Abstract
Retina differentiation involves the acquisition of a precise layered arrangement, with RPE cells in the first layer in intimate contact with photoreceptors in the second layer. Here, we developed an in vitro coculture model, to test the hypothesis that RPE cells play a pivotal role in organizing the spatial structure of the retina. We cocultured rat retinal neurons with ARPE-19 epithelial cells under various experimental conditions. Strikingly, when seeded over RPE cells, photoreceptors attached to their apical surfaces and proceeded with their development, including the increased synthesis of rhodopsin. Conversely, when we seeded RPE cells over neurons, the RPE cells rapidly detached photoreceptors from their substrata and positioned themselves underneath, thus restoring the normal in vivo arrangement. Treatment with the metalloproteinase inhibitor TIMP-1 blocked this reorganization, suggesting the involvement of metalloproteinases in this process. Reorganization was highly selective for photoreceptors because 98% of photoreceptors but very few amacrine neurons were found to redistribute on top of RPE cells. Interestingly, RPE cells were much more efficient than other epithelial or nonepithelial cells in promoting this reorganization. RPE cells also promoted the growth of photoreceptor axons away from them. An additional factor that contributed to the distal arrangement of photoreceptor axons was the migration of photoreceptor cell bodies along their own neurites toward the RPE cells. Our results demonstrate that RPE and photoreceptor cells interact in vitro in very specific ways. They also show that in vitro studies may provide important insights into the process of pattern formation in the retina.
Keywords: spatial reorganization, metalloproteinases, axonal outgrowth
Interactions between photoreceptors and RPE cells are essential for development and differentiation, and for the maintenance of the visual function (Bok and Hall, 1971; Hollyfield and Witkovsky, 1974; Bok, 1993; Jeffery, 1998; Marmorstein et al., 1998a; Marmorstein, 2001). RPE cells are among the major supplier of nutrients for photoreceptors (Bok, 1993; Rizzolo, 1997). In the eye, both cell types depend on each other; mutations in genes expressed in RPE cells lead to photoreceptor degeneration; conversely, mutations in genes expressed in photoreceptors induce degenerative changes in RPE cells (Bok and Hall, 1971; Gu et al., 1997; Mata et al., 2000; Glazer and Dryja, 2002; Strauss, 2005). RPE cell grafts can prevent photoreceptor degeneration in Royal College of Surgeons rats (Li and Turner, 1991), even beyond the area of donor cell distribution, indicating the release of diffusible survival factors for photoreceptors (Wang et al., 2005). RPE cells release several well-established survival factors, such as fibroblast growth factor (FGF) 1, 2, and 5; ciliary neurotrophic factor; transforming growth factor-beta; insulin-like growth factor 1; vascular endothelial growth factor; and pigment epithelium-derived factor (Steele et al., 1992; Tanihara et al., 1997; Jablonski et al., 2000; Strauss, 2005), several of which protect photoreceptors from light-induced damage (Abe et al., 2005). In addition, RPE cells play a crucial role in preserving the levels of the major polyunsaturated fatty acid in the retina, docosahexaenoic acid, critical for photoreceptor development, survival, and differentiation (Rotstein et al., 1996, 1997, 1998; Politi et al., 2001a, 2001b; Insua et al., 2003).
The mutual benefits obtained from the interaction between RPE and photoreceptors depend on the establishment of a very precise spatial arrangement. A failure in achieving this arrangement may lead to an insufficient supply of trophic molecules, impairment in the recycling of molecules involved in the visual cycle, and a failure in neurotransmission, thus hampering visual function. During development, photoreceptors selectively localize into the outer nuclear layer, build and direct their outer segments toward the RPE cells, and extend their axons toward the inner nuclear layer, thus integrating into the layered structure of the mature retina. On the other hand, RPE cells develop their basolateral–apical polarity, and contact primordial photoreceptors through their immature apical membranes (Bok, 1993; Marmorstein et al., 1998a; Marmorstein, 2001). This key interaction is believed to promote the differentiation of both RPE apical membrane and photoreceptor outer segments. In rodents, the maturation of the retina into its characteristic layered structure starts during early embryonic development and is completed during the first month of postnatal life. The mechanisms through which interactions with RPE might determine the spatial orientation of photoreceptor outer segments and axons remain largely unknown. The relevance for the proper function of the retina of attaining a correct spatial organization of both cell types during development and the broad spectrum of protective effects of RPE cells on photoreceptors lead us to hypothesize here that RPE cells could play a critical role in the acquisition of the correct spatial orientation of both cell types during early development of the retina. Most available evidence on RPE-photoreceptor interactions during retinal development derives from studies in the whole retina, in which many factors interact simultaneously; however, direct analysis of the mutual influences between retina neurons and epithelial cells is still lacking.
Here, we developed an in vitro system coculturing ARPE-19 cells, a human RPE cell line, with rat retina neurons to test the hypothesis that RPE cells might contribute to establishing the architecture of the retina. This in vitro system allowed us to modify the normal spatial arrangement between photoreceptors and RPE cells found in vivo, to test hypotheses on how these cells develop their physiological orientation. Our results demonstrate that photoreceptor-RPE cell interactions play a key role in this process. They also provide strong evidence that cocultures of RPE cells and retinal neurons constitute a powerful model, complementary to in vivo approaches, to investigate the role of retinal cell interactions during early retinal development.
MATERIALS AND METHODS
Materials
Two-day-old albino Wistar rats bred in our own colony were used in all the experiments. All proceedings concerning animal use were done in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, revised 1985), and the principles presented in the “Guidelines for the Use of Animals in Neuroscience Research” by the Society for Neuroscience. Plastic 35-mm-diameter culture dishes were from NUNC. Fetal bovine serum (FBS) was from Gensa (Buenos Aires, Argentina). Dulbecco’s modified Eagle medium (DME) was from Gibco. Trypsin, trypsin inhibitor, transferrin, hydrocortisone, putrescine, insulin, polyornithine, selenium, gentamycin, DAPI (4′,6-diamidino-2-phenylindole), fluorescein-conjugated secondary antibodies, paraformaldehyde, tetrarhodamine isothiocyanate (TRITC)-labeled phalloidin, the primary antibody anti-Syntaxin (HPC-1) and tissue inhibitor of metalloproteinases (TIMP)-1 were from Sigma (St. Louis, MO). TO-PRO3- and Alexa 488–conjugated-goat anti-mouse secondary antibody were from Molecular Probes (Eugene, OR). Cy3 was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Tyramine was from NEN Life Science Products and ABC reagents were from Vector Laboratories (Burlingame, CA). Monoclonal anti-opsin (Rho4D2) and polyclonal anti-CRX were generous gifts from Drs. R. Molday (University of British Columbia) and Drs. X. Zhu and C. Craft (Zhu and Craft, 2000), respectively; anti-CD147 was from BD Pharmingen (Becton Drive Franklin Lakes, NJ). Anti-MCT-1 and anti-MCT-4 were the gift of Dr. N. Philp (Philp et al., 2003). All other reagents used were analytical grade.
ARPE-19 and Caco-2 cell lines were obtained from American Type Culture Collection, and the BC3H-1 cell line was the gift of Dr. Patrick (Patrick et al., 1977).
Cell Cultures
Retinal cultures
Amacrine- and photoreceptorenriched cultures were obtained following previously established procedures (Politi et al., 1996; Rotstein et al., 1996, 1997). In brief, rat retinas were dissected and dissociated by trypsin digestion followed by mechanical dissociation. Cells were then resuspended in a chemically defined medium (neuronal medium), as previously described, and seeded on 35-mm-diameter dishes, sequentially pretreated with polyornithine (0.1 mg/mL) and schwannoma-conditioned medium (Adler, 1982). Cultures were incubated at 36°C in a humidified atmosphere of 5% CO2. To obtain the neuronal enriched cultures we seeded the cells at a low density of about 800,000 cells/35-mm-diameter dish. Under these conditions, 95% of the neuronal progenitors developed and differentiated as amacrine and photoreceptor neurons. Photoreceptor cells represented about 60%–70% of the neurons. The remaining 5% of cells were presumptive bipolar and ganglion cells. A few contaminant glial cells were sometimes observed, which did not proliferate under the present culture conditions.
Epithelial cell cultures
ARPE-19 cells, a spontaneously arising human RPE cell line, were routinely passaged by dissociation in 0.25% trypsin and 5 mM EDTA, in Hanks’ balanced salt solution without calcium and magnesium (HBSS), followed by replating at a split ratio 1:3. ARPE-19 cells were maintained in a growth medium (DME high glucose [DME-HG] with 10% FBS). For short-term coculture experiments, ARPE-19 cells were differentiated in 25-cm2 flasks maintained during 4–5 weeks after confluence in the growth medium. For long-term coculture experiments, cells were seeded at 50% confluence on plastic 35-mm-diameter culture dishes; after reaching 70% of confluence, the medium was replaced with a differentiation medium (DME-HG, 1% FBS, plus 25 nM all-trans retinoic acid) for 2–3 weeks before being used (Dunn et al., 1996).
Caco2 cells, an epithelial cell line derived from human colorectal adenocarcinoma, were grown in DME-HG with 10% FBS and 1% (v/v) nonessential amino acids. After reaching confluence, cells were allowed to differentiate for two more weeks before use (Lenaerts et al., 2007). Cells from the BC3H-1 muscle cell line were grown in DME with 10% FBS. Before reaching confluence, the culture medium was replaced by neuronal medium for 24 hr and then the cells were detached from their substrata, gently dissociated with a glass pipette and resuspended in neuronal medium.
RPE-neuronal cocultures
To evaluate the spatial reorganization processes occurring during development, cocultures of ARPE-19 cells and neurons were obtained following two alternative procedures, either by seeding retinal neurons on top of RPE cells or vice versa.
Cocultures of neurons seeded over RPE cells
ARPE-19 cells were grown until differentiation (between 3–4 weeks), as previously described and then incubated in neuronal medium during 24 hr. Retinas were dissected and neuronal cell suspensions were prepared in neuronal medium as described above and immediately seeded over ARPE-19 cell cultures. The resulting cocultures were incubated at 368C in a humidified atmosphere of 5% CO2 for 8 days.
