Abstract
Increased oxidative stress occurs in the lungs and systemically in COPD, which plays a role in many of the pathogenic mechanisms in COPD. Hence, targeting local lung and systemic oxidative stress with agents that modulate the antioxidants/redox system or boost endogenous antioxidants would be a useful therapeutic approach in COPD. Thiol antioxidants (N-acetyl-L-cysteine and N-acystelyn, carbocysteine, erdosteine, and fudosteine have been used to increase lung thiol content. Modulation of cigarette smoke induced oxidative stress and its consequent cellular changes have also been reported to be effected by synthetic molecules, such as spin traps (α-phenyl-N-tert-butyl nitrone), catalytic antioxidants (superoxide dismutase [ECSOD] mimetics), porphyrins, and lipid peroxidation and protein carbonylation blockers/inhibitors (edaravone and lazaroids/tirilazad). Pre-clinical and clinical trials have shown that these antioxidants can reduce oxidative stress, affect redox and glutathione biosynthesis genes, and pro-inflammatory gene expression. In this review the approaches to enhance lung antioxidants in COPD and the potential beneficial effects of antioxidant therapy on the course of the disease are discussed.
Keywords: Cigarette smoke, antioxidants, oxidants, glutathione, thiols, Nrf2, Chronic Obstructive Pulmonary Disease
Introduction
The lungs due to their high blood supply and large surface area are constantly in a high-oxygen environment. In addition the lung epithelium is also constantly exposed to oxidants generated endogenously during respiration from mitochondrial electron transport, from activated inflammatory cells that influx into the lungs and exogenously from cigarette smoke (CS) and air pollutants, such as ozone, nitrogen dioxide, and combustion particulates, as a result of its exposure to the environment.
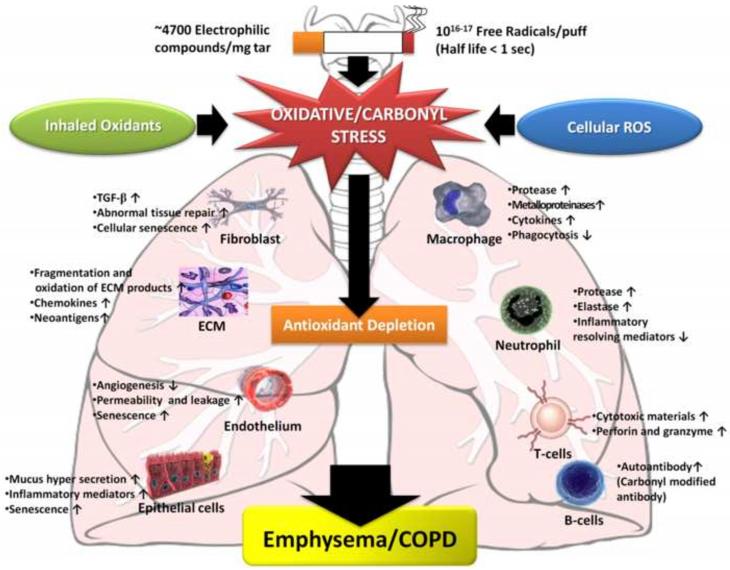
When the the resident antioxidants are insufficient or fail to upregulate sufficiently to neutralize an increased oxidant burden oxidative stress occurs. Reactive oxygen species (ROS), either non-radical, such as hydrogen peroxide (H2O2) or oxygen radicals, such as superoxide anion (O2•−) and the hydroxyl radical (•OH) that are highly unstable species with unpaired electrons are capable of initiating oxidation, and together with reactive nitrogen species (RNS) result in a variety of adverse consequences ranging from cell necrosis, senescence, apoptosis, autophagy, lipid peroxidation and protein carbonylation, inflammatory responses, epigenetic changes, remodeling of extracellular matrix and blood vessels, endothelial dysfunction, inactivation of antiproteases, mucus hypersecretion, and impaired tissue repair [1]. COPD is also a disease associated aging, which has been shown to result in a decline in the endogenous antioxidant defenses resulting in less protection against oxidative stress. The pathogenesis of COPD involves several processes as described above. All of these processes are intimately associated with oxidative stress (Fig 1) [1•].
Figure 1. Consequences of oxidative stress in COPD.
The pathogenesis of COPD involves several oxidative stress-induced cellular and molecular processes. Oxidative stress imposed by inhaled oxidants or produced from endogenous sources can lead to depletion of antioxidants. An oxidant/antioxidant imbalance in favor of oxidants leads to activation of various cellular processes which result in cellular and molecular events involved in pathogenesis of COPD.
Rationale for antioxidants therapy in COPD
Cigarette smoke is the main etiological factor in pathogenesis of COPD and contains more than 1015-17 oxidant/free radical molecules per puff [2], which increases oxidant burden in the lungs in current smokers. Since many of the pathogenic mechanisms in COPD involve oxidative stress, oxidative stress should be a target for treatment that may have an effect on underlying disease processes in this condition. This could be achieved either by decreasing the generation of oxidants or by enhancing antioxidants.
Clinical testing of several small-molecular weight compounds that target oxidant/redox signaling, or quench oxidants and reactive aldehydes are currently being conducted. Antioxidant agents, such as thiol compounds/donors and their analogs (GSH and mucolytic drugs, such as N-acetyl-L-cysteine, carbocysteine, erdosteine, and fudosteine all effectively scavenge/detoxify free radicals/oxidants, increase intracellular thiol levels and control NF-κB activation, and hence inhibit inflammatory gene expression. Enzyme mimetics that can either enhance the expression/activity of the antioxidant enzymes or mimic their function are currently being developed. In the ensuing sections, we discuss the beneficial effects of a wide variety of pharmacological antioxidants that are potential therapeutic agents in COPD (Table 1). The efficacy of these antioxidant molecules can be assessed by i) improving symptoms or function, ii) modifying the course of the disease by reducing the decline in lung function or decreasing exacerbation frequency, and iii) decreasing the oxidant burden or biomarkers of oxidative stress in patients with COPD.
Table 1. A list of thiol antioxidants and Nrf2 activators.
| Antioxidant | Structure |
|---|---|
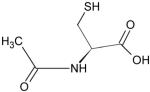
| N-acetyl-L-cysteine |
|
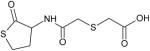
| Erdosteine |
|
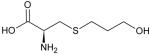
| Fudosteine |
|
| Carbocysteine (S-Carboxymethyl-L-cysteine) |
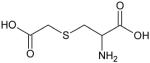
|
| Sulforaphane |
|
|
Triterpenoids- 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) Other analogs of CDDO -1[2-Cyano-3,12-dioxooleana-1,9(11)-dien-28- oyl]imidazole (CDDO-Im) Dihydro-CDDO-Trifluoroethyl Amide CDDO Methyl Amide |
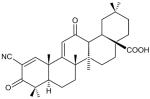
|
Small molecule thiol antioxidants
N-acetyl-L-cysteine (NAC)
NAC is an acetyl derivative of the amino acid, cysteine, and is strong reducing agent (Table 1). NAC is a mucolytic agent that reduces mucus viscosity, thereby improving mucociliary clearance. NAC is deacetylated to cysteine in the gastrointestinal tract which serves as precursor of glutathione. By reducing disulfide bonds, NAC is able to neutralize oxidant species. Since NAC can reduce intracellular cystine to cysteine, it can increase intracellular GSH in vivo in lungs.
