipital lobe, were significantly thicker in SBM. Medial and lateral inferior parietal regions, as well as lateral superior temporal and medial temporal regions, were significantly thinner. The posterior cingulate regions were thinner in SBM, whereas the rostral anterior cingulate was thicker. Group differences in cortical thickness were bilateral with the exception of the right fusiform gyrus and the left temporal pole. Results are generally consistent with those previously reported (Juranek et al. 2008; Juranek and Salman 2010).
Table 3.
Simple effect comparisons of cortical thickness between SBM and TD groups
| Cortical region | Left hemisphere |
Right hemisphere |
||||
|---|---|---|---|---|---|---|
| SBM | TD | p | SBM | TD | p | |
| Temporal lobe | ||||||
| Bank of STS | 2.66 (0.27) | 2.94 (0.27) | <0.001 | 2.51 (0.29) | 2.94 (0.25) | <0.001 |
| Entorhinal | 3.11 (0.38) | 3.59 (0.34) | <0.001 | 3.28 (0.42) | 3.50 (0.46) | 0.033 |
| Fusiform | 2.98 (0.28) | 3.10 (0.16) | 0.044 | 2.94 (0.20) | 2.99 (0.17) | 0.262 |
| Inferior temporal | 3.20 (0.23) | 3.18 (0.22) | 0.611 | 3.18 (0.24) | 3.15 (0.25) | 0.590 |
| Middle temporal | 3.18 (0.23) | 3.29 (0.22) | 0.036 | 3.11 (0.23) | 3.33 (0.25) | <0.001 |
| Parahippocampal | 2.63 (0.45) | 2.85 (0.24) | 0.024 | 2.68 (0.44) | 2.92 (0.31) | 0.016 |
| Superior temporal | 2.96 (0.18) | 3.14 (0.19) | <0.001 | 2.80 (0.22) | 3.21 (0.18) | <0.001 |
| Temporal pole | 3.74 (0.48) | 3.90 (0.33) | 0.142 | 3.59 (0.51) | 3.83 (0.49) | 0.046 |
| Transverse temporal | 2.38 (0.34) | 2.50 (0.28) | 0.114 | 2.60 (0.50) | 2.41 (0.33) | 0.083 |
| Cortical region | SBM | TD | p | |||
| Frontal lobe | ||||||
| Caudal middle frontal | 2.95 (0.21) | 2.86 (0.17) | 0.070 | |||
| Frontal pole | 3.39 (0.41) | 3.25 (0.27) | 0.056 | |||
| Lateral orbitofrontal | 3.31 (0.22) | 3.14 (0.19) | 0.001 | |||
| Medial orbitofrontal | 3.14 (0.22) | 2.85 (0.20) | <0.001 | |||
| Parsopercularis | 3.01 (0.21) | 2.97 (0.17) | 0.307 | |||
| Parsorbitalis | 3.58 (0.25) | 3.24 (0.27) | <0.001 | |||
| Parstriagularis | 3.00 (0.27) | 2.87 (0.21) | 0.023 | |||
| Precentral | 2.61 (0.20) | 2.70 (0.12) | 0.014 | |||
| Rostral middle frontal | 2.88 (0.24) | 2.73 (0.22) | 0.005 | |||
| Superior frontal | 3.34 (0.22) | 3.18 (0.16) | 0.002 | |||
| Parietal lobe | ||||||
| Inferior parietal | 2.70 (0.26) | 2.86 (0.23) | 0.005 | |||
| Paracentral | 2.66 (0.33) | 2.68 (0.23) | 0.843 | |||
| Postcentral | 2.33 (0.19) | 2.23 (0.18) | 0.023 | |||
| Precuneus | 2.50 (0.31) | 2.73 (0.27) | 0.001 | |||
| Superior parietal | 2.60 (0.29) | 2.47 (0.25) | 0.036 | |||
| Supramarginal | 2.69 (0.24) | 2.89 (0.20) | <0.001 | |||
| Occipital lobe | ||||||
| Cuneus | 2.17 (0.24) | 2.07 (0.18) | 0.048 | |||
| Lateral occipital | 2.69 (0.24) | 2.49 (0.18) | <0.001 | |||
| Lingual | 2.22 (0.31) | 2.20 (0.15) | 0.693 | |||
| Pericalcarine | 1.77 (0.18) | 1.62 (0.11) | <0.001 | |||
| Cingulate cortex | ||||||
| Caudal anterior | 3.17 (0.41) | 3.06 (0.22) | 0.104 | |||
| Isthmus | 2.44 (0.49) | 3.05 (0.25) | <0.001 | |||
| Posterior | 2.65 (0.37) | 3.01 (0.21) | <0.001 | |||
| Rostral anterior | 3.54 (0.30) | 3.31 (0.22) | 0.001 | |||
Note. Values are mean (SD) in millimeters. The higher mean between the 2 groups, and p < 0.05, are in bold.
SBM, spina bifida myelomeningocele; STS, superior temporal sulcus; TD, typically developing.
Figure 2.
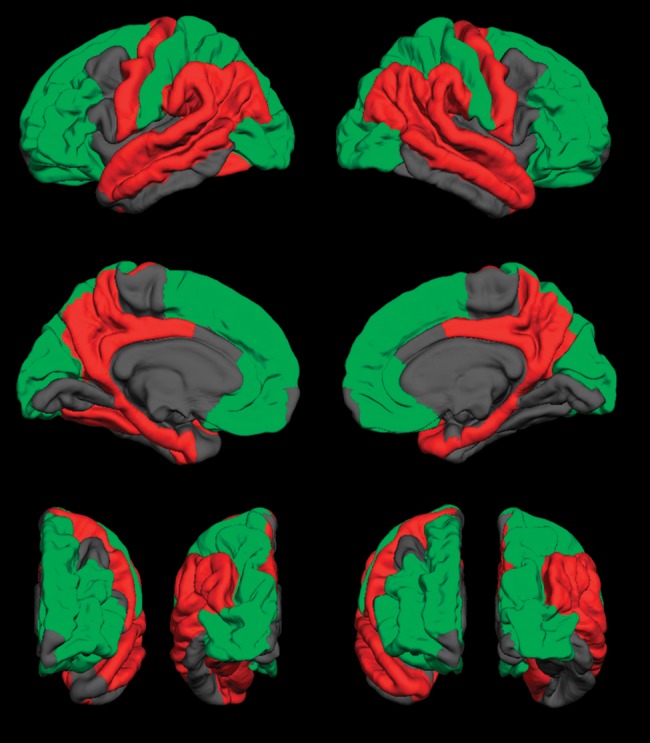
Visual representation of group differences in cortical thickness. Green regions indicate thicker cortex in spina bifida myelomeningocele (SBM); red regions indicate thinner cortex in SBM; gray regions indicate no significant difference in thickness between SBM and typically developing groups. First row: lateral surface; second row: medial surface; bottom row: anterior (left) and posterior (right) surfaces of the left and right hemispheres, respectively. p< 0.05.
