Summary
Objectives
Vascular graft infections arise from bacterial colonization of either the external or internal graft surfaces. We assessed whether methicillin-resistant Staphylococcus aureus (MRSA) and Escherichia coli could translocate through pores of ePTFE grafts.
Methods
To assess translocation from the internal to the external surface, we placed 105 cfu of bacterial suspension inside ePTFE graft segments and suspended them in sterile broth for 72 h. To assess translocation from the external to the internal surface, we placed sterile broth inside ePTFE segments, and incubated them for 72 h in a bacterial suspension (105 cfu/mL). At 72 h, in addition to culturing the sterile broth and bacterial suspensions, the external and internal surfaces were first qualitatively cultured separately and then quantitatively cultured by sonication.
Results
At 72 h, the sterile broth remained sterile. The bacterial suspensions yielded 107–109 cfu/mL. Graft cultures indicated that colonization of one surface with either organism did not result in bacterial translocation to the other surface. Quantitative bacterial counts of the external vs. internal surfaces were significantly different (p < 0.01).
Conclusions
MRSA and E. coli do not translocate across ePTFE graft surfaces. These in-vitro findings help elucidate the pathogenesis of graft infections and prompt conduction of validation studies in-vivo.
Keywords: ePTFE grafts, Staphylococcus aureus, Escherichia coli, Translocation
Introduction
Open vascular bypass surgery has long been the standard-of-care for surgical treatment of peripheral vascular disease. Over 85,000 open vascular bypass surgeries have been performed annually in the United States between 1996 and 2005.1 Despite routine peri-operative antibiotic prophylaxis, 0.5–5% of all vascular grafts become infected. Different graft surfaces may vary in their propensity to bacterial colonization and infection.2 Dacron and expanded polytetrafluoroethylene (ePTFE) are the two most clinically used synthetic grafts for correction of vascular disease. Dacron grafts are composed of knitted or woven polyethylene terephthalate fiber, whereas ePTFE grafts have a characteristic node-fibril lattice structure with an average inter-nodal distance (pore size) of 30 μm for a standard graft.3 Pore size can affect the rate of development of the neointima and endothelialization, which is believed to protect against infection.4 Graft infections can originate either from bacterial colonization of the external surface or from endovascular seeding of the internal surface, but it is unknown if bacteria can translocate from one surface of these porous grafts to another. This question merits assessment because it is critical for understanding the pathogenesis of graft infections. In the current investigation, we examined translocation of methicillin-resistant Staphylococcus aureus (MRSA) and Escherichia coli across ePTFE graft surfaces.
Materials and methods
To assess bacterial translocation, we tested clinical isolates of MRSA and E. coli that had caused catheter-associated bacteraemia. We chose these two organisms in our experimental design to provide data for both gram-positive cocci and gram-negative bacilli commonly implicated in graft infections. We utilized Exxcel Soft standard wall ePTFE vascular grafts (Boston Scientific/Medi-Tech, Wayne, NJ). Six sets of each of the two experiments mentioned below were conducted for each MRSA and E. coli.
