Abstract
Background
Infection is a common complication of ventricular-assist devices (VADs) and is associated with re-hospitalization, thromboembolic events, VAD malfunction, delay in heart transplantation, and a high mortality rate. The objectives of this study were to investigate the frequency of fungal VAD infections and assess various risk factors and their effects on mortality as compared to bacterial VAD infections.
Methods
We conducted a retrospective chart review of patients with infected VADs at a single tertiary care center. The frequency, risk factors and outcomes of fungal vs. bacterial VAD infections were compared.
Results
Of the 300 patients who received a VAD, 108 (36%) developed VAD infection, including 85 bacterial and 23 fungal infections. Most common bacterial causes of infection were Staphylococcus aureus, coagulase-negative staphylococci, enterococci and Pseudomonas aeuruginosa. Most common fungal etiologic agent was Candida albicans. Only the use of TPN was associated with the development of a fungal VAD infection in multivariate analysis (OR 6.95, 95% CI 1.71–28.16, p=0.007). Patients who suffered from fungal VAD infection were less likely to be cured (17.4% vs. 56.3%, p=0.001) and had greater mortality (91% vs. 61%, p=0.006), as compared with those who experienced bacterial VAD infections.
Conclusions
Fungi were responsible for approximately one-fifth of VAD infections and were associated with a mortality rate of 91%. Restriction of TPN use is essential in decreasing the rate of fungal VAD infection. Trials are needed for investigating the use of echinocandins or lipid formulations of amphotericin B for prevention and/or treatment of fungal VAD infections.
Keywords: ventricular-assist device, cardiac device, prosthesis infection
BACKGROUND
Ventricular-assist devices (VADs) are essential for the modern management of end-stage heart failure and are associated with longer survival and better quality of life. Although VADs were initially designed for patients awaiting heart transplantation, the higher survival rates observed among VAD recipients motivated their use as destination therapy as well.[1, 2] Unfortunately, the use of VADs to provide mechanical circulatory support can be associated with multiple complications, including thromboembolic events, cardiac arrhythmias, device malfunction, and infection.
Infection is a common complication observed in VAD recipients and is associated with increased likelihood of re-hospitalization, thromboembolic events, VAD malfunction requiring multiple revisions, delay in heart transplantation, and removal or exchange of the infected device.[3, 4] The need to remove the infected VAD is attributed to the presence of infecting organisms within the biofilm, which comprises a complex population of slowly multiplying organisms and their products as well as host elements that include platelets and host tissue ligands. Not only does the biofilm provide a barrier to host defense mechanisms (leukocytes, antibodies, and complement), but it also can impair the penetration and/or the activity of antimicrobial agents. In that regard, the MIC of some antibiotics are up to 500 times higher for bacteria embedded within the biofilm matrix than their planktonic (free-floating) counterpart.[5–7]
The main pathogenesis of VAD infection has invariably focused on the driveline (percutaneous tunnel for VAD power cables) as the port of entry for organisms which then migrate via this conduit into subcutaneous tissues and the pump site leading to pocket infection. This can result in infection of the pump-housing, pump inflow, outflow tracts and/or the bloodstream, and eventually cause a disseminated infection. Alternatively, the pump inflow and outflow tracts may be hematogenously infected, as is the case with endocarditis.[8] The most common organisms associated with VAD infection are gram-positive bacteria, particularly the staphylococci which are responsible for 57% of all VAD-related infections and 62% of all VAD-related bloodstream infection (BSI). Other causes of VAD infections include Pseudomonas aeruginosa, Escherichia coli, and enterococci. Candida infections are less frequent but are responsible for the highest mortality rates.[8, 9] Infection of the VAD is the most common cause of death among patients who require this device for long-term mechanical circulatory support.[10]
There is substantial literature describing VAD infections but most of these describe bacterial VAD infections. There is very little data describing fungal VAD infections and none comparing the risk factors and outcomes to bacterial VAD infections. The main goals of this study were to investigate the frequency of fungal VAD infections in the era of broad-spectrum prophylactic antimicrobial use, assess risk factors for infection, and compare the outcomes of fungal vs. bacterial VAD infections.
METHODS
Study design
We performed a retrospective chart review of all patients that had received a VAD at Saint Luke’s Episcopal Hospital in Houston, Texas from January 1992 through December 2007. The study was approved by the Institutional Review Boards (IRB) of Baylor College of Medicine and Saint Luke’s Episcopal Hospital which determined that patients’ informed consent was not required.