Group Differences in LGI
The mixed model analyses of LGI yielded a significant region by hemisphere by group interaction for the temporal lobe, F(8,704) = 3.56, p < 0.001, and the occipital lobe, F(3,264) = 2.66, p = 0.049. The 3-way interaction was not significant in the remaining lobes or the cingulate cortex (all p > 0.05). To follow-up the significant 3-way interaction, we examined the region by group interaction within each hemisphere. For both the temporal and occipital lobes, the region by group interaction was significant within both the left (temporal: F(8,704) = 14.66, p < 0.001; occipital: F(3,264) = 20.09, p < 0.001) and right (temporal: F(8,704) = 6.78, p < 0.001; occipital: F(3,264) = 35.03, p < 0.001) hemispheres. These results indicate that the pattern and magnitude of differences in LGI between groups varied significantly between cortical regions and between hemispheres. As a result, follow-up analyses in the temporal and occipital lobes consisted of simple effect comparisons of LGI between groups at each cortical region within each hemisphere.
To follow-up the nonsignificant 3-way interactions in the frontal and parietal lobes and the cingulate cortex, we examined the region by group interaction collapsing across the left and right hemispheres. The region by group interaction collapsing across hemisphere was significant in each lobe and the cingulate cortex (frontal: F(9,792) = 33.46, p < 0.001; parietal: F(5,440) = 52.93, p < 0.001; cingulate cortex: F(3,264) = 21.45, p < 0.001). These results indicate that the pattern and magnitude of differences in LGI between groups varied significantly between cortical regions, but the pattern was comparable in the 2 hemispheres. As a result, follow-up analyses in the frontal and parietal lobes and the cingulate cortex consisted of simple effect comparisons of LGI between groups at each cortical region collapsing across hemisphere.
Table 4 presents LGI values and results of simple effect comparisons. Figure 3 provides a visual representation of group differences in LGI. Large regions of significantly higher LGI in SBM were located in the middle frontal lobe and in the inferior parietal and supramarginal regions of the parietal lobe stretching down into the inferior temporal lobe. Significantly lower LGI in SBM was evident in the entire posterior medial surface of the brain, beginning superiorly in the superior parietal and lateral occipital cortices, spanning the medial surfaces of the parietal, temporal, and occipital lobes, and stretching inferiorly to affect the anterior superior regions of the temporal lobe and the inferior regions of the frontal lobe. Group differences in LGI were bilateral with the exception of the right fusiform, right entorhinal, left middle temporal, and left lateral occipital cortices. Results are generally consistent with those previously reported (Juranek and Salman 2010).
Table 4.
Simple effect comparisons of LGI between SBM and TD groups
| Cortical region | Left hemisphere |
Right hemisphere |
||||
|---|---|---|---|---|---|---|
| SBM | TD | p | SBM | TD | p | |
| Temporal lobe | ||||||
| Bank of STS | 3.79 (0.41) | 3.47 (0.30) | <0.001 | 4.18 (0.45) | 3.59 (0.32) | <0.001 |
| Entorhinal | 2.28 (0.16) | 2.41 (0.11) | <0.001 | 2.31 (0.17) | 2.38 (0.15) | 0.069 |
| Fusiform | 2.37 (0.19) | 2.45 (0.12) | 0.048 | 2.41 (0.16) | 2.43 (0.13) | 0.554 |
| Inferior temporal | 2.64 (0.20) | 2.52 (0.11) | 0.008 | 2.67 (0.17) | 2.43 (0.11) | <0.001 |
| Middle temporal | 3.26 (0.34) | 3.24 (0.23) | 0.760 | 3.49 (0.33) | 3.18 (0.20) | <0.001 |
| Parahippocampal | 2.31 (0.25) | 2.54 (0.17) | <0.001 | 2.40 (0.20) | 2.53 (0.20) | 0.010 |
| Superior temporal | 3.83 (0.32) | 4.13 (0.33) | <0.001 | 3.89 (0.36) | 4.07 (0.26) | 0.019 |
| Temporal pole | 2.28 (0.17) | 2.32 (0.13) | 0.322 | 2.29 (0.16) | 2.30 (0.14) | 0.787 |
| Tranverse temporal | 4.50 (0.38) | 4.78 (0.50) | 0.006 | 4.51 (0.42) | 4.77 (0.35) | 0.007 |
| Occipital lobe | ||||||
| Cuneus | 2.39 (0.29) | 2.78 (0.22) | <0.001 | 2.48 (0.30) | 2.99 (0.24) | <0.001 |
| Lateral occipital | 2.39 (0.19) | 2.44 (0.15) | 0.178 | 2.43 (0.18) | 2.52 (0.16) | 0.048 |
| Lingual | 2.33 (0.30) | 2.59 (0.18) | <0.001 | 2.46 (0.28) | 2.71 (0.21) | <0.001 |
| Pericalcarine | 2.37 (0.30) | 2.67 (0.21) | <0.001 | 2.48 (0.31) | 2.86 (0.23) | <0.001 |
| Cortical region | SBM | TD | p | |||
| Frontal lobe | ||||||
| Caudal middle frontal | 3.43 (0.27) | 3.24 (0.22) | 0.002 | |||
| Frontal pole | 1.84 (0.10) | 1.92 (0.10) | 0.002 | |||
| Lateral orbitofrontal | 2.34 (0.18) | 2.60 (0.14) | <0.001 | |||
| Medial orbitofrontal | 1.99 (0.12) | 2.00 (0.09) | 0.778 | |||
| Parsopercularis | 3.90 (0.32) | 4.29 (0.32) | <0.001 | |||
| Parsorbitalis | 2.73 (0.25) | 3.00 (0.28) | <0.001 | |||
| Parstriagularis | 3.41 (0.31) | 3.75 (0.30) | <0.001 | |||
| Precentral | 3.58 (0.24) | 3.59 (0.25) | 0.761 | |||
| Rostral middle frontal | 2.90 (0.17) | 2.73 (0.18) | <0.001 | |||
| Superior frontal | 2.22 (0.14) | 2.18 (0.12) | 0.221 | |||
| Parietal lobe | ||||||
| Inferior parietal | 3.41 (0.24) | 3.23 (0.21) | 0.001 | |||
| Paracentral | 2.24 (0.18) | 2.37 (0.17) | 0.002 | |||
| Postcentral | 3.66 (0.26) | 3.62 (0.25) | 0.485 | |||
| Precuneus | 2.54 (0.27) | 2.90 (0.22) | <0.001 | |||
| Superior parietal | 2.82 (0.18) | 3.01 (0.20) | <0.001 | |||
| Supramarginal | 4.15 (0.35) | 3.67 (0.24) | <0.001 | |||
| Cingulate cortex | ||||||
| Caudal anterior | 1.97 (0.18) | 1.90 (0.11) | 0.042 | |||
| Isthmus | 2.47 (0.28) | 2.70 (0.19) | <0.001 | |||
| Posterior | 2.16 (0.21) | 2.21 (0.16) | 0.351 | |||
| Rostral anterior | 2.04 (0.16) | 2.00 (0.11) | 0.244 | |||
Note. Values are mean (SD) LGI. The higher mean between the 2 groups, and p < 0.05, are in bold.
