Abstract
We studied thyroid function in 81 long term survivors of allo-SCT (median follow-up 84 months, range 45–166). Median age at transplant was 35 years (range 6–66). Seventy two received a total body irradiation conditioning regimen (12Gy 23; 13Gy 49). Twenty one (25.9%) had subclinical hypothyroidism and 9 (11.1%) developed overt hypothyroidism at a median of 28 months (range 3–78) after allo-SCT. Multivariate logistic regression analysis showed prolonged immunosuppressive therapy (IST) was significantly associated with subclinical (OR 3.8) and overt (OR 2.6) hypothyroidism. Anti-Thyroglobulin and Thyroid Peroxidase Antibody were detected in 12 of 60 (20%) patients tested. There was no correlation between the occurrence of thyroid antibodies and hypothyroidism (p=0.13) or cGVHD (p=0.55). In conclusion, thyroid dysfunction is relatively common after SCT and is more likely to occur in patients receiving prolonged IST for cGVHD. However, thyroid dysfunction did not appear to be related to an antibody-mediated autoimmune process.
Keywords: Hypothyroidism, thyroid antibodies, GVHD, long term survivors, stems cell transplantation
Introduction
Nearly 90% of patients alive 2 years after allogenic stem cell transplantation (allo- SCT) will become long term survivors,1 shifting the focus of care from cure of the original disease to the identification and treatment of delayed and long-term complications that may affect quality of life.2–4 Thyroid failure (both overt and subclinical) is a recognized long-term complication after allo- SCT which can affect quality of life and predispose to cardiac and metabolic complications.5 Approximately 15 % surviving patients are reported to develops hypothyroidism and twice number (30–40%) develop compensated hypothyroidism. 6–8 Times to hypothyroidism after allo-SCT is reported to vary from 1 to 10 year.6–8 Thyroid failure has been linked to total body irradiation (TBI), but is also described after non- TBI conditioning regimens. 7;8 As transplant centers increasingly use non-TBI conditioning (with inclusion of older adults); it is important to define risk factors other than the conditioning regimen predisposing to thyroid failure. A few studies have linked thyroid dysfunction with chronic GVHD, but no consistent link has been found; furthermore, a relationship between anti-thyroid antibodies, cGVHD and thyroid dysfunction is not clearly established.9 Here we studied thyroid function test in patients surviving more than 3 year post transplantation; to identify factors associated with hypothyroidism and determine the relative contribution of conditioning regimen, autoantibodies and chronic GVHD to thyroid dysfunction.
Study Design, Patients and methods
Four hundred seventeen patients with hematological disorders received SCT from an HLA identical sibling between 1993 and 2003. Patients at a minimum of 3 years post-transplantation were enrolled between 04/2005–10/2006 in an IRB-approved long-term evaluation protocol (NHLBI 05-H-0130; ClinicalTrials.gov identifier NCT00106925). Of the 111 patients surviving 3 or more years at study initiation in 2005, 84 patients gave written informed consent according to principles outlined in the Declaration of Helsinki. Thyroid function tests were performed in 81. Transplant conditioning mainly consisted of total body irradiation (TBI) 12–13.6 Gy and cyclophosphamide (± fludarabine), followed by an allogeneic SCT (n=72). Nine received a reduced-intensity conditioning (RIC) of fludarabine and cyclophosphamide, followed by an allogeneic SCT. All patients received cyclosporine as GVHD prophylaxis. Acute and chronic GVHD were graded by previously described criteria.10;11 Patient characteristics are shown in Table 1
Table 1.
