Abstract
The primate cerebral cortex is characterized by regional variation in the structure of pyramidal neurons, with more complex dendritic arbors and greater spine density observed in prefrontal compared with sensory and motor cortices. Although there are several investigations in humans and other primates, virtually nothing is known about regional variation in the morphology of pyramidal neurons in the cerebral cortex of great apes, humans' closest living relatives. The current study uses the rapid Golgi stain to quantify the dendritic structure of layer III pyramidal neurons in 4 areas of the chimpanzee cerebral cortex: Primary somatosensory (area 3b), primary motor (area 4), prestriate visual (area 18), and prefrontal (area 10) cortex. Consistent with previous studies in humans and macaque monkeys, pyramidal neurons in the prefrontal cortex of chimpanzees exhibit greater dendritic complexity than those in other cortical regions, suggesting that prefrontal cortical evolution in primates is characterized by increased potential for integrative connectivity. Compared with chimpanzees, the pyramidal neurons of humans had significantly longer and more branched dendritic arbors in all cortical regions.
Keywords: area 10, dendrites, evolution, Golgi, primate cerebral cortex
Introduction
Neuromorphological studies of the primate cerebral cortex have revealed remarkable variation in the structure of pyramidal neurons across different cortical areas. Specifically, it has been shown that in humans and other primates the extent and branching complexity of dendrites, as well as the number and density of spines, increases from primary sensory and motor to higher order processing areas, such as the prefrontal cortex (Elston and Rosa 1997, 1998; Elston et al. 1999a, 1999b, 2001, 2005a, 2005b, 2006; Elston 2000; Jacobs et al. 1997, 2001). Additionally, of all the cortical regions examined, the prefrontal cortex is characterized by the greatest inter- and intraspecific neuromorphological variation in Old World monkeys (Elston et al. 2011). The pyramidal neurons of the human prefrontal cortex, furthermore, have been shown to exhibit a greater total number of dendritic spines compared with the prefrontal cortex of other primates, while in other cortical regions interspecific differences in the pyramidal neuron phenotype are not as pronounced (Elston et al. 2001, 2003, 2006). The prefrontal cortex is a heteromodal association region that subserves high-order cognitive functions (Fuster 1997) and displays anatomical specializations in humans compared with other great apes (Semendeferi et al. 2001, 2011; Smaers et al. 2011), such as microstructural changes indicating increased neuropil fraction and spacing among cortical minicolumns (Semendeferi et al. 2011; Spocter et al. 2012). Taken together, these data suggest that the evolution of the prefrontal cortex of primates, and especially of humans, has been characterized by substantial reorganization of wiring for enhanced neuronal integration.
Although studies of cortical neuronal morphology have been conducted in Old World monkeys (i.e. macaque monkeys, vervets, baboons; Elston and Rosa 1997, 1998; Elston et al. 1999a, 2005a, 2005b, 2011; Elston 2000), New World monkeys (Elston et al. 1999b) and strepsirrhine primates (Elston et al. 2005c), there are currently no data concerning regional differences in dendritic complexity of any great ape species (i.e. chimpanzees, bonobos, gorillas, and orangutans), humans' closest living relatives. Such data are critical to determine what features of pyramidal neuron morphology in humans have been uniquely modified in evolution since the last common ancestor shared with great apes. In the current study, we used rapid Golgi staining to quantify differences in the neuromorphology of supragranular pyramidal neurons in 4 areas of the chimpanzee cerebral cortex, including primary somatosensory (area 3b), primary motor (area 4), prestriate visual (area 18), and rostral prefrontal (area 10) cortex. We expected that the prefrontal cortex (area 10), which is involved in higher cognitive processing, would contain neurons with longer dendrites, greater dendritic branching, and higher spine number and density than regions such as somatosensory, motor, and visual areas (areas 3b, 4, and 18). We also sought to compare our results in chimpanzees to data from human pyramidal neurons in order to examine whether dendritic arbors in the prefrontal cortex (area 10) appear disproportionately elaborated in human evolution.
Materials and Methods
Specimens
Formalin-fixed samples were obtained from the left hemisphere of the brains of common chimpanzees (Pan troglodytes; see Table 1 for details) that were donated by the Yerkes National Primate Research Center (Atlanta, GA), the MD Anderson Cancer Center of the University of Texas, and the Maryland Zoo in Baltimore. Chimpanzees were housed according to each institution's guidelines; none was euthanized for the purpose of this study and all had died from natural causes. None of the animals used in this study showed evidence of neurological disorder and all brains appeared normal by routine assessment for pathology or in magnetic resonance images.
Table 1.
Demographic information for chimpanzee (N = 7) and human samples (N = 8).
| Age (years) | Sex | PMIa (h) | Length of fixation | |
|---|---|---|---|---|
| Chimpanzee # | ||||
| 1 | 23 | F | >14 | 13 days |
| 2 | 28 | M | 12 | 14 days |
| 3 | 30 | M | ∼4 | 4 months |
| 4 | 30 | M | ∼4 | 2 months |
| 5 | 31 | M | ∼4 | 4 months |
| 6 | 39 | M | ∼4 | 3 months |
| 7 | 43 | F | ∼4 | 3 months |
| Human # | ||||
| 1 | 14 | M | 14 | 43 days |
| 2 | 22 | F | 1 | 23 days |
| 3 | 23 | M | 12 | 57 days |
| 4 | 32 | F | 20 | 14 days |
| 5 | 32 | M | 9 | 21 days |
| 6 | 34 | F | 14.5 | 42 days |
| 7 | 50 | M | 22 | 20 days |
| 8 | 69 | M | 7 | 8 days |
aPost-mortem interval.
Age, sex, post-mortem interval (PMI), and length of fixation of the chimpanzee (N = 7) and human samples (N = 8).
