Abstract
Differentiation and clonal expansion of Ag-activated naive T cells play a pivotal role in the adaptive immune response. T cell Ig mucin (Tim) proteins influence the activation and differentiation of T cells. Tim-3 and Tim-2 clearly regulate Th1 and Th2 responses, respectively, but the precise influence of Tim-1 on T cell activation remains to be determined. We now show that Tim-1 stimulation in vivo and in vitro induces polyclonal activation of T cells despite absence of a conventional TCR-dependent signal 1. In this model, Tim-1-induced proliferation is dependent on strong signal 2 costimulation provided by mature dendritic cells. Ligation of Tim-1 upon CD4+ T cells with an agonist anti-Tim-1 mAb elicits a rise in free cytosolic calcium, calcineurin-dependent nuclear translocation of NF-AT, and transcription of IL-2. Because Tim-4, the Tim-1 ligand, is expressed by mature dendritic cells, we propose that interaction between Tim-1+ T cells and Tim-4+ dendritic cells might ensure optimal stimulation of T cells, when TCR-derived signals originating within an inflamed environment are weak or waning.
T cell Ig mucin (Tim)4-1 protein belongs to a family of regulatory cell surface glycoproteins that modulate immune responses. Ligation of Tim-1 molecule transmits a potent stimulatory signal that results in enhancement of T cell proliferation and cytokine production. Consequently, Tim-1 has been depicted as positive costimulatory molecule acting in concert with TCR ligation (1). Recent data, however, challenge the costimulatory role for Tim-1 and suggest that Tim-1 can directly activate T cells without concomitant TCR engagement (2). For example, overexpression of Tim-1 via gene transfer induces T cell activation (2, 3). De Souza et al. (3) observed robust transcription of IFN-γ following overexpression of Tim-1 in Jurkat cells despite the absence of TCR stimulation. More recently, Binne et al. (2) showed that ligation of Tim-1 on resting Tim-1+ transfectant Jurkat cells by an agonist anti-Tim-1 mAb stimulates phosphorylation of ZAP70 and IL-2-inducible T cell kinase, key proteins in the early TCR signaling pathway. Involvement of proximal TCR signaling complex elements was illustrated by the observation that interaction of T cells with Tim-4, a ligand for Tim-1, induces T cell expansion and phosphorylation of Tim-1, linker of activated T cells, Akt, and ERK 1/2 (4). Likewise, Tim-1 transfection into resting human CD4+ T cells resulted in the activation of downstream TCR signaling components and bolstered the production of both Th1- and Th2-type cytokines (2). Finally, stimulation with an agonist anti-Tim-1 mAb induces up-regulation of activation markers (CD69 and CD25) by naive murine CD4+ T cells in the absence of TCR stimulus (5). Moreover: 1) Tim-1 engagement intensifies CD3 capping (6), and 2) Tim-1 and CD3 colocalize after T cell activation (2). Given these recent observations, we hypothesize that ligation of Tim-1 molecules upon T cells may trigger activation of the signal 1 pathway, and thereby serve to heighten and sustain T cell responses.
Materials and Methods
Mice
C57BL/6 (H-2b), B6.129S4-Cd80tm1Shr Cd86tm1Shr/J, B6.129P2-Cd40tm1Kik/J, B.6SJL-PtprcaPep3b/BoyJ (H-2b/CD45.1+), B6.129S2-H2dlAb1-Ea/J, and DBA/2 (H-2d) mice were purchased from The Jackson Laboratory. OX40L knockout (KO) mice were provided by M. Sayegh (Harvard Medical School, Boston, MA). All mice were housed and cared in the animal facility at the Harvard Institutes of Medicine, according to the guidelines established by the Animal Care Committee at Harvard Medical School.
Monoclonal Abs
The following mAbs were purchased from eBiosciences: PE-B220 (clone RA3-6B2), PE-CD3e (clone 145-2C11), PE-CD8a (clone 53-6.7), PECD11b (clone M1/70), PE-CD25 (clone PC61.5), PE-Ter-119 (clone Ter-119), CyChrome-CD4 (GK1.5), and isotype control Abs. The agonist anti-Tim-1 mAb (clone 3B3) was produced by R. DeKruyff and S. Umetsu and characterized in previous studies (6, 7). The isotype control mAb used for the in vivo experiment was produced by Bio × Cell. Endotoxin levels in anti-Tim-1 mAb and isotype control mAb were below 2 EU/mg (Limulus amebocyte lysate test).
Cell preparation
After RBC lysis (Invitrogen), spleen and lymph node (inguinal, axillary, and cervical lymph nodes) single-cell suspensions were enriched for T cells using mouse CD3 T cell enrichment columns (R&D Systems), followed by incubation for 15 min at 4°C with the following mixture of PE Abs: anti-Ter119, anti-CD11b, anti-B220, anti-CD25, anti-NK1.1, and anti-CD8. Cells were then washed, and anti-PE magnetic beads (Miltenyi Biotec) were added for 15 min at 4°C. The cells were again washed, and CD4+CD25− T cells were obtained by negative selection. The purity of the CD4+CD25− T cells as assessed by FACS analysis was greater than 95%.