Cocultures of RPE cells seeded over neuronal cells
ARPE-19 cells were grown during 4–5 weeks in DME-HG, 10% FBS, and then incubated in neuronal medium for 24 hr. The epithelial cells were then detached from their substrata with a 0.25% trypsin solution during 1–2 min followed by a 1-min treatment with 0.25% trysin inhibitor and DME addition. Cells were centrifuged at 800 rpm for 5 min and resuspended in neuronal medium. This cell suspension was seeded over 1-day neuronal-enriched cultures, obtained as described above. The cocultures were incubated for 24 or 48 hr before fixation.
Cocultures of retinal neurons with Caco2 or BC3H-1 cells
To investigate whether other cell lines showed a reorganization process similar to that of RPE cells and photoreceptors, Caco-2 epithelial cells were differentiated as described above, incubated in neuronal medium for 24 hr and then detached from their substrata, centrifuged at 800 rpm for 5 min, washed with DME, and resuspended in neuronal medium. This cell suspension was seeded over 1-day neuronal-enriched cultures and cocultured for another 24 hr.
Similarly, nonconfluent BC3H-1 cells were resuspended in neuronal medium and seeded over 1-day neuronal-enriched cultures. The resulting cocultures were incubated for another 24 hr before fixation.
Addition of TIMP-1
To inhibit metalloproteinases, RPE-neuronal cocultures were treated with a solution of TIMP-1, a metalloproteinase pan-inhibitor in HBSS (150 ng/mL, final concentration in the culture medium), immediately after seeding RPE cells over neurons. The same volume of HBSS was added to control cocultures. Cocultures were analyzed at different times to evaluate the relative localization of neurons and RPE cells.
Immunocytochemical Methods
Cultures were fixed for at least 1 hr with 2% paraformaldehyde in phosphate-buffered saline (PBS), followed by permeation with Triton X-100 (0.1%) for 15 min. Neuronal cell types were identified by their morphology by using phase contrast microscopy and by immunocytochemistry, using the monoclonal antibodies Rho4D2 and HPC-1, which selectively react with photoreceptor or amacrine neurons, respectively (Barnstable, 1980; Hicks and Barnstable, 1987; Kljavin et al., 1994). Photoreceptor progenitors were identified by an antibody against CRX, a transcription factor required for photoreceptor development (Zhu and Craft, 2000), which is an early marker of photoreceptors in vitro (Garelli et al., 2006).
Alexa 488-conjugated-goat anti-mouse was used as the secondary antibody. Tyramide signal amplification was occasionally used to improve visualization, following the procedure described by the manufacturers.
For CD147, MCT-1 and MCT-4, double immunostaining, nonpermeabilized cells were sequentially incubated with 0.1% Tween-20 Tris buffer (TNT) with nonfat dry milk 2% for 30 min, then with the CD147 antibody diluted in the same buffer and finally with Alexa 488 secondary antibody. After the first staining, cells were permeated with 0.1% Triton X-100 and incubated with MCT-1 or MCT-4 antibodies for 1 hr and then with Cy3-conjugated secondary antibody.
Controls for immunocytochemistry were done by omitting either the primary or the secondary antibody. Cultures were then analyzed by phase and fluorescence microscopy with a Nikon Eclipse E600 microscope and by a laser scanning confocal microscope (Leica DMIRE2) with a 63 water objective. x–y (top to bottom) and x–z sections were collected and processed with LCS software (Leica) and Photoshop 8.0 (Adobe Systems, San Jose, CA). In some cases, phase pictures were contrasted as pseudo-Nomarsky by processing them with the XN-View computer program.
Photoreceptors have a small round cell body of about 5 lm, with a single neurite at one end, which usually ends in a conspicuous synaptic “spherule”; sometimes they display a connecting cilium at the opposite end, but they fail to develop their characteristic outer segments; opsin is diffusely distributed over their cell body, which is usually darker than that of amacrine neurons. To be identified as photoreceptors, the cells had to display at least three of the above-described criteria. Amacrine neurons are larger than photoreceptors (7–20 lm) and have multiple neurites.
Actin Staining
To improve visualization of photoreceptors and RPE cells and confirm the localization of photoreceptors on the apical surface of epithelial cells, in cocultures obtained by seeding ARPE-19 cells over neuronal cultures, cells were permeated with Triton X-100, washed with PBS, and then incubated with TRITC-labeled phalloidin for 20 min to stain actin fibers.
Statistical Analysis
The results represent the average of at least three separate experiments (±SD), unless specifically indicated, and each experiment was performed in triplicate. For cytochemical studies, 10 fields per sample were analyzed in each case. Statistical significance was determined by either Student’s twotailed t-test or one-way repeated measures ANOVA with Bonferroni test as a post hoc test.
RESULTS
Spatial Reorganization of Retinal Neurons and RPE Cells During Their Early Development in Vitro
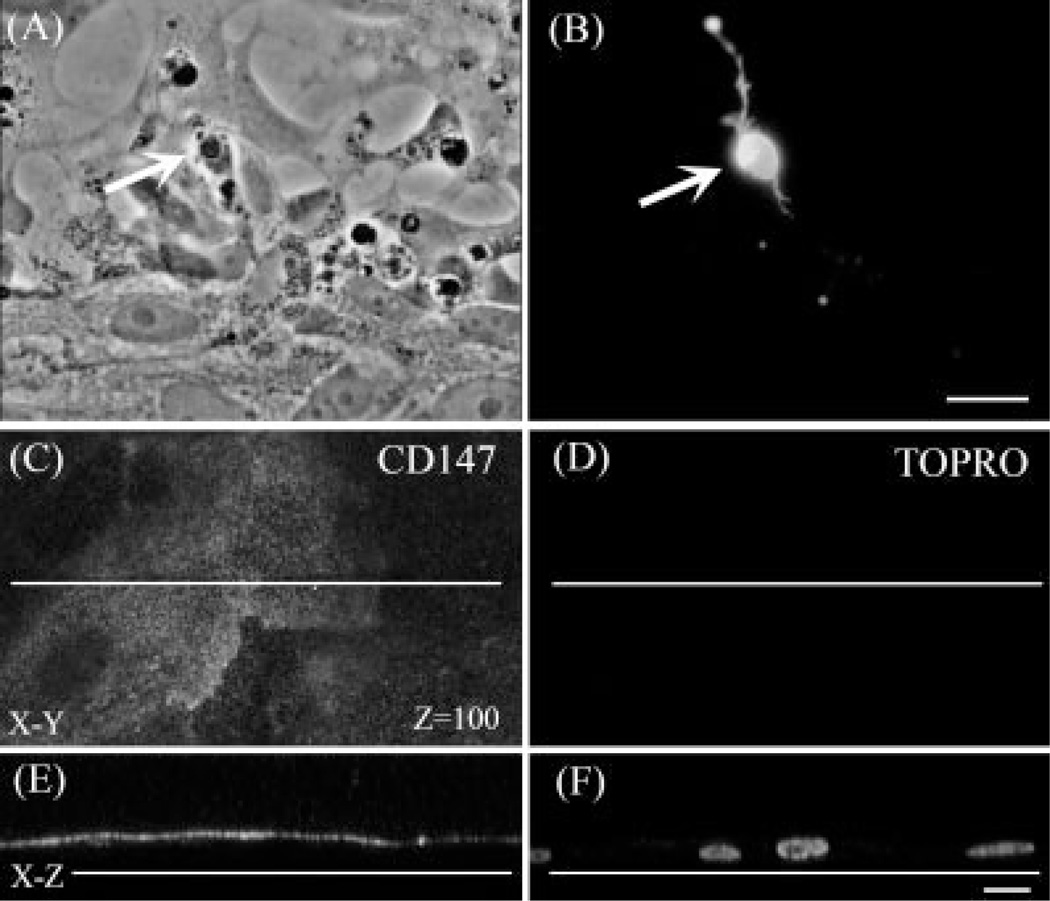
During normal eye development, RPE cells and photoreceptors establish a very precise topological organization, essential for proper visual function. The microvilli at the apical surface of RPE cells closely intermingle with the photoreceptor outer segments; this arrangement is necessary for the performance of key cooperative functions such as the visual cycle and phagocytosis of shed photoreceptor outer segments by RPE. To further our understanding of how these interactions are established, we cocultured ARPE-19 cells and retinal neurons (Fig. 1A). Cultured ARPE-19 cells broadly expressed the lactate transporter MCT-1 and its chaperone CD147 at the apical surface and the lactate transporter MCT-4 at the basolateral membrane (Fig 1C,E), as previously reported (Deora et al., 2005). These experiments indicate that ARPE-19 cells seeded directly on the dish surface rapidly developed their characteristic polarity, with the membrane exposed to the culture media becoming the apical surface.
Fig. 1.
Expression of opsin in photoreceptors and development of polarity in ARPE-19 in retina neurons cocultured with differentiated RPE cells. Phase (A) and fluorescence (B–F) photomicrographs of retina neurons seeded on ARPE-19 epithelial cells, after 8 days in coculture. Photoreceptor cells (white arrows in A,B) were labeled with Rho4D2 monoclonal antibody (B) for immunochemical detection of opsin. Photoreceptors seeded on epithelial cells recognized epithelial apical surfaces and expressed opsin (B). Confocal images of RPE cells double stained with the apical marker CD147 (C,E) and TO-PRO3 (D,F), to label cell nuclei. C,D: x-y sections at z 5 100 showing that CD147 was widely and selectively expressed in RPE cell membranes exposed to the culture media (C). x-z section obtained at the level indicated by the white lines in (E,F). Note that the apical marker CD147 was absent from RPE membranes contacting the substrata (E). Scale bars = 10 µm.
When we seeded retinal neurons over these polarized RPE cells, neuroblasts attached to their apical surface (Fig. 1A), attaining a spatial organization corresponding to the one found in the retina in vivo. Under these conditions, ARPE-19 cells rapidly promoted pho-toreceptor differentiation; two days later, photoreceptors began to express opsin, and maintained this expression even after 8 days (white arrows in Fig. 1A,B). This suggests that neuronal cells recognized RPE apical membranes as a “natural,” adequate substratum, suitable for proceeding with their development.