Experiment 1
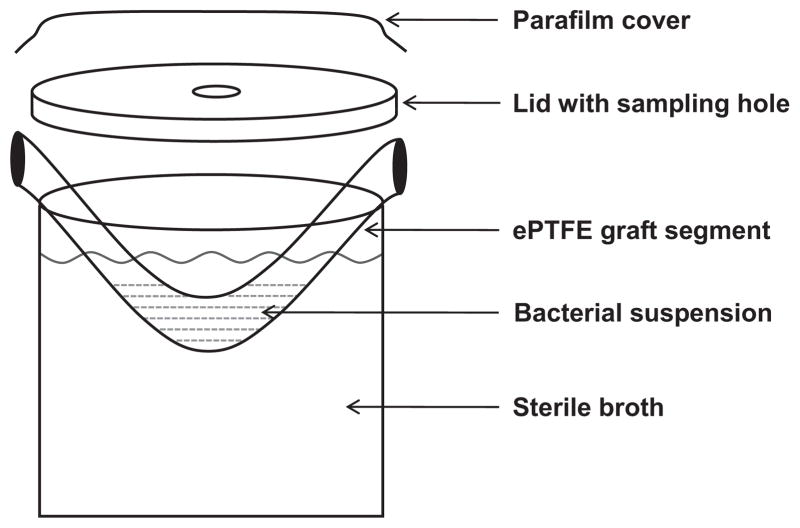
To assess bacterial translocation from the internal surface to the external surface of ePTFE grafts, 1 mL aliquot of bacterial suspension of MRSA or E. coli containing 105 cfu/mL was placed inside the lumen of a 5-cm segment of ePTFE graft. We used a bacterial concentration of 105 cfu based on previous studies of biofilm creation on vascular catheters.5 The graft segments were suspended at 37 °C for 72 h in sterile containers filled with sterile tryptic soy broth (TSB) such that the ends of the graft segment were secured underneath the lid of the container, forming an ‘U’ shape (Fig. 1). This ensured that there was no direct communication between the bacteria within the graft lumen and the TSB in the container. The assembly was sealed with parafilm to avoid external contamination. The sterile TSB around the graft segment was assessed for cloudiness indicative of bacterial growth and 0.1 mL samples were collected at 2, 4, 6, 24, 48 and 72 h intervals. At 72 h, the graft segment was removed from the sterile container and a 0.1 mL aliquot of the bacterial suspension inside the graft was collected to quantitatively assess bacterial counts. The external surface of each graft segment was cultured by roll-plating thrice onto tryptic soy agar (TSA) plates that contained 5% sheep blood (Remel, Lenexa KS) and the internal surface was cultured thrice onto TSA blood agar plates using three sterile foam swabs. Each graft segment was then washed thrice in 45 mL of sterile normal saline (NS) to remove planktonic organisms, sectioned into three 1-cm sections, which were then sonicated in 1 mL of sterile NS for 10 min. Aliquots of the sonicate (0.1 mL) and successive dilutions were plated onto TSA blood plates and colony counts were assessed 24 h after incubation (limit of detection = 10 cfu/mL). The median colony count from the three sections was considered as the representative observation of each set of experiments.
Figure 1.
Experimental assembly assessing bacterial translocation from the internal to the external graft surface.
Experiment 2
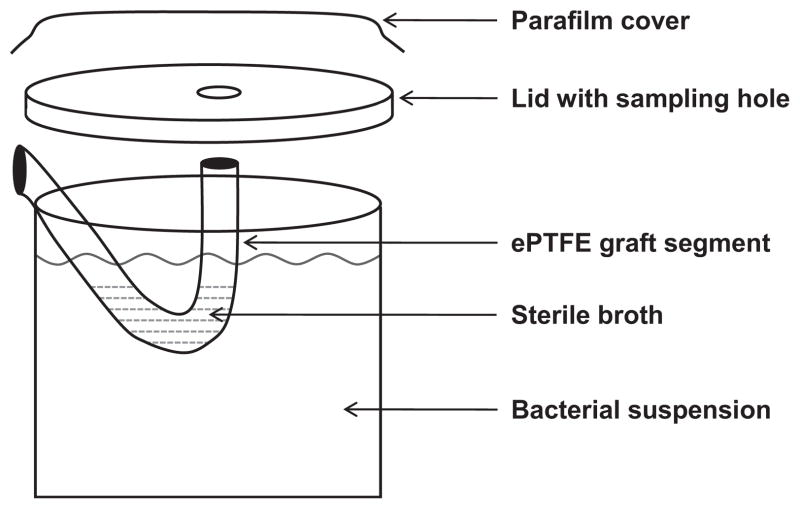
To investigate the possibility of bacterial translocation from the external to the internal graft surface, we placed 1 mL of sterile TSB inside 5-cm segments of ePTFE grafts and suspended them in sterile containers filled with 125 mL of either MRSA or E. coli suspension containing 105 cfu/mL, as described above (Fig. 2). The assembly was sealed with parafilm to avoid external contamination. Seventy-two hours after incubation of the assembly at 37 °C, the sterile TSB and the bacterial suspension were cultured quantitatively. The external surface of each graft segment was cultured by roll-plating thrice on to TSA blood agar plates and the internal surface was swabbed thrice. Each graft segment was then washed thrice in 45 mL of sterile NS, cut into three 1-cm sections, sonicated and quantitatively cultured as described above. The median colony count of the three sections was considered the representative observation of each set of experiments.
