Abstract
Scurfy mice have a deletion in the Foxp3 gene, resulting in a failure to generate Foxp3+ regulatory T cells, and they subsequently develop severe CD4+ T-cell-mediated autoimmune inflammation. Multiple organs are involved, but the skin is one of the main organs affected. During the course of disease, Scurfy mice develop autoantibodies; however, the targeted antigens are unknown. In this study, we show that Scurfy mice develop autoantibodies directed against skin antigens. Using western blot analysis, we found that Scurfy serum reacted with proteins in total skin lysate, as well as in a keratinocyte lysate. Most of the Scurfy sera tested identified a major band at 50 kDa. Transfer of Scurfy CD4+ T cells into nu/nu mice yielded autoantibodies with similar reactivity. Further analysis using 2D western blots, followed by peptide mass fingerprinting, identified several keratins as targets. To confirm this observation, we chose one of the identified targets, keratin 14, and prepared recombinant proteins encompassing the N-terminal, middle, and C-terminal portions of the keratin 14 protein. Scurfy serum predominantly recognized the C-terminal fragment. Sera from patients with immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, the human disease resulting from FOXP3 mutations, also recognized skin antigens, including keratin 14. Thus, the results of our study indicate that autoantibodies in Scurfy mice and patients with IPEX target keratins.
INTRODUCTION
Regulatory T cells (Treg) maintain peripheral tolerance by suppressing autoreactive T cells (Maloy and Powrie, 2001; Sakaguchi et al., 2001; Shevach, 2002). The transcription factor Foxp3 is important for the development and function of naturally occurring Treg cells (Fontenot et al., 2003; Gavin et al., 2007). Scurfy mice lack functional Treg because of disruption of the Foxp3 gene by a 2 bp insertion and develop a lymphoproliferative disease with multiorgan inflammation (Godfrey et al., 1991a, b). Autoreactive CD4+ effector T cells infiltrate tissues, secrete cytokines, recruit other inflammatory cells, and ultimately lead to the destructive pathology present in liver, lung, and skin. B cells are also activated in the Scurfy mouse and high antibody titers are present in the serum (Godfrey et al., 1991b). However, none of the antigenic targets recognized by autoreactive T cells and autoantibodies have thus far been identified.
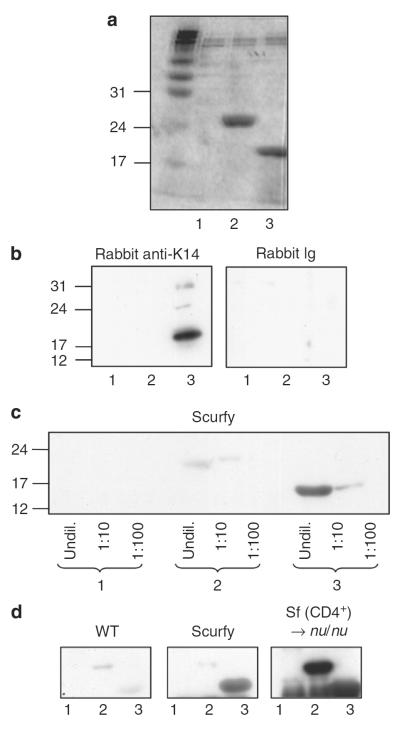
We asked if an autoimmune response directed against skin antigens exists in Scurfy mice. We screened Scurfy sera for reactivity to skin proteins and identified several keratins as antigenic targets. We showed by adoptive transfer experiments involving nu/nu mice that Scurfy CD4+ T cells provided T cell help to autoreactive B cells present in the normal B cell pool, leading to the generation of autoantibodies similar to the ones present in Scurfy serum. For one of the target antigens, keratin 14, we localized the predominant epitope to the C-terminal part of the protein.
Patients with the immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) (Wildin and Freitas, 2005; Ochs et al., 2007), which resembles the disease in Scurfy mice (Bennett et al., 2001; Wildin et al., 2001) and is also caused by mutations in FOXP3 gene (Bennett et al., 2001), often present with severe skin disease (Wildin et al., 2002; Gambineri et al., 2008; Halabi-Tawil et al., 2009). We tested sera from IPEX patients with and without skin disease using western blot for their reactivity to skin antigens and identified strong reactivity in one of the patients with eczema. Taken together, these data identify keratins as antigenic targets in autoimmune disease and should facilitate the investigation of the specificity of autoreactive T cells in autoimmune skin diseases.
RESULTS
Scurfy mice develop severe skin inflammation
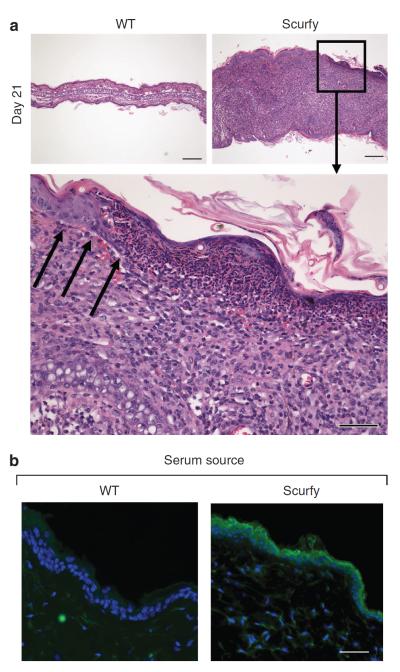
Between days 10 and 14, Scurfy mice develop visible skin disease. At day 21, large inflammatory infiltrates in the dermis lead to severe thickening of the ear and the dermoepidermal junction is effaced (Figure 1a). Inflammatory cells also infiltrate the epidermis and in some areas completely replace it (Figure 1a, lower panel). We stained skin sections from RAG−/− mice with Scurfy or wild-type (WT) littermate sera by immunohistochemistry. Scurfy serum recognized mainly epidermal structures (Figure 1b). Some of the sera from individual Scurfy mice reacted predominantly with the basal layer of keratinocytes, whereas other sera reacted with the upper layers or with the entire epidermis. No staining was observed with control sera (Figure 1b).
