Summary
The dorsal anterior cingulate cortex (dACC) has a near-ubiquitous presence in the neuroscience of cognitive control. It has been implicated in a diversity of functions, from reward processing and performance monitoring to the execution of control and action selection. Here, we propose that this diversity can be understood in terms of a single underlying function: allocation of control based on an evaluation of the expected value of control (EVC). We present a normative model of EVC that integrates three critical factors: the expected payoff from a controlled process, the amount of control that must be invested to achieve that payoff, and the cost in terms of cognitive effort. We propose that dACC integrates this information, using it to determine whether, where and how much control to allocate. We then consider how the EVC model can explain the diverse array of findings concerning dACC function.
The dorsal anterior cingulate cortex (dACC), spanning the cingulate gyrus and sulcus from the plane of the anterior commissure to the genu of the corpus callosum (Figure 1), is one of the most heavily studied regions of the brain and yet, remains one of the least clearly understood. Although has recently been an explosion of research on the role of dACC in cognition and behavior, this has led to a proliferation of diverging theories concerning its function. The dACC has been proposed to play a key role in pain processing, performance monitoring, value encoding, decision making, emotion, learning, and motivation. A precise and coherent account of dACC function seems as elusive now as it did in the earliest days of theory development.
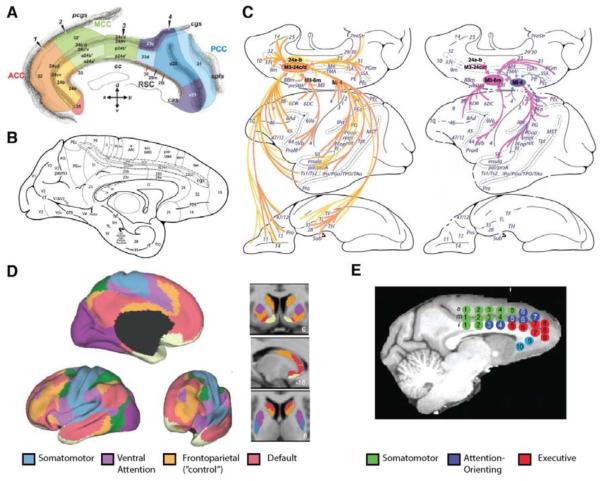
Figure 1. Anatomy and connectivity of the dorsal anterior cingulate cortex (dACC).
A-B) Cytoarchitectonic subdivisions of human (A) and macaque (B) medial prefrontal cortices. The cingulate sulcus (cgs) has been opened up in both. dACC typically refers to areas 24a-d and the dorsal extent of area 32 (32’ in A, 32(s) in B). Panel A focuses specifically on paracingulate regions of the medial surface, and the color-coding reflects Vogt et al.’s (2004) four-region model. The region referred to as human dACC throughout the main text is the anterior portion of mid-cingulate cortex (aMCC), encompassing an area Picard and Strick (1996) referred to as the rostral cingulate zone (RCZ).. C) Cortical projections to regions of dACC (left; areas 24a-b in yellow, areas 24c-d in orange) and more posterior regions of dorsomedial PFC (right; supplementary and primary motor cortices in pink and purple, respectively) in the macaque (cf. panel B). Relative to the more posterior regions, projections to dACC are much more widespread and include regions of orbital and rostrolateral PFC, temporal and parietal cortices, and insula. D-E) Patterns of resting-state functional connectivity estimated in human (D) and macaque (E) brains using fMRI. The colors in panel D label seven networks within which activity between the regions (of a given color) is highly correlated at rest. Under this parcellation scheme, regions of dACC span the frontoparietal network (orange; often referred to as the “control” network) and the ventral attention network (violet). Panel E shows a parcellation of resting-state connectivity networks focused on the connectivity of cingulate cortex regions-of-interest with the rest of the brain. Patterns of connectivity for the “executive” network (shown in red) and the “attention-orienting” network (dark blue), particularly within lateral PFC, suggest potential homologues with human frontoparietal and ventral attention networks. However, exact boundaries and homologies between dACC across species remain ambiguous (see, e.g., Cole et al., 2009). Panel A reprinted from Palomero-Gallagher et al. (2009) by permission of John Wiley and Sons; panels B-C reprinted from Morecraft et al. (2012), © (2012), modified with permission from Elsevier; panel D cortical and striatal connectivity reprinted from Yeo et al. (2011) and Choi et al. (2012), respectively, with permission from The American Physiological Society; panel E reprinted from Hutchison et al. (2011), by permission of Oxford University Press.
Two opposing tendencies appear to have slowed progress toward an integrated understanding of dACC function. One has been to base theoretical analyses on too narrow a subset of empirical findings, while another has been to embrace a wide range of empirical findings, but to reduce them to a single basic computation at the cost of oversimplifying dACC function. Here, we propose an integrative account of dACC function that strives to avoid these pitfalls.
We build on one observation which appears to be widely and consistently agreed upon: that dACC is engaged by tasks that demand cognitive control. Broadly, this can be defined as the set of mechanisms required to pursue a goal, especially when distraction and/or strong (e.g., habitual) competing responses must be overcome. Numerous meta-analyses of the neuroimaging literature have confirmed the dACC’s involvement in control-demanding tasks (Nee et al., 2007; Niendam et al., 2012; Ridderinkhof et al., 2004; Shackman et al., 2011) and these have been supplemented by evidence of a causal relationship between dACC and cognitive control. For instance, using diffusion tensor imaging (DTI), Metzler-Baddeley and colleagues (2012) showed that older adults with lower white matter integrity in the anterior cingulum bundle (the white matter bundle projecting to/from dACC) performed more poorly on control-demanding tasks.
Despite the strong consensus that dACC is involved in cognitive control, there is little agreement about the specific function(s) it subserves. Here, we synthesize a number of existing proposals concerning the role of dACC into a single theoretical construct and show how this can be reconciled with empirical findings concerning dACC function. Specifically, we propose that the dACC integrates information about the rewards and costs that can be expected from a control-demanding task, in order to estimate a quantity we refer to as the expected value of control (EVC). Put simply, EVC represents the net value associated with allocating control to a given task. We propose that dACC estimates this quantity in order to determine whether it is worth investing control in a task, how much should be invested and, when several potential tasks are in contention, which is the most worthwhile. We assume that this information is used to select among competing tasks and allocate the appropriate amount of control to performance of the one selected. This proposal ascribes to dACC a specific decision making function regarding the allocation of control that is distinct from other control-related functions, such as the valuative ones that provide input to the decision and the implementational ones responsible for executing it; these are presumed to be subserved by other neural mechanisms.
We begin by establishing some foundational points concerning cognitive control and its constituent functions that are necessary for framing the EVC theory and our consideration of dACC. We then introduce the basic elements of the EVC theory. Finally, we review key findings and existing theoretical proposals from the dACC literature, relating these to the EVC theory.
1. The computational basis of cognitive control
Processes that demand control are often distinguished from automatic processes, which involve associations that are sufficiently strong as to be resistant to distraction or interference (Botvinick and Cohen, in press; Cohen et al., 1990; Norman and Shallice, 1986; Posner and Snyder, 1975; Shiffrin and Schneider, 1977). A classic illustration of the distinction between controlled and automatic processing is provided by the Stroop task. Participants are shown a color word but asked to name the color of the font in which it is displayed. When the two dimensions disagree (e.g., GREEN), participants find it harder to name the color than when the two agree (e.g., RED). However, this interference effect does not occur when the task is, instead, to simply read the word. This difference between task conditions is explained by assuming that word reading is automatic (allowing the word to be processed even when the task is color naming), whereas color naming is controlled (preventing the color from being processed unless the task is to do so). This explanation is reinforced by the observation that, when presented with a conflict stimulus in the absence of a specific task instruction, people invariably read the word, illustrating the automatic, or “default”, nature of verbal responses to words. Verbally responding to the color requires an instruction and/or intention to do so, at least in the presence of conflicting word information.
1.1 A simple model of cognitive control
A computational model of the mechanisms underlying the Stroop task is shown in Figure 2A (Cohen et al., 1990). The model takes the form of a neural network, with units encoding stimulus features projecting forward to intermediate (associative) units, and then to output units representing verbal responses. The automaticity of the response to words is captured by strong connection weights along the pathway from word identity to verbal response. These also make it the default response (i.e., the response generated in the absence of any instruction). However, without any additional apparatus, the model would not be able to respond to the color of a conflicting stimulus. To address this, the model also includes a set of control units that represent the current task. When the unit representing the color naming task is active, this provides top-down support for units in the pathway from color to verbal response, priming these units and thereby permitting a response to the color even when there is conflicting information arriving along the word pathway. Thus, in this context, color naming can be considered to be a controlled process to the extent that a correct response to the color depends on activation of the color naming task unit.
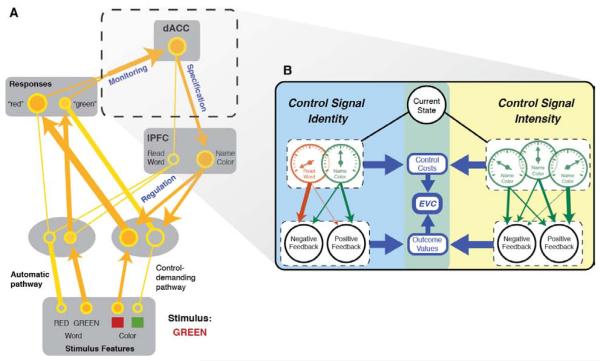
Figure 2. The Expected Value of Control (EVC) model applied to the Stroop task.
A) A model of the Stroop task illustrating the three major components of cognitive control: monitoring, specification and regulation. Thickness of the connections and size of the units denote the amplitude of the signal along each processing pathway. The figure also illustrates how the model can be extended to include conflict monitoring that, in turn, can be used to specify the strength of the control signal needed to support processing in the task-relevant pathway. Note that the model ascribes to dACC roles in monitoring and specification, and to the lPFC a role in regulation. B) The EVC model. Control signal specification involves choosing a control signal that maximizes EVC. For illustration, we diagram here the processes underlying specification for the Stroop color-naming task (illustrated in panel A). The objective is to select a control signal that maximizes the EVC. This, in turn, requires comparison of signals that differ in their identity (here, word-reading vs. color-naming task sets) and intensity (represented in the figure by meters). In both cases, the EVC estimation takes into account both the costs of each candidate signal and its expected payoffs and expected outcomes (arrow weights indicate transition probabilities). Although identity and intensity are segregated here for the purposes of illustration, they are fully integrated in the EVC estimation as specified in Eq. 1, and likely reflect the operation of a common set of mechanisms (see Section 5.3). Moreover, unlike specifying the intensity of a given control signal (right), the process of specifying control signal identities (left) does not require mutual exclusivity (i.e., multiple control signal identity-intensity pairings can be simultaneously specified as a single array).
The model shown in Figure 2A also includes a unit that serves a ‘conflict monitoring’ function, responding to coactivation of the network’s response units (see Botvinick et al., 2001). Such conflict is an indicator of inadequate control. For example, if the color naming unit is insufficiently activated, then activation of the response to the color will be weaker and compete less effectively with activation of the response to a conflicting word, allowing the latter to become more active. This coactivation will have two potentially adverse consequences for behavior. At best it will slow responding, since the correct response unit must overcome inhibitory competition from the incorrect one. At worst it will produce an error. These dangers can be ameliorated by increasing the activity of the color naming task unit. Thus, conflict serves as an indicator of the need for additional allocation of control.
1.2 Three component functions of cognitive control
This simple model of the Stroop task and conflict monitoring is of course not intended as a comprehensive model of cognitive control. However, the architecture of the model illustrates three core component functions of cognitive control (Figure 2A).
1. Regulation
The sine qua non feature of control is its capacity to govern or influence lower level information processing mechanisms, a function we refer to as regulation. In the language of engineering, activity of a task-demand unit represents a control signal, which determines the parameters for more basic processes (in this case, the sensitivity of the associative units). Note that this signal has two defining characteristics: its identity and its intensity (the strength of the signal, both in literal terms — e.g., level of activation of the task demand unit — and in terms of its impact on information processing). Control signals can determine a wide range of processing parameters, including thresholds and/or biases for responding (governing speed-accuracy tradeoffs; Bogacz et al., 2006; Wiecki and Frank, 2013), templates for attention or memory search (Desimone and Duncan, 1995; Olivers et al., 2011; Polyn et al., 2009), and modulators of emotion (Johns et al., 2008; McClure et al., 2006). In each case, a distinction can be made between signal identity (the parameter targeted) and signal intensity (the degree to which the parameter is displaced from its default value).
2. Specification
In order for regulation to occur, a critical step is for an appropriate control signal to be chosen: Control requires a decision on which, if any, controlled task should be undertaken, and on how intensively it should be pursued. We refer to this decision-making function as control signal specification, which must determine the identity and intensity of the desired control signal. Importantly, control signal specification should be distinguished from regulation which consists of implementing the specified control signal so as to actually effect the changes in information processing required for the task. This distinction between specification (the decision process) and regulation (that mediates its effects) is central to the EVC theory. While both are essential components of the control system, the EVC theory ascribes to dACC a role in specification but not regulation, as we discuss below.
3. Monitoring
In order to specify the appropriate control signal, and deploy regulative functions in an adaptive manner, the system must have access to information about current circumstances and how well it is serving task demands. Detecting and evaluating these requires a monitoring mechanism. The conflict-detection component in the Stroop model provides one example of such a monitoring function and how it can guide specification: the occurrence of response conflict indicates that insufficient control is being allocated to the current task (see Botvinick, 2007; Botvinick et al., 2001; Botvinick et al., 2004). In this instance, conflict indicates the need to re-specify control signal intensity. However, conflict is just one among many signals that can indicate the need to adjust intensity. Others include response delays, errors, negative feedback and the sensation of pain, and these signals all carry information about performance within a task and how to specify control signal intensity. Monitoring must also consider information relevant to the specification of control signal identity; that is, to task choice. Such information can come from external sources (e.g., explicit instructions, cues indicating new opportunities for reward, or the sudden appearance of a threat) or internal ones (e.g., diminishing payoffs from the current task indicating it is no longer worth performing, recollection of another task that needs to be performed, etc.). In all of these cases, monitoring must be responsive to, but should be distinguished from, the sensory and valuative processes that represent the actual information relevant to specification. Thus, just as we distinguish between specification and regulation on the efferent side of control, we distinguish between monitoring and valuation on the afferent side. In each case, the EVC theory ascribes to dACC a role in the former, but not the latter.
1.3 Optimization, motivation, and the cost of cognitive control
Research on control focused initially on regulative and monitoring mechanisms, but growing attention is being paid to the problem of control-signal specification. Work in this area has been driven increasingly by ideas from research on reward-based decision making and reinforcement learning. One emerging trend has involved reframing control-signal specification as an optimization problem, shaped by learning or planning mechanisms that serve to maximize long-term expected reward (Bogacz et al., 2006; Dayan, 2012; Hazy et al., 2007; O’Reilly and Frank, 2006; Yu et al., 2009). Under this view, cognitive control can be defined as the set of mechanisms responsible for configuring behavior in order to maximize the attainment of reward. This definition accords well with the definition of control in other fields, most notably control theory in engineering, and from this perspective, cognitive control can be viewed not only as adaptive, but also as motivated.