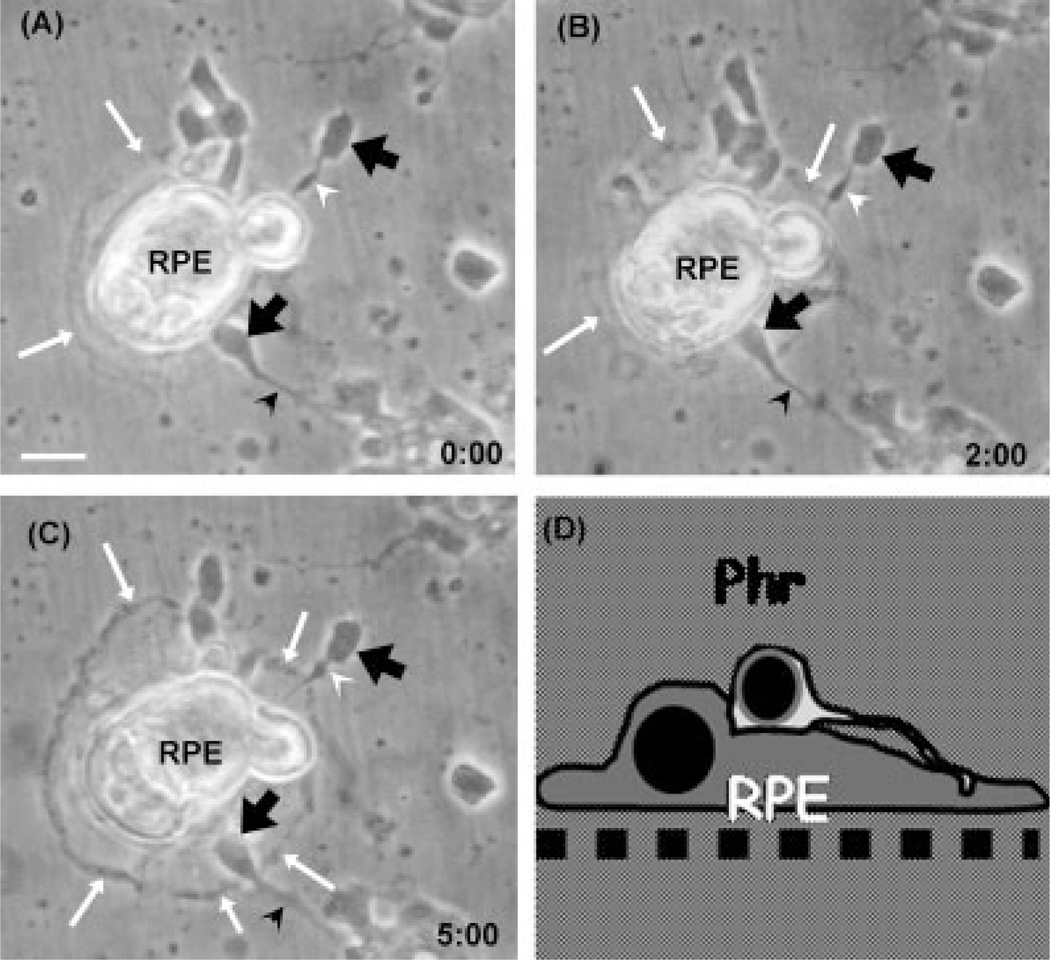
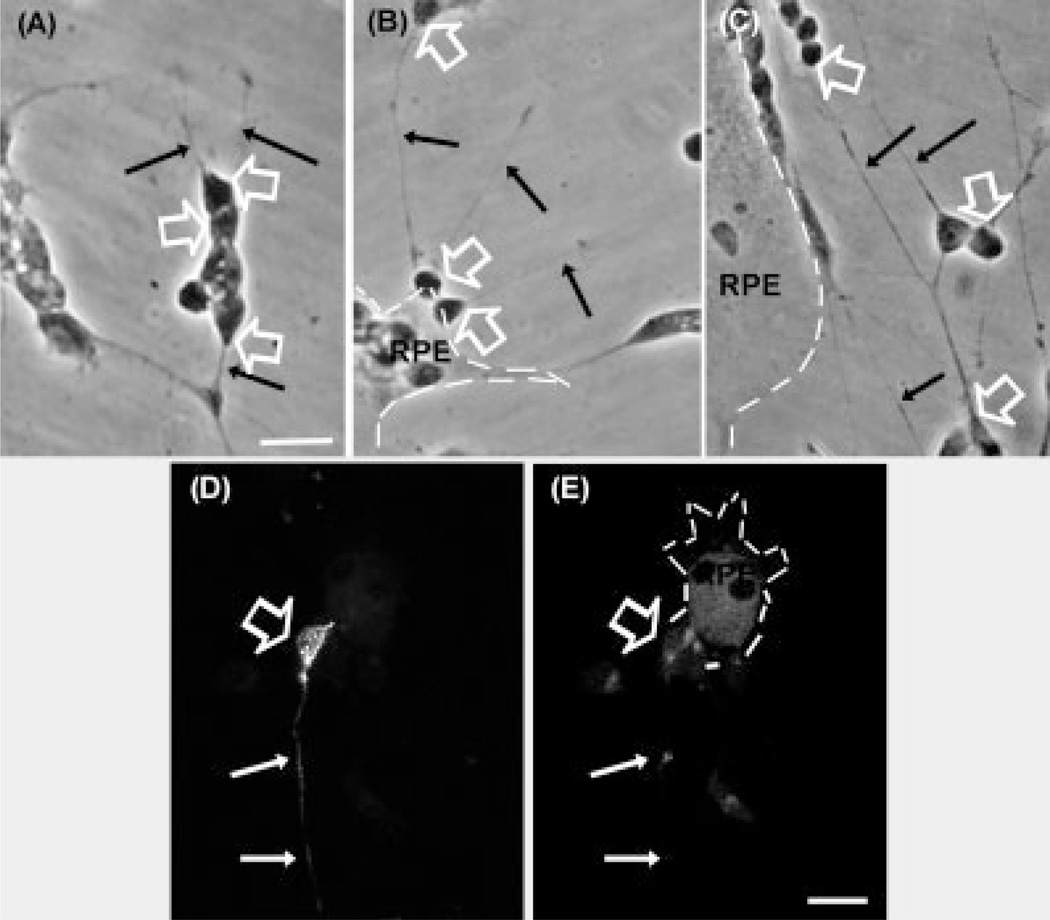
In a second set of experiments, neuronal cells were seeded first and RPE cells were then seeded on top of them. Neurons rapidly attached to the plastic surfaces of the culture dishes, and many of them began to develop their neurites (arrowheads in Fig. 2A). When after 24 hr RPE cells were seeded over the neurons, many of these cells attached directly onto the dish because neurons were seeded at low density, while other RPE cells set over the neurons presumably via their basolateral membranes (Fig. 2A), a configuration opposite to that found in vivo. Strikingly, RPE extended their growing lamellipodia (white arrows in Fig. 2B) below the neurons, detaching them from their substrata (Fig. 2B), thus promoting the establishment of a normal configuration, with most neurons on top of RPE cells (Fig. 2C). This process was fast; just 2 hr after seeding ARPE-19 cells, their outgrowing lamellipodia had reached many neurons in the cocultures (Fig. 2B) and after 5 hr the reorganization process was virtually completed, with most RPE cells being now attached to the dish surface, acting as substratum for the neuronal cells lying on top of them (Fig. 2C).
Fig. 2.
Spatial reorganization of retinal neurons and RPE cells in coculture. Retinal neurons were seeded first and ARPE-19 cells were seeded on top of them 1 day later. Photomicrographs show the cocultures at 0 (A), and after 2 hr (B) and 5 hr (C). The cartoon in (D) represents the relative spatial arrangement of photoreceptors and RPE cells at the end of the reorganization process. Note that after 2 and 5 hr, lamellipodia edges (white arrows in B,C) grew and extended until they reached neuronal cell bodies (black arrows in B,C) or axons (arrowheads in B,C). RPE cells ended beneath neurons (C,D), resembling the relative spatial arrangement found in vivo. Note that the axons that were oriented toward the epithelial cells underwent a rapid retraction (compare white arrowheads in B,C). Scale bars = 10 µm.