Figure 2.
Experimental assembly assessing bacterial translocation from the external to the internal graft surface.
To assess the significance of bacterial translocation, the Fisher’s exact test (SAS v9.0, Cary, NC) was used to compare the frequency of translocation from the external and internal graft surfaces.
Results
Experiment 1
The results were similar for both MRSA and E. coli. Samples of the TSB surrounding the grafts segments taken at 2, 4, 6, 24, 48 and 72 h were all sterile. At 72 h after incubation, cultures of the luminal bacterial inocula yielded 107–109 cfu/mL of MRSA or E. coli (Table 1). Roll-plate culture of the external surface did not yield any bacterial growth. On visualization of the internal surface after washing with saline, an adherent layer of biofilm was noted. Swab cultures of the internal surface showed confluent bacterial growth. Quantitative sonication cultures of the graft segments yielded 103–107 cfu of MRSA or E. coli, which were presumably embedded within the layer of biofilm along the luminal surface of the graft (Table 2). Comparisons of bacterial counts from the sterile TSB outside the graft vs. the luminal bacterial suspensions as well as the external vs. internal surface of the graft segments yielded significant differences (p < 0.01) at 72 h for both tested organisms.
Table 1.
Bacterial counts obtained from the external sterile tryptic soy broth (TSB) and bacterial suspensions inside the graft segments after 72 h of incubation (Experiment 1).
| Experiment number | MRSA
|
Escherichia coli
|
||
|---|---|---|---|---|
| External TSB | Luminal bacterial suspensiona | External TSB | Luminal bacterial suspensiona | |
| 1 | 0 | 1.66 × 108 | 0 | 1.9 × 109 |
| 2 | 0 | 8.2 × 107 | 0 | 3.02 × 109 |
| 3 | 0 | 3.67 × 107 | 0 | 1.62 × 109 |
| 4 | 0 | 4.2 × 107 | 0 | 1.61 × 109 |
| 5 | 0 | 1.74 × 107 | 0 | 1.28 × 109 |
| 6 | 0 | 4 × 107 | 0 | 3.22 × 109 |
Two-sided Fisher’s exact test p < 0.01 for comparison of bacterial counts of the luminal bacterial suspensions and the external TSB.
Table 2.
Results of qualitative cultures of the external graft surface and quantitative sonication cultures of the internal graft surface after 72 h of incubation (Experiment 1).
| Experiment number | MRSA
|
Escherichia coli
|
||
|---|---|---|---|---|
| External surface | Internal surfacea,b | External surface | Internal surfacea,b | |
| 1 | 0 | 2.9 × 105 | 0 | 1.23 × 105 |
| 2 | 0 | 6.4 × 103 | 0 | 3.1 × 106 |
| 3 | 0 | 7 × 104 | 0 | 1.5 × 107 |
| 4 | 0 | 5.9 × 103 | 0 | 7 × 106 |
| 5 | 0 | 2.2 × 104 | 0 | 3.5 × 105 |
| 6 | 0 | 1.6 × 104 | 0 | 1.9 × 105 |
Each observation represents the median colony count for three sections of a unique graft.
Two-sided Fisher’s exact test p < 0.01 for comparison of bacterial colony counts obtained from the internal and the external graft surfaces.