NAC is the most widely studied thiol molecule in vitro and in vivo. In preclinical studies oral administration of NAC has been shown to attenuate elastase-induced emphysema in rats [3]. NAC also protects against the oxidation of Z α1-antitrypsin by cigarette smoke in an early-onset emphysema mouse model [4]. In view of the importance of glutathione (GSH) as an antioxidant in the lungs, NAC has mainly been used to enhance lung GSH in patients with COPD [5]. Clinical studies of the beneficial effects of NAC and other thiols in patients with COPD have yielded mixed results (Table 2) [5-19].
Table 2. Clinical trials conducted for the efficacy of thiol antioxidants in smokers and COPD.
| Clinical Trial | Antioxidant | Study Aim | Outcome | Reference |
|---|---|---|---|---|
| BRONCUS | NAC | Effect of NAC on FEV1 | No effect. A reduction in lung over inflation in patients with severe COPD without inhaled glucocorticoids No change on decline in FEV1. Decrease of exacerbation if NAC and Inhaled corticosteroids combined. |
[6-7] |
| Systematic Cochrane review of 23 randomized, controlled trails |
NAC (2 months of oral therapy) |
Effect of NAC and antibiotics on number of days of disability |
No difference in lung function. Significant reduction in days of disability (0.65 day per patient per month) and 29% reduction in exacerbations. |
[8-9] |
| Systematic Cochrane review of randomized, controlled trials; 11 of 39 retrieved trials |
NAC | Use of validated score to evaluate the quality of each study |
9 trials showed prevention of exacerbation and 5 of which addressed improvement of symptoms compared with 34.6% of patients receiving placebo. |
[10] |
| Meta-analysis of published trials |
NAC | Assess possible prophylactic benefits of prolonged treatment |
23% decrease in number of acute exacerbations | [11] |
| - | NAC (600 mg/d, 5 days and 600 mg, 3/d. 5days) |
Effect of NAC on GSH and cysteine |
Increase of GSH at day 5 and cysteine (plasma) at day 5 |
[5] |
| - | NAC (600 mg once a day for 12 months) |
Effect of NAC on H2O2 and TBARS in EBC |
No change in TBARS levels Reduce H2O2 levels |
[12] |
| - | NAC (600 mg/d, 7days) |
Effect of NAC on FEV1, breathlessness |
No difference compared to placebo group | [13] |
| - | NAC (600 mg /d) | Effect of NAC on cytokine and exhaled breath condensate (EBC) |
Decrease IL-8 and ECP level in NAC group | [14] |
| - | NAC (600 mg × 2/d × 2 months) |
Effect of NAC on H2O2 | Decrease H2O2 level in NAC group | [15] |
| EQUALIFE studies | Vectrine-thiol compound, 300mg b.i.d for months |
Effect on exacerbation rate, hospitalization, lung function and quality of life |
Decreased exacerbations and fewer days in hospital. No loss of lung function and improvement in health-related quality of life. |
[16] |
| Small scale UK studies | Carbocysteine | Effect of 2.25–3.00 g carbocysteine daily along with placebo in chronic bronchitis |
Heterogeneous results on alterations in FEV1, peak flow rate and dyspnea scores |
[27-30] |
| An Italian multi-center, prospective, double-blind RCT involving 662 outpatients with a chronic bronchitis |
Carbocysteine | Effect of 2.7 g S-CMC- Lys once daily for 6 months on COPD patients |
No significant difference in baseline FEV1 between the groups. Mean time to first exacerbation was significantly prolonged and significant reduction in mean days of acute respiratory illness per patient. |
[31] |
| A double–blind, parallel- group study in the UK in 109 patients with chronic bronchitis over 6 winter months |
Carbocysteine | Effect of 750 mg carbocysteine three times daily compared with placebo on peak flow and exacerbation rate. |
No significant difference in exacerbation rate. Significant increases in peak flow from baseline in both placebo and intervention groups |
[32] |
| RCTs in Tokyo in 156 patients with COPD over a 12-month period |
Carbocysteine | Effect of 1.5 g carbocysteine daily with placebo |
No significant differences in severity of COPD. Significant reduction in the number of common colds and reduction in rate of exacerbation |
[33-34] |
| RCTs in Japan involving 142 patients with COPD conducted over a 12- month period |
Carbocysteine | Treatment with 500mg carbocysteine three times a day |
Consistent reduction in exacerbation frequency. No change in lung function |
[26] |
| PEACE study EQUALIFE studies |
Carbocysteine (carbocisteine) Vectrine-thiol compound, 300mg bid for months |
Effect on rate of exacerbations Effect on exacerbation rate, hospitalization, lung function, and quality of life |
Long-term (one year) use of carbocysteine (1500 mg/day) produced reduction in numbers of exacerbations in patients with COPD Decreased exacerbations and fewer days in hospital. No loss of lung function and improvement in health-related quality of life |
[17] [23] |
BRONCUS = Bronchitis Randomized on N-acetyl-L-cysteine Cost Utility Study, bid= Twice daily; FEV1 = Forced expiratory volume in 1 second, TBARS = Thiobarbituric acid reactive substances, NAC = N-acetyl-L-cysteine, EBC = Exhaled Breath Condensate, RCTs: Randomized placebo-controlled trials.
A randomized, double-blind, placebo controlled trial of 6-month of 600 mg NAC, twice daily reduced various plasma and BAL fluid oxidative biomarkers in smokers [18]. NAC 600 mg twice daily for 2 months was shown to reduce the oxidant burden in the airways of stable COPD patients [15], and was associated with reduced risk of exacerbations and improved lung symptoms in patients with chronic bronchitis [10]. Another study has shown a beneficial effect of NAC on muscle function by demonstrating an increase in quadriceps endurance time in severe COPD patients associated with a decrease in markers of systemic oxidative stress [20].
A Cochrane systematic review and other meta-analyses [9] showed a decrease in number of exacerbations by 29% . However, the large multicenter trial, the Bronchitis Randomized on NAC Cost-Utility Study (BRONCUS) showed no effect on exacerbation frequency or decline in FEV1 [7••]. However, this study showed a reduction in overinflation and in exacerbation frequency in patients with COPD not treated with inhaled glucocorticoids [7].
NAC has to be deacetylated in the gut to cysteine to act as a precursor of GSH and as such is not very bioavailable to increase GSH. Thus further studies may be warranted using NAC at higher doses (1200 or 1800 mg/day) or using other thiol agents that have a greater bioavailability in order to observe any clinical benefit in COPD.
Carbocysteine
S-carboxymethylcysteine (carbocysteine or S-CMC), which has mucoactive, antioxidant and anti-inflammatory properties, is a thiol derivative of amino-acid, L-cysteine (Table 1). Oral preparations of carbocysteine both as S-CMC and its lysine salt (S-CMC-lys) are available. The lysine residue in S-CMC-lys is cleaved in the gastrointestinal tract to yield the active drug S-CMC. The mucoactive action of carbocysteine differs from other thiol mucolytics, such as NAC and erdosteine since it increases the sialomucin content which influences the rheological properties of mucus via the inhibition of kinins [21]. Carbocysteine also facilitates muco-ciliary clearance velocity, particularly in patients with chronic bronchitis who have slow clearance before treatment [21].