LGI, 3D local gyrification index; SBM, spina bifida myelomeningocele; STS, superior temporal sulcus; TD, typically developing.
Figure 3.
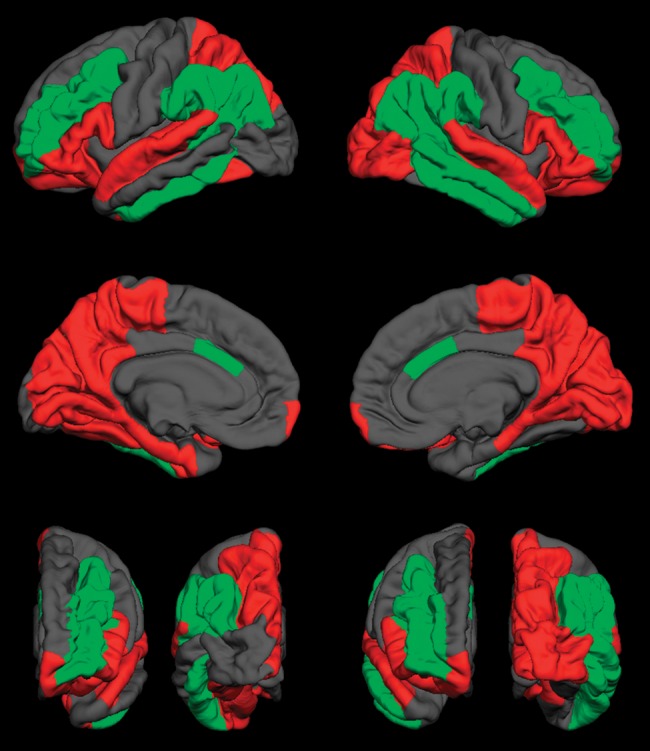
Visual representation of group differences in 3D LGI. Green regions indicate more gyrified cortex in spina bifida myelomeningocele (SBM); red regions indicate less gyrified cortex in SBM; gray regions indicate no significant difference in LGI between SBM and typically developing groups. First row: lateral surface; second row: medial surface; bottom row: anterior (left) and posterior (right) surfaces of the left and right hemispheres, respectively. p< 0.05.
Relations Between Cortical Thickness and LGI
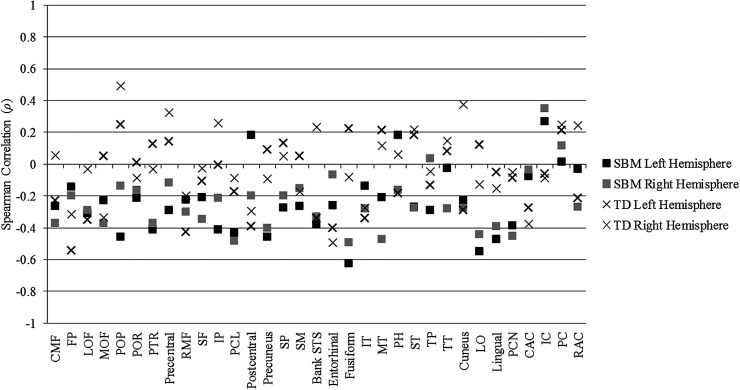
Spearman rank-order partial correlations examining the relations between cortical thickness and LGI in all cortical regions, while controlling for age and gender, are presented in Figure 4 for both the SBM and TD groups. In the group with SBM, cortical thickness and LGI were negatively correlated in 59 of the total 66 cortical regions, indicating that higher cortical thickness was associated with lower LGI and lower cortical thickness was associated with higher LGI in the large majority of cortical regions. Association sizes of the negative correlations were small in 19 regions, medium in 28 regions, and large in 9 regions. Cortical thickness and LGI were positively correlated in 7 of 66 cortical regions in SBM, indicating that higher cortical thickness was associated with higher LGI and lower cortical thickness was associated with lower LGI in this small number of regions. Association sizes of the positive correlations were small in 3 regions and medium in 2 regions.
Figure 4.
Spearman rank-order partial correlation coefficients, controlling for age and gender, between cortical thickness and 3D LGI in all cortical regions in spina bifida myelomeningocele (SBM) and typically developing (TD) groups. CAC, caudal anterior cingulate; CMF, caudal middle frontal; FP, frontal pole; IC, isthmus cingulate; IP, inferior parietal; IT, inferior temporal; LO, lateral occipital; LOF, lateral orbitofrontal; MOF, medial orbitofrontal; MT, middle temporal; PC, posterior cingulate; PCL, paracentral; PCN, pericalcarine; PH, parahippocampal; POP, parsopercularis; POR, parsorbitalis; PTR, parstriangularis; RAC, rostral anterior cingulate; RMF, rostral middle frontal; SF, superior frontal; SM, supramarginal; SP, superior parietal; ST, superior temporal; STS, superior temporal sulcus; TP, temporal pole; TT, transverse temporal.
Correlations between cortical thickness and LGI were less consistent in the TD group. Cortical thickness and LGI were negatively correlated in 39 of the total 66 cortical regions, with 18 small, 13 medium, and 2 large association sizes. Cortical thickness and LGI were positively correlated in 27 of 66 cortical regions in the TD group, with 20 small, 3 medium, and 1 large effect sizes.
