Abstract
The role of subcortical structures in language function is complex and dependent on language task, with studies increasingly showing subcortical involvement for the production of formulaic language, including recited speech. Individuals with Parkinson's disease (PD), with (n = 6) and without (n = 7) surgical treatment, deep brain stimulation (DBS), were compared to healthy adults (n = 14) to determine whether individuals with subcortical dysfunction produce more errors during a recitation speech task. Participants were asked to recite poems, prayers, and rhymes familiar to them in order to determine the effects of subcortical disease on recited speech ability. When compared with healthy controls, the DBS-OFF group produced significantly more error words, suggesting that deficits in recitation arise with severe states of subcortical dysfunction. Individuals with DBS in the ON or OFF conditions did not differ significantly during the recited speech task. Results support a model of language where large units of overlearned language are at least partially modulated by subcortical structures.
Keywords: Parkinson's disease, Dual-process model, Recited speech, Formulaic language
1. Background
Neural organization and allocation of speech and language function have long been attributed to cortical structures in the left cerebral hemisphere; however, current research provides additional support for subcortical and right-hemisphere involvement in specific aspects of verbal communication, including those reflecting pragmatic competence. John Hughlings Jackson (1874/1958) described two forms of speech, propositional and non-propositional, that are differentially affected by aphasia. The study of non-propositional or automatic speech was seemingly overshadowed throughout much of the 20th century by the generative grammar movement (Chomsky, 1957, 1965) and emphasis was placed on novel, newly created utterances (Van Lancker, 1975). However, cases were described by aphasiologists through the mid-to-late 20th century that showed selective preserved production of automatic speech (e.g., yes/no, counting, reciting the days of the week), fixed or formulaic expressions (e.g., yes, hello, well and you know, pause fillers including uh and um), and even longer units of overlearned language (e.g., songs, poems, rhymes, quotations) following damage to the left cortical regions from stroke or hemispherectomy (see Riese, 1949; as cited in Critchley, 1967; Critchley, 1970; Espir & Rose, 1970; Smith, 1966; Smith & Burklund, 1967; Zangwill, 1967). The selective preservation of these overlearned structures, formulaic as well as recited language, following damage to the left-cerebral hemisphere suggests the role of the right-hemisphere and, likely, subcortical regions in the production of at least some forms of language (Code, 1982; Espir & Rose, 1970; Van Lancker Sidtis, 2004, 2006, 2012; Wray & Perkins, 2000; Zangwill, 1967). This evidence of preserved overlearned language units following left-hemisphere damage suggests a dual-process model of language production, whereby formulaic language production is modulated by a subcortical-right hemisphere circuit and novel language is modulated by the left-hemisphere (Sidtis, Canterucci, & Katsnelson, 2009; Van Lancker Sidtis, 2004; Wray & Perkins, 2000). Based in current theories, this work is motivated by a desire to discover whether longer units of overlearned linguistic material, namely recited speech, also fall under the purview of subcortical structures, thereby providing further evidence for the dual process model of language production.
The involvement of subcortical structures in language production can be examined through the study of individuals with selective neural damage to or preservation of functional areas of the brain (e.g., cortical and subcortical stroke, Alzheimer's disease, Parkinson's disease). Recitation speech is thought to be modulated by subcortical structures, such as the basal ganglia, and can be examined by studying patients with Parkinson's disease, who have subcortical dysfunction. By examining performance in individuals with Parkinson's disease, more can be learned about the nature of the neural control of this type of routinized language task.
1.1. Idiopathic Parkinson's disease
Idiopathic Parkinson's disease (PD) is a progressive neurodegenerative disease caused by deterioration of the substantia nigra in the midbrain. This deterioration causes upstream imbalances in the functioning of subcortical and cortical areas, with specifically devastating effects in the complex pathways of the basal ganglia (Bhatnagar, 2008; Graybiel, 1996). Impairments due to dopaminergic imbalances in the basal ganglia result in heterogeneous patient profiles of symptoms associated with PD, but some symptoms are commonly observed. These symptoms can include bradykinesia (slowed movements), akinesia (reduced initiation of movements), disturbances in gait, cogwheel rigidity, resting tremor, and hypokinetic dysarthria (Duffy, 2012).
1.2. Involvement of subcortical structures in formulaic language
A common language symptom observed with various types of subcortical dysfunction is a sparseness of formulaic language (see below). Current thought indicates that formulaic language falls under the pragmatic domain of language, as it consists of commonly used phrases that aid in pragmatic aspects of communication, and it has been proposed to be modulated in part by a subcortical-right-hemisphere circuit (Sidtis et al., 2009; Van Lancker, 1987; Van Lancker Sidtis, 2004, 2006, 2012).