Figure 1. Scurfy mice develop severe skin inflammation with destruction of the dermoepidermal junction.
(a) Ear sections from Scurfy mice and WT littermate controls were taken on day 21. Hematoxylin and eosin (H&E) staining; bar = 200 μm. The lower panel shows the dermoepidermal junction from a Scurfy mouse ear at higher magnification. H&E staining; bar = 50 μm, black arrows indicate the dermoepidermal junction. (b) Frozen sections of skin from RAG−/− mice were stained with either Scurfy or WT littermate control serum as primary antibody, followed by anti-mouse IgG Alexa Fluor 488, DAPI counterstain in blue. Bar = 50 μm.
Scurfy serum contains autoantibodies against skin antigens
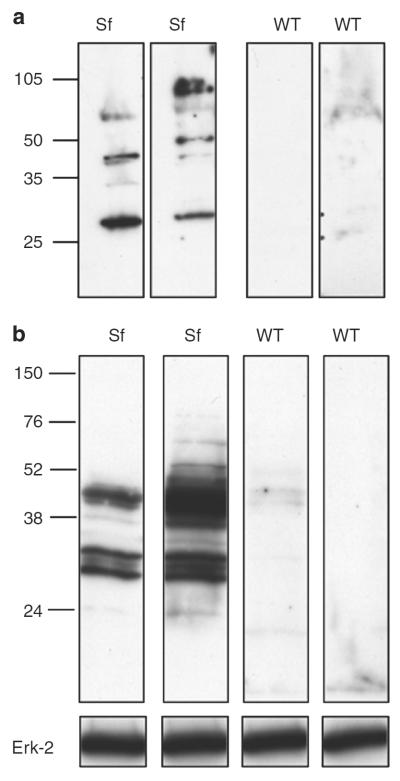
We screened the reactivity of Scurfy sera and sera from littermate controls with total skin lysates using western blot analysis. To exclude the possibility that protein–antibody complexes might already be present in skin lysates, we prepared lysates from RAG−/− skin for all experiments. Scurfy sera recognized proteins in the skin lysate, whereas control sera did not (Figure 2a). We then isolated keratinocytes from newborn mouse skin as previously described (Lichti et al., 2008) and prepared keratinocyte lysates for western blot analysis. In contrast to control sera, Scurfy sera recognized several proteins in the keratinocyte lysate (Figure 2b).
Figure 2. Scurfy autoantibodies recognize target antigens in keratinocytes.
(a) Skin lysate from RAG−/− mice was separated on SDS-PAGE, followed by western blot. Single strips were cut and then stained individually with either Scurfy or WT littermate control sera as primary antibody, followed by anti-mouse-horseradish peroxidase (HRP). Each lane represents staining with serum of an individual mouse. One representative experiment of at least three with similar results is shown. (b) Lysates from freshly isolated keratinocytes were separated on SDS-PAGE, followed by western blot using either Scurfy or WT control sera as described in (a). One representative experiment of at least five with similar results is shown.
Autoantibodies in Scurfy sera target keratinocytes
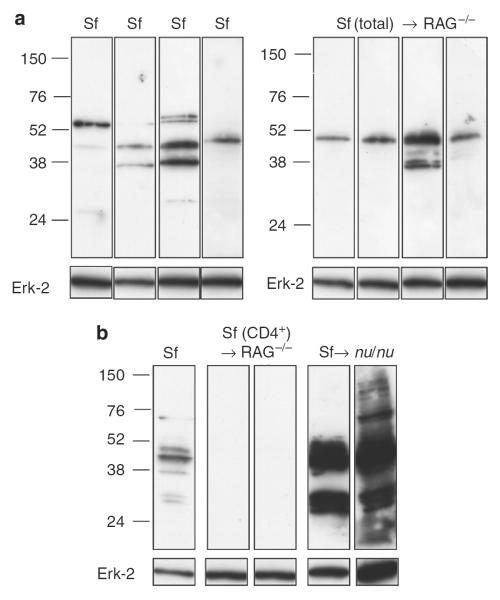
To rule out a possible abnormality in scurfy skin that predisposes to autoantibody production, we determined if Scurfy B cells also mounted antibody responses to keratinocyte antigens expressed in the skin of mice not harboring the Scurfy mutation. Total cells from lymph nodes and spleens of Scurfy mice are transferred into RAG−/− recipients (Huter et al., 2008). All recipient mice developed skin pathology very similar to that seen in Scurfy mice (data not shown). The sera of the recipients obtained 4 weeks after transfer reacted with the same pattern of keratinocyte antigens as those recognized by Scurfy sera (Figure 3a).