Relations of Cortical Thickness and LGI with IQ and Fine Motor Dexterity
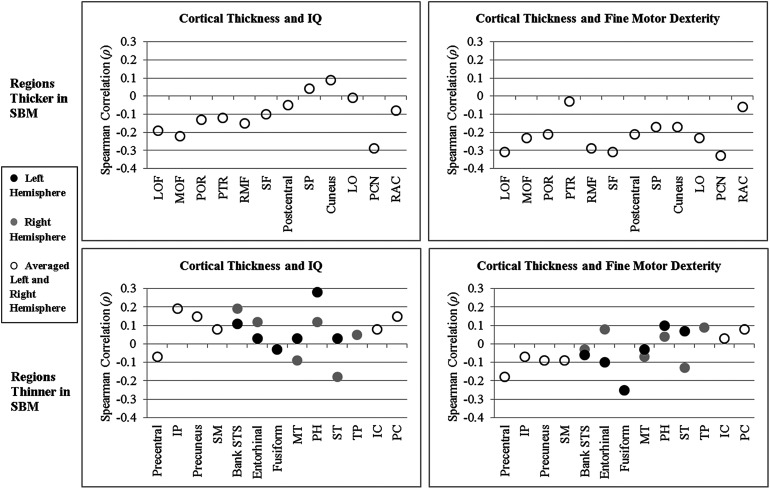
Spearman rank-order partial correlations, controlling for SES, of cortical thickness with IQ and fine motor dexterity in the group with SBM were investigated in 6 regions of the left hemisphere, 6 regions of the right hemisphere, and in 18 regions averaged across left and right hemispheres, that showed significant group differences (p < 0.05 in Table 3). Figure 5 plots correlation coefficients for regions that were significantly thicker or thinner in SBM. Of note, the direction of the relations of thickness with IQ and fine motor dexterity scores in the majority of regions appear to be related to whether the cortical region was thicker or thinner in SBM relative to the TD group. In 22 of the total 24 correlations computed for regions that were significantly thicker in SBM, cortical thickness was negatively correlated with IQ and/or fine motor dexterity, indicating that higher cortical thickness was associated with poorer cognitive and motor outcomes. In contrast, in 21 of the total 36 correlations computed for regions that were significantly thinner in SBM, cortical thickness was positively correlated with IQ and/or fine motor dexterity, indicating that lower cortical thickness was associated with poorer cognitive and motor outcomes. In terms of the strength of relations between cortical thickness and IQ, association sizes were small in 22 regions and medium in 2 regions. In terms of the strength of relations between cortical thickness and fine motor dexterity, association sizes were small in 20 regions and medium in 5 regions.
Figure 5.
Spearman rank-order partial correlation coefficients, controlling for socioeconomic status, of cortical thickness with IQ and fine motor dexterity in spina bifida myelomeningocele (SBM). IC, isthmus cingulate; IP, inferior parietal; LO, lateral occipital; LOF, lateral orbitofrontal; MOF, medial orbitofrontal; MT, middle temporal; PC, posterior cingulate; PCN, pericalcarine; PH, parahippocampal; POR, parsorbitalis; PTR, parstriangularis; RAC, rostral anterior cingulate; RMF, rostral middle frontal; SF, superior frontal; SM, supramarginal; SP, superior parietal; ST, superior temporal; STS, superior temporal sulcus; TP, temporal pole.
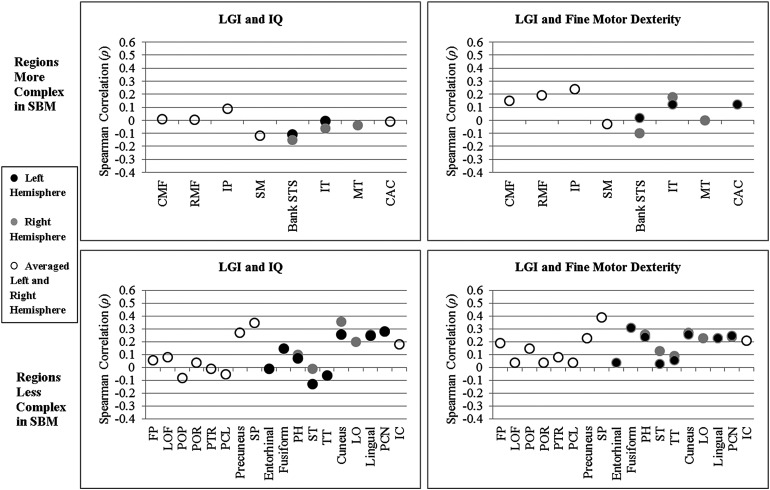
Spearman rank-order partial correlations, controlling for SES, of LGI with IQ and fine motor dexterity in the group with SBM were investigated in 10 regions of the left hemisphere, 10 regions of the right hemisphere, and in 14 regions averaged across left and right hemispheres, that demonstrated significant group differences in LGI (p < 0.05 in Table 4). Figure 6 plots correlation coefficients for regions that were significantly more gyrified or less gyrified in SBM. Similar to the analyses of cortical thickness, LGI was positively correlated with IQ and/or fine motor dexterity in 40 of the total 48 correlations computed for regions that were significantly less gyrified in SBM, indicating that lower LGI was associated with poorer cognitive and motor outcomes. LGI in the few regions that were more gyrified in SBM was unrelated to IQ or fine motor dexterity; of the total 20 correlations computed, 10 were positive, and 10 were negative, in direction. In terms of the strength of relations between LGI and IQ, association sizes were small in 17 regions and medium in 8 regions. In terms of the strength of relations between LGI and fine motor dexterity, association sizes were small in 20 regions and medium in 6 regions.
Figure 6.
Spearman rank-order partial correlation coefficients, controlling for socioeconomic status, of 3D LGI with IQ and fine motor dexterity in spina bifida myelomeningocele (SBM). CAC = caudal anterior cingulate. CMF, caudal middle frontal; FP, frontal pole; IC, isthmus cingulate; IP, inferior parietal; IT, inferior temporal; LO, lateral occipital; LOF, lateral orbitofrontal; MT, middle temporal; PCL, paracentral; PCN, pericalcarine; PH, parahippocampal; POP, parsopercularis; POR, parsorbitalis; PTR, parstriangularis; RMF, rostral middle frontal; SM, supramarginal; SP, superior parietal; ST, superior temporal; STS, superior temporal sulcus; TT, transverse temporal.