Patient information
Information was collected only from patients who developed a VAD infection and included demographics (age, gender, and race), presence of co-morbidities, underlying cardiomyopathy, use of preoperative antimicrobial prophylaxis, type of implanted VAD, operative data, type of VAD infection, non-VAD infections, hospital course, pertinent laboratory data, and results of microbiological tests. Data was collected from the time of admission for VAD implantation to last outpatient/inpatient follow-up or death.
VAD recipients received perioperative antibiotics that generally consisted of vancomycin and cefepime. After 2000, fluconazole was added to the antibiotic regimen. The duration of postoperative antimicrobial prophylaxis was variable and the decision to discontinue antimicrobial prophylaxis was made by the surgical and infectious disease teams. After discharge, patients were followed weekly in the clinic. Care of the driveline exit site consisted of daily cleansing with chlorhexidine/iodine solution and placement of an occlusive dressing. All patients who developed symptoms and/or signs of infection were evaluated to identify the source of infection. Pertinent cultures were obtained from the driveline exit site and, if indicated, blood cultures were obtained. Samples were also sent for culture if surgical drainage was performed. Imaging of the pocket site with ultrasound or computed tomography was also performed as needed.
Definitions of outcomes
VAD infections were classified into 4 categories: (1) driveline infection was defined as the presence of purulent drainage from the driveline exit site and isolation of at least one pathogen from this site, (2) pocket infection was defined by a fluid collection around the ventricular pump which was detected on imaging studies and at least one pathogen isolated from that fluid collection, (3) VAD-related bloodstream infection (BSI) was defined as either the isolation of the same microorganism from more than 1 set of blood cultures and from the driveline exit site and/or the VAD pocket, or the presence of more than one positive blood culture with no evidence for another source of infection besides the ventricular device, and, (4) VAD-related infective endocarditis (IE) was defined clinically as the isolation of one or more organisms from more than one set of blood cultures associated with the development of one or more embolic events. Although most patients had histopathologic evidence of infection of the inflow/outflow conduits of the explanted ventricular device, these findings were not a requisite for the diagnosis of VAD-related infective endocarditis.
If a patient had a fungal isolate that was related to the VAD, that patient was counted among the fungal infection category. Patients in the bacterial infection category had a purely bacterial infection. Among patients with bacterial infections, only the first episode was considered for analysis. Clinical cure was defined as resolution of the clinical features of a VAD infection. Microbiological cure meant that repeat cultures following clinical resolution of the VAD infection were negative. Recurrence was defined as growth of an identical organism with the same antimicrobial susceptibility pattern that caused the initial VAD infection at any time following clinical resolution of that first infection.
Statistical analysis
Categorical variables were evaluated by using univariate analysis (chi square or Fisher’s exact test, as appropriate) and multivariate logistic regression analysis. Continuous variables were compared by using a Student’s t-test. A 2-tailed p-value <0.05 was considered significant. For survival analysis, the groups were compared by performing a log-rank test on the Kaplan-Meier estimates. Data were analyzed using SAS version 9.2 (SAS Institute, Cary, North Carolina) and Stata version 8.2 (Statacorp, College Station, Texas).
RESULTS
Patients
We identified 476 patients who underwent VAD placement during the study period, but the charts for only 300 were available for review (the remaining 176 charts were either incomplete, missing or had been destroyed due to flooding in 2001). Of the 300 available charts, 108 (36%) had VAD infections. The 108 infected patients had received one of 5 different types of VADs, including HeartMate IP (n=34), HeartMate XVE (n=30), HeartMate II (n=23), Thoratec (n=9) [these 4 types of VADs were manufactured by Thoratec Corporation, Pleasanton, California], and Jarvik (n=12) [Jarvik Heart, Inc., New York, New York].
Infections
A total of 108 patients had VAD infections. Fifty eight patients had a single VAD infection, 31 patients had 2 episodes, 13 patients had 3 episodes and 6 patients had 4 episodes of VAD infection. Of all patients with > 1 episode of infection, 28 (26%) had a recurrent infection with the same organism. The median time to diagnosis of a VAD infection from implantation was 36 days (25–75% interquartile range, IQR, 28–94.5 days). Of the 108 patients with a VAD infection, 85 had a bacterial VAD infection and 23 had a fungal infection (7/23 had a bacterial VAD infection that preceded the fungal VAD infection). There was no difference in time from VAD implantation to infection between the fungal and bacterial groups. Median duration of follow-up from the time of VAD implantation for patients with a bacterial VAD infection was 315 days (IQR 150–1061 days) and for a fungal VAD infection was 130 days (IQR 54–257 days; p=0.007).