In preclinical studies Carbocysteine has been shown to protect against emphysema induced by cigarette smoke in rats [22]. Treatment of COPD patients with S-CMC-Lys for a 6-months significantly decreased the levels of the lipid peroxidation product 8-isoprostane and the pro-inflammatory cytokine: IL-6, indicating that the drug has both antioxidant and anti-inflammatory properties [23].
Due to its ability to reduce bacterial respiratory tract infections in COPD [24-25], it has been suggested that carbocysteine may act via the inhibition of pathogen adherence to cells. This is supported by in vitro studies, where carbocysteine treatment has been shown to reduce in the adherence of Moraxella catarrhalis (a bacteria commonly found in exacerbations of COPD) to pharyngeal epithelial cells, of both healthy subjects and those with chronic bronchitis, when compared to placebo treated group [24]. Similarly, carbocysteine can significantly reduce attachment of Streptococcus pneumoniae to pharyngeal epithelial cells [25]. Carbocysteine could also reduce the frequency of common colds and associated exacerbations in COPD patients, a property that has been attributed to its ability to decrease ICAM-1 expression in the respiratory tract [26].
Clinical studies of carbocysteine in COPD patients are now available (Table 2) [17,26-34]. The PEACE study investigated the effect of treatment of 709 Chinese COPD subjects for 3 years with carbocysteine (250 mg t.d.s) and found that COPD patients treated with carbocysteine experienced fewer numbers of exacerbations per year [17••]. Of note the majority of these patients were not receiving corticosteroids.
Erdosteine
Erdosteine is a mucoactive thiol antioxidant (Table 1). The drug was originally used as a mucolytic agent and acts by breaking the disulfide bonds of mucus glycoproteins, affecting the physical properties of the mucus, thus leading to increased mucus clearance [35]. It also has antioxidant, anti-inflammatory, and antibacterial activity.
‘Equalife’, a randomized, placebo-controlled clinical study involving oral administration of 300 mg erdosteine twice daily for 8 months produced a significant improvement in quality of health and a reduction in exacerbations compared to placebo [16]. Erdosteine has also been reported to be beneficial in patients suffering from repeated, prolonged or severe exacerbations of COPD [36-37]. In one study treatment with Erdosteine reduced the rate of severe exacerbations requiring hospital admission [35]. Administration of erdosteine 300 mg twice a day for 7 - 10 days also improved symptoms and reduced the duration of hospitalization in patients presenting with an exacerbation of COPD [38]. Erdosteine (600 mg/day) treatment with procysteine has been shown to improve cigarette smoke-induced ROS production by alveolar macrophages and the levels of the chemotactic cytokines IL-6 and IL-8 in bronchial secretions of current smokers with COPD [37]. The anti-inflammatory properties of erdosteine have also been shown by a reduction in the levels of proinflammatory eicosanoids in blood in COPD patients [39].
Fudosteine
Fudosteine, [(−)-(R)-2-amino-3-(3-hydroxypropylthio)] propionic acid (Table 1), has been used and a mulcolytic and antioxidant. It has greater bioavailability than NAC and acts as an antioxidant by increasing intracellular cysteine levels. In preclinical studies, fudosteine inhibits mucin hypersecretion by downregulating MUAC5AC gene expression [40]. Expression levels of p-p38 MAPK and p-ERK in rat in vivo and of p-ERK in a bronchial epithelial cell line in vitro are decreased by fudosteine [40]. Fudosteine has also been shown to inhibit peroxynitrite-induced airway nitrative stress in lung epithelial cells by direct scavenging of this free radical [41]. Hence, fudosteine may be used in the treatment of chronic respiratory diseases, such as bronchial asthma, chronic bronchitis, COPD, and bronchiectasis as a mucoactive agent [40,42].
Nrf2 activators
Nuclear factor erythroid 2 p45-related factor 2 (Nrf2) is a basic-leucine zipper (b-ZIP) transcription factor present in the cytoplasm of normal cells that plays an important protective role against electrophiles and ROS. In response to oxidative and electrophilic stresses, Nrf2 detaches from its cytosolic inhibitory subunit, Kelch-like ECH-associated protein 1 (Keap1), and translocates into the nucleus where it binds to the antioxidant response element (ARE) of target genes [43-45]. Nrf2 regulates almost all the antioxidants and phase II cytoprotective genes, such as NAD(P)H/quinone oxidoreductase 1(NQO1), glutamate cysteine ligase modifier subunit (GCLM), glutamate cysteine synthase, glutathione peroxidase (GPx), and several members of the glutathione S-transferase family [43].
Studies with Nrf2 null mice have shown greater susceptibility of these mice to cigarette smoke-induced emphysema compared with wild-type mice [46-47] indicating a protective role for Nrf2. Loss of Nrf2 positive regulator DJ-1 (stabilizer of Nrf2) and posttranslational modifications of the Keap1–Bach1 equilibrium results in downregulation of Nrf2 in the lungs of patients with COPD [48-51•]. The Nrf2 activator CDDO-imidazolide (Table 1) has been shown to protect mice against CS-induced emphysema [45•]. Activation of Nrf2 by sulforaphane (present in broccoli and cruciferous vegetables) can also have a beneficial effect in attenuating some of biochemical alterations that occur in smokers/COPD [52••].
Novel compounds which are potent activators of Nrf2 or which stabilize Keap1/DJ-1/Maf proteins can be developed. Chalcones have anti-inflammatory effects due to their ability to inhibit the NF-κB pathway [53-54] and simultaneously activate the Nrf2/ARE pathway thus inducing phase II detoxifying enzyme expression [55]. Currently, various derivatives of chalcones are being developed with a potential therapeutic role in COPD [56•]. However, the pharmacokinetics, bioavailability, and toxicity of these compounds in the lungs are as yet unknown.
Lipid peroxidation and protein carbonylation inhibitors/blockers
Edaravone (MC-186)
Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) is a potent free-radical and protein carbonyl scavenger and inhibitor of lipid peroxidation [57-58]. Protein carbonylation and carbonyl stress via aldehydes occur in COPD and hence edaravone has the potential to protect the lungs against the effects of these oxidative products [59-60]. Edaravone has been shown to ameliorate the lung injury, inflammation, oxidative stress, and mortality induced by intestinal ischemia/reperfusion in rats [61]. Given the antioxidant, anti-inflammatory properties of edaravone, it has potential as a treatment in COPD.
Lazaroids
Lazaroids (21-aminsteroids, U75412E or tirilazad mesylate) are a group of non-glucocorticoid analogues of methyl-prednisolone which are able to penetrate hydrophobic regions of the cell membrane, specifically to prevent peroxidation of membrane lipids [62]. The protective effects of lazaroids have been reported in many animal models of lung injury [63-64], including the effects of cigarette smoke [65]. Their protective effect is mainly by inhibition of lipid peroxidation. In a smoke-induced lung injury model lazaroids inhibited the formation of free radicals and the release of tumor necrosis factor-α by alveolar macrophages [66-67]. Further studies are required to evaluate the efficacy of lazaroids as therapeutic strategy in COPD.