Patients* Characteristics
| Variable (n=81) | N (%) |
|---|---|
| Age at transplant | Median 35 years (range 6–66) |
| Gender | |
| Male | 47(58) |
| Female | 34 (42) |
| Disease distribution | |
| AML/MDS | 25 (30.8) |
| ALL | 3 (3.7) |
| CML | 49 (60.5) |
| Others | 4 (4.9) |
| Stem cell source | |
| BMT | 15 (18.5) |
| PBSCT | 66 (81.5) |
| Conditioning regimen | |
| MST | 72 (90) |
| RIC | 9 (10) |
| Acute GVHD (grades) | |
| I–IV | 35 (43.2) |
| II–IV | 15 (18.5) |
| Chronic GVHD | 66 (81.5) [L50; E 16] |
| Prolonged IST (> 3 year) | 14 (17) |
| Mortality beyond 3 year follow up | 2 (2.5) |
| Hypothyroidism (time to hypothyroidism-median, range) | |
| All cases (28 mo, 3–78) | 30 (37) |
| Subclinical (20 mo, 3–38) | 21 (25.9) |
| Overt (32 mo, 9–78) | 9 (11.1) |
| Total no of patients on treatment | 20 (24.7) |
| Positive anti-thyroid antibodies (n=60) | 12 (20) |
Follow-up period, median 84 mo (range 25–166); AML-acute myeloid leukemia, ALL-acute lymphoblastic leukemia, CML-chronic myeloid leukemia, L-limited; E-extensive; MST-TBI based myeloablative conditioning; RIC-reduced intensity conditioning; IST-immunosuppressive therapy, mo-months
Thyroid function tests and definitions
TSH, T4 and T3 levels were measured pre-transplantation; 3, 6 months and then annually post-transplantation, and were checked earlier and more frequently if clinically indicated. In the last 60 patients studied anti-thyroid antibodies (Anti-Thyroglobulin and Thyroid Peroxidase Antibody) were measured.
Subclinical hypothyroidism was defined as elevated TSH with normal T4, T3 levels in asymptomatic patients, while overt hypothyroidism was defined if elevated TSH and low T4 and or T3 levels with or without clinical features. Hypothyroidism was defined as subclinical or overt, whichever occurred first for this analysis.
Statistical analysis
Clinical and transplant characteristics were used to evaluate risk factors for hypothyroidism. Variables included in analysis are listed in Table 2. In univariate analyses, Chi-squared or Fisher's exact tests were used for categorical variables and Mann-Whitney U-test for continuous variables. Summary statistics, such as proportions, means, standard deviations, 95% confidence intervals, medians, and ranges, were used to describe the patient characteristics, pre-transplant variables, and post-transplant outcomes. Kaplan-Meier curves were used to display the distributions of events among subgroups of patients. Logistic regression was used for multivariate analysis of associated variables. Statistical significance was accepted at P<0.05. Data analysis was performed using SPSS 15 for Windows (SPSS Inc., Chicago, IL) software.
Table 2.
Factors associated with hypothyroidism
| Factor (N=) | Subclinical N (%) | P value | Overt N (%) | P value | Overall N (%) | P value |