Discussion
The cortex is atypically organized in individuals with SBM (Juranek et al. 2008; Juranek and Salman 2010). The present study explored the limits and functional consequences of atypical cortical organization by investigating relations of deviant levels of cortical thickness and gyrification with cognitive and motor function in children and young adults with SBM. Relative to a TD group, individuals with SBM had anomalous cortical thickness and gyrification at both ends of the size continuum, supporting the first hypothesis. Although some interactions involving hemisphere were statistically significant, the patterns across lobes and the cingulate cortex were generally bilateral, with interactions emerging because of small differences in magnitude in the temporal lobes for cortical thickness, and both the temporal and occipital lobes for LGI. Consistent with the second hypothesis, individuals with SBM exhibited generally significant and negative associations between cortical thickness and gyrification. As predicted by the third hypothesis, lower IQ and fine motor dexterity scores characteristic of SBM were associated with bidirectionally anomalous cortical thickness and gyrification. Whereas cortical thickness and gyrification tended to be negatively associated with cognitive and motor outcomes in cortical regions that were thicker or more gyrified in SBM, the associations tended to be positive in regions that were thinner or less gyrified in SBM. In effect, the more deviant the levels of cortical thickness and gyrification in SBM—whether higher or lower relative to the TD comparison individuals—the more impaired the cognitive and motor outcomes. Together, the results suggest that extremes of atypical cortical organization in SBM are functionally maladaptive, rather than adaptive.
The complex atypical cortical organization in SBM, involving aberrations in cortical thickness and gyrification, is likely principled in ways that are currently not well understood. Atypical cortical organization may be related to the variable effects of hydrocephalus and/or other disruptive developmental processes underlying the ontogeny of the cortical malformations observed in SBM. In congenital hydrocephalus, the likely primary pathological mechanism is tissue-damaging mechanical forces from expansion of the ventricles causing gradual destruction of periventricular white matter axons (see reviews by Del Bigio 2004, 2010). Our findings of lower cortical thickness and higher gyrification tracing the path of the lateral and third ventricles on both the lateral and medial surfaces of the brain highlight the possible contributions of hydrocephalus and disrupted underlying white matter connections to the cortex in SBM (a subcortical disconnection syndrome; Del Bigio 2010). Additional secondary reactive changes in congenital hydrocephalus might also contribute to aberrations in cortical development, including abnormal expression of trophic factors and/or reduced proliferation and migration of germinal cells away from the subventricular zone (Del Bigio 2004, 2010). Findings in animal models of congenital hydrocephalus also provide support for disruption of cortical development (Hale et al. 1992; Kriebel et al. 1993; Kriebel and McAllister 2000).
Alternatively, or additionally, atypical cortical organization in SBM may involve underlying malformative processes that affect the development of white matter, which are distinct from the effects of hydrocephalus (Del Bigio 2010). Diffusion tensor imaging of the association fibers in SBM shows higher transverse diffusivity values in SBM relative to TD controls, suggestive of long-term effects on myelination, including possible degeneration and failure of reparative mechanisms (Hasan et al. 2008). In addition, hypogenesis of the corpus callosum, which likely occurs prenatally in SBM, is difficult to explain as a mechanical effect of hydrocephalus and suggests additional malformative processes affecting the white matter (Barkovich 2005). Although our findings provide evidence of extreme atypical cortical organization in SBM, it is difficult to separate the effects of hydrocephalus from longer term effects on white matter development. Because key congenital anomalies characteristic of SBM are typically not seen in individuals with other forms of congenital hydrocephalus, such as from aqueductal stenosis, quantitative neuroimaging evaluations in such populations would help to address these questions.
The negative association between cortical thickness and gyrification in SBM might suggest another mechanical process contributing to atypical cortical organization, whereby excesses or reductions in 1 cortical property compromise the level of the other cortical property in the same region, but in the opposite direction. For example, the cortex may be excessively gyrified in the inferior parietal and supramarginal cortices because the cortex is aberrantly thinned in these regions and, as a result, may fold more compactly. Emerging hypotheses concerning the cellular mechanisms underlying gyrification may also support the inverse relation of thickness and gyrification in aberrant conditions. The degree of cortical thickness appears to be determined, at least partially, by the number of underlying neural connections (Huttenlocher and Dabholkar 1997; Van Essen 1997; Chklovskii et al. 2004; Sur and Rubenstein 2005), which may have an effect on the degree of gyrification; however, whether the relations between the number of underlying neural connections and the level of gyrification would be predicted to be an inverse relation remains under investigation (White et al. 2010).
Our finding of a generally negative association between cortical thickness and gyrification in many cortical regions in the TD group is one of the first reports of a significant association between cortical thickness and gyrification in a TD sample. The relation between cortical thickness and gyrification was less consistent in this group relative to the SBM group, possibly because of the relatively smaller sample size (n = 26) of TD individuals. As a result, this finding will require confirmation in a larger sample. Our results add to previous findings of inconsistent results in TD individuals (Thompson et al. 2005; Lin et al. 2007; Bearden et al. 2009). Variability in results between studies may be attributable to the various methods by which the cortical properties (especially gyrification) were quantified, the various statistical methods by which their relation was analyzed, and our exploratory approach.
The present study answers the broad question about the functional significance of atypical cortical organization in SBM by demonstrating regions of atypical cortical organization that are associated with poorer, not better, function. More specifically, the study highlights bidirectional deviance in thickness and gyrification as a cause of poorer outcome. Our findings illustrate the “Goldilocks principle,” recently applied to neurodevelopmental disorders by Dennis (2009). Referencing the fairytale of a girl who entered the house of 3 bears, the Goldilocks principle is applied across many disciplines (including economics, astrobiology, and medicine) to explain optimal circumstances falling between less-optimal extremes. Applied to neurodevelopmental disorders, the principle proposes that brain development needs to be within homeostatic limits (e.g. “not too big, not too small, but just right”) to support normative cognitive and motor functioning (Dennis 2009).