Enzymatic antioxidants
Cellular ROS can be effectively neutralized by antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase, whose expression and activities are altered in various disease conditions involving oxidative stress. Restoration of altered antioxidant enzyme activity can be achieved by small molecules possessing catalytic properties which can mimic the activity of the enzyme.
SOD mimetics
SOD mimetics are of three classes. The first class includes several manganese-based macrocyclic ligands, such as M40401, M40403, and M40419 [68-69]. The second class includes manganese-metaloporphyrins, such as AEOL-10113 and AEOL-10150 [70-71], and the third category is comprised of “Salens” (manganese based SOD mimetic). Salens have an additional advantage as they are also reported to have catalase-like activity, and therefore can also neutralize H2O2, and ONOO− [72]. Until now only the second class of SOD mimetics has been studied in animal models of airway inflammation. A significant decrease in the lungs of markers of oxidative stress, and emphysema has been observed in response to the SOD mimetic M40419 in animal models [68]. The SOD mimetic AEOL10150 has also been shown to inhibit cigarette smoke-induced lung inflammation in the rat and to decreased lipid peroxidation and the generation of ONOO− [71]. The ability of recombinant SOD treatment to prevent neutrophil influx into the lungs and decrease IL-8 release induced by cigarette smoking [73], indicates its potential as an antioxidant an anti-inflammatory in COPD.
Broad antioxidant properties and the ability to scavenge superoxide, lipid peroxides, ONOO-, and H2O2 have been attributed to the metalloporphyrin-based catalytic antioxidant, MnTE-2-PyP [Manganese (III) Meso-Tetrakis-(NMethlypyridinium-2-yl) porphyrin] [74-76]. Administration of MnTE-2-PyP has been shown to decrease inflammation and injury induced by wide variety of factors [77-78]. Its anti-inflammatory properties have been attributed to its ability to reduce NF-κB signaling [77]. Therefore, these compounds may have potential for therapeutic use in COPD.
Extracellular superoxide dismutase (ECSOD or SOD3) is highly expressed in lungs and is located in the extracellular matrix in the junctions of airway epithelial cells, the surface of airway smooth muscle, and the lining of blood vessels of the lungs [79]. SOD3 directly scavenges O2 •−, and may therefore play an important role in protecting against oxidative lung damage. SOD3 protects against cigarette smoke/elastase induced mouse models of emphysema via reduction of oxidative ECM fragmentation and oxidative posttranslational modifications of elastin fragments (leading to autoantibody production) [80••]. A recent study has revealed that SOD3 can decrease CS-induced oxidative stress in mouse macrophages [81]. SOD3 also attenuates lung inflammation and emphysema by decreasing oxidative fragmentation of ECM, such as heparin sulfate and elastin [80]. Therefore, the development of pharmacological mimetics to replenish/augment SOD3 in the lung would be a rational therapeutic intervention for COPD/emphysema.
Glutathione peroxidase (GPx) mimetics
Ebselen is a selenium-based organic complex that mimics the activity of glutathione peroxidase. Ebselen is strong antioxidant and is also known to have strong neutralizing effect against the peroxynitrite radical [82]. Ebselen inhibits the activation of NF-κB/AP-1, and hence the expression of pro-inflammatory genes in human leukocytes treated with peroxynitrite. Ebselen has been shown in vivo in animal models to prevent LPS-induced airway inflammation [83-84]. However, no reports are available as the protective effect of ebselen against cigarette smoke-induced lung inflammation.
Spin traps and iNOS inhibitors
Spin traps are chemical agents which can quench free radicals to form measurable stable end products. Most spin traps have a nitrone- or nitroxide-nucleus and are derivatives of these moieties. Spin traps have been widely used for in vitro studies and their therapeutic effects in vivo have also been investigated in models of lung inflammation using α-phenyl-N-tert-butyl nitrone [85]. Early spin traps had extremely small half lives and generated toxic hydroxyl radicals on decay. This problem has now been overcome by the introduction of electron withdrawing moieties around the core pyrroline ring [86]. Isoindole-based nitrones [87] and azulenyl-based nitrones [88], such as STANZ have strong antioxidant properties and can inhibit lipid peroxidation in vitro. Phenyl-base nitrone spin trap (PBN) derivatives, such as NXY-059 (PBN-2,4,disulfonate), have been shown to have benefits in a wide variety of animal models of lung diseases (http://www.nitrone.com/).
Recent studies have suggested that inhibition of iNOS by various chemical inhibitors [N(6)-(1-iminoethyl)-L-lysine (L-NIL), G-nitro-L-arginine-methyl ester or L-NAME attenuated animal models of emphysema [89-91]. It is possible that selective inhibition of iNOS [90•] along with supplementation of other antioxidants may provide a strategy in the management of COPD.
Redox sensors: Enzymatic
Thioredoxin
Thioredoxin (Trx) and redox effector factor-1 (Ref-1), belong to oxidoreductase family of redox sensors. Trx, is primarily bound to proteins, such as hepatopoietin [92] and the apoptosis signal regulating kinase (ASK-1) [93], that are released from these complexes during oxidative stress [92]. After dissociation, Trx reduces a key thiol group within the p65/NF-κB subunit leading to transcriptional activation [94]. Inhibiting Trx (in the nucleus) with MOL-294 (a small molecular weight inhibitor of Trx), blocks nuclear activation of both NF-κB and AP-1-dependent transcription that results in diminished neutrophil influx and TNF-α production in an animal model [95]. Activation of Trx by synthetic small molecules attenuated oxidative stress [96]. Overexpression of thioredoxin-1 (Trx-1), primarily due to its antioxidant property attenuates CS-mediated oxidative stress and emphysema [97•], however the effects in COPD still remain to be investigated.
Conclusions
Increased oxidative/carbonyl stress occurs in COPD as is thought to be an important mechanism in the pathogenesis of this condition. Targeting oxidative stress with pharmacological antioxidants or boosting the endogenous levels of antioxidants is likely to be beneficial as a treatment in COPD. Antioxidant therapy may affect important outcomes in COPD, such as overcoming steroid resistance, mucus hypersecretion, inflammation, and ECM remodeling. Several small molecule antioxidant compounds have been investigated in pre-clinical and clinical trials. Although thiol antioxidant treatments have shown promising effects in targeting ROS and adverse oxidant-mediated cellular responses, development of novel wide-spectrum small molecule antioxidants with a good bioavailability and potency are needed for clinical trials for COPD. However, the clinical trials have been limited and there is a lack of information on pharmacokinetics, bioavailability, toxicity, and absorption of various exogenous antioxidants and activators of endogenous antioxidants.
Highlights.
Cigarette smoke causes oxidative stress in COPD.
Antioxidants can modulate intracellular redox system.
Thiols, enzyme mimetics, spin traps, and redox sensors are therapeutic agents.
Lipid peroxidation and protein carbonylation inhibitors block oxidative processes.
Antioxidants have pharmacological beneficial effects in management of COPD.