Experiment 2
The results were similar for both MRSA and E. coli. At 72 h after incubation, the TSB inside the graft segments remained sterile and culture of the bacterial suspensions surrounding the external surfaces of the grafts yielded 107–109 cfu/mL of MRSA or E. coli (Table 3). On visualization, the external surface was noted to have an adherent layer of biofilm that persisted after washing. Roll-plate cultures of the external surface of the graft segments showed confluent growth of bacteria, but swab cultures of the internal graft surface did not yield any bacterial growth. Quantitative sonication cultures of the graft segments yielded 105–108 cfu of MRSA or E. coli, which were presumably embedded within the layer of biofilm along the external graft surface (Table 4). Comparisons of bacterial counts from the bacterial suspension surrounding the graft segments vs. the sterile TSB inside the lumen of the graft as well as the external vs. internal surface of the graft segments yielded significant differences (p < 0.01) for both tested organisms at 72 h.
Table 3.
Bacterial counts obtained from the external bacterial suspensions and sterile tryptic soy broth (TSB) inside the graft segments after 72 h of incubation (Experiment 2).
| Experiment number | MRSA
|
Escherichia coli
|
||
|---|---|---|---|---|
| External bacterial suspensiona | Luminal TSB | External bacterial suspensiona | Luminal TSB | |
| 1 | 2.09 × 107 | 0 | 1.78 × 109 | 0 |
| 2 | 6.6 × 106 | 0 | 1.74 × 109 | 0 |
| 3 | 1.75 × 107 | 0 | 1.45 × 109 | 0 |
| 4 | 9.6 × 107 | 0 | 1.31 × 109 | 0 |
| 5 | 7.4 × 107 | 0 | 1.46 × 109 | 0 |
| 6 | 4 × 107 | 0 | 1.34 × 109 | 0 |
Two-sided Fisher’s exact test p < 0.01 for comparison of bacterial counts of the external bacterial suspensions and the luminal TSB.
Table 4.
Results of qualitative cultures of the internal graft surface and the quantitative sonication cultures of the external graft surface after 72 h of incubation (Experiment 2).
| Experiment number | MRSA
|
Escherichia coli
|
||
|---|---|---|---|---|
| External surfacea,b | Internal surface | External surfacea,b | Internal surface | |
| 1 | 1.51 × 106 | 0 | 3.72 × 107 | 0 |
| 2 | 3.92 × 105 | 0 | 1.13 × 108 | 0 |
| 3 | 1.43 × 105 | 0 | 1.01 × 108 | 0 |
| 4 | 1.54 × 106 | 0 | 2.46 × 108 | 0 |
| 5 | 1.47 × 105 | 0 | 6.6 × 107 | 0 |
| 6 | 2.77 × 106 | 0 | 8.4 × 108 | 0 |
Each observation represents the median colony count for three sections of a unique graft.
Two-sided Fisher’s exact test p < 0.01 for comparison of bacterial colony counts obtained from the external and the internal graft surfaces.
Discussion
Several studies have assessed biofilm formation on graft surfaces by quantitative cultures and microscopy techniques, but the ability of bacteria to translocate across graft pores has not been assessed.6,7 We investigated this issue as it can have significant impact on interventional strategies such as the use of antimicrobial coating of graft surfaces for the prevention of graft infection. Our results demonstrate that MRSA and E. coli do not translocate across the ePTFE graft surface, despite visible biofilm formation on the graft surface exposed to the organisms. Since the qualitative cultures of the graft surface in contact with the TSB did not show any bacterial growth and the TSB itself remained sterile at 72 h, the quantitative bacterial counts from our sonication cultures can be presumed to have been derived from the biofilm on the graft surface in contact with the bacterial suspension. Although one would expect that bacteria, which have an average diameter of 0.5–2 μm, would pass through the 30-μm pores of ePTFE grafts, it is possible that sessile bacteria in biofilms are unable to pass through the ePTFE graft pores. It is unclear whether the graft wall thickness (0.2–0.4 mm) impedes the ability of bacteria to traverse across the wall or if the cross-linking of the fibrils is such that the pores are not lined up, thus constituting a physical barrier.