|---|---|---|---|---|---|---|
| Age (< vs. > M) | 0.526 | 0.484 | 0.557 | |||
| ≤Median (41) | 11 (26.8) | 4 (9.7) | 15 (36.5) | |||
| >Median (40) | 10 (25) | 5 (12.5) | 15 (37.5) | |||
| Age quartiles | 0.161 | 0.037 | 0.476 | |||
| First three (61) | 18 (29.5) | 4 (6.5) | 22 (36) | |||
| Oldest quartiles (20) | 3 (15) | 5 (25) | 8 (40) | |||
| Gender | 0.193 | 0.428 | 0.335 | |||
| Male (47) | 10 (21.2) | 3 (8.8) | 16 (34) | |||
| Female (34) | 11 (32.3) | 6 (12.7) | 14 (41.1) | |||
| Type of SCT | 0.413 | 0.528 | 0.494 | |||
| BMT (15) | 3(20) | 2(11.7) | 5 (33.3) | |||
| PBSCT (66) | 18 (27.2) | 7 (10.6) | 25 (37.8) | |||
| TBI * | 0.573 | 0.261 | 0.442 | |||
| Yes (72) | 19 (26.3) | 7 (9.7) | 26 (36.1) | |||
| No (9) | 2 (22.2) | 2 (22.2) | 4 (44.4) | |||
| aGVHD – grade | 0.184 | 0.214 | 0.494 | |||
| 0–1 (66) | 19 (28.7) | 6 (9) | 25 (37.8 | |||
| 2–4 (15) | 2 (13.3) | 3 (20) | 5 (33.3) | |||
| Chronic GVHD | 0.192 | 0.432 | 0.365 | |||
| Y (66) | 15 (23) | 8 (12.1) | 23 (34.8) | |||
| N (15) | 6 (40) | 1 (6.7) | 7 (46.6) | |||
| Prolonged IST ** | 0.031 | 0.044 | 0.001 | |||
| Y (14) | 7 (50) | 4 (28.5) | 11 (78.5) | |||
| N (67) | 14 (20.8) | 5 (7.4) | 19 (28.3) |
there was no significant difference when patients were analyzed with TBI dose of 12 vs. 13.6 Gy;
immunosuppressive therapy beyond 3 years post-transplant
Results
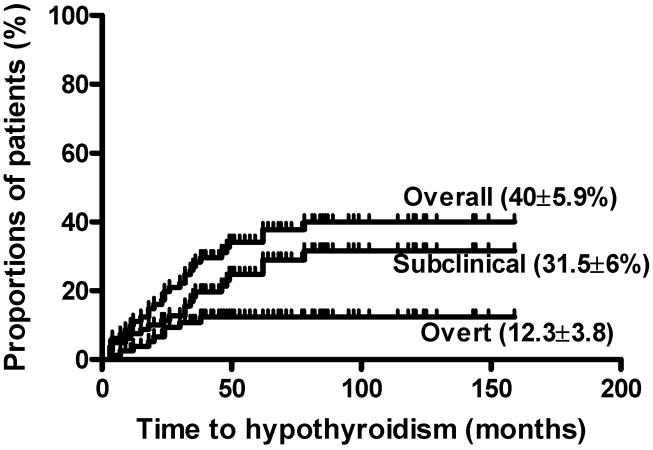
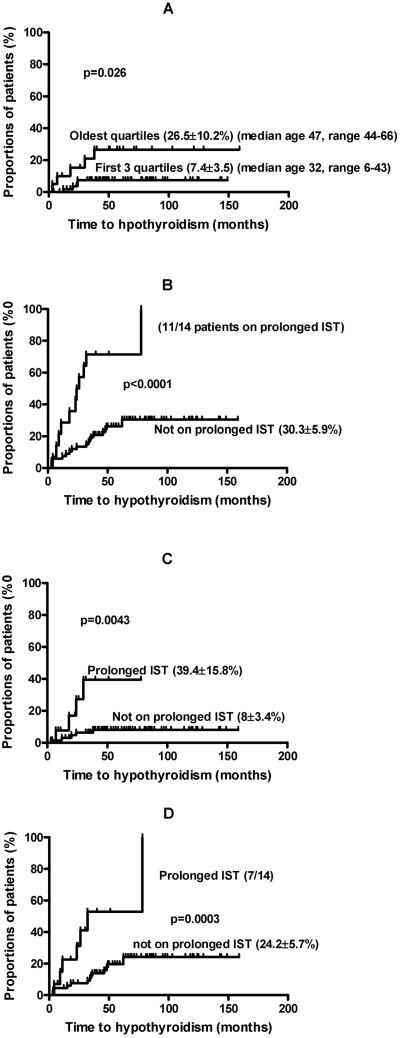
Table 1, summarizes patient characteristics and Table 2 shows univariate analysis of factors associated with subclinical and overt hypothyroidism. Hypothyroidism occurred in 30 patients with a cumulative incidence in these three year survivors of 40 ± 5.9% (Figure 1). Median time to hypothyroidism was 28 months, range 3–78 (subclinical 20, 3–38 and overt 32, 9–78). Hypothyroidism was more likely to occur in older patients (Table 2, Figure 2A), or in patients requiring prolonged immunosuppressive therapy (IST) for longstanding chronic GVHD (cGVHD) (Table 2, Figure 2 B, C, D). To further explore the relationship between cGVHD, prolonged IST and hypothyroidism, we analyzed the cGVHD group separately. There were 52 patients developing cGVHD requiring treatment for less than 3 years. Of these 13 (25%) developed hypothyroidism compared with 11 of 14 (78.5%) patients with cGVHD persisting beyond 3 years and requiring IST (p<0.0001). Our data showed, there was no significant difference in rate of hypothyroidism (p=0.088) and positive thyroid antibodies (p=0.5) among patients with limited GVHD vs. extensive GVHD. Thus cGVHD duration appeared to be a more important factor than cGVHD occurrence or severity. This data must be interpreted with caution as small number of patients developed extensive cGVHD. More than half of patients (11 of 21) with subclinical hypothyroidism eventually required thyroid