An emphasis on motivation also aligns with the ubiquitous observation that the exertion of cognitive control carries an inherent subjective cost. From the earliest definitions, controlled processing was described as effortful, and like physical effort, mental effort is assumed to carry intrinsic disutility. That is, people spontaneously seek to minimize it. Recent empirical work bears out this assumption, linking effort specifically to the exertion of cognitive control (Kool and Botvinick, in press; Kool et al., 2010). Human decision-makers show a bias against tasks demanding top-down control, and within certain bounds they will delay task goals or even forego reward in order to avoid such tasks (Dixon and Christoff, 2012; Kool et al., 2010; Westbrook et al., in press). These effects imply an intrinsic ‘cost of control’, which scales with the intensity of the control required to perform the task (Dixon and Christoff, 2012; Kool et al., 2010). These ideas, combined with the idea that control signals are specified based on the reward potential of the task they support, suggest that the allocation of control is driven by a cost-benefit analysis, weighing potential payoffs against attendant costs, including those inherently associated with the exertion of control itself.
1.4 The neural substrates of cognitive control
Previous work has established links between components of the Stroop model and specific neural structures involved in cognitive control. In particular, lateral prefrontal cortex (lPFC) together with associated structures (e.g., basal ganglia and brainstem dopaminergic nuclei) have been proposed to implement the regulative component of the model (Braver and Cohen, 2000; Cohen and Servan-Schreiber, 1992; Frank et al., 2001; Miller and Cohen, 2001), while dACC has been proposed to implement the monitoring component (Botvinick, 2007; Botvinick et al., 2001; Botvinick et al., 2004). According to this mapping, the key step of control-signal specification arises in the communication from dACC to lPFC (Botvinick et al., 2001; Kerns et al., 2004). That is, the model assigns to the dACC responsibility for monitoring and specification, evaluating current demands for control and using the relevant information to decide how to allocate control. The specified control signals are then implemented by lPFC and associated structures, which are assumed to be responsible for the regulative function of control — that is, actually effecting the changes in processing required to perform the task. The EVC model elaborates this proposal, structuring it in a normative description of how both the identity and the intensity of control signals are determined and placing new emphasis on optimization (i.e., reward maximization) in understanding the relationship, within dACC, between monitoring and specification.
2. Control-signal specification based on the expected value of control
The operation of cognitive control, as we have characterized it, involves deciding what control signal should be selected (i.e., its identity) and how vigorously this control signal should be engaged (i.e., its intensity) (Figure 2B). We propose that the brain makes this two-part decision in a rational or normative manner to maximize expected future reward. To make this idea precise, we will express the choice of what and how much to control in formal terms, borrowing approaches from reinforcement learning and optimal control theory to analogous problems of motor action selection.
We begin by defining a control signal to be an array variable with two components: identity (e.g., ‘respond to color’ or ‘respond to word’) and intensity. Determining the expected value of each control signal requires integration over two sources of value-related information. First, it must consider the overall payoff that can be expected from engaging this control signal, taking into account both positive and negative outcomes that could result from performing the corresponding task. Second, as discussed above, it must take into account the fact that there is an intrinsic cost to engaging control itself, which scales with the intensity of the signal required. Taken together, these two components determine what we will refer to as the expected value of control (EVC), which can be formalized as follows (see also Figures 2B and 4A-B):
| Eq.1 |
As indicated by the arguments on the left hand side, the EVC is a function of two variables, signal and state. Signal refers to a specific control signal (e.g., designating a particular task representation and its intensity). State refers to the current situation, spanning both environmental conditions and internal factors (e.g., motivational state, task difficulty, etc.). Finally, outcomes are subsequent states that result from the application of a particular control signal in the context of the current state, each with a particular probability (Pr); for example the occurrence of a correct response or of an error. Since outcomes are themselves states, the terms ‘state’ and ‘outcome’ in Eq. 1 can also be thought of as ‘current state’ and ‘future state.’ The Value of an outcome is defined recursively as follows:
| Eq.2 |
where ImmediateReward can be either positive or negative (for example, in the case of an error, monetary loss or pain; the term ‘reward’ is borrowed from reinforcement learning models but can be understood more colloquially as ‘value’). Note that the maximization of EVC in the final term is over all feasible control signals (indexed by i), with outcome serving in place of the current state. The estimation of outcome value thus folds in the EVC of control signals implemented in future states. The parameter γ is a discount factor, between zero and one, controlling how much the current decision weighs future rewards relative to more immediate ones. The significance of this final term is that it links outcome value (and thus the EVC) not only to immediate rewards, but also to predictable future events and their associated rewards.
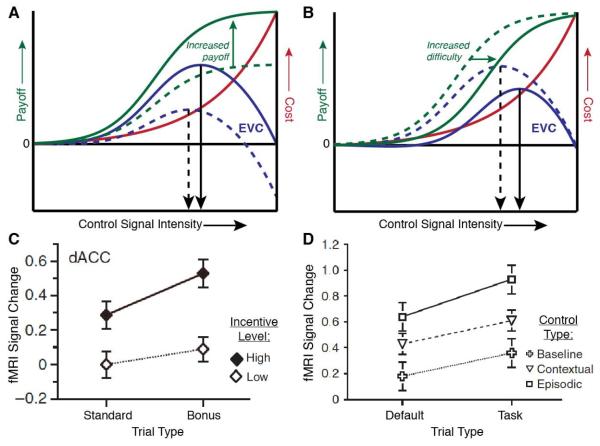
Figure 4. The influence of incentives and task difficulty on control allocation and dACC activity.
The EVC model predicts shifts in control intensity in response to changes in task incentives (panel A) and in task difficulty (B). In each case, control intensity is specified based on a maximization of the EVC (blue curves). As indicated in Eq.s 1 and 2, the EVC depends, in turn, on both the expected payoffs and costs for candidate control signals (see also Figure 2B). Payoffs (green curves) can vary with signal intensity due to resulting changes in task performance. For example, a stronger control signal might yield more accurate performance, and therefore greater payoffs. However, the inherent cost of control (red curves) also rises with control signal intensity. A) An increase in task incentives affects the payoff curve. Here, we consider a laboratory scenario in which monetary reward for each correct response shifts from a lower amount (dashed green curve) to a higher amount (solid). When integrated with cost information (red curve), this results in a shift in the EVC function (dashed blue curve to solid blue curve), and a resulting shift in the signal intensity that maximizes the EVC (dashed to solid black arrow). B) An increase in task difficulty reduces the expected payoff for any given control signal intensity (shift from dashed to solid green curve). In the present scenario this is due to a reduction in the probability, for any given signal intensity, of a correct response. The shift in the payoff curve, when integrated with cost (red curve), again yields a change in the EVC function (dashed to solid blue) and a shift in the EVC-maximizing control signal intensity (dashed to solid black arrow). C-D) Kouneiher and colleagues (2009) found that dACC activity tracked both of these EVC-relevant variables. They had participants perform a letter discrimination task and showed that dACC activity increased with the overall incentive level for the current block (C), whether or not the higher incentive was available for the current trial (Standard trials did not offer additional incentives for correct performance but Bonus trials did). They also found that dACC was modulated by the difficulty of a given trial (D; Default trials always had the same response mapping, obviating any additional letter discrimination, Task trials required using a letter discrimination rule based on the letter color, and this color-rule mapping was either stable throughout the session [Baseline/Contextual] or varied by block [Episodic]). See Sections 6 and 7 for additional details. Panels C-D reprinted by permission from Macmillan Publishers Ltd: Nature Neuroscience, Kouneiher et al. (2009), © (2009).
The final term in Eq. 1 captures the intrinsic cost of control, which is presumed to be a monotonic function of control-signal intensity (although for a richer model, see Kool and Botvinick, in press). In sum, Eq. 1 says that the EVC of any candidate control signal is the sum of its anticipated payoffs (weighted by their respective probabilities) minus the inherent cost of the signal (a function of its intensity).
Control-signal specification involves the identification of a combination of signal identity and intensity that will yield the greatest value. We propose that the control system accomplishes this by comparing the EVC across a set of candidate control signals, and seeking the optimum:
| Eq.3 |
Once it has been specified, the optimal control signal (signal*) is implemented and maintained by mechanisms responsible for the regulative component of control, which guide information processing in the service of task performance. This continues until a change in the current state — detected through monitoring — indicates that the previously specified control signal is no longer optimal (either in terms of its identity or intensity), and a new signal* should be specified.
3. An EVC model of dorsal anterior cingulate function
Drawing upon the theoretical constructs laid out above, we suggest that dACC function can be understood in terms of monitoring and control-signal specification. Specifically, we propose that the dACC monitors control-relevant information, using this to estimate the EVC of candidate control signals, selecting an optimum from among these, and outputting the result to other structures that are directly responsible for the regulative function of control (such as lPFC). Critically, we propose that the dACC’s output serves to specify both the identity and intensity of the optimal control signal. Thus, the dACC influences both the specific content of control (e.g., what task should be performed, or parameter should be adjusted) and also, by way of its intensity, the balance between controlled and automatic processing, taking into account the inherent cost of a control signal of the specified intensity.
The EVC model, shares elements both with our own and other theories concerning the mechanisms underlying cognitive control and action selection, as we shall emphasize. The value of the EVC model lies not in the novelty of its individual ingredients, but in its explicit formalization of these ingredients in a way that allows for their integration within a single coherent framework. The theory helps synthesize central concepts in theories of cognitive control — the distinction between controlled and automatic processing, and the relationship between the monitoring and specification functions of control, the relevance of both identity and intensity in control signal specification, and the costs of control — into an overarching account that is mechanistically explicit, computationally coherent, and that does justice to the wide array of findings that have been reported concerning dACC function.
4. Monitoring functions of the dACC: Supporting the calculation of EVC
Estimates of EVC require two key pieces of information: the current state (i.e. information concerning current task demands, processing capacity and motivational state) and the value of potential outcomes that may occur given each candidate control signal, taking into account their likelihood of occurrence and anticipated worth.
The EVC model proposes that dACC monitors such present-state and outcome-value information, garnered from other regions (such as orbitofrontal, ventromedial prefrontal and insular cortex), as a basis for computing and maximizing the EVC. A range of empirical evidence is consistent with the idea that dACC is responsive to each of these two types of information.
4.1. dACC and the monitoring of state information
Computing the intensity and the identity of the optimal control signal requires different types of information about present state. For example, the presence of conflict may indicate the need to increase the intensity of the control signal, whereas an unexpected environmental cue may indicate the need to change the identity of the control signal (e.g., to perform a more rewarding task). The evidence strongly suggests that dACC is sensitive to state information that serves both of these needs.
State information relevant to control signal intensity: conflict monitoring
As noted above, conflict can provide important information about the demands of the current task and the intensity of control that should be allocated. Increasing control intensity will generally improve performance. However, specifying the optimal control-signal must also take into account the cost of control, which also increases with intensity (Eq. 1). That is, control signals should be just strong enough to accomplish task objectives but no stronger (Figure 4). Given this, it is critical to determine the control demands of a task. Explicit outcomes provide one source of such information (e.g., feedback concerning performance); however, such information is not always available. Conflicts that arise during processing represent one source of internally available information useful for this purpose. As illustrated by the Stroop model, conflict during processing can provide an indication of the need to allocate additional control, much as an overt error would do. In fact, conflict serves as an earlier, and potentially more sensitive, signal of the need for control than explicit error feedback (Yeung et al., 2004).
Both empirical and computational modeling work strongly support the role of dACC in conflict monitoring. The first imaging study of the Stroop task (Pardo et al., 1990) reported dACC activity in response to conflict stimuli. A number of subsequent studies provided additional evidence that dACC activity is specifically associated with the presence of conflict in processing (Carter et al., 1998; Carter et al., 2000), and that it can be dissociated from the regulative functions of control (Botvinick et al., 1999; Egner and Hirsch, 2005a, b; Kerns, 2006; Kerns et al., 2004; Macdonald et al., 2000; however, see Figure 3 and Section 7.2). These studies focused on tasks that involved conflict among competing responses, using classic paradigms such as the Stroop task, Simon task, and Eriksen flanker task (see meta-analyses in Laird et al., 2005; Nee et al., 2007; Ridderinkhof et al., 2004). However, subsequent studies have extended the association between dACC and conflict processing to a much wider range of tasks, showing that it is also sensitive to conflicts that arise in perceptual discriminations (Ho et al., 2009; Krebs et al., 2012; Woolgar et al., 2011), language processing (Barch et al., 2000; Snyder et al., 2011), value-based decisions (Blair et al., 2006; Marsh et al., 2007; Pochon et al., 2008), moral judgments (Greene et al., 2004), social judgment (Cunningham et al., 2004), memory retrieval (Guerin and Miller, 2011), and strategy selection (Venkatraman et al., 2009).
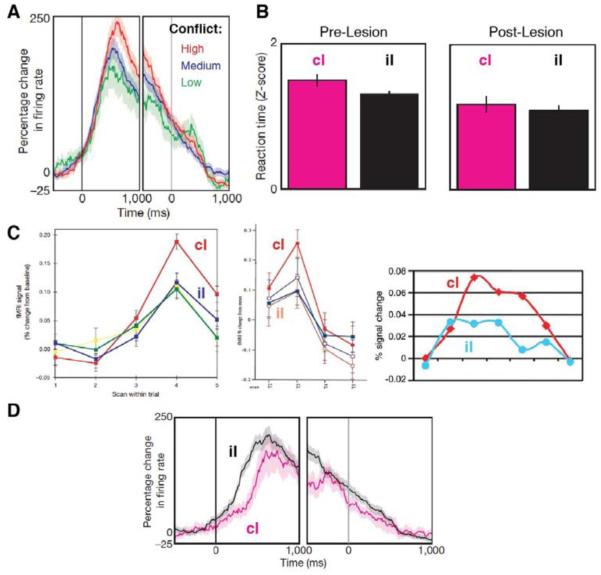
Figure 3. The relationship of conflict adaptation effects in human dACC to control monitoring versus specification.
A) Measuring from single units in human dACC, Sheth et al. (2012) found a parametric effect of current trial conflict (example neuron shown), an effect that has been widely reported in neuroimaging studies (see Section 4.1). Left and right sides of this figure plot firing rate changes aligned to stimulus and response onsets, respectively. Note that this effect alone can be indicative of either monitoring of demands and/or specification of different intensities of control accordingly. B) Left: This group also found evidence of conflict adaptation (Gratton et al., 1992), with high-conflict (incongruent) trials requiring greater control, and therefore exhibiting longer RTs, when following a low-conflict (congruent) trial (cI) than when following another incongruent trial (iI). Right: This behavioral effect was abolished after these individuals underwent cingulotomy. C) Previous fMRI studies have tied sequential adjustment effects to a particular pattern of responses in dACC: greater activity on cI than iI trials. This pattern has been observed in numerous experiments using different tasks and manipulations, including Botvinick et al., 1999 (left), Carter et al., 2000 (center), and Kerns et al., 2004 (right). It has been interpreted as reflecting a monitoring function, since greater dACC activity was observed under conditions of high conflict but low control. D) Strikingly, single unit recording data from Sheth et al. show the opposite pattern, with higher firing rates on iI than cI trials, a pattern consistent with control-signal specification. The presence of both monitoring and specification signals in dACC is consistent with the EVC theory. Determining why one function manifests in fMRI and the other in single-unit recording presents an important challenge for further research, and the EVC model may be of use in guiding such investigations. Panels A, B, and D reprinted by permission from Macmillan Publishers Ltd: Nature, Sheth et al. (2012), © (2012). Panel C: [left] reprinted by permission from Macmillan Publishers Ltd: Nature, Botvinick et al. (1999), © (1999); [center] from Carter et al. (2000), © (2000) National Academy of Sciences, USA; [right] from Kerns et al. (2004), with permission from AAAS.