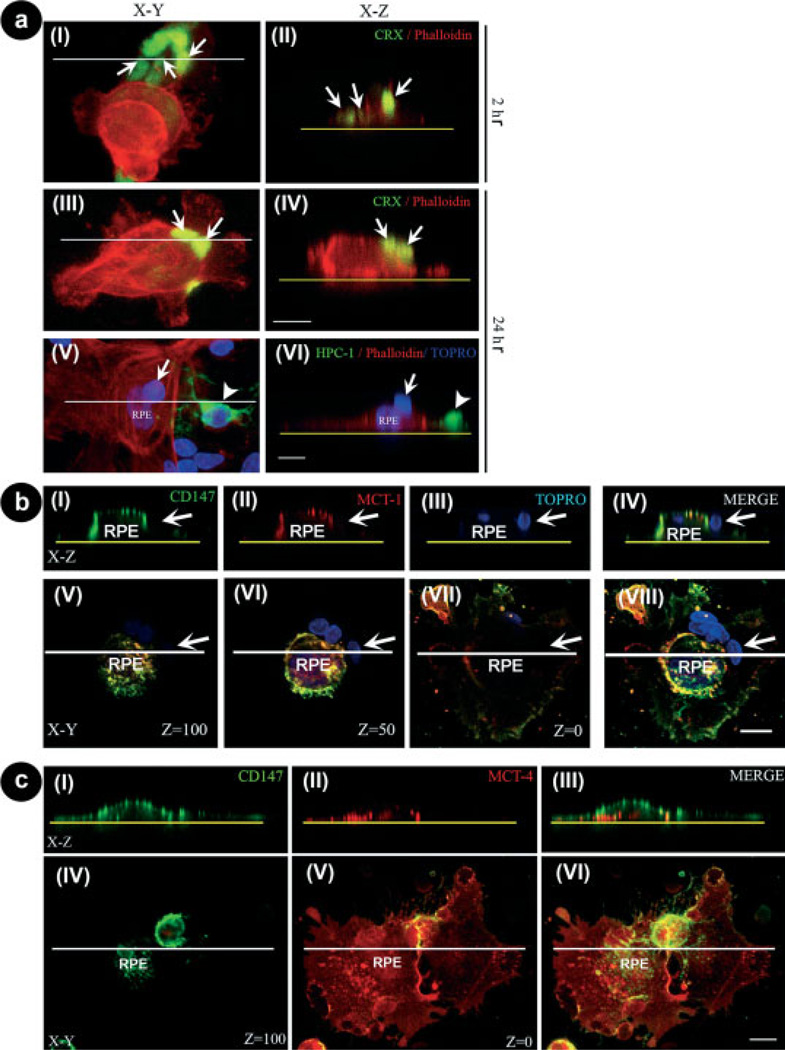
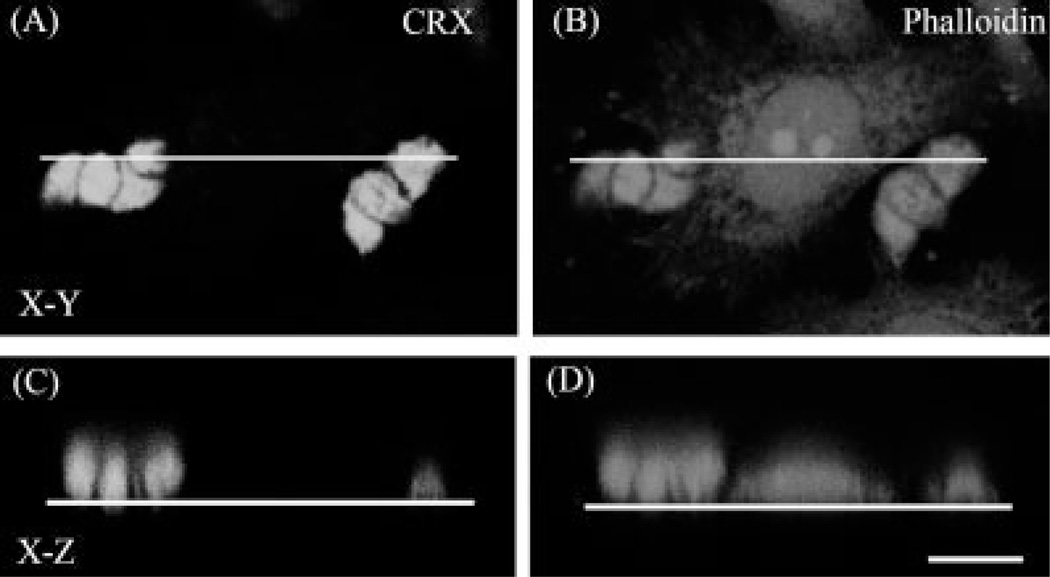
To understand how this spatial reorganization was achieved, 2 and 24 hr cocultures were double-labeled with CRX, to label photoreceptors and with phalloidin, to evidence the actin cytoskeleton in the cells. Confocal images obtained from different x-y sections and x-z sections of the cocultures showed that as early as 1 hr after seeding epithelial cells over neurons, many photoreceptors were already visible on top of the RPE cells (Fig. 3a). The number of photoreceptors that were found on top of RPE cells was about 6,000 and 28,000, 1 and 2 hr after seeding RPE cells, respectively (Table I), indicating that the reorganization process was extremely rapid. This rearrangement continued very actively, and after 24 hr in coculture about 88,000 photoreceptors lied on top of the RPE cells (Table I), i.e., 98.2% of the total photoreceptors found in the close proximity of RPE cells. The amount of photoreceptors over RPE cells increased significantly at every time analyzed, when compared with 0 hr (Table I). However, the difference in these amounts was not significant between 5 and 24 hr, suggesting that the reorganization process was nearly complete after the first 5 hr in coculture.
Fig. 3.
Photoreceptors colocalization with apical markers of RPE cells. a: Confocal images of 2 hr (I,II) and 24 hr (III–VI) cocultures of RPE cells seeded over neurons stained with phalloidin to label actin filaments (red staining); anti-CRX (green staining in I–IV), to identify photoreceptors (arrows); anti-HPC-1 (green staining in V,VI), to selectively recognize amacrine neurons (arrowheads), and TO-PRO3 (blue staining in V,VI). x-y projections (I,III,V) and x-z projections (II,IV,VI) obtained at the levels indicated by the white lines in I, III, and V. Yellow dotted lines in II, IV, and VI indicate the substratum. After 2 hr in coculture, most photoreceptors remained attached to the substratum while some of them were already reorganized (arrows in II); after 24 hr in cocultures, most photoreceptors were found on top of RPE cells (arrows in IV and VI), while amacrine neurons remained attached to the substratum (arrowheads in VI). Scale bars 5 10 µm. b: Confocal images of 24-hr co-cultures of RPE cells seeded over neurons. (I–IV) x-z sections of photoreceptors (white arrows) and RPE cells stained with the apical surface markers, anti-CD147 (I), and anti-MCT-1 (II), and with TO-PRO3 (III). Merge images are shown in (IV). The yellow dotted lines in I–IV represent the bottom of the culture dish. Arrows show photoreceptor nuclei. V–VII:x-y merge images obtained at different levels (z = 100; z = 50; and z = 0) of the z-axis. The image projection of the above pictures is shown in (VIII). (I–IV) x-z section obtained at the level indicated by the white lines in (V–VIII). Note that at 24 hr in coculture, most photoreceptors were already localized on top of RPE cells at their apical surface, as indicated by the colocalization of CD147 and MCT-1 staining (I–IV). Scale bar 5 10 µm. c: Confocal images of 24-hr cocultures of neurons seeded over RPE cocultures. (I–III) x-z sections of RPE cells stained with anti-CD147 (I), anti-MCT-4 (II), and the merged image x-z (III). The yellow dotted lines in (I–III) represent the bottom of the culture dish. (IV–VI) pictures represent x-y merge images obtained at different levels (z = 100 and z = 0) of the z axis. The image projection of the above pictures is shown in (VI). (I–III) x-z section obtained at the level indicated by the white lines in (IV–VI). Scale bar = 10 µm.
TABLE I.
Spatial Reorganization of Retinal Neurons and RPE Cells in Co-Culture
| Neurons over RPE cells/dish |
Photoreceptors over RPE cells/dish |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number |
(%) |
Number |
(%) |
|||||||
| Time (hrs) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 0 | 0.0 ± 0.0 | a | 0.0 ± 0.0 | 0.0 ± 0.0 | a | 00.0 ± 0.00 | ||||
| 1 | 06621.0 ± 13137.0 | a | 06.3 ± 12.6 | 06016.0 ± 11931.1 | a | 06.3 ± 11.7 | ||||
| 2 | 30267.3 ± 5464.50 | b | 28.8 ± 6.30 | 28312.6 ± 8244.70 | b | 31.6 ± 8.40 | ||||
| 5 | 74110.9 ± 10634.4 | c | 70.5 ± 10.1 | 71420.5 ± 10763.7 | c | 81.2 ± 18.2 | ||||
| 24 | 90715.4 ± 10535.3 | c | 86.3 ± 10.0 | 88004.0 ± 11793.2 | c | 98.2 ± 2.50 | ||||
Retinal neurons were seeded on the plastic dishes and RPE cells were seeded over them 1 day later. The data represent the amount of neurons and photoreceptors, respectively, found on top of RPE cells at different times in co-culture. The values were calculated from the total number of cells found in the close proximity of RPE cells (i.e., less than 100 lm from each other). Data are the mean ± SD of 5 experiments. Statistical significance was calculated by using One-Way Repeated Measures ANOVA test for each column. At the 0.05 level, the population means are significantly different. Mean values were compared using Bonferroni t statistics. Letter (a–c) indicate significant differences ( p < 0.05) among the cases analyzed.
Confocal images of 24 hr-cocultures projected onto the x-z-axes confirmed that the upper and lateral surfaces of the RPE cells coexpressed the apical markers CD147 and MCT-1 (Fig. 3b, I,II). They also showed that the TO-PRO3-labeled nuclei of photoreceptors were on top of RPE cells and in close proximity to these apical markers (Fig. 3b, III,IV). Projections of these images obtained at different levels of the x-y-axes (i.e., z = 100 and z = 50) confirmed this localization of photoreceptors (white arrows in Fig. 3b, V–VIII). RPE cell membranes in contact with the dish surface expressed the MCT-4 basal marker (Fig. 3c, II,V); the confocal images obtained of the x-z projection at z = 0 showed that MCT-4 was widely distributed in the basal membranes (Fig. 3c, II, V), while it was normally absent in the cell membranes exposed to the culture media (Fig. 3c, IV). In contrast, confocal images obtained from different levels of x-y projections showed the CD147 apical marker concentrated in the apical and lateral membranes (Fig. 3c, I,VI), and excluded from the basal membranes (Fig. 3c). Hence, although MCT-1 and CD147 usually colocalized on the cell surface exposed to the culture medium (Fig. 3b IV), the expression of the apical and basal markers, MCT-1 and MCT-4 respectively, were mutually exclusive, suggesting that RPE cells in coculture preserved their polarity after their spatial reorganization.
Nearly all the epithelial and photoreceptor cells that were in close proximity, i.e., less than 100 lm from each other, switched their spatial distribution. Timelapse experiments showed that about 70% of the neurons switched their position during the first 5 hr in coculture; after 24 hr in coculture no photoreceptors remained beneath RPE cells (Figs. 2C, 3a, and Table I).
We then investigated whether the spatial reorganization induced by RPE cells was specific for photoreceptors. After 24 hr in coculture, over 90,000 neurons were over RPE cells (Table I). At this time, photoreceptors had mostly switched their position and were over RPE cells; in contrast, most amacrine cells remained attached to the plastic substratum (white arrowheads in Fig. 3a, V,VI). A quantitative analysis revealed that 88.9% ± 2.6 (n = 6) of the neurons lying on top of RPE cells were CRX positive, i.e., photoreceptors, while only 3.6% ± 1.4% (n = 4) were HPC-1-positive, amacrine neurons; less than 6% were undetermined (not shown). These results suggest that the reorganization process promoted by RPE cells was highly specific for photoreceptors.