Generation of bone marrow-derived dendritic cells (BM-DC)
Bone marrow cells were flushed from the femurs and tibiae of various types of mice. RBC were lysed, and the cells were stained with PE anti-B220, anti-Gr1, and anti-Ter119 cells. B cells, granulocytes, and erythrocyte progenitors were then removed by positive selection with anti-PE MACS beads, and the remaining cells were plated at a density of 5 × 105 cells/ml in RPMI 1640 medium containing 5% FBS and 20 ng/ml GM-CSF. The medium was replaced on days 2 and 4, and cells were harvested on day 6. To obtain mature dendritic cells (DC), LPS was added to culture at a final concentration of 40 ng/ml on day 5. Cells were collected on day 6, and mature myeloid DC were positively selected with PE anti-CD86 and anti-CD40 Abs, as described above. DC purified from CD80 and CD86 double-KO mice or from CD40 KO mice were positively selected for CD40 or CD86 expression, respectively. Immature DC were obtained from non-LPS-treated cultures and were negatively selected by removing GR1+ and CD86+ cells with anti-PE MACS beads. Mature DC were fixed with 2% paraformaldehyde in PBS for 10 min on ice or permeabilized and fixed using perm/fix from BD Pharmingen for 30 min on ice. The cells were washed in PBS and resuspended in RPMI 1640 containing 0.1 M lysine for 20 min at room temperature. The cells were washed in complete medium and used in the proliferation assay.
In vitro MLR
Standard MLR were performed using 1 × 105 cells/well of C57BL/6 CD4+CD25− T cells and 2.5 × 104 cells/well of the indicated BM-DC in 96-well flat-bottom plates in a final volume of 250 μl of complete medium. The cells were cultured at 37°C in 5% CO2 for 2 days. IgG2a or anti-Tim-1 mAb (1–10 μg/ml) were titrated into the culture system, as indicated. Tritiated thymidine (1 μCi/well) was added for the last 6 h of culture. The radiolabeled cells were harvested using a Tomtec 96-well harvester, and incorporation of [3H]thymidine was measured via a beta plate scintillation counter (PerkinElmer Wallac). Data are presented as mean cpm in quadruplicate wells. Cyclosporine, a calcineurin inhibitor, was added to some replicates at a final concentration of 100 ng/ml. Complete medium consisted of 1× MEM (Life Technologies/Invitrogen) supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 10% FBS, 0.3% sodium bicarbonate, 19 mM HEPES, 50 μg/ml gentamicin, 5.5 × 10−5 2-ME, and 100 U/ml penicillin/streptomycin.
In vivo analysis of CFSE-labeled cells
Splenic leukocytes from C57BL/6 CD45.1 mice were prepared and labeled with the vital dye CFSE (Molecular Probes-Invitrogen), as previously described (8). CFSE-labeled cells (~6–8 × 107 cells) were then injected via the tail vein into DBA/2 or C57BL/6 CD45.2 mice that were irradiated (1000 rad) with a Gammacell Exactor (Kanata) shortly before receiving donor cells. Recipient mice were administered 250 μg of 3B3 anti-Tim-1 mAb or isotype IgG2a control mAb (i.p.). Three days later, the host mice were sacrificed. The spleens were harvested and counterstained with annexin V, per the manufacturer's instructions (BD Biosciences). The cell division history of CFSE-labeled donor CD4+CD45.1+ cells in the host spleen was examined by flow cytometry and analyzed using FlowJo software (Tree Star). FlowJo Proliferation Platform was used to evaluate the percentage of divided cells, which represents the percentage of cells in the original sample that have divided (assuming that no divided cells died during the culture).
Calcium ion influx assay using flow cytometry
After isolation, CD4+CD25− T cells were resuspended at 5 × 106/ml in HBSS containing 1% FBS, 3 μg/ml fluo-3-acetoxymethyl ester (fluo-3-AM), and 0.02% Pluronic F127 (Invitrogen Molecular Probes). The cells were incubated at 37°C in 5% CO2 for 30 min, and then centrifuged at 1400 rpm for 5 min before gentle resuspension at 3 × 106/ml in HBSS containing 1% FBS. The cells were left at room temperature for 20 min. All analyses were performed using a LSRII flow cytometer with FacsDIVA software (BD Biosciences). Fluorescence intensity of fluo-3-AM was collected for 2 min (background level of fluorescence); then anti-Tim-1 mAb or IgG2a (10 μg/ml) was added and data were collected for an additional 5 min.
Real-time quantitative PCR
Total RNA was extracted from cells using the RNeasy Mini Kit (Qiagen). Reverse transcription was conducted with 1 μg of RNA using ABI Prism TaqMan reverse-transcription reagents with random hexamers as primers. The expression of the genes of interest was analyzed using TaqMan primer-probe sets purchased as Assay-on-Demand from Applied Biosystems and normalized to the expression of GAPDH. Transcript levels were calculated according to the 2−ddCt method, as described by the manufacturer.