The Goldilocks principle may generalize to other disorders involving aberrant neurodevelopment. Evidence in support of the “too big” component of the Goldilocks principle has been reported in children born preterm (Kesler et al. 2006). Higher gyrification in the temporal lobes, relative to full-term comparison individuals, was negatively correlated with reading abilities in these regions. Evidence in support of the “too small” component of the Goldilocks principle has been demonstrated in individuals with bipolar disorder, in which reduced prefrontal gyrification, relative to comparison individuals, was positively correlated with IQ in this region (McIntosh et al. 2009). To our knowledge, however, the present study is the first investigation to show both positive and negative associations (i.e. “too small” and “too big”) of either cortical thickness or gyrification with cognitive and motor outcomes that depend on the direction of the cortical deviation within a single disorder. Future research should investigate whether the same trends of cortical thickness or gyrification with cognitive and motor functioning are found in other conditions in which both excesses and reductions have been identified, such as in 22q11.2 deletion syndrome (Bearden et al. 2007, 2009; Schaer et al. 2009), autism spectrum disorder (Hyde et al. 2010; Jiao et al. 2010), schizophrenia (White and Hilgetag 2011), temporal lobe epilepsy (Lin et al. 2007; Voets et al. 2011), and Turner syndrome (Raznahan et al. 2010).
To be sure, functional outcome is broader than the measures we have used in this study. Deviant levels of cortical thickness, gyrification, or some other neural property might be associated with aspects of intact cognitive and motor functioning not examined here. For example, word recognition—a strength in many children with SBM—was examined in a combined functional and volumetric neuroimaging study of children with SBM and TD children (Simos et al. 2011). Despite comparable word recognition between groups, individuals with SBM showed lower activation in left supramarginal and bilateral angular gyri, as well as a trend for lower surface area/gray matter volume in the left angular gyrus, relative to a TD group. However, individuals with SBM also showed higher gyrification in the left superior and middle temporal gyri and in the left middle frontal gyrus. Higher gyrification in these regions was positively associated with outcome, and may also represent an atypical but effective basis for word recognition.
It is also unclear whether atypical brain organization principles are similar for cortical and infratentorial regions. For instance, the area of the midsagittal cerebellar vermis is expanded in those individuals with SBM who show preserved functionality of eye movements, whereas normative ranges of midsagittal cerebellar vermis area are associated with eye movement deficits (Salman et al. 2009). Future investigations should delineate directionally aberrant brain development in relation to a range of outcomes in SBM.
Given our exploratory analyses and the fact that we used a cross-sectional age design, it is not clear how far the present results can be generalized across cortical development. Future investigations in disordered or TD samples should examine the longitudinal trajectories of cortical thickness and gyrification in relation to cognitive and motor outcomes in order to provide a fuller understanding of their interrelations throughout development.
The present investigation provides significant new information about the functional significance of atypical cortical organization for a common neurodevelopmental disorder, and about the discordance between structure and function. We have shown that atypical organization of the cortex in SBM is extensive; that there is an inverse relation between cortical thickness and gyrification in several cortical regions of the brain in individuals with SBM; and that bidirectional deviations—too little or too much cortical thickness and gyrification—are dysfunctional. This underscores the fact that, in neurodevelopmental disorders, similar cognitive and motor outcomes may be the result of neuroanatomical mechanisms that differ between and within individuals. The broader implication of the data is that atypical cortical organization need not be universally adaptive, and structural adaptations need not always preserve or enhance functionality. While circuit plasticity in animals may rearrange brain connectivity after a brain lesion, the anomalous synaptic circuitry can also be responsible for permanent functional deficits (Schneider and Jhaveri 1974; Isaacson 1975; Schneider 1979; Goldman-Rakic 1980; Giza and Prins 2006). The data reported here provide modern MRI evidence of this principle in human individuals with a neurodevelopmental disorder and add the important new information that bidirectional deviations, not just a loss or excess of neural tissue, may be associated with cognitive and motor dysfunction.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant (P01 HD35946-06, “Spina Bifida: Cognitive and Neurobiological Variability”). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
Notes
Conflict of Interest: None declared.
References
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Awate SP, Win L, Yushkevich P, Schultz RT, Gee JC. 3D cerebral cortical morphometry in autism: Increased folding in children and adolescents in frontal, parietal, and temporal lobes. Med Image Comput Comput Assist Interv. 2008;11:559–567. doi: 10.1007/978-3-540-85988-8_67. [DOI] [PubMed] [Google Scholar]
- Barkovich J. Pediatric neuroimaging. Philadelphia (PA): Lippincott, Williams & Wilkins; 2005. [Google Scholar]
- Batty MJ, Liddle EB, Pitiot A, Toro R, Groom MJ, Scerif G, Liotti M, Liddle PF, Paus T, Hollis C. Cortical gray matter in attention-deficit/hyperactivity disorder: a structural magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49:229–238. doi: 10.1016/j.jaac.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, van Erp TG, Dutton RA, Lee AD, Simon TJ, Cannon TD, Emanuel BS, McDonald-McGinn D, Zackai EH, Thompson PM. Alterations in midline cortical thickness and gyrification patterns mapped in children with 22q11.2 deletions. Cereb Cortex. 2009;19:115–126. doi: 10.1093/cercor/bhn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, van Erp TG, Dutton RA, Tran H, Zimmermann L, Sun D, Geaga JA, Simon TJ, Glahn DC, Cannon TD, et al. Mapping cortical thickness in children with 22q11.2 deletions. Cereb Cortex. 2007;17:1889–1898. doi: 10.1093/cercor/bhl097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Araque J, Giedd J, Rumsey JM. Reduced brain size and gyrification in the brains of dyslexic patients. J Child Neurol. 2004;19:275–281. doi: 10.1177/088307380401900407. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cognitive Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain Pathol. 2004;14:317–324. doi: 10.1111/j.1750-3639.2004.tb00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR. Neuropathology and structural changes in hydrocephalus. Dev Disabil Res Rev. 2010;16:16–22. doi: 10.1002/ddrr.94. [DOI] [PubMed] [Google Scholar]