Figure 3. Autoreactive B cells recognizing skin antigens are present in nu/nu mice.
(a) Total lymph node and spleen cells from a Scurfy mouse were transferred into RAG−/− recipients. At 4 weeks after transfer, mice were bled and sera were analyzed individually by western blot analysis on keratinocyte lysate as described earlier. The left panel shows staining with four Scurfy sera as controls. (b) CD4+ T cells were purified from Scurfy lymph nodes and transferred into either RAG−/− or nu/nu recipients. The recipient mice were bled 4 weeks after transfer, and the sera were analyzed individually as described in (a). Sera from two mice of four in each group are shown.
In this model, the Scurfy B cells received T cell help from Scurfy T cells transferred into the RAG−/− recipient. To determine if autoreactive B cells targeting the same epidermal proteins as Scurfy B cells are present in the B cell repertoire of normal mice, we transferred CD4+ Scurfy T cells into either RAG−/− or nu/nu mice and bled the mice after 4 weeks. The sera of the RAG−/− recipients reconstituted with Scurfy CD4+ T cells did not contain any autoantibodies (Figure 3b), whereas the sera of the nu/nu recipients showed strong reactivity with bands of a similar molecular weight as the Scurfy serum (Figure 3b). Thus, autoreactive B cells recognizing epidermal targets are present in the normal B cell repertoire and these B cells can be activated and receive help from Scurfy T cells.
Identification of several keratins as targets for autoantibodies in Scurfy mice
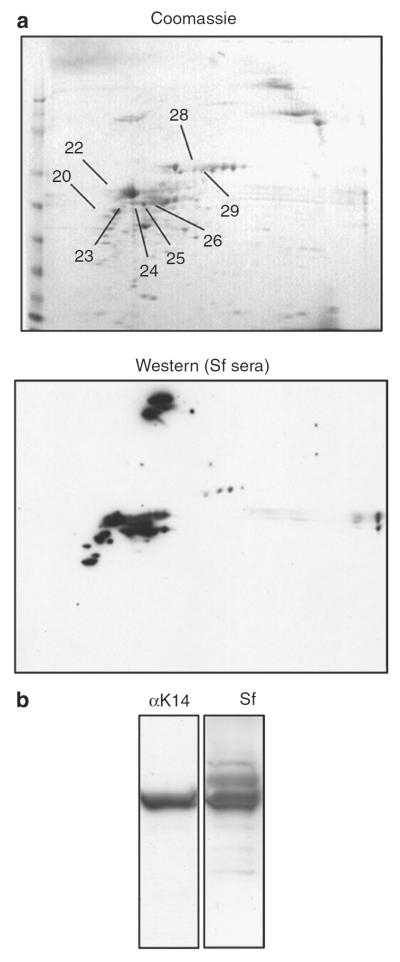
We resolved keratinocyte or whole-skin lysates using 2D SDS-PAGE, stained with pooled Scurfy sera, and subjected the spots reactive with Scurfy sera to protein identification using peptide mass fingerprinting (PMF) after tryptic digestion (Figure 4a). In both sets of experiments (keratinocyte and total skin lysate), most of the proteins identified were keratins with keratin 1, keratin 14, and keratin 2 yielding the highest scores (Figure 4a; Table 1). As human keratins are a common source of contamination, we also ran the resulting PMF against the human database; human keratins gave drastically lower scores than mouse keratins, strongly suggesting that the keratins identified are of mouse origin (data not shown).
Figure 4. Scurfy autoantibodies target keratins.
(a) Mouse ear lysate was subjected to 2D gel electrophoresis, followed by Coomassie stain (left) or western blot analysis using a pool of five Scurfy sera as primary antibody (right). The numbers on the Coomassie-stained gel refer to spots identified by PMF as shown in Table 1. (b) Total keratin was extracted from freshly isolated keratinocytes from newborn mice, separated by SDS-PAGE, and analyzed by western blot using either a monoclonal mouse anti-human keratin 14 antibody (LL001), which is cross-reactive to mouse keratin 14, or a Scurfy serum as primary antibody.
Table 1.
Results of protein identification
| Spot number | ID | Score1 | Number of peptides identified | Coverage (%) |
|---|---|---|---|---|
| 6 | Albumin (Mus musculus) | 1,262 | 29 | 48 |
| 9 | Striated-muscle α-tropomyosin | 296 | 7 | 27 |
| 11 | Keratin | 91 | 5 | 8 |
| 15 | ATP synthase β-subunit | 63 | 5 | 11 |
| 20 | Keratin 15; | 264 | 7 | 17 |
| Vimentin (Mus musculus) | 69 | 3 | 6 | |
| 22 | Keratin complex 1, acidic, gene 10 (Mus musculus) | 1,175 | 25 | 36 |
| 23 | Keratin 14 (Mus musculus) | 413 | 14 | 24 |
| 24 | Keratin 14 (Mus musculus) | 143 | 8 | 16 |
| 25 | Keratin 2 (Mus musculus) | 392 | 10 | 15 |
| Keratin 14 (Mus musculus) | 99 | 6 | 13 | |
| 26 | Keratin 2 (Mus musculus) | 68 | 3 | 5 |
| 28 | Keratin 2 (Mus musculus) | 152 | 6 | 9 |
| 29 | Keratin complex 2, basic, gene 17 (Mus musculus) | 135 | 6 | 10 |
Representative set of results of one protein identification of two with similar results is shown.
