Abstract
The field of nanoscience has produced more hype than probably any other branch of materials science and engineering in its history. Still, the potentials of this new field largely lay undiscovered ahead of us; what we have learnt so far with respect to the peculiarity of physical processes on the nanoscale is only the tip of an iceberg. Elaborated in this critical review is the idea that the surge of interest in physical chemistry of phenomena at the nanoscale presents a natural consequence of the spatial refinement of the human ability to controllably manipulate the substratum of our physical reality. Examples are given to illustrate the sensitivity of material properties to grain size on the nanoscale, a phenomenon that directly contributed to the rise of nanoscience as a special field of scientific inquiry. Main systemic challenges faced by the present and future scientists in this field are also mentioned. In part, this perspective article resembles standing on the constantly expanding seashore of the coast of nanoscience and nanoengineering and envisioning the parts of the island where the most significant advances may be expected to occur and where, therefore, most of the attention of scientist in this field is to be directed: (a) crossing the gap between life science and materials science; (b) increasing experimentation sensitivity; (c) crisscrossing theory and experiments; and (d) conjoining top-down and bottom-up synthetic approaches. As for materials and the application areas discussed, a special emphasis is placed on calcium phosphate nanoparticles and their usage in controlled drug delivery devices and other applications of biomedical relevance. It is argued that the properties of nanoparticles as drug carriers often comprise the critical determinant for the efficacy of the drug therapy. Therefore, the basic properties of nanoparticles to be optimized for the purpose of maximizing this efficacy are discussed: size, size distribution, morphology, polymorphic nature, crystallinity, biocompatibility, biodegradability, drug elution profiles, and aggregation propensity.
Keywords: Calcium Phosphate, Drug Delivery, Nanoparticles, Nanoscience, Nanotechnology
INTRODUCTION: HOW NANOSCIENCE SPRANG INTO LIFE
Although nanoparticles have existed on the planet Earth since the earliest days of its cosmic conception, nanoscience stands for one of the newest and most prosperous subfields of materials science. Unequivocal beliefs are shared in its potential to revolutionize all fields of natural sciences. This section will explicate the causes behind the birth of this scientific discipline. Since the total body of its knowledge is comparable to the tip of an iceberg, it can be considered to still exist in the embryonic stage of its development. The following sections in the discourse will focus on the formulation of some of the central systemic challenges that will be faced on the path of its growth and maturation.
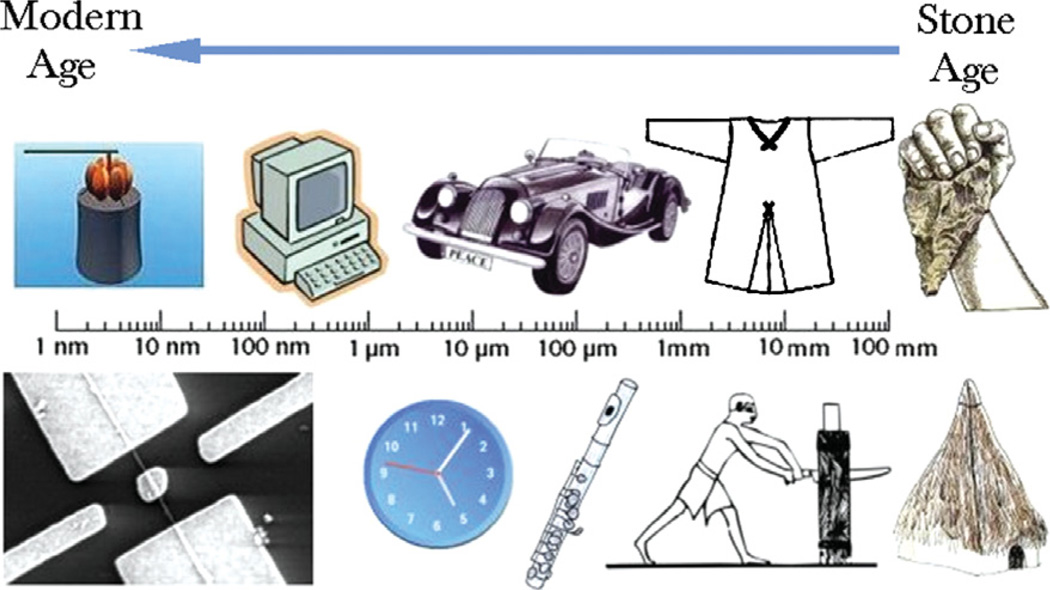
The progress of humanity throughout the history has corresponded to a gradual increase in the fineness with which we are able to controllably reshape the material substrate of our environment.1 As shown in Figure 1, critical boundaries incorporated within the products of human creativity have thus been created at an ever finer scale. As the result, we have witnessed miniaturization of devices in our everyday surrounding. The natural consequence of this is that, as of a few decades ago, materials science has entered the era of controlling physical processes at the nanoscale. Potentials for producing ever more potent technological and biomedical applications have thus arisen. Yet, as the limits in controllability are stretched more than ever, questions have been posed: how far would we be able to go from now on? Certainly, in the realm of nanoscience, advances are not only possible, but seem inevitable too. The discourse that follows is organized around an attempt to outline some of the provinces within the kingdom of nanoscience where the most critical advances may be expected to be made in the near future. At the same time, those could be seen as currently the most exciting frontiers in the investigation of physical phenomena at the nanoscale.
Figure 1.
Critical length scales for some of the key inventions from the history of humanity. A shift across this scale towards ever smaller dimensions paralleled the advancement of humanity from the stone age to the modern age. Hence, as the products of the most sophisticated technologies of the day progressed from prehistoric huts, cutting tools and garments to modern musical instruments, automobiles and mechanical clocks to computers, nanocopters and nanomotors, the critical lengths shifted from millimeter to micrometer to nanometer scale, respectively.