ACKNOWLEDGEMENTS
This work was supported by the NIH 1R01HL085613, 1R01HL097751, 1R01HL09284, and NIEHS Environmental Health Science Center grant P30-ES01247.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
•• of outstanding interest
- 1•.Yao H, Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol Appl Pharmacol. 2011;254:72–85. doi: 10.1016/j.taap.2009.10.022. A comprehensive review highlighting the antioxidant targets for cigarette smoke-mediated oxidative damage in COPD.
- 2.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubio ML, Martin-Mosquero MC, Ortega M, Peces-Barba G, Gonzalez-Mangado N. Oral N-acetylcysteine attenuates elastase-induced pulmonary emphysema in rats. Chest. 2004;125:1500–1506. doi: 10.1378/chest.125.4.1500. [DOI] [PubMed] [Google Scholar]
- 4.Alam S, Li Z, Janciauskiene S, Mahadeva R. Oxidation of Z alpha1-antitrypsin by cigarette smoke induces polymerization: a novel mechanism of early-onset emphysema. Am J Respir Cell Mol Biol. 2011;45:261–269. doi: 10.1165/rcmb.2010-0328OC. [DOI] [PubMed] [Google Scholar]
- 5.Bridgeman MM, Marsden M, Selby C, Morrison D, MacNee W. Effect of N-acetyl cysteine on the concentrations of thiols in plasma, bronchoalveolar lavage fluid, and lung tissue. Thorax. 1994;49:670–675. doi: 10.1136/thx.49.7.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decramer M, Dekhuijzen PN, Troosters T, van Herwaarden C, Rutten-van Molken M, van Schayck CP, Olivieri D, Lankhorst I, Ardia A. The Bronchitis Randomized On NAC Cost-Utility Study (BRONCUS): hypothesis and design. BRONCUS-trial Committee. Eur Respir J. 2001;17:329–336. doi: 10.1183/09031936.01.17303290. [DOI] [PubMed] [Google Scholar]
- 7••.Decramer M, Rutten-van Molken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, van Schayck CP, Olivieri D, Del Donno M, De Backer W, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 8.Poole PJ, Black PN. Oral mucolytic drugs for exacerbations of chronic obstructive pulmonary disease: systematic review. BMJ. 2001;322:1271–1274. doi: 10.1136/bmj.322.7297.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poole PJ, Black PN. Preventing exacerbations of chronic bronchitis and COPD: therapeutic potential of mucolytic agents. Am J Respir Med. 2003;2:367–370. doi: 10.1007/BF03256664. [DOI] [PubMed] [Google Scholar]
- 10.Stey C, Steurer J, Bachmann S, Medici TC, Tramer MR. The effect of oral N-acetylcysteine in chronic bronchitis: a quantitative systematic review. Eur Respir J. 2000;16:253–262. doi: 10.1034/j.1399-3003.2000.16b12.x. [DOI] [PubMed] [Google Scholar]
- 11.Grandjean EM, Berthet P, Ruffmann R, Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther. 2000;22:209–221. doi: 10.1016/S0149-2918(00)88479-9. [DOI] [PubMed] [Google Scholar]
- 12.Kasielski M, Nowak D. Long-term administration of N-acetylcysteine decreases hydrogen peroxide exhalation in subjects with chronic obstructive pulmonary disease. Respir Med. 2001;95:448–456. doi: 10.1053/rmed.2001.1066. [DOI] [PubMed] [Google Scholar]
- 13.Black PN, Morgan-Day A, McMillan TE, Poole PJ, Young RP. Randomised, controlled trial of N-acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344] BMC Pulm Med. 2004;4:13. doi: 10.1186/1471-2466-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Overveld FJ, Demkow U, Gorecka D, de Backer WA, Zielinski J. New developments in the treatment of COPD: comparing the effects of inhaled corticosteroids and N-acetylcysteine. J Physiol Pharmacol. 2005;56:135–142. [PubMed] [Google Scholar]
- 15.De Benedetto F, Aceto A, Dragani B, Spacone A, Formisano S, Pela R, Donner CF, Sanguinetti CM. Long-term oral n-acetylcysteine reduces exhaled hydrogen peroxide in stable COPD. Pulm Pharmacol Ther. 2005;18:41–47. doi: 10.1016/j.pupt.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Moretti M, Bottrighi P, Dallari R, Da Porto R, Dolcetti A, Grandi P, Garuti G, Guffanti E, Roversi P, De Gugliemo M, et al. The effect of long-term treatment with erdosteine on chronic obstructive pulmonary disease: the EQUALIFE Study. Drugs Exp Clin Res. 2004;30:143–152. [PubMed] [Google Scholar]
- 17••.Zheng JP, Kang J, Huang SG, Chen P, Yao WZ, Yang L, Bai CX, Wang CZ, Wang C, Chen BY, et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled study. Lancet. 2008;371:2013–2018. doi: 10.1016/S0140-6736(08)60869-7. This study showed that COPD patients treated with carbocysteine (25 mg t.d.s for 3 years) experienced fewer numbers of exacerbations per year.