The microbiologic findings are described in Table 1. Gram-positive bacteria accounted for 58.3%, gram-negative bacteria 26.8%, and fungal 14.8% of the total 209 microbiologic isolates. Common pathogens included Staphylococcus aureus (21.1%), coagulase-negative staphylococci (19.1%), enterococci (14.4%), and P. aeruginosa (10%). Twenty three of the 108 (21%) patients had fungal infections, with Candida albicans being the most common fungal pathogen. 21.3% of infections were polymicrobial. Of all VAD infections, 46 (44.4%) involved the driveline, 34 (31.5%) affected the pocket, 57 (52.8%) involved the bloodstream, and 17 (15.7%) were associated with infective endocarditis. The relative distribution of these infections by bacterial and fungal groups is shown in Table 2. 65.2% of all fungal infections involved the bloodstream whereas bacterial infections were more likely to involve the driveline than fungal infections (49.4% vs. 26%, p=0.046).
Table 1.
Microbiology of 108 patients with VAD infections.
| Microorganism | N (% of total 209 isolates) | Driveline (n) | Pocket (n) | * BSI (n) | ^ IE (n) |
|---|---|---|---|---|---|
| Bacterial isolates | 178 (85.2%) | 62 | 49 | 53 | 14 |
|
| |||||
| Staphylococcus aureus | 44 (21.1%) | 16 | 11 | 13 | 4 |
| α MRSA | 23 (11%) | 7 | 7 | 8 | 1 |
|
| |||||
| Coagulase-negative staphylococci | 40 (19.1%) | 14 | 8 | 15 | 3 |
|
| |||||
| Enterococcus species | 30 (14.4%) | 5 | 12 | 12 | 1 |
| β VRE | 19 (9.1%) | 1 | 10 | 8 | |
|
| |||||
| Streptococcus viridans | 3 (1.4%) | 1 | 2 | ||
|
| |||||
| Corynebacterium species | 2 (1%) | 1 | 1 | ||
|
| |||||
| Diptheroids | 1 (0.5%) | 1 | |||
|
| |||||
| Bacillus species | 1 (0.5%) | 1 | |||
|
| |||||
| Lactobacillus species | 1 (0.5%) | 1 | |||
|
| |||||
| Pseudomonas aeruginosa | 21 (10%) | 11 | 3 | 5 | 2 |
|
| |||||
| Klebsiella pneumoniae | 9 (4.3%) | 2 | 3 | 3 | 1 |
|
| |||||
| Enterobacter cloacae | 7 (3.3%) | 3 | 2 | 2 | |
|
| |||||
| Citrobacter freundi | 4 (1.9%) | 1 | 2 | 1 | |
|
| |||||
| Serratia marsescens | 4 (1.9%) | 1 | 1 | 2 | |
|
| |||||
| Proteus mirabilis | 2 (1%) | 1 | 1 | ||
|
| |||||
| Escherichia coli | 1 (0.5%) | 1 | |||
|
| |||||
| Bacteroides species | 6 (2.9%) | 3 | 2 | 1 | |
|
| |||||
| Eikinella species | 1 (0.5%) | 1 | |||
|
| |||||
| Prevotella species | 1 (0.5%) | 1 | |||
|
| |||||
| Fungal isolates | 31 (14.8%) | 5 | 6 | 15 | 5 |
|
| |||||
| Candida albicans | 14 (6.7%) | 3 | 3 | 5 | 3 |
|
| |||||
| Candida glabrata | 7 (3.3%) | 2 | 4 | 1 | |
|
| |||||
| Candida kruseii | 6 (2.9%) | 2 | 1 | 2 | 1 |
|
| |||||
| Candida parapsilosis | 3 (1.4%) | 3 | |||
|
| |||||
| § Candida antigen + | 1 (0.5%) | 1 | |||
|
| |||||
| Total no. of isolates | 209 | 67 | 55 | 68 | 19 |
BSI is bloodstream infection
IE is infective endocarditis
MRSA is methicillin-resistant S. aureus
VRE is vancomycin-resistant Enterococcus species.
This refers to a positive serum latex agglutination test of circulating antigen in patients with systemic infection due to Candida albicans, C. tropicalis and C. parapsilosis.
A single patient may have a positive culture from more than one site.
23 patients had polymicrobial infections.
6 patients were culture negative.
Table 2.