Tissue Preparation and Golgi Staining
Within 14 h of each subject's death, the brain was removed and immersed in 10% formalin. The brain was transferred to 0.1 M phosphate-buffered saline with 0.1% sodium azide solution within 4 months and stored at 4 °C. The regions of interest were chosen because of their inclusion in previous human studies (Jacobs et al. 1997, 2001). Blocks of 3–5 mm in thickness, each containing one region of interest from the left hemisphere, were removed and randomly coded to prevent experimenter bias. The portion of area 4 that was sampled corresponded to the region of hand representation, located within the “knob” of the precentral gyrus (Hopkins et al. 2010); the part of area 3b that was sampled was located on the inner surface of the postcentral gyrus adjacent to the “knob”; the dorsal part of area 18 was used in the analyses.
Modified rapid Golgi staining (Scheibel and Scheibel 1978) was performed using the same protocol described previously for humans (Jacobs et al. 1997). Tissue blocks containing the regions were first incubated in an osmic acid solution for ∼72 h, followed by incubation in silver nitrate for 24 h. Blocks were then sectioned on a vibratome at 120 μm, mounted, coverslipped, and stored flat and protected from light at 4 °C. Of 9 individuals that were initially processed, 2 had to be discarded due to incomplete impregnation.
Morphological Analysis
Ten relatively isolated layer III pyramidal neurons were selected per region, for a total of 40 neurons per individual (N = 280). Selection criteria followed Jacobs et al. (1997, 2001). Briefly, neurons had to be well impregnated, located within the center of the section, and be as complete as possible, typically showing at least 2 complete basilar dendrites with multiple branching, and a single apical dendrite extending from the cell body to the pial surface. Higher order dendritic branches were determined to be complete when the terminal ends narrowed and then tapered off and could be found within the 120-μm section thickness. To avoid sampling neurons from different cortical layers, an ongoing tally of average soma depth was recorded, and neurons were selected within a range that spanned across cortical layer III (mean soma depth for area 3b: 796 ± 209 μm, area 4: 707 ± 249 μm, area 18: 692 ± 241 μm, area 10: 734 ± 224 μm). No distinction was made between the sublaminae of layer III (a, b, c). Neurons meeting criteria were traced using a Zeiss Axioplan 2 photomicroscope (Zeiss, Thornwood, NY, United States of America) equipped with a Ludl XY motorized stage (Ludl Electronics, Hawthorne, NY, United States of America), Heidenhain z-axis encoder, and an Optronics MicroFire color videocamera (Optronics, Golenta, CA, United States of America) coupled to a Dell PC workstation running Neurolucida software (MBF Bioscience, Williston, VT, United States of America). Neuron tracing was performed on a computer screen under Koehler illumination using a ×40 objective (Zeiss Plan-Apochromat, N.A. 0.75), by following dendrites along their entire length through the Z-plane, and counting all visible spines. Once tracing was complete, neuron morphology was quantified in NeuroExplorer (MBF Bioscience), according to the following measurements of dendritic structure (Jacobs et al. 2001): (1) total dendritic length (TDL)—the sum of the individual lengths of all dendritic segments; (2) dendritic segment count (DSC)—the number of all dendritic segments; (3) mean segment length (MSL)— TDL/DSC, (4) dendritic spine number (DSN)—the number of all spines marked on the dendritic arbor; and (5) dendritic spine density (DSD)—the ratio of spines per unit (1 μm) of dendritic length. Cell body cross-sectional area was also recorded, as well as cell soma depth in the cortex. Dendritic measurements were taken for both basilar and apical dendrites. However, because apical dendrites were often incomplete due to sectioning, quantitative comparisons of dendritic length and spine number across cortical regions were performed only for basilar dendrites. Nonetheless, spine density, which is a relative measure (count by length), was calculated for both basilar and apical dendrites. In addition, a Sholl analysis was also performed to examine the number of intersections per 20 μm of basilar dendritic length.
Statistical Analysis
All tracings were performed blind to the regions of interest by C.D.S. and S.B., who were normed with another rater (A.L.B.) and checked by C.C.S. To ensure accuracy, intrarater reliability was assessed by tracing the same dendritic branch 10 times. Coefficients of variation calculated by S.B. (cell soma area = 5.1%, TDL = 1.8%, and DSN = 2.6%) and C.D.S. (cell soma area = 9.16%, TDL = 1.93%, DSN = 5.72%) showed little variation, and a split-plot design revealed no significant differences between the first and the second half of the tracings (P > 0.05). As assessed by the coefficient of intraclass correlation, interrater reliability was high: Cell soma area = 0.90, TDL = 0.98, DSN = 0.91 (A.L.B and S.B.), and cell soma = 0.58, TDL = 0.99, and DSN = 0.99 (S.B and C.D.S.).
Data for the 6 variables of interest (soma area, TDL, DSC, MSL, DSN, DSD) were analyzed separately among regions. Specifically, we used a nested ANOVA design (IBM SPSS 18.0), in which each neuron was nested within region (areas 3b, 4, 10, and 18), which was nested within individual brain. We did not consider sex differences in the analysis because of the relatively small sample size (2 females, 5 males). Pairwise contrasts were then performed using a Bonferroni correction for multiple comparisons.
As dendrites may be incomplete due to sectioning, to ensure that the most complete cells were compared across regions, analyses were run on the entire data set (N = 280), as well as on a subset of neurons showing at least 3 dendritic trees and fourth-order branches (area 3b n = 37, area 4 n = 50, area 10 n = 53, area 18 n = 43). The latter was also used to compare the current data on chimpanzee dendritic morphology with existing Golgi data from humans (Jacobs et al. 1997, 2001), collected for areas 3b, 4, 10, and 18 by using the same staining protocol, section thickness, and experimental procedure as that employed in the current study. The human sample comprised 8 individuals (individual details are provided in Table 1). Combined data from chimpanzees and humans were analyzed by performing a mixed model repeated-measures ANOVA with species as a between-subject factor and cortical region as a within-subject factor using the mean data from each cortical region.