Probability-based Mowse score; in general, ion scores of 54 or more indicated identity or intensive homology (P<0.05).
Keratins comprise intermediate filaments that are expressed in epithelial cells, including keratinocytes (Lane and McLean, 2004; Moll et al., 2008). Keratin 14 is expressed mainly in the basal layer of the stratified epithelium in skin and as keratinocytes differentiate on their way to the stratum corneum, keratin 14 expression is reduced and finally lost (McMillan et al., 2003). In contrast, other keratins, such as keratin 10, are not found in basal keratinocytes, but their expression is upregulated during differentiation (Ishida-Yamamoto et al., 1992; Kartasova et al., 1993). We focused on keratin 14 for further analysis, because keratin 14 might be a dominant autoantigen not only important for skin disease, but also for inflammation developing in Scurfy liver and lung as both organs are affected in Scurfy mice and expression of keratin 14 in liver and lung has been reported (Bisgaard et al., 1994; Moll et al., 2008).
We extracted solubilized keratin filaments from mouse keratinocytes using urea (Steinert et al., 1979). Keratin 14 could be readily identified in this keratin extract as an ~50 kDa band when the lysate was probed with a mouse monoclonal anti-human keratin 14 antibody, which is known to cross-react with murine keratin 14 (Figure 4b, left). When Scurfy serum was used on the same keratin extract, a major band of approximately the same molecular weight (~50 kDa) was visible (Figure 4b, right), strongly suggesting that Scurfy serum also recognizes keratin 14.
Scurfy autoantibodies recognize keratin 14
To conclusively show that keratin 14 is targeted by Scurfy autoantibodies, we divided the mouse keratin 14 gene into three fragments of equal size (~480 bp each) and expressed them in a bacterial system. The C-terminal (no. 3) and middle fragments (no. 2) were expressed, but the N-terminal fragment (no. 1) was not visualized on the Coomassie stain (Figure 5a). The C-terminal fragment (no. 3) was reactive with a rabbit polyclonal antibody against a synthetic peptide from the C-terminal part of mouse keratin 14 (Figure 5b). Similarly, Scurfy sera also recognized the C-terminal fragment (no. 3) of keratin 14 (Figure 5c and d). Sera from nu/nu recipients that had been reconstituted with Scurfy CD4+ T cells recognized the C-terminal (no. 3) and middle fragment (no. 2), whereas littermate controls did not recognize any fragment (Figure 5d).
Figure 5. Scurfy sera recognize epitopes in the C-terminal fragment of keratin 14.
(a) Three protein fragments of keratin 14 were expressed and separated by SDS-PAGE, followed by Coomassie staining. (b) Fragments 1–3 were separated by SDS-PAGE, followed by western blot using a polyclonal rabbit anti-mouse keratin 14 antibody (AF64) or the corresponding isotype control as primary antibody. (c) The keratin 14 fragments were used either undiluted or at a 1:10 or 1:100 dilution for western blot with Scurfy serum as primary antibody. (d) The keratin 14 fragments were used for western blot as in (b), but with Scurfy, WT littermate, or nu/nu recipient serum as primary antibody. One representative experiment of at least three experiments with similar results is shown.
CD4+ T cells from Scurfy mice recognize keratins
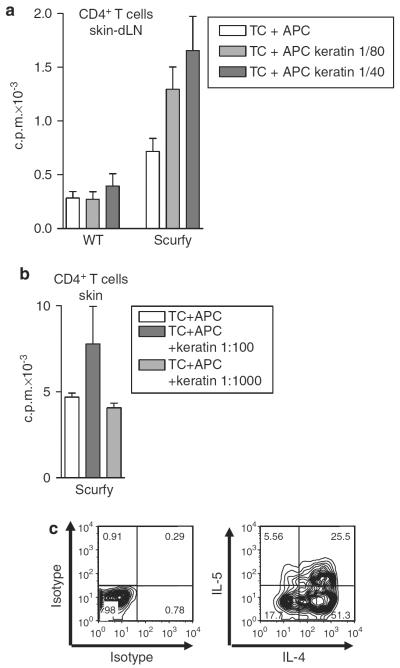
To show directly the presence of keratin-reactive T cells in Scurfy mice, we isolated CD4+ T cells from skin-draining lymph nodes of sick Scurfy mice and stimulated the cells in vitro with the keratin extract used in Figure 4b. Scurfy CD4+ T cells, but not CD4+ T cells from normal mice, proliferated in response to this extract (Figure 6a). We then sorted CD3+CD4+ T cells directly from inflamed skin and stimulated them with anti-CD3/anti-CD28 and IL-2 to increase cell numbers. After a resting phase in IL-2 alone, the expanded Scurfy skin cells also proliferated when challenged with the keratin extract (Figure 6b). When the expanded cells were restimulated with phorbol 12-myristate 13-acetate and ionomycin and analyzed for intracellular expression of cytokines, a high percentage produced Th2 cytokines, IL-4 and IL-5, which are characteristic of the atopic dermatitis-like disease in Scurfy skin (Figure 6c).