The majority of evidence linking dACC to conflict monitoring has come from human neuroimaging studies. However, two recent studies have used direct neuronal recordings to test this relationship. In one study (Sheth et al., 2012), patients awaiting cingulotomy performed a Stroop-like interference task. fMRI identified conflict-related activity in a dACC region targeted for surgical resection. During the surgery itself, single-unit recording within the same region revealed firing-rate responses to conflict, providing unusually direct evidence for dACC involvement in conflict monitoring (Figure 3).
Another study provided evidence for neuronal responses to conflict in the macaque (Amemori and Graybiel, 2012). In this experiment, monkeys made choices between receiving a small reward or a larger one paired with an aversive stimulus (air-puff to the eye). Neuronal responses in pregenual ACC — a region potentially homologous to conflict-associated regions of human dACC (see Figure 1E; Hutchison et al., 2011) — tracked the subjective similarity of a given set of option values (and thus decision conflict). Variation in decision conflict accounted for variance in the firing rate of neurons in this area independently of reward, air puff magnitude, overall expected utility, or response time.
Computational modeling work has provided convergent support for the idea that dACC activity is responsive to the degree of conflict elicited by the task (Botvinick et al., 2001). Models of conflict monitoring have accounted not only for task conditions that elicit dACC activity, but also the dynamics of that activity (Cockburn and Frank, 2011; Yeung et al., 2004).
The idea that conflict monitoring provides an internal index of task difficulty is also consistent with the ubiquitous observation that dACC activity is closely associated with the cognitive demands of a task (Botvinick, 2007; Duncan, 2010; Nachev et al., 2007; Paus et al., 1998; Venkatraman and Huettel, 2012). This includes demands that are increased by responses that are sequential or depend on complex rule structure versus simple and isolated ones (e.g., Kouneiher et al., 2009; Shima and Tanji, 1998); novel versus familiar or habitual responses (e.g., Procyk et al., 2000); larger vs. smaller option sets (e.g., Barch et al., 2000; Marsh et al., 2007; Snyder et al., 2011); the accumulation of evidence over the course of making a decision (e.g., Gluth et al., 2012; Landmann et al., 2007); or the requirement for internally generated responses versus externally cued/guided ones (e.g., Fleming et al., 2012; Shima and Tanji, 1998; Walton et al., 2004).
Despite the wealth of evidence that dACC is responsive to conflicts in processing, this idea has not been without controversy (Cole et al., 2009; Ito et al., 2003; Mansouri et al., 2009; Nachev, 2011; Nakamura et al., 2005). Early debates focused on whether dACC is responsive to conflict versus explicit failures in performance (i.e., errors) and/or negative feedback. There now seems to be general consensus that, consistent with the EVC model, dACC is responsive to both (e.g., Nee et al., 2011). However, recently it has been suggested that dACC activity reflects “time-on-task” irrespective of conflict, errors, or even error likelihood (Grinband et al., 2011b), and that it is more closely tied to task maintenance or attention that endures over the course of even simple tasks. However, the theoretical analyses that have led to this conclusion have been challenged (Brown, 2011; Yeung et al., 2011; see also Grinband et al., 2011a). Furthermore, we note that their interpretation of dACC function, more closely aligned with the regulative component of control, is difficult to square with much of the literature we will review in the remaining sections. For instance, it fails to account for dACC responses to the value of outcomes or for conditions in which dACC activity is uncorrelated, or even negatively correlated with, RT (e.g., Cavanagh et al., 2011; Gluth et al., 2012; Guerin and Miller, 2011; Sheth et al., 2012; Van Maanen et al., 2011). In contrast, while the EVC model predicts that dACC responses reflecting its monitoring function may correlate with RT, it also predicts conditions under which this should not necessarily occur, as discussed further below.
State information relevant to control signal identity
So far, our consideration has focused on state information relevant to deciding how much control to allocate; that is, the specification of control signal intensity. However, knowledge concerning the current situation or state is equally important for deciding which task to pursue.; that is, the specification of control signal identity. The dACC sits in an ideal location for gathering such state information (Morecraft et al., 2012; Shackman et al., 2011; Weston, 2012; Figure 1C). Inputs from cortical areas associated with high-level perception give it immediate access to information about external task cues, and inputs from structures such as the amygdala and insula give it access to information about motivational states that may favor particular lines of behavior. Although the impact of such inputs on dACC activity has been relatively little studied, the information they carry would be of obvious relevance to selection among control signal identities. Consistent with this, dACC appears to differentiate representations of signal identity, including representations of response rules (Dixon and Christoff, 2012; Durstewitz et al., 2010; Johnston et al., 2007; Matsuzaka et al., 2012; Womelsdorf et al., 2010), task sets (Forstmann et al., 2006; Haynes et al., 2007) and specific actions (Hampton and O’Doherty, 2007; Isomura et al., 2003; for reviews see Morecraft and Tanji, 2009; Rushworth et al., 2004; Sakai, 2008). Taken together, such findings support the idea that the dACC registers state information directly relevant to the specification of control-signal identity.
4.2. dACC monitoring of outcome information
Estimation of the EVC requires not only information about the present state, but also information about potential outcomes and, critically, the positive or negative value associated with those outcomes. In order to be sensitive to such information, the dACC should register both the anticipated value of outcomes ahead of their occurrence and their value when they actually occur.
Negative-valued outcomes
Numerous neuroimaging findings have demonstrated dACC responses to negative outcomes. These range from the most concrete, such as pain (reviewed in Shackman et al., 2011), errors in task performance (e.g. Brown and Braver, 2005; Holroyd and Coles, 2002), monetary loss (Blair et al., 2006; Kahnt et al., 2009; Liu et al., 2011), and the presentation of threatening stimuli (e.g., Mobbs et al., 2010), to more abstract outcomes such as social rejection (Eisenberger and Lieberman, 2004; Kawamoto et al., 2012), a loss by a favored sports team (Cikara et al., 2011), pain experienced by another individual (Botvinick et al., 2005; Lamm et al., 2010), and even the hypothetical death of strangers (Shenhav and Greene, 2010). These findings are paralleled by direct neuronal recordings in non-human species, which have demonstrated responses in dACC to errors (Amiez et al., 2005; Ito et al., 2003; Niki and Watanabe, 1979; Totah et al., 2009), losses or less-than-anticipated gains (Ito et al., 2003; Kennerley et al., 2011), and cues predictive of aversive outcomes (Gabriel and Orona, 1982; Amemori and Graybiel, 2012).
Conflict, in addition to its role in signaling task difficulty, may itself constitute an aversive outcome (Botvinick, 2007; Fritz and Dreisbach, 2013; Hirsh et al., 2012). Amemori and Graybiel’s (2012) study provided evidence in support of this assertion, showing that patterns of activity for ACC neurons that coded positively for conflict functionally clustered with those that coded for magnitude of punishment.
Positive-valued outcomes
There is also a growing accumulation of findings indicating that dACC is also responsive to positive outcomes. Direct neuronal recordings have consistently identified responses to rewarding events, often among units interdigitated with those responsive to negative outcomes. This includes neurons responsive to the magnitude and probability of rewards, including to hypothetical rewards (for a recent review see Wallis and Kennerley, 2011). Human neuroimaging studies have also provided evidence for reward-related signals in dACC (Knutson et al., 2005; Kouneiher et al., 2009; meta-analysis in Bartra et al., 2013).
Control-relevance of outcome value
A simple interpretation of the findings above might be that dACC responds to the value of any event. However, the EVC model makes a more specific claim: dACC should be selectively responsive to the value of events that are relevant to the allocation of control. To engage dACC, a valenced event need not necessarily pertain to the current task, but it should pertain to some potential control-demanding task that could currently be executed. Although this prediction has not been well-tested in the literature, there is evidence that dACC is more sensitive to outcomes when they are tied to actions, or stimuli that demand an action, than when they are only tied to non-imperative stimuli (for reviews see Rangel and Hare, 2010; Rushworth et al., 2011; Wallis and Kennerley, 2011). Furthermore, there is evidence that dACC responses to outcomes diminish when there is a decline in demand for control. For example, fMRI studies have shown that dACC engagement falls progressively with extended practice on a cognitive task (Chein and Schneider, 2005; Chein and Schneider, 2012). Similarly, feedback-related dACC activity is observed in tasks that require subjects to search for the correct response from a set of options, but is diminished when they are allowed to repeat the correct response a number of times before outcome contingencies change (reviewed in Khamassi et al., 2010). Landmann and colleagues (2007) found the same pattern of dACC activity in a task for which participants had to progressively discover the correct sequence of button presses, also through trial and error (see also Procyk et al., 2000). They showed that dACC activity was greater during search than after discovery of the correct sequence, and that during search it correlated with the amount of information carried by feedback at each step of the current sequence.
Note that, insofar as the dACC takes account of control-relevant outcome information in estimating EVC, it should therefore predict subsequent shifts in control based on such information. There is robust evidence for such a link, as will be discussed below.
Reward-prediction error signals
As articulated in Eq. 2, the value term in the EVC expression refers not only to the immediate reward associated with an outcome, but also to the expected future rewards. This is important, because it allows control-signal specification to be based on delayed outcomes. Readers familiar with reinforcement learning will recognize this particular formulation of value from that context (Sutton and Barto, 1998). In reinforcement learning models, estimates of state value are typically shaped not directly by raw representations of reward, but instead by reward-prediction errors (PE), signals indicating the extent to which experienced rewards are better or worse than expected.
A number of findings indicate the occurrence of PE signals in the dACC. The earliest evidence came from EEG recordings demonstrating an event related potential (ERP) with a frontomedial source that occurs in response to negative outcomes. This was dubbed the feedback-related negativity (FRN; Miltner et al., 1997), referring to its occurrence in response to negative feedback such as the indication of an error in task performance or a monetary loss following a gamble (Gehring and Willoughby, 2002). Critically, the FRN has been found to be sensitive to the expectations established by local context (Holroyd et al., 2004a; Jessup et al., 2010; Nieuwenhuis et al., 2005b). For example, in a gambling task, when the range of outcomes is from negative to neutral, the FRN is observed for losses but not neutral outcomes. However, when outcomes range from neutral to positive, the FRN is now observed for neutral outcomes, but not gains. Thus, expectations established by context dictates whether the FRN is elicited by a neutral outcome (see also Jessup et al., 2010). This provides strong evidence that the FRN reflects a PE, rather than a direct representation of absolute reward. Although the source of the FRN has not been definitively localized to dACC, neuroimaging studies have demonstrated activity in dACC under conditions that mimic those in which the FRN is observed (Holroyd et al., 2004b).
The FRN is closely related to another commonly observed ERP, the error related negativity (ERN). This occurs following errors in speeded response trials even when explicit feedback is not provided. There is direct evidence that the ERN has its source in the dACC: Simultaneous recording of EEG and fMRI has shown that the magnitude of the ERN correlates with the BOLD signal from dACC on a trial-by-trial basis (Debener et al., 2005). As suggested earlier, direct neuronal recordings from regions of dACC have also revealed error-related responses in humans (Wang et al., 2005), monkeys (Emeric et al., 2008; Ito et al., 2003), and rats (Narayanan and Laubach, 2008) (however, see Cole et al., 2009 for discussion of homology). One influential interpretation of the ERN is that it reflects negative PEs (associated with the commission of an error) that are used to drive learning in the service of improving action selection (Holroyd and Coles, 2002). The EVC model is consistent with this hypothesis. However, it has been proposed that the ERN can also reflect post-response conflict, under conditions in which error information is not immediately available (Yeung et al., 2004). In this context, the ERN may reflect the role of dACC in conflict monitoring and control-signal specification, as discussed above.
In addition to these EEG findings, evidence for positive and negative PEs in dACC has been found with both direct neuronal recordings (Kennerley et al., 2011; Matsumoto et al., 2007; Quilodran et al., 2008) and fMRI (Amiez et al., 2012; Kahnt et al., 2011). Interestingly, this has suggested that dACC can also respond to PEs in an unsigned manner — that is, comparably to both positive and negative PEs. This has been shown through direct neuronal recordings (Bryden et al., 2011; Hayden et al., 2011a), and is consistent with EEG and fMRI studies showing elevated dACC activity in response to surprising outcomes (Cavanagh et al., 2012; Landmann et al., 2007; Nee et al., 2011; Wessel et al., 2012) and, more generally, following unanticipated shifts in task contingencies (Alexander and Brown, 2011; Behrens et al., 2007; Bland and Schaefer, 2011). These observations have inspired a recent model of dACC function by Alexander and Brown (2011), which suggests that dACC stores predicted associations between stimuli and response-outcome (RO) conjunctions, and signals any violations of these predicted S-RO relationships. Although this proposal is potentially compatible with the EVC model, the latter makes more direct contact with data concerning the consequences of surprise signaling, as we discuss further on.
5. Specification function of the dACC: Maximizing the expected value of control
The EVC model proposes that information provided by monitoring is used to determine when and how control signals should be adjusted in order to maximize the opportunity for reward, by specifying the optimal control-signal identity and intensity. While the mechanisms implementing specification along each of these dimensions may be tightly coupled or even the same, different circumstances may rely differentially upon these two dimensions of specification. For example, switching between two control-demanding tasks would rely more heavily on selecting a signal identity, while responding to increasing demands of the current task by augmenting control would rely more heavily on adjustments of intensity (Figure 2B).
The literature provides evidence that dACC is engaged in both types of specification, though most studies have focused on only one or the other. Accordingly, most theories have tended to ascribe to dACC a role in either task selection (identity specification) or modulation of control (intensity specification). The EVC model integrates these accounts, proposing that they refer to different dimensions of the same function. Accordingly, dACC should be responsive to circumstances that engage either or both. In the two sections that follow, we review the literature concerning the association of dACC with each of these two dimensions of control specification.
5.1. Specification of control signal identity
Among the earliest theories of dACC function were ones that proposed a role in action selection (Devinsky et al., 1995; Matsumoto et al., 2003; Rangel and Hare, 2010; Rushworth et al., 2007; Rushworth et al., 2004). More recent theories have elaborated this idea to include task selection (Holroyd and Yeung, 2012; Kouneiher et al., 2009; O’Reilly, 2010). These are commensurate with the role of dACC in the specification of control signal identity proposed by the EVC model. Some evidence for this comes from studies showing dACC selectivity for different control signal identities, including rules and task sets. However, the EVC model also requires that control signals be specified based on their expected value. This predicts that the dACC should exhibit responses that are both selective for a particular line of behavior and sensitive to the value of outcomes associated with that behavior. This prediction is consistent with the findings of several recent studies.
For example, when monkeys were required to choose between targets in a visual saccade task, overlapping populations of dACC neurons were found to encode the value and direction of the saccade chosen on a given trial (Cai and Padoa-Schioppa, 2012; Hayden and Platt, 2010). Kaping and colleagues (2011) demonstrated similar effects in a task involving covert shifts of visual attention, rather than explicit eye movements. In their study, a colored fixation cue at the start of each trial indicated which of two subsequently presented colored visual stimuli should be attended. The monkeys were then rewarded if they correctly reported whether the stimulus with the corresponding color rotated clockwise or counterclockwise. The amount of reward earned by a correct response was signaled by the color of the initial fixation cue. As in previous studies, overlapping neuronal populations in rostral dACC were found to encode the target of the attentional shifts and the value of those targets, independently of any overt saccade used to report movement direction.