Results
The tissue appeared generally well impregnated. Morphologically, layer III pyramidal neurons in chimpanzees were characterized by a dendritic “skirt” of basilar dendrites emerging from the base of a triangular-shaped cell body, and 1 vertical apical dendrite, with oblique secondary branches ascending perpendicularly toward the pial surface. All neurons were sampled from layer III, at an overall average cortical depth of 737 ± 324 μm (Table 2). There were no significant differences among regions in the depth of the sampled neurons.
Table 2.
Dependent measures in areas 3b, 4, 18, and 10 in chimpanzees and humans.
| Area 3b | Area 4 | Area 18 | Area 10 | |
|---|---|---|---|---|
| Chimpanzee | ||||
| Cell soma area (μm2) | 183 ± 88 | 185 ± 62 | 167 ± 75 | 190 ± 63 |
| Cell soma depth (μm) | 796 ± 209 | 707 ± 247 | 692 ± 241 | 733 ± 224 |
| Total dendritic length (TDL, μm) | 894 ± 429 | 966 ± 377 | 851 ± 406 | 1329 ± 705 |
| Mean segment length (MSL, μm) | 27.5 ± 8.2 | 46.7 ± 11.7 | 39.9 ± 11.8 | 49.1 ± 15.7 |
| Dendritic segment count (DSC, μm) | 21 ± 8 | 21 ± 6 | 21 ± 7 | 27 ± 10 |
| Dendritic spine number (DSN, μm) | 148 ± 78 | 166 ± 101 | 169 ± 84 | 401 ± 203 |
| Dendritic spine density (DSD, μm) | 0.16 ± 0.04 | 0.17 ± 0.07 | 0.19 ± 0.04 | 0.33 ± 0.22 |
| Dendritic trees count | 4.18 ± 0.88 | 4.49 ± 1.09 | 4.28 ± 1.16 | 4.88 ± 1.43 |
| Human | ||||
| Cell soma area (μm2) | 235 ± 79 | 273 ± 88 | 212 ± 89 | 288 ± 81 |
| Cell soma depth (μm) | 847 ± 164 | 822 ± 191 | 785 ± 156 | 784 ± 166 |
| Total dendritic length (TDL, μm) | 3179 ± 1071 | 3563 ± 1191 | 3465 ± 760 | 4187 ± 1329 |
| Mean segment length (MSL, μm) | 62.4 ± 9.8 | 66.9 ± 12.3 | 63.8 ± 9.5 | 70.7 ± 12.8 |
| Dendritic segment count (DSC, μm) | 51 ± 15 | 53 ± 15 | 54 ± 13 | 59 ± 16 |
| Dendritic spine number (DSN, μm) | 738 ± 457 | 818 ± 326 | 1023 ± 424 | 1340 ± 600 |
| Dendritic spine density (DSD, μm) | 0.17 ± 0.06 | 0.19 ± 0.05 | 0.23 ± 0.09 | 0.26 ± 0.1 |
Values of cell soma area, total dendritic length, mean segment length, dendritic segment count, dendritic spine number and density in areas 3b, 4, 18, and 10 in chimpanzees and humans.
Regional Differences in Pyramidal Neuron Morphology in the Chimpanzee Cerebral Cortex
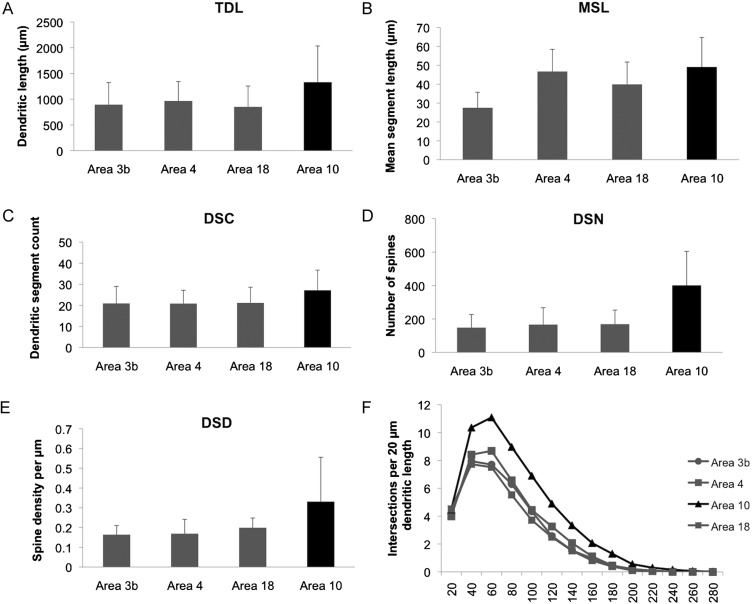
Significant regional differences were found for all variables (Figs 1–3) (cell body area: F(21,252)= 3.21, P< 0.001; TDL: F(21,252)= 5.57, P< 0.001; MSL: F(21,252)= 4.82, P< 0.001; DSC: F(21,252)= 3.78, P< 0.001; DSN: F(21,252)= 13.04, P< 0.001; DSD: F(21,252)= 5.59, P< 0.001). Pairwise comparisons indicated that prefrontal area 10 differed from the other cortical areas in having neurons with greater dendritic length (P< 0.001), spine number (P< 0.001), spine density (P< 0.001), and MSL (P= 0.001 for area 3b, P < 0.001 for area 18; but no differences with area 4, P= 0.93; Table 2). Compared with the average for neurons in areas 3b, 4, and 18, TDL and MSL were, respectively, 47% and 31% greater in area 10, the spine number was more than double (150%), and spine density was 86% higher. Although the mean number of dendritic trees per neuron was the same across regions (∼4), the number of dendritic segments in the basilar arbors of prefrontal neurons was 30% greater than other cortical areas (P< 0.001), indicating increased structural complexity of neurons in this region. This pattern was also confirmed by a Sholl analysis, which revealed that pyramidal neurons in prefrontal area 10 had more intersections per 20 μm of dendritic length compared with the other regions (F(21,252)= 6.62, P < 0.001). In addition, pyramidal neurons in area 10 had a larger