Abstract
Defective DNA mismatch repair (MMR) occurs in approximately 15% of sporadic colorectal cancers (CRCs). Multiple retrospective studies have shown that patients with MMR-deficient CRCs have a more favorable stage-adjusted prognosis compared with those who have MMR-proficient tumors. Evidence also indicates that patients with MMR-deficient colon cancers do not benefit from treatment with adjuvant 5-fluorouracil chemotherapy. Furthermore, recent studies, including a pooled analysis, have validated the prognostic and predictive impact of MMR status in patients with stage II and III colon cancer who were treated in adjuvant chemotherapy trials. Given these data, it can be recommended that MMR status be determined and used to inform clinical decision-making for adjuvant chemotherapy in patients with stage II colon cancer.
Introduction
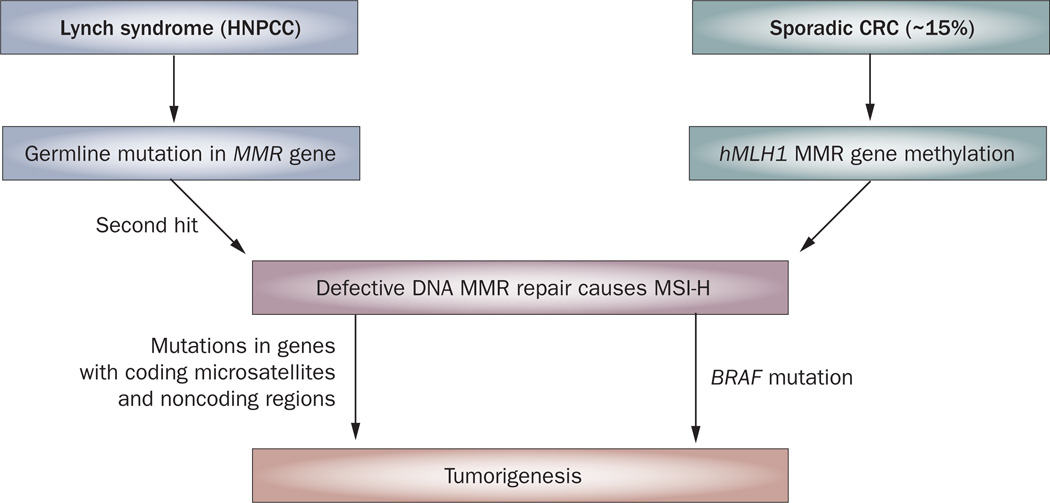
While most colorectal cancers (CRCs) show chromosomal instability, approximately 15% of CRCs arise as a result of defective DNA mismatch repair (MMR).1,2 These tumors show high-frequency microsatellite in stability (MSI-H) that occurs due to the inability of cells to repair single nucleotide DNA mismatches. MSI-H is a hallmark of Lynch syndrome (also referred to as hereditary nonpolyposis colorectal cancer [HNPCC]) that results from germline mutation in MMR genes (MLH1, MSH2, MSH6, PMS2)1 that are highly penetrant, yet account for less than 5% of all CRCs (Figure 1). Amsterdam II criteria and the revised Bethesda guidelines were developed to identify individuals with Lynch syndrome and thus, likely to carry a germline mutation in one of the known MMR genes, most commonly MLH1 or MSH2.3 The majority of MSI-H CRCs, however, are sporadic non Lynch syndrome cases that result from epigenetic inactivation of the MLH1 gene promoter by DNA hypermethylation (Figure 1).2 These sporadic MSI-H CRCs often arise in the setting of a specific pathway of DNA hypermethylation, known as the CpG island methylator phenotype (CIMP) with CIMP-related silencing of MLH1.4 Patients with MSI-H tumors have distinct clinical and pathological features, irrespective of their germline or sporadic origins, which include proximal colon predominance, frequent poor differentiation and mucinous histology, and increased numbers of tumor-infiltrating lymphocytes.5,6 In addition to being molecularly distinct from Lynch syndrome cases, patients with sporadic MSI-H cancers have associated epidemiological features, including older age at diagnosis, female gender and cigarette smoking.2
Figure 1.
Distinct molecular pathways for the development of defective DNA MMR and MSI-H in CRCs. Lynch syndrome CRCs arise due to germline mutations in MMR genes whereas epigenetic inactivation of the hMLH1 gene characterizes sporadic CRCs with MMR deficiency and MSI-H. Abbreviations: CRC, colorectal cancer; HNPCC, hereditary nonpolyposis colorectal cancer; MMR, mismatch repair; MSI-H, high-frequency microsatellite instability.