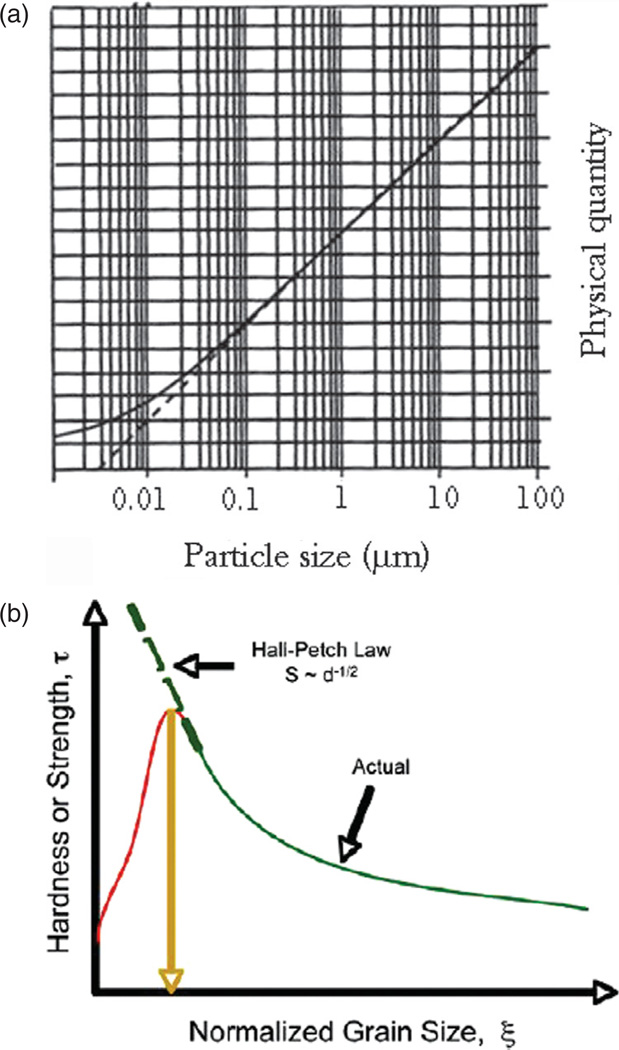
One of the underlying premises of materials science is that properties of materials can be deduced from the known microstructure, the term that describes ways in which the perfect symmetry of an ideal crystal of a given composition is disrupted by imperfections. In addition to intracrystalline defects, these intrinsic imperfections are also directly proportional to the concentration of grain boundaries, which is, in turn, inversely proportional to the grain size. Consequently, many are physical properties that could be described as a function of the size of the grains, alongside their shape, orientation, spatial distribution, etc. Nanoscience as a special field of inquiry spontaneously emerged a couple of decades ago as a branch of physical chemistry of solid state from the need to explain peculiarities observed upon extrapolation of specific physical qualities that are a function of the grain size and entrance into the size region lower than 100 nm (Fig. 2(a)). Namely, the competition between high interfacial energy and the quantum nature of many physical properties at such a small scale produces drastic changes in the properties of materials following negligible modifications of the particle size, polydispersity, inter-particle interaction or morphology. The first observation of such deviations from a law that describes a physical property of a material as a function of its grain size came from the failure of the Hall-Petch law.2 Namely, although the latter suggests inverse proportionality between the grain size and yield strength (the stress under which the material begins to deform plastically) due to the ability of grain boundaries to impede the movement of dislocations, Figure 2(b) demonstrates a typical drastic drop in microhardness at sufficiently low, sub-100-nm particle sizes and the consequent deviation from the function predicted by the Hall-Petch relationship.
Figure 2.
(a) A hypothetic curve illustrating deviation (—) from a theoretically predicted dependence of a physical quantity on the particle size of a material (- - -) once the latter enters the domain of nanosizes (< 100 nm); (b) hardness or strength of a material as a function of normalized grain size, demonstrating the deviations of experimentally obtained values from those predicted by the Hall-Petch law at sufficiently low, sub-100-nm grain sizes. Reprinted with permission from [5], C. S. Pande and K. P. Cooper, Nanomechanics of hall-petch relationship in nanocrystalline materials. Progress Mat. Sci. 54, 689 (2009). © 2009, Elsevier.
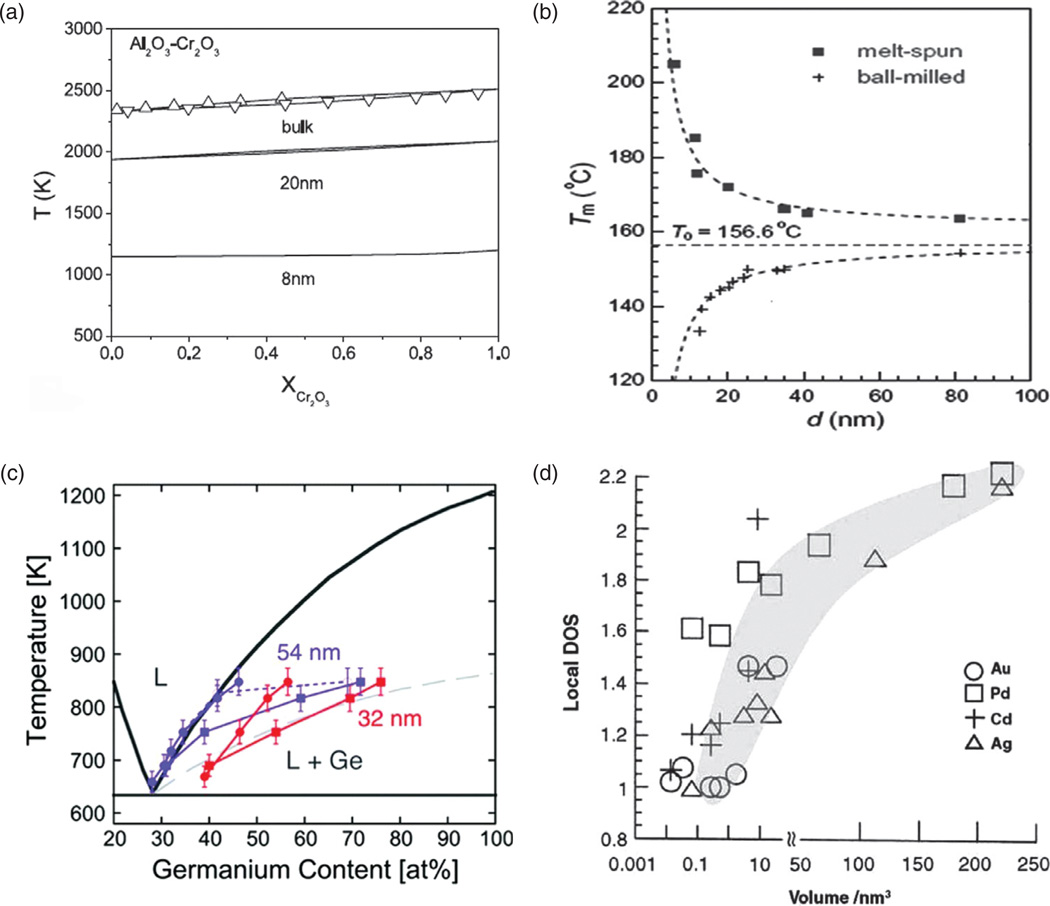
Other examples of such dramatic changes in material properties upon seemingly minor variations in the particle size are given in Figures 3–7. In Figure 3(a), shown is the melting point of Al2O3–Cr2O3 alloy, approximately constant with respect to the chromate content, but greatly depending on the particle size when the latter reaches the nanosized range6 (< 100 nm by convention,3 although this limit is often taken to be 300 nm in the biomedical milieu4). A greater difference in the melting point is produced by a drop in the particle size from 20 to 8 nm than by reducing the particle diameter from 100 to 20 nm, demonstrating that not only does a micro-to-nano transition in the grain size of a material entail drastic changes in the material properties, but also that a minor change in the grain size at the nanoscale can have far more drastic effects on the materials properties than the very transition from bulk to nano. Figure 3(b) shows the melting temperature of indium as dependent not only on the particle size, but on the method of preparation too; thus, a sample prepared by ball-milling exhibits the melting point that exponentially decreases with a decrease in the particle size, while an opposite trend is displayed by the sample synthesized by melt-spinning7 (Fig. 3(b)). This highlights another truism of particularly critical relevance for the field of nanoscience: the method of preparation oftentimes has a crucial effect on the materials properties.
Figure 3.