soma area than pyramidal neurons in area 18 (P= 0.048), as well as greater spine density of apical dendrites (F(21,252)= 8.36, P < 0.001). Although substantial interindividual variability was observed for all the variables, the pattern of regional differences defined by greater dendritic length, increased spine density, and more elaborate branching complexity in prefrontal area 10 characterized most subjects. In contrast, no significant differences were observed among areas 3b, 4, or 18, which showed similar values. Regional differences were also confirmed when only the largest and most complete cells, as defined by at least 3 dendritic trees and fourth-order dendritic branches, were included in the analysis (TDL: F(21,155)= 3.82, P < 0.001; MSL: F(21,155)= 3.49, P < 0.001; DSC: F(21,155)= 2.56, P= 0.001; DSN: F(21,155)= 8.23, P < 0.001; DSD: F(21,155)= 2.90, P < 0.001). Area 10 showed longer dendrites than area 3b (P < 0.001), area 4 (P = 0.003), and area 18 (P < 0.001); greater MSL than area 18 (P < 0.001), but not area 4 (P = 1.000), and area 3b (P = 0.057); a higher number of dendritic segments than area 3b (P = 0.001), area 4 (P < 0.001), and area 18 (P < 0.001), as well as greater spine number (P < 0.001) and density (area 3b, and 4: P < 0.001; area 18: P < 0.006).
Figure 1.
(A–E) Regional differences in TDL (in μm), MSL (in μm), DSC, DSN, and DSD in areas 3b, 4, 18, and 10. (F) Results from a Sholl analysis, reporting the mean number of intersections per 20 μm of dendrite length. Error bars indicate standard deviations.
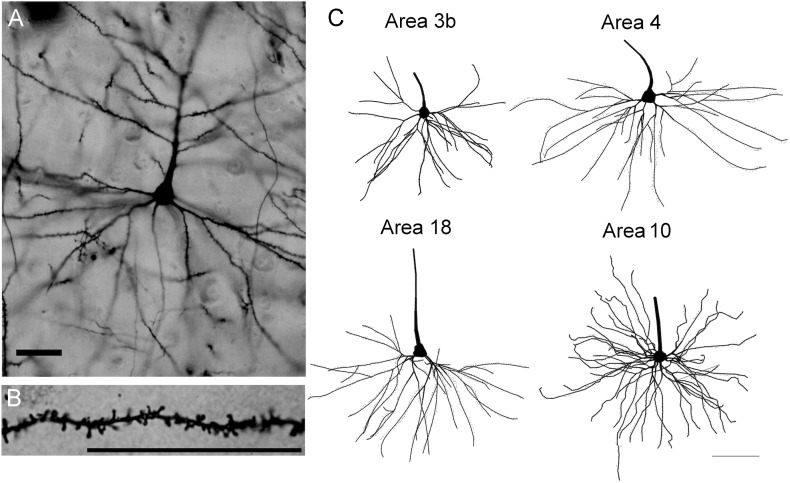
Figure 2.
(A and B) Photomicrographs of pyramidal neurons in area 10 of chimpanzees; (C) close-up of a basilar dendritic shaft of a pyramidal neuron in area 10. Scale bar in (A and B) = 100 μm and scale bar in (C) = 50 μm.
Figure 3.
Tracings of chimpanzee pyramidal neurons in areas 3b, 4, 18, and 10. Scale bar = 100 μm.
Comparison of Regional Differences in Dendritic Morphology Between Chimpanzees and Humans
As expected, analysis of the combined human and chimpanzee data revealed a main effect of cortical region for each variable (cell soma area: F(3,39) = 3.27, P= 0.03; TDL: F(3,39)= 11.39, P < 0.001; MSL: F(3,39)= 6.91, P = 0.004; DSC: F(3,39)= 7.71, P < 0.001; DSN: F(3,39)= 15.03, P < 0.001; DSD: F(3,39)= 13.27, P < 0.001). Pairwise comparisons confirmed that area 10 was significantly different from the other regions for both humans and chimpanzees, showing longer dendrites than areas 3b and 18 (P < 0.001), and approaching significance for area 4 (P= 0.094); greater MSL than area 3b (P = 0.018) and area 18 (P < 0.001); higher number of dendritic segments than area 3b (P = 0.008), area 4 (P = 0.026), and area 18 (P = 0.012); greater spine number than area 3b (P = 0.002), area 4 (P = 0.004), and area 18 (P < 0.001), as well as greater spine density than area 3b (P = 0.003) and area 4 (P = 0.001).
A main effect of species was also found for most variables, revealing that humans had larger dendritic arbors, with longer dendritic segments and more spines than chimpanzees (cell soma area: F(1,13)= 12.80, P = 0.003, TDL: F(1,13)= 222.35, P < 0.001; MSL: F(1,13)= 66.78, P < 0.001; DSC: F(1,13)= 141.45, P < 0.001; DSN: F(1,13)= 45.94, P < 0.001). For spine density, however, no species effect was found (F(1,13)= 0.002, P = 0.96).
No significant interaction was found between species and region for any measure of dendritic morphology (cell soma area: F(3,39)= 1.19, P= 0.33, TDL: F(3,39)= 1.43, P =0.25, MSL: F(3,39)= 0.78, P = 0.47; DSC: F(3,39)= 0.79, P = 0.39; DSN: F(3,39)= 2.43, P = 0.13, DSD: F(3,39)= 1.754, P = 0.17).