Molecular Mechanisms Involved in RPE Cell–Photoreceptor Reorganization
Neurons are firmly adhered to the dish surface in neuronal-enriched cultures; hence, their detachment from their substratum by RPE cells was rather startling and suggested that epithelial cells might carry it out by enzymatic, rather than by nonenzymatic, mechanic means. RPE cells synthesize and release several metalloproteinases (Padgett et al., 1997; Plantner et al., 1998; Hunt et al., 1993), capable of remodeling tissues by detaching cells from their substrata. To investigate whether these enzymes were involved in cell detachment, RPE cells were seeded over 1-day neuronal cells, and the cocultures were incubated with or without TIMP-1. As previously described, in cocultures lacking the inhibitor photoreceptors and RPE cells completely switched their positions after 24 hr; almost all photoreceptors in contact with RPE cells ended on top of these cells (Fig. 4A,C) and extended their neurites to make contacts with other neurons. Phalloidin staining clearly showed that the actin cytoskeletons of photoreceptors were on the apical surface of epithelial cells (Fig. 4C).
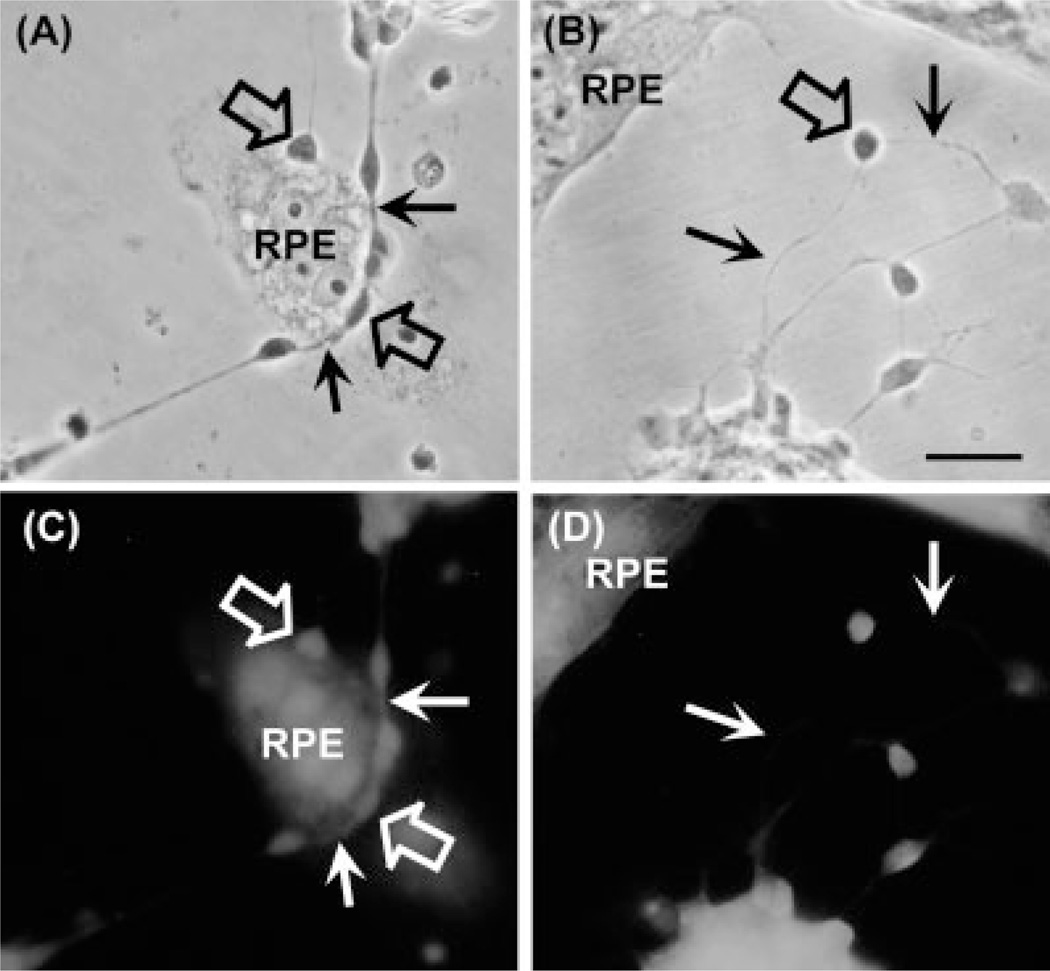
Fig. 4.
Inhibition of the spatial reorganization of retinal neurons and RPE cells by the metalloproteinase inhibitor TIMP-1. (A,B) Phase and (C,D) fluorescence photomicrographs of neuroepithelial cocultures without (A,C) or with (B,D) TIMP-1 (150 ng/mL), added immediately after seeding RPE cells over a 1-day neuronal culture (open arrows). After 24 hr, the cocultures were fixed and labeled with phalloidin (C,D). In the absence of TIMP-1, RPE cells promoted the spatial rearrangement of neuronal cells (open arrows in A,C), which ended on top of RPE cells. Phalloidin staining showed the occurrence of neurites on top of RPE cells in TIMP-lacking cultures (white arrows in C). In cultures treated with TIMP-1, neuronal cell bodies (open arrow in B) and their neurites (thin arrows in B, D) remained attached to the dish surface. Scale bar = 10 µm.
On the contrary, in cocultures incubated with TIMP-1 no cell rearrangement was observed; RPE cells extended their lamellipodia until they reached retinal neurons, but were unable to detach neurons from their substrata; both cell types retained their original arrangement, and no neurons were switched to rest over epithelial cells (Fig. 4B,D). These results implicate that enzymatic degradation by metalloproteinases mediated photoreceptor detachment.
To assess whether the ability to detach neurons and promote cell reorganization was inherent to epithelial cells or also found in other cell types, cocultures of retinal neurons and BC3H-1, a muscle cell line, were prepared. In these cocultures there was no cell rearrangement after 24 hr (not shown). We then investigated whether all epithelial cells shared this capacity for promoting reorganization. When cells from the epithelial colorectal cell line Caco2 were seeded on top of neuronal cells, most neurons remained next to, or underneath Caco2 cells without changing their spatial orientation (Fig. 5); after 24 hr in coculture only 12.7% ± 7.2% (n = 3) of the neurons ended on top of Caco2 cells. Hence, Caco2 epithelial cells were not as competent as RPE cells to promote neuronal spatial reorganization. Notably, 88.2% ± 2.0% of the neurons that switched their spatial organization were CRX-positive photoreceptor cells, as occurred in neuronal–RPE cocultures. This suggests that the mechanisms involved in the reorganization process depend to a certain extent in specific interactions between photoreceptors and epithelial cells, even when epithelial cells derive from nonretinal tissues.
Fig. 5.
Scarce spatial reorganization of retinal neurons and Caco-2 cells in coculture. Confocal images of 24 hr cocultures of Caco-2 cells seeded on top of neuronal cultures, and stained with anti-CRX (A,C) and phalloidin (B,D). (A,B) x-y projections and (C,D) x-z sections obtained at the levels indicated by the white lines in (A,B). Note that photoreceptors remained adhered to the substratum (C). Scale bar = 10 µm.
Effect of RPE Cells on Orientation and Growth of Photoreceptor Axons
RPE cells had remarkable effects on neurite outgrowth and orientation. In neuronal-enriched cultures, photoreceptor axons oriented randomly (Fig. 6A, black thin arrows); this lack of orientation was also observed in neuron-RPE cocultures when neurons and RPE cells were more than 100 µm apart (not shown). However, at shorter distances, RPE cells clearly influenced neurite orientation. Neurons less than 100 µm apart from RPE cells usually oriented their neurites parallel to the epithelial edges (Fig. 6C, black thin arrows), and neurite growth preferentially adopted a radial distribution, heading away from RPE cells in photoreceptors placed either on the apical surface of RPE cells or immediately next to them (Fig. 6B, black thin arrows).
Fig. 6.
Effect of RPE cells on neurite orientation in photoreceptors. Phase (A–C) and fluorescence (D,E) photomicrographs of neuronalenriched cultures (A) or cocultures (B–E) of ARPE-19 cells seeded on top of neuronal cultures, showing RPE lamellipodia edges (dashed lines) and the orientation of outgrowing axons (thin arrows). Photo-receptors (open arrows) were identified by their morphology or with the Rho4D2 monoclonal antibody (white thin arrows in D). In neuronal-enriched cultures, neurites grew randomly oriented (A), but in cocultures, neurite growth adopted a radial distribution pointing away from (B,D,E) or parallel to RPE cells (C). Rho4D2 staining of cocultures (D) and phalloidin staining (E) observed under confocal microscopy confirmed that most photoreceptors ended on top of RPE cells with their axons pointing away from these cells. Scale bars = 10 µm.
Double staining of the neuronal-RPE cocultures with phalloidin and Rho4D2 confirmed that photoreceptors initially seeded on the plastic surface of the dish ended on top of RPE cells (Fig. 6D,E) and extended their axons away from RPE cells. Photoreceptor neurites occasionally approached epithelial cells but when they reached certain proximity they changed their orientation and went on growing parallel to the epithelial edges (not shown). Quantitative analysis revealed that 71.3% ± 11.8% (n = 3) of them pointed away from epithelial cells, thus suggesting that epithelial cells release cues that repel axons, keeping them away.
The proximity of RPE cells also induced neurite retraction. As is clearly visible in Figure 2, soon after RPE cells were seeded over neuronal culture, both neurons with axons pointing toward RPE cells and others with neurites exhibiting the opposite orientation (white arrowheads in Fig. 2A – C) were observed in the cocultures. Notably, although neurites pointing away from the RPE cells continued their outgrowth in the same direction (black arrowheads in Fig. 2), those oriented toward epithelial cells underwent a noticeable retraction (compare white arrowheads in Fig. 2B,C). This retraction began soon after the outgrowing RPE lamellipodia contacted neurites (Fig. 2B, white arrowheads); only 2 hr after seeding the RPE cells, this nearness forced neurites to retract (Fig. 2B).