Vascular graft infections are one of the most feared complications of arterial re-vascularization surgeries. Aortic graft infections are associated a one-year mortality of 37% and a limb-amputation rate of 17%, whereas inguinal graft infections have a 18% mortality rate with a 40% amputation rate over the same time period.8,9 Complete eradication of infection requires removal of the infected prosthetic material and extensive debridement of devitalized tissue.10 Early graft infections that occur within the first 3 months after implantation are more common than late graft infections and are thought to arise from intra-operative contamination or bacterial spread from post-operative wound infections to the external graft surface.8 Such graft infections are usually monomicrobial and often have a fulminant course. Bacterial seeding of the luminal surface from haematogenous spread is thought to account for majority of late graft infections, typically caused by indolent organisms such as Staphylococcus epidermidis.7,8 This is reminiscent of the literature on biofilm formation around implanted vascular catheters.11 Vulnerability to endovascular seeding declines with endothelialization and neointimal development, and the rates of neointimization and patency are associated with pore size.3,12 Despite the porous nature of grafts, studies show that endothelialization in humans may be incomplete for several years after implantation and is usually limited to 1–2 cm of each anastomosis.4,13
Our demonstration of the absence of bacterial translocation through ePTFE graft pores in-vitro represents a crucial step in elucidating the pathogenesis of vascular graft infections and prompts further experiments with other graft materials and various pathogens. Since it is possible that bacterial translocation may occur from one surface to another at the site of anastomoses, we plan to further validate our results in a rabbit model of graft infection. Since translocation could not be detected when bacteria were suspended in culture media in-vitro, we anticipate that translocation will also not occur in physiologic conditions that utilize serum/plasma. Improved understanding of bacterial translocation across and adherence to graft surfaces will help design interventions directed at preventing graft infection such as selective surface modification of vascular graft surfaces with antimicrobial and/or anti-adherence agents, as has been accomplished with other vascular devices.14 Since contamination of the external surface accounts for the vast majority of early graft infections, and the internal surface is relatively protected due to endothelialization, exclusively coating the external surface of grafts with antimicrobial and/or anti-biofilm agents may constitute a viable approach for decreasing graft infection. The anticipated minimal compromise of the surface properties of the endovascular surface would ensure the safety of this intervention. Coating the internal graft surface is a much less desirable alternative, because the blood circulation would constantly leach away the anti-infective coating, thus making the residual anti-infective activity against late graft infections occurring years after implantation minimal. In addition, alteration of the surface properties of the luminal surface carries the risk of increased thrombogenicity of the graft. The lack of translocation across these porous devices will allow us to independently assess bacterial adherence to the external vs. internal graft surfaces, which is the next step in research directed towards surface modification.
We chose to use ePTFE grafts in this study because they are widely used in repairing medium-sized blood vessels, including aortofemoral and femoropopliteal grafts and the risk of vascular graft infection is anatomically greatest at the inguinal region.6 The results of our study are not generalizable to other graft materials, such as Dacron, that have a bigger pore size. We assessed the possible translocation of MRSA and E. coli to serve as examples of gram-positive cocci and gram-negative bacilli because they are clinically relevant pathogens with variations in size and shape which could theoretically affect bacterial translocation across vascular grafts. Though we have not included S. epidermidis, a commonly implicated pathogen in late graft infections in this study, since it is comparable in size and lack of motility to S. aureus, and has similar numbers of organisms in biofilm state, we expect that it will behave similar to S. aureus and be unable to translocate through the intact ePTFE graft pores.
We plan to direct our future studies towards in-vivo validation of the results of this study as well as investigating if coating the external ePTFE graft surface with a combination of antimicrobial/anti-biofilm agents would be an effective strategy in preventing bacterial adherence and preventing early graft infection.
Acknowledgments
Portions of this work were presented at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy (Abstract K-306), San Francisco, CA, USA in September 2009 and at the 47th Infectious Diseases Society of America Meeting (Late-breaker-30), Philadelphia, PA, USA in October 2009.
We would like to express our sincere gratitude to Mike Spratt of Medical Media Section of Education Service Line at Michael E. DeBakey VA Medical Center for his technical help with the illustrations.