Discussion
The present study demonstrates that layer III pyramidal neurons in prefrontal cortex (area 10) of chimpanzees are more branched and spiny than pyramidal neurons in other cortical regions (i.e. areas 3b, 4, and 18). These results are consistent with previous observations in humans (Jacobs et al. 1997, 2001) and macaque monkeys (Elston 2000; Elston et al. 2001, 2006), and suggest that amplification of neuronal integration in the prefrontal cortex is a common feature that characterizes Old World anthropoid primates. In addition, when human and chimpanzee layer III pyramidal neurons were directly compared, the results showed that elaboration of dendritic branching distinguishes human neurons across all the cortical regions that were examined. However, there was not a disproportionate increase in the dendritic extent of human pyramidal neurons selectively in prefrontal cortex (area 10) (Fig. 4).
Figure 4.
(A) Photomicrograph of a pyramidal neuron in area 10 of the human prefrontal cortex; (B) close-up of a basilar dendritic branch of a pyramidal neuron in area 10 in the human prefrontal cortex; (C) tracings of human neurons in areas 3b, 4, 18, and 10. Note that only the basilar dendrites were traced in these neurons. Scale bars: (A) = 50 μm; (B) = 50 μm; (C) = 100 μm.
Methodological Considerations
Since the pioneering work by Ramón y Cajal, the Golgi stain has been widely employed to visualize different neuron types in the mammalian cerebral cortex. More recently, techniques based on florescent dyes, such as those utilizing Lucifer Yellow intracellular injections, have also proven effective to visualize dendritic arbors and spines in a range of species (see Elston et al. 2009). Compared with the rapid Golgi method, intracellular dye injection techniques are more predictable and allow for acquisition of data from physiological or tract tracing experiments in conjunction with morphological visualization of a targeted selection of neurons (Hanani 2012). In contrast to the Golgi stain, however, intracellular injections do not provide a random sampling of neurons, and appears to be problematic when applied to autopsy tissue (Jacobs et al. 1997). For these reasons, Golgi impregnation techniques remain a primary tool for examining neuronal morphology in postmortem human tissue, as well as tissue from other species, such as hominoids, for which invasive procedures are not possible, tissue availability is scarce, and fixation lengths may be relatively long.
Before discussing the implications of our findings, methodological limitations of the rapid Golgi method should be considered. For instance, the values reported by the current study for spine number and dendritic length are likely to be an underestimation of the actual biological values. This is because spines situated immediately above and below the dendritic shaft may be obscured under traditional light microscopy and a section thickness of 120 μm does not encompass the entire dendritic array. In fact, differences in section thickness have been shown to affect estimates of TDL (Koenderink and Uylings 1996; Jacobs et al. 1997), thus making it difficult to compare results from Golgi studies in which a different section thickness (i.e. 160–200 μm) is used (e.g. Petanjek et al. 2008; Petanjek et al. 2011; Vuksić et al. 2011).
In order to hold constant the potential interactions of Golgi staining with technical factors in specimen preservation, preparation, microscopy, and quantification method, our analyses were designed to examine patterns of regional differences among neuronal populations in a within-subject design for chimpanzees. In addition, direct comparisons with previously published data were limited to those studies that used a rapid Golgi staining technique, a section thickness of 120 μm, and the same experimental procedure. As a consequence, we were able to perform quantitative comparison of our results with those of previous rapid Golgi studies in humans (Jacobs et al., 1997, 2001) but not with other studies from other species that have used different experimental methods (Elston et al. 2001). Even so, it should be noted that the human data to which our results are compared were collected by using the Neurolucida Lucivid system, while the current chimpanzee tracings were performed with a digital camera on a computer screen. Although no differences in measures of dendritic length have been reported between the 2 data collection systems, quantification of spine number appears greater in Neurolucida camera tracings (Anderson et al. 2010), making it possible that interspecific differences in spine density are actually greater than those reported here.
Regional Differences in Pyramidal Neuron Morphology in the Cerebral Cortex of Chimpanzees
Pyramidal neurons are the most common neuronal type in the primate cerebral cortex, constituting up to 70–90% of the total number of neurons depending on the region and species (Elston 2007). Their typical structure, with a skirt of basilar dendrites and a single, vertical apical dendrite, allows for signaling among neighboring neurons across and between layers of the cortex by regulating the convergence and divergence of inputs (Purves 1988), and by providing loci for excitatory synapses on spines (Yuste and Tank 1996; Spruston 2008). Dendritic arbors are also extremely plastic structures, which undergo lifespan changes (Jacobs et al. 1993, 1997; Jacobs and Schiebel 1993; Koenderink et al. 1994; Anderson and Rutledge 1996; de Brabander et al. 1998; Travis et al. 2005; Petanjek et al. 2008, 2011). Spines, in particular, are remodeled by experience during development, and provide support for the formation of life-long memories in adulthood (Diamond 1967; Jacobs et al. 1993; van Praag et al. 2000; Grutzendler et al. 2002; Radley et al. 2008; Kabaso et al. 2009; Yang et al. 2009; Bose et al. 2010; Bloss et al. 2011; Yadav et al. 2012). As such, the geometric arrangement and total length of dendrites and their branching patterns are instrumental in determining the computational power of the neuron itself, and in regulating processes of learning and memory (Moser et al. 1994; Sin et al. 2002).