NF-AT expression
NF-AT nuclear translocation occurring upon stimulation with anti-Tim-1 mAb was assayed using the TransAM NF-ATc1 kit (Active Motif), per the manufacturer's instructions. Briefly, naive CD4+CD25− T were cultured with mature syngeneic BM-DC and anti-Tim-1 mAb or isotype control mAb in the presence or absence of cyclosporine (100 ng/ml) for 21 h. The nuclear extracts were harvested from each culture. The protein concentrations were measured using Bradford reagent. Equal amounts of nuclear extracts were added to each well of the 96-well plate, a plate precoated with an oligonucleotide containing the NF-AT consensus binding site. The NF-ATc1 protein contained in the nuclear extract binds specifically to the NF-AT consensus binding site oligonucleotide and is detected through use of an Ab directed against NF-ATc1. The final readout is a sensitive colorimetric reaction quantified by spectrophotometry.
Results
Tim-1 provides signal 1 for T cell proliferation
We sought to determine whether ligation of Tim-1 in the absence of obvious TCR stimulation stimulates T cell activation. Agonist anti-Tim-1 mAb (clone 3B3) or an IgG2a isotype control was placed into cultures of naive C57BL/6 CD4+CD25− T cells and mature syngeneic BM-DC (Fig. 1A). In this system, Tim-1 is primarily engaged by the interaction with agonist anti-Tim-1 mAb. The DC used in this experiment do not express Tim-4, the ligand for Tim-1, and neither do normal T cells (1, 9). Proliferation was not induced in isotype control cultures, reflecting the inability of self MHC-peptide complexes to activate resting T cells. However, addition of agonist anti-Tim-1 mAb resulted in a rapid and dose-dependent T cell proliferation (Fig. 1A). Perhaps, ligation of Tim-1 triggers a TCR-induced signal 1. Alternatively, Tim-1 may lower the threshold for TCR signal activation by endogenous peptides presented by mature syngeneic BM-DC. To test this possibility, we harvested mature syngeneic BM-DC from MHC class II KO mice and used these MHC class II-deficient DC in the anti-Tim-1 mAb-driven proliferation assay (Fig. 1B). These MHC class II null BM-DC have a defect in Ag presentation. However, MHC class II-deficient mature syngeneic DC were similarly effective in supporting Tim-1-dependent activation of naive CD4+ T cells, as were DC from wild-type C57BL/6 (Fig. 1B). Taken together, these data suggest that Tim-1 ligation on T cells provides a functional signal 1 that stimulates T cell proliferation, at least when Tim-1 is engaged in concert with signal 2 stimulation.
FIGURE 1.
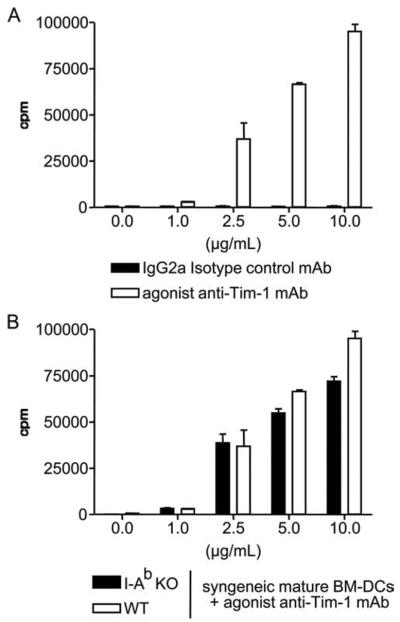
Anti-Tim-1 mAb plus mature DC stimulate naive T cells to proliferate in the absence of MHC class II-derived signals. Naive CD4+ CD25− T cells were cultured with mature syngeneic BM-DC from wild-type mice (A) or MHC class II (I-Ab)-deficient mice (B), in the presence of increasing doses of anti-Tim-1 mAb or isotype control mAb. Tritiated thymidine incorporation (cpm) was measured after 48 h of culture. Bars represent the mean ± SEM of one of four consecutive experiments yielding similar results.