- Dennis M. The Goldilocks dilemma in neurodevelopmental disorders: A functional brain that is not too big, not too small, but just right. Annual Meeting of the International Neuropsychological Society; 2009 Feb 11–14; Atlanta, GA. J Int Neuropsychol Soc. 2009;15(Suppl 1):1–249. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, Reiss AL. Children and adolescents with velocardiofacial syndrome: A volumetric MRI study. Am J Psychiatr. 2000;157:409–415. doi: 10.1176/appi.ajp.157.3.409. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Copeland K, Frederick JA, Blaser SE, Kramer LA, Northrup H, Hannay HJ, Brandt ME, Francis DJ, Villarreal G, et al. Spinal lesion level in spina bifida: A source of neural and cognitive heterogeneity. J Neurosurg. 2005;102:268–279. doi: 10.3171/ped.2005.102.3.0268. [DOI] [PubMed] [Google Scholar]
- Gaser C, Luders E, Thompson PM, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Bellugi U, Galaburda AM, Korenberg JR, et al. Increased local gyrification mapped in Williams syndrome. Neuroimage. 2006;33:46–54. doi: 10.1016/j.neuroimage.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giza CC, Prins ML. Is being plastic fantastic? Mechanisms of altered plasticity after developmental traumatic brain injury. Dev Neurosci. 2006;28:364–379. doi: 10.1159/000094163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Morphological consequences of prenatal injury to the primate brain. Prog Brain Res. 1980;53:1–19. [PubMed] [Google Scholar]
- Hale PM, McAllister JP, 2nd, Katz SD, Wright LC, Lovely TJ, Miller DW, Wolfson BJ, Salotto AG, Shroff DV. Improvement of cortical morphology in infantile hydrocephalic animals after ventriculoperitoneal shunt placement. Neurosurgery. 1992;31:1085–1096. doi: 10.1227/00006123-199212000-00015. [DOI] [PubMed] [Google Scholar]
- Hannay HJ, Dennis M, Kramer L, Blaser S, Fletcher JM. Partial agenesis of the corpus callosum in spina bifida meningomyelocele and potential compensatory mechanisms. J Clin Exp Neuropsychol. 2009;31:180–194. doi: 10.1080/13803390802209954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartberg CB, Lawyer G, Nyman H, Jonsson EG, Haukvik UK, Saetre P, Bjerkan PS, Andreassen OA, Hall H, Agartz I. Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatr Res. 2010;182:123–133. doi: 10.1016/j.pscychresns.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Eluvathingal TJ, Kramer LA, Ewing-Cobbs L, Dennis M, Fletcher JM. White matter microstructural abnormalities in children with spina bifida myelomeningocele and hydrocephalus: A diffusion tensor tractography study of the association pathways. J Magn Reson Imaging. 2008;27:700–709. doi: 10.1002/jmri.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Sankar A, Halphen C, Kramer LA, Ewing-Cobbs L, Dennis M, Fletcher JM. Quantitative diffusion tensor imaging and intellectual outcomes in spina bifida: Laboratory investigation. J Neurosurg Pediatr. 2008;2:75–82. doi: 10.3171/PED/2008/2/7/075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven (CT): Yale University; 1975. [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Lerch JP, Zatorre RJ, Griffiths TD, Evans AC, Peretz I. Cortical thickness in congenital amusia: When less is better than more. J Neurosci. 2007;27:13028–13032. doi: 10.1523/JNEUROSCI.3039-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Samson F, Evans AC, Mottron L. Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Hum Brain Mapp. 2010;31:556–566. doi: 10.1002/hbm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Yoon U, Shin YW, Hong SB, Kim IY, Kwon JS, Kim SI. Fractal dimension in human cortical surface: Multiple regression analysis with cortical thickness, sulcal depth, and folding area. Hum Brain Mapp. 2006;27:994–1003. doi: 10.1002/hbm.20238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson RL. The myth of recovery from early brain damage. In: Ellis NR, editor. Aberrant development in infancy: Human and animal studies. Hillsdale (NJ): Lawrence Erlbaum Associates; 1975. [Google Scholar]
- Jiao Y, Chen R, Ke X, Chu K, Lu Z, Herskovits EH. Predictive models of autism spectrum disorder based on brain regional cortical thickness. Neuroimage. 2010;50:589–599. doi: 10.1016/j.neuroimage.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J, Fletcher JM, Hasan KM, Breier JI, Cirino PT, Pazo-Alvarez P, Diaz JD, Ewing-Cobbs L, Dennis M, Papanicolaou AC. Neocortical reorganization in spina bifida. Neuroimage. 2008;40:1516–1522. doi: 10.1016/j.neuroimage.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J, Salman MS. Anomalous development of brain structure and function in spina bifida myelomeningocele. Dev Disabil Res Rev. 2010;16:23–30. doi: 10.1002/ddrr.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Ad-Dab'bagh Y, Haier RJ, Deary IJ, Lyttelton OC, Lepage C, Evans AC, Group TBDC. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2009;37:145–155. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Folley BS, Lanham DC, Capone GT, Kaufmann WE. Cerebral growth in Fragile X syndrome: review and comparison with Down syndrome. Microsc Res Tech. 2002;57:159–167. doi: 10.1002/jemt.10068. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Vohr B, Schneider KC, Katz KH, Makuch RW, Reiss AL, Ment LR. Increased temporal lobe gyrification in preterm children. Neuropsychologia. 2006;44:445–453. doi: 10.1016/j.neuropsychologia.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Kivitie-Kallio S, Autti T, Salonen O, Norio R. MRI of the brain in the Cohen syndrome: a relatively large corpus callosum in patients with mental retardation and microcephaly. Neuropediatrics. 1998;29:298–301. doi: 10.1055/s-2007-973581. [DOI] [PubMed] [Google Scholar]
- Kriebel RM, McAllister JP., 2nd Pathology of the hippocampus in experimental feline infantile hydrocephalus. Neurol Res. 2000;22:29–36. doi: 10.1080/01616412.2000.11741035. [DOI] [PubMed] [Google Scholar]
- Kriebel RM, Shah AB, McAllister JP., 2nd The microstructure of cortical neuropil before and after decompression in experimental infantile hydrocephalus. Exp Neurol. 1993;119:89–98. doi: 10.1006/exnr.1993.1009. [DOI] [PubMed] [Google Scholar]
- Lafayette Instruments. Purdue pegboard model #32020 instructions and normative data. Lafayette(IN): Lafayette Instruments; 1999. [Google Scholar]