One way in which dendritic morphology relates to cognition is by providing an anatomical substrate for increased corticocortical connectivity (Jacobs et al. 2001). In humans, it has been demonstrated that heteromodal association areas of the cortex, which integrate inputs from numerous other cortical and subcortical areas, display greater dendritic complexity than unimodal sensory or motor regions (Jacobs et al. 1997, 2001). The prefrontal cortex is a supramodal association area that subserves “executive” functions by integrating inputs from diverse regions of the cortex (Rao et al. 1997; Miller 1999). The greater dendritic length, more complex branching patterns, and the higher spine number and density exhibited by layer III pyramidal neurons in the chimpanzee prefrontal cortex supplement current evidence from other primates, suggesting that increased corticocortical wiring in this region may have been important in the evolution of executive cognitive capacities (Jacobs et al. 1997, 2001; Elston et al. 2001, 2006; but see Clemo and Meredith 2012 for findings showing similar or lower spine density in heteromodal vs. unimodal sensory regions). Indeed, comparative evidence suggests that several nonhuman anthropoids possess socio-cognitive skills that depend on prefrontal cortex functions, which are highly sophisticated (Premack 2007), including some form of “mentalizing” (Byrne and Whiten 1992; Hare et al. 2001; but see Call and Tomasello 2008), the ability to establish and transmit cultural traditions, frequently involving the use of tools (Whiten et al. 1999), and capacity for delayed gratification and inhibitory control (Beran et al. 1999; Amici et al. 2008; Wobber et al. 2010).
Comparison Between Chimpanzees and Humans
Previous studies have claimed that the prefrontal cortex of humans displays particular anatomical specializations when compared with other primates (Deacon 1997; Elston et al. 2001; Semendeferi et al. 2001). Specifically, evidence suggests that the human prefrontal cortex may have evolved molecular and neuronal specializations that enhance its capacity for integrative activity and synaptic plasticity. The human forebrain, and especially the prefrontal cortex, for instance, shows evidence of upregulation of thrombospondin (THB4)— a protein involved in synaptogenesis and dendrite outgrowth (Cáceres et al. 2007)— as well as acceleration in change of gene expression regulating metabolic activities (Uddin et al. 2004; Fu et al. 2011).
Consistent with the possibility that evolutionary modifications occurred in the morphology of the cytoskeleton of neurons and the cell energetic demand of the human prefrontal cortex, investigations of its cytoarchitecture indicate that, compared with other cortical areas, prefrontal area 10 is characterized by greater spacing among cortical minicolumns (Semendeferi et al. 2011), as well as an increased neuropil fraction (Spocter et al. 2012) in humans but not in chimpanzees. These observations suggest that prefrontal cortex of humans contains a greater proportion of dendrites, axons, synapses, glial cell processes, and microvasculature relative to the space occupied by neuronal and glial somata. In the current study, we sought to determine whether disproportionate elaboration of prefrontal cortex (area 10) pyramidal neuron morphology also characterizes humans compared with chimpanzees. We found that chimpanzees and humans are similar in sharing the same pattern of relative differences in pyramidal neuron complexity among cortical regions, as indicated by the lack of a significant interaction effect between species and cortical region in all analyses of measures of dendritic morphology. Thus, the current results cannot explain the relative amplification of neuropil space and minicolumn widths in the prefrontal cortex that appears to be unique to humans (Semendeferi et al. 2011; Spocter et al. 2012). Consequently, additional species-specific differences in microstructure, such as the spread of the dendritic skirt of pyramidal neurons or specializations within other cortical layers, may underlie the relatively increased neuropil in human prefrontal cortex.
While being similar to humans in sharing the same pattern of relative regional differences in dendritic morphology, chimpanzees differed in exhibiting pyramidal neurons with dendritic arbors that were absolutely smaller and had fewer spines across all regions of interest. However, it is notable that no interspecific differences in spine density were found (Table 2). Larger brains are generally characterized by neurons with greater dendritic arbors (Purves 1988), yet the scaling of dendritic morphology in relation to brain size remains poorly understood (Changizi 2001). In fact, data comparing dendritic morphology across primates of different brain size indicate that regional functional specializations rather than allometry may account for a considerable amount of inter-specific variation in dendritic complexity (Elston et al. 2001). Because our analyses did not find an interaction effect between species and cortical region, the current results suggest that the extent of basilar dendrites in humans is larger by a similar proportion among cortical regions, without selective specialized elaboration of prefrontal neurons (Table 2). Given the variability of the Golgi stain, however, we are limited in our ability to interpret the degree of those absolute quantitative differences in dendritic morphology between chimpanzees and humans.
In conclusion, the current study provides further evidence that the evolution of executive cognitive functions in primates involved correlated changes in the microstructure of prefrontal cortex underlying the capacity for greater neuronal connectivity. Moreover, by examining regional dendritic differences in a nonhuman great ape species for the first time, these data support the conclusion that increased dendritic complexity in the prefrontal cortex is a shared trait that was likely to have been present in the common ancestor of humans and chimpanzees.
Funding
This work was supported by the National Science Foundation (grants BCS-0515484, BCS-0549117, BCS-0824531, DGE-0801634), the National Institutes of Health (grant number NS042867, and the Yerkes National Primate Research Center, RR000165), the James S. McDonnell Foundation (grants 22002078 and 220020293), and the Kavli Institute for Brain and Mind, The University of California, San Diego.
Notes
Conflict of interest: none declared.