MSI testing can be performed on paraffin-embedded tumor tissue using a PCR-based assay for detection of instability at selected microsatellite loci.5,7 A panel of five microsatellites have been validated and recommended as a reference panel.8 CRCs can be characterized on the basis of: MSI-H, if two or more of the five microsatellite markers show instability (that is, have insertion/deletion mutations), low-frequency MSI (MSI-L) if only one of the five markers shows instability, and microsatellite stable (MSS) if none of the markers show instability.8 MSI testing requires a molecular laboratory, whereas analysis of MMR protein expression by immunohistochemistry (IHC) is an alternative test that is widely available. IHC has the advantage of identifying the affected gene by detecting loss of its protein product. MSI testing and IHC are complimentary, and loss of MMR protein expression by IHC has been shown to be highly concordant with DNA-based MSI testing.7 Using IHC, only loss of hMLH1 protein expression has been described in sporadic CRCs.9 Tumors that demonstrate MSI-H or loss of an MMR protein can be collectively referred to as MMR-deficient. MMR-proficient tumors include those that are micro satellite stable (MSS) and MSI-low (MSI-L) or tumors with intact MMR proteins. Of the 147,000 CRCs expected to have been diagnosed in 2009 in the US, it can be estimated that approximately 15,000 cases would show defective MMR if tested.
Impact of MMR deficiency
When MSI was first identified in CRCs, it was noted that patients with MSI-H tumors had better survival rates compared with those who had MSS or MSI-L tumors.5 Moreover, patients with MSI-H tumors were found to have lower tumor stage at diagnosis and rarely had metastatic disease. Since the initial discovery of MSI in CRC, substantial data have accumulated that demonstrate the better outcome of patients with MMR-deficient CRC compared with patients who have MMR-proficient tumors. These data are largely from retrospective studies,10–13 but also include a population-based study14 and a meta-analysis15 where patients with MMR-deficient CRCs had a more favorable stage-adjusted survival compared with patients who had MMR-proficient tumors. The meta-analysis included 32 studies of 1,277 patients with MMR-deficient CRCs; a 35% reduction in the risk of death for patients with MMR-deficient tumors versus those with MMR-proficient tumors was demonstrated.15 This analysis included untreated patients as well as patients treated with 5-fluorouracil (5-FU)-based adjuvant therapy in phase III randomized trials. More recently, 1,436 patients with stage II CRCs were treated in the QUASAR adjuvant therapy trial; the tumors of these patients were analyzed for MMR status and used as a validation cohort.12 Multivariate analysis revealed that MMR deficiency was an independent prognostic variable for improved survival (hazard ratio [HR] = 0.31, 95% CI 0.15–0.63; P <0.001).16 Despite these findings, some studies,17,18 have not found an association of MMR deficiency with a favorable outcome, including a cooperative group trial18 whereby no survival differences were found for patients with MMR-deficient colon cancers compared with those who had MMR-proficient tumors. Factors that may account for these discrepant data include an insufficient sample size because patients with MMR-deficient tumors represent a relatively small subset of cases, and the modest magnitude of the MMR prognostic effect. Furthermore, a selection bias can be introduced into adjuvant clinical trials because tissue samples are only available for a subset of patients. In addition, the MSI markers that are used to detect MSI-H cases have been variable and their sensitivity and specificity to detect MSI differ.8 It is possible, therefore, that false-positive MSI results may dilute an already modest prognostic impact.