Costimulation by mature DC is required for Tim-1-induced T cell proliferation
We next investigated whether a costimulatory type signal 2 was necessary for anti-Tim-1 mAb-induced T cell proliferation. We first compared the ability of immature (CD86−CD40−) and mature (CD86+CD40+) BM-DC to support T cell proliferation in this assay system (Fig. 2A). In contrast to their mature MHC class II+ counterpart, MHC class II-deficient DC that had not been previously activated by LPS did not support anti-Tim-1-triggered proliferation of naive syngeneic CD4+CD25− T cells (Fig. 2A), suggesting that costimulation was critical for the Tim-1 mitogenic effect. We then sought to identify costimulatory molecules expressed by mature BM-DC that are required to support the T cell proliferation induced by agonist anti-Tim-1 mAb. To this end, we produced mature BM-DC from select KO mice to study the importance of various costimulatory molecules (e.g., CD40, CD80, CD86, CD80 and CD86, or OX40L). Although mature syngeneic BM-DC from CD40 KO mice supported T cell proliferation as well as wild-type DC (Fig. 2B), mature BM-DC from either CD80 and CD86 double-KO or OX40L KO mice were defective in their ability to support anti-Tim-1-driven proliferation of syngeneic naive CD4+CD25− T cells (Fig. 2, C and D). Similar results were obtained when highly mature BM-DC from CD80 or CD86 KO mice were used (data not shown). Of note, LPS-stimulated BM-DC derived from the CD80 or CD86 or OX40L KO mice did up-regulate MHC class II molecules and all the other studied costimulatory molecules (data not shown). Moreover, upon LPS stimulation, these BM-DC secreted similar amounts of a variety of cytokines (e.g., IL-2, IL-4, IL-6, IFN-γ, TNF-α) (data not shown). Perhaps the activated BM-DC provide support for Tim-1-driven activation through the provision of other soluble factors. To address this possibility, mature DC from C57BL/6 MHC class II KO mice were fixed with 2% paraformaldehyde and then used in the anti-Tim-1 mAb-driven proliferation assay. Fixed DC cannot: 1) transmit intracellular signals; 2) synthesize or insert new molecules into their cell membranes; and 3) secrete proteins (our unpublished data). Nonetheless mature fixed MHC class II KO DC strongly support the anti-Tim-1 mAb-triggered T cell proliferation (Fig. 3), indicating that soluble factors produced by mature DC do not play a major role in anti-Tim-1 mAb-driven T cell activation. In summary, these data indicate that fully mature DC support Tim-1 activation through the provision of an indispensable costimulatory signal 2 involving at least the CD80/86-CD28 and OX40-OX40L pathways.
FIGURE 2.
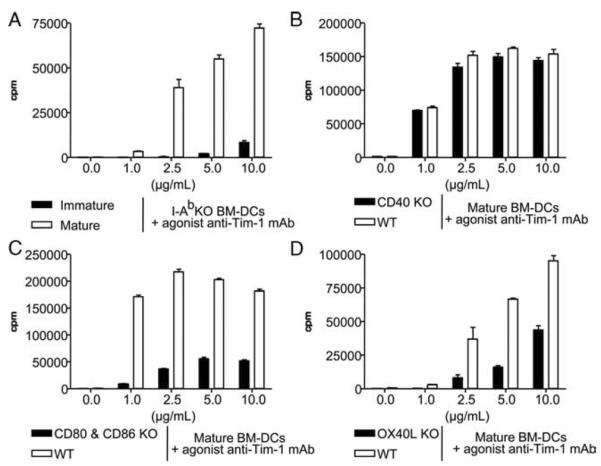
Anti-Tim-1-induced T cell proliferation requires costimulatory signals. Naive CD4+CD25− T cells were cultured in medium containing various doses of anti-Tim-1 mAb or isotype control mAb with immature or mature syngeneic BM-DC purified from MHC class II KO mice (A). Naive CD4+CD25− T cells were cultured with mature syngeneic BM-DC from CD40 KO mice (B), CD80 plus CD86 double-KO mice (C), or OX40L KO mice (D). Tritiated thymidine incorporation (cpm) was measured after 48 h of culture. Bars represent the mean ± SEM of one of three consecutive experiments yielding similar results.
FIGURE 3.
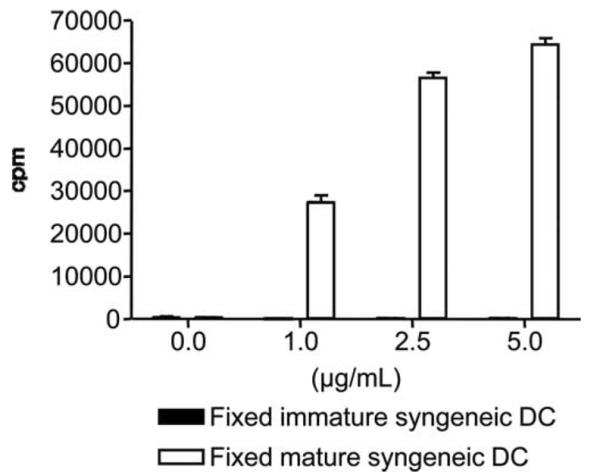
Fixed mature BM-DC function in the anti-Tim-1 mAb proliferation assay. Naive CD4+CD25− T cells purified from C57BL/6 mice were cultured with immature or mature MHC class II-deficient DC, in the presence of increasing dose of anti-Tim-1 mAb. Immature and mature MHC class II-deficient DC were fixed with 2% paraformaldehyde in PBS prior addition to the culture. Tritiated thymidine incorporation (cpm) was measured after 48 h of culture. Bars represent the mean ± SEM of one of three consecutive experiments yielding similar results.