(a) The melting point of Al2O3-Cr2O3 alloy as a function of Cr2O3 content and the particle size; (b) the melting point of indium as a function of the particle size for two different preparation procedures; (c) the phase diagram for a germanium-based alloy and two different particle sizes—54 and 32 nm; (d) local density of states, a measure of the band gap, decreasing in direct proportion to the particle volume and indicating a metal-insulator transition atV≈ 0.01–1 nm3 in case of three different metals: Au, Cd and Ag, but not Pd. Reprinted with permissions from [6], L. H. Liang, et al., Size-dependent continuous binary solution phase diagram. Nanotech. 14, 438 (2003). © 2003, IOP Publishing; From [7], K. Lu and Z. H. Jin, Melting and superheating of low-dimensional materials. Curr. Op. Solid State Mat. Sci. 5, 39 (2001). © 2001, Elsevier; From [8], E. A. Sutter and P. W. Sutter, Size-dependent phase diagram of nanoscale alloy drops used in vapor-liquid-solid growth of semiconductor nanowires. ACS Nano 4, 4943 (2010). © 2010, American Chemical Society.
Figure 7.
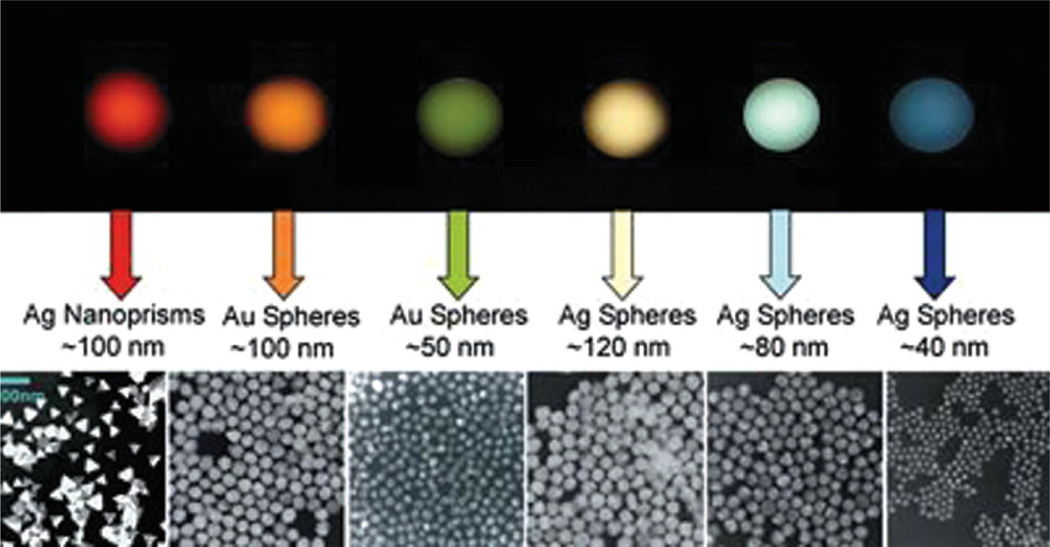
Gold nanoparticles whose color is defined by their size and shape, demonstrating that size-dependent properties are the clue to the tremendous potential of nanoscale objects. Reprinted with permission from [20], Retrieved from http://www.discovernano.northwestern.edu/whatis/index_html/sizematters_html (2012). © 2012, Northwestern University.
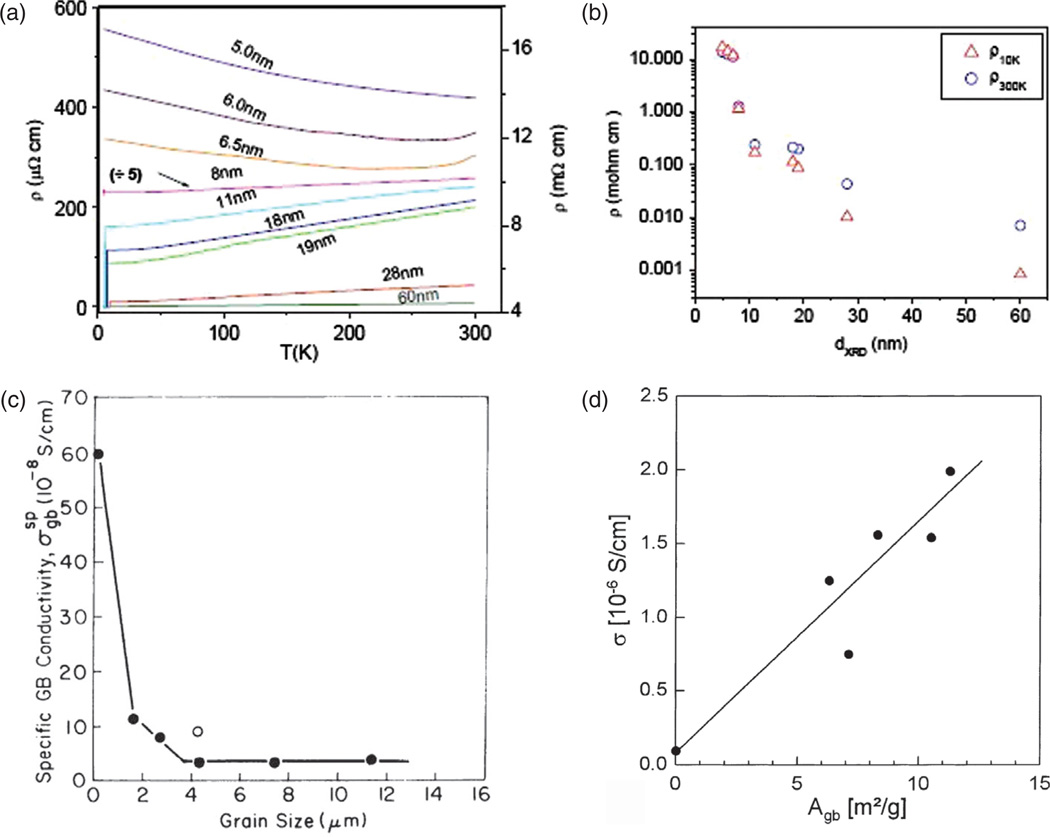
The phase diagram for a germanium-based alloy is shown to drastically differ depending on whether the particle size is 32 or 54 nm8 (Fig. 3(c)). Local density of states, a measure of the band gap of a conductive material, is then shown to depend on the particle size and reaches values around 1, indicating the virtual nonexistence of the band gap and thus the loss of conductivity of metallic particles (Fig. 3(d)). A similar conductor-to-insulator transition induced by a decrease in the particle size is displayed in Figure 4(a)–(b) for the case of nanostructured Nb films.9 The logarithmic dependence of resistivity on the particle size shown in Figure 4(b) dictates that the transition from micrograins to 10-nm-sized particles produces the same magnitude of increase in resistivity as the transition from 10-nm-sized particles to 5-nm-sized ones does.
Figure 4.
The particle size effect on: (a) the resistivity of nanostructured Nb films at different temperatures (the scale on the left corresponds to the films withd≥ 8 nm and the scale on the right to the films with d < 8 nm); (b) the resistivity of the same material at 10 and 300 K; (c) specific conductivity of CaO as a function of the grain size; (d) specific conductivity of Gd-doped CeO2 as a function of the grain boundary surface area per unit mass. Reprinted with permissions from [9], S. Bose, et al., Size induced metal insulator transition in nanostructured Niobium thin films: Intragranular and intergranular contributions. J. Phys: Condens. Matt. 18, 4553 (2006). © 2006, IOP Publishing; From [10], H. L. Tuller, Ionic conduction in nanocrystalline materials. Solid State Ionics 131, 143 (2000). © 2000, Elsevier; From [11], A. Tschöpe, et al., Grain size-dependent electrical conductivity of polycrystalline cerium oxide I: Experiments. Solid State Ionics 139, 255 (2001). © 2001, Elsevier.