Figure 6. CD4+ T cells from Scurfy mice target keratins.
(a) CD4+ T cells were isolated from lymph nodes of Scurfy mice or normal controls and were stimulated in vitro with antigen-presenting cell (APC) in the absence or presence of different dilutions of keratin. Proliferation was measured after 4 days by 3H-thymidine uptake. Results are shown as mean±SD of triplicate wells. One representative experiment of three is shown. (b) CD3+CD4+ T cells were FACS-sorted from inflamed Scurfy ears, stimulated in vitro with plate-bound anti-CD3/anti-CD28, rested in IL-2 for 7 days, and restimulated in vitro with APC and keratin. Proliferation was measured as above. One representative experiment of two is shown. (c) The cells were restimulated for 4 hours with phorbol 12-myristate 13-acetate (PMA)/ionomycin and stained for intracellular cytokines.
IPEX patients harbor autoantibodies recognizing skin antigens
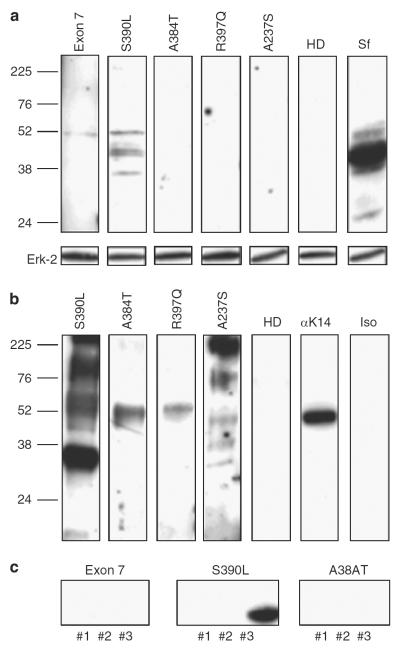
To determine if IPEX patients also develop autoantibodies against skin antigens, we used sera from four IPEX patients. We first analyzed the reactivity of the sera to murine keratinocyte lysate and found reactive autoantibodies in the serum of one patient (S390L) who had moderate eczema (Figure 7a). We then used a human keratin lysate for western blot analysis and found that the serum from the same patient (S390L), which was reactive to the murine lysate (Figure 7b), was strongly reactive with human keratin. Interestingly, the sera from the other patients, but not from four normal controls, also reacted with a protein of ~50 kDa, which may correspond to keratin 14 (Figure 7b; data not shown). To confirm that keratin 14 is contained in the human keratin lysate, we performed western blot analysis with a monoclonal anti-human keratin 14 antibody (Figure 7b). The serum of IPEX patient S390L was used to perform protein identification using PMF. Human keratin lysate was subjected to 2D gel electrophoresis followed by western blot analysis, using serum of patient S390L as primary antibody. The spots identified by PMF are shown in Table 2 and include keratin 14, keratin 10, and keratin 1 (Table 2). Keratin 14 and keratin 10 were found to yield the highest scores in the protein identification with Scurfy sera, indicating that the same antigenic targets are recognized. In the human protein identification, one spot (no. 1.4) matched keratin 14 from pan troglodytes. As there is a high degree of homology of troglodyte keratin to human keratin 14 (94%), it is likely that human keratin 14 is also a target. To conclusively demonstrate that keratin 14 is recognized by human autoantibodies, we analyzed the reactivity of the sera to the recombinant murine keratin 14 fragments. The middle fragment (no. 2) shows a high degree of homology with the human middle part of keratin 14, and the C-terminal fragment (no. 3) is 100% homologous to the C-terminal portion of human keratin 14. The N-terminal fragment was not expressed and again served as negative control. Because serum of IPEX patient S390L reacted strongly to bacterial proteins present in the recombinant fragment solution, we subjected the sera from IPEX patients to a preabsorption step on blotted pure Escherichia coli protein extract before using the sera as primary antibody for western blot analysis. The serum from IPEX patient S390L recognized the C-terminal fragment of keratin 14, whereas the other two sera tested did not show reactivity (Figure 7c).
Figure 7. Autoantibodies in sera from IPEX patients recognize skin antigens.
(a) Murine keratinocyte lysate was separated by SDS-PAGE, followed by western blot using sera of different immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) patients or of a healthy donor. Serum from a Scurfy mouse served as positive control. One experiment of two with similar results is shown. (b) Human keratin lysate was separated by SDS-PAGE, followed by western blot using sera of different IPEX patients. The two right lanes show staining with either mouse anti-human keratin 14 antibody (LL001) or isotype control. One experiment of four with similar results is shown. (c) After preabsorption of the diluted IPEX sera on Escherichia coli proteins blotted onto polyvinylidene difluoride membranes, the sera were used separately for western blot analysis on the different fragments of murine keratin 14.
Table 2.