Sidtis et al. (2009) studied the production of formulaic language in the spontaneous speech of individuals with focal basal ganglia stroke and compared it to individuals with left- and right-hemisphere stroke, as well as healthy controls. They found that individuals with basal ganglia stroke had significantly fewer formulaic expressions in their spontaneous speech than individuals with left-hemisphere damage and healthy adults, implicating the basal ganglia (and right-hemisphere) in formulaic language production. Clinical observations of aphasia (language impairment due to left-hemisphere cortical damage) and research on the language of people with Alzheimer's disease (Bridges & Van Lancker Sidtis, 2013) also support the importance of intact subcortical structures in the production of fixed and formulaic expressions, as these are frequently preserved when novel language is impaired. Illes, Metter, Hanson and Iritani (1988) studied the spontaneous speech of healthy adults and those with Parkinson's disease and found that individuals with Parkinson's disease had fewer modals and interjections (e.g., “Ah,” “Well”), which are types of formulaic expressions (see Sidtis et al., 2009). Additionally, preliminary findings of formulaic language in Parkinson's disease indicate reduced frequency in spontaneous speech (Rogers, Sidtis & Sidtis, 2009).
While there is some evidence of subcortical involvement in the production of formulaic language, given preservation with left cortical damage and reductions in subcortical impairment, the role of subcortical structures in the memorization and recitation of larger units of language (recited speech) has only been sparsely investigated. Recitation ability is an aspect of linguistic competence of general interest to language users. The production of lengthy, overlearned, unitary expressions is observed in many cultures of the world with both oral and written language traditions (Hunter, 1985). Nursery rhymes are long overlearned units of language that are recited to children and learned at very early ages. Sufficient knowledge of these memorized rhymes is related to children's later phonological and reading skills (Bryant, Bradley, Maclean & Crossland, 1989). Even into adulthood, knowledge of long recited speech units is maintained and in the population of individuals with neurological disease, the production of these unitary expressions may or may not be affected depending on location of damage (cortical versus subcortical). Often, clinical evaluation of language function includes in part, recitation of overlearned material (e.g., rhymes and songs). Stahl, Kotz, Henseler, Turner and Geyer (2011) studied adults with non-fluent aphasia on the production of recitation material (German nursery rhymes), formulaic language, and novel speech. They found that the recited materials, whether sung or spoken, were more accurately produced than the production of formulaic language or novel speech (Stahl et al., 2011), implicating the role of non-damaged neural regions (cortical and subcortical right-hemisphere). In a case study of a man who had stroke in the caudate nucleus of the basal ganglia, Speedie, Wertman, Ta'ir, & Heilman (1993) provide some evidence for subcortical control of this language function. Poststroke, this individual had a significantly impaired ability to say prayers that had been memorized and recited since childhood despite largely intact function on other language tasks (Speedie et al., 1993). In a case study of a woman with a left-temporal lobe tumor, Shinoura et al. (2010) described an increase in the spontaneous production of Sutra, a Buddhist prayer, and deficits in other aspects of language as the neoplastic condition worsened. Researchers performed a Wada test, where the woman's left-hemisphere was ‘inactivated’ by injecting propofol into the left internal carotid artery, which induced recitation of Sutra (Shinoura et al., 2010). Shinoura and colleagues attribute Sutra production to right-hemisphere cortical function, although it may be the case that right-hemisphere cortical as well as subcortical structures were involved.
1.3. Deep brain stimulation and PD
Although pharmacological (levadopa) treatments have been available for PD for several decades and ameliorate symptoms, they do not halt the disease progression (Kent, Duffy, Slama, Kent, & Clift, 2001) and they lead to serious side effects. Electrical stimulation of brain structures has been used in research since the early 1900s and was a method of determining function localization prior to neural surgery (Schaltenbrand, 1965). Schaltenbrand described the effects of exploratory electrical stimulation of subcortical nuclei on behaviors in individuals with parkinsonian syndrome. In recent years, neurosurgical techniques (i.e., lesioning, brain stimulation) have returned as a favored treatment of Parkinson's disease, with deep brain stimulation (DBS) having the most promising results in symptom management and in possibly halting disease progression (Benabid, Chabardes, Mitrofanis, & Pollak, 2009). DBS was initially believed to act as a ‘reversible lesion, ’ due to the ability for the stimulation to be turned on and off (Volkmann, 2004). When in the ON condition, the stimulators have a lesion effect whereby the neural region in which it is implanted is in a sense ‘inactivated. ’ However, recent evidence suggests a more complicated mechanism (Benabid et al., 2009; Vitek, 2008) that is characterized by autonomic changes that affect global cerebral blood flow (Sidtis et al., 2012). Although DBS has targeted various nuclei, DBS of the subthalamic nucleus appears to be the optimal site for many individuals with PD in symptom attenuation. The goal of DBS of the subthalamic nucleus is to inactivate inhibitory function thereby allowing the remaining pathways to function, despite reduced activation from the degenerated substantia nigra (McIntyre, Savasta, Kerkerian-Le Goff, & Vitek, 2004; Trost et al., 2006). Despite great success in reducing tremor, rigidity, and hypokinesia (Henderson & Dunnett, 1998), there may be a disconnect between positive outcomes for speech and nonspeech movements (see Kent et al., 2001). Clinical anecdotes, preliminary evidence, and patient reports suggest speech may not improve or may even worsen post-DBS in some people. However, any effects of DBS on language function, and specifically on the production of fixed/formulaic expressions or longer recited units of language, are even less well understood.
In summary, it is known that in addition to gross and fine motor function, subcortical structures have a prominent role in speech function, as evidenced by hypokinetic dysarthria characteristic of Parkinson's patients. However, the role of subcortical structures in language production is only recently emerging as a contributor, being especially prominent in automatic and formulaic language tasks (as evidenced by individuals with damage due to subcortical stroke). The role of deep brain stimulation in the speech and language function of people with Parkinson's disease is of increasing interest, as there are anecdotal reports of negative communicative outcomes secondary to DBS.