These findings are consistent with a role for dACC in specifying control signal identity based on its expected value. However, an alternative interpretation is possible: they could instead reflect the state and/or outcome monitoring functions of dACC without reflecting a role in specification. Weighing against this more restrictive interpretation is the observation that activity of dACC neurons in the studies described above typically anticipated switches between actions and/or tasks (though see Cai and Padoa-Schioppa, 2012). Similarly, Womelsdorf and colleagues (2010) have shown that local field potentials (LFPs) in the theta band observed within macaque dACC could discriminate which of two stimulus-response mapping rules (pro- vs. anti-saccade) would be used prior to appearance of the stimulus. Furthermore, this rule selectivity was absent prior to error trials, consistent with the hypothesis that activity in dACC was required to specify the identity of the task-appropriate control signal. Interestingly, when rule-selective activity reemerged prior to a correct trial following an error, the selectivity was seen earlier than on correct trials that followed a previous correct one (see also Johnston et al., 2007). A subsequent study from this group used a similar task to provide causal support for this control specification role (Phillips et al., 2011). They found that stimulating dACC during the response preparation period significantly facilitated antisaccade performance (accelerating responses without increasing error rate), but had a less consistent influence on prosaccade performance, a complement to the impairments (slowing) previously found in human dACC lesion patients performing an antisaccade task (Gaymard et al., 1998).
Additional evidence consistent with identity specification comes from one of the most comprehensive analyses to date of human patients with focal brain lesions (Gläscher et al., 2012). This study combined data from four different set-shifting tasks into a single “cognitive control factor” and found that the poorest performance along this factor was associated with lesions in rostral dACC. These findings are consistent with a causal role for dACC in specifying control identities. It is also consistent with its role in specifying the intensity of those control signals.
5.2. Specification of control signal intensity
Motivation
A role in specifying control intensity is consistent with the earliest observations regarding dACC function, which ascribed to it a function in ‘motivation,’ driven in part by the observation that medial frontal damage can lead to gross deficits in motivated behavior (e.g., abulia; see Holroyd & Yeung, 2012). More recent proposals have suggested that dACC motivates or ‘energizes’ action or task engagement based on current incentives (Holroyd and Yeung, 2012; Kouneiher et al., 2009; Stuss and Alexander, 2007). In support of this, circumscribed lesions that encompass dACC produce longer overall reaction times (e.g., Alexander et al., 2007; Fellows and Farah, 2005), and higher false alarm rates (e.g., Løvstad et al., 2012; Tsuchida and Fellows, 2009). These are consistent with a role for dACC in specifying control intensity.
Adaptive adjustments in control intensity
Beyond this broad relationship to motivation, the EVC model makes the more specific proposal that the dACC is responsible for adjusting the intensity of control adaptively based on task demands. That is, not only must the control system determine what task is best to perform, but also the amount of control that must be allocated to that task so as to optimize EVC. This follows from the assumption that control is costly, as discussed earlier (see Figure 4). There is longstanding evidence for adaptive adjustments in control in the behavioral literature, for example changes in the speed-accuracy tradeoff observed following errors in simple decision tasks (see Danielmeier and Ullsperger, 2011). Gratton et al. (1992) suggested that such adaptive adjustments extend to the allocation of attention, showing that the response to an incongruent stimulus is faster when it follows another incongruent stimulus than when it follows a congruent one. This was interpreted as evidence of an enhancement of attention to the task-relevant dimension in response to the interference produced by a prior incongruent one.
In computational work, Botvinick et al. (2001) demonstrated that the behavioral effects described above could be explained by a mechanism that monitors conflict elicited by lapses in performance and/or interference and uses this to adjust the intensity of the task-relevant control signals in order to maintain task performance. However, the EVC model makes a stronger claim: that such adjustments serve to optimize the allocation of control. A modest, but growing corpus of work has begun to address this stronger claim and its relation to dACC function.
Optimization of control intensity
The most extensive analyses of control optimization have focused on simple two-alternative choice tasks, such as those used to demonstrate adaptive changes in the speed-accuracy tradeoff mentioned above. Such tasks have been modeled extensively using simple accumulator models, in which the intensity of the control signal influences two parameters of the decision process: the decision threshold and initial bias. Together, these determine the speed-accuracy tradeoff. Botvinick et al. (2001) showed that monitoring response conflict in such models and using this to adjust the intensity of the control signal accurately accounted for adaptive changes in the speed-accuracy tradeoff observed in behavior. In that model, the intensity of the control signal determined the decision threshold. More recently, formal analyses by Bogacz et al. (2006) have shown that there is an optimal threshold (i.e., speed-accuracy tradeoff) that maximizes reward rate for a given set of task conditions and similarly for initial bias. Furthermore, behavioral studies show that participants adapt their behavior to changes in task conditions in ways that often approximate adoption of the optimal threshold and bias (reviewed in Cohen and Holmes, in press). While most work addressing optimality of control has focused on adjustments of threshold and/or bias in simple two-alternative forced-choice tasks, at least one theoretical effort has addressed the optimal allocation of attention in a conflict task. Yu et al. (2009) determined the optimal strength with which attention should be allocated to the target stimulus in the Erisken flanker task. They showed that this could be approximated by within-trial adjustments in the strength of attention based on conflict monitoring, and that this in turn accurately reproduced the dynamics of attentional allocation observed in the task.
Role of dACC in adaptive adjustments of control intensity
The findings of these theoretical and behavioral studies are consistent with the idea that the intensity of the control signal is adjusted to maximize EVC. The EVC model proposes that dACC mediates these adjustments, by monitoring for the conditions that require them, and specifying the necessary adjustments for others systems responsible for implementing them. This makes two predictions: first, that dACC should be responsive to conditions indicating the need to adjust control intensity; and, second, that it should be associated with the engagement of neural systems responsible for implementing these adjustments (i.e., the regulative function of control).
There is extensive evidence in support of the first prediction, indicating that dACC is responsive to conditions requiring adjustments of threshold and/or response bias, such as increases in time pressure and changes in prior probabilities (Bogacz et al., 2010; Forstmann et al., 2010; Forstmann et al., 2008; Ivanoff et al., 2008; Mulder et al., 2012; Van Maanen et al., 2011); as well as conditions requiring changes in the degree of attention, such as the cases of processing conflict described earlier.
There is also evidence in support of the second prediction. Several studies have shown that dACC interacts directly with structures proposed to implement changes of threshold, such as the subthalamic nucleus (Aron et al., 2007; Aron and Poldrack, 2006; Cavanagh et al., 2011; Jahfari et al., 2011; Wiecki and Frank, 2013), as well as those thought to influence response biases, such as dorsal striatum (Bogacz et al., 2010; Jahfari et al., 2011; Wiecki and Frank, 2013). There is also evidence that dACC is associated with adjustments in the strength of attention in conflict tasks. Several human neuroimaging studies have demonstrated a direct association between dACC responses to conflict on one trial, and subsequent increases in the activity of regions thought to be responsible for regulating attention and corresponding improvements in performance on the next trial (e.g., Cavanagh et al., 2009; Kerns, 2006; Kerns et al., 2004; King et al., 2010; Macdonald et al., 2000). In a recent study, Danielmeier and colleagues (2011) used a variant of the Simon task to study the relationship of dACC responses to conflict, performance, and activity in stimulus-specific regions of visual cortex. As had previously been found, dACC activity associated with errors predicted response slowing on the subsequent trial. Critically, however, it also predicted the degree to which activity increased in task-relevant and decreased in task-irrelevant processing regions, consistent with an increase in the control signal following errors. EEG studies have provided similar evidence, linking indicators of dACC responses (such as the ERN) to sequential adjustments in behavior following conflict and/or errors (Crottaz-Herbette and Menon, 2006; Forster et al., 2011). Such effects can also be found within a given trial. For example, Sohn et al. (2007) found that when participants were explicitly informed about the amount of conflict likely to arise on a given trial of a problem-solving task, anticipatory dACC activity predicted how efficiently conflict was resolved on that trial (see also Aarts et al., 2008). Finally, studies in non-human species have also provided evidence that responses in dACC predict changes in the amount of attention subsequently paid to a given stimulus or task dimension (Bryden et al., 2011; Narayanan and Laubach, 2008; Totah et al., 2009).
These studies provide convergent evidence for a correlation of responses in dACC with subsequent changes in performance and task-specific neural activity indicative of adjustments in the intensity of control. Sheth et al. (2012) provided evidence that dACC contributes causally to these adjustments. Patients about to undergo cingulotomy were studied using both fMRI and intracranial recordings while performing a conflict task. Preoperatively, participants exhibited the standard conflict adaptation effects in both behavior (e.g., faster RTs on high-conflict trials that followed a high-conflict versus a low-conflict trial; Figure 3B, left) and neuronal activity (differential dACC firing rates for this same contrast; though see Figure 3C-D for discussion of a surprising divergence from previous neuroimaging findings). Importantly, following cingulotomy this adaptation effect was no longer apparent, consistent with a causal role for this region in adaptively influencing control intensities (Figure 3B, right).
Attention for learning
Another context in which the dACC appears to play a role in specifying control intensity relates to its responses to surprising events. Research demonstrating unsigned PE signals in dACC has highlighted a potential connection with the Pearce-Hall model of learning, in which surprising outcomes trigger an intensification of attention, that in turn facilitates learning. To test this, Bryden and colleagues (2011) had rats poke their nose into a port to receive an odor instructing them where to obtain a reward, and found that rats were faster to poke their nose into this port on trials that followed a surprising outcome (increases or decreases from expected reward). Critically, they found that responses in dACC to surprise on the preceding trial predicted the degree to which the animal hastened or slowed this orienting response on the trial that followed. Also consistent with an ‘attention for learning’ account, Behrens and colleagues (2007) showed that dACC responses to changes in task contingencies – specifically, to their volatility, which reflects the expected frequency of surprise – were associated with more robust learning. Such findings, and the attention-for-learning account overall, are consistent with the EVC model of dACC, insofar as the signals driving top-down attention and/or increases in learning rates may be considered as control signals.
5.3. Default override
Thus far we have treated the specification of identity and intensity separately. In reality, however, the identity and intensity of the control signal must be jointly specified (see Figure 2). For example, to perform color naming in the Stroop task, the control system must specify both the identity of the control signal (the color naming task), as well as the intensity needed to overcome any conflict from the word. Such circumstances are representative of a broader class of conditions often described as default override. In general, this refers to situations in which the task to be performed is less automatic than the default behavior in that circumstance; that is, the behavior that would normally occur in absence of control and is the source of conflict. Under such circumstances, adequate control is needed to override the default response, and execute the specified task (see, e.g., Shah and Oppenheimer, 2008).
Some of the earliest neuroimaging studies of cognitive control established a role for dACC in overriding default behavior (e.g., Paus et al., 1993). The dACC’s involvement in overriding defaults has been seen not only when the participant is explicitly instructed to perform the non-default behavior, but also when they voluntarily make a choice that runs counter to current task- or context-defined defaults, including choices to go against gain vs. loss frames (De Martino et al., 2006), against the status quo (Fleming et al., 2010), against Pavlovian response-outcome associations (Cavanagh et al., 2013), or against a group decision (Tomlin et al., 2013). Three additional circumstances that involve default override, and that have begun to attract considerable attention, are exploration, foraging and intertemporal choice.
Exploration
This refers to behavior that favors information gathering with the prospect that this will yield greater future reward, over the pursuit of behavior with known and usually more immediate reward (i.e., exploitation). People generally exhibit a strong bias toward the pursuit of more immediate reward, so exploitation can be considered the default. Choosing to explore therefore requires overriding this default, and thus allocating control. Accordingly, the EVC model predicts that exploration should engage dACC (for related accounts, see Aston-Jones and Cohen, 2005; Khamassi et al., 2010). This prediction was borne out in a study carried out by Daw et al. (2006). Participants chose among four options providing probabilistic rewards that varied gradually over time. Initially they sampled the different options (exploration) to determine which currently provided the greatest mean payoff, after which they repeatedly selected that one (exploitation) until it began to decline in value, or they suspected others may have increased in value. At that point, they chose to sample the other options again (exploration). Decisions to explore were associated with increased dACC activity. This association between dACC and exploratory behavior has been replicated in humans (Amiez et al., 2012; Cavanagh et al., 2012), and also demonstrated in monkeys (Procyk et al., 2000; Quilodran et al., 2008) and rodents (Karlsson et al., 2012).
Foraging
Like exploration, foraging involves searching for an alternative source of reward. However, in this case it typically involves an initial cost and is also usually driven by knowledge of the reward structure of the environment (whereas exploration is directed at acquiring such knowledge). Nevertheless, like exploration, foraging involves overriding current pursuit of more immediate reward to pursue an alternative that promises greater future reward, and thus relies on the allocation of control. Accordingly, the EVC model predicts that foraging should also engage the dACC. This prediction is supported by a number of studies. For instance, Kolling et al. (2012) had participants make a series of choices between pairs of options that yielded probabilistic payoffs with known means. However, before each choice, participants were given the opportunity to switch the pair of options in front of them to a different pair that could yield higher average rewards, but at a cost for the switch. This was designed to be analogous to situations in which an animal’s decision to forage carries a near term cost but a long term benefit. Activity in dACC was found to closely track the extent to which the mean value of the alternative options was greater than that of the current options, and to correlate with the decision to switch option sets in such cases (see also Boorman et al., 2013; Rushworth et al., 2012; Wunderlich et al., 2009). This is consistent with the EVC model, which predicts that dACC should track the value of control-demanding behavior, and its selection over the current default. Animal studies have provided convergent findings. For example, Hayden et al. (2011b) found that macaque dACC neurons also track the value of foraging, and Li et al. (2012) found that dACC-lesioned rats forage for food substantially less than non-lesioned animals, while continuing to engage normally in other habitual or automatic behavior.
Intertemporal choice
Finally, it is worth noting that, insofar as both exploration and foraging involve the comparative evaluation of longer term versus immediate payoffs, they both involve intertemporal choice. One universally observed finding in the literature on intertemporal choice is that people (like all other species) exhibit a strong immediacy bias. That is, they favor rewards that are immediate, even when later ones are worth considerably more. In this respect, behavior associated with immediate reward can be considered the default behavior in general, and thus should require control to be overcome. Consistent with this view, neuroimaging studies of intertemporal choice, beyond those focused on exploration or foraging, suggest that patient behavior (i.e., choices for longer term over immediate reward) rely on neural mechanisms associated with cognitive control (Figner et al., 2010; McClure et al., 2007; McClure et al., 2004), including the dACC. In these cases, as in general, the EVC model proposes that the role of dACC is to determine the EVC of the control-demanding behavior, and specify the control signal needed to pursue it. This assumes that it has access to information about the value of the options in contention that is represented in other structures, such as ventral regions of mPFC (Floresco et al., 2008; Haber and Knutson, 2010; Prévost et al., 2010; Rangel and Hare, 2010; Rushworth et al., 2011).
6. dACC and the cost of cognitive control
The expected value of a control-demanding behavior depends not only on the rewards it promises, but also on the expenditures needed to procure those rewards; that is, it depends on the cost of control (Cost(signal) in Eq. 1). As reviewed earlier, behavioral evidence supports the idea that the exertion of control is associated with subjective disutility manifest as the avoidance of control-demanding tasks (Kool and Botvinick, in press; Kool et al., 2010). The EVC model proposes that the dACC registers the costs of control in a manner that is proportional to the intensity of control and that it specifies control signals in a way that is sensitive to such costs. This proposal generates several predictions concerning dACC function and its relation to behavior.