Results of protein identification
| Spot number | ID | Score1 | Number of peptides identified | Coverage (%) |
|---|---|---|---|---|
| 1.3 | Keratin 10 (Homo sapiens) | 285 | 8 | 13 |
| Keratin 9 (Homo sapiens) | 180 | |||
| Keratin 1 (Homo sapiens) | 108 | |||
| 1.4 | Keratin, type I cytoskeletal 14 (cytokeratin-14) (Pan troglodytes) | 316 | 13 | 9 |
| Keratin 9 (Homo sapiens) | 300 | |||
| Keratin 10 (Homo sapiens) | 280 | |||
| 1.5 | Keratin 1 (Homo sapiens) | 265 | 7 | 10 |
| Keratin, type I cytoskeletal 14 (cytokeratin-14) (Pan troglodytes) | 245 | |||
| Keratin 9 (Homo sapiens) | 217 |
Probability-based Mowse score; in general, ion scores of 54 or more indicated identity or intensive homology (P<0.05).
DISCUSSION
Scurfy mice develop severe autoimmune disease and die between days 14 and 28 (Godfrey et al., 1991a, b). The skin is affected in 100% of Scurfy mice, but the target structures for the autoreactive T cells are not known. As an initial approach to identify the targets in the skin, we characterized the reactivity of autoantibodies that develop in Scurfy mice. We found that keratins were the main proteins recognized in the epidermis. The epitopes recognized by keratin-specific antibodies are likely to be linear and not conformational as the sera recognize both SDS-denatured and presumably native keratin in cryosections. As cryosections were carried out on normal ear skin, it is unlikely that they contain denatured keratin.
Keratins represent a family of intermediate filaments expressed in epithelia and typically form heteropolymeric filaments by pairing type I and type II keratin molecules (Moll et al., 2008). Keratin 14, which is usually expressed in the basal layer of stratified epithelia, has a major role in maintaining the stability of the skin (Troy and Turksen, 1999; Lane and McLean, 2004). Keratin 14 forms a heterodimer with keratin 5 and mutations in the genes encoding either one have been shown to account for a group of blistering diseases in humans, termed epidermolysis bullosa (Lane and McLean, 2004). In mice, the absence of keratin 14 also leads to severe blistering and finally death early in life (Troy and Turksen, 1999).
Given the functional importance of keratin 14 for maintenance of skin function, it seems reasonable that Treg cells might contribute to peripheral tolerance to this autoantigen. Our analysis of the skin inflammation developing in Scurfy mice strongly suggests that keratin 14 may be a target. First, the initial inflammatory infiltrate was localized in the epidermis and in some areas the dermoepidermal border was effaced (Figure 1a), consistent with a target antigen in the basal layer of the epidermis. Second, the autoantibodies in Scurfy sera recognize epidermal keratin 14 on western blot analysis of total skin, of keratinocyte lysates, and by reactivity with a fragment derived from recombinant keratin 14 (Figures 4 and 5). The autoantibodies themselves are not pathogenic, as transfer of serum from a sick Scurfy mouse does not induce disease in WT mice (data not shown). Scurfy mice also harbor skin-reactive T cells, as CD4+ T cells from both skin and draining lymph nodes proliferated specifically when stimulated with the bulk preparation of keratins. Further studies will be needed to define the fine specificity of CD4+ T cells present in Scurfy mice.
In human autoimmune skin diseases keratins might also be potential autoantigenic targets. Using a similar approach as we did, one study previously identified keratin 10-specific antibodies in sera of psoriasis patients by protein identification (El-Rachkidy et al., 2008). Another recent study reported anti-keratin autoantibodies in patients with rheumatoid arthritis (Quismorio et al., 2008). In this study, we identified different keratins as antigenic targets for the autoantibodies in sera from IPEX patients. Two of the IPEX patients tested had skin disease and the serum from one patient, who had moderate eczema (S390L), showed strong reactivity against skin antigens, including keratin 14. Thus, our identification of keratin 14 as an antigenic target should facilitate the analysis of the specificity of autoreactive T cells in human skin diseases and thereby provide potential impact on future therapeutic strategies.
MATERIALS AND METHODS
Mice
Female heterozygous B6.Cg-Foxp3sf/J (Scurfy) mice were purchased from Jackson Laboratories (Bar Harbour, ME) and bred to C57BL/6 WT male mice to generate hemizygous male B6 (Cg-Foxp3sf/Y (Scurfy) offspring); C57BL/6 WT male littermates were used as controls. C57BL/6 RAG−/− mice and C57BL/6 nu/nu mice were purchased from Taconic (Rockville, MD). All mice were held under specific pathogen-free conditions and cared for in accordance with guidelines approved by the NIAID Animal Care and Use Committee.
Antibodies and reagents
Monoclonal anti-keratin 14 antibody (clone LL001) and the corresponding isotype control (mouse IgG2a) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit polyclonal antikeratin 14 antibody (AF64) was purchased from Abcam (Cambridge, MA).
Histology
Ears were fixed in 4% neutral buffered formalin. Fixed tissues were embedded in paraffin and stained with hematoxylin and eosin by American Histolabs (Gaithersburg, MD).
Immunohistochemistry
Back skin of C57BL/6 RAG−/− mice was snap-frozen in OCT compound embedding medium (Tissue-Tek, Sakura Finetek, Torrance, CA). Sections were cut on a cryostat, air-dried, fixed in ice-cold acetone, and stored at −20 °C until further use. The sections were rehydrated two times, and blocked with 10% goat serum in 5% skim milk powder in phosphate-buffered saline (PBS) at room temperature (RT). Slides were incubated with Scurfy or WT serum at a 1:70 dilution for 1 hour at RT, washed three times for 5 minutes with PBS and incubated with goat anti-mouse IgG Alexa Fluor 488 (Invitrogen, Carlsbad, CA) 1:500 in PBS for 1 hour at RT in the dark. After washing three times for 5 minutes with PBS, slides were mounted with ProLong Gold antifade reagent with DAPI counterstain (Invitrogen).