1.4. Aims and hypotheses
The aim of this exploratory study was to determine the role of subcortical structures in a specific form of non-novel language, recited speech, by studying individuals with Parkinson's disease, and to explore the potential differential effects of the surgical procedure, deep brain stimulation, on recitation ability. The study was guided by these questions: What is the extent of subcortical involvement in the production of recited speech items, and will recitation in individuals with Parkinson's disease, who are treated with DBS, differ as a function of stimulation state (ON vs. OFF)? With respect to the first question, as noted earlier, there is evidence of subcortical involvement in the production of formulaic language (reduced incidence in patients with basal ganglia stroke and preserved in aphasic and Alzheimer patients), and it is postulated that recitation speech (e.g., prayers, poems, rhymes) may be considered larger segments of formulaic expressions. It is expected that individuals with Parkinson's disease (without DBS, DBS-OFF and DBS-ON) will produce more error words during recitation. If this were observed, it would provide further evidence for the role of the basal ganglia in the production of overlearned, routinized language (i.e., automatic speech, formulaic language), but it would go a step farther in showing that the basal ganglia is involved in the production of even larger segments of overlearned verbal information. The secondary question, determining whether there are differential effects of DBS on recited speech, is exploratory in nature, as it is unknown what the language effects are with DBS, and speech effects are variable. By studying DBS-ON and -OFF conditions, it may be possible to extend our knowledge of the effects of this therapy from motor speech control to language use, and to provide additional information about risks and benefits of DBS on language function (specifically routinized language) to individuals contemplating undergoing this treatment. Additionally, it could also contribute to further clarification of the nature of the DBS mechanism on different types of language function.
2. Method
2.1. Participants
Recitation speech samples were collected from thirteen individuals with idiopathic Parkinson's disease (9 male, 4 female, mean age = 60.85). Six of these individuals (all male) diagnosed with idiopathic Parkinson's disease were treated by the surgical procedure, deep brain stimulation (DBS; mean age = 58.17 years). Seven individuals (3 male, 4 female) diagnosed with idiopathic Parkinson's disease were not treated with DBS (PD; mean age = 63.14 years). Fourteen healthy consecutive volunteers with no history of neurological disease (HC; 6 male, 8 female, mean age = 55.50 years) participated and served as a control group in this study. All participants were right-handed, native English-speaking adults from the Northeastern United States, who were recruited as participants from a larger umbrella study. Individuals with DBS were all treated by the same surgical team who implanted the deep brain stimulators in the subthalamic nuclei, bilaterally. All PD participants underwent a clinical examination by a neurologist to assure the accuracy of Parkinson's diagnoses. Informed consent was obtained from all participants and all elements of this study adhered to the guidelines of ethical research.
Demographic information regarding age, years of education, relevant medical history, time since PD diagnosis (for DBS and PD groups), and duration of DBS therapy is provided in Table 1. Individuals with PD (no DBS), those with DBS, and HC participants had a mean of 15.86, 16.17, and 16.00 years of education, respectively. For DBS participants, recitation samples were collected on two separate occasions within one week of each other: once with the stimulators turned on and once turned off. DBS participants had a mean of 17 months with DBS. For all individuals with Parkinson's disease (DBS-ON, DBS-OFF and PD groups), the motor section of the Unified Parkinson's Disease Rating Scale (UPDRS) (Fahn, Elton & UPDRS Program Members, 1987) was administered. The UPDRS is a scale used to rate the symptom severity of individuals with Parkinson's disease. It integrates parts of other scales to provide a cohesive clinical rating of disease progression. The UPDRS scale ranges from 1 to 30, with scores from 1 to 10 indicating mild impairment, 11-20 moderate impairment and 21-30 severe or advanced impairment. Individuals with PD had a mean UPDRS score of 20.50. For individuals with DBS, UPDRS was administered twice. The mean UPDRS score for DBS-OFF was 27.83 and for DBS-ON was 18.08, indicating reduction in negative symptoms in the ON condition. For all means and standard deviations for all demographic data, see Table 1.
Table 1.
Mean age, years of education, years since diagnosis, UPDRS score, and duration (in months) since implantation for participant groups: PD-DBS, PD (without DBS), and HC.