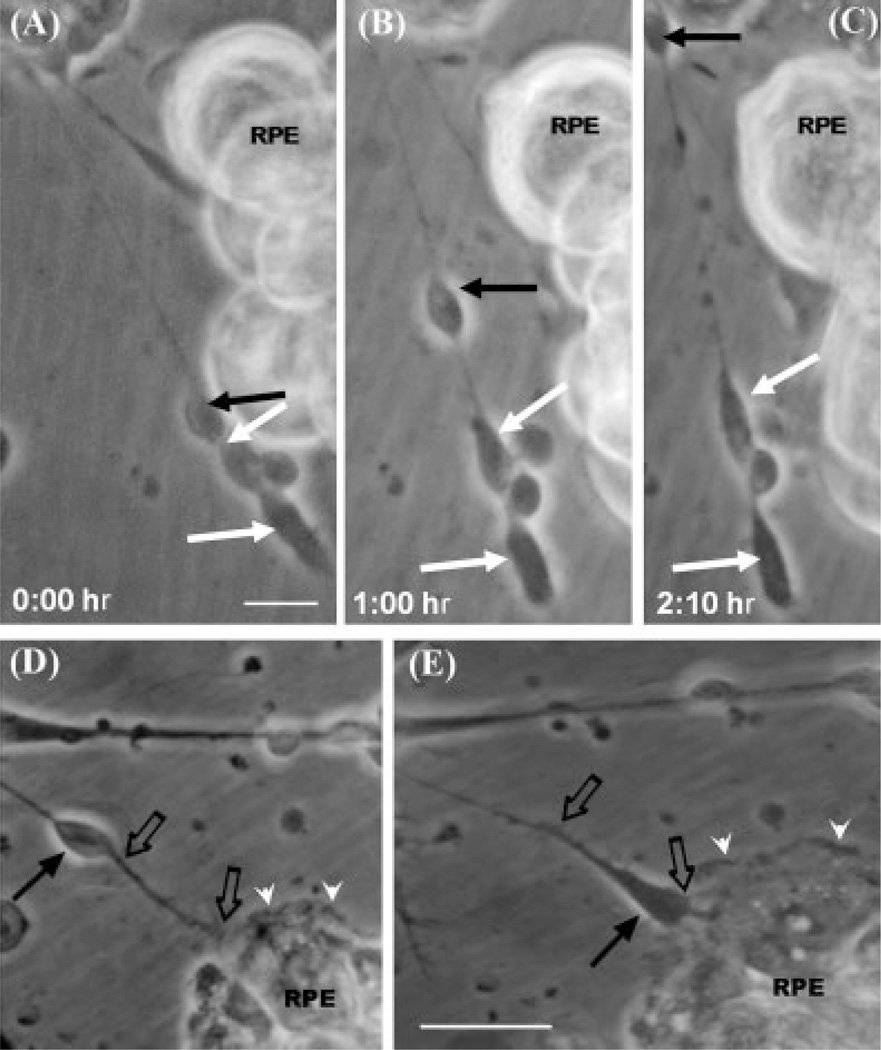
Remarkably, many photoreceptors moved very actively during the first hours of development in coculture, traveling along their own neurites (Fig. 7, solid black arrows). Photoreceptor cell bodies migrated swiftly from one end to the other of their neurites by using them as guidance tracks (Fig. 7A–C, black filled arrows). Thus, neurons having their outgrowing neurites pointing toward RPE cells (black arrow in Fig. 7D) and with their cell bodies farther away (black filled arrow in Fig. 7D), moved cell bodies along their own neurites toward RPE cells (black empty arrows in Fig. 7E). Through these rearrangements, photoreceptor cell bodies ended next to the epithelial cells with axonal endings pointed away from RPE cells (black arrow in Fig. 7E), thus resembling the spatial organization of the retina in vivo. This reorientation was quite rapid because cell bodies covered distances of about 10–20 µm per hour (black arrows in Fig. 7D,E). This migration might also play a role in the reorientation of photoreceptor axons during development.
Fig. 7.
Active migration of photoreceptor cell bodies. Photomicrographs of RPE cells seeded over neuronal cells, taken at 0 hr (A), 1 hr (B), and 2 hr (C) after seeding RPE cells on top of retinal neurons. A photoreceptor cell body (A–C, black solid arrows) is visible rapidly moving along its own neurite, migrating parallel to RPE cells, while other photoreceptors (white arrows) were left behind. Photomicrographs after 2 hr (D) and 3 hr (E) of seeding RPE cells on top of neuronal cells show that RPE lamellipodia (white arrowheads) reached the neuronal processes (black empty arrows, D) and extended beneath neurons (E). Some neurons (black thin arrows in D,E) initially extended their neurites (empty arrows in D,E) toward the RPE cells, but then changed their orientation by displacing their cell bodies toward the epithelium (black thin arrow in E). Scale bars = 10 µm.
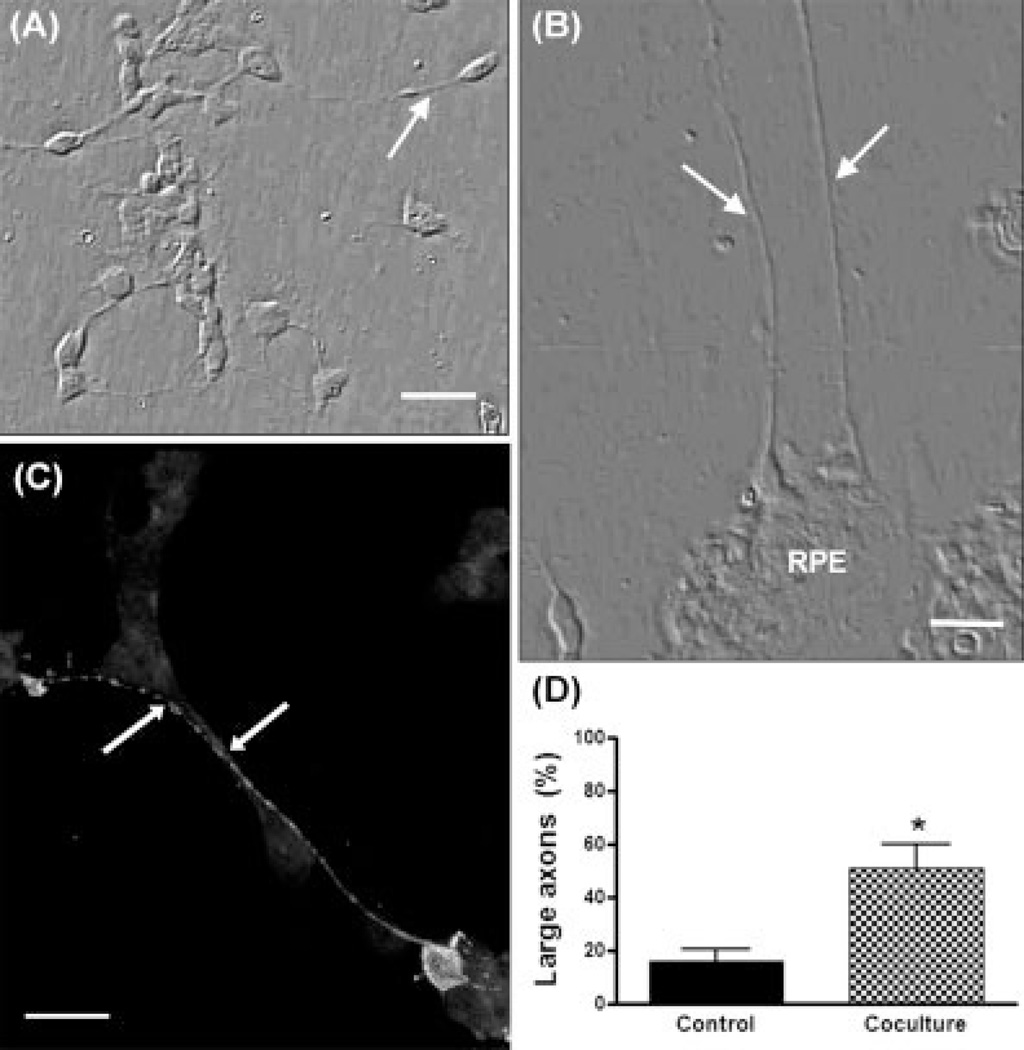
RPE cells also influenced axonal outgrowth. In neuronal-enriched cultures, photoreceptor axons were usually short (Fig. 8A); only about 20% of photoreceptors had axons longer than three times their cell body lengths (Fig. 8D). Coculture of retinal neurons with RPE cells led to a marked increase in axonal length (Fig. 8B,C); about 50% of photoreceptor axons ranged between 4 to 10 times the cell body length (Fig. 8D). Hence, RPE cells seemed to release both molecules that promoted axonal outgrowth and repulsive axon guidance cues.
Fig. 8.
Effect of RPE cells on axonal outgrowth of photoreceptors. Phase (A, B) and fluorescence (C) confocal photomicrographs double stained with phalloidin (background fluorescence) and Rho4D2 (intense fluorescence) of neuronal-enriched cultures (A) or neuronepithelial cocultures (B,C) showing photoreceptor axons (arrows). Photoreceptors were identified by their morphology or with the Rho4D2 antibody (intense fluorescence in C). Quantitative analysis of axon length is shown in (D). The bars show the percentage of photoreceptors having “long” axons (i.e., defined as longer than three times the cell body diameter). *Statistically significant differences compared with control (P < 0.01). Scale bars = 10 µm.
