Abstract
Studies suggest that neuropsychological measures may provide prognostic information regarding SSRI treatment response, yet it is unclear which specific cognitive domains are the most effectual predictors. The aim of this study was to characterize the cognitive profile associated with SSRI nonresponse using a comprehensive set of neuropsychological tests. Participants (N = 32) met criteria for current major depressive episode. Assessment followed pre-treatment medication washout. Clinical response was measured after 3-month open-label SSRI treatment. Groups did not differ by demographic characteristics, intelligence or depression severity. Responders outperformed nonresponders across all cognitive domains, with the largest differences observed in executive, language and working memory functions. Results indicate poorer global cognitive functioning is predictive of treatment nonresponse. Deficits were most pronounced in tests demanding greater mental search and manipulation rather than speeded motor output. Cognitive slowing may mediate the working memory and executive function deficits found in nonresponders. These findings can inform exploration for pharmacogenetic endophenotypes.
Keywords: Neuropsychology, Depression, SSRI, Response
Introduction
No single treatment has been found to be uniformly effective for major depressive disorder (Fava et al. 2003). Only 40% of patients achieve remission with their initial antidepressant trial (Rush et al. 2006), and the need for successive interventions because of poor clinical response prolongs the period of illness. Although there is limited empirical information to guide clinicians’ choice of medication, the selective serotonin reuptake inhibitor (SSRI) medications are commonly used as the first line of treatment given their relatively low toxicity and high tolerability (Rush et al. 2006). Predictors of efficacy of individual antidepressants or classes of antidepressants would be of considerable clinical value.
Several studies suggest neuropsychological measures can be used as markers for SSRI response in depression. A range of cognitive deficits has been reported in patients who subsequently demonstrate poor clinical response to SSRI treatment. However, the essential neuropsychological profile related to SSRI nonresponse remains unknown, due to variability in sample composition, test selection, and treatment administration. In an open-treatment medication study, nonresponding patients were found to have poorer baseline motor performance relative to responders (Caligiuri et al. 2003). Similarly designed studies, though, find poor SSRI efficacy associated with deficits in higher-level cognitive domains such as information integration (Kampf-Sherf et al. 2004). Results of placebo-controlled clinical trials fail to clarify the nature of the cognitive impairment predictive of SSRI nonresponse. Dunkin et al. (2000) found patients whose symptoms did not remit following 8 weeks of fluoxetine treatment had lower baseline scores on executive function measures such as the Wisconsin Card Sorting Test (WCST). Conversely, Taylor et al. (2006) suggest a deficit in psychomotor speed distinguishes SSRI nonresponse. In a 12-week fluoxetine trial, nonresponders had poorer pre-treatment performance on timed tests of Letter Fluency and Stroop Color Naming, but there were no group differences on the WCST. In depressed geriatric samples, executive dysfunction and verbal memory deficits appear to increase the risk of poor treatment outcome with significantly lower scores on the Stroop Color Word Test and verbal fluency (Alexopoulos et al. 2005; Baldwin et al. 2004) and uneven performance on tests of planning (Tower of London) and verbal learning (WMS Verbal Paired Associates) (Marcos et al. 2005).
Though prior studies have made claims regarding the specificity of cognitive deficits that predict SSRI nonresponse, few have compared the overall profiles of neuropsychological functioning between responders and nonresponders. The goal of the current study was to provide this broader examination of the cognitive markers of treatment nonresponse in depression. A comprehensive neuropsychological battery assessing a wide range of cognitive abilities was administered to depressed subjects before initiation of 3-month open-label treatment. Test scores were grouped into seven domains, and, at the end of the 3-month period, profiles of cognitive functioning were contrasted between patients classified as SSRI responders and nonresponders using a multivariate analysis.