Keratin isolation
Keratinocytes were isolated from newborn C57BL/6 mice as previously described (Lichti et al., 2008). The keratin intermediate filaments were isolated using 8 m urea as previously described (Steinert et al., 1979). Keratin solution from human epidermis was purchased from Sigma-Aldrich (St Louis, MO).
Western blot
Ears from C57BL/6 RAG−/− mice or freshly isolated keratinocytes from 1-day-old C57BL/6 mice were homogenized on ice in lysis buffer containing 5 mm Tris (pH 8.0), 150 mm NaCl 1% NP-40, and protease inhibitor cocktail (Roche, Indianapolis, IN). The lysates were spun for 10 minutes at 4 °C at 12,000 r.p.m. in an Eppendorf table-top centrifuge and supernatants were stored at −80 °C. Samples were diluted in NuPAGE 4× sample buffer (Invitrogen) supplemented with 5% β-mercaptoethanol, boiled for 5 minutes at 95 °C, put on ice for 1 minute, spun down, separated by 10 or 12% SDS-PAGE gels, and blotted onto Hybond-ECL nitrocellulose membranes (Amersham, Piscataway, NJ). The membrane was blocked (5% skim milk in PBS 0.1% Tween 20) for 1 hour at RT with shaking, followed by incubation in block with either Scurfy or WT serum or IPEX patient or healthy control serum overnight at 4 °C with shaking. After washing five times in PBS 0.1% Tween 20, the membrane was incubated with horseradish-peroxidase-conjugated sheep anti-mouse IgG (dilution 1:10,000; Amersham), horseradish-peroxidase-conjugated sheep anti-rabbit IgG (dilution 1:10,000; Amersham), or horseradish-peroxidase-conjugated goat anti-human IgG(γ) (dilution 1:5,000; Invitrogen) for 1 hour at RT, washed five times in PBS 0.1% Tween 20, and developed with enhanced chemiluminescence reagent (Pierce, Rockford, IL) and autoradiography (Amersham).
Protein identification by mass spectrometry
Keratinocyte or total skin lysates were separated on 2D SDS-PAGE gels. Protein identification of 2D gel-separated proteins was performed on reduced and alkylated, trypsin-digested samples prepared by standard mass spectrometry protocols. Tryptic digests were analyzed by coupling the Nanomate (Advion BioSciences, Ithaca, NY), an automated chip-based nanoelectrospray interface source, to a quadrupole time-of-flight mass spectrometer, QStar XL MS/MS System (Applied Biosystems/Sciex, Foster City, CA). Computer-controlled data-dependent automated switching to MS/MS provided peptide sequence information. AnalystQS software (Applied Biosystems/Sciex) was used for data acquisition. Data processing and databank searching were performed with Mascot software (Matrix Science, Boston, MA). The NCBInr protein database from The National Center for Biotechnology Information, NLM/NIH was used for the search analysis.
Statistics
Ion score was −10×log(P), where P is the probability that the observed match is a random event. Individual ions scores ≥54 indicate identity or extensive homology (P<0.05). Protein scores are derived from ions scores as a nonprobabilistic basis for ranking protein hits.
Keratin 14 expression
A vector with the complete mouse keratin 14 cDNA was purchased from OriGene (Austin, TX). The following primer pairs were used to amplify three ~480 bp cDNA fragments encoding for the N-terminal, middle, and C-terminal parts of keratin 14: primer 1 (forward), TGCACGGCTAGCGCCACCTGCAGCCGCCAGTTCACCTCCTCCAGC; primer 2 (reverse), TGCACGAAGCTTTTAGTCTTTGATCTCAGTGGGCCGCTGCCTCTG; primer 3 (forward), TGCACGGCTAGCTACCAGAGGCAGCGGCCCACTGAGATCAAAGAC; primer 4 (reverse), TGCACGAAGCTTTTACTTGCCGCTCTGCACCAGCTCGCTGTTGGT; primer 5 (forward), TGCACGGCTAGCGCCACCAACAGCGAGCTGGTGCAGAGCGGCAAG; primer 6 (reverse), TGCACGAAGCTTTTAGTTCTTGGTGCGCAGGACCTGCTCGTGGGT.
The PCR fragments were cloned into the pET-21b vector (Novagen, Darmstadt, Germany) and used to transform BL21 (DE3)-competent cells (Novagen). Bacterial expression was induced by addition of 1 m IPTG to bacterial cultures. After 2.5 hours, bacteria were pelleted and lysed with lysis buffer (BUGBuster Protein Extraction Reagent+Benzolase+Lysozyme; all from Novagen). After additional centrifugation, the pellet containing insoluble inclusion bodies was washed twice in TE and stored at −20 °C until further use.
Cell isolation and transfer
CD4+ T cells were isolated from lymph nodes through the positive-select program on AutoMACS (Miltenyi, Auburn, CA). The cells were washed, resuspended in PBS, and injected i.v. into either C57BL/6 RAG−/− or C57BL/6 nu/nu recipients. Total lymph node and spleen cells from 10- to 14-day-old Scurfy mice were pooled, washed, resuspended in PBS, and injected i.v. into C57BL/6 RAG−/− recipients.