Triggering Tim-1 on the surface of T cells directly elicits a Ca2+-dependent signaling cascade
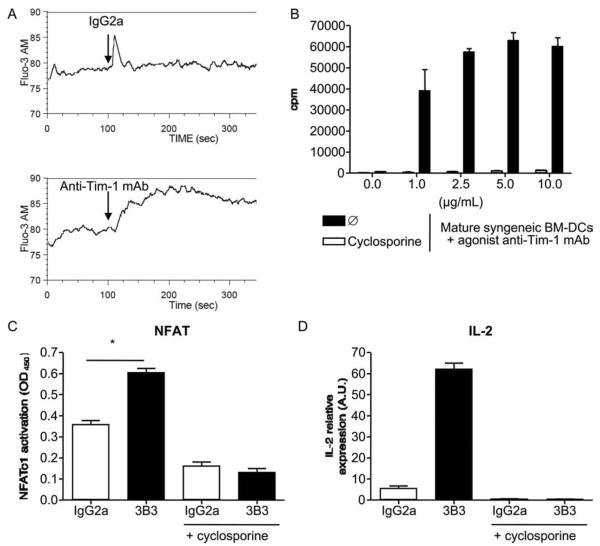
A rise in intracellular free Ca2+ is a crucial second messenger in the TCR pathway (10). Stimulation of purified CD4+CD25− T cells by anti-Tim-1 mAb, but not an isotype control Ab, induces a rapid rise in intracellular free Ca2+ (Fig. 4A). An important downstream target of cytoplasmic Ca2+ is the Ca2+/calmodulin-dependent serine phosphatase, calcineurin (11). Cyclosporine, a potent immunosuppressive drug, acts by inhibiting the enzymatic activity of calcineurin (12, 13). To test the requirement for calcineurin activity in Tim-1-triggered T cell proliferation, naive CD4+CD25− T cells were cultured with mature syngeneic BM-DC and agonist anti-Tim-1 mAb in the presence or in the absence of cyclosporine. Cyclosporine abrogates the proliferative effect of anti-Tim-1 mAb upon T cells (Fig. 4B). Calcineurin cleaves phosphate from NF-AT, thereby enabling translocation of the transcription factors to the nucleus. The interaction of NF-AT with the NF-AT/AP-1 site in the IL-2 gene induces IL-2 expression (14). Because activation of NF-AT is calcineurin dependent, we tested whether agonist anti-Tim-1 mAb triggered the activation of NF-AT (Fig. 4C). CD4+CD25− T cells were cultured with 3B3 anti-Tim-1 mAb in the presence of syngeneic mature BM-DC. Activation of NF-AT was analyzed. Agonist anti-Tim-1 mAb induced nuclear translocation of NF-AT in T cells, a process that is inhibited by cyclosporine (Fig. 4C). In contrast, anti-Tim-1 mAb does not elicit translocation of NF-AT in isolated mature BM-DC (data not shown). Because the transcription factor NF-AT was initially identified through its ability to induce IL-2 production by activated T cells (15), we also analyzed the effect of anti-Tim-1 mAb on the IL-2 gene transcription. Activation of CD4+CD25− T cells with agonist anti-Tim-1 mAb in the presence of syngeneic mature BM-DC results in a robust expression of IL-2 transcripts (Fig. 4D). Thus, ligation of T cell surface Tim-1 molecules directly elicits a Ca2+ signal, which in turn activates calcineurin and NF-AT. This process leads to IL-2 gene transcription. Hence, Tim-1 signaling pathway uses downstream components of the TCR/CD3 signal 1 pathway.
FIGURE 4.
Activation of Tim-1 on the surface of T cells directly delivers a positive signal that triggers Ca2+/calmodulin/NF-AT pathway. A, Naive CD4+CD25− T were labeled with fluo-3-AM and IgG2a or agonist anti-Tim-1 mAb was added at the time indicated by (↓). Data are representative of three consecutive experiments yielding similar results. B, Naive CD4+CD25− T were cultured with mature syngeneic BM-DC and anti-Tim-1 mAb in the presence or in the absence of cyclosporine (100 ng/ml). Tritiated thymidine incorporation (cpm) was measured after 48 h of culture. Bars represent the mean ± SEM of one of three consecutive experiments yielding similar results. C, Naive CD4+CD25− T were cultured with mature syngeneic BM-DC and anti-Tim-1 mAb or isotype control mAb in the presence or absence of cyclosporine (100 ng/ml) for 21 h. NF-AT activity was measured from the nuclear extract of the various experimental settings. Bars represent the mean ± SEM of one of three consecutive experiments yielding similar results. *, p < 0.05 (Mann-Whitney U test). D, Naive CD4+CD25− T were cultured with mature allogeneic BM-DC in the presence of IgG2a isotype control mAb or anti-Tim-1 mAb (5 μg/ml), and in the absence or the presence of cyclosporine (100 ng/ml), for 2 days. Expression of IL-2 transcripts was then quantified by quantitative RT-PCR. Bars represent the mean ± SEM of three consecutive experiments.