- 18.Van Schooten FJ, Besaratinia A, De Flora S, D’Agostini F, Izzotti A, Camoirano A, Balm AJ, Dallinga JW, Bast A, Haenen GR, et al. Effects of oral administration of N-acetyl-L-cysteine: a multi-biomarker study in smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:167–175. [PubMed] [Google Scholar]
- 19.Gerrits CM, Herings RM, Leufkens HG, Lammers JW. N-acetylcysteine reduces the risk of re-hospitalisation among patients with chronic obstructive pulmonary disease. Eur Respir J. 2003;21:795–798. doi: 10.1183/09031936.03.00063402. [DOI] [PubMed] [Google Scholar]
- 20.Koechlin C, Couillard A, Cristol JP, Chanez P, Hayot M, Le Gallais D, Prefaut C. Does systemic inflammation trigger local exercise-induced oxidative stress in COPD? Eur Respir J. 2004;23:538–544. doi: 10.1183/09031936.04.00069004. [DOI] [PubMed] [Google Scholar]
- 21.Braga PC, Allegra L, Rampoldi C, Ornaghi A, Beghi G. Long-lasting effects on rheology and clearance of bronchial mucus after short-term administration of high doses of carbocysteine-lysine to patients with chronic bronchitis. Respiration. 1990;57:353–358. doi: 10.1159/000195871. [DOI] [PubMed] [Google Scholar]
- 22.Hanaoka M, Droma Y, Chen Y, Agatsuma T, Kitaguchi Y, Voelkel NF, Kubo K. Carbocisteine protects against emphysema induced by cigarette smoke extract in rats. Chest. 2011;139:1101–1108. doi: 10.1378/chest.10-0920. [DOI] [PubMed] [Google Scholar]
- 23.Carpagnano GE, Resta O, Foschino-Barbaro MP, Spanevello A, Stefano A, Di Gioia G, Serviddio G, Gramiccioni E. Exhaled Interleukine-6 and 8-isoprostane in chronic obstructive pulmonary disease: effect of carbocysteine lysine salt monohydrate (SCMC-Lys) Eur J Pharmacol. 2004;505:169–175. doi: 10.1016/j.ejphar.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Zheng CH, Ahmed K, Rikitomi N, Martinez G, Nagatake T. The effects of S-carboxymethylcysteine and N-acetylcysteine on the adherence of Moraxella catarrhalis to human pharyngeal epithelial cells. Microbiol Immunol. 1999;43:107–113. doi: 10.1111/j.1348-0421.1999.tb02381.x. [DOI] [PubMed] [Google Scholar]
- 25.Cakan G, Turkoz M, Turan T, Ahmed K, Nagatake T. S-carboxymethylcysteine inhibits the attachment of Streptococcus pneumoniae to human pharyngeal epithelial cells. Microb Pathog. 2003;34:261–265. doi: 10.1016/s0882-4010(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 26.Tatsumi K, Fukuchi Y. Carbocisteine improves quality of life in patients with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2007;55:1884–1886. doi: 10.1111/j.1532-5415.2007.01373.x. [DOI] [PubMed] [Google Scholar]
- 27.Aylward M. An assessment of S-carboxymethylcysteine in the treatment of chronic bronchitis. Curr Med Res Opin. 1974;2:387–394. doi: 10.1185/03007997409112654. [DOI] [PubMed] [Google Scholar]
- 28.Edwards GF, Steel AE, Scott JK, Jordan JW. S-carboxymethylcysteine in the fluidification of sputum and treatment of chronic airway obstruction. Chest. 1976;70:506–513. doi: 10.1378/chest.70.4.506. [DOI] [PubMed] [Google Scholar]
- 29.Miskoviti G, Szule P, Mescaros K. Double blind study of carbocysteine against placebo in chronic bronchitis; Mucoregulation in respiratory tract disorders. Proc R Soc Med. 1982;5:1–3. [Google Scholar]
- 30.Puchelle E, Girard F, Zahm JM. [Rheology of bronchial secretions and mucociliary transport (author’s transl)] Bull Eur Physiopathol Respir. 1976;12:771–779. [PubMed] [Google Scholar]
- 31.Allegra L, Cordaro CI, Grassi C. Prevention of acute exacerbations of chronic obstructive bronchitis with carbocysteine lysine salt monohydrate: a multicenter, double-blind, placebo-controlled trial. Respiration. 1996;63:174–180. doi: 10.1159/000196540. [DOI] [PubMed] [Google Scholar]
- 32.Grillage M, Barnard-Jones K. Long-term oral carbocisteine therapy in patients with chronic bronchitis. A double blind trial with placebo control. Br J Clin Pract. 1985;39:395–398. [PubMed] [Google Scholar]
- 33.Yasuda H, Yamaya M, Sasaki T, Inoue D, Nakayama K, Tomita N, Yoshida M, Sasaki H. Carbocisteine reduces frequency of common colds and exacerbations in patients with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2006;54:378–380. doi: 10.1111/j.1532-5415.2005.00592_9.x. [DOI] [PubMed] [Google Scholar]
- 34.Yasuda H, Yamaya M, Sasaki T, Inoue D, Nakayama K, Yamada M, Asada M, Yoshida M, Suzuki T, Nishimura H, et al. Carbocisteine inhibits rhinovirus infection in human tracheal epithelial cells. Eur Respir J. 2006;28:51–58. doi: 10.1183/09031936.06.00058505. [DOI] [PubMed] [Google Scholar]
- 35.Moretti M. Pharmacology and clinical efficacy of erdosteine in chronic obstructive pulmonary disease. Expert Rev Respir Med. 2007;1:307–316. doi: 10.1586/17476348.1.3.307. [DOI] [PubMed] [Google Scholar]
- 36.Cazzola M, Floriani I, Page CP. The therapeutic efficacy of erdosteine in the treatment of chronic obstructive bronchitis: a meta-analysis of individual patient data. Pulm Pharmacol Ther. 2010;23:135–144. doi: 10.1016/j.pupt.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Dal Negro RW. Erdosteine: antitussive and anti-inflammatory effects. Lung. 2008;186(Suppl 1):S70–73. doi: 10.1007/s00408-007-9065-3. [DOI] [PubMed] [Google Scholar]
- 38.Moretto N, Facchinetti F, Southworth T, Civelli M, Singh D, Patacchini R. alpha, beta-Unsaturated aldehydes contained in cigarette smoke elicit IL-8 release in pulmonary cells through mitogen-activated protein kinases. Am J Physiol Lung Cell Mol Physiol. 2009;296:L839–848. doi: 10.1152/ajplung.90570.2008. [DOI] [PubMed] [Google Scholar]
- 39.Dal Negro RW, Visconti M, Tognella S, Micheletto C. Erdosteine affects eicosanoid production in COPD. Int J Clin Pharmacol Ther. 2011;49:41–45. doi: 10.5414/cpp49041. [DOI] [PubMed] [Google Scholar]
- 40.Rhee CK, Kang CM, You MB, Yoon HK, Kim YK, Kim KH, Moon HS, Park SH, Song JS. Effect of fudosteine on mucin production. Eur Respir J. 2008;32:1195–1202. doi: 10.1183/09031936.00018508. [DOI] [PubMed] [Google Scholar]
- 41.Osoata GO, Hanazawa T, Brindicci C, Ito M, Barnes PJ, Kharitonov S, Ito K. Peroxynitrite elevation in exhaled breath condensate of COPD and its inhibition by fudosteine. Chest. 2009;135:1513–1520. doi: 10.1378/chest.08-2105. [DOI] [PubMed] [Google Scholar]
- 42.Komatsu H, Yamaguchi S, Komorita N, Goto K, Takagi S, Ochi H, Okumoto T. Inhibition of endotoxin- and antigen-induced airway inflammation by fudosteine, a mucoactive agent. Pulm Pharmacol Ther. 2005;18:121–127. doi: 10.1016/j.pupt.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 44.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47, 59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, et al. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci U S A. 2009;106:250–255. doi: 10.1073/pnas.0804333106. First experimental study showing utility of synthetic Nrf2 activator in experimental emphysema in a mouse model.
- 46.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, et al. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells. 2005;10:1113–1125. doi: 10.1111/j.1365-2443.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 48.Goven D, Boutten A, Lecon-Malas V, Marchal-Somme J, Amara N, Crestani B, Fournier M, Leseche G, Soler P, Boczkowski J, et al. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax. 2008;63:916–924. doi: 10.1136/thx.2007.091181. [DOI] [PubMed] [Google Scholar]
- 49.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2008;39:673–682. doi: 10.1165/rcmb.2007-0424OC. [DOI] [PubMed] [Google Scholar]
- 51•.Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–488. doi: 10.1152/ajplung.00361.2007. First experimental cell culture based evidence showing that Nrf2 undergoes oxidative post-translational modifications by cigarette smoke extract exposure.
- 52••.Malhotra D, Thimmulappa RK, Mercado N, Ito K, Kombairaju P, Kumar S, Ma J, Feller-Kopman D, Wise R, Barnes P, et al. Denitrosylation of HDAC2 by targeting Nrf2 restores glucocorticosteroid sensitivity in macrophages from COPD patients. J Clin Invest. 2011;121:4289–302. doi: 10.1172/JCI45144. First experimental ex-vivo study showing that a broccoli containing compound sulforaphane (Nrf2 activator) restored dexamethasone sensitivity in alveolar macrophages isolated from patients with COPD. The effect of sulforaphane was due to restoration of intracellular glutathione levels.