DISCUSSION
RPE Cells Promote the Spatial Orientation of Developing Photoreceptors
During development of the retina in vivo, photo-receptors complete their differentiation through their interaction with the apical surfaces of highly polarized RPE cells and orient their axons pointing away from the pigment epithelium (Bok, 1993; Marmorstein et al., 1998a; Marmorstein, 2001). Given the complexity of the factors occurring during retina development, it is difficult to establish the roles of the different neuronal and nonneuronal cell types in setting up the cytoarchitecture of the retina. To overcome this difficulty we developed cocultures of RPE cells and retinal neurons, which enabled us to examine the interactions between these two cell types during their development. Thus, we determined that both cell types have an active crosstalk at early stages of development that allows them to acquire their specific topological arrangement. Photoreceptors and RPE cells in vitro recognized which was their correct relative orientation and had mechanisms to modify their spatial arrangement when it was not the physiological one. RPE cells rapidly developed their characteristic polarity once in contact with the dish surface; shortly after being seeded, they expressed the apical markers CD147 and MCT-1 in those membranes exposed to the culture medium, and the basal marker MCT-4 in the membranes exposed to the substrata, as previously described (Deora et al., 2005). Hence, photoreceptors seeded over RPE cells contacted the epithelial apical surfaces, recognized them as their adequate substratum, adhered to them and proceeded with their differentiation. By contrast, when RPE cells were seeded over neurons, and epithelial basal membranes contacted photoreceptors, in a nonphysiological orientation, a rapid reorganization process took place. RPE cells promoted the detachment of photoreceptors from the dish surface and their relocalization on the apical epithelial surface, thus establishing the spatial arrangement and polarity occurring in vivo.
The spatial reorganization appeared to require the activity of metalloproteinases, probably released by RPE cells because TIMP-1, a pan-inhibitor of these enzymes, blocked photoreceptor detachment without inhibiting the development of RPE cells and the outgrowth of their lamellipodia. Matrix metalloproteinases (MMP) are zinc-dependent endopeptidases, capable of degrading extracellular matrix proteins. The presence of MMP 1, 2, 3, and 9 has been described in the interphotoreceptor matrix (IPM) of the retina (Plantner et al., 1998), and MMPs have been found in RPE cells, which secrete them from their apical surfaces (Padgett et al., 1997). MMPs have been proposed to degrade most of the glycoproteins and/or proteoglycans found in the IPM and their expression is regulated by signaling from ECM receptors (Werb et al., 1989; Boudreau et al., 1995). Our results suggest that interaction of photoreceptors with the basal membranes of RPE cells might trigger the release of metalloproteinases, which would then detach photoreceptors from their substrata and thus initiate their rearrangement. Noteworthy, RPE cells can also secrete TIMP-1 from their apical membrane (Padgett et al., 1997), which might provide a tool to autoregulate the degradation process.
Once detached, photoreceptors adhered onto the apical surfaces of RPE cells, thus achieving the correct topological orientation. How photoreceptors recognized the apical surface of RPE cells in culture is still unclear. RPE cells selectively allocate certain proteins in their apical membranes (Marmorstein et al., 1996, 1998b; Bonilha et al., 1999), and this distribution changes during development, suggesting that it depends on the establishment of adult interactions between RPE cells and retina cells (Marmorstein et al., 1996). Integrins undoubtedly play a role in these interactions; specific integrins participate in migration and attachment of RPE cells to their substrata (Jin et al., 2000) and in outer segment phagocytosis (Finnemann et al., 1997). The cell adhesion molecule N-CAM has been proposed to regulate adhesive contacts with photoreceptors and with the IPM. Cadherins have also been shown to mediate apical adhesions between retina epithelial cells; differential expression of N-cadherin contributes to the spatial organization of retina cells in the Drosophila eye (Hayashi and Carthew, 2004). Because RPE cells expressed characteristic apical and basal markers, they might also express other apical proteins naturally occurring in RPE membranes, some of which might be relevant as recognition sites and to dock photoreceptors on top of epithelial cells during development in vitro. Recognition of epithelial apical membranes by photoreceptors might favor the release of molecular cues or trigger a signaling pathway, essential to proceed with photoreceptor development and differentiation.
The ability to promote the spatial reorganization of retinal neurons appears to be specific for epithelial cells because the spatial rearrangement of retinal neurons was not observed in cocultures with the muscle cell line BC3H-1. However, not every epithelial cell had the same skill to carry on this reorganization; although nearly all photoreceptors ended on top of RPE cells in RPE-neuronal cocultures, only a small percentage of them underwent a similar rearrangement when cocultured with Caco2 cells, evidencing that RPE cells were much more efficient than the later in promoting the spatial reorganization of photoreceptors. Caco2 cells have also been shown to release MMP 9, which is inhibited by TIMP-1 (Gan et al., 2001). Therefore, epithelial cells might share the molecular tools required for switching the topological organization of neighbor cells, in order to prevent their erroneous localization. This ability might have evolved to a very efficient, highly specific mechanism for the selective interactions between photoreceptors and RPE cells, which might be useful for establishing the layer structure of the mammalian retina. Previous evidence suggests that RPE cells might be involved in the acquisition of retina cytoarchitecture; embryonic chick retinal cells reaggregated when they were grown in the presence of RPE cells, forming stratified retinal spheres, with rods and cones integrated into a structure similar to the outer nuclear layer of the normal retina (Rothermel and Layer, 2001).
The specificity of the interactions between photoreceptors and RPE cells was also supported by the finding that photoreceptors switched their topological orientation much more efficiently than amacrine cells. About 70% of the neuronal cells in these cocultures were photoreceptors and 30% were amacrine neurons. However, there was an overwhelming prevalence of photoreceptors on the apical surface of ARPE-19 cells, and notably on Caco2 cells too, with very few (less than 5%) amacrine neurons. The reasons for this specificity remain to be established. It might arise on a differential ability of MMPs to detach photoreceptors and amacrine neurons from their substrata, arising in differences in the proteins involved in this attachment in both cell types. It might also depend on the presence of docking cues for photoreceptors, and not for amacrine neurons, on the apical surface of epithelial cells, that favor photoreceptor adhesion once detached. In this context, it is relevant to point out that in the retina in vivo, RPE cells have little probability of interacting with amacrine cells, which are in the inner layer, and do not contact epithelial cells.
Photoreceptors appear to contribute to establish the spatial rearrangement. In those cases in which the polarity of photoreceptors was opposite to the normal arrangement found in the eye (i.e., photoreceptor cell bodies were distant from RPE cells and their neurites were oriented toward RPE cells), these neurons reversed their polarity moving their cell bodies toward RPE cells. By using their own neurites as guidance tracks, they ended with their neurite pointing away from RPE cells, thus restoring the physiological polarity. Hence, rather than remaining static, both neurons and epithelial cells displayed a considerable activity after being seeded.
RPE Cells Promote Differentiation of Photoreceptors
RPE cells also influenced other features of photoreceptor differentiation. They markedly enhanced neurite outgrowth because photoreceptor axons were much longer in the cocultures than in neuronal-enriched cultures. Epithelial cells also influenced axonal orientation; over 70% of photoreceptor axons pointed parallel to or away from RPE cells, thus mimicking the situation in vivo. RPE cells release several molecules that promote neurite outgrowth, such as insulinlike growth factor 1, FGF-1 and −2, ciliary neurotrophic factor, and pigment epithelium-derived factor (Gaur et al., 1992; Jablonski et al., 2000; reviewed in Strauss, 2005), some of which might promote axonal outgrowth in neuroepithelial cocultures. In addition, RPE cells actively synthesize and release docosahexaenoic acid (Wang and Anderson, 1993), which promotes the elongation of photoreceptor axons (Garelli et al., 2006). RPE effect on neurite orientation appeared to depend on the closeness to RPE cells; when neurites grew in the close proximity of epithelial cells, their growth cones usually diverged from them, suggesting RPE cells might release molecules that repelled axonal outgrowth. This correlates with the situation in vivo; during normal development of the retina, photoreceptors must orient their axons toward the inner nuclear layer, away from RPE cells, to establish their adequate connections. This orientation might be determined not only by attractive cues released by target cells in inner layers of the retina, but also by the repulsive signals derived from molecules secreted by RPE cells.
Taken together, our results suggest that RPE cells play a critical role in building the architecture of the retina. Whether the mechanism for reorganizing cell arrangement in vitro described here has a role in the setting up of the spatial pattern in the retina in vivo is an intriguing question. Whatever the answer, our results show that RPE cells have mechanisms that might help to avoid the occurrence of abnormal configurations while developing the architecture of the retina. The pattern formation of the retina requires organizing its different cell types into a precise spatial arrangement. After their last mitotic division, retinal neuroblasts migrate toward their final position in the layered structure of the retina, a process constituting an important mechanism for developing the cytoarchitecture of the retina. Our findings suggest that RPE cells might provide a means for reorganizing the position of abnormally localized cells and exert a surveillance activity aimed to achieve a correct positioning of photoreceptors on the apical surfaces of RPE cells, thus playing a role in defining the spatial organization of the retina.
ACKNOWLEDGMENTS
L. Politi and N. Rotstein are CONICET researchers and O.L. German is a CONICET postdoctoral fellow. We thank Nancy Philp (Thomas Jefferson University, Philadelphia) for supplying the antibodies CD147, MCT-1, and MCT-4; and Horacio DeGenaro and Beatriz de los Santos (Instituto de Investigaciones Bioquimicas de Bahia Blanca) for technical assistance.
Contract grant sponsors: FONCyT, CONICET, and Universidad Nacional del Sur (to L.P.).