Costs of control and control intensity
First, and most simply, the dACC should be sensitive to demands for control and/or to the intensity of the current control signal. As reviewed in the preceding sections, there is abundant evidence in support of this point. Second, the dACC should encode the exertion of control as costly. Evidence consistent with this idea has come from several recent studies. For example, Botvinick and colleagues (2009a) found that, during performance of a cognitively demanding task, a greater dACC response predicted decreased subsequent responses in nucleus accumbens to monetary rewards, interpreted as “payment” for the task. This effect is consistent with cognitive effort discounting; that is, a reduction in the subjective value attached to a reward based on cognitive costs borne to attain it. Other studies have shown that dACC responses to such costs predict subsequent decisions about control. In one, Magno and colleagues (2006) presented participants with a series of attentionally-demanding search arrays and, for each array, gave them the choice to identify the presence or absence of a target or to indicate that they would like to forgo the search on that trial. They found that dACC activity was highest on trials the participant actively chose to forgo rather than engage for potential reward (or loss). Similarly, McGuire and Botvinick (2010) found that the degree to which performance of a cognitively demanding task engaged dACC predicted the extent to which that same task would later be avoided. Collectively, these findings are consistent not only with dACC encoding of control costs, but also with a role for dACC in cost-sensitive control signal specification.
Figure 4 illustrates how the optimal control signal intensity predicted by the EVC model is determined by the relationships of control costs and payoffs to control signal intensity. These relationships determine the function relating EVC to intensity, and the optimum occurs at a point where the slope of that function is zero. Under plausible assumptions about the shape of the payoff and cost functions (see Kool and Botvinick, in press), the optimal control signal intensity will rise with the magnitude of task incentives (see Figure 4A-B). This predicts that dACC activity should grow both with task difficulty and with the stakes associated with task performance. This dual effect was reported by Kouneiher and colleagues (2009), who had participants perform a series of trials in which a colored letter cued them to perform a letter discrimination task or to simply press a single key unrelated to letter identity (‘default’ trials). Each trial was also cued with whether or not a correct response would carry a monetary bonus, and the value of these bonuses differed by trial block. The authors found that dACC activity increased with the difficulty of the trial as well as with the average incentive level for the trial block (regardless of whether a bonus was available on a particular trial; see Figure 4C-D).
Control signal intensity and willingness to pay
The prediction of a monotonic relationship between control-signal intensity and the cost of control means that the output of the dACC can be interpreted in either of two ways: as directly reflecting the specified intensity of the current control signal, or as indirectly reflecting the cost that has been licensed for this control signal. The latter follows from the assumption of the EVC model that the dACC specifies the optimal control signal; accordingly, its intensity should indicate the amount of control that was determined to be ‘worth’ the expected payoff. This relationship between intensity and cost can be understood by analogy to the economic concept known as willingness-to-pay, which refers to the amount worth trading for a good. Recent work has characterized orbitofrontal cortex as carrying a willingness-to-pay signal during economic choice (Levy and Glimcher, 2012; Padoa-Schioppa, 2011; Plassmann et al., 2007). The EVC theory suggests that the output of dACC can be thought of as a willingness-to-pay signal in the currency of cognitive control.
Thinking of dACC output in these terms provides another way of understanding impairments of cognition and behavior following medial frontal damage. As discussed above, this is commonly associated with acquired apathy or an inability/lack of energy to perform willed actions, as well as subtler deficits such as response slowing, perseverative errors, and failures to speed or slow current trial performance based on information from the previous trial (e.g., Stuss, 2011; Stuss and Alexander, 2007). All of these may reflect a failure to specify the required “willingness-to-pay” for initiating effortful control, particularly when the incentives for doing so are minimal.
Other forms of costs
In considering the costs of executing controlled behavior, we have focused on the cost of control itself, but this reflects only one possible cost that must be factored into computing EVC. Other costs — such as any physical effort involved — are equally relevant. The EVC model predicts that dACC should be responsive to such costs as well, and there is an abundance of evidence that dACC is responsive to the physical effort required by an action and revises its estimate of expected reward downward in order to reflect the cost of exerting this effort (e.g., Croxson et al., 2009; Hillman and Bilkey, 2010; Walton et al., 2007). Neurons in dACC have been found to track the effort demands of a prospective action, whether this involves lever presses (Kennerley et al., 2011; Kennerley et al., 2009) or physical obstacles that need to be overcome along a path (Cowen et al., 2012; Hillman and Bilkey, 2010). The same has been found in human neuroimaging studies when varying, for instance, how many visuomotor targets would need to be detected on a task (Croxson et al., 2009) or how much force needs to be exerted on a handgrip (Prévost et al., 2010). As with cognitive demands, the dACC also signals the degree to which these motor requirements reduce the value of an action. That is, dACC activity signals the overall value of potential actions. .
7. The cascade of control: dACC in relation to other control-related structures
The proposal that dACC integrates information relevant to evaluating EVC places it at the heart of a broader network of systems that support control-demanding behaviors. Specifically, it places it at the juncture between structures involved in valuation from which it receives input, and structures responsible for regulation to which it provides its output. Importantly, the EVC model makes a clear distinction between these functions and those of monitoring and control signal specification that the dACC is proposed to subserve. Nevertheless, the full span of control functions is likely to reflect a continuous cascade of processing, from valuation to monitoring and estimation of EVC, to control signal specification and finally regulation. Thus, in practice it may be difficult to dissociate these individual functions. It is not surprising, therefore, that structures commonly associated with valuation and regulation have been found to co-activate and/or share structural and functional connectivity with dACC (Figure 1; Beckmann et al., 2009; Haber and Knutson, 2010; Morecraft et al., 2012; Power and Petersen, 2013; Touroutoglou et al., 2012; Vincent et al., 2008; Yeo et al., 2011). In fact, finding evidence for dissociations of function among these structures has been something of a challenge for this area of research. In this section, we review the literature addressing such efforts, and what it has to say about the division of labor between dACC and other structures that have been implicated in valuation and the implementation of cognitive control.
7.1 Interactions with insula, ventral PFC, striatal and midbrain structures involved in valuation
The EVC model makes a fundamental distinction between the primary representation of value — whether of internal signals or external ones— and the monitoring of these for use in estimating the EVC of candidate control signals. While the model proposes that dACC subserves the latter, it assumes that the primary representation of value is subserved by other structures that project to dACC, including other cortical areas (e.g., insula and ventral/medial regions of PFC) and subcortical ones (e.g., basal ganglia and dopaminergic midbrain structures).
Insula and detection of affective salience
One of the regions most commonly co-activated with dACC is the anterior insula, and these two regions share robust reciprocal connections. While some have suggested that the insula might take part in a regulative role in maintaining task sets (Dosenbach et al., 2006), others have proposed that the interaction between insula and dACC may reflect a sequential process of registering motivationally salient stimuli that engender adaptive adjustments of processing. On this account, the insula supports representations of affective/motivational significance. These are then conveyed to the dACC in order to appropriately modify processing to influence internal autonomic states as well as changes in overt behavior, including emotional expressions (Bush et al., 2000; Craig, 2009; Medford and Critchley, 2010; Shackman et al., 2011; Singer et al., 2009; Ullsperger et al., 2010). The dACC can also register the extent to which these affective/autonomic states interfere with ongoing task performance and therefore require additional cognitive control. This division of labor is supported by differences in the patterns of connectivity of insula and dACC with other regions, and evidence that the insula is more consistently tied to the conscious experience of emotion while the dACC is more closely tied to overt responses to emotion-eliciting stimuli. These observations have led Craig (2002; 2009) to refer to the insula and dACC as limbic sensory and limbic motor cortices, respectively.
This division of labor between primary valuation and adaptive responding is generally consistent with the EVC model. However, the model distinguishes between the function of dACC in specifying adaptations, and their implementation by other structures that actually regulate processing. The model also asserts that the dACC’s involvement should be specific to adaptations that involve controlled but not automatic responses. This includes situations in which automatic responses to emotionally salient events must be overridden or overcome.
Ventral PFC, midbrain, amygdala, striatum, and valuation
In addition to inputs from the insula, the dACC also receives extensive inputs from OFC/vmPFC, the dopaminergic midbrain, and the amygdala. Along with the striatum, these are all areas that have been consistently implicated in the representation of value and/or prediction error signals. Thus, inputs from these areas are consistent with the state and outcome monitoring functions of dACC proposed by the EVC model. Furthermore, the dACC projects to both ventral and dorsomedial regions of the striatum (Choi et al., 2012; Haber and Knutson, 2010). As noted earlier, fMRI evidence implicates the dACC in modulating reward signals in ventral striatum, effectively deducting the cost of cognitive control.
7.2. Interactions with lPFC and subcortical structures involved in regulation
The EVC model also distinguishes sharply between control-signal specification and direct regulation of information processing. Specifically, the model proposes that the dACC is responsible for the decision process — evaluating EVC and using this to specify the optimal control signal — while the specified control signal itself is implemented in other structures that are responsible for the top-down regulation of processing. At the broadest level, a distinction can be made between two kinds of regulative functions: One type that identifies and supports the execution of specific tasks, and is subserved primarily by cortical structures together with parts of the basal ganglia; and another that sets processing parameters more broadly by global modulation of processing and is subserved primarily by subcortical structures.
lPFC and task-specific regulation
Perhaps the structure most commonly associated with cognitive control is lPFC. A dominant view of lPFC function is that it supports the active maintenance of task representations that bias processing in pathways of posterior cortex responsible for executing specific control-demanding tasks, consistent with a regulative function in control (see Figure 2A). Thus, according to the EVC model, lPFC can be seen as implementing the control signal to support a given task, as specified by dACC. There is a growing consensus about this distribution of functions between dACC and lPFC (Banich, 2009; Cavanagh et al., 2009; Holroyd and Yeung, 2012; Johnston et al., 2007; Kerns et al., 2004; Kouneiher et al., 2009; Macdonald et al., 2000; O’Reilly, 2010; Ridderinkhof et al., 2007; Rothé et al., 2011; Venkatraman and Huettel, 2012).
Given the close relationship between specification and regulation, it is perhaps not surprising that lPFC is another region frequently co-activated with dACC in control-demanding tasks (Duncan, 2010; Niendam et al., 2012). For example, sustained activity during task performance has been observed in both dACC and lPFC. While this may reflect the role of lPFC in active maintenance (i.e., regulation), for dACC it could reflect its role in continuous online evaluation of interference or changes in payoff and corresponding adjustments in control signal intensity that drive the level of activity in lPFC. Conversely, lPFC has often been found to track response conflict (Laird et al., 2005; Nee et al., 2007), though this would be expected if it is responsible for augmenting control in response to the dACC’s detection of conflict and re-specification of control signal intensity. While these interpretations of the findings are consistent with the division of labor proposed by the EVC model, some investigators have taken a different view.
One widely considered account suggests that the dACC itself plays a regulative function in cognitive control, in addition to or instead of the roles in monitoring and specification proposed by the EVC model (e.g., Danielmeier et al., 2011; Dosenbach et al., 2006; Posner et al., 1988; Power and Petersen, 2013; Weissman et al., 2005). For example, Dosenbach and colleagues (2008; 2006) have argued that the dACC and anterior insula comprise a core network for task-set maintenance, responsible for sustaining attention to a task over extended periods (see also Holroyd and Yeung, 2012). In support of this, they analyzed imaging data from a large number of participants performing a diverse array of cognitive tasks. They showed that dACC and anterior insula are the only two regions that exhibit not only phasic responses to salient events, but also tonically increased responses throughout task performance consistent with a maintenance (i.e., regulative) function (but see Sridharan et al., 2008). Further evidence that dACC may support a regulative function comes from studies such as that of Danielmeier and colleagues (2011), in which dACC is shown to predict changes in attention in the absence of lPFC involvement (although, again, this could also be viewed as reflecting specification rather than regulation).
The tight coupling between specification and regulation may make it difficult to produce qualitative dissociations in responses between dACC and lPFC. This may be especially so for findings from methods with limited temporal resolution, such as fMRI. One approach to this challenge is to look for quantitative biases in effects, using methods with better temporal resolution. A study by Johnston and colleagues (2007) provided such evidence from single unit recordings in monkeys. The animals were trained to fixate a cue for over a second prior to performing a pro- or antisaccade to a stimulus. Neurons were found in both dACC and lPFC that, during this pre-stimulus preparatory period, exhibited selectivity for the task rule that would be implemented on the upcoming trial (as in Womelsdorf et al., 2010). Importantly, they found that dACC task selectivity was greater and onset earlier than lPFC immediately following a task switch, but that this relationship reversed (i.e., dACC task selectivity gradually weakened and began later than lPFC) with more trials of the same rule associations. This pattern would seem to be consistent with a role for dACC in control signal specification, and for lPFC in maintenance of the control signal in the service of regulation.
Another recent study has provided even finer-grained evidence for a dissociation between the specification and regulation functions of control. Measuring local field potentials (LFPs) in both the dACC and lPFC of macaques, Rothé and colleagues (2011) showed that transient increases in the high-gamma LFP within dACC signaled salient events (errors and first correct feedback; see also Quilodran et al., 2008), that were followed shortly by more sustained responses in lPFC. Moreover, while high-gamma activity was always correlated between the two regions, the lag in activity between them was only found for feedback during search periods and not when the animal was allowed to repeat the behavior for the same reward. This is consistent with the engagement of dACC in response to events calling for a re-evaluation and specification of the control signal, and the engagement of lPFC for the representation and maintenance of that signal once specified, in the service of regulating controlled behavior.
Despite the challenges involved, some human imaging studies have also produced evidence for dissociations of responses in dACC and lPFC. For example, MacDonald and colleagues (2000) showed that dACC was more sensitive to response conflict and less so to the implementation of task set instructions, whereas the reverse was true for lPFC. Furthermore, while many studies have found that activity in dACC is consistently associated with the occurrence of an event that triggers adaptive responding activity in lPFC appears to be more closely associated with the adaptations that occur after such events (e.g., Egner and Hirsch, 2005a; Egner and Hirsch, 2005b; Kerns, 2006; Kerns et al., 2004).Additional evidence for this dissociation comes from the study by Kouneiher and colleagues (2009), in which participants switched between two task rules. While the authors found that regions of dACC tracked the incentives for control, they found that lPFC discriminated the task required for the current trial. Furthermore, functional connectivity analyses showed that the connectivity between dACC and lPFC varied with incentive level.
The findings above are largely consistent with the division of labor between dACC and lPFC proposed by the EVC model, but they are not definitive. One alternative is that topographic dissociations exist within dACC itself, such that some subregions support specification and others regulation. Consistent with this possibility, findings both from humans (Orr and Weissman, 2009) and macaques (Kaping et al., 2011) have suggested that patterns of sustained activity can be localized to separate regions within dACC than transient responses signaling salient events. In both studies, a more anterior region of dACC was associated with transient responses (consistent with monitoring and specification), whereas a more posterior region was associated with sustained responses (consistent with regulation). However, as Kaping and colleagues point out for their findings, and as noted above, sustained dACC responses could alternatively represent continuous online evaluation of interference or changes in payoff and/or corresponding adjustments required in the intensity of the control signal, consistent with monitoring and/or specification rather than regulation. Clearly, this is an area that is in need of continued, detailed study.