- 53.Lee JH, Jung HS, Giang PM, Jin X, Lee S, Son PT, Lee D, Hong YS, Lee K, Lee JJ. Blockade of nuclear factor-kappaB signaling pathway and anti-inflammatory activity of cardamomin, a chalcone analog from Alpinia conchigera. J Pharmacol Exp Ther. 2006;316:271–278. doi: 10.1124/jpet.105.092486. [DOI] [PubMed] [Google Scholar]
- 54.Liu YC, Hsieh CW, Wu CC, Wung BS. Chalcone inhibits the activation of NF-kappaB and STAT3 in endothelial cells via endogenous electrophile. Life Sci. 2007;80:1420–1430. doi: 10.1016/j.lfs.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 55.Foresti R, Hoque M, Monti D, Green CJ, Motterlini R. Differential activation of heme oxygenase-1 by chalcones and rosolic acid in endothelial cells. J Pharmacol Exp Ther. 2005;312:686–693. doi: 10.1124/jpet.104.074153. [DOI] [PubMed] [Google Scholar]
- 56•.Kumar V, Kumar S, Hassan M, Wu H, Thimmulappa RK, Kumar A, Sharma SK, Parmar VS, Biswal S, Malhotra SV. Novel chalcone derivatives as potent Nrf2 activators in mice and human lung epithelial cells. J Med Chem. 2011;54:4147–4159. doi: 10.1021/jm2002348. A synthetic chemical approach in identifying a quantitative structure-activity relationship of various novel Nrf2 activators, which act independent of ROS or redox changes in the cell.
- 57.Tajima S, Bando M, Ishii Y, Hosono T, Yamasawa H, Ohno S, Takada T, Suzuki E, Gejyo F, Sugiyama Y. Effects of edaravone, a free-radical scavenger, on bleomycin-induced lung injury in mice. Eur Respir J. 2008;32:1337–1343. doi: 10.1183/09031936.00164407. [DOI] [PubMed] [Google Scholar]
- 58.Kikuchi K, Uchikado H, Miyagi N, Morimoto Y, Ito T, Tancharoen S, Miura N, Miyata K, Sakamoto R, Kikuchi C, et al. Beyond neurological disease: New targets for edaravone (Review) Int J Mol Med. 2011;28:899–906. doi: 10.3892/ijmm.2011.795. [DOI] [PubMed] [Google Scholar]
- 59.Aldini G, Vistoli G, Regazzoni L, Benfatto MC, Bettinelli I, Carini M. Edaravone inhibits protein carbonylation by a direct carbonyl-scavenging mechanism: focus on reactivity, selectivity, and reaction mechanisms. Antioxid Redox Signal. 2010;12:381–392. doi: 10.1089/ars.2009.2814. [DOI] [PubMed] [Google Scholar]
- 60.Aldini G, Dalle-Donne I, Facino RM, Milzani A, Carini M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med Res Rev. 2007;27:817–868. doi: 10.1002/med.20073. [DOI] [PubMed] [Google Scholar]
- 61.Ito K, Ozasa H, Horikawa S. Edaravone protects against lung injury induced by intestinal ischemia/reperfusion in rat. Free Radic Biol Med. 2005;38:369–374. doi: 10.1016/j.freeradbiomed.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 62.Braughler JM, Pregenzer JF, Chase RL, Duncan LA, Jacobsen EJ, McCall JM. Novel 21-amino steroids as potent inhibitors of iron-dependent lipid peroxidation. J Biol Chem. 1987;262:10438–10440. [PubMed] [Google Scholar]
- 63.Miniati M, Cocci F, Monti S, Filippi E, Sarnelli R, Ferdeghini M, Gattai V, Pistolesi M. Lazaroid U-74389F attenuates phorbol ester-induced lung injury in rabbits. Eur Respir J. 1996;9:758–764. doi: 10.1183/09031936.96.09040758. [DOI] [PubMed] [Google Scholar]
- 64.Shenkar R, Abraham E. Effects of treatment with the 21-aminosteroid, U7438F, on pulmonary cytokine expression following hemorrhage and resuscitation. Crit Care Med. 1995;23:132–139. doi: 10.1097/00003246-199501000-00022. [DOI] [PubMed] [Google Scholar]
- 65.Wang S, Lantz RC, Rider ED, Chen GJ, Breceda V, Hays AM, Robledo RF, Tollinger BJ, Dinesh SV, Witten ML. A free radical scavenger (Lazaroid U75412E) attenuates tumor necrosis factor-alpha generation in a rabbit model of smoke-induced lung injury. Respiration. 1997;64:358–363. doi: 10.1159/000196704. [DOI] [PubMed] [Google Scholar]
- 66.Wang S, Lantz RC, Vermeulen MW, Chen GJ, Breceda V, Robledo RF, Hays AM, Young S, Witten ML. Functional alterations of alveolar macrophages subjected to smoke exposure and antioxidant lazaroids. Toxicol Ind Health. 1999;15:464–469. doi: 10.1177/074823379901500501. [DOI] [PubMed] [Google Scholar]
- 67.Tanigaki T, Suzuki Y, Heimer D, Sussman HH, Ross WG, Raffin TA. Attenuation of acute lung injury and oxygen radical production by the 21-aminosteroid, U-78518F. J Appl Physiol. 1993;74:2155–2160. doi: 10.1152/jappl.1993.74.5.2155. [DOI] [PubMed] [Google Scholar]
- 68.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol. 2003;29:88–97. doi: 10.1165/rcmb.2002-0228OC. [DOI] [PubMed] [Google Scholar]
- 69.Muscoli C, Sacco I, Alecce W, Palma E, Nistico R, Costa N, Clementi F, Rotiroti D, Romeo F, Salvemini D, et al. The protective effect of superoxide dismutase mimetic M40401 on balloon injury-related neointima formation: role of the lectin-like oxidized low-density lipoprotein receptor-1. J Pharmacol Exp Ther. 2004;311:44–50. doi: 10.1124/jpet.104.068205. [DOI] [PubMed] [Google Scholar]
- 70.Chang LY, Crapo JD. Inhibition of airway inflammation and hyperreactivity by an antioxidant mimetic. Free Radic Biol Med. 2002;33:379–386. doi: 10.1016/s0891-5849(02)00919-x. [DOI] [PubMed] [Google Scholar]
- 71.Smith KR, Uyeminami DL, Kodavanti UP, Crapo JD, Chang LY, Pinkerton KE. Inhibition of tobacco smoke-induced lung inflammation by a catalytic antioxidant. Free Radic Biol Med. 2002;33:1106–1114. doi: 10.1016/s0891-5849(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 72.Sharpe MA, Ollosson R, Stewart VC, Clark JB. Oxidation of nitric oxide by oxomanganese-salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics. Biochem J. 2002;366:97–107. doi: 10.1042/BJ20020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nishikawa M, Kakemizu N, Ito T, Kudo M, Kaneko T, Suzuki M, Udaka N, Ikeda H, Okubo T. Superoxide mediates cigarette smoke-induced infiltration of neutrophils into the airways through nuclear factor-kappaB activation and IL-8 mRNA expression in guinea pigs in vivo. Am J Respir Cell Mol Biol. 1999;20:189–198. doi: 10.1165/ajrcmb.20.2.3305. [DOI] [PubMed] [Google Scholar]
- 74.Day BJ, Shawen S, Liochev SI, Crapo JD. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced endothelial cell injury, in vitro. J Pharmacol Exp Ther. 1995;275:1227–1232. [PubMed] [Google Scholar]