Demographics and clinical characteristics of patients with VAD infections. Laboratory data refers to data recorded on the day of diagnosis of VAD infection. Temperature refers to the maximum temperature within 24 hours of the diagnosis of VAD infection. Types of infections included all sites and thus the percentages are > 100%.
| Variable | Bacterial infection (n=85) | Fungal infection (n=23) | P value |
|---|---|---|---|
| Pre-operative characteristics | |||
|
| |||
| Mean Age (years) | 49.3 | 50.3 | 0.78 |
|
| |||
| Male | 76 (89.4%) | 23 (100%) | 0.2 |
|
| |||
| Race | 0.4 | ||
| Caucasian | 45 (52.9%) | 15 (65.2%) | |
| African-American | 28 (32.9%) | 4 (17.4%) | |
| Hispanic | 11 (12.9%) | 3 (13.0%) | |
| Asian | 1 (1.2%) | 1 (4.4%) | |
|
| |||
| Diabetes mellitus | 21 (25%) | 7 (30.4%) | 0.6 |
|
| |||
| * Obesity (BMI>30) | 26 (30.6%) | 8 (34.8%) | 0.7 |
|
| |||
| Ischemic cardiomyopathy | 37 (45.5%) | 13 (56.5) | 0.27 |
|
| |||
| ≈ AICD present | 25 (29.4%) | 8 (34.8%) | 0.62 |
|
| |||
| Previous VAD | 4 (4.7%) | 2 (8.7%) | 0.61 |
|
| |||
| Intraaortic balloon pump present | 48 (59.3%) | 19 (82.6%) | 0.02 |
|
| |||
| Infection present | 37 (43.5%) | 9 (39.1%) | 0.67 |
| Pneumonia | 3 (3.6%) | 0 | 1 |
|
| |||
| Bloodstream infection | 18 (21.4%) | 6 (26.1%) | 0.46 |
| Abdominal infection | 2 (2.4%) | 0 | 1 |
| Other | 13 (15.5%) | 3 (13.0%) | 1 |
|
| |||
| On hemodialysis | 7 (8.8%) | 5 (21.7%) | 0.09 |
|
| |||
| On ventilator | 10 (12.4%) | 4 (17.4%) | 0.5 |
|
| |||
| Median length of stay prior to VAD placement (IQR)§ | 11 (4–19) | 9 (6–15) | 0.34 |
|
| |||
| Total parenteral nutrition | 8 (11.0%) | 11 (47.8) | 0.0001 |
|
| |||
| Surgical characteristics | |||
|
| |||
| Duration of surgery in hours (mean ± SD)^ | 7.0 (± 1.6) | 8.1 (± 1.8) | 0.01 |
|
| |||
| Number of packed red blood cell transfusions (mean ± SD) | 10.9 (± 9.0) | 16.7 (± 10.9) | 0.01 |
|
| |||
| Post-operative characteristics | |||
|
| |||
| Mechanical ventilation ≥ 1 week | 15 (18.5%) | 7 (30.4%) | 0.22 |
|
| |||
| Hemodialysis | 11 (13.6%) | 10 (45.5%) | 0.001 |
|
| |||
| Vasopressor use | 42 (51.9% | 14 (60.9%) | 0.44 |
|
| |||
| Mean no. of invasive devices (± SD) | 1.45 (± 1.6) | 3 (± 1.7) | < 0.001 |
|
| |||
| Delayed chest wall closure | 17 (20.2%) | 9 (39.1%) | 0.06 |
|
| |||
| Abdominal surgery | 18 (21.7%) | 10 (43.5%) | 0.036 |
|
| |||
| Mediastinal exploration | 51 (61.5%) | 12 (52.2%) | 0.42 |
|
| |||
| Tracheostomy | 15 (17.7%) | 7 (30.4%) | 0.21 |
|
| |||
| VAD malfunction | 15 (17.7%) | 6 (26.1%) | 0.38 |
|
| |||
| Post-operative non-VAD infection | 44 (53.7%) | 15 (65.2%) | 0.25 |
| Bloodstream infection | 17 (20%) | 1 (4.3%) | 0.02 |
| Abdominal infection | 14 (16.5%) | 6 (26.1%) | 0.64 |
| Pulmonary infection | 12 (14.1%) | 7 (30.4%) | 0.29 |
|
| |||
| Antifungal prophylaxis | 51 (65.4%) | 14 (70%) | 0.7 |
|
| |||
| Infection | |||
|
| |||
| WBC count (mean ± SD) | 9.9 (± 5.0) | 9.53 (± 4.2) | 0.74 |
|
| |||