REFERENCES
- Abe T, Saigo Y, Kano T, Wakusawa R, Tokita Y, Tamai M. Protection of photoreceptor cells from phototoxicity by transplanted retinal pigment epithelial cells expressing different neurotrophic factors. Cell Transplant. 2005;14:799–808. doi: 10.3727/000000005783982549. [DOI] [PubMed] [Google Scholar]
- Adler R. Regulation of neurite growth in purified retina cultures. Effects of PNPF, a substratum-bound neurite-promoting factor. J Neurosci Res. 1982;8:165–177. doi: 10.1002/jnr.490080207. [DOI] [PubMed] [Google Scholar]
- Barnstable C. Monoclonal antibodies which recognize different cell types in the rat retina. Nature. 1980;286:231–235. doi: 10.1038/286231a0. [DOI] [PubMed] [Google Scholar]
- Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993;17:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- Bok D, Hall M. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol. 1971;49:664–682. doi: 10.1083/jcb.49.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha V, Finnemann S, Rodrίguez-Boulan E. Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J Cell Biol. 1999;47:1533–1548. doi: 10.1083/jcb.147.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Myers C, Bissell M. From laminin to lamin: regulation of tissue-specific gene expression by the ECM. Trends Cell Biol. 1995;5:1–4. doi: 10.1016/s0962-8924(00)88924-2. [DOI] [PubMed] [Google Scholar]
- Deora A, Philp N, Hu J, Bok D, Rodriguez-Boulan E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc Natl Acad Sci U S A. 2005;102:16245–16250. doi: 10.1073/pnas.0504419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K, Aotaki-Keen A, Putkey F, Hjelmeland L. ARPE-19, a Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Finnemann S, Bonilla V, Marmorstein A, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci U S A. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X, Wong B, Wright S, Cai T. Production of matrix metalloproteinase-9 in CaCO-2 cells in response to inflammatory stimuli. J Interferon Cytokine Res. 2001;2:93–98. doi: 10.1089/107999001750069953. [DOI] [PubMed] [Google Scholar]
- Garelli A, Rotstein N, Politi L. Docosahexaenoic acid promotes photoreceptor differentiation without altering CRX expression. Invest Ophthalmol Vis Sci. 2006;47:3017–3027. doi: 10.1167/iovs.05-1659. [DOI] [PubMed] [Google Scholar]
- Gaur V, Lis Y, Turner J. RPE conditioned medium stimulates photoreceptor cell survival, neurite outgrowth and differentiation in vitro. Exp Eye Res. 1992;54:645–659. doi: 10.1016/0014-4835(92)90020-s. [DOI] [PubMed] [Google Scholar]
- Glazer L, Dryja T. Understanding the etiology of Stargardt’s disease. Ophthalmol Clin North Am. 2002;15:93–100. doi: 10.1016/s0896-1549(01)00011-6. [DOI] [PubMed] [Google Scholar]
- Gu S, Thompson D, Srikumari C, Lorenz B, Finckh U, Nicoletti A, Murthy K, Rathmann M, Kumaramanickavel G, Denton M, Gal A. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Carthew R. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- Hicks D, Barnstable C. Different rhodopsin monoclonal antibodies reveal different binding patterns on developing and adult rat retina. J Hystochem Cytochem. 1987;35:1317–1328. doi: 10.1177/35.11.3655327. [DOI] [PubMed] [Google Scholar]
- Hollyfield J, Witkovsky P. Pigmented retinal epithelium involvement in photoreceptor development and function. J Exp Zool. 1974;189:357–378. doi: 10.1002/jez.1401890309. [DOI] [PubMed] [Google Scholar]
- Hunt R, Fox A, al Pakalnis V, Sigel M, Kosnosky W, Choudhry P, Black E. Cytokines cause cultured retinal pigment epithelial cells to secrete metalloproteinases and to contract collagen gels. Invest Ophthalmol Vis Sci. 1993;34:3179–3186. [PubMed] [Google Scholar]
- Insua M, Garelli A, Rotstein N, German O, Arias A, Politi E. Cell cycle regulation in retinal progenitors by gliaderived neurotrophic factor and docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2003;44:2235–2244. doi: 10.1167/iovs.02-0952. [DOI] [PubMed] [Google Scholar]
- Jablonski M, Tombran-Tink J, Mrazek D, Iannaccone A. Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. J Neurosci. 2000;20:7149–7157. doi: 10.1523/JNEUROSCI.20-19-07149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery G. The retinal pigment epithelium as a developmental regulator of the neural retina. Eye. 1998;12:499–503. doi: 10.1038/eye.1998.137. [DOI] [PubMed] [Google Scholar]
- Jin M, He S, Worpel V, Ryan SJ, Hinton DR. Promotion of adhesion and migration of RPE cells to provisional extracellular matrices by TNF-alpha. Invest Ophthalmol Vis Sci. 2000;41:4324–4332. [PubMed] [Google Scholar]
- Kljavin I, Lagenaur C, Bixby J, Reh T. Cell adhesion molecules regulating neurite growth from amacrine and rod photoreceptor cells. J Neurosci. 1994;14:5035–5049. doi: 10.1523/JNEUROSCI.14-08-05035.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts K, Bouwman F, Lamers WH, Renes J, Mariman E. Comparative proteomic analysis of cell lines and scrapings of the human intestinal epithelium. BMC Genomics. 2007;8:91–105. doi: 10.1186/1471-2164-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Turner J. Optimal conditions for long-term photoreceptor cell rescue in RCS rats: the necessity for healthy RPE transplants. Exp Eye Res. 1991;52:669–679. doi: 10.1016/0014-4835(91)90019-b. [DOI] [PubMed] [Google Scholar]
- Marmorstein A. The polarity of the retinal pigment epithelium. Traffic. 2001;2:867–872. doi: 10.1034/j.1600-0854.2001.21202.x. [DOI] [PubMed] [Google Scholar]
- Marmorstein A, Bonilha V, Chiflet S, Neill J, Rodriguez-Bouland E. The polarity of the plasma membrane protein RET-PE2 in retinal pigment ephitelium is developmentally regulated. J Cell Sci. 1996;109:3025–3034. doi: 10.1242/jcs.109.13.3025. [DOI] [PubMed] [Google Scholar]
- Marmorstein A, Finnemann S, Bonilha V, Rodriguez-Boulan E. Morphogenesis of the retinal pigment epithelium: toward understanding retinal degenerative diseases. Ann N Y Acad Sci. 1998a;857:1–12. doi: 10.1111/j.1749-6632.1998.tb10102.x. [DOI] [PubMed] [Google Scholar]
- Marmorstein A, Gan Y, Bonilha V, Finnemann S, Csaky K, Rodriguez-Boulan E. Apical Polarity of N-CAM and EMMPRIN in Retinal Pigment Epithelium Resulting from Suppression of Basolateral Signal Recognition. J Cell Biol. 1998b;142:697–710. doi: 10.1083/jcb.142.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata N, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett L, Lui G, Werb Z, LaVail M. Matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 in the retinal pigment epithelium and interphotoreceptor matrix: vectorial secretion and regulation. Exp Eye Res. 1997;64:927–938. doi: 10.1006/exer.1997.0287. [DOI] [PubMed] [Google Scholar]
- Patrick J, McMillan H, Wolfson H, O’Brien CJ. Acetylcholine receptor metabolism in a nonfusing muscle cell line. J Biol Chem. 1977;252:2143–2153. [PubMed] [Google Scholar]
- Philp NJ, Wang D, Yoon H, Hjelmeland LM. Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophtahlmol Vis Sci. 2003;44:1716–1721. doi: 10.1167/iovs.02-0287. [DOI] [PubMed] [Google Scholar]
- Plantner J, Smine A, Quinn T. Matrix metalloproteases and metalloprotease inhibitors in human interphotoreceptor matrix and vitreous. Curr Eye Res. 1998;17:132–140. doi: 10.1076/ceyr.17.2.132.5610. [DOI] [PubMed] [Google Scholar]
- Politi L, Bouzat C, de los Santos E, Barrantes F. Heterologous retinal cultured neurons and cell adhesion molecules induce clustering of acetylcholine receptors and polynucleation in mouse muscle BC3H-1 clonal cell line. J Neurosci Res. 1996;43:639–651. doi: 10.1002/(SICI)1097-4547(19960315)43:6<639::AID-JNR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Politi L, Rotstein N, Carri N. Effects of GDNF on neuroblast proliferation and photoreceptor survival: additive protection with docosahexaenoic acid. Invest Ophtahlmol Vis Sci. 2001a;42:3008–3015. [PubMed] [Google Scholar]
- Politi L, Rotstein N, Carri N. Effects of docosahexaenoic acid on retinal development: cellular and molecular aspects. Lipids. 2001b;36:927–935. doi: 10.1007/s11745-001-0803-8. [DOI] [PubMed] [Google Scholar]
- Rizzolo L. Polarity and the development of the outer bloodretinal barrier. Histol Histopathol. 1997;12:1057–1067. [PubMed] [Google Scholar]
- Rothermel A, Layer P. Photoreceptor plasticity in reaggregates of embryonic chick retina: rods depend on proximal cones and on tissue organization. Eur J Neurosci. 2001;13:949–958. doi: 10.1046/j.1460-9568.2001.01469.x. [DOI] [PubMed] [Google Scholar]
- Rotstein N, Aveldaño M, Barrantes F, Politi L. Docosahexaenoic acid is required for the survival of rat retinal photoreceptors in vitro. J Neurochem. 1996;66:1851–1859. doi: 10.1046/j.1471-4159.1996.66051851.x. [DOI] [PubMed] [Google Scholar]
- Rotstein N, Aveldaño M, Barrantes F, Roccamo A, Politi L. Apoptosis of retinal photoreceptors during development in vitro: protective effect of docosahexaenoic acid. J Neurochem. 1997;69:504–513. doi: 10.1046/j.1471-4159.1997.69020504.x. [DOI] [PubMed] [Google Scholar]
- Rotstein N, Politi L, Aveldaño M. Docosahexaenoic acid promotes differentiation of developing photoreceptors in culture. Invest Ophthalmol Vis Sci. 1998;39:2750–2758. [PubMed] [Google Scholar]
- Steele F, Chader G, Johnson L, Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A. 1992;90:1526–1530. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Tanihara H, Inatani M, Honda Y. Growth factors and their receptors in the retina and pigment epithelium. Prog Retin Eye Res. 1997;16:271–301. [Google Scholar]
- Wang N, Anderson R. Synthesis of docosahexaenoic acid by retina and retinal pigment epithelium. Biochemistry. 1993;32:13703–13709. doi: 10.1021/bi00212a040. [DOI] [PubMed] [Google Scholar]
- Wang S, Lu B, Wood P, Lund R. Grafting of ARPE-19 and Schwann Cells to the Subretinal Space in RCS Rats. Invest Ophthalmol Vis Sci. 2005;46:2552–2560. doi: 10.1167/iovs.05-0279. [DOI] [PubMed] [Google Scholar]
- Werb Z, Tremble P, Behrendtsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Craft C. Modulation of CRX transactivation activity by phosducin isoforms. Mol Cell Biol. 2000;20:5216–5226. doi: 10.1128/mcb.20.14.5216-5226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]