| PD with DBS (n = 6) | PD without DBS (n = 7) | Total PD (n = 13) | HC (n = 14) | ||
|---|---|---|---|---|---|
| Age | 58.17 (range: 49–62) | 63.14 (range: 44–73) | 60.85 (range: 44–73) | 55.50 (range: 36–72) | |
| Education | 16.17 (SD = 1.60) | 15.86 (SD = 2.12) | 16.00 (SD = 1.83) | 16.00 (n = 13) (SD = 1.87) | |
| Years since diagnosis | 12.67 (SD = 3.45) | 10.29 (SD = 3.09) | 11.38 (SD = 3.36) | – | |
| UPDRS score | Off 27.83 (SD = 13.65) |
On 18.08 (SD = 9.95) |
20.50 (SD = 6.63) | 23.88 (SD = 10.68) | – |
| Duration of DBS (months) | 17.00 (SD = 22.11) | – | – | ||
| Levadopa dosage (mg) | 516.67 (n = 6, SD = 132.92) | 550.00 (n = 2, SD = 212.13) | – | ||
2.2. Procedure
Participants were asked to recite various rhymes, poems and prayers (henceforth “recitation speech”) during a clinical speech and language exam. The recitation speech samples were elicited by a trained speech and language researcher who informed the participants that they would be asked to recite some childhood nursery rhymes, poems or prayers as best they could. Recited items included Humpty Dumpty, Twinkle Twinkle Little Star, Mary had a Little Lamb, Jack and Jill, Roses are Red, Sticks and Stones, The Lord's Prayer, and The Pledge of Allegiance. All participants endorsed familiarity with the particular items chosen for recording. Participants were audio-recorded while producing the recited speech items.
The audio recordings were transcribed by a native English-speaking graduate student by recording all words said during the task (by both participants as well as examiners), including pause fillers, “Uh” and “Um” as well as interjections, “Oh I don't know.” A second native English-speaking graduate student verified the accuracy of the transcriptions and conferred with the initial transcriber concerning any discrepancies. There were very few instances of discrepancies in the transcriptions, and in such cases, both transcribers came to a mutual agreement to determine the final accepted transcript.
2.3. Data analysis
Recited speech samples from twenty-seven individuals (6 with DBS, 7 without DBS, 14 healthy adults) were analyzed. The individuals with DBS provided recitation samples twice: once in the ON condition and once in the OFF condition. Only recited items that were spoken were included for analysis; That is, two individuals (1 DBS and 1 PD) produced “Twinkle Twinkle Little Star” as a song, and thus this item was excluded from analysis. Participants recited between 2 and 8 items with total recited word counts ranging from 53 to 291 words per individual. As the total number of recited items varied (and therefore total word counts), percentages were used for all comparisons.
A determination was made of the exact words comprising the target rhyme, prayer or poem by obtaining transcripts through internet searches of the various items. The transcription chosen as the target was one agreed upon by two native-English speaking researchers from the United States who determined that the target was accurate for the culture. As some recited items have different versions (e.g., “Twinkle, Twinkle Little Star,” “Mary had a Little Lamb” and “The Lord's Prayer”), participants varied in the number of verses and versions produced. Therefore, the personally familiar target was determined for each participant and used for comparison to each person's actual production. The target number of words for “Twinkle Twinkle Little Star” ranged from 21 to 32 words (with the majority of participants' intended targets at 22 words), “Mary had a Little Lamb” ranged from 18 to 59 words (with the majority of participants' intended targets at 22 words), and “The Lord's Prayer” ranged from 53 to 71 words (with the majority of participants' intended targets at 56 words). Additionally, some target transcriptions had alternate versions (e.g., Sticks and stones may break my bones but NAMES/WORDS will never HURT/HARM me). Individuals were given credit for correct productions including alternate versions after two consultants and internet searches verified that the variation was acceptable to the target form for the culture. Ultimately, the use of percentages for comparisons accounted for differences in verses and versions.
Once the target transcript for each recited item was identified, data were analyzed by determining the total number of words corresponding to the speech target, and the total number of non-target words. Then from these non-target words, which included interjected words (e.g., “I don't know”), pause fillers, and repetitions of correct words, the number of error words were determined per recited item.
The number of error words produced was recorded for each recited item. Error words included incorrect words added to the recitation (e.g., incorrect verses) and incorrect word substitutions (e.g., “Jack and MARY went up the hill to fetch a pail of water”) that did not qualify as acceptable variations of the target. Percentages were obtained for the number of wrong words out of the total number of non-target words produced for each recited item. Means were then obtained for each participant, and for each of the three participant groups to use for comparisons.
3. Results
3.1. Demographic comparisons
Comparisons were made between the three groups (DBS, PD, and HC) for age, education and gender. One-way ANOVAs revealed that age and education did not differ significantly between the three participant groups. A chi-square revealed a significant gender by subject group association [Pearson X2(df = 2) = 6.17, p = .046], as the DBS group consisted of only males, and the PD and HC groups consisted of males and females (see Table 1).
Additional demographic comparisons were made between the DBS and PD groups for mean years since diagnosis with PD and mean levadopa dosage. Significant differences were not found between PD and DBS for years since PD diagnosis, or mean levadopa dosage.
Finally, within the DBS group, the DBS-ON and DBS-OFF UPDRS scores were compared to each other and to the PD group. Independent samples t-tests failed to reveal significant differences between the DBS-OFF and PD group and the DBS-ON and PD group for UPDRS scores. However, a paired samples t-test revealed significant differences in UPDRS scores for the DBS-OFF and DBS-ON conditions [t(5) = 2.90, p = .034], where the DBS-OFF condition had significantly higher UPDRS scores (M = 27.83) than the DBS-ON condition (M = 18.08), indicating a higher degree of motor dysfunction in the DBS-OFF condition, with the non-DBS PD group falling between the two (M = 20.50). The lowered UPDRS scores in the DBS-ON condition further indicate the therapeutic benefits on motor function.