replacement therapy. Multivariate logistic regression analysis showed that prolonged IST was independently associated with both overt and subclinical hypothyroidism (OR for overt 2.6, 95% CI 1.1–21.4, p=0.04; subclinical 3.8, 95% CI 1.2–14.4, p=0.03). In the sub-group of 11 patients receiving prolonged IST, initially diagnosed with subclinical hypothyroidism, all became symptomatic and required thyroid replacement therapy.
Figure 1.
Cumulative incidence of hypothyroidism.
Figure 2.
A. Age and overt hypothyroidism; B. prolonged immunosuppressive therapy (IST) and hypothyroidism (all cases); prolonged IST and overt (c) and subclinical (D) hypothyroidism.
Relation between anti-thyroid antibodies, cGVHD and hypothyroidism
Sixty of 81 patients were tested for anti- thyroid antibodies (Anti-Thyroglobulin and Thyroid Peroxidase Antibody). Twelve (20%) were positive for either or both antibodies. However, there was no significant association between the occurrence of anti- thyroid antibodies and hypothyroidism: antibodies were found in 7/24 (29%) patients with hypothyroidism and 5/36 (14%) without (p=0.13). Similarly, there was no association between cGVHD and positive anti-thyroid antibodies: 10/48 (20.8%) with a history of cGVHD were positive compared with 2/12 (16.6%) patients without cGVHD (p=0.55) (Table 2). To further explore the relationship between prolonged IST and anti-thyroid antibodies we analyzed patients with history of cGVHD separately. Four of 12 (33%) of patients requiring prolonged IST for cGVHD (>3 years) were positive for anti-thyroid antibodies compared to 6 of 36 (17%) with cGVHD (<3 years) (p=0.202). These results further confirm a lack of association of anti-thyroid antibodies with cGVHD requiring prolonged IST.
Discussion
Our study demonstrates that hypothyroidism is a significant late complication for long-term allo- SCT survivors; occurring in almost 40% of patients surviving 3 or more years from SCT. In contrast to previous studies,7;12–15 we found prolonged IST for ongoing cGVHD (both overt and subclinical hypothyroidism) and increasing age (overt hypothyroidism) as the only risk factors for hypothyroidism in patients followed up for a median of 7 years.
Previous studies6;16 of thyroid function tests in children and adolescents receiving allo-SCT showed younger children (<10 years) more likely have thyroid dysfunction compared to older children. Our studies do not contradict these observations since it included only 2 patients under 10 years at transplant. The majority were adults including 21 patients over 44 years. Patients in the age range 44–66 years had a significantly higher rate of overt hypothyroidism in univariate analysis. However the effect of age was confounded by the fact that 8 of 14 patients in this older cohort received prolonged IST for cGVHD.