T-cell proliferation assays
T cells were magnetically sorted with CD4 microbeads (Miltenyi) or were FACS-sorted. T cells were stimulated with irradiated (3,000 rad) T-depleted WT splenocytes in the absence or presence of the indicated dilutions of keratin extract. Staining for intracellular cytokines was performed as previously described (Stummvoll et al., 2008).
ACKNOWLEDGMENTS
We thank Dr U. Lichti, Dr J. Anders, Dr S. Yuspa, and Dr M.C. Udey for helpful discussions. We also thank Dr C. Hammer, Dr M.A. Robinson, Dr M. Zhao, and Dr R. Martin (Mass Spectrometry Laboratory, Protein Biochemistry Section at the National Institute of Allergy and Infectious Diseases) for the help with protein identification. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Abbreviations
- IPEX
immunodysregulation, polyendocrinopathy, enteropathy, X-linked
- PMF
peptide mass fingerprinting
- Sf
scurfy
- Treg
regulatory T cells
Footnotes
CONFLICT OF INTEREST The authors state no conflict of interest.
REFERENCES
- Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Bisgaard HC, Nagy P, Ton PT, et al. Modulation of keratin 14 and alpha-fetoprotein expression during hepatic oval cell proliferation and liver regeneration. J Cell Physiol. 1994;159:475–84. doi: 10.1002/jcp.1041590312. [DOI] [PubMed] [Google Scholar]
- El-Rachkidy RG, Young HS, Griffiths CE, et al. Humoral autoimmune responses to the squamous cell carcinoma antigen protein family in psoriasis. J Invest Dermatol. 2008;128:2219–24. doi: 10.1038/jid.2008.71. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Gambineri E, Perroni L, Passerini L, et al. Clinical and molecular profile of a new series of patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: inconsistent correlation between forkhead box protein 3 expression and disease severity. J Allergy Clin Immunol. 2008;122:1105–12. e1. doi: 10.1016/j.jaci.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Gavin MA, Rasmussen JP, Fontenot JD, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–5. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- Godfrey VL, Wilkinson JE, Rinchik EM, et al. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci USA. 1991a;88:5528–32. doi: 10.1073/pnas.88.13.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey VL, Wilkinson JE, Russell LB. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol. 1991b;138:1379–87. [PMC free article] [PubMed] [Google Scholar]
- Halabi-Tawil M, Ruemmele FM, Fraitag S, et al. Cutaneous manifestations of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Br J Dermatol. 2009;160:645–51. doi: 10.1111/j.1365-2133.2008.08835.x. [DOI] [PubMed] [Google Scholar]
- Huter EN, Punkosdy GA, Glass DD, et al. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–21. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, McGrath JA, Judge MR, et al. Selective involvement of keratins K1 and K10 in the cytoskeletal abnormality of epidermolytic hyperkeratosis (bullous congenital ichthyosiform erythroderma) J Invest Dermatol. 1992;99:19–26. doi: 10.1111/1523-1747.ep12611391. [DOI] [PubMed] [Google Scholar]
- Kartasova T, Roop DR, Holbrook KA, et al. Mouse differentiation-specific keratins 1 and 10 require a preexisting keratin scaffold to form a filament network. J Cell Biol. 1993;120:1251–61. doi: 10.1083/jcb.120.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane EB, McLean WH. Keratins and skin disorders. J Pathol. 2004;204:355–66. doi: 10.1002/path.1643. [DOI] [PubMed] [Google Scholar]
- Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- McMillan JR, Akiyama M, Shimizu H. Epidermal basement membrane zone components: ultrastructural distribution and molecular interactions. J Dermatol Sci. 2003;31:169–77. doi: 10.1016/s0923-1811(03)00045-8. [DOI] [PubMed] [Google Scholar]
- Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129:705–33. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs HD, Gambineri E, Torgerson TR. IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol Res. 2007;38:112–21. doi: 10.1007/s12026-007-0022-2. [DOI] [PubMed] [Google Scholar]
- Quismorio FP, Jr, Kaufman RL, Beardmore T, et al. Reactivity of serum antibodies to the keratin layer of rat esophagus in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:S69–74. doi: 10.1002/art.23360. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Steinert PM, Idler WW, Poirier MC, et al. Subunit structure of the mouse epidermal keratin filament. Biochim Biophys Acta. 1979;577:11–21. doi: 10.1016/0005-2795(79)90003-5. [DOI] [PubMed] [Google Scholar]
- Stummvoll GH, DiPaolo RJ, Huter EN, et al. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J Immunol. 2008;181:1908–16. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy TC, Turksen K. In vitro characteristics of early epidermal progenitors isolated from keratin 14 (K14)-deficient mice: insights into the role of keratin 17 in mouse keratinocytes. J Cell Physiol. 1999;180:409–21. doi: 10.1002/(SICI)1097-4652(199909)180:3<409::AID-JCP12>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Wildin RS, Freitas A. IPEX and FOXP3: clinical and research perspectives. J Autoimmun. 2005;25(Suppl):56–62. doi: 10.1016/j.jaut.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–45. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]