3.2. Effects of subcortical disease & DBS
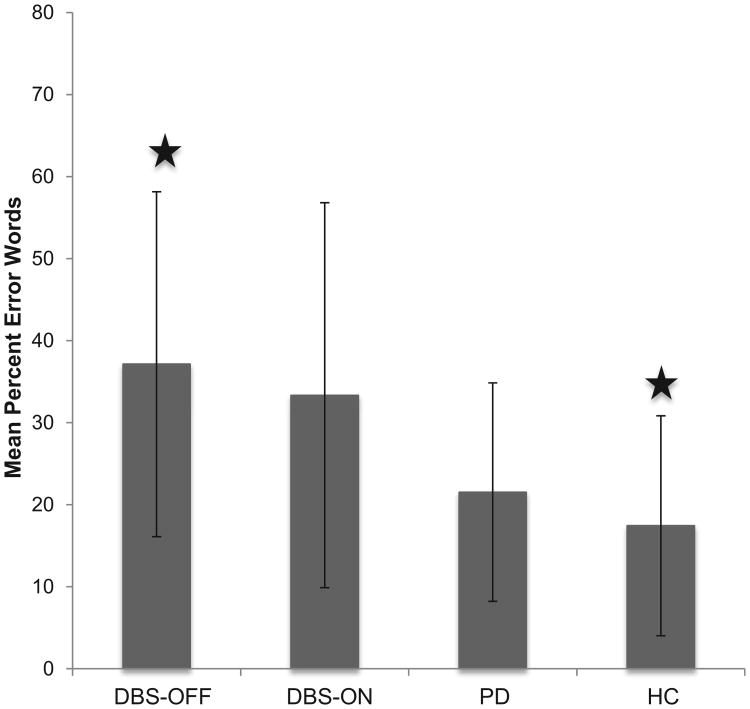
In order to determine the role of subcortical structures in recited speech, individuals with PD [with (n = 6) and without DBS (n = 7)] were compared with healthy controls (n = 14) on the percentage of error words produced during the task. Individuals with DBS-OFF produced an average of 37.13% error words (SD = 23.47), and when in the ON condition, they had an average of 33.34% (SD = 21.03) error words. A paired-samples t-test indicated differences were not significant between the DBS-OFF and DBS-ON conditions. The PD group produced an average of 21.53% error words (SD = 13.31), while the HC group had 17.44% error words (SD = 13.41) during the task. A one-way ANOVA comparing DBS-OFF, PD, and HC revealed a significant moderate effect [F(2,24) = 3.52, p = .046, ]. Post-hoc analysis using Tukey's HSD found that the individuals with DBS-OFF produced significantly more error words during the task than the HC group (p = .037). The PD participants performed as an intermediate group between the DBS-OFF and the HC, but the differences with either group did not reach significance (refer to Fig. 1).
Fig.1.
Mean percent error words produced by individuals with PD DBS-OFF, DBS-ON, without DBS (PD) and HC. Error bars represent standard deviation. Significant differences were found between DBS-OFF and HC.
4. Discussion
4.1. General discussion
To evaluate whether basal ganglia function is related to language ability during recitation speech tasks, the DBS group (in the ON and OFF conditions) was compared to PD participants and healthy controls. It was expected that individuals with subcortical disease in general would perform more poorly during recitation speech than healthy controls (as evidenced by more errors during the task), because recitation is hypothesized to be at least to some extent subcortically modulated. Data partially support this hypothesis: individuals with DBS in the OFF condition, those with the most severe disease state as reflected in motor deficits (evidenced by the lowest UPDRS scores), had significantly more error words out of their non-target words than healthy control participants. These results provide further support for the involvement of intact subcortical structures on successful recitation ability of overlearned lengthy units of language, as people with the most severe state of subcortical dysfunction show deficits in this language task.
The present study provides corroborating evidence derived from group data to Speedie et al.'s (1993) case study of a man, post-basal ganglia stroke, who had marked deficits in the ability to recite prayers that had been said daily since youth. Thus, subcortical disease from both basal ganglia stroke and Parkinson's disease has been seen to negatively impact the ability to recite longer, overlearned units of linguistic material. These current findings can be extended to highlight the importance of intact subcortical structures in recitation ability, and converge with additional evidence of preserved recitation skills in Alzheimer's disease. Hoblitzelle (2008), in a detailed chronicle of her husband's decline with Alzheimer's disease, described that even in the late-stages (characterized by severe cognitive impairment associated with cortical degeneration), he was able to recite large passages of poetry that he had memorized over many years as an English professor (Hoblitzelle, 2008). As Alzheimer's disease is a cortical degenerating process, leaving the basal ganglia and related structures intact until very late in the disease progression, it can be inferred that the relatively preserved basal ganglia contributed to the preserved production of even large units of recited speech. Schaltenbrand's (1965) early use of electrical brain stimulation for stereotactic surgery also found evidence of both stimulation as well as an interruption of counting and recitation ability for neurologically-disordered individuals (with Parkinsonism, dystonia, tremor, epileptic) when specific subcortical structures (anterior corpus callosum, fornix, anterior commissure, amygdala, pallidum internum, and ventro-oral thalamic nuclei) were stimulated. Although Schaltenbrand did not specifically stimulate the subthalamic nucleus, his early work provides evidence of recited and automatic speech being modulated by subcortical structures, as has been shown in the present study.