| Creatinine (mean ± SD) | 1.5 (± 0.9) | 1.9 (± 1.2) | 0.07 |
|
| |||
| Glucose (mean ± SD) | 128.2 (± 53.33) | 143.3 (± 48.7) | 0.23 |
|
| |||
| Bilirubin (mean ± SD) | 4.0 (± 6.1) | 2.5 (± 3.2) | 0.28 |
|
| |||
| Albumin (mean ± SD) | 3.21 (± 0.6) | 3.15 (± 0.6) | 0.62 |
|
| |||
| Hemoglobin (mean ± SD) | 11.6 (± 1.8) | 11.04 (± 2.3) | 0.22 |
|
| |||
| Temperature in °F (mean ± SD) | 98.9 (± 1.6) | 98.7 (± 1.7) | 0.59 |
|
| |||
| Embolic events | 14 (16.5%) | 4 (17.4%) | 1 |
|
| |||
| Valvular symptoms | 6 (7.1%) | 4 (17.4%) | 0.22 |
|
| |||
| Type of infection | |||
| Driveline | 42 (49.4%) | 6 (26%) | 0.046 |
| 29 (34.1%) | 5 (21.7%) | 0.26 | |
| VAD bloodstream infection | 42 (49.4%) | 15 (65.2%) | 0.18 |
| VAD infective endocarditis | 12 (14.1%) | 5 (21.7%) | 0.35 |
|
| |||
| Polymicrobial infection | 15 (17.6%) | 8 (34.8%) | 0.09 |
All comparisons, except for the following, remained similar in statistical significance when analysis was repeated with a modified fungal group that excluded 7 patients who had a preceding bacterial VAD infection prior to development of a fungal VAD infection: delayed chest wall closure, abdominal surgery, and driveline infections.
BMI is body mass index
AICD is automatic implantable cardioverter defibrillator
IQR is interquartile range (25–75%ile)
SD is standard deviation
Risk factors for fungal infection
As shown in Table 2, factors significantly associated with the development of fungal VAD infection in univariate analysis included pre-operative use of an intraaortic balloon pump (IABP), receipt of total parenteral nutrition (TPN), longer operative time for the initial VAD surgery, greater number of blood transfusions, post-operative need for hemodialysis, greater number of invasive devices, and abdominal surgery. Multivariate analysis showed that only use of TPN was significantly associated with the development of a fungal VAD infection (OR 6.95, 95% CI 1.71–28.16, p=0.007). Number of invasive devices tended towards significance (OR 1.39, 95% CI 0.98–1.96, p=0.06).
Fungal infections
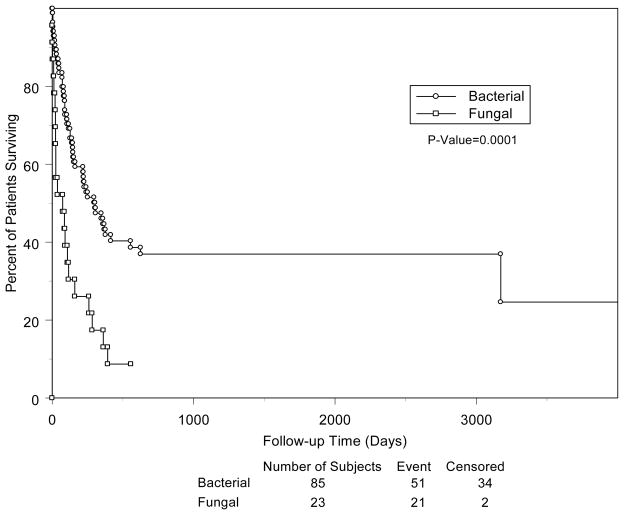
Antifungal prophylaxis (generally with fluconazole) was administered to 70% of patients who subsequently developed fungal VAD infections and 65.5% of bacterial infections. The use of antifungal prophylaxis was unrelated to the development of a fungal VAD infection (p=0.7). The rate of fungal VAD infections prior to the routine use of antifungal prophylaxis was 6.6% vs. 8% after the use of prophylaxis. We were unable to obtain details regarding antifungal susceptibilities of the fungal isolates. Patients with a fungal VAD infection were less likely to achieve a clinical and microbiological cure and had a greater number of infections when compared to those with bacterial infections (Table 3). More patients with fungal vs. bacterial VAD infection died (91% vs. 61%, p=0.006). Figure 1 illustrates the survival analysis of patients with fungal and bacterial VAD infections.
Table 3.