Tim-1 substitutes for signal 1 in vivo and sustains T cell survival after activation
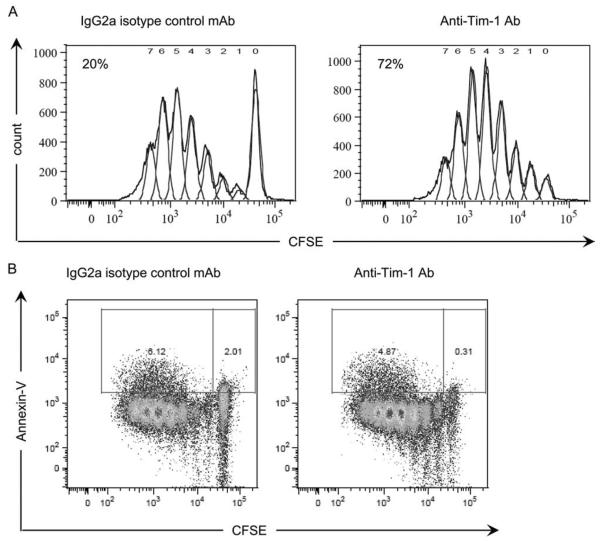
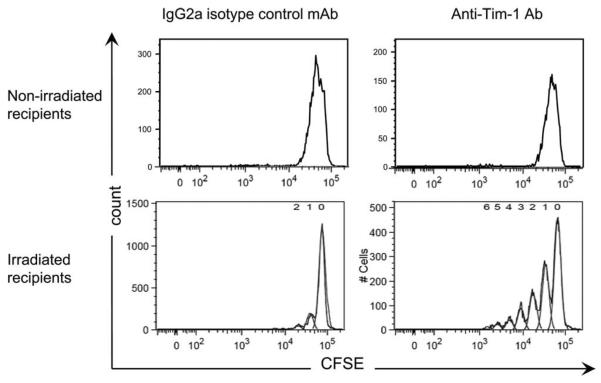
To determine whether our observations on anti-Tim-1 activation in vitro were pertinent in vivo, we turned to a graft-vs-host-like model. CFSE-labeled C57BL/6 spleen cells (graft, H-2b) were transfused into irradiated DBA/2 mice (host, H-2d) treated with 3B3 anti-Tim-1 mAb or IgG2a isotype control Ab (Fig. 5A). Three days later, the CFSE-labeled CD4+ T cells were recovered, and the percentage of dividing cells was examined by flow cytometry analysis of CFSE dilution. In this system, an average of 20% of CD4+ T cells isolated from mice treated with the isotype control entered the cell cycle by day 3. By contrast, 72% of cells had proliferated at the same time point under the influence of the anti-Tim-1 mAb, thereby confirming the immunostimulatory consequences of activation of the Tim-1 pathway in an in vivo system. Interestingly, the proportion of dividing CD4+ cells positive for annexin V remained low, despite the increase of proliferation observed when the host was treated with the anti-Tim1 mAb (Fig. 5B). To address whether ligation of Tim-1 could also substitute for signal 1 in vivo, congeneic CFSE-labeled C57BL/6 CD45.1 spleen cells (graft, H-2b) were transfused into irradiated C57BL/6 mouse (host, H-2b) (Fig. 6). Mirroring the effects of Tim-1 ligation in the syngeneic in vitro MLR, cell proliferation was not detected in this system unless the host had been treated with 3B3 anti-Tim-1 mAb (Fig. 6). Finally, we reasoned that irradiation in this system might induce a substitute for signal 2 insofar as irradiation induces cell death and triggers inflammatory cytokine release, and create the inflammatory environment necessary for Tim-1 to exert its stimulatory activity. Consistent with this hypothesis, we observed that anti-Tim-1 mAb had virtually no stimulatory effect when congeneic cells were transferred into a nonirradiated host (Fig. 6).
FIGURE 5.
In vivo stimulation of Tim-1 induces polyclonal T cell proliferation. CFSE-labeled splenocytes from C57BL/6 mice were injected into irradiated allogeneic DBA/2 hosts treated with agonist anti-Tim-1 mAb or IgG2a control mAb (250 μg/ml). Three days later, proliferation was assessed by analysis of the CFSE profile of CFSE-labeled CD4+ T cells (A). Proliferative T cells that are programmed for apoptosis were identified by flow cytometry as the annexin V+ CFSE-labeled CD4+ T cells (B). Data are representative of four consecutive experiments yielding similar results.
FIGURE 6.
In vivo Tim-1-induced CD4+ T cell proliferation occurs in the absence of MHC class II-derived signals. CFSE-labeled splenocytes from C57BL/6 CD45.1 mice were injected into nonirradiated or irradiated C57BL/6 CD45.2 hosts treated with an agonist anti-Tim-1 mAb or the IgG2a control mAb. Three days later, proliferation was assessed by analysis of the CFSE profile of CFSE-labeled CD4+ CD45.1+ T cells. Data are representative of four consecutive experiments yielding similar results.