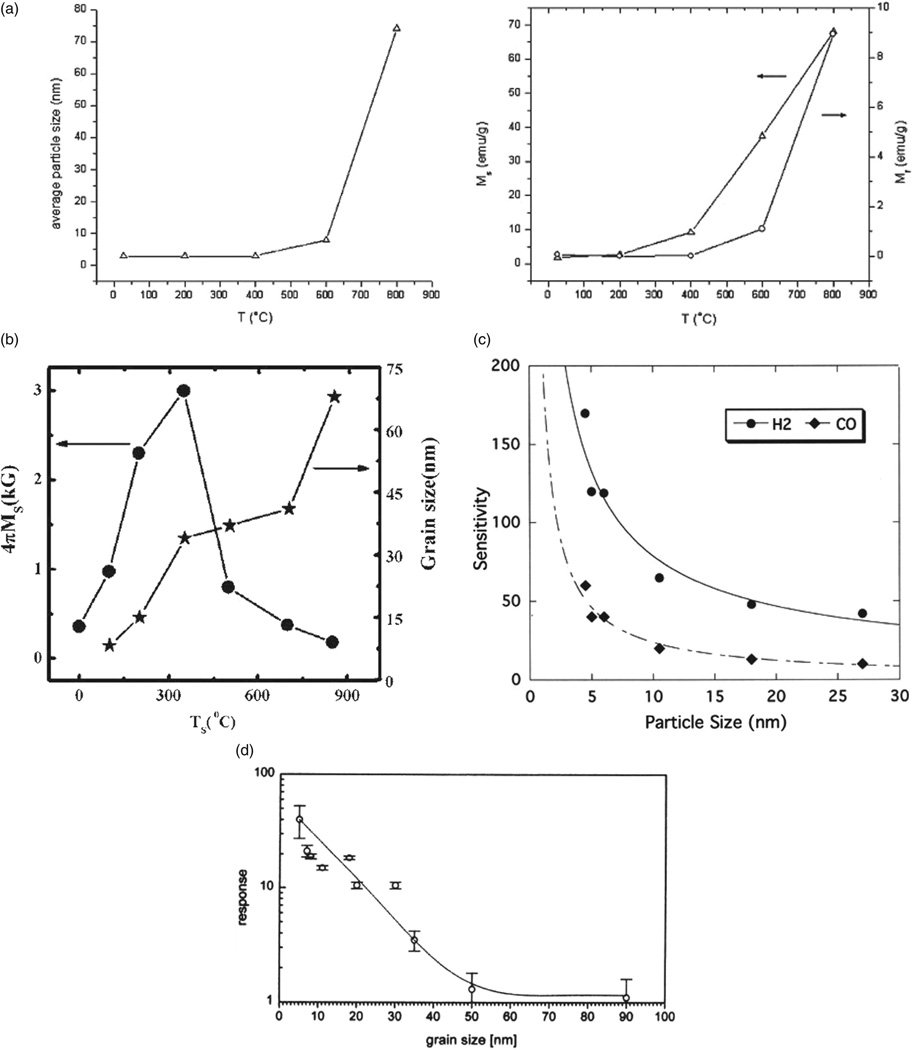
The peculiarities entailed by micro-to-nano structural transitions are further illustrated by the fact that most metals become insulators when their particles size reaches nano scale, and that as the result of the reduced free path of electrons traversing disordered grain boundary. On the other hand, some ceramics undergo the opposite transition and become conductive in the nanoparticulate form, the effect that is either associated with increased concentration of vacancies along the grain boundary with decreasing grain size, contributing to higher lattice entropy and higher ionic conductivity, as in the case of CaO10 (Fig. 4(c)) and ZrO2-Y2O3,12 or is due to electronic conductivity resulting from the accumulation of free electrons in the space charge layer at the grain boundary, as in the case of CeO2 (Fig. 4(d)). Figure 4(d) shows how electrical conductivity of Gd-doped cerium oxide increases in direct proportion to the grain boundary surface area per unit mass.11 How dramatic this change in the overall conductivity of the material can be is demonstrated by the case of rutile,13 TiO2, where a fivefold reduction in the particle size, from 260 to 50 nm, led to a few thousands of times higher conductivity, from 1.4 · 10−6 to 4 · 10−3 Ω−1 cm −1. As for magnetic properties of materials, ferromagnetic compounds in their bulk form typically transform to one of two forms of paramagnetism as their magnetic domain size begins to exceed the particle size: (a) regular paramagnetism in cases when the combination of surface relaxation, lattice expansion and incorporation of impurities interferes with the magnetic ordering to a drastic enough extent, or (b) superparamagnetism in cases when magnetic ordering in the particle core is still sufficient to enable significant collective dipole orientation in the external field (Fig. 5(a)). On much rarer occasions, however, observable is the opposite effect: transformation of a paramagnetic material to a ferromagnetic one paralleling the bulk-to-nano transition, as exemplified by the case of ZnFe2O4 (Fig. 5(b)). Figure 5(c)–(d) furthermore shows the effect of increased sensitivity of sensory surfaces of SnO2 and In2O3 to different gases as a result of the smaller particle size and increased surface area. The increased reactivity of nanoscale surfaces is an effect utilized in various biomedical microelectromechanical systems (Bio-MEMS) for detection of physicochemical agents, including disease biomarkers, at ultralow concentrations.14,15
Figure 5.
The parallel increase in nickel–zinc ferrite particle size ((a), left), saturation magnetization ((a), right, left Y -axis) and remanence ((a), right, right Y -axis) with an increase in the annealing temperature, indicative of ferromagnetic-tosuperparamagnetic transition that entails a drop in the particle size below circa 5 nm; magnetization and particle size of zinc ferrite thin films as a function of the substrate temperature, indicative of the paramagnetic-to-ferromagnetic transition following the transformation of the material from bulk to nanosized form (b); the effect of particle size on sensitivity of SnO2 (c) and In2O3 sensors (d) in detecting CO/H2 (a) and NO2 gases. Reprinted with permissions from [16], V. Uskokovic´ and M. Drofenik, Synthesis of lanthanum-strontium manganites by oxalate-precursor co-precipitation methods in solution and in reverse micellar microemulsion. J. Magn. Magn. Mater. 303, 214 (2006). © 2006, Elsevier; From [17], M. Bohra, et al., Large room temperature magnetization in nanocrystalline zinc ferrite thin films. Appl. Phys. Lett. 88, 262506 (2006). © 2006, American Institute of Physics; From [18], N. Yamazoe, New approaches for improving semiconductor gas sensors. Sensors Actuators B: Chem. 5, 7 (1991). © 1991, Elsevier; From [19], A. Gurlo, et al., Grain size control in nanocrystalline In2O3 semiconductor gas sensors. Sensors Actuators B: Chem. 44, 327 (1997). © 1997, Elsevier.