Basal ganglia and task-specific regulation
There is longstanding evidence that much of prefrontal cortex (including lPFC) is reciprocally connected to the basal ganglia (and thalamus) in a series of topographically organized loops and that these structures are commonly engaged, together with prefrontal cortex, in cognitive control tasks (see Figure 1D; Choi et al., 2012; Scimeca and Badre, 2012). Frank and colleagues (Frank et al., 2001; O’Reilly and Frank, 2006; Wiecki and Frank, 2013) have proposed that these corticostriatal loops may serve as a gating mechanism, regulating action implementation as well as updating of control representations in prefrontal cortex (for related models, also see Bogacz et al., 2010; O’Reilly et al., 2002; Reynolds and O’reilly, 2009; Rougier et al., 2005). A similar gating mechanism could play an intermediary role between the dACC’s selection of candidate control signals and their implementation by lPFC (e.g., through dorsal striatum). Though speculative, such a mechanism might account for cases in which the response latency between the two regions is longer than expected for a direct cortico-cortical projection (e.g., over 100ms in the study by Rothé and colleagues).
Subcortical structures and global regulation
Thus far, our discussion of the relationship between specification and regulation has focused on circumstances in which control is responsible for selecting and supporting the execution of a particular task, but there are also instances in which control must specify other parameters of processing — for example response threshold in simple decision tasks or the bias to explore rather exploit. It has been proposed that these forms of regulation are implemented by subcortical structures, through more global modulatory mechanisms. Such global influences are presumed to interact with the task-specific ones discussed above, to jointly select a particular processing pathway (lPFC), and the parameters that will apply to it (subcortical mechanisms). For the latter, the EVC model proposes a similar division of labor as for the former, with dACC responsible for monitoring and specification, and the relevant subcortical structures responsible for regulation. The literature is largely consistent with this prediction.
For example, Frank and colleagues (Cavanagh et al., 2011; Frank, 2006; Wiecki and Frank, 2013) have proposed that projections from dACC to STN specify the threshold for evidence accumulation before initiating a motor or cognitive response, and that efferents from STN implement this threshold. To test this, Cavanagh and colleagues (2011) used scalp and intracortical EEG to measure dACC and STN activity in patients with Parkinson’s Disease while undergoing deep brain stimulation (DBS) to the STN. On each trial, patients chose between pairs of stimuli that they had learned to associate with either similar or different rewards (high- and low-conflict trials, respectively). Activity in both dACC and STN tracked the level of decision conflict for a given choice. Furthermore, greater dACC activity associated with high conflict trials predicted slower more accurate responses (reflecting a higher threshold). In contrast, when DBS was applied to STN (interfering with its function), responses on these trials became more impulsive and error-prone (reflecting lower decision thresholds), and the relationship between dACC activity and slower responding was lost. Taken together, these findings provide support for the role of dACC in specifying adaptive adjustments in threshold that are then implemented by STN.
Similarly, Aston-Jones and Cohen (2005) have proposed that dACC is involved in monitoring behavioral outcomes and deciding when it is appropriate to explore versus exploit, and that this is conveyed to LC which implements the decision by means of its broad modulatory projections to the thalamus and neocortex. This division of labor is consistent with strong anatomic connections from dACC to LC and is also supported by imaging studies implicating dACC in the decision to explore and recent behavioral and psychophysiological studies suggesting a role for LC in mediating these decisions by regulating the balance between exploration and exploitation (Gilzenrat et al., 2010; Jepma and Nieuwenhuis, 2011; Murphy et al., 2011; Nieuwenhuis et al., 2005a).
The projections of dACC to subcortical modulatory structures, together with its efferents to other cortical areas, puts the dACC in a position to specify control signals of a variety of types, and over a variety of domains of processing, from signals required to regulate specific tasks (e.g., in lPFC) to broader, modulatory ones needed to influence a wide range of tasks (e.g., in STN and LC). This centralized responsibility for specifying such a wide range of control signals may also explain why dACC appears to be so consistently associated with cognitive control, and more so than other candidate structures like lPFC (e.g., Danielmeier et al., 2011; Dosenbach et al., 2006). Insofar as most of those other structures are responsible for regulation, they are dedicated either to the support of specific tasks or to specific modulatory forms of control. Accordingly, they will be engaged only when the demands for control involve that particular regulative function. In contrast, as the central hub in control specification, the dACC would be expected to be engaged in any circumstance demanding the specification of a control signal.
7.3. Control Hierarchies
Several recent studies have proposed that separate regions within the lPFC might encode information pertinent to different levels of task structure, with higher-level processes engaging more anterior regions (Badre and D’esposito, 2009; Koechlin et al., 2003; though see Crittenden and Duncan, in press; Reynolds et al., 2012). Similar proposals have been made regarding the organization of dACC. For example, Kouneiher and colleagues (2009) showed that regions within dACC and pre-SMA differentially encode task incentives for a block of trials versus individual trials within a block. Furthermore, the patterns of connectivity between dACC and lPFC were found to be modulated by motivation type, with anterior regions of dACC and lPFC being engaged by block-level incentives and more posterior regions exhibiting a similar pattern for trial-level incentives.
Evidence that the dACC is topographically organized to represent the motivation for control at different levels of temporal abstraction is broadly consistent with a proposal by Holroyd and Yeung (2011, 2012), according to which the dACC is specifically involved in the control of hierarchical, temporally extended, actions. This account is theoretically motivated by hierarchical reinforcement learning (HRL; Botvinick et al., 2009b), and has found support in the recent finding that prediction error signals specifically anticipated by HRL are observed within dACC (Ribas-Fernandes et al., 2011). A related account suggests that representations within dACC may be organized by the level of abstraction or complexity of a task (Venkatraman and Huettel, 2012; see Nachev et al., 2008 for an analogous account). For example, Venkatraman and colleagues (2009) showed that progressively anterior regions of dACC signaled increasingly complex task demands, from conflicts between specific motor actions at the posterior extent to conflicts between high-level strategies at the anterior extent. This group has further shown that these regions within dACC show differential patterns of resting-state functional connectivity with lPFC regions that Koechlin and colleagues (Koechlin et al., 2003; Kouneiher et al., 2009) have shown to be involved in regulative aspects of control at similarly increasing levels of temporal abstractness (Taren et al., 2011; Venkatraman and Huettel, 2012; Venkatraman et al., 2009).
The EVC model does not speak directly to the issue of hierarchical organization of control. According to the EVC model, the dACC should be engaged by control-demanding behaviors irrespective of their level of abstractness or temporal extent, whether these involve individual motor actions, more abstract strategies, or temporally extended tasks. The primary determinant of dACC engagement is whether the behavior involves processes that cannot rely fully on pre-specified parameters — that is, that are not automatic. In this respect, the EVC model provides a role for dACC in a broader range of control-demanding behaviors than is predicted by theories linking it to more hierarchically-organized or temporally extended behaviors. Furthermore, in addition to the idea that dACC is involved in specifying task identity, the EVC model integrates the idea that dACC is also involved in specifying control intensity, a function not typically addressed by the theories discussed above.
The findings by Venkatraman and colleagues are intriguing in part because they lend credence to an implicit assumption of the EVC model: that cognitive control signals are analogous to motor control signals and therefore undergo a similar process of optimization. Anatomic studies have revealed increasingly motor-related cytoarchitecture and patterns of connectivity as one traverses dorsomedial PFC from anterior dACC to pre-SMA and SMA (Figure 1C; Morecraft et al., 2012; Nachev et al., 2008; Vogt et al., 2003). This suggests that, rather than supporting a heterogeneity of functions, these regions may serve a similar set of functions applied over a range of abstractness of control signals. Accordingly, while we have focused on the evaluation of cognitive control signals in the EVC model, the same notation has been applied in different areas of the action selection and motor control literature. The EVC term itself generalizes what is referred to as a Q-value in the reinforcement learning literature (mapping the value of an action in a given state; Balleine et al., 2008; Sutton and Barto, 1998), and previous work has already described how the strength (i.e., vigor) of a motor action can be weighed against the attendant physical effort costs (Niv, 2007; Niv et al., 2006).
8. Conclusions
The challenge of understanding the role of the dACC in cognition and behavior is daunting. The experimental evidence accumulated to date contains a remarkable diversity of findings that have lent themselves to a wide range of interpretations. Here, we have proposed an account of dACC function that attempts to accommodate this diversity, while at the same time organizing it into a coherent picture. In particular, we have proposed that the dACC leverages a wide range of information in order to estimate the EVC, a quantity integrating the expected payoffs and costs of candidate control signals. Based on the results of this computation, the dACC specifies both the identity and intensity of the control signals that maximize estimated EVC. These are then implemented by regulatory structures that are responsible for actually effecting the changes in information processing in the rest of the brain required to perform the specified task(s).
The EVC model owes many of its components to previous theoretical proposals, both from our own labs and from others. By fitting these pieces together, and accommodating some new ones within a single integrative account, we hope that the EVC model provides a useful theoretical reference point for future research. Like any model, it raises new questions alongside the ones it attempts to answer: How is a set of candidate control signals initially learned? How might the EVC be feasibly (and perhaps only approximately) estimated by neural mechanisms? If cognitive control is inherently costly, what exact form does the cost function assume? And what costs might attach to the estimation of EVC itself? Finally, how are the component functions proposed by the model implemented and organized within the neural architecture of the dACC? Given the fast pace of research in this area, we feel confident that the next few years will yield data pertinent to these questions, and to the expected value of the EVC model itself.
Acknowledgments
This work is supported by the C.V. Starr Foundation (A.S), the National Institute of Mental Health R01MH098815-01 (M.M.B), and the John Templeton Foundation. The opinions expressed in this publication are those of the authors and do not necessarily reflect the views of the John Templeton Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts E, Roelofs A, van Turennout M. Anticipatory Activity in Anterior Cingulate Cortex Can Be Independent of Conflict and Error Likelihood. J. Neurosci. 2008;28:4671–4678. doi: 10.1523/JNEUROSCI.4400-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S. Regional frontal injuries cause distinct impairments in cognitive control. Neurology. 2007;68:1515–1523. doi: 10.1212/01.wnl.0000261482.99569.fb. [DOI] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat. Neurosci. 2011;14:1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemori K-I, Graybiel AM. Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nat. Neurosci. 2012;15:776–785. doi: 10.1038/nn.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Joseph J-P, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. Eur. J. Neurosci. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb. Cortex. 2006;16:1040–1055. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Sallet J, Procyk E, Petrides M. Modulation of feedback related activity in the rostral anterior cingulate cortex during trial and error exploration. Neuroimage. 2012;63 doi: 10.1016/j.neuroimage.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TEJ, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Ann. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Badre D, D’esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat. Rev. Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Daw ND, O’Doherty JP. Multiple forms of value learning and the function of dopamine. In: Glimcher P, Camerer CF, Fehr E, Poldrack RA, editors. Neuroeconomics: Decision Making and the Brain. Academic Press; London: 2008. pp. 367–385. [Google Scholar]
- Banich MT. Executive Function: The Search for an Integrated Account. Curr. Dir. Psychol. Sci. 2009;18:89–94. [Google Scholar]
- Barch D, Braver T, Sabb F, Noll D. Anterior cingulate and the monitoring of response conflict: Evidence from an fMRI study of overt verb generation. J. Cogn. Neurosci. 2000;12:298–309. doi: 10.1162/089892900562110. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J. Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Walton ME, Rushworth MFS. Learning the value of information in an uncertain world. Nat. Neurosci. 2007;10:1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Blair KS, Marsh AA, Morton J, Vythilingam M, Jones M, Mondillo K, Pine DC, Drevets WC, Blair RJR. Choosing the lesser of two evils, the better of two goods: specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate in object choice. J. Neurosci. 2006;26:11379–11386. doi: 10.1523/JNEUROSCI.1640-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland AR, Schaefer A. Electrophysiological correlates of decision making under varying levels of uncertainty. Brain Res. 2011;1417:55–66. doi: 10.1016/j.brainres.2011.08.031. [DOI] [PubMed] [Google Scholar]
- Bogacz R, Brown E, Moehlis J, Holmes P, Cohen JD. The physics of optimal decision making: A formal analysis of models of performance in two-alternative forced-choice tasks. Psychol. Rev. 2006;113:700–765. doi: 10.1037/0033-295X.113.4.700. [DOI] [PubMed] [Google Scholar]
- Bogacz R, Wagenmakers E-J, Forstmann BU, Nieuwenhuis S. The neural basis of the speed-accuracy tradeoff. Trends Neurosci. 2010;33:10–16. doi: 10.1016/j.tins.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Boorman ED, Rushworth MF, Behrens TE. Ventromedial Prefrontal and Anterior Cingulate Cortex Adopt Choice and Default Reference Frames during Sequential Multi-Alternative Choice. J. Neurosci. 2013;33:2242–2253. doi: 10.1523/JNEUROSCI.3022-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–319. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn. Affect. Behav. Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD. The computational and neural basis of cognitive control: Charted territory and new frontiers. Cogn. Sci. doi: 10.1111/cogs.12126. in press. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire J. Effort discounting in human nucleus accumbens. Cogn. Affect. Behav. Neurosci. 2009a;9:16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Niv Y, Barto AC. Hierarchically organized behavior and its neural foundations: A reinforcement learning perspective. Cognition. 2009b;113:262–280. doi: 10.1016/j.cognition.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. In: S M, J D, editors. Attention and Performance XVIII; Control of cognitive processes. MIT Press; Cambridge, MA: 2000. pp. 713–737. [Google Scholar]
- Brown JW. Medial prefrontal cortex activity correlates with time-on-task: what does this tell us about theories of cognitive control? Neuroimage. 2011;57:314–315. doi: 10.1016/j.neuroimage.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Bryden DW, Johnson EE, Tobia SC, Kashtelyan V, Roesch MR. Attention for Learning Signals in Anterior Cingulate Cortex. J. Neurosci. 2011;31:18266–18274. doi: 10.1523/JNEUROSCI.4715-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cai X, Padoa-Schioppa C. Neuronal Encoding of Subjective Value in Dorsal and Ventral Anterior Cingulate Cortex. J. Neurosci. 2012;32:3791–3808. doi: 10.1523/JNEUROSCI.3864-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AM, Botvinick MM, Ross LL, Stenger A, Noll D, Cohen JD. Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJB. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J. Neurosci. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Figueroa CM, Cohen MX, Frank MJ. Frontal Theta Reflects Uncertainty and Unexpectedness during Exploration and Exploitation. Cereb. Cortex. 2012;22:2575–2586. doi: 10.1093/cercor/bhr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat. Neurosci. 2011;14:1462–1467. doi: 10.1038/nn.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Eisenberg I, Guitart-Masip M, Huys Q, Frank MJ. Frontal Theta Overrides Pavlovian Learning Biases. J. Neurosci. 2013;33:8541–8548. doi: 10.1523/JNEUROSCI.5754-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Cogn. Brain Res. 2005;25:607–623. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Chein JM, Schneider W. The Brain’s Learning and Control Architecture. Curr. Dir. Psychol. Sci. 2012;21:78–84. [Google Scholar]