Discussion
T cell activation and clonal expansion is a multistep process requiring at least three signals. The first signal is delivered to the T cell when the TCR binds to the peptide/MHC complexes upon activated DC (for review, see Huang and Wange (16)). The second signal is delivered, at least in part, to the T cell by binding of the CD80/CD86 costimulatory molecules expressed on the activated DC to CD28 expressed on T cells. Stimulation by signals 1 plus 2 leads to IL-2 gene activation and production of IL-2 (17) and to the expression of high-affinity receptors for IL-2 and other T cell growth factors (for review, see Refs. 18 and 19). The binding of IL-2 and other T cell growth factors to their high-affinity receptors delivers the third stimulatory signal to T cells. We found that ligation of Tim-1 in the presence of mature DC triggers polyclonal T cell activation. Anti-Tim-1 mAb-triggered proliferation of cultured T cells was achieved in the absence of an obvious TCR stimulant. Robust polyclonal activation of T cells is achieved only in the presence of mature APC, which bear essential costimulatory proteins (CD80, CD86, and OX40L). In a graft-vs-host-like model, CFSE-labeled T cells proliferate following transfer into allogeneic, not syngeneic, irradiated hosts, but administration of anti-Tim-1 mAb promotes a proliferative response in the syngeneic model.
Little is known about the intracellular signaling pathway that it is spawned by ligation of Tim-1 cell surface proteins with agonistic anti-Tim-1 mAb, but these events may resemble those induced by interaction with Tim-4Ig (4). The administration of agonist anti-Tim-1 mAb (clone 3B3) enhances T cell activation and proliferation and blocks development of tolerance (1, 20). Tim-1 associates with the TCR following T cell activation, and cross-linking with agonist anti-Tim-1 mAb leads to engagement of tyrosine signaling motifs (2, 3). We now show that activation of Tim-1 elicits a rise in intracellular Ca2+. As a consequence, Tim-1 ligation triggers the cyclosporine-sensitive calcineurin/NF-AT pathway. It is noteworthy that De Souza et al. (3) have found that ligation of Tim-1 on Jurkat or D10 T cells triggers tyrosyl phosphorylation of intracellular domain of Tim-1 and the recruitment of NF-AT/AP-1 elements. Our results are also consistent with a recent report showing that interaction with Tim-4, which is expressed on activated APC, triggers tyrosyl phosphorylation of the linker of activated T cells (4), one of the most important T cell adapter proteins (21). The elicitation of T cell activation and proliferation without TCR ligation has been previously reported by others. Luhder et al. (22) found that superagonist anti-CD28 mAb bypass the requirement of direct TCR-triggered signal 1 in T cell activation. Moreover, the requirement of potent costimulatory signals to elicit Tim-1-induced T cell activation has been suggested by others. Indeed, Rodriguez-Manzanet et al. (4) have shown that Chinese hamster ovary-Tim-4 transfectants induce T cell proliferation only in the presence of anti-CD28. Within the TCR complex, Tim-1 intimately associates with CD3 (2). Given this physical association, it seems reasonable that ligation of Tim-1 produces effects similar to those produced by cross-linking CD3. In this scenario, the signal is initiated by ligation of Tim-1, not the TCR. Therefore, this pathway constitutes an unorthodox means to generate signal 1.
Given the ability of Tim-1-derived signals to elicit T cell proliferation, expression of Tim-1 and Tim-4, the Tim-1 ligand, is likely to be tightly regulated to preclude uncontrolled activation of T cells. Expression of Tim-4 by myeloid splenic DC is induced through TLR-4-induced signaling events. LPS stimulation induces the expression of Tim-4 by myeloid splenic DC, and expression of Tim-4 is correlated with the expression of costimulatory molecules (i.e., CD80 and CD86; data not shown). In resting T cells, Tim-1 is weakly expressed on the membrane and accumulates at intracellular vesicles (23). Activation by TCR ligation or treatment with ionomycin/PMA induces a marked increase of Tim-1 expression on the cell surface (our unpublished data) (23). We suspect that under physiological conditions, the interaction between Tim-4 expressed upon DC and Tim-1 serves to heighten T cell activation in vivo only in the presence of danger signals. This may be of particular importance in sustaining T cell activation.
Activation of Tim-1 pathway not only heightens T cell proliferation, but also promotes antiapoptotic properties. The frequency of apoptotic cells remains low despite vigorous proliferation of CD4+ T cells induced by in vitro or in vivo by agonist anti-Tim-1 mAb stimulation (Fig. 4) (20). IL-2 promotes T cell viability by several mechanisms, including activation of the PI3K/AKT pathway and induction of the antiapoptotic genes bcl-xL and bcl-2. Following stimulation with 3B3 anti-Tim-1 mAb, increased expression of IL-2 and bcl-2 is noted (Fig. 4) (20). Antiapoptotic protein Akt was found to be phosphorylated in T cells upon engagement of Tim-1 by Tim-4 (4). Thus, activation of the Tim-1 pathway spawns growth and survival signals that enable expansion and survival of these activated T cells. Manipulating the immune system using anti-Tim-1 mAb may offer new strategies to activate or heighten the immune response against tumors, microbes, and vaccines.