Materials and methods
Subjects
The sample consisted of 32 adults participating in protocols within the Conte Center for the Study of Suicidal Behavior at Columbia University Medical Center. All subjects met DSM-IV criteria for current major depressive episode, with consensus Axis I diagnosis made using the Structured Clinical Interview for DSM-IV patient edition (SCID I) (First et al. 1994). Two subjects were classified with Bipolar Disorder-currently depressed, three with Bipolar Disorder NOS-currently depressed, and the remainder with Major Depressive Disorder. All subjects were proficient in English. Subjects had a minimum 17-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960) score of 16 at time of study entry. Clinical histories, as well as physical and laboratory exams, were used to rule out neurological disease and acute medical conditions. Urine toxicology analyses were used to screen for current illicit substance use and were negative for all subjects.
All participants gave written informed consent for protocol participation, which was approved by the local institutional review board.
Measures and procedures
Neuropsychological assessment was conducted following two weeks of washout (6 weeks for fluoxetine) from all psychotropic medications. Testing was performed by Master’s level technicians under the supervision of authors of the current study (M. G. and J. G. K.). The battery incorporates standardized paper and pencil tests as well as several computerized measures developed in our lab, and has demonstrated success for characterizing deficits seen in depression and suicidal behavior (Keilp et al. 2001) and impulsiveness (Keilp et al. 2005). Detailed information about the computerized measures has been described previously (Keilp et al. 2005).
Each test score was converted into a z-score using values obtained from a normative sample, to correct for age, sex and education effects. The z-scores were then grouped into seven cognitive domains, and averaged. The domains and their composite tests were as follows: motor (Finger Tapping, Stroop Color Naming, Stroop Word Reading), psychomotor (WAIS-III Digit Symbol, Trailmaking A and B), attention (Continuous Performance Task, Stroop Color-Word), memory (Buschke Selective Reminding Test), working memory (N-Back, A not B Reasoning Test), language (Letter and Category Fluency) and executive (Wisconsin Card Sorting Test—number of categories, Trailmaking B–A). The battery also included the WAIS-III Vocabulary subtest as an estimate of general intelligence.
Subjects were provided with open-label treatment following completion of research protocols, with medication selection and dose determined by clinical judgment. Treatment was provided at subjects’ discretion by study psychiatrists or private psychiatrists in the community. All subjects in this sample received an SSRI medication [paroxetine (15 responders, 6 nonresponders; 10–60 mg QD), fluoxetine (1 nonresponder; 40 mg QD), sertraline (2 responders, 2 nonresponders; 50–200 mg QD), citalopram (1 responder, 3 nonresponders; 20–40 mg QD), or escitalopram (1 responder, 1 nonresponder; 10 mg QD)]. Since treatment was not administered within the context of a controlled clinical trial, only 15 subjects (9 responders, 6 nonresponders) were treated exclusively with an SSRI. The remaining subjects received adjunctive anxiolytics (2 responders, 3 nonresponders; lorazepam, alprazolam, clonazepam), mood stabilizers (4 responders, 2 nonresponders; valproic acid, lithium, gabapentin), and/or non-SSRI antidepressants (4 responders, 5 nonresponders; bupropion, venlafaxine, trazodone, mirtazapine, nortriptyline). No subjects were treated with antipsychotic medications or ECT in addition to an SSRI.
Clinical status was re-assessed following 3 months of treatment. Treatment outcome was determined by HDRS score. Responders (N = 19) were defined as having a decrease in HDRS score of at least 50% from baseline, plus a total follow-up HDRS score ≤ 10. Responders and nonresponders were compared on demographic and clinical variables using Student’s t tests and chi-square analyses. A MANOVA was used to determine differences in overall cognitive performance associated with medication response. Student’s t tests were used to compare specific neuropsychological domain and test scores between groups.
Results
As shown in Table 1, responders and nonresponders had similar baseline clinical and demographic characteristics. Both groups were equally depressed at baseline, and had an equivalent number of prior depressive episodes. Groups also had comparable ethnicity and gender compositions, and similar proportions of subjects with past suicide attempts and substance dependence (alcohol or substance dependence as defined by DSM-IV TR criteria). There were equal proportions of participants who received adjunctive medications among the responders and nonresponders. Groups did not differ in number of patients who met criteria for melancholic depression. The number of subjects with Bipolar Disorder also did not differ between the groups.