These present findings of increased error word production during recited speech for individuals with subcortical disease expand on previous formulaic language studies by showing that the production of even large units of overlearned, routinized language is related to subcortical function, lending further support for the dual process model of language production (Van Lancker Sidtis, 2004, 2012; Wray & Perkins, 2000). Formulaic language, which consists of relatively smaller structures of routinized, overlearned, semi-‘automatic’ language gestures, has been shown to be partially dependent on intact subcortical structures. In a preliminary study of individuals with PD, Rogers et al. (2009) found a reduction in the incidence of formulaic expressions for individuals with long-duration Parkinson's disease. Similarly, Sidtis et al. (2009) found reduced incidence of formulaic language in the spontaneous speech of individuals who have had subcortical strokes when compared to healthy adults. Findings from studies of the speech of individuals with Alzheimer's disease show relative preservation of formulaic language even when novel language production deteriorates due to cortical degeneration (Bridges & Van Lancker Sidtis, 2013), providing evidence that intact subcortical structures may be involved in the production of this language form.
Another exploratory aspect of this study was to evaluate whether the direct manipulation of the actions of subcortical structures improve or interfere with routinized language, specifically recited speech. There have been anecdotal reports of communicative deficits (specifically within the speech realm) associated with DBS, so a better understanding of this mechanism is desirable. To examine this, the language ability of people with PD (using recitation speech) was analyzed to uncover any potential effects of DBS in the ON and OFF conditions. Differences in error word production were not significant between individuals with DBS in the ON and OFF conditions during recitation speech. Although speech may be affected by DBS (see Van Lancker Sidtis, Rogers, Godier, Tagliati, & Sidtis, 2010), the results of this study fail to support that language, specifically the production of recited material, is impacted by DBS.
4.2. Limitations and future directions
Although efforts were made to obtain matched samples of DBS, PD, and HC participants, groups differed on gender (the DBS group was all male). Gender should be considered in future studies, as familiarity with recited items (in this study, several nursery rhymes) may differ between males and females, and thus recitation ability may differ as a function of gender and disease state.
Another noteworthy observation from this study is the great variability in performance on the recitation of this overlearned linguistic material, even for the healthy control participants. Consecutive volunteers were recruited as healthy control participants, and as such, they can be expected to represent the general population of healthy adults in their performance on this task. Considerable variability in performance on this task, accessing childhood and other experiences, is to be expected. The healthy adults in this study ranged in the production of error words during recitation from 0% to around 33% error words (of non-target words). Further controlled evaluation of recitation ability is desirable to lend additional support to the results of this exploratory study.
4.3. General conclusions
The primary finding of this study further illustrates the importance of subcortical structures in the production of overlearned linguistic material. As subcortical structures have been shown to be involved in procedural memory for syntax production (Ullman, 2008; Zanini et al., 2004), recitation speech as a task may also be procedural, driven by subcortical neural substrates controlling automatic, overlearned motor sequences. Similar to findings of formulaic language (see Bridges & Van Lancker Sidtis, 2013; Rogers et al., 2009 for discussion of formulaic language in Alzheimer's disease and Parkinson's disease), successful recitation speech is also associated with an intact subcortex, as deficits are observed with severe subcortical dysfunction (in the participants with DBS turned OFF). Overall, these findings provide support for the dual process model of language production involving a subcortical-right-hemisphere circuit in addition to the left-hemisphere (Van Lancker Sidtis, 2004, 2006, 2012; Wray & Perkins, 2000). A different kind of memory process is required for the production of formulaic expressions and longer units of recited speech. These language structures are holistically produced as a complete gesture, possibly benefiting from the motor organization and chunking processes of the basal ganglia (Graybiel, 1998). Observations of preserved formulaic expressions in advanced Alzheimer's disease (see Bridges & Van Lancker Sidtis, 2013), as well as the reduction in formulaic language (Rogers et al., 2009; Sidtis et al., 2009) and impaired performance during recited speech (associated with basal ganglia dysfunction; see Speedie et al., 1993) as observed in this study, are dramatic and support the dual-process model of language production, implicating the intact subcortex.
Acknowledgments
Thank you to Maria Grigos and Christina Reuterskiöld for comments on earlier editions of this paper, and thank you to all Brain and Behavior Laboratory members for contribution at different stages of this project.
Ethical approval: This study was approved by the institutional review board at the Nathan Kline Institute and all principles of ethical research were adhered to during the completion of this work.
Funding: This research was supported by NIDCD grant R01 DC007658 to John J. Sidtis & Diana Van Lancker Sidtis and New York University's Steinhardt Dean's Grant for Student Research to Kelly Bridges.
The funding source had no role in collecting, analyzing, interpreting, writing the paper, or submitting this work for publication.
Contributor Information
Kelly A. Bridges, Email: kab516@nyu.edu.
Diana Van Lancker Sidtis, Email: diana.sidtis@nyu.edu.
John J. Sidtis, Email: john.sidtis@nyu.edu.