Acknowledgments
We thank Fabienne Haspot and Alberto Sanchez-Fueyo for stimulating discussions and critical reading of this manuscript.
Footnotes
Disclosures The authors have no financial conflict of interest.
This work was supported by the Société de Néphrologie (to C.M.), the Société Francophone de Transplantation (to C.M.), the National Institute of Allergy and Infectious Disease (to X.X.Z., D.T.U., and T.B.S.), and the Juvenile Diabetes Research Foundation Center for Immunologic Tolerance at Harvard (to T.B.S.).
Abbreviations used in this paper: Tim, T cell Ig mucin; BM-DC, bone marrow-derived dendritic cell; DC, dendritic cell; fluo-3-AM, fluo-3-acetoxymethyl ester; KO, knockout.
References
- 1.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, Zheng XX, Umetsu DT, DeKruyff RH, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat. Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 2.Binne LL, Scott ML, Rennert PD. Human TIM-1 associates with the TCR complex and up-regulates T cell activation signals. J. Immunol. 2007;178:4342–4350. doi: 10.4049/jimmunol.178.7.4342. [DOI] [PubMed] [Google Scholar]
- 3.De Souza AJ, Oriss TB, O'Malley JK, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc. Natl. Acad. Sci. USA. 2005;102:17113–17118. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Manzanet R, Meyers JH, Balasubramanian S, Slavik J, Kassam N, Dardalhon V, Greenfield EA, Anderson AC, Sobel RA, Hafler DA, et al. TIM-4 expressed on APCs induces T cell expansion and survival. J. Immunol. 2008;180:4706–4713. doi: 10.4049/jimmunol.180.7.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Souza AJ, Oak JS, Jordanhazy R, DeKruyff RH, Fruman DA, Kane LP. T cell Ig and mucin domain-1-mediated T cell activation requires recruitment and activation of phosphoinositide 3-kinase. J. Immunol. 2008;180:6518–6526. doi: 10.4049/jimmunol.180.10.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao S, Najafian N, Reddy J, Albin M, Zhu C, Jensen E, Imitola J, Korn T, Anderson AC, Zhang Z, et al. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J. Exp. Med. 2007;204:1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, Berry GJ, Nagumo H, Freeman GJ, Umetsu DT, DeKruyff RH. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat. Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 8.Auchincloss H, Jr., Lee R, Shea S, Markowitz JS, Grusby MJ, Glimcher LH. The role of “indirect” recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice. Proc. Natl. Acad. Sci. USA. 1993;90:3373–3377. doi: 10.1073/pnas.90.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilker PR, Sedy JR, Grigura V, Murphy TL, Murphy KM. Evidence for carbohydrate recognition and homotypic and heterotypic binding by the TIM family. Int. Immunol. 2007;19:763–773. doi: 10.1093/intimm/dxm044. [DOI] [PubMed] [Google Scholar]
- 10.Bootman MD, Berridge MJ. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol. Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 12.Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 13.Emmel EA, Verweij CL, Durand DB, Higgins KM, Lacy E, Crabtree GR. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989;246:1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- 14.Masuda ES, Imamura R, Amasaki Y, Arai K, Arai N. Signalling into the T-cell nucleus: NFAT regulation. Cell. Signal. 1998;10:599–611. doi: 10.1016/s0898-6568(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 15.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Wange RL. T cell receptor signaling: beyond complex complexes. J. Biol. Chem. 2004;279:28827–28830. doi: 10.1074/jbc.R400012200. [DOI] [PubMed] [Google Scholar]
- 17.Lanier LL, O'Fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K, Ito D, Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 18.Ozaki K, Leonard WJ. Cytokine and cytokine receptor pleiotropy and redundancy. J. Biol. Chem. 2002;277:29355–29358. doi: 10.1074/jbc.R200003200. [DOI] [PubMed] [Google Scholar]
- 19.Waldmann TA. The interleukin-2 receptor. J. Biol. Chem. 1991;266:2681–2684. [PubMed] [Google Scholar]
- 20.Degauque N, Mariat C, Kenny J, Zhang D, Gao W, Vu MD, Alexopoulos S, Oukka M, Umetsu DT, Dekruyff RH, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J. Clin. Invest. 2007;118:735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosselut R, Zhang W, Ashe JM, Kopacz JL, Samelson LE, Singer A. Association of the adaptor molecule LAT with CD4 and CD8 coreceptors identifies a new coreceptor function in T cell receptor signal transduction. J. Exp. Med. 1999;190:1517–1526. doi: 10.1084/jem.190.10.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luhder F, Huang Y, Dennehy KM, Guntermann C, Muller I, Winkler E, Kerkau T, Ikemizu S, Davis SJ, Hanke T, Hunig T. Topological requirements and signaling properties of T cell-activating, anti-CD28 antibody superagonists. J. Exp. Med. 2003;197:955–966. doi: 10.1084/jem.20021024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santiago C, Ballesteros A, Tami C, Martinez-Munoz L, Kaplan GG, Casasnovas JM. Structures of T cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity. 2007;26:299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]