- 75.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys. 1997;347:256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 76.Ferrer-Sueta G, Vitturi D, Batinic-Haberle I, Fridovich I, Goldstein S, Czapski G, Radi R. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. J Biol Chem. 2003;278:27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- 77.Tse HM, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med. 2004;36:233–247. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 78.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, Samulski TV, Fridovich I, Dewhirst MW, Anscher MS. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33:857–863. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 79.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 80••.Yao H, Arunachalam G, Hwang JW, Chung S, Sundar IK, Kinnula VL, Crapo JD, Rahman I. Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM. Proc Natl Acad Sci U S A. 2010;107:15571–15576. doi: 10.1073/pnas.1007625107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tollefson AK, Oberley-Deegan RE, Butterfield KT, Nicks ME, Weaver MR, Remigio LK, Decsesznak J, Chu HW, Bratton DL, Riches DW, et al. Endogenous enzymes (NOX and ECSOD) regulate smoke-induced oxidative stress. Free Radic Biol Med. 2010;49:1937–1946. doi: 10.1016/j.freeradbiomed.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jozsef L, Filep JG. Selenium-containing compounds attenuate peroxynitrite-mediated NF-kappaB and AP-1 activation and interleukin-8 gene and protein expression in human leukocytes. Free Radic Biol Med. 2003;35:1018–1027. doi: 10.1016/s0891-5849(03)00439-8. [DOI] [PubMed] [Google Scholar]
- 83.Haddad el B, McCluskie K, Birrell MA, Dabrowski D, Pecoraro M, Underwood S, Chen B, De Sanctis GT, Webber SE, Foster ML, et al. Differential effects of ebselen on neutrophil recruitment, chemokine, and inflammatory mediator expression in a rat model of lipopolysaccharide-induced pulmonary inflammation. J Immunol. 2002;169:974–982. doi: 10.4049/jimmunol.169.2.974. [DOI] [PubMed] [Google Scholar]
- 84.Zhang M, Nomura A, Uchida Y, Iijima H, Sakamoto T, Iishii Y, Morishima Y, Mochizuki M, Masuyama K, Hirano K, et al. Ebselen suppresses late airway responses and airway inflammation in guinea pigs. Free Radic Biol Med. 2002;32:454–464. doi: 10.1016/s0891-5849(01)00825-5. [DOI] [PubMed] [Google Scholar]
- 85.Chabrier PE, Auguet M, Spinnewyn B, Auvin S, Cornet S, Demerle-Pallardy C, Guilmard-Favre C, Marin JG, Pignol B, Gillard-Roubert V, et al. BN 80933, a dual inhibitor of neuronal nitric oxide synthase and lipid peroxidation: a promising neuroprotective strategy. Proc Natl Acad Sci U S A. 1999;96:10824–10829. doi: 10.1073/pnas.96.19.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi H, Timmins G, Monske M, Burdick A, Kalyanaraman B, Liu Y, Clement JL, Burchiel S, Liu KJ. Evaluation of spin trapping agents and trapping conditions for detection of cell-generated reactive oxygen species. Arch Biochem Biophys. 2005;437:59–68. doi: 10.1016/j.abb.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 87.Bottle SE, Micallef AS. Synthesis and EPR spin trapping properties of a new isoindole-based nitrone: 1,1,3-trimethylisoindole N-oxide (TMINO) Org Biomol Chem. 2003;1:2581–2584. doi: 10.1039/b300642e. [DOI] [PubMed] [Google Scholar]
- 88.Becker DA, Ley JJ, Echegoyen L, Alvarado R. Stilbazulenyl nitrone (STAZN): a nitronyl-substituted hydrocarbon with the potency of classical phenolic chain-breaking antioxidants. J Am Chem Soc. 2002;124:4678–4684. doi: 10.1021/ja011507s. [DOI] [PubMed] [Google Scholar]
- 89.Valenca SS, Rueff-Barroso CR, Pimenta WA, Melo AC, Nesi RT, Silva MA, Porto LC. L-NAME and L-arginine differentially ameliorate cigarette smoke-induced emphysema in mice. Pulm Pharmacol Ther. 2011;24:587–594. doi: 10.1016/j.pupt.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 90•.Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, Milger K, Egemnazarov B, Turowska A, Fuchs B, et al. Inducible NOS Inhibition Reverses Tobacco-Smoke-Induced Emphysema and Pulmonary Hypertension in Mice. Cell. 2011;147:293–305. doi: 10.1016/j.cell.2011.08.035. Important study showing that inhibition of NO generation by iNOS inhibitors can lead to reversal of experimental emphysema in a mouse model.
- 91.Brindicci C, Kharitonov SA, Ito M, Elliott MW, Hogg JC, Barnes PJ, Ito K. Nitric oxide synthase isoenzyme expression and activity in peripheral lung tissue of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:21–30. doi: 10.1164/rccm.200904-0493OC. [DOI] [PubMed] [Google Scholar]
- 92.Li Y, Liu W, Xing G, Tian C, Zhu Y, He F. Direct association of hepatopoietin with thioredoxin constitutes a redox signal transduction in activation of AP-1/NF-kappaB. Cell Signal. 2005;17:985–996. doi: 10.1016/j.cellsig.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 93.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qin J, Clore GM, Kennedy WM, Huth JR, Gronenborn AM. Solution structure of human thioredoxin in a mixed disulfide intermediate complex with its target peptide from the transcription factor NF kappa B. Structure. 1995;3:289–297. doi: 10.1016/s0969-2126(01)00159-9. [DOI] [PubMed] [Google Scholar]
- 95.Souza DG, Vieira AT, Pinho V, Sousa LP, Andrade AA, Bonjardim CA, McMillan M, Kahn M, Teixeira MM. NF-kappaB plays a major role during the systemic and local acute inflammatory response following intestinal reperfusion injury. Br J Pharmacol. 2005;145:246–254. doi: 10.1038/sj.bjp.0706190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bachnoff N, Trus M, Atlas D. Alleviation of oxidative stress by potent and selective thioredoxin-mimetic peptides. Free Radic Biol Med. 2011;50:1355–1367. doi: 10.1016/j.freeradbiomed.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 97•.Sato A, Hoshino Y, Hara T, Muro S, Nakamura H, Mishima M, Yodoi J. Thioredoxin-1 ameliorates cigarette smoke-induced lung inflammation and emphysema in mice. J Pharmacol Exp Ther. 2008;325:380–388. doi: 10.1124/jpet.107.134007. First experimental study highlighting the important of redox sensor molecule in experimental emphysema in mouse.