Table 1.
Demographic and clinical data for responder and nonresponder patients
| Responders N = 19 |
Nonresponders N = 13 |
P value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age | 41.90 | 13.3 | 42.50 | 13.7 | 0.92 |
| Education | 15.50 | 1.5 | 15.70 | 3.1 | 0.84 |
| GAF Score | 55.30 | 9.4 | 58.40 | 9.0 | 0.36 |
| Baseline Hamilton DRS (24 items) | 21.40 | 8.8 | 22.30 | 7.8 | 0.77 |
| Baseline Beck Depression Inventory | 22.50 | 10.4 | 23.80 | 9.3 | 0.70 |
| Follow-up Hamilton DRS (3 months) | 4.80 | 3.1 | 17.80 | 8.0 | <0.001 |
| Follow-up Beck (3 months) | 4.60 | 3.9 | 15.30 | 9.6 | <0.001 |
|
| |||||
| % | N | % | N | P value | |
|
| |||||
| Gender (%female) | 52.60 | 10 | 61.50 | 8 | 0.62 |
| Race (%Caucasian) | 68.40 | 13 | 69.20 | 9 | 0.80 |
| Hx substance dependence | 36.80 | 7 | 15.40 | 2 | 0.19 |
| Hx suicide attempt | 36.80 | 7 | 38.50 | 5 | 0.93 |
| Melancholic depression | 21.10 | 4 | 25.00 | 3 | 0.80 |
| Atypical depression | 10.50 | 2 | 25.00 | 3 | 0.29 |
| Medication (% w/adjunctive agents) | 52.60 | 10 | 53.80 | 7 | 0.95 |
Neuropsychological domain z-scores for responders and nonresponders are shown in Table 2 and Fig. 1. Responders generally outperformed nonresponders in all domains, though the groups did not differ in Vocabulary score, an estimate of general intellectual functioning. The responder group’s domain scores were all around or above zero. Nonresponders’ domain scores were between 0.2 and 1 SD below the means of the nondepressed normative sample. In a MANOVA comparing the groups’ cognitive performance profiles, there was a significant main effect for treatment response [F(1,30) = 4.51, P = 0.042]. Effect for domain [F(6, 180) = 1.39, P = 0.220] and the interaction of response group by domain [F(6, 180) = 0.31, P = .933] were not significant.
Table 2.
Neuropsychological domain and test scores for responder and nonresponder patients
| RespondersN = 19 |
NonrespondersN = 13 |
t | P value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| WAIS-III vocabulary | 13.28 | 2.80 | 12.08 | 4.94 | 0.86 | 0.40 |
| Motor domain | 0.32 | 2.25 | −0.24 | 1.97 | 0.73 | 0.47 |
| Tapping—dominant | 0.18 | 1.32 | 0.01 | 1.51 | 0.34 | 0.74 |
| Tapping—nondominant | −0.20 | 1.55 | −0.03 | 1.48 | −0.29 | 0.77 |
| Stroop color naming | −0.23 | 1.44 | 0.33 | 0.98 | −1.22 | 0.23 |
| Stroop word reading | −0.43 | 1.30 | 0.13 | 1.22 | −1.22 | 0.23 |
| Psychomotor domain | 0.02 | 1.10 | −0.39 | 1.05 | 1.05 | 0.30 |
| Digit symbol | 0.37 | 1.17 | −0.05 | 0.92 | 1.08 | 0.29 |
| Trailmaking A (T score) | −0.91 | 1.24 | −0.81 | 0.91 | −0.24 | 0.81 |
| Trailmaking B (T score) | −0.35 | 1.09 | −1.08 | 0.82 | 2.05 | 0.04 |
| Attention domain | 0.03 | 0.83 | −0.41 | 0.83 | 1.49 | 0.15 |