Predictive role of 5-FU therapy
Results from randomized phase III clinical trials and a prospective study have shown that MMR status is predictive of response to adjuvant 5-FU-based chemotherapy in patients with CRC.19–21 Patients with MMR-deficient tumors did not derive benefit from adjuvant 5-FU-based chemotherapy, whereas those with MMR-proficient tumors had a significant survival benefit with this treatment.19–21 Similarly, a meta-analysis that included patients with stage II and III CRCs who participated in randomized phase III clinical trials also demonstrated that patients with MSI-H tumors did not derive benefit from adjuvant 5-FU therapy compared with non-MSI-H cases.15 These data are consistent with preclinical studies, which have demonstrated that human CRC cell lines with MSI-H display resistance to compounds that induce DNA damage, such as 5-FU.22 Resistance to 5-FU has been shown to be overcome by the restoration of normal DNA MMR function in CRC cells.23 The predictive utility of MSI, however, has generated controversy given that some retrospective studies have failed to demonstrate a predictive impact of MSI,17,18 and some reports24,25 have suggested that patients with MMR-deficient CRCs may have a greater benefit from adjuvant 5-FU-based treatment compared with those who have MMR-proficient tumors. In addition to a potential selection bias in these studies, it is important to recognize that the favorable prognosis of patients with MMR-deficient tumors may result in an equivalent outcome as observed for patients with MMR-proficient tumors, given the lack of benefit from 5-FU in MMR-deficient cases. In a recent study, aimed at validating the prognostic and predictive impact of MMR status, tumors from a large series of patients with stage II and III colon cancers from North American and European adjuvant therapy trials were analyzed for MMR status.26 Study data were pooled and among patients who received surgery alone (n = 515), those with MMR-deficient tumors had a 49% improvement in disease-free survival (DFS) and better overall survival compared to those with MMR proficient tumors.26 Furthermore, this study confirmed that patients with MMR-deficient tumors do not benefit from 5-FU therapy compared to patients with MMR-deficient tumors who were randomized to untreated control arms.26
In an adjuvant chemotherapy trial (CALGB 89803),27 the impact of MSI status upon clinical outcome was studied in patients with stage III colon cancer treated with irinotecan, 5-FU, and leucovorin (LV), known as IFL, versus 5-FU/LV. Patients with MMR-deficient tumors who were treated with IFL had a significantly improved 5-year DFS compared with those who had MMR-proficient tumors who were treated with IFL. However, a similar relationship was not observed for patients treated with 5-FU/LV. In patients with MMR-deficient tumors, a trend toward longer DFS was seen with IFL versus 5-FU/LV; median DFS or overall survival, however, has not yet been reached in this trial. These data suggest that the addition of irinotecan may improve the clinical outcome of patients with MMR-deficient colon cancers. However, data from the Pan European Trial Adjuvant Colon Cancer (PETACC-3), which was designed to determine whether the addition of irinotecan to 5-FU/LV could improve DFS, failed to show a benefit with irinotecan in patients with MSI-H colon cancers.28 The results from this study contrast with the findings reported in the CALGB 89803 trial. Since all patients received 5-FU in the PETACC-3 study, the benefit, or lack thereof, of 5-FU therapy alone in patients with MSI-H tumors cannot be addressed, except to indicate that MSI-H remained prognostic despite treatment in this study.28 It is important to note that neither study found an overall survival benefit with the addition of irinotecan to 5-FU/LV. The addition of irinotecan in the CALGB 89803 trial was associated with increased lethal and nonlethal toxic effects, with deaths that were primarily attributed to neutropenic sepsis or vascular thromboembolic events.29 Thus, irinotecan cannot be recommended as an adjuvant therapy regimen for patients with CRC, although it is an effective treatment for patients with metastatic CRC.
Therapy and patient selection
The use of adjuvant chemotherapy in patients with curatively resected stage II (lymph-node negative) colon cancer remains controversial, and represents a dilemma for medical oncologists with regard to the potential benefits versus risks of treatment. It is estimated that one-third of all patients with stage II disease receive adjuvant therapy in the US. Current evidence supports the use of MMR testing (MSI DNA-based analysis or IHC for MMR proteins) for decision-making regarding the use of adjuvant therapy in patients with stage II colon cancer. Specifically, we recommend that patients with stage II MMR-deficient colon cancers should not receive adjuvant chemotherapy on the basis that these patients have a favorable prognosis and data indicate that 5-FU is ineffective in these cases. Thus, patients with stage II MSI-H colon cancers can be spared from unnecessary treatment-related toxic effects and reduced quality of life during chemotherapy. However, MMR proficiency does not, by itself, indicate a high-risk stage II tumor nor does it provide a rationale for adjuvant chemotherapy. Decisions regarding adjuvant therapy should be individualized for patients with MMR-proficient stage II colon cancers. The impact of MMR status upon treatment decisions in patients with stage III disease awaits further study. The standard of care for patients with resected stage III (lymph-node positive) CRC is adjuvant 5-FU/LV and oxaliplatin (FOLFOX).30 At present, available data do not justify the exclusion of patients with stage III MMR-deficient CRC from receiving adjuvant FOLFOX chemotherapy since the responsiveness of these tumors to oxaliplatin or FOLFOX is unknown and remains an important research question. A phase III clinical trial assessing the FOLFOX regimen in patients with stage II colon cancers (ECOG 5202) is ongoing; patients are categorized into low-risk (MSI-H or chromosome 18q intact) or high-risk (MSS, MSI-L or 18q allelic imbalance) groups. Low-risk patients receive observation, whereas high-risk patients are treated with FOLFOX with or without the anti-VEGF antibody, bevacizumab. Bevacizumab has been shown to enhance the efficacy of 5-FU-based therapy in patients with metastatic CRC.31 Regrettably, this trial will not provide data on the responsiveness of MSI-H tumors to FOLFOX therapy, since patients with MSI-H tumors are not being treated.