- Choi EY, Yeo BTT, Buckner RL. The Organization of the Human Striatum Estimated By Intrinsic Functional Connectivity. J. Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikara M, Botvinick MM, Fiske ST. Us versus them: social identity shapes neural responses to intergroup competition and harm. Psychol. Sci. 2011;22:306–313. doi: 10.1177/0956797610397667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn J, Frank MJ. Reinforcement learning, conflict monitoring, and cognitive control: An integrative model of cingulate-striatal interactions and the ERN. In: Rushworth MFS, Yeung N, editors. Neural basis of motivational and cognitive control. MIT Press; Cambridge: 2011. pp. 311–331. [Google Scholar]
- Cohen JD, Dunbar K, Mcclelland JL. On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychol. Rev. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Holmes P. Optimality and some of its discontents. Trends Cogn. Sci. doi: 10.1111/tops.12084. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol. Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Cole MW, Yeung N, Freiwald WA, Botvinick MM. Cingulate cortex: diverging data from humans and monkeys. Trends Neurosci. 2009;32:566–574. doi: 10.1016/j.tins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen SL, Davis GA, Nitz DA. Anterior cingulate neurons in the rat map anticipated effort and reward to their associated action sequences. J. Neurophysiol. 2012:1–55. doi: 10.1152/jn.01012.2011. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig ADB. How do you feel — now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crittenden BM, Duncan J. Task Difficulty Manipulation Reveals Multiple Demand Activity but no Frontal Lobe Hierarchy. Cereb. Cortex. 2012 Nov 6; doi: 10.1093/cercor/bhs333. in press. [Epub ahead of print]. doi: 10.1093/cercor/bhs333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J. Cogn. Neurosci. 2006;18:766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TEJ, Rushworth MFS. Effort-based cost-benefit valuation and the human brain. J. Neurosci. 2009;29:4531. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Chris Gatenby J, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychol. Sci. 2004;15:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Danielmeier C, Eichele T, Forstmann BU, Tittgemeyer M, Ullsperger M. Posterior Medial Frontal Cortex Activity Predicts Post-Error Adaptations in Task-Related Visual and Motor Areas. J. Neurosci. 2011;31:1780–1789. doi: 10.1523/JNEUROSCI.4299-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, Ullsperger M. Post-error adjustments. Front. Psychol. 2011;2:233. doi: 10.3389/fpsyg.2011.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P. How to set the switches on this thing. Curr. Opin. Neurobiol. 2012;22:1068–1074. doi: 10.1016/j.conb.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J. Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Ann. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dixon ML, Christoff K. The Decision to Engage Cognitive Control Is Driven by Expected Reward-Value: Neural and Behavioral Evidence. PLoS ONE. 2012;7:e51637. doi: 10.1371/journal.pone.0051637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N, Fair D, Cohen A, Schlaggar B, Petersen S. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn. Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Vittoz NM, Floresco SB, Seamans JK. Abrupt Transitions between Prefrontal Neural Ensemble States Accompany Behavioral Transitions during Rule Learning. Neuron. 2010;66:438–448. doi: 10.1016/j.neuron.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat. Neurosci. 2005a;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. NeuroImage. 2005b;24:539–547. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Eisenberger N, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn. Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex. J. Neurophysiol. 2008;99:759–772. doi: 10.1152/jn.00896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain. 2005;128:788–796. doi: 10.1093/brain/awh405. [DOI] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. Lateral prefrontal cortex and self-control in intertemporal choice. Nat. Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Huijgen J, Dolan RJ. Prefrontal Contributions to Metacognition in Perceptual Decision Making. J. Neurosci. 2012;32:6117–6125. doi: 10.1523/JNEUROSCI.6489-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Thomas CL, Dolan RJ. Overcoming status quo bias in the human brain. Proc. Natl. Acad. Sci. U.S.A. 2010;107:6005–6009. doi: 10.1073/pnas.0910380107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn. Affect. Behav. Neurosci. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Forster SE, Carter CS, Cohen JD, Cho RY. Parametric manipulation of the conflict signal and control-state adaptation. J. Cogn. Neurosci. 2011;23:923–935. doi: 10.1162/jocn.2010.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Anwander A, Schäfer A, Neumann J, Brown S, Wagenmakers E-J, Bogacz R, Turner R. Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc. Natl. Acad. Sci. U.S.A. 2010;107:15916–15920. doi: 10.1073/pnas.1004932107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Brass M, Koch I, Von Cramon D. Voluntary selection of task sets revealed by functional magnetic resonance imaging. J. Cogn. Neurosci. 2006;18:388–398. doi: 10.1162/089892906775990589. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, Wagenmakers E-J. Striatum and pre-SMA facilitate decision-making under time pressure. Proc. Natl. Acad. Sci. U.S.A. 2008;105:17538–17542. doi: 10.1073/pnas.0805903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006;19:1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn. Affect. Behav. Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Fritz J, Dreisbach G. Conflicts as aversive signals: Conflict priming increases negative judgments for neutral stimuli. Cogn. Affect. Behav. Neurosci. 2013;13:311–317. doi: 10.3758/s13415-012-0147-1. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Orona E. Parallel and serial processes of the prefrontal and cingulate cortical systems during behavioral learning. Brain Res. Bull. 1982;8:781–785. doi: 10.1016/0361-9230(82)90107-1. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Rivaud S, Cassarini JF, Dubard T, Rancurel G, Agid Y, Pierrot-Deseilligny C. Effects of anterior cingulate cortex lesions on ocular saccades in humans. Exp. Brain Res. 1998;120:173–183. doi: 10.1007/s002210050391. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn. Affect. Behav. Neurosci. 2010;10:252–269. doi: 10.3758/CABN.10.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 2012;109:14681–14686. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluth S, Rieskamp J, Buchel C. Deciding When to Decide: Time-Variant Sequential Sampling Models Explain the Emergence of Value-Based Decisions in the Human Brain. J. Neurosci. 2012;32:10686–10698. doi: 10.1523/JNEUROSCI.0727-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. Optimizing the use of information: strategic control of activation of responses. J. Exp. Psychol. Gen. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J. Conflict, error likelihood, and RT: Response to Brown & Yeung et al. Neuroimage. 2011a;57:320–322. doi: 10.1016/j.neuroimage.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage. 2011b;57:303–311. doi: 10.1016/j.neuroimage.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin SA, Miller MB. Parietal cortex tracks the amount of information retrieved even when it is not the basis of a memory decision. Neuroimage. 2011;55:801–807. doi: 10.1016/j.neuroimage.2010.11.066. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, O’Doherty JP. Decoding the neural substrates of reward-related decision making with functional MRI. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1377–1382. doi: 10.1073/pnas.0606297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Heilbronner SR, Pearson JM, Platt ML. Surprise Signals in Anterior Cingulate Cortex: Neuronal Encoding of Unsigned Reward Prediction Errors Driving Adjustment in Behavior. J. Neurosci. 2011a;31:4178–4187. doi: 10.1523/JNEUROSCI.4652-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Pearson JM, Platt ML. Neuronal basis of sequential foraging decisions in a patchy environment. Nat. Neurosci. 2011b;14:933–939. doi: 10.1038/nn.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Platt ML. Neurons in anterior cingulate cortex multiplex information about reward and action. J. Neurosci. 2010;30:3339–3346. doi: 10.1523/JNEUROSCI.4874-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J-D, Sakai K, Rees G, Gilbert S, Frith C, Passingham RE. Reading hidden intentions in the human brain. Curr. Biol. 2007;17:323–328. doi: 10.1016/j.cub.2006.11.072. [DOI] [PubMed] [Google Scholar]
- Hazy T, Frank M, O’Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2007;362:1601. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman KL, Bilkey DK. Neurons in the Rat Anterior Cingulate Cortex Dynamically Encode Cost-Benefit in a Spatial Decision-Making Task. J. Neurosci. 2010;30:7705–7713. doi: 10.1523/JNEUROSCI.1273-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman KL, Bilkey DK. Neural encoding of competitive effort in the anterior cingulate cortex. Nat. Neurosci. 2012;15:1290–1297. doi: 10.1038/nn.3187. [DOI] [PubMed] [Google Scholar]
- Hirsh JB, Mar RA, Peterson JB. Psychological entropy: A framework for understanding uncertainty-related anxiety. Psychol. Rev. 2012;119:304–320. doi: 10.1037/a0026767. [DOI] [PubMed] [Google Scholar]
- Ho TC, Brown S, Serences JT. Domain general mechanisms of perceptual decision making in human cortex. J. Neurosci. 2009;29:8675–8687. doi: 10.1523/JNEUROSCI.5984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Larsen JT, Cohen JD. Context dependence of the event-related brain potential associated with reward and punishment. Psychophysiology. 2004a;41:245–253. doi: 10.1111/j.1469-8986.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MGH, Cohen JD. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat. Neurosci. 2004b;7:497–498. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. An integrative theory of anterior cingulate cortex function: Option selection in hierarchical reinforcement learning. In: Mars RB, Sallet J, Rushworth MFS, Yeung N, editors. Neural Basis of Motivational and Cognitive Control. MIT Press; Cambridge, MA: 2011. pp. 333–349. [Google Scholar]
- Holroyd CB, Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn. Sci. 2012;16:121–127. doi: 10.1016/j.tics.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Leung LS, Menon RS, Everling S. Resting-State Connectivity Identifies Distinct Functional Networks in Macaque Cingulate Cortex. Cereb. Cortex. 2011;22:1294–1308. doi: 10.1093/cercor/bhr181. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Ito Y, Akazawa T, Nambu A, Takada M. Neural coding of “attention for action” and “response selection” in primate anterior cingulate cortex. J. Neurosci. 2003;23:8002–8012. doi: 10.1523/JNEUROSCI.23-22-08002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Ivanoff J, Branning P, Marois R. fMRI evidence for a dual process account of the speed-accuracy tradeoff in decision-making. PLoS ONE. 2008;3:e2635. doi: 10.1371/journal.pone.0002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Waldorp L, Van Den Wildenberg WPM, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective Connectivity Reveals Important Roles for Both the Hyperdirect (Fronto-Subthalamic) and the Indirect (Fronto-Striatal-Pallidal) Fronto-Basal Ganglia Pathways during Response Inhibition. J. Neurosci. 2011;31:6891–6899. doi: 10.1523/JNEUROSCI.5253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepma M, Nieuwenhuis S. Pupil diameter predicts changes in the exploration-exploitation trade-off: evidence for the adaptive gain theory. J. Cogn. Neurosci. 2011;23:1587–1596. doi: 10.1162/jocn.2010.21548. [DOI] [PubMed] [Google Scholar]
- Jessup R, Busemeyer J, Brown JW. Error effects in anterior cingulate cortex reverse when error likelihood is high. J. Neurosci. 2010;30:3467–3472. doi: 10.1523/JNEUROSCI.4130-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M, Inzlicht M, Schmader T. Stereotype threat and executive resource depletion: Examining the influence of emotion regulation. J. Exp. Psychol. Gen. 2008;137:691–705. doi: 10.1037/a0013834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K, Levin H, Koval M, Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Kahnt T, Grueschow M, Speck O, Haynes J-D. Perceptual Learning and Decision-Making in Human Medial Frontal Cortex. Neuron. 2011;70:549–559. doi: 10.1016/j.neuron.2011.02.054. [DOI] [PubMed] [Google Scholar]
- Kahnt T, Park S, Cohen MX, Beck A, Heinz A, Wrase J. Dorsal striatal-midbrain connectivity in humans predicts how reinforcements are used to guide decisions. J. Cogn. Neurosci. 2009;21:1332–1345. doi: 10.1162/jocn.2009.21092. [DOI] [PubMed] [Google Scholar]
- Kaping D, Vinck M, Hutchison RM, Everling S, Womelsdorf T. Specific Contributions of Ventromedial, Anterior Cingulate, and Lateral Prefrontal Cortex for Attentional Selection and Stimulus Valuation. PLoS Biol. 2011;9:e1001224. doi: 10.1371/journal.pbio.1001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MP, Tervo DGR, Karpova AY. Network Resets in Medial Prefrontal Cortex Mark the Onset of Behavioral Uncertainty. Science. 2012;338:135–139. doi: 10.1126/science.1226518. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Onoda K, Nakashima K.i., Nittono H, Yamaguchi S, Ura M. Is dorsal anterior cingulate cortex activation in response to social exclusion due to expectancy violation? An fMRI study. Front. Evol. Neurosci. 2012;4:1–10. doi: 10.3389/fnevo.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Behrens TEJ, Wallis JD. Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat. Neurosci. 2011;14:1581–1589. doi: 10.1038/nn.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. Neurons in the frontal lobe encode the value of multiple decision variables. J. Cogn. Neurosci. 2009;21:1162–1178. doi: 10.1162/jocn.2009.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG. Anterior cingulate and prefrontal cortex activity in an FMRI study of trial-to-trial adjustments on the Simon task. Neuroimage. 2006;33:399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Khamassi M, Wilson CRE, Rothé M, Quilodran R, Dominey PF, Procyk E. Meta- learning, cognitive control, and physiological interactions between medial and lateral prefrontal cortex. In: Mars R, Sallet J, Rushworth MFS, Yeung N, editors. Neural Bases of Motivational and Cognitive Control. MIT Press; Cambridge, MA: 2010. pp. 351–370. [Google Scholar]
- King JA, Korb FM, von Cramon DY, Ullsperger M. Post-error behavioral adjustments are facilitated by activation and suppression of task-relevant and task-irrelevant information processing. J. Neurosci. 2010;30:12759–12769. doi: 10.1523/JNEUROSCI.3274-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J. Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The Architecture of Cognitive Control in the Human Prefrontal Cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kolling N, Behrens TEJ, Mars RB, Rushworth MFS. Neural Mechanisms of Foraging. Science. 2012;336:95–98. doi: 10.1126/science.1216930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool W, Botvinick MM. A Labor/Leisure Tradeoff in Cognitive Control. J. Exp. Psychol. Gen. 2012 Dec 10; doi: 10.1037/a0031048. in press. Advance online publication. doi: 10.1037/a0031048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool W, McGuire JT, Rosen ZB, Botvinick MM. Decision making and the avoidance of cognitive demand. J. Exp. Psychol. Gen. 2010;139:665–682. doi: 10.1037/a0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat. Neurosci. 2009;12:939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Roberts KC, Song AW, Woldorff MG. The involvement of the dopaminergic midbrain and cortico-striatal-thalamic circuits in the integration of reward prospect and attentional task demands. Cereb. Cortex. 2012;22:607–615. doi: 10.1093/cercor/bhr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. A Comparison of Label-Based Review and ALE Meta-Analysis in the Stroop Task. Hum. Brain Mapp. 2005;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2010;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Landmann C, Dehaene S, Pappata S, Jobert A, Bottlaender M, Roumenov D, Le Bihan D. Dynamics of prefrontal and cingulate activity during a reward-based logical deduction task. Cereb. Cortex. 2007;17:749–759. doi: 10.1093/cercor/bhk028. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Curr. Opin. Neurobiol. 2012;22:1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Li M, Cao W, Xu Y, Luo Y, Zhong X, Zhang J, Dai R, Zhou X-F, Li Z, Li C. Anterior cingulate cortical lesion attenuates food foraging in rats. Brain Res. Bull. 2012;88:602–608. doi: 10.1016/j.brainresbull.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løvstad M, Funderud I, Meling T, Krämer UM, Voytek B, Due-Tønnessen P, Endestad T, Lindgren M, Knight RT, Solbakk AK. Anterior cingulate cortex and cognitive control: Neuropsychological and electrophysiological findings in two patients with lesions to dorsomedial prefrontal cortex. Brain Cogn. 2012;80:237–249. doi: 10.1016/j.bandc.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. J. Neurosci. 2006;26:4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat. Rev. Neurosci. 2009;10:141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Blair KS, Vythilingam M, Busis S, Blair RJR. Response options and expectations of reward in decision-making: the differential roles of dorsal and rostral anterior cingulate cortex. Neuroimage. 2007;35:979–988. doi: 10.1016/j.neuroimage.2006.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Suzuki W, Tanaka K. Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science. 2003;301:229–232. doi: 10.1126/science.1084204. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Matsumoto K, Abe H, Tanaka K. Medial prefrontal cell activity signaling prediction errors of action values. Nat. Neurosci. 2007;10:647–656. doi: 10.1038/nn1890. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Akiyama T, Tanji J, Mushiake H. Neuronal activity in the primate dorsomedial prefrontal cortex contributes to strategic selection of response tactics. Proc. Natl. Acad. Sci. U.S.A. 2012;109:4633–4638. doi: 10.1073/pnas.1119971109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S, Ericson K, Laibson D, Loewenstein G, Cohen J. Time discounting for primary rewards. J. Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Botvinick MM, Yeung N, Greene JD, Cohen JD. Conflict monitoring in cognition-emotion competition. In: Gross JJ, editor. Handbook of Emotion Regulation. Guilford; New York: 2006. pp. 1–45. [Google Scholar]