References
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. The Lancet Neurology. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. http://dx.doi.org/10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- Bhatnagar SC. Neuroscience for the study of communicative disorders. 3rd. Baltimore, MD: Wolters Kluwer; 2008. [Google Scholar]
- Bridges KA, Van Lancker Sidtis D. Formulaic language in Alzheimer's disease. Aphasiology. 2013:1–12. doi: 10.1080/02687038.2012.757760. http://dx.doi.org/10.1080/02687038.2012.757760. Advanced online publication. [DOI] [PMC free article] [PubMed]
- Bryant PE, Bradley L, Maclean M, Crossland J. Nursery rhymes, phonological skills and reading. Journal of Child Language. 1989;16:407–428. doi: 10.1017/s0305000900010485. http://dx.doi.org/10.1017/S0305000900010485. [DOI] [PubMed] [Google Scholar]
- Chomsky N. Syntactic structures. The Hague/Paris: Mouton; 1957. [Google Scholar]
- Chomsky N. Aspects of a theory of syntax. Cambridge, MA: MIT Press; 1965. [Google Scholar]
- Code C. Neurolinguistic analysis of recurrent utterance in aphasia. Cortex. 1982;18:141–152. doi: 10.1016/s0010-9452(82)80025-7. http://www.ncbi.nlm.nih.gov/pubmed/6197231. [DOI] [PubMed] [Google Scholar]
- Critchley E. Speech origins and development. Springfield, IL: Charles C. Thomas; 1967. [Google Scholar]
- Critchley M. Aphasiology and other aspects of language. London, UK: Edward Arnold; 1970. [Google Scholar]
- Duffy JR. Motor speech disorders: Substrates, differential diagnosis, and management. 3rd. St. Louis, MO: Mosby-Year Book, Inc; 2012. [Google Scholar]
- Espir L, Rose F. The basic neurology of speech. Oxford, UK: Blackwell Scientific Publications; 1970. [Google Scholar]
- Fahn S, Elton RL, UPDRS program members . Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson's disease. Vol. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- Graybiel AM. Basal ganglia: new therapeutic approaches to Parkinson's disease. Current Biology: Dispatch. 1996;4:368–371. doi: 10.1016/s0960-9822(02)00497-9. http://dx.doi.org/10.1016/S0960-9822(02)00497-9. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiology of Learning and Memory. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. http://dx.doi.org/10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Dunnett SB. Targeting the subthalamic nucleus in the treatment of Parkinson's disease. Brain Research Bulletin. 1998;46:467–474. doi: 10.1016/s0361-9230(97)00449-8. http://dx.doi.org/10.1016/S0361-9230(97)00449-8. [DOI] [PubMed] [Google Scholar]
- Hoblitzelle OA. Ten thousand joys and ten thousand sorrows. New York, NY: Penguin Group; 2008. [Google Scholar]
- Hunter IML. Lengthy verbatim recall: the role of text. In: Ellis AW, editor. Progress in the psychology of language. Vol. 1. London, UK: Lawrence Erlbaum Associates; 1985. pp. 207–235. [Google Scholar]
- Illes J, Metter EJ, Hanson WR, Iritani S. Language production in Parkinson's disease: acoustic and linguistic considerations. Brain and Language. 1988;33:146–160. doi: 10.1016/0093-934x(88)90059-4. http://dx.doi.org/10.1016/0093-934X(88)90059-4. [DOI] [PubMed] [Google Scholar]
- Jackson JH. On the nature of the duality of the brain. In: Taylor J, editor. Selected writings of John Hughlings Jackson. Vol. 1. London, UK: Hodder and Stoughton; 1874/1958. ReprintedJ. [Google Scholar]
- Kent RD, Duffy JR, Slama A, Kent JF, Clift A. Clinicoanatomic studies in dysarthria: review, critique and directions for research. Journal of Speech Language and Hearing Research. 2001;44:535–551. doi: 10.1044/1092-4388(2001/042). http://dx.doi.org/10.1044/1092-4388(2001/042) [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clinical Neurophysiology. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. http://dx.doi.org/10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Riese W. Aphasia in brain tumors. Its appearance in relation to the natural history of the lesion. Confinia Neurologica. 1949;9:64–79. [PubMed] [Google Scholar]
- Rogers T, Sidtis D, Sidtis J. Formulaic language in Alzheimer and Parkinson speech; Poster presented at the 47th Annual Academy of Aphasia Meeting; Boston, MA. 2009. [Google Scholar]
- Schaltenbrand G. The effects of stereotactic electrical stimulation in the depth of the brain. Brain. 1965;88:835–840. doi: 10.1093/brain/88.4.835. http://dx.doi.org/10.1093/brain/88.4.835. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Onodera T, Kurokawa K, Tsukada M, Yamada R, Tabei Y, et al. Damage of left temporal lobe resulting in conversion of speech to Sutra, a Buddhist prayer stored in the right hemisphere. Neurocase: The Neural Basis of Cognition. 2010;16(4):317–320. doi: 10.1080/13554790903559689. http://dx.doi.org/10.1080/13554790903559689. [DOI] [PubMed] [Google Scholar]