- Lin JJ, Salamon N, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Luders E, Toga AW, Engel J, Jr, Thompson PM. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex. 2007;17:2007–2018. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Andreasen NC, Schultz SK, Harris G, Cizadlo T, Heckel D, Nopoulos P, Flaum M. Quantitative in vivo measurement of gyrification in the human brain: Changes associated with aging. Cereb Cortex. 1999;9:151–160. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Mangin JF, Jouvent E, Cachia A. In-vivo measurement of cortical morphology: Means and meanings. Curr Opin Neurol. 2010;23:359–367. doi: 10.1097/WCO.0b013e32833a0afc. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Moorhead TW, McKirdy J, Hall J, Sussmann JE, Stanfield AC, Harris JM, Johnstone EC, Lawrie SM. Prefrontal gyral folding and its cognitive correlates in bipolar disorder and schizophrenia. Acta Psychiatr Scand. 2009;119:192–198. doi: 10.1111/j.1600-0447.2008.01286.x. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Burgess MP, Gidley Larson JC. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130:2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW. Widespread neocortical abnormalities in temporal lobe epilepsy with and without mesial sclerosis. Neuroimage. 2009;46:353–359. doi: 10.1016/j.neuroimage.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Lagercrantz H, Hutton C. Effects of preterm birth on cortical thickness measured in adolescence. Cereb Cortex. 2010;21:300–306. doi: 10.1093/cercor/bhq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Lin J, Kim J, Phillips OR, Del'homme M, Caplan R, Toga AW, McCracken JT, Levitt JG. Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:1014–1022. doi: 10.1097/CHI.0b013e3181b395c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Oyegbile T, Hansen R, Magnotta V, O'Leary D, Bell B, Seidenberg M, Hermann BP. Quantitative measurement of cortical surface features in localization-related temporal lobe epilepsy. Neuropsychology. 2004;18:729–737. doi: 10.1037/0894-4105.18.4.729. [DOI] [PubMed] [Google Scholar]
- Rae C, Joy P, Harasty J, Kemp A, Kuan S, Christodoulou J, Cowell CT, Coltheart M. Enlarged temporal lobes in Turner syndrome: An X-chromosome effect? Cereb Cortex. 2004;14:156–164. doi: 10.1093/cercor/bhg114. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Cutter W, Lalonde F, Robertson D, Daly E, Conway GS, Skuse DH, Ross J, Lerch JP, Giedd JN, et al. Cortical anatomy in human X monosomy. Neuroimage. 2010;49:2915–2923. doi: 10.1016/j.neuroimage.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. How does your cortex grow? J Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddon JR, Gill DM, Gauk SE, Maerz MD. Purdue Pegboard: Test–retest estimates. Percept Mot Skill. 1988;66:503–506. doi: 10.2466/pms.1988.66.2.503. [DOI] [PubMed] [Google Scholar]
- Salman MS, Dennis M, Sharpe JA. The cerebellar dysplasia of Chiari II malformation as revealed by eye movements. Can J Neurol Sci. 2009;36:713–724. doi: 10.1017/s0317167100008325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 2008;27:161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- Schaer M, Debbane M, Bach Cuadra M, Ottet MC, Glaser B, Thiran JP, Eliez S. Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): A cross-sectional and longitudinal study. Schizophr Res. 2009;115:182–190. doi: 10.1016/j.schres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Schneider GE. Is it really better to have your brain lesion early? A revision of the “Kennard principle”. Neuropsychologia. 1979;17:557–583. doi: 10.1016/0028-3932(79)90033-2. [DOI] [PubMed] [Google Scholar]
- Schneider GE, Jhaveri SR. Neuroanatomical correlations of spared or altered function after brain lesions in the newborn hamster. In: Stein DG, Rosen JJ, Butters N, editors. Plasticity and recovery of function in the central nervous system. New York: Academic Press; 1974. [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Papanicolaou AC, Castillo EM, Juranek J, Cirino PT, Rezaie R, Fletcher JM. Brain mechanisms for reading and language processing in spina bifida meningomyelocele: A combined magnetic source- and structural magnetic resonance imaging study. Neuropsychology. 2011;25:590–601. doi: 10.1037/a0023694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield AC, Moorhead TW, Harris JM, Owens DG, Lawrie SM, Johnstone EC. Increased right prefrontal cortical folding in adolescents at risk of schizophrenia for cognitive reasons. Biol Psychiatry. 2008;63:80–85. doi: 10.1016/j.biopsych.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Subramaniam B, Naidu S, Reiss AL. Neuroanatomy in Rett syndrome: Cerebral cortex and posterior fossa. Neurology. 1997;48:399–407. doi: 10.1212/wnl.48.2.399. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Tan WH, Bacino CA, Skinner SA, Anselm I, Barbieri-Welge R, Bauer-Carlin A, Beaudet AL, Bichell TJ, Gentile JK, Glaze DG, et al. Angelman syndrome: Mutations influence features in early childhood. Am J Med Genet. 2011;155A:81–90. doi: 10.1002/ajmg.a.33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Eckert MA, Bellugi U, Galaburda AM, Korenberg JR, Mills DL, et al. Abnormal cortical complexity and thickness profiles mapped in Williams syndrome. J Neurosci. 2005;25:4146–4158. doi: 10.1523/JNEUROSCI.0165-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike R, Hagen E, Sattler J. The Stanford–Binet intelligence scale. Itasca (IL): Riverside; 1986. [Google Scholar]
- Tiffen J. Purdue Pegboard test. Chicago: Scientific Research Associates; 1968. [Google Scholar]
- Vachha B, Adams RC, Rollins NK. Limbic tract anomalies in pediatric myelomeningocele and Chiari II malformation: anatomic correlations with memory and learning—initial investigation. Radiology. 2006;240:194–202. doi: 10.1148/radiol.2401050674. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Voets NL, Bernhardt BC, Kim H, Yoon U, Bernasconi N. Increased temporolimbic cortical folding complexity in temporal lobe epilepsy. Neurology. 2011;76:138–144. doi: 10.1212/WNL.0b013e318205d521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Weber G, Neuhold A, Rett A. Down syndrome: MR quantification of brain structures and comparison with normal control subjects. Am J Neuroradiol. 1991;12:1207–1211. [PMC free article] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 2003;54:418–426. doi: 10.1016/s0006-3223(03)00065-9. [DOI] [PubMed] [Google Scholar]
- White T, Hilgetag CC. Gyrification and neural connectivity in schizophrenia. Dev Psychopathol. 2011;23:339–352. doi: 10.1017/S0954579410000842. [DOI] [PubMed] [Google Scholar]
- White T, Su S, Schmidt M, Kao CY, Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010;72:36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobrock T, Gruber O, McIntosh AM, Kraft S, Klinghardt A, Scherk H, Reith W, Schneider-Axmann T, Lawrie SM, Falkai P, et al. Reduced prefrontal gyrification in obsessive-compulsive disorder. Eur Arch Psychiatry Clin Neurosci. 2010;260:455–464. doi: 10.1007/s00406-009-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH. Abnormal cerebral cortex structure in children with ADHD. Hum Brain Mapp. 2009;30:175–184. doi: 10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhou Y, Yu C, Lin L, Li C, Jiang T. Reduced cortical folding in mental retardation. Am J Neuroradiol. 2010;31:1063–1067. doi: 10.3174/ajnr.A1984. [DOI] [PMC free article] [PubMed] [Google Scholar]