There was no significant impact of conditioning regimen intensity (Table 2). Nevertheless, we cannot exclude a contribution of TBI to the development of hypothyroidism because only a minority of patients received a non-myeloablative conditioning regimen. Our data suggest that the thyroid may be susceptible to alloimmune attack associated with prolonged GVHD. However, we could not confirm that cGVHD per se is a provocative factor for thyroid damage since we did not find a relationship between hypothyroidism, anti-thyroid antibodies, and cGVHD. It may be that thyroid damage is simply T cell mediated or that prolonged IST per se damages the thyroid gland. Importantly we found that all patients with subclinical hypothyroidism requiring prolonged IST developed symptomatic hypothyroidism and required replacement therapy. This might indicate the need for early replacement therapy, especially in this patient group who are seen infrequently at our clinic. There is continued a debate on whether to treat patients with subclinical hypothyroidism.6;17;18 Initially we and others6 did not treat subclinical hypothyroidism, in contrast to other investigators.18 An important reason to treat subclinical hypothyroidism is to diminish the risk of thyroid adenoma and carcinoma19;20 and in young patients to prevent growth failure and delayed development. Stem cell transplant recipients are at increased risk of developing second malignancies21;22 An EBMT study showed thyroid cancer was the most common secondary cancer with a standardized incidence ratio (SIR) approaching 50 among long term survivors after SCT. Similar to our study the risk factors for developing secondary cancer were extensive cGVHD and IST for cGVHD. Thyroid cancer and hyperthyroidism has not occurred in any of our patients to date.
Thyroid dysfunction following allo-SCT has been linked to an autoimmune process; however the true incidence of clinically significant autoimmune thyroid dysfunction after allo-SCT is largely unknown. It has been reported that thyroid damage after allo-SCT, causing transient subclinical hypothyroidism and low titer thyroid antibodies may be common.23 In small case series, autoimmune thyroid dysfunction has been described in up to 3% of the allo- SCT survivors.9;24 However, in our study there was no correlation between the development of thyroid autoantibodies and hypothyroidism. Thus, while an alloimmune response may contribute to thyroid dysfunction after SCT, it does not appear to be mediated through the classical autoantibody pathway. Further investigation is needed to determine how the thyroid might be affected by the cGVHD process.
Acknowledgments
(This work was supported by the intramural research program of the NHLBI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of commercial interest: None
ClinicalTrials.gov Identifier NCT00106925
Reference List
- 1.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N.Engl.J.Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 2.Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J.Clin.Oncol. 2005;23:6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 3.Savani BN, Montero A, Srinivasan R, et al. Chronic GVHD and pretransplantation abnormalities in pulmonary function are the main determinants predicting worsening pulmonary function in long-term survivors after stem cell transplantation. Biol.Blood Marrow Transplant. 2006;12:1261–1269. doi: 10.1016/j.bbmt.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savani BN, Donohue T, Kozanas E, et al. Increased risk of bone loss without fracture risk in long-term survivors after allogeneic stem cell transplantation. Biol.Blood Marrow Transplant. 2007;13:517–520. doi: 10.1016/j.bbmt.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 5.Tichelli A, Bhatia S, Socie G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. Br.J.Haematol. 2008;142:11–26. doi: 10.1111/j.1365-2141.2008.07165.x. [DOI] [PubMed] [Google Scholar]