References
- Amici F, Aureli F, Call J. Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr Biol. 2008;18:1415–1419. doi: 10.1016/j.cub.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Anderson B, Rutledge V. Age and hemisphere effects on dendritic structure. Brain. 1996;119:1983–1990. doi: 10.1093/brain/119.6.1983. [DOI] [PubMed] [Google Scholar]
- Anderson K, Yamamoto E, Kaplan J, Hannan M, Jacobs B. Neurolucida Lucivid versus Neurolucida camera: A quantitative and qualitative comparison of three-dimensional neuronal reconstructions. J Neurosci Methods. 2010;186:209–214. doi: 10.1016/j.jneumeth.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Savage-Rambaugh ES, Pate JL, Rumbaugh DM. Delay of gratification in chimpanzees. Devel Psychobiol. 1999;34:119–127. doi: 10.1002/(sici)1098-2302(199903)34:2<119::aid-dev5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose M, Muñoz-Llancao P, Roychowdhury S, Nichols JA, Jakkamsetti V, Porter B, Byrapureddy R, Salgado H, Kilgard MP, Aboitiz F, et al. Effect of the environment on the dendritic morphology of the rat auditory cortex. Synapse. 2010;64:97–110. doi: 10.1002/syn.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne RW, Whiten A. Cognitive evolution in primates: Evidence from tactical deception. Man. 1992;27:609–627. [Google Scholar]
- Cáceres M, Suwyn C, Maddox M, Thomas JW, Preuss TM. Increased cortical expression of two synaptogenic thrombospondins in human brain evolution. Cereb Cortex. 2007;17:2312–2321. doi: 10.1093/cercor/bhl140. [DOI] [PubMed] [Google Scholar]
- Call J, Tomasello M. Do chimpanzees have a theory of mind? 30 years later. Trends Cogn Sci. 2008;15:187–192. doi: 10.1016/j.tics.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Changizi MA. Principles underlying mammalian neo-cortical scaling. Biol Cybern. 2001;84:207–215. doi: 10.1007/s004220000205. [DOI] [PubMed] [Google Scholar]
- Clemo HR, Meredith MA. Dendritic spine density in multisensory versus primary sensory cortex. Synpase. 2012;66:714–724. doi: 10.1002/syn.21560. [DOI] [PubMed] [Google Scholar]
- Deacon TW. New York: W.W. Norton & Company; 1997. The symbolic species: The coevolution of language and the brain. [Google Scholar]
- De Brabander M, Kramers RJK, Uylings HBM. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur J Neurosci. 1998;10:1261–1269. doi: 10.1046/j.1460-9568.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- Diamond M. Extensive cortical depth measurements and neuron number increases in the cortex of environmentally enriched rats. J Comp Neurol. 1967;131:357–364. [Google Scholar]
- Elston GN. Cortex, cognition and the cell: New insights into the pyramidal neuron and prefrontal function. Cereb Cortex. 2003;13:1124–1138. doi: 10.1093/cercor/bhg093. [DOI] [PubMed] [Google Scholar]
- Elston GN. Pyramidal cells of the frontal lobe: All the more spinous to think with. J Neurosci. 2000;20:RC95. doi: 10.1523/JNEUROSCI.20-18-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN. Specializations in pyramidal cell structure during primate evolution. In: Kaas JH, Preuss TM, editors. Evolution of nervous systems. Oxford: Academic Press; 2007. pp. 191–242. [Google Scholar]
- Elston GN, Tomofumi O, Fujita I. J Neurosci. 2009;29:3271–3275. doi: 10.1523/JNEUROSCI.5216-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, DeFelipe J. A study of pyramidal cell structure in the cingulate cortex of the macaque monkey with comparative notes on inferotemporal and primary visual cortex. Cereb Cortex. 2005a;15:64–73. doi: 10.1093/cercor/bhh109. [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, DeFelipe J. The pyramidal cell in cognition: A comparative study in human and monkey. J Neurosci. 2001;21:RC163. doi: 10.1523/JNEUROSCI.21-17-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, Elston A, Manger PR, DeFelipe J. Pyramidal cells in prefrontal cortex of primates: Marked differences in neuronal structure among species. Front Neuroanat. 2011;5:2. doi: 10.3389/fnana.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, Elston A, Manger PR, DeFelipe J. Specialization in pyramidal cell structure in the sensory-motor cortex of the Chacma baboon (Papio ursinus) with comparative notes on macaque and vervet monkeys. Anat Rec. 2005b;286:854–865. doi: 10.1002/ar.a.20217. [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, Elston A, Zietsch B, DeFelipe J, Manger P, Casagrande V, Kaas JH. Specializations of the granular prefrontal cortex of primates: Implications for cognitive processing. Anat Rec A. 2006;288:26–35. doi: 10.1002/ar.a.20278. [DOI] [PubMed] [Google Scholar]
- Elston GN, Elston A, Kaas JH, Casagrande V. Regional specialization in pyramidal cell structure in the visual cortex of the galago: An intracellular injection study of striate and extrastriate areas with comparative notes on new world and old world monkeys. Brain Behav Evol. 2005c;66:10–21. doi: 10.1159/000085044. [DOI] [PubMed] [Google Scholar]
- Elston GN, Rosa MG. Morphological variation of layer III pyramidal neurones in the occipitotemporal pathway of the macaque monkey visual cortex. Cereb Cortex. 1998;8:278–294. doi: 10.1093/cercor/8.3.278. [DOI] [PubMed] [Google Scholar]
- Elston GN, Rosa MG. The occipitoparietal pathway of the macaque monkey: Comparison of pyramidal cell morphology in layer III of functionally related cortical visual areas. Cereb Cortex. 1997;7:432–452. doi: 10.1093/cercor/7.5.432. [DOI] [PubMed] [Google Scholar]