| CPT (d’) | −0.08 | 1.37 | −0.60 | 1.27 | 1.10 | 0.28 |
| Stroop interference | −0.14 | 0.90 | 0.22 | 0.91 | −1.11 | 0.27 |
| Memory domain | 0.18 | 0.94 | −0.21 | 1.53 | 0.88 | 0.39 |
| Buschke (total recall) | 0.23 | 0.94 | −0.21 | 1.53 | 0.99 | 0.33 |
| Working memory domain | −0.10 | 0.76 | −1.02 | 1.24 | 2.61 | 0.01 |
| N back (d’) | −0.11 | 0.94 | −1.09 | 1.12 | 2.18 | 0.04 |
| A not B (RT) | −0.02 | 0.93 | 1.15 | 1.78 | −2.43 | 0.02 |
| Language domain | 0.15 | 0.64 | −0.46 | 0.99 | 2.12 | 0.04 |
| Letter fluency | 0.09 | 0.76 | −0.51 | 0.88 | 2.03 | 0.05 |
| Category fluency | 0.30 | 1.11 | −0.43 | 1.39 | 1.54 | 0.14 |
| Executive function domain | −0.03 | 0.72 | −0.80 | 0.92 | 2.67 | 0.01 |
| WCST (categories) | −0.53 | 0.82 | −0.38 | 0.68 | −0.53 | 0.60 |
| Trailmaking B–A | 0.46 | 1.06 | −1.14 | 1.25 | 3.90 | <0.01 |
Fig. 1.
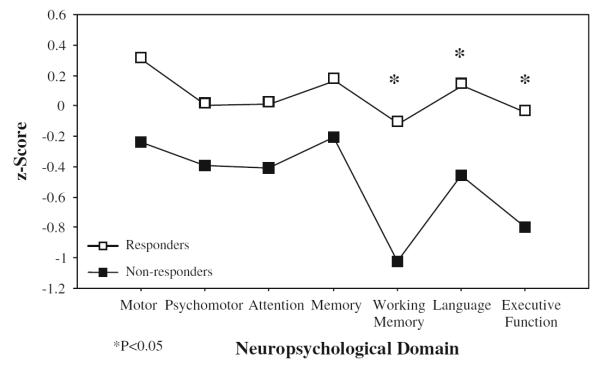
Neuropsychological domain scores of responders and nonresponders
Although there was no interaction in the multivariate analysis, group differences in individual domain tests were most pronounced in the domains of Working Memory [t(30) = 2.61, P = 0.014], Language [t(30) = 2.12, P = 0.042] and Executive Functioning [t(30) = 2.67, P = 0.012].
Several specific neuropsychological tests within the domains differed between groups. Nonresponders had significantly poorer scores on both Working Memory tests [A not B: t(30) = −2.43, P = 0.022; N-Back: t(30) = 2.19, P = 0.037]. Within the Language domain, Letter Fluency [t(30) = 2.04, P = 0.051] but not Category Fluency [t(26) = 1.54, P = 0.135] differed significantly. Though the mean difference in Category Fluency performance was larger than that of Letter Fluency, it did not reach significance due to greater score variability. In Executive Functioning, only the Trailmaking B–A [t(30) = 3.91, P < 0.001] score differentiated responders and nonresponders; the number of WCST categories achieved did not [t(29) = −0.53, P = 0.599].
Discussion
Depressed patients exhibiting cognitive deficits may represent a mood disorder subgroup for which SSRI treatment is less effective. Consistent with prior reports (Baldwin et al. 2004; Dunkin et al. 2000; Marcos et al. 2005; Taylor et al. 2006) patients in the current study who failed to respond to SSRI medications performed more poorly on baseline measures of cognitive ability relative to treatment responders. Nonresponders in the this sample had lower scores in all neuropsychological domains relative to the responder group, though there were no differences in baseline depression ratings. The performance differences were also not attributable to differences in premorbid functioning, as similar levels of educational attainment and estimated intelligence scores were found in both groups.