CRCs with deficient DNA MMR
Our group developed a model to predict MMR deficiency using clinical and pathological data available in routine clinical practice in order to identify patients with sporadic MMR-deficient CRC and to utilize such information for clinical decision-making. Data from 982 patients with stage II and III colon cancer who participated in six adjuvant 5-FU-based therapy trials was analyzed; MMR deficiency was detected in 4% of patients with distal tumors, as defined relative to splenic flexure.32 Therefore, the author recommends that testing for MMR status by MSI analysis or IHC be performed on all resected, stage II cancers of the proximal colon in patients where adjuvant therapy is a consideration. It could be argued that the cost of MMR testing may be offset by more-efficient use of adjuvant chemotherapy.33
MMR status in patients with stage III disease is of research interest, but should not be used to inform adjuvant treatment decisions at the present time. Since loss of hMLH1 protein expression can be due to promoter hypermethylation or a germline mutation, MMR testing should also include hMLH1 promoter methylation analysis, which is a sensitive test.2 Somatic BRAF (V600E) mutation analysis should also be performed since activation of the BRAF oncogene, a member of the RAS/RAF family of kinases, by the V600E mutation is characteristic of sporadic colon cancers with MSI, and has been shown to associate with the silencing of the MLH1 gene by hypermethylation.34 By contrast, activating mutations in BRAF are not found in Lynch syndrome tumors and therefore, these analyses can be used to distinguish Lynch syndrome from sporadic CRC cases.35 It is important to emphasize that loss of MMR proteins other than MLH1, as detected by IHC, suggests Lynch syndrome. Specifically, only the MLH1 MMR gene has been shown to be methylated in sporadic CRCs with MMR deficiency and loss of MSH2, MSH6 or PMS2 proteins suggests a germline mutation in the genes encoding these proteins and Lynch syndrome.9 Patients with suspected hereditary CRC should be referred for genetic counseling, where the identification of germline mutations and evaluation/screening of family members can be appropriately addressed.
Conclusions
The determination of MMR status in patients with CRC can provide valuable prognostic and predictive information, and can help in clinical decision-making. Specifically, the favorable prognosis of patients with stage II MMR-deficient CRCs and the lack of benefit from adjuvant 5-FU-based therapy, indicate that these patients should not receive adjuvant chemotherapy. While data for patients with stage III MMR-deficient CRCs suggest a lack of benefit from 5-FU alone, the benefit of the current standard FOLFOX regimen in this patient subgroup awaits further investigation. Use of MMR status to guide patient management represents a further step toward personalized cancer care.
Footnotes
Competing interests
The author declares no competing interests.