- 6.Ishiguro H, Yasuda Y, Tomita Y, et al. Long-term follow-up of thyroid function in patients who received bone marrow transplantation during childhood and adolescence. J Clin Endocrinol Metab. 2004;89:5981–5986. doi: 10.1210/jc.2004-0836. [DOI] [PubMed] [Google Scholar]
- 7.Berger C, Le-Gallo B, Donadieu J, et al. Late thyroid toxicity in 153 long-term survivors of allogeneic bone marrow transplantation for acute lymphoblastic leukaemia. Bone Marrow Transplant. 2005;35:991–995. doi: 10.1038/sj.bmt.1704945. [DOI] [PubMed] [Google Scholar]
- 8.Tauchmanova L, Selleri C, Rosa GD, et al. High prevalence of endocrine dysfunction in long-term survivors after allogeneic bone marrow transplantation for hematologic diseases. Cancer. 2002;95:1076–1084. doi: 10.1002/cncr.10773. [DOI] [PubMed] [Google Scholar]
- 9.Au WY, Lie AK, Kung AW, et al. Autoimmune thyroid dysfunction after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35:383–388. doi: 10.1038/sj.bmt.1704766. [DOI] [PubMed] [Google Scholar]
- 10.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 11.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin.Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 12.Boulad F, Bromley M, Black P, et al. Thyroid dysfunction following bone marrow transplantation using hyperfractionated radiation. Bone Marrow Transplant. 1995;15:71–76. [PubMed] [Google Scholar]
- 13.Thomas BC, Stanhope R, Plowman PN, Leiper AD. Endocrine function following single fraction and fractionated total body irradiation for bone marrow transplantation in childhood. Acta Endocrinol (Copenh) 1993;128:508–512. doi: 10.1530/acta.0.1280508. [DOI] [PubMed] [Google Scholar]
- 14.Michel G, Socie G, Gebhard F, et al. Late effects of allogeneic bone marrow transplantation for children with acute myeloblastic leukemia in first complete remission: the impact of conditioning regimen without total-body irradiation--a report from the Societe Francaise de Greffe de Moelle. J Clin Oncol. 1997;15:2238–2246. doi: 10.1200/JCO.1997.15.6.2238. [DOI] [PubMed] [Google Scholar]
- 15.Toubert ME, Socie G, Gluckman E, et al. Short- and long-term follow-up of thyroid dysfunction after allogeneic bone marrow transplantation without the use of preparative total body irradiation. Br J Haematol. 1997;98:453–457. doi: 10.1046/j.1365-2141.1997.2433060.x. [DOI] [PubMed] [Google Scholar]
- 16.Sanders JE, Hoffmeister PA, Woolfrey AE, et al. Thyroid function following hematopoietic cell transplantation in children: 30 years experience. Blood. 2008 doi: 10.1182/blood-2008-08-173005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson GR, Curry RW., Jr. Subclinical thyroid disease. Am.Fam.Physician. 2005;72:1517–1524. [PubMed] [Google Scholar]
- 18.Borgstrom B, Bolme P. Thyroid function in children after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1994;13:59–64. [PubMed] [Google Scholar]
- 19.Rivas M, Santisteban P. TSH-activated signaling pathways in thyroid tumorigenesis. Mol.Cell Endocrinol. 2003;213:31–45. doi: 10.1016/j.mce.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 20.Field JB, Bloom G, Chou MC, et al. Effects of thyroid-stimulating hormone on human thyroid carcinoma and adjacent normal tissue. J.Clin.Endocrinol.Metab. 1978;47:1052–1058. doi: 10.1210/jcem-47-5-1052. [DOI] [PubMed] [Google Scholar]
- 21.Kolb HJ, Socie G, Duell T, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann.Intern.Med. 1999;131:738–744. doi: 10.7326/0003-4819-131-10-199911160-00004. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 23.Kami M, Tanaka Y, Chiba S, et al. Thyroid function after bone marrow transplantation: possible association between immune-mediated thyrotoxicosis and hypothyroidism. Transplantation. 2001;71:406–411. doi: 10.1097/00007890-200102150-00012. [DOI] [PubMed] [Google Scholar]
- 24.Au WY, Lie AKW, Kung AWC, Liang R. Autoimmune thyroid dysfunction after stem cell transplantation: a revisit. Bone Marrow Transplant. 2004;33:S213–S214. doi: 10.1038/sj.bmt.1704766. [DOI] [PubMed] [Google Scholar]