- Sidtis D, Canterucci G, Katsnelson D. Effects of neurological damage on production of formulaic language. Clinical Linguistics and Phonetics. 2009;23(4):270–284. doi: 10.1080/02699200802673242. http://dx.doi.org/10.1080/02699200802673242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidtis JJ, Tagliati M, Alterman R, Sidtis D, Dhawan V, Eidelberg D. Therapeutic high-frequency stimulation of the subthalamic nucleus in Parkinson's disease produces global increases in cerebral blood flow. Journal of Cerebral Blood Flow and Metabolism. 2012;32:41–49. doi: 10.1038/jcbfm.2011.135. http://dx.doi.org/10.1038/jcbfm.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Speech and other functions after left (dominant) hemispherectomy. Journal of Neurology, Neurosurgery & Psychiatry. 1966;29:467–471. doi: 10.1136/jnnp.29.5.467. http://dx.doi.org/10.1136/jnnp.29.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Burklund CW. Nondominant hemispherectomy: neurophysiological implications for human brain functions. Proceedings of the American Psychological Association. 1967;2:103–104. http://psycnet.apa.org/psycinfo/1967-13181-001. [Google Scholar]
- Speedie LJ, Wertman E, Ta'ir J, Heilman KM. Disruption of automatic speech following a right basal ganglia lesion. Neurology. 1993;43(9):1768–1774. doi: 10.1212/wnl.43.9.1768. http://dx.doi.org/10.1212/WNL.43.9.1768. [DOI] [PubMed] [Google Scholar]
- Stahl B, Kotz SA, Henseler I, Turner R, Geyer S. Rhythm in disguise: why singing may not hold the key to recovery from aphasia. Brain. 2011;134(10):3083–3093. doi: 10.1093/brain/awr240. http://dx.doi.org/10.1093/brain/awr240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost M, Su S, Su P, Yen RF, Tseng HM, Barnes A, et al. Network modulation by the subthalamic nucleus in the treatment of Parkinson's disease. Neuroimage. 2006;31:301–307. doi: 10.1016/j.neuroimage.2005.12.024. http://www.ncbi.nlm.nih.gov/pubmed/16466936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman MT. The role of memory systems in disorders of language. In: Stemmer B, Whitaker HA, editors. The handbook of the neuroscience of language. Amsterdam, The Netherlands: Associated Press; 2008. pp. 189–198. [Google Scholar]
- Van Lancker D. Heterogeneity in language and speech: Neurolinguistic studies Working papers in phonetics. Los Angeles, CA: University of California Los Angeles; 1975. [Google Scholar]
- Van Lancker D. Nonpropositional speech: neurolinguistic studies. In: Ellis A, editor. Progress in the psychology of language. Vol. 3. Hillsdale, NJ: Lawrence Erlbaum; 1987. pp. 49–118. [Google Scholar]
- Van Lancker Sidtis D. When novel sentences spoken or heard for the first time in the history of the universe are not enough: toward a dual-process model of language. International Journal of Language and Communication Disorders. 2004;39(1):1–44. doi: 10.1080/13682820310001601080. http://dx.doi.org/10.1080/13682820310001601080. [DOI] [PubMed] [Google Scholar]
- Van Lancker Sidtis D. Where in the brain is nonliteral language? Metaphor and Symbol. 2006;21(4):213–244. http://dx.doi.org/10.1207/s15327868ms2104_2. [Google Scholar]
- Van Lancker Sidtis D. Two track mind: formulaic and novel language support a dual process model. In: Faust M, editor. Advances in the neural substrates of language: Toward a synthesis of basic science and clinical research. London, UK: Blackwell Publishing; 2012. pp. 342–367. [Google Scholar]
- Van Lancker Sidtis D, Rogers T, Godier V, Tagliati M, Sidtis JJ. Voice and fluency changes as a function of speech task and deep brain stimulation. Journal of Speech, Language, and Hearing Research. 2010;53:1167–1177. doi: 10.1044/1092-4388(2010/09-0154). http://dx.doi.org/10.1044/1092-4388(2010/09-0154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek JL. Deep brain stimulation: how does it work? Cleveland Clinic Journal of Medicine. 2008;75:S59–S65. doi: 10.3949/ccjm.75.suppl_2.s59. http://dx.doi.org/10.3949/ccjm.75.Suppl_2.S59. [DOI] [PubMed] [Google Scholar]
- Volkmann J. Deep brain stimulation for the treatment of Parkinson's disease. Journal of Clinical Neurophysiology. 2004;21:6–17. doi: 10.1097/00004691-200401000-00003. http://dx.doi.org/10.1097/00004691-200401000-00003. [DOI] [PubMed] [Google Scholar]
- Wray A, Perkins MR. The functions of formulaic language: an integrated model. Language & Communication. 2000;20:1–28. http://dx.doi.org/10.1016/S0271-5309(99)00015-4. [Google Scholar]
- Zangwill OL. Speech and the minor hemisphere. Acta Neurologica et Psychiatrica Belgica. 1967;67:1013–1020. http://psycnet.apa.org/psycinfo/1969-00358-001. [PubMed] [Google Scholar]
- Zanini S, Tavano A, Vorano L, Schiavo F, Gigli GL, Aglioti SM, et al. Greater syntactic impairments in native language in bilingual Parkinsonian patients. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75:1678–1681. doi: 10.1136/jnnp.2003.018507. http://dx.doi. org/10.1136/jnnp.2003.018507. [DOI] [PMC free article] [PubMed] [Google Scholar]