Comparison of outcomes for patients with bacterial vs. fungal VAD infections.
| Outcomes | Bacterial infection N=85 | Fungal infection N=23 | p-value |
|---|---|---|---|
| Exchange/explantation of VAD | 22 (25.9%) | 8 (34.8%) | 0.4 |
| Incision and drainage of VAD | 30 (35.3%) | 11 (47.8%) | 0.27 |
| Clinical Cure | 45 (56.3%) | 4 (17.4%) | 0.001 |
| Microbiological cure | 47 (61.8%) | 5 (22.7%) | 0.001 |
| Number of VAD infections (mean ± SD) | 1.56 (± 0.75) | 2.17 (± 1.19) | 0.003 |
| Heart transplant | 31 (36.5%) | 4 (17.4%) | 0.08 |
| Death related to VAD infection | 27 (31.8%) | 15 (65.2%) | 0.004 |
| All-cause mortality | 52 (61.9%) | 21 (91.3%) | 0.007 |
All comparisons, except for number of VAD infections, remained similar in statistical significance when analysis was repeated with a modified fungal group that excluded 7 patients who had a preceding bacterial VAD infection prior to development of a fungal VAD infection.
Figure 1.
Kaplan-Meier survival analysis of bacterial versus fungal VAD infections.
Risk factors for death
Factors associated with all-cause mortality in univariate analysis were presence of a fungal VAD infection, VAD-related infective endocarditis, use of TPN, non-VAD related BSI, non-VAD related infection, and presence of diabetes mellitus (DM). Multivariate analysis showed only the following independent risk factors to be significantly associated with mortality: Fungal VAD infection (OR 6.33, 95% CI 1.33–30.06, p=0.02), presence of a non-VAD related infection (OR 3.45, 95% CI 1.38–8.65, p=0.008) and presence of DM (OR 3.95, 95% CI 1.16–13.42, p=0.028).
DISCUSSION
VAD infection is a relatively frequent and life-threatening complication.[9, 11, 12]. The overall prevalence of VAD infections in this study was 36%, which falls within the wide range of 17.7–72% that is reported in the literature.[9, 11–13] The median time to diagnosis of VAD infection in our study was 36 days, as previously described in the literature.[9]
About half of VAD infections in this study were associated with a BSI, which occurred at a rate similar to a previous report [9] but higher than in another study.[12] In general, BSI leads to a higher rate of complications than seen with driveline or pocket infections alone. As almost half of the infections were associated with the driveline, it is likely that the driveline was the major portal of entry for the infecting pathogens. Since VAD infections tend to clinically manifest early in the post-operative period, some infections may be due to perioperative inoculation of organisms, and, thus, meticulous application of sterile surgical techniques would be essential for limiting the rates of infection. In addition, immobilization of the driveline to minimize trauma, and daily cleansing and dressing exchange of the driveline exit site in the early post-operative stage are considered standard of care practices that are intended to decrease contamination and colonization of the device and, therefore, device-related infection. Antimicrobial coating or dipping of these devices, especially the driveline, in antimicrobial/antibiofilm solution has the potential of decreasing the incidence of early infection as well.[14]
Fungal VAD infections occurred in 7.6% of all 300 VAD recipients and accounted for 21% of all VAD infections (inclusive of 6.5% of patients that had a bacterial infection prior to development of a fungal VAD infection). One previous study done in the era before routine antifungal prophylaxis noted that 35% of all VAD recipients developed a fungal VAD infection.[15]
Patients with VADs may have iatrogenic immunologic dysfunction that places them at an even higher risk of developing infection. The device induces an aberrant state of T-cell activation that leads to programmed cell death among CD4-bearing T cells.[16] This results in defects in cellular immunity that may predispose to certain types of infection, including fungal infections. In a case-control study, the risk of developing disseminated candidiasis was found to be markedly increased (28% vs. 3%; p=0.003) in VAD recipients vs. control patients who received medical management of heart failure without VAD placement.[17]
Use of TPN is associated with candidemia in numerous study populations, including neonates, critically ill patients and immunocompromised hosts.[18] In this study, we documented that use of TPN was the only risk factor in multivariate analysis that was associated with development of a fungal VAD infection. In fact, almost half of patients with fungal VAD infections had received TPN. Given the poor outcome of this infection, it is imperative that we optimize the nutritional status of VAD recipients via enteral feeding and avoid TPN.