Altogether, results indicate that neuropsychological measures are capable of identifying the patients who may need a different course of care for their depression, as they are unlikely to promptly improve with an SSRI alone. Poor symptom response extends the period of functional impairment and increases the risk for suicidal behavior. Alternate treatments could be implemented sooner if SSRI response markers were available. Neuropsychological testing could also serve an essential role in psychoeducation and treatment planning. Patients who present with depression and concurrent cognitive impairment are less likely to experience symptom remission within the first few months of medication administration. Such information may help the treating psychiatrist to convey to the patient realistic expectations for rate of recovery, and that may reduce treatment noncompliance.
Prior studies in this area have hypothesized that there are specific neuropsychological deficits that predict SSRI response, and focused their test selection accordingly. In contrast, the broader-based assessment battery used in the current study suggests the cognitive deficit in nonresponders may be more global in scope. A diffuse cognitive deficit in nonresponders means that administering select tests to a modest-sized sample can give rise to misleading results regarding particular cognitive differences, so discrete deficits in this group must be viewed within the context of overall cognitive impairment. The multivariate analysis conducted with the current sample size was capable of detecting a responder/nonresponder difference across cognitive functioning scores, but a domain by response interaction did not reach significance. Further studies to confirm the nature of nonresponders’ cognitive deficits will need larger samples as well as a comprehensive battery to have the necessary statistical power to detect domains with exceptional impairment.
Examination of the profiles of test scores in the current study suggests that a specific group of cognitive deficits may best characterize patients with poor SSRI treatment outcome. Though nonresponders had lower scores in all tests, the most pronounced differences between responders and nonresponders were found in the working memory, language and executive function domains. This cluster of cognitive processing impairments in nonresponders was detected despite nonsystematic administration of pharmacological treatment. This is also the first study to include a set of tests of working memory to investigate SSRI treatment response, and to report group differences in this area of neuropsychological functioning. Poorer verbal fluency among nonresponders has been reported across studies (Kalayam and Alexopoulos 1999; Taylor et al. 2006) and this test is found to be highly predictive of nonresponse (Taylor et al. 2006). Prior findings with the WCST were equivocal (Dunkin et al. 2000; Taylor et al. 2006) and group differences were not detected in the current sample, but nonresponder deficits in other tests typically considered measures of executive functions have been reported (Alexopoulos et al. 2005; Kampf-Sherf et al. 2004; Marcos et al. 2005).
The detection of poorer working memory in nonresponders is a critical finding. Working memory appears to be the aspect of cognition maximally compromised in this group. It involves both storage of information as well as the manipulation of these cognitive contents by executive processes (Nebes et al. 2000). Because this is a dynamic process conducted to support simultaneous cognitive operations, working memory is negatively impacted by reduced cognitive processing speed (Salthouse 1992), and plays a key role in the execution of higher-level functions (Gathercole 1994). SSRI nonreponders may be experiencing a type of cognitive slowing that affects working memory, and this impairment is therefore best detected by tests that contain greater mental search, manipulation and analysis demands. Nonresponders did not have deficits of the same magnitude on tests that required speeded output of motor responses but lacked a substantial cognitive processing load. This pattern of function identifies reduced mental processing speed as the core cognitive weakness predictive of SSRI nonresponse, but does not necessarily include motor slowing as concluded by Taylor et al. (2006). Working memory deficits may then mediate the executive dysfunction noted in nonresponders in this study as well as in other reports (Baldwin et al. 2004; Dunkin et al. 2000; Marcos et al. 2005). Slowed thinking can interfere with the capacity to mentally maintain the information needed to formulate efficient strategies, and manage the planning, sequencing and reasoning demands of complex executive function tasks. In addition, verbal fluency deficits in depression have been found to reflect slowed processing speed rather than executive dysfunction (Henry and Crawford 2005). Reduced cognitive pace in nonresponders suggesting diffuse changes in brain functioning, rather than a circumscribed area of deficit, is associated with poor treatment response to SSRIs.