References
- 1.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 2.Poynter JN, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol. Biomarkers Prev. 2008;17:3208–3215. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piñol V, et al. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA. 2005;293:1986–1994. doi: 10.1001/jama.293.16.1986. [DOI] [PubMed] [Google Scholar]
- 4.Toyota M, et al. CpG island methylator phenotype in colorectal cancer. Proc. Natl Acad. Sci. USA. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science (New York, NY) 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 6.Jass JR, et al. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42:673–679. doi: 10.1136/gut.42.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindor NM, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J. Clin. Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 8.Boland CR, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 9.Herman JG, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc. Natl Acad. Sci. USA. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinicrope FA, et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology. 2006;131:729–737. doi: 10.1053/j.gastro.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Gafà R, et al. Sporadic colorectal adenocarcinomas with high-frequency microsatellite instability. Cancer. 2000;89:2025–2037. [PubMed] [Google Scholar]
- 12.Halling KC, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J. Natl Cancer Inst. 1999;91:1295–1303. doi: 10.1093/jnci/91.15.1295. [DOI] [PubMed] [Google Scholar]
- 13.Lanza G, et al. Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectal cancer patients. J. Clin. Oncol. 2006;24:2359–2367. doi: 10.1200/JCO.2005.03.2433. [DOI] [PubMed] [Google Scholar]
- 14.Samowitz WS, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol. Biomarkers Prev. 2001;10:917–923. [PubMed] [Google Scholar]
- 15.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 16.Kerr DJ, et al. A quantitative multigene RT-PCR assay for prediction of recurrence in stage II colon cancer: selection of the genes in four large studies and results of the independent, prospectively designed QUASAR validation study [abstract] J. Clin. Oncol. 2009;27(Suppl. 15S):a4000. [Google Scholar]
- 17.Lamberti C, et al. Microsatellite instability did not predict individual survival of unselected patients with colorectal cancer. Int. J. Colorectal Dis. 2007;22:145–152. doi: 10.1007/s00384-006-0131-8. [DOI] [PubMed] [Google Scholar]
- 18.Kim GP, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J. Clin. Oncol. 2007;25:767–772. doi: 10.1200/JCO.2006.05.8172. [DOI] [PubMed] [Google Scholar]
- 19.Benatti P, et al. Microsatellite instability and colorectal cancer prognosis. Clin. Cancer Res. 2005;11:8332–8340. doi: 10.1158/1078-0432.CCR-05-1030. [DOI] [PubMed] [Google Scholar]
- 20.Ribic CM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jover R, et al. The efficacy of adjuvant chemotherapy with 5-fluorouracil in colorectal cancer depends on the mismatch repair status. Eur. J. Cancer. 2009;45:365–373. doi: 10.1016/j.ejca.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Carethers JM, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology. 1999;117:123–131. doi: 10.1016/s0016-5085(99)70558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold CN, Goel A, Boland CR. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int. J. Cancer. 2003;106:66–73. doi: 10.1002/ijc.11176. [DOI] [PubMed] [Google Scholar]
- 24.Hemminki A, Mecklin JP, Jarvinen H, Aaltonen LA, Joensuu H. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology. 2000;119:921–928. doi: 10.1053/gast.2000.18161. [DOI] [PubMed] [Google Scholar]
- 25.Elsaleh H, et al. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355:1745–1750. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 26.Sargent DJ, et al. Confirmation of deficient mismatch repair (dMMR) as a predictive marker for lack of benefit from 5-FU based chemotherapy in stage II and III colon cancer (CC): A pooled molecular reanalysis of randomized chemotherapy trials [abstract] J. Clin. Oncol. 2008;26(Suppl.):a4008. [Google Scholar]
- 27.Bertagnolli MM, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J. Clin. Oncol. 2009;27:1814–1821. doi: 10.1200/JCO.2008.18.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tejpar SB, et al. Microsatellite instability (MSI) in stage II and III colon cancer treated with 5FU-LV or 5FU-LV and irinotecan (PETACC 3-EORTC 40993-SAKK 60/00 trial) [abstract] J. Clin. Oncol. 2009;27(Suppl. 15S):a4001. [Google Scholar]
- 29.Saltz LB, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J. Clin. Oncol. 2007;25:3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 30.de Gramont A, et al. Oxaliplatin/5FU/LV in adjuvant colon cancer: Updated efficacy results of the MOSAIC trial, including survival, with a medial follow-up of six years. J. Clin. Oncol. 2007;25(Suppl. 18S):a4007. [Google Scholar]
- 31.Saltz LB, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 32.Sinicrope FA, et al. Model-based prediction of defective DNA mismatch repair using clinicopathological variables in sporadic colon cancer patients. Cancer. doi: 10.1002/cncr.24913. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrow E, McMahon R, Evans DG, Levine E, Hill J. Cost analysis of biomarker testing for mismatch repair deficiency in node-positive colorectal cancer. Br. J. Surg. 2008;95:868–875. doi: 10.1002/bjs.6172. [DOI] [PubMed] [Google Scholar]
- 34.Deng G, et al. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin. Cancer Res. 2004;10:191–195. doi: 10.1158/1078-0432.ccr-1118-3. [DOI] [PubMed] [Google Scholar]
- 35.Domingo E, et al. BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. J. Med. Genet. 2004;41:664–668. doi: 10.1136/jmg.2004.020651. [DOI] [PMC free article] [PubMed] [Google Scholar]