Increased number of invasive devices tended towards significance in its association with fungal VAD infections. Other studies have shown that severity of illness, presence of central venous catheters, and the need for mechanical ventilation are all associated with invasive candidiasis.[19, 20] Extubation and removal of indwelling catheters and drainage tubes as soon as possible after device implantation may decrease the rate of fungal infection. Alternatively, duration of antifungal prophylaxis may need to be extended until all lines have been removed. Given the poor outcomes of fungal VAD infections, identification of risk factors is crucial for identification of factors that are amenable to manipulation and improvement before and after VAD implantation.
Approximately 70% of patients in our study had received antifungal prophylaxis with fluconazole; however, it did not appear to reduce the rate of fungal VAD infections. One study noted that continuation of antifungal prophylaxis until extubation and discontinuation of antibiotics was not cost-effective in VAD patients.[21] It may be reasonable to investigate the use of extended antifungal prophylaxis in VAD recipients until removal of all invasive devices or prophylaxis with agents other than fluconazole, such as echinocandins or amphotericin B.
An important finding of this study is the abysmal survival rate of patients who develop a fungal VAD infection. All-cause mortality was 91% in this population and VAD infection-related mortality was 65%. An amalgation of various factors may play a role. This high mortality may reflect, at least in part, the general debilitation and poor immune status of patients that develop fungal VAD infections.[17] In other words, sicker patients tend to develop fungal infections and are also more likely to die from the infection. The high mortality may also be related to the difficulty in eradicating Candida species when present within the biofilm on the surface of devices. Studies have demonstrated drug resistance, especially to the azoles, by biofilm-embedded Candida organisms grown on a variety of synthetic materials.[22] Use of amphotericin B lipid complex and caspofungin in animal models of vascular catheter infections have demonstrated significant decrease in biofilm fungal burden, and thus, these may be better options for the treatment of fungal VAD infections rather than azoles.[23, 24]
We found that diabetes was associated with an increased risk of death regardless of the type of VAD infection. One study found that the presence of diabetes in VAD recipients was associated with an increased risk of developing VAD endocarditis, which in turn, was associated with an increased risk of death versus other types of VAD infections.[12] Another series noted that diabetic patients were more likely to develop VAD-related BSI.[9] It is unclear at this point whether optimal blood sugar control in the peri-operative period would lead to a decreased risk of infection or mortality and certainly opens up avenues for further research.
There are several limitations to our study. A large number of charts were unavailable for review as they were incomplete, missing or destroyed via flooding. Since this was a retrospective chart review, there may be data-collection bias. We were also unable to abstract data regarding the specific number of antibiotic-days as well as the exact duration of stay in the intensive care unit. Additionally, the fungal infection group contained 7 patients that had a bacterial infection prior to developing a fungal VAD infection. However, we conducted risk factor and outcome analysis with the exclusion of these patients with similar results (not shown here) and thus do not believe that inclusion of these patients have had significant impact on the results. Since this is a single center study, the results may not be generalizable to other centers.
In summary, fungi were responsible for approximately one-fifth of VAD infections and were associated with high mortality. Since infection is the most common cause of death in patients with VADs, it is important to decrease the incidence of this complication and its consequences. Restriction of TPN use is essential in decreasing the rate of fungal VAD infection. Trials are needed for investigating the use of echinocandins or lipid formulations of amphotericin B for prevention and/or treatment of fungal VAD infections. By identifying and controlling risk factors, it is theoretically possible to reduce the occurrence of fungal VAD infections and/or increase the index of suspicion and treat such infections at an early stage. Such an approach may ameliorate outcomes, lead to clinical benefits and incur sizable cost-savings.
Summary.
Fungal VAD infections are associated with receipt of total parenteral nutrition and a mortality rate of 91%.
Acknowledgments
This work was supported by funds from Baylor College of Medicine, Houston, Texas. R.O.D. has ongoing funding support through VA Cooperative Study # 535 and NIH/NICHHD T32 HD049350; and NIH/NIDDK 1K23DK078828-01 A2 (to S.A.).
Footnotes
All authors deny any financial conflicts of interest relevant to the research described in this paper.