Slowed thinking and working memory deficits have been associated with dopaminergic disturbance in other clinical populations (Gilbert et al. 2005; Goldman-Rakic et al. 2004; Lewis et al. 2005). Taylor et al. (2006) likewise suggest that altered dopaminergic functioning in depressed nonresponders leads to a specific profile of cognitive impairment related to generalized slowing. More pronounced cognitive slowing in depression may then attest to the need to consider pharmacotherapy that addresses a broad range of neurochemical deficits. It is also possible that these patients would be more responsive to antidepressant medications with a different mechanism of action than that of SSRIs. Prior studies provide some indication that clinical ratings of psychomotor disturbance, which includes slowed thinking, are associated with positive treatment response to tricyclic agents (Downing and Rickels 1972; Joyce and Paykel 1989; Sobin and Sackeim 1997) but with poor response to SSRIs (Flament et al. 1999; Simpson et al. 1998).
Our findings additionally suggest that cognitive test performance may predict clinical response across a range of SSRI medications. This study is limited by its small sample size and lack of medication control. Nonetheless, the results lend support to the need for further work to explore the role of cognition in the prediction of antidepressant response, using larger samples treated within controlled clinical trials. Including a set of tests of cognitive function within antidepressant medication studies might offer essential information regarding moderators of treatment efficacy, and subsequently inform treatment planning.
Conclusion
While depression is associated with a range of cognitive deficits, patients with more severe impairments appear to be at risk for poor treatment outcome with SSRI medications.
The administration of a comprehensive battery rather than select tests of a few neuropsychological functions reveals a global cognitive deficit in nonresponder patients, unrelated to premorbid intellectual functioning or depression severity. Previously unreported differences in working memory, however, further suggest that nonresponders are distinguished by a generalized cognitive slowing which impairs their performance on tests with processing speed, mental manipulation and strategic planning demands. To the extent that slowed thinking is associated with poor clinical response to SSRIs, and has been connected to dopaminergic disturbance in other clinical populations, reduced cognitive pace measured by neuropsychological test performance may attest to the need to consider pharmacotherapy that addresses a broad range of neurochemical deficits. Further studies using cognitive assessment within controlled treatment trials may clarify the nature of cognitive deficits in SSRI nonresponder patients.
Acknowledgments
This research project was supported by National Institute of Mental Health grants MH-062155 and MH-062185, as well as awards from NARSAD and the American Foundation for Suicide Prevention to Dr. Keilp, and an award from the American Foundation for Suicide Prevention to Dr. Gorlyn. The authors thank Micky Gerchak for editing the manuscript.
Contributor Information
Marianne Gorlyn, Department of Neuroscience, New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY, USA; Unit 42, 1051 Riverside Drive, New York, NY, USA.
John G. Keilp, Department of Neuroscience, New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY, USA
Michael F. Grunebaum, Department of Neuroscience, New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY, USA
Bonnie P. Taylor, Department of Biopsychology, New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY, USA
Maria A. Oquendo, Department of Neuroscience, New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY, USA
Gerard E. Bruder, Department of Biopsychology, New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY, USA
Jonathan W. Stewart, Department of Therapeutics, New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY, USA
Gil Zalsman, Department of Neuroscience, New York State Psychiatric Institute, New York, NY, USA; Department of Psychaitry, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