- McClure SM, Laibson D, Loewenstein G, Cohen J. Separate Neural Systems Value Immediate and Delayed Monetary Rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McGuire JT, Botvinick MM. Prefrontal cortex, cognitive control, and the registration of decision costs. Proc. Natl. Acad. Sci. U.S.A. 2010;107:7922–7926. doi: 10.1073/pnas.0910662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct. Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Jones DK, Steventon J, Westacott L, Aggleton JP, O’Sullivan MJ. Cingulum Microstructure Predicts Cognitive Control in Older Age and Mild Cognitive Impairment. J. Neurosci. 2012;32:17612–17619. doi: 10.1523/JNEUROSCI.3299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Ann. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-Related Brain Potentials Following Incorrect Feedback in a Time-Estimation Task: Evidence for a “Generic” Neural System for Error Detection. J. Cogn. Neurosci. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20582. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, Mcneal DW, Pandya DN. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res. Bull. 2012;87:457–497. doi: 10.1016/j.brainresbull.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Tanji J. Cingulofrontal interactions and the cingulate motor areas. In: Vogt BA, editor. Cingulate Neurobiology and Disease. Oxford University Press; Oxford: 2009. pp. 113–144. [Google Scholar]
- Mulder MJ, Wagenmakers E-J, Ratcliff R, Boekel W, Forstmann BU. Bias in the Brain: A Diffusion Model Analysis of Prior Probability and Potential Payoff. J. Neurosci. 2012;32:2335–2343. doi: 10.1523/JNEUROSCI.4156-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, Robertson IH, Balsters JH, O’Connell RG. Pupillometry and P3 index the locus coeruleus-noradrenergic arousal function in humans. Psychophysiology. 2011;48:1532–1543. doi: 10.1111/j.1469-8986.2011.01226.x. [DOI] [PubMed] [Google Scholar]
- Nachev P. The blind executive. Neuroimage. 2011;57:312–313. doi: 10.1016/j.neuroimage.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O’Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36:T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Roesch MR, Olson CR. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J. Neurophysiol. 2005;93:884–908. doi: 10.1152/jn.00305.2004. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. J. Neurophysiol. 2008;100:520–525. doi: 10.1152/jn.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Kastner S, Brown JW. Functional heterogeneity of conflict, error, task-switching, and unexpectedness effects within medial prefrontal cortex. Neuroimage. 2011;54:528–540. doi: 10.1016/j.neuroimage.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cogn. Affect. Behav. Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012;12:141–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Gilzenrat MS, Holmes BD, Cohen JD. The Role of the Locus Coeruleus in Mediating the Attentional Blink: A Neurocomputational Theory. J. Exp. Psychol. Gen. 2005a;134:291–307. doi: 10.1037/0096-3445.134.3.291. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, Alting von Geusau NJ, Mars RB, Holroyd CB, Yeung N. Activity in human reward-sensitive brain areas is strongly context dependent. NeuroImage. 2005b;25:1302–1309. doi: 10.1016/j.neuroimage.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res. 1979;171:213–224. doi: 10.1016/0006-8993(79)90328-7. [DOI] [PubMed] [Google Scholar]
- Niv Y. Cost, Benefit, Tonic, Phasic: what do response rates tell us about dopamine and motivation? Ann. N. Y. Acad. Sci. 2007;1104:357–376. doi: 10.1196/annals.1390.018. [DOI] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Dayan P. How fast to work: Response vigor, motivation and tonic dopamine. Adv. Neural Inf. Process. Syst. 2006;18:1019. [Google Scholar]
- Norman DA, Shallice T. Attention to action: Willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and Self-Segulation: Vol. 4. Advances in research and theory. Plennum Press; New York: 1986. pp. 1–18. [Google Scholar]
- O’Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC. The What and How of prefrontal cortical organization. Trends Neurosci. 2010;33:355–361. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, Noelle DC, Braver TS, Cohen JD. Prefrontal cortex and dynamic categorization tasks: Representational organization and neuromodulatory control. Cereb. Cortex. 2002;12:246–257. doi: 10.1093/cercor/12.3.246. [DOI] [PubMed] [Google Scholar]
- Olivers CN, Peters J, Houtkamp R, Roelfsema PR. Different states in visual working memory: When it guides attention and when it does not. Trends Cogn. Sci. 2011;15:327–334. doi: 10.1016/j.tics.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Orr JM, Weissman DH. Anterior cingulate cortex makes 2 contributions to minimizing distraction. Cereb. Cortex. 2009;19:703–711. doi: 10.1093/cercor/bhn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Neurobiology of economic choice: a good-based model. Ann. Rev. Neurosci. 2011;34:333–359. doi: 10.1146/annurev-neuro-061010-113648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum. Brain Mapp. 2009;30:2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc. Natl. Acad. Sci. U.S.A. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport. 1998;9:R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J. Neurophysiol. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Johnston K, Everling S. Effects of anterior cingulate microstimulation on pro- and antisaccades in nonhuman primates. J. Cogn. Neurosci. 2011;23:481–490. doi: 10.1162/jocn.2010.21482. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb. Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty JP, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J. Neurosci. 2007;27:9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon J-B, Riis J, Sanfey AG, Nystrom LE, Cohen JD. Functional imaging of decision conflict. J. Neurosci. 2008;28:3468–3473. doi: 10.1523/JNEUROSCI.4195-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyn SM, Norman KA, Kahana MJ. A context maintenance and retrieval model of organizational processes in free recall. Psychol. Rev. 2009;116:129–156. doi: 10.1037/a0014420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, Fox PT, Raichle ME. Localization of cognitive operations in the human brain. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Power JD, Petersen SE. Control-related systems in the human brain. Curr. Opin. Neurobiol. 2013;23:223–228. doi: 10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévost C, Pessiglione M, Météreau E, Cléry-Melin M, Dreher J. Separate valuation subsystems for delay and effort decision costs. J. Neurosci. 2010;30:14080–14090. doi: 10.1523/JNEUROSCI.2752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procyk E, Tanaka YL, Joseph JP. Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nat. Neurosci. 2000;3:502–508. doi: 10.1038/74880. [DOI] [PubMed] [Google Scholar]
- Quilodran R, Rothé M, Procyk E. Behavioral shifts and action valuation in the anterior cingulate cortex. Neuron. 2008;57:314–325. doi: 10.1016/j.neuron.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Rangel A, Hare TA. Neural computations associated with goal-directed choice. Curr. Opin. Neurobiol. 2010;20:262–270. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, O’Reilly RC, Cohen JD, Braver TS. The Function and Organization of Lateral Prefrontal Cortex: A Test of Competing Hypotheses. PLoS ONE. 2012;7:e30284. doi: 10.1371/journal.pone.0030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, O’reilly RC. Developing PFC representations using reinforcement learning. Cognition. 2009;113:281–292. doi: 10.1016/j.cognition.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Fernandes José J.F., Solway A, Diuk C, Mcguire Joseph T., Barto Andrew G., Niv Y, Botvinick Matthew M. A Neural Signature of Hierarchical Reinforcement Learning. Neuron. 2011;71:370–379. doi: 10.1016/j.neuron.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Nieuwenhuis S, Braver TS. Medial frontal cortex function: an introduction and overview. Cogn. Affect. Behav. Neurosci. 2007;7:261–265. doi: 10.3758/cabn.7.4.261. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rothé M, Quilodran R, Sallet J, Procyk E. Coordination of high gamma activity in anterior cingulate and lateral prefrontal cortical areas during adaptation. J. Neurosci. 2011;31:11110–11117. doi: 10.1523/JNEUROSCI.1016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier NP, Noelle DC, Braver TS, Cohen JD, O’Reilly RC. Prefrontal cortex and flexible cognitive control: Rules without symbols. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7338. doi: 10.1073/pnas.0502455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn. Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Kolling N, Sallet J, Mars RB. Valuation and decision-making in frontal cortex: one or many serial or parallel systems? Curr. Opin. Neurobiol. 2012;22:946–955. doi: 10.1016/j.conb.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Noonan MaryAnn P., Boorman Erie D., Walton Mark E., Behrens Timothy E. Frontal Cortex and Reward-Guided Learning and Decision-Making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman D. Action sets and decisions in the medial frontal cortex. Trends Cogn. Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Sakai K. Task set and prefrontal cortex. Ann. Rev. Neurosci. 2008;31:219–245. doi: 10.1146/annurev.neuro.31.060407.125642. [DOI] [PubMed] [Google Scholar]
- Scimeca JM, Badre D. Striatal Contributions to Declarative Memory Retrieval. Neuron. 2012;75:380–392. doi: 10.1016/j.neuron.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AK, Oppenheimer DM. Heuristics made easy: an effort-reduction framework. Psychol. Bull. 2008;134:207–222. doi: 10.1037/0033-2909.134.2.207. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Greene JD. Moral Judgments Recruit Domain-General Valuation Mechanisms to Integrate Representations of Probability and Magnitude. Neuron. 2010;67:667–677. doi: 10.1016/j.neuron.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488:218–221. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. J. Neurophysiol. 1998;80:3247–3260. doi: 10.1152/jn.1998.80.6.3247. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Snyder HR, Banich MT, Munakata Y. Choosing Our Words: Retrieval and Selection Processes Recruit Shared Neural Substrates in Left Ventrolateral Prefrontal Cortex. J. Cogn. Neurosci. 2011;23:3470–3482. doi: 10.1162/jocn_a_00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn M-H, Albert MV, Jung K, Carter CS, Anderson JR. Anticipation of conflict monitoring in the anterior cingulate cortex and the prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 2007;104:10330–10334. doi: 10.1073/pnas.0703225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT. Functions of the frontal lobes: relation to executive functions. J. Int. Neuropsychol. Soc. 2011;17:759–765. doi: 10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning: An Introduction. MIT Press; Cambridge, MA: 1998. [Google Scholar]
- Taren AA, Venkatraman V, Huettel SA. A Parallel Functional Topography between Medial and Lateral Prefrontal Cortex: Evidence and Implications for Cognitive Control. J. Neurosci. 2011;31:5026–5031. doi: 10.1523/JNEUROSCI.5762-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin D, Nedic A, Prentice DA, Holmes P, Cohen JD. The Neural Substrates of Social Influence on Decision Making. PLoS ONE. 2013;8:e52630. doi: 10.1371/journal.pone.0052630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totah NKB, Kim YB, Homayoun H, Moghaddam B. Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. J. Neurosci. 2009;29:6418–6426. doi: 10.1523/JNEUROSCI.1142-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Barrett LF. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage. 2012;60:1947–1958. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Lesion Evidence That Two Distinct Regions within Prefrontal Cortex are Critical for n-Back Performance in Humans. J. Cogn. Neurosci. 2009;21:2263–2275. doi: 10.1162/jocn.2008.21172. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain Struct. Funct. 2010;214:629–643. doi: 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maanen L, Brown SD, Eichele T, Wagenmakers E-J, Ho T, Serences J, Forstmann BU. Neural Correlates of Trial-to-Trial Fluctuations in Response Caution. J. Neurosci. 2011;31:17488–17495. doi: 10.1523/JNEUROSCI.2924-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Huettel SA. Strategic control in decision-making under uncertainty. Eur. J. Neurosci. 2012;35:1075–1082. doi: 10.1111/j.1460-9568.2012.08009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Rosati AG, Taren AA, Huettel SA. Resolving Response, Decision, and Strategic Control: Evidence for a Functional Topography in Dorsomedial Prefrontal Cortex. J. Neurosci. 2009;29:13158–13164. doi: 10.1523/JNEUROSCI.2708-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. J. Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SWG. Structural and functional dichotomy of human midcingulate cortex. Eur. J. Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Vogt LJ. Cingulate gyrus. In: Paxinos G, Mai J, editors. The Human Nervous System. Elsevier; Amsterdam: 2004. pp. 915–949. [Google Scholar]
- Wallis JD, Kennerley SW. Heterogeneous reward signals in prefrontal cortex. Curr. Opin. Neurobiol. 2010;20:191–198. doi: 10.1016/j.conb.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Kennerley SW. Contrasting reward signals in the orbitofrontal cortex and anterior cingulate cortex. Ann. N. Y. Acad. Sci. 2011;1239:33–42. doi: 10.1111/j.1749-6632.2011.06277.x. [DOI] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MFS. Interactions between decision making and performance monitoring within prefrontal cortex. Nat. Neurosci. 2004;7:1259–1265. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Walton ME, Rudebeck PH, Bannerman D, Rushworth MFS. Calculating the cost of acting in frontal cortex. Ann. N. Y. Acad. Sci. 2007;1104:340–356. doi: 10.1196/annals.1390.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J. Neurosci. 2005;25:604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb. Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Danielmeier C, Morton JB, Ullsperger M. Surprise and Error: Common Neuronal Architecture for the Processing of Errors and Novelty. J. Neurosci. 2012;32:7528–7537. doi: 10.1523/JNEUROSCI.6352-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook A, Kester D, Braver TS. What is the subjective cost of cognitive effort? Load, trait, and aging effects revealed by economic preference. PloS ONE. doi: 10.1371/journal.pone.0068210. in press. doi: 10.1371/journal.pone.0068210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CSE. Another major function of the anterior cingulate cortex: The representation of requirements. Neurosci. Biobehav. Rev. 2012;36:90–110. doi: 10.1016/j.neubiorev.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. A computational model of executive control in frontal cortex and basal ganglia: multiple levels of analysis. Psychol. Rev. 2013;120:329–355. doi: 10.1037/a0031542. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Johnston K, Vinck M, Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc. Natl. Acad. Sci. U.S.A. 2010;107:5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolgar A, Hampshire A, Thompson R, Duncan J. Adaptive Coding of Task-Relevant Information in Human Frontoparietal Cortex. J. Neurosci. 2011;31:14592–14599. doi: 10.1523/JNEUROSCI.2616-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich K, Rangel A, O’Doherty JP. Neural computations underlying action-based decision making in the human brain. Proc. Natl. Acad. Sci. U.S.A. 2009;106:17199–17204. doi: 10.1073/pnas.0901077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B, Krienen F, Sepulcre J, Sabuncu M, Lashkari D, Hollinshead M, Roffman J, Smoller J, Zöllei L, Polimeni J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol. Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Yeung N, Cohen JD, Botvinick MM. Errors of interpretation and modeling: A reply to Grinband et al. Neuroimage. 2011;57:316–319. doi: 10.1016/j.neuroimage.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AJ, Dayan P, Cohen JD. Dynamics of attentional selection under conflict: Toward a rational Bayesian account. J. Exp. Psychol. Hum. Percept. Perform. 2009;35:700–717. doi: 10.1037/a0013553. [DOI] [PMC free article] [PubMed] [Google Scholar]