Funding
This study was supported by Baylor College of Medicine and the Michael E. DeBakey VA Medical Center, Houston, TX. S.A. has received support from NIH/NIDDK 1K23DK078828-01 A2.
This work was not funded by industry.
Footnotes
Conflict of interest statement
All authors have no relevant financial conflicts and have nothing to declare.
References
- 1.Rowe VL, Lee W, Weaver FA, Etzioni D. Patterns of treatment for peripheral arterial disease in the United States: 1996–2005. J Vasc Surg. 2009;49(4):910–7. doi: 10.1016/j.jvs.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 2.Wilson SE. New alternatives in management of the infected vascular prosthesis. Surg Infect. 2001;2(2):171–5. doi: 10.1089/109629601750469492. [DOI] [PubMed] [Google Scholar]
- 3.Xue L, Griesler HP. Biomaterials in the development and future of vascular grafts. J Vasc Surg. 2003;37(2):472–80. doi: 10.1067/mva.2003.88. [DOI] [PubMed] [Google Scholar]
- 4.Hazama K, Miura H, Shimada T, Okada Y, Murashita T, Nishibe T. Relationship between fibril length and tissue in-growth in the healing of expanded polytetrafluoroethylene grafts. Surg Today. 2004;34:685–9. doi: 10.1007/s00595-004-2774-9. [DOI] [PubMed] [Google Scholar]
- 5.Aslam S, Trautner BW, Ramanathan V, Darouiche RO. Combination of tigecycline and N-acetylcysteine reduces biofilm-embedded bacteria on vascular catheters. Antimicrob Agents Chemother. 2007;51(4):1556–8. doi: 10.1128/AAC.00893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmacht D, Armstrong P, Johnson B, Pierre K, Back M, Honeyman A, et al. Graft infectivity of rifampin and silver-bonded polyester grafts to MRSA contamination. Vasc Endovascular Surg. 2005;39(5):411–20. doi: 10.1177/153857440503900505. [DOI] [PubMed] [Google Scholar]
- 7.Kaebnick HW, Bandyk DF, Bergamini TW, Towne JB. The microbiology of explanted vascular prostheses. Surgery. 1987;102(4):756–62. [PubMed] [Google Scholar]
- 8.Chambers ST. Diagnosis and management of staphylococcal infections of vascular grafts and stents. Intern Med J. 2005;35:S72–S78. doi: 10.1111/j.1444-0903.2005.00981.x. [DOI] [PubMed] [Google Scholar]
- 9.Quinones-Baldrich WJ, Hernandez JJ, Moore WS. Long-term results following surgical management of aortic graft infection. Arch Surg. 1991;126:507–11. doi: 10.1001/archsurg.1991.01410280111018. [DOI] [PubMed] [Google Scholar]
- 10.Bunt TJ. Vascular graft infections: an update. Cardiovasc Surg. 2001;9:225–33. doi: 10.1016/s0967-2109(00)00104-6. [DOI] [PubMed] [Google Scholar]
- 11.Raad I. Intravascular-catheter-related infections. Lancet. 1998;351:893–8. doi: 10.1016/S0140-6736(97)10006-X. [DOI] [PubMed] [Google Scholar]
- 12.Campbell CD, Goldfarb D, Roe R. A small arterial substitute: expanded polytetrafluoroethylene: patency versus porosity. Ann Surg. 1975;182(2):138–43. doi: 10.1097/00000658-197508000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathiasen SR, Wu HD, Sauvage LR, Usui Y, Walker MW. An experimental study of eight current arterial prosthesis. J Vasc Surg. 1986;4:33–41. doi: 10.1067/mva.1986.avs0040033. [DOI] [PubMed] [Google Scholar]
- 14.Darouiche RO, Raad II, Heard SO, Thornby JI, Wenker OC, Gabrielli A, et al. A comparison of two antimicrobial-impregnated central venous catheters. Catheter Study Group. N Engl J Med. 1999;340(1):1–8. doi: 10.1056/NEJM199901073400101. [DOI] [PubMed] [Google Scholar]