J. John Mann, Department of Neuroscience, New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY, USA.
References
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Baldwin R, Jeffries S, Jackson A, Sutcliffe C, Thacker N, Scott M, Burns A. Treatment response in late-onset depression: relationship to neuropsychological, neuroradiological and vascular risk factors. Psychol Med. 2004;4:125–136. doi: 10.1017/s0033291703008870. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Gentili V, Eberson S, Kelsoe J, Rapaport M, Gillin JC. A quantitative neuromotor predictor of antidepressant non-response in patients with major depression. J Affect Disord. 2003;77:135–141. doi: 10.1016/s0165-0327(02)00107-6. [DOI] [PubMed] [Google Scholar]
- Downing RW, Rickels K. Predictors of amitriptyline response in outpatient depressives. J Nerv Ment Dis. 1972;154:248–263. doi: 10.1097/00005053-197204000-00003. [DOI] [PubMed] [Google Scholar]
- Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord. 2000;60:13–23. doi: 10.1016/s0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, Quitkin FM, Wisniewski S, Lavori PW, Rosenbaum JF, Kupfer DJ. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psych Clin North Am. 2003;26:457–494. doi: 10.1016/s0193-953x(02)00107-7. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders (SCID) New York State Psychiatric Institute, Biometrics Research; New York, NY: 1994. [Google Scholar]
- Flament MF, Lane RM, Zhu R, Ying Z. Predictors of an acute antidepressant response to fluoxetine and sertraline. Int Clin Psychopharmacol. 1999;14:259–275. [PubMed] [Google Scholar]
- Gathercole SE. Neuropsychology and working memory: a review. Neuropsychology. 1994;8:494–505. [Google Scholar]
- Gilbert B, Belleville S, Bherer L, Chouinard S. Study of verbal working memory in patients with Parkinson’s disease. Neuropsychology. 2005;19:106–114. doi: 10.1037/0894-4105.19.1.106. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J, Crawford JR. A meta-analytic review of verbal fluency deficits in depression. J Clin Exp Neuropsychol. 2005;27:78–101. doi: 10.1080/138033990513654. [DOI] [PubMed] [Google Scholar]
- Joyce PR, Paykel ES. Predictors of drug response in depression. Arch Gen Psychiatry. 1989;46:89–99. doi: 10.1001/archpsyc.1989.01810010091014. [DOI] [PubMed] [Google Scholar]
- Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56:713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- Kampf-Sherf O, Zlotogorski Z, Gilboa A, Speedie L, Lereya J, Rosca P, Shavit Y. Neuropsychological functioning in major depression and responsiveness to selective serotonin reuptake inhibitors antidepressants. J Affect Disord. 2004;82:453–459. doi: 10.1016/j.jad.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Brodsky BS, Oquendo MA, Malone KM, Mann JJ. Neuropsychological dysfunction in depressed suicide attempters. Am J Psychiatry. 2001;158:735–741. doi: 10.1176/appi.ajp.158.5.735. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Mann JJ. Correlates of trait impulsiveness in performance measures and neuropsychological tests. Psychiatry Res. 2005;135:191–201. doi: 10.1016/j.psychres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia. 2005;43:823–832. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Marcos T, Portella MJ, Navarro V, Gasto C, Rami L, Lazaro L, Salamero M. Neuropsychological prediction of recovery in late-onset major depression. Int J Geriatr Psychiatry. 2005;20:790–795. doi: 10.1002/gps.1363. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, Reynolds CF., III Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med. 2000;30:679–691. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, Ritz L, Biggs MM, Warden D, Luther JF, Shores-Wilson K, Niederehe G, Fava M. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354:1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Influence of processing speed on adult age differences in working memory. Acta Psychol (Amst) 1992;79:155–170. doi: 10.1016/0001-6918(92)90030-h. [DOI] [PubMed] [Google Scholar]
- Simpson S, Baldwin RC, Jackson A, Burns AS. Is subcortical disease associated with a poor response to antidepressants? Neurological, neuropsychological and neuroradiological findings in late-life depression. Psychol Med. 1998;28:1015–1026. doi: 10.1017/s003329179800693x. [DOI] [PubMed] [Google Scholar]
- Sobin C, Sackeim HA. Psychomotor symptoms of depression. Am J Psychiatry. 1997;154:4–17. doi: 10.1176/ajp.154.1.4. [DOI] [PubMed] [Google Scholar]
- Taylor BP, Bruder GE, Stewart JW, McGrath PJ, Halperin J, Ehrlichman H, Quitkin FM. Psychomotor slowing as a predictor of fluoxetine nonresponse in depressed outpatients. Am J Psychiatry. 2006;163:73–78. doi: 10.1176/appi.ajp.163.1.73. [DOI] [PubMed] [Google Scholar]