- Elston GN, Tweedale R, Rosa MG. Cortical integration in the visual system of the macaque monkey: Large-scale morphological differences in the pyramidal neurons in the occipital, parietal and temporal lobes. Proc R Soc B. 1999a;266:1367–1374. doi: 10.1098/rspb.1999.0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN, Tweedale R, Rosa MG. Cellular heterogeneity in cerebral cortex: A study of the morphology of pyramidal neurones in visual areas of the marmoset monkey. J Comp Neurol. 1999b;415:33–51. doi: 10.1002/(sici)1096-9861(19991206)415:1<33::aid-cne3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Fu X, Giavalisco P, Liu X, Catchpole G, Fu N, Ning Z-B, Guo S, Yan Z, Somel M, Pääbo S, et al. Rapid metabolic evolution in human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:6181–6186. doi: 10.1073/pnas.1019164108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: Anatomy, physiology and neurospychology of the frontal lobe. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 1997. [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Hanani M. Lucifer Yellow-an angel rather than the devil. J Cell Mol Med. 2012;16:21–31. doi: 10.1111/j.1582-4934.2011.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. Do chimpanzee know what conspecifics know? Anim Behav. 2001;61:139–151. doi: 10.1006/anbe.2000.1518. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Taglialatela JP, Russell JL, Nir TM, Schaeffer J. Cortical representation of lateralized grasping in chimpanzees (Pan troglodytes): A combined MRI and PET study. PLoS One. 2010;5(10):e13383. doi: 10.1371/journal.pone.0013383. doi:10.1371/journal.pone.0013383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: A quantitative Golgi study. J Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, Jacobs J, Ford K, Wainwright M, Treml M. Regional dendritic and spine variation in human cerebral cortex: A quantitative Golgi study. Cereb Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Schall M, Scheibel A. A quantitative dendritic analysis of Wernicke's area in humans II. Gender, hemispheric and environmental factors. J Comp Neurol. 1993;327:97–111. doi: 10.1002/cne.903270108. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Schiebel A. A quantitative dendritic analysis of Wernicke area in humans I. Life span changes. J Comp Neurol. 1993;327:83–96. doi: 10.1002/cne.903270107. [DOI] [PubMed] [Google Scholar]
- Kabaso D, Coskren PJ, Henry BI, Hof PR, Wearne SL. The electrotonic structure of pyramidal neurons contributing to prefrontal cortical circuits in macaque monkeys is significantly altered in aging. Cereb Cortex. 2009;19:2248–2268. doi: 10.1093/cercor/bhn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenderink MJ, Uylings HB. Morphometric dendritic field analysis of pyramidal neurons in the human prefrontal cortex: Relation to section thickness. J Neurosci Methods. 1996;64:115–122. doi: 10.1016/0165-0270(95)00117-4. [DOI] [PubMed] [Google Scholar]
- Koenderink MJ, Uylings HB, Mrzljak L. Postnatal maturation of the layer III pyramidal neurons in the human prefrontal cortex: A quantitative Golgi analysis. Brain Res. 1994;653:173–182. doi: 10.1016/0006-8993(94)90387-5. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex: Complex neural properties for complex behavior. Neuron. 1999;22:15–17. doi: 10.1016/s0896-6273(00)80673-x. [DOI] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci USA. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Kostovic I, Uylings HB. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: A layer-specific pattern. Cereb Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D. Human and animal cognition: Continuity and discontinuity. Proc Natl Acad Sci USA. 2007;104:13861–13867. doi: 10.1073/pnas.0706147104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Body and brain. A trophic theory of neural connections. Cambridge, MA: Harvard University Press; 1988. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. The methods of Golgi. In: Robertson RT, editor. Neuroanatomical research techniques. New York: Academic Press; 1978. pp. 89–114. [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: A comparative study of area 10. Am J Phys Anthropol. 2001;114:224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Teffer K, Buxhoeveden DP, Park MS, Bludau S, Amunts K, Travis K, Buckwalter J. Spatial organization of neurons in the frontal pole sets humans apart from great apes. Cereb Cortex. 2011;21:1485–1497. doi: 10.1093/cercor/bhq191. [DOI] [PubMed] [Google Scholar]
- Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- Smaers JB, Steele J, Case CR, Cowper A, Amunts K, Zilles K. Primate prefrontal cortex evolution: Human brains are the extreme of a lateralized ape trend. Brain Behav Evol. 2011;77:67–78. doi: 10.1159/000323671. [DOI] [PubMed] [Google Scholar]
- Spocter MA, Hopkins WD, Barks SK, Bianchi S, Hehmeyer AE, Anderson SM, Stimpson CD, Fobbs AJ, Hof PR, Sherwood CC. Neuropil distribution in the cerebral cortex differs between humans and chimpanzees. J Comp Neurol. 2012;520:2917–2919. doi: 10.1002/cne.23074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: Dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Travis K, Ford K, Jacobs B. Regional dendritic variation in neonatal human cortex: A quantitative Golgi study. Dev Neurosci. 2005;27:277–287. doi: 10.1159/000086707. [DOI] [PubMed] [Google Scholar]
- Uddin M, Wildman DE, Liu G, Xu W, Johnson RM, Hof PR, Kapatos G, Grossman LI, Goodman M. Sister grouping of chimpanzees and humans as revealed by genome-wide phylogenetic analysis of brain gene expression profiles. Proc Natl Acad Sci USA. 2004;101:2957–2962. doi: 10.1073/pnas.0308725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Vuksić M, Petanjek Z, Kostovic I. Development of pyramidal layer III neurons in infants with Down syndrome. Transl Neurosci. 2011;2:225–232. [Google Scholar]
- Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. Chimpanzee culture. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- Wobber V, Wrangham R, Hare B. Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Curr Biol. 2010;20:226–230. doi: 10.1016/j.cub.2009.11.070. [DOI] [PubMed] [Google Scholar]
- Yadav A, Gao YZ, Rodriguez A, Dickstein DL, Wearne SL, Luebke JI, Hof PR, Weaver CM. Morphologic evidence for spatially clustered spines in apical dendrites of monkey neocortical pyramidal cells. J Comp Neurol. 2012;520:2888–2902. doi: 10.1002/cne.23070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Tank DW. Dendritic integration in mammalian neurons a century after Cajal. Neuron. 1996;16:701–716. doi: 10.1016/s0896-6273(00)80091-4. [DOI] [PubMed] [Google Scholar]