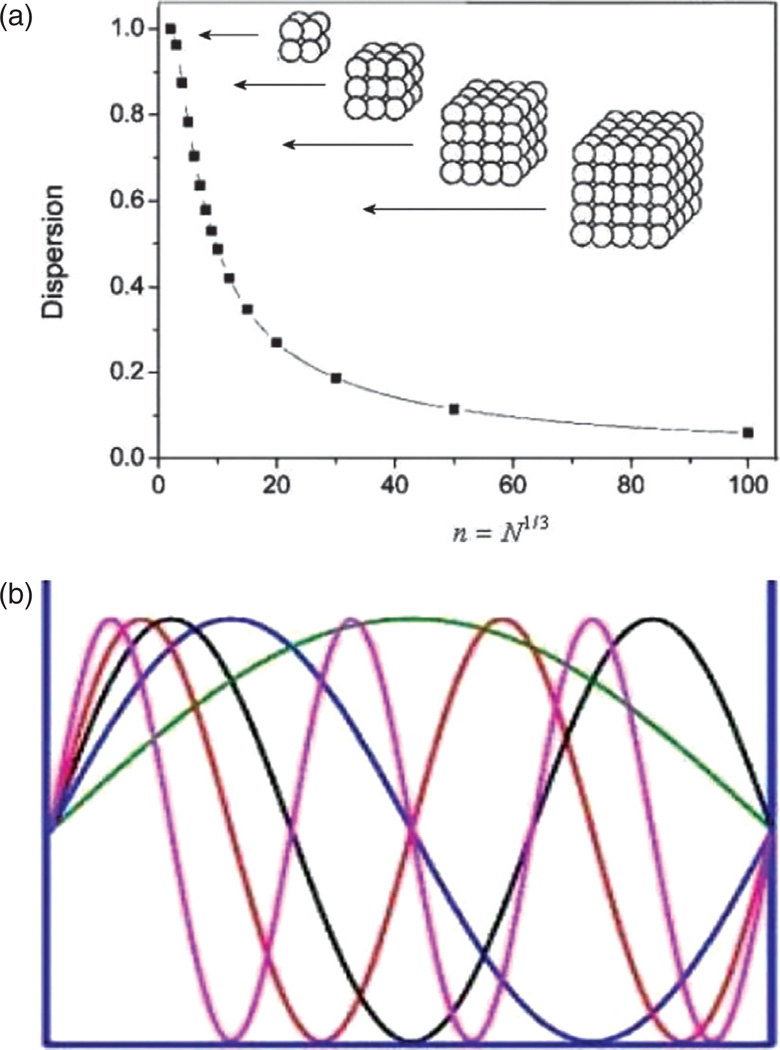
Surface effects are, however, only one reason behind the appearance of novel properties of materials when their grain sizes become reduced to nano scale. Namely, as the particle size decreases, more and more atoms find themselves positioned on the comparatively disordered particle surface and exposed to greater interfacial energies than those experienced by the atoms in the interior of the particle (Fig. 6(a)). For example, a half of the atoms comprising a spherical particle with 3 nm in diameter reside on the particle surface. Another effect coupled to the surface one comes from the quantum confinement apparent when the critical dimensions for a physical quality, often defined by the particle size limits, become comparable to the wavelength of the wave function used to describe the given quality in quantum terms. Quantum confinement effect is demonstrated in Figure 6(b) on standing waves that fit within a resonance box only in specific wavelengths, without permitting any intermediate wavelengths to occur. In other words, as the space in which the physical quality describable by the wavelength of electromagnetic radiation becomes limited, the given quality tends to adopt very specific and well defined states. Quantization, that is, formation of discrete, precisely determined and oftentimes tunable energy levels takes place under such conditions of confinement. Figure 7 thus demonstrates how a seemingly minor change in the size of Ag and Au particles modifies their quantum states and thereby the optical response. The color of the particle sols correspondingly becomes tunable by controlling the particle size as well as their shape.
Figure 6.
(a) Dispersion as a measure of the percentage of atoms located on the particle surface as a function of the number of atoms comprising the particle; (b) quantum confinement effect illustrated on standing waves that fit within a resonance box only in specific wavelengths.
All in all, systematic observation of these and other peculiarities occurring at the nanoscale and a belief that they could be explained within a single framework of thought led to the rise of a special new scientific discipline: nanoscience, or the science of the small, as it has been informally baptized.
CROSSING THE GAP BETWEEN LIFE SCIENCE AND MATERIALS SCIENCE
If you think that the first line in this section has been inadvertently indented, you are wrong. It was intentionally drawn to signify nothingness: pure blankness in space and time.
This is how this section will start. And then a question: “What results from our staring for too long at a single patch of reality?” Blindness, you might say. For, to observe qualities in physical systems, we need to compare their features with some remote standards. For this reason, Rudyard Kipling lamented, “Who knows England who only England knows.” 21 This is why I will draw a bridge (Fig. 8), place materials scientists on one coast, life scientists on the other, and invite you to walk towards the opposite coast from the one that you have inhabited. The hypothesis I offer here is that great things could be learned not only of the side onto which we step, but also of the one that we have temporarily left behind.
Figure 8.
A bridge joining the coasts of materials science and life science on which some of the most exciting research in the field of nanoscience is about to take place.
As we climb from the complexity level of fundamental physics to those of chemistry, biochemistry and molecular biology, biology, psychology, anthropology, ecology, sociology and other social sciences, scientific approaches become less intricate despite the fact that the systems subjected to our scrutiny are, in reality, more complex. This impoverishment of methodological intricacy is naturally reflected in a lesser emphasis on rigorous and ultrafine quantification. Peering into a biochemistry lab for the first time, physical chemists gotten used to exceptional precision in experimentation thus often become surprised realizing how approximate concentrations of chemicals used in reactions are. Partially, this is in accordance with the highly pronounced qualitative character of molecular biology, naturally arising from the fundamental reliance on molecular recognition effects in the research methodology that is paradigmatic in life science labs. On the other hand, it is a logical response of scientists to dealing with highly unpredictable and hardly controllable systems, which even the simplest conceivable biological structures are. For, with this ascent from the atomic scale to the macroscopic scale, the systems subjected to scientific scrutiny become so complex that they require significant simplification thereof lest the practicality of the given scientific methodologies be threatened. And as investigated systems become less complex in the schemes of their investigators, so does the complexity of the exploratory approach applied become spontaneously reduced. The mathematical representations of physicochemical phenomena in the range of materials science, dealing with finer and less dynamic details of Nature, are thus markedly more intricate than those used to represent the biochemical phenomena where the statistical regimes and approximations that limit the ability to scrupulously study these phenomena on the quantum scale begin to reign. Needless to add, all these things contribute to the tremendous gap between materials scientists and life scientists of the modern day, despite the fact that the most exciting research takes place exactly along their intersections. However, as is the case with all the interdisciplinary areas of our inquiry, they demarcate both the riskiest and the most potentially rewarding areas of research. This, on the other hand, explains why the majority of scientists—naturally inclined to stay in the safety of their narrow fields of specialization and remain diligent paradigm-builders instead of groundbreaking paradigm-shifters—decide to avoid those challenging interdisciplinary encounters. Today, they are still entered mainly by those whose curiosity lives up to the norm ‘who dares, wins.’