References
- 1.DeRose JJ, Jr, Umana JP, Argenziano M, et al. Implantable left ventricular assist devices provide an excellent outpatient bridge to transplantation and recovery. J Am Coll Cardiol. 1997;30:1773–7. doi: 10.1016/s0735-1097(97)00396-3. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 3.Frazier OH, Rose EA, Oz MC, et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186–95. doi: 10.1067/mtc.2001.118274. [DOI] [PubMed] [Google Scholar]
- 4.Chinn R, Dembitsky W, Eaton L, et al. Multicenter experience: prevention and management of left ventricular assist device infections. Asaio J. 2005;51:461–70. doi: 10.1097/01.mat.0000170620.65279.aa. [DOI] [PubMed] [Google Scholar]
- 5.Trautner BW, Darouiche RO. Catheter-associated infections: pathogenesis affects prevention. Arch Intern Med. 2004;164:842–50. doi: 10.1001/archinte.164.8.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aslam S. Effect of antibacterials on biofilms. Am J Infect Control. 2008;36:S175, e9–11. doi: 10.1016/j.ajic.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Donlan R, Costerton JW. Biofilms: Survival Mechanisms of Clinically Relevant Microoganisms. Clin Micro Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis. 2006;6:426–37. doi: 10.1016/S1473-3099(06)70522-9. [DOI] [PubMed] [Google Scholar]
- 9.Simon D, Fischer S, Grossman A, et al. Left ventricular assist device-related infection: treatment and outcome. Clin Infect Dis. 2005;40:1108–15. doi: 10.1086/428728. [DOI] [PubMed] [Google Scholar]
- 10.Holman WL, Park SJ, Long JW, et al. Infection in permanent circulatory support: experience from the REMATCH trial. J Heart Lung Transplant. 2004;23:1359–65. doi: 10.1016/j.healun.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Poston RS, Husain S, Sorce D, et al. LVAD bloodstream infections: therapeutic rationale for transplantation after LVAD infection. J Heart Lung Transplant. 2003;22:914–21. doi: 10.1016/s1053-2498(02)00645-9. [DOI] [PubMed] [Google Scholar]
- 12.Monkowski DH, Axelrod P, Fekete T, Hollander T, Furukawa S, Samuel R. Infections associated with ventricular assist devices: epidemiology and effect on prognosis after transplantation. Transpl Infect Dis. 2007;9:114–20. doi: 10.1111/j.1399-3062.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- 13.Morgan JA, John R, Rao V, et al. Bridging to transplant with the HeartMate left ventricular assist device: The Columbia Presbyterian 12-year experience. J Thorac Cardiovasc Surg. 2004;127:1309–16. doi: 10.1016/j.jtcvs.2003.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez MD, Mansouri MD, Aslam S, Zeluff B, Darouiche RO. Efficacy of Combination of N-acetylcysteine, Gentamicin, and Amphotericin B for Prevention of Microbial Colonization of Ventricular Assist Devices. Infect Control Hosp Epidemiol. 2008 doi: 10.1086/593205. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DJ, el-Amir NG, Ashton RC, Jr, et al. Fungal infections in left ventricular assist device recipients. Incidence, prophylaxis, and treatment. Asaio J. 1995;41:873–5. [PubMed] [Google Scholar]
- 16.Ankersmit HJ, Edwards NM, Schuster M, et al. Quantitative changes in T-cell populations after left ventricular assist device implantation: relationship to T-cell apoptosis and soluble CD95. Circulation. 1999;100:II211–5. doi: 10.1161/01.cir.100.suppl_2.ii-211. [DOI] [PubMed] [Google Scholar]
- 17.Ankersmit HJ, Tugulea S, Spanier T, et al. Activation-induced T-cell death and immune dysfunction after implantation of left-ventricular assist device. Lancet. 1999;354:550–5. doi: 10.1016/s0140-6736(98)10359-8. [DOI] [PubMed] [Google Scholar]
- 18.Zilberberg MD, Shorr AF. Fungal infections in the ICU. Infect Dis Clin North Am. 2009;23:625–42. doi: 10.1016/j.idc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994;220:751–8. doi: 10.1097/00000658-199412000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser VJ, Jones M, Dunkel J, Storfer S, Medoff G, Dunagan WC. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin Infect Dis. 1992;15:414–21. doi: 10.1093/clind/15.3.414. [DOI] [PubMed] [Google Scholar]
- 21.Skinner JL, Harris C, Aaron MF, et al. Cost-benefit analysis of extended antifungal prophylaxis in ventricular assist devices. Asaio J. 2000;46:587–9. doi: 10.1097/00002480-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–6. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee PK, Long L, Kim HG, Ghannoum MA. Amphotericin B lipid complex is efficacious in the treatment of Candida albicans biofilms using a model of catheter-associated Candida biofilms. Int J Antimicrob Agents. 2009;33:149–53. doi: 10.1016/j.ijantimicag.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 24.Lazzell AL, Chaturvedi AK, Pierce CG, Prasad D, Uppuluri P, Lopez-Ribot JL. Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. J Antimicrob Chemother. 2009;64:567–70. doi: 10.1093/jac/dkp242. [DOI] [PubMed] [Google Scholar]