A vital challenge for this brave new type of scientists that dwell on any of these two mainstream sides of the contemporary research is to build a bridge that connects them and do exciting research either right on it or on their own respective coasts while carrying precious goods and insights across this bridge. The symptoms of the gap between the two fields, however, could be recognized in different methods and vocabularies that biological and materials scientists employ to describe and fundamentally understand the subjects of their inquiry. Whether the former talk about polymerase chain reactions, various blots and in vitro assays, or the latter refer to photonics, semiconductors or crystal growth phenomena, most core materials and life scientists find the terms from an opposite field incomprehensible. Even identical terms sometimes have completely different connotations, as the example of the Latin phrase ‘in situ’ can demonstrate. While it signifies RNA expression assays for a life scientist, it stands for real-time sensors or syntheses that yield a desired material instantaneously, with no need for post-processing treatments, among materials scientists.
While the former mostly tackle highly specific molecular recognition properties of biomolecules, the latter rely on more robust, math-based and classical physical concepts. Yet, it is an inevitable challenge for the frontiers of materials science, and especially its nano fields, to adopt synthetic and characterization approaches applied in life sciences. Biomedical contexts of a large extent of the modern materials science research dictate that.22–24 Many biocombinatorial screening techniques, including selective adsorption using phage display panning techniques, successfully applied to assess specificity of protein-nanomaterial interactions, have led to important and highly functional organic-inorganic interfaces, alongside room temperature procedures for preparation of oxides, ranging from copper oxide25 to calcium molybdate26 to germanium oxide,27 all of which would have otherwise required high annealing temperatures for their formation. However, although numerous highly specific interactions between polypeptides and inorganic surfaces were elucidated and applied using this technique, it still does not allow for discerning the aspects of crystal formation on which the proteins have the most decisive influence: nucleation, diffusion or surface-controlled crystal growth, aggregation of subunits, morphological evolution during aging, et cetera (strictly speaking, all of these aspects are intertwined and affecting one of them is hardly possible without affecting all the other). Clearly, a successful rapprochement of the language of life scientists and materials scientist, the former of whom would bring in the knowledge of the kinetics of protein conformational changes, adsorption and assembly and the latter of whom would contribute with the knowledge on the crystal faceting and growth, conditions yielding answers to these and similar questions.
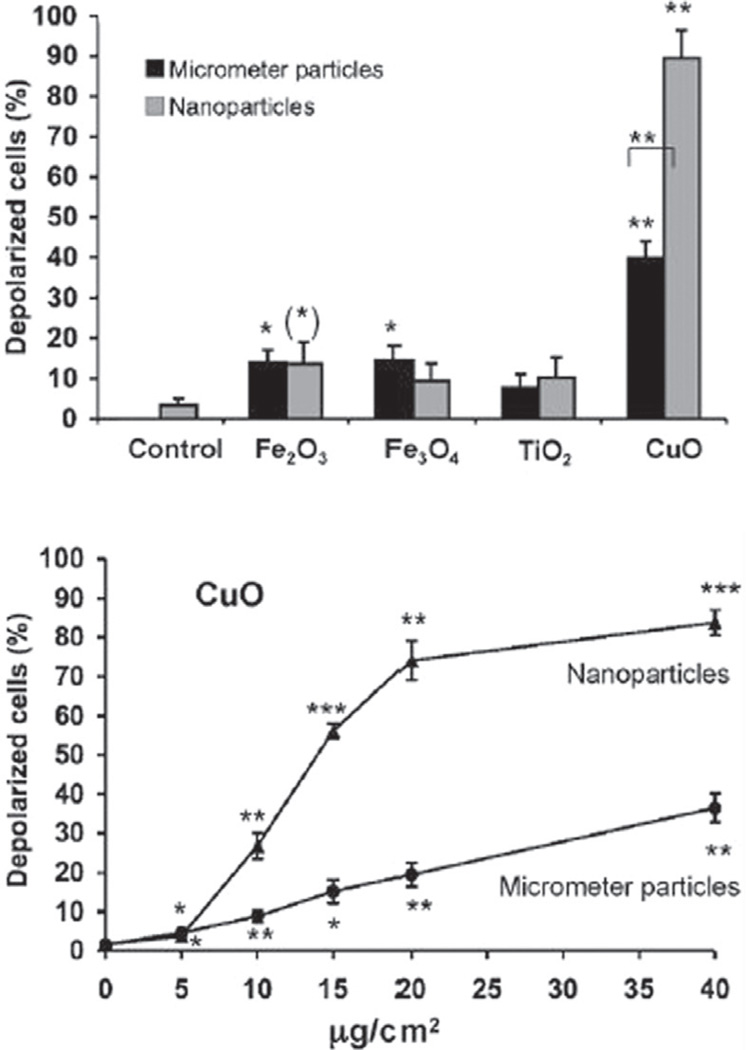
Standing on the bridge that connects these two fields, one would also be able to understand better the currently odd and unexpected effects of nanoparticles on cells and organisms.29 Namely, depending on the particle composition, surface chemistry and stiffness, as well as the experimental context in which they are assessed, nanoparticles can be more or less toxic than their microsized counterparts. For example, 20-nm sized polytetrafluoroethylene particles turn out to be toxic and the 130-nm ones harmless,30 whereas 25-nm sized CeO2 particles are safe and 250-nm ones are toxic.31 Also, minor changes in the particle size sometimes have dramatic effects on their biodistribution or cellular uptake profiles. Dendrimers with 3–6 nm in size were thus rapidly excreted through the kidneys, while the 6–8 nm sized particles localized in tumor tissues and 15-nm sized ones were taken up by the macrophages.32 In another study, the uptake efficiency peaked for 50-nm sized gold nanoparticles and decreased in a size-dependent manner at both smaller and larger size ranges.33 Other studies came to conclusion that 20–25 nm is the optimal size for the cellular uptake of gold nanoparticles, indicating that smaller is not necessarily better.34,35 The uptake of 50-nm sized polystyrene nanoparticles by human intestinal Caco-2 cells was, on the other hand, smaller than for any particles in the 0.1–1 µm range,36 suggesting that not only are countless particle properties, including, naturally, the synthesis method,37 involved in defining the nanoparticlecell interaction, but the cell type and metabolism too. Then, displayed in Figure 9 is the percentage of depolarized human alveolar basal epithelial cells, directly indicative of mitochondrial damage, following incubation with micro- and nano-particles of different chemical composition. Interestingly, only in the case of copper oxide did nanoparticles induce a significantly more damaging effect—reflected in DNA damage and reduced cell viability too—than their microsized counterparts. Although most commercially applied nanoparticles are either confined within functional devices or coated with protective layers, which minimizes their exposure to air and thus mitigates their possible toxic effects,38 toxicity assessments are expected to attract more funding in future. This is especially so since it is known that not only are material properties often subject to dramatic changes as the grain size decreases down to the nanoscale, but the same effect applies to the biological response to them.39 In fact, the field of nanoscience arose to explain peculiar effects that nanosized particles exhibited in comparison with their bulk counterparts in the context of physics and chemistry, and assessing the same from different angles may lead to the birth of many new natural and social science fields of inquiry.
Figure 9.
Percentage of depolarized adenocarcinomic human alveolar basal epithelial cells (A549) after incubation with micro- and nano-particles of different chemical composition. Reprinted with permission from [28], H. L. Karlsson, et al., Size-dependent toxicity of metal oxide particles—A comparison between nano- and micrometer size. Toxicol. Lett. 188, 112 (2009). © 2009, Elsevier.
Central to the development of biomedicine are, similarly, not only physical pathologies, but the necessity of successful medical treatments to induce an inflammatory response at first. Namely, a medical body of knowledge in bone repair using exogenous implants could remind us that every successful implantation of a biomaterial is followed by a certain degree of inflammation. Avoiding the latter would imply a complete inertness of the body to the foreign material, which would signify all but a healthy and sensitive response. Mild inflammation attracts leukocytes and macrophages and stimulates blood vessels, thus promoting regeneration of the tissue. The more of the regenerative growth, the less of the fibroblast accumulation and the lesser the chance for the formation of scar at the site of the wound repair. Similarly, for gene transfection to be effectively performed, required is a carrier that exhibits moderate levels of cytotoxicity, able to reversibly rupture the cell membrane and invasively penetrate the cell prior to releasing a plasmid that will travel to the nucleus. Then, just as shoveling disturbs the soil but helps seeds be sown deeply into it, so must drug delivery devices maybe be mildly harmful in order to intrude into the organism and distribute a benevolent agent to it effectively. Oral drug delivery carriers are thus being designed so as to reversibly disrupt the tight junction between epithelial cells in the intestine—an effect otherwise associated with pathological inflammation—so as to enable the permeation of drugs otherwise large enough to be able to permeate the epithelium per se.40 Years after asbestos was banned from usage, research is done on similarly elongated silicon-based nanoparticles for the purpose of drug delivery across the epithelium, assuming that they can be optimized to produce just enough damage to the body to enable an effective delivery of therapeutic agents.39
Furthermore, it is known that bone is a continuously regenerated organ, but it is less acknowledged that in order for the cellular regeneration to be triggered, a fracture needs to occur first. Although the latter mostly appears in form of microscopic cracks, this still shows the importance of imperfection and fragileness for the sake of preserving regenerative potentials. Since the inflammation is an integral part of a healthy response of the organism to implanted foreign material and since most effective drug delivery approaches utilize vehicles that interfere with the integrity of the targeted tissue to a certain extent (i.e., they are harmful to just about the right extent), any problematic effect that nanoparticles are found to exert on the body could be turned into an advantageous one with a little bit of imagination. This could be directly inferred from the hereby exposed viewpoint that witnesses the emergence of nanoscience and its applicative findings from the puzzling peculiarities observed at the nanoscale. For example, some time after it had been found out that nanoparticles have a tendency to accumulate in the lymph nodes,41 this effect began to be utilized in preparation of nanoparticle formulations for targeting lymph node dendritic cells and delivering adjuvants, suspensions of antibodies used to boost immune response, thereto.42,43 Therefore, just as shelled mollusks form calcium carbonate pearls as a response to irritating particles of sand and just as binocular creatures turn the binocular disparity from the cause of disorienting double vision to the source of stereoscopic vision, so could we expect the future to bring many instances of transformation of the harmful and perplexing into the remedial and enlightening in the domain of nanoscience.
IT’S ALL IN A PARTICLE: DRUG DELIVERY APPLICATION AS AN EXAMPLE
As life science and materials science coalesce to an ever greater extent, biomedical therapeutic techniques are expected to be ever more affected by finely tuned and controllable drug delivery methods. In that sense, it is worth recalling that the properties of the particle used for drug delivery largely predetermine the pharmacokinetic efficacy and potency of the drug. Many hydrophobic drugs that are orally unavailable in their nascent forms but are easily deliverable when encapsulated within polymeric particles neatly illustrate that even though one may be in possession of a most pharmaceutically potent chemical, without the right form of delivery its potency would not be of much use. Another example of this effect comes from cholesterol.44 Namely, the popularly mistaken dichotomy of “good” and “bad’ cholesterol refers to an identical chemical structure of cholesterol molecules, although bound to different cholesterol-transferring lipoprotein complexes in the bloodstream: high-density ones in case of the “good” and low-density ones in case of the “bad.” Hence, the “particle” to which cholesterol is bound defines whether it will act as a beneficial or health-threatening agent.
The following subsections are named after the key properties of particles applied in drug delivery that should be optimized for a given application in the body.
Aggregation Propensity
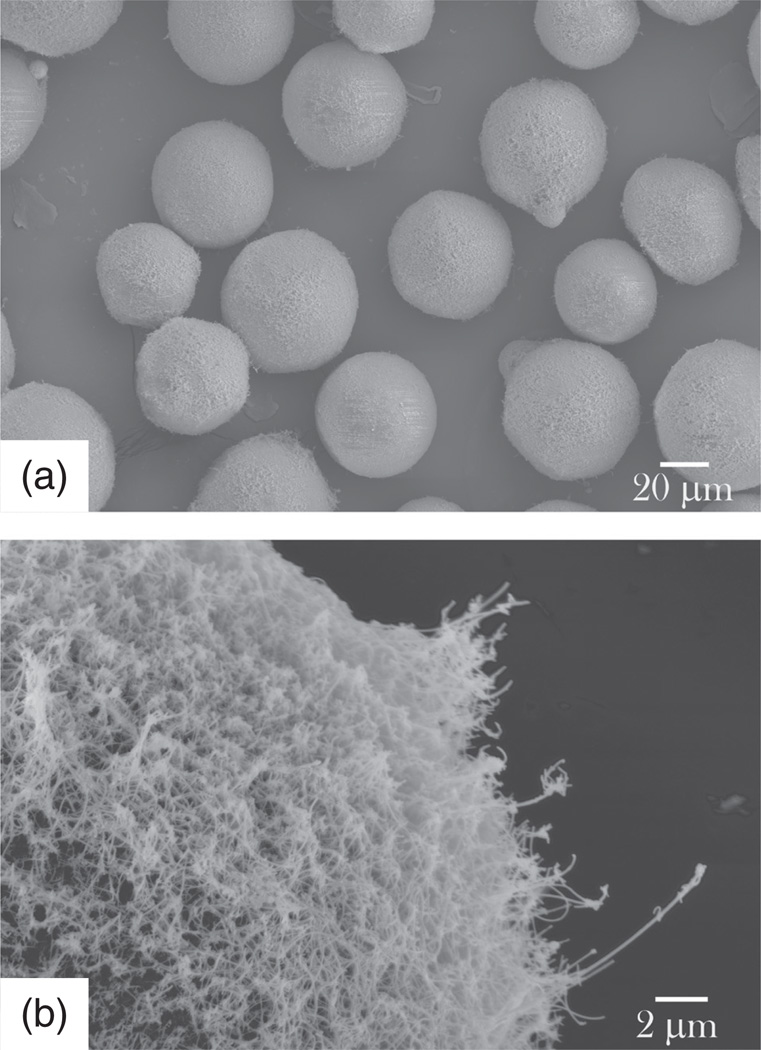
Ever since the definition of a nanomaterial as a solid physical structure whose grain size does not exceed 100 nm along at least one axis was proposed, discussions have been held among nanoscientists over whether agglomerated nanoparticles can be considered as nanosystems or not.45 Sometimes such agglomerated powders retain the properties of their nanoparticulate units. Sometimes, however, they lose the properties of the discrete units and begin to resemble a highly defective bulk crystal. Whether the former or the latter is the case greatly depends on the property in question; thus, for example, whereas only mild agglomeration of magnetic and luminescent nanoparticles, which would have comprised a superparamagnetic material if sufficiently separated, leads to appearance of unintended ferro- or ferri-magnetism,46 aggregation of these particulate subunits need not markedly modify their photoluminescence. And while nanoscientists were busy quarreling over the purely etymological issue of whether agglomerates of nanosized particles should be considered as nanosystems or not, eyes have become more and more focused on controllably and symmetrically aggregated assemblies of nanoparticles, nowadays known as colloidal crystals or superlattices.47,48 Just like the organization of atoms in crystals leads to delocalization of valence electrons between atomic orbitals of adjacent lattice sites, resulting in energy drops that exceed the thermal energy of the given atoms by two orders of magnitude on average, yielding effective properties untraceable to the constituent units alone, so does organization of nanocrystals within symmetrically ordered superlattices lead to electronic coupling between nanocrystals that counteracts the quantum confinement, yielding new and often unexpected properties to the overall system.49 Still, the effect of controlled aggregation has been utilized to a minor extent only, even though such new ways to control the aggregation properties of nanoparticles may lead to novel applications in the field of biomaterials and drug delivery. An example of how controlled aggregation can lead to sustained released of pharmaceutics comes from the recently utilized method for preparation of calcium phosphate particles loaded with antibiotics, proteins or small organic molecules.50 Efficient loading and sustained release of the drug were shown to be consequential to the drying-induced aggregation of calcium phosphate nanoparticles and their gradual breaking in the solution, respectively (Figs. 10, 11).
Figure 10.
Nanosized spherical particles of calcium phosphate (left) acting as subunits in microscopic blocks of material (right) formed by aggregation during desiccation of the powder.
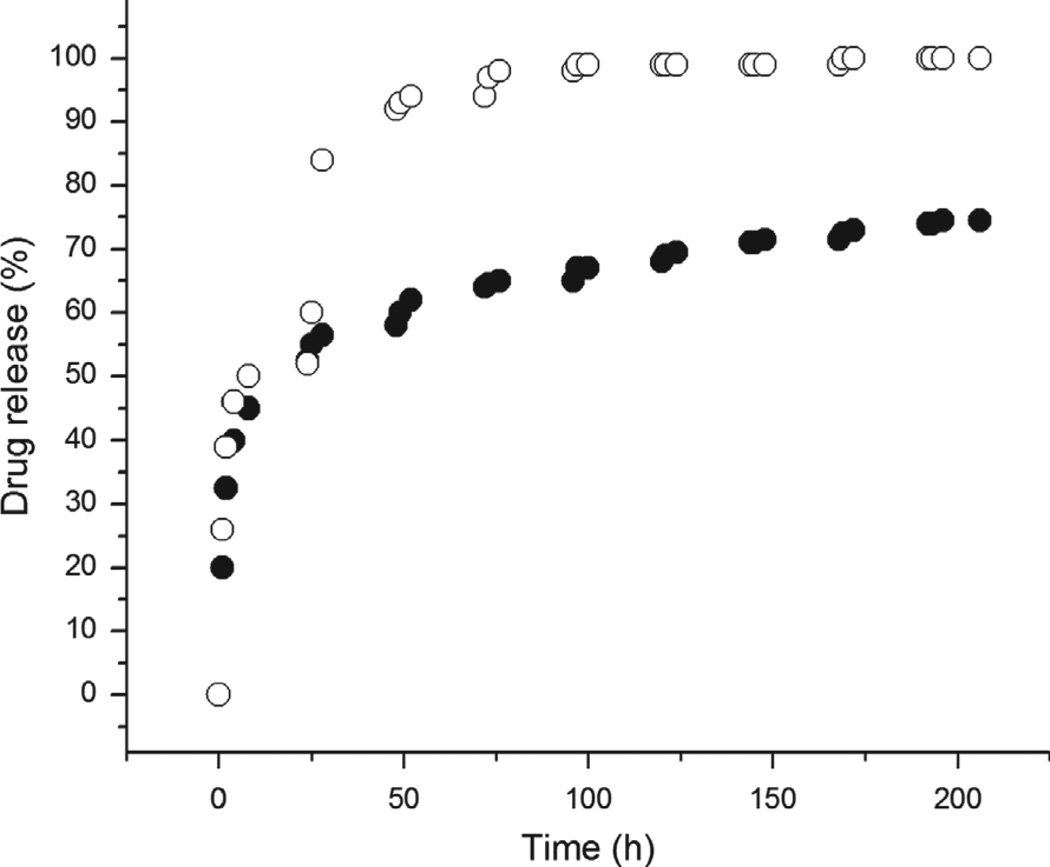
Figure 11.
Comparison of the release of a 376 Da organic molecule, fluorescein, from calcium phosphate nanoparticles (○) and their agglomerates (●) shown in Figure 10. While non-aggregated particles exhibit burst release of the entire amount of the drug in a short span of time, the agglomerated ones display a more sustained release thereof.
Nanoparticles are increasingly utilized for drug delivery purposes because of multiple benefits they offer. Firstly, they tend to increase the stability of drugs. A paradigmatic example is provided by paclitaxel nanoparticles stabilized with Pluronic F68, stable for years, in contrast with the same drug in dissolved form, undergoing complete degradation in less than 48 h.51 Secondly, nanoparticles as drug carriers allow for biodistribution of insoluble drugs and increase of their pharmacokinetic half-life. Thirdly, the degradation of nanoparticles in the body can be fine-tuned until prolonged release of the drug is reached; the need for repetitive dosages is thus overcome, enabling more sustained and leveled drug concentrations in the target area. Aside from the improved stability and sustained therapeutic release, drug encapsulation within nanoparticles can also enhance bioavailability of drugs administered via routes other than intravenous (IV). Both insoluble and soluble drugs can thus be incorporated within nanoparticulate sols and made transferable through blood in stable form for longer periods of time. Some corrosion-resistant metals form a protective oxide layer on their surface, and sensitive drug molecules encapsulated within particles often undergo a similar effect. Even if particles are made of drug only, the molecules on the surface may undergo degradation when exposed to air or solvent, protecting those in the interior of the particle. Additionally, drug delivery using fine particles offers the benefits of tissue-specific targeting via particle functionalization with appropriate moieties.52 This all explains why nanoparticulate colloids rather than solutions of drugs are often chosen as a more appropriate delivery form in pharmaceutics.53
Still, there are no perfectly stable colloidal suspensions.54 When the attribute of stability is ascribed to a sol, it merely means that it takes comparatively long periods of time for the system to destabilize itself. In other words, aggregation of particulate units of one such system is sufficiently slow. Hence, although novices in the realm of chemistry often presume the precipitated powders to preserve their form once the crystal growth is over, that is quite an incorrect assumption. For, the “dance” of their molecules and incessantly ongoing phase transformations along the interface occur long after the preparation is over, all the way through their storage or aging. The same thermodynamic arguments applicable to colloidal systems can be used with respect to nanoparticles. The large surface area predisposes them to be unstable; when found in a medium in which a transfer of matter facilely occurs, they tend to undergo Ostwald ripening and minimize the surface area in contact with the environment by dissolution of smaller particles and growth of the bigger ones. If we were to translate the language of thermodynamics to the one of chemistry, we would convert the interfacial free energy to supersaturation and arrive at the original Ostwald’s observation: small particles have higher saturation limit than the bigger ones, which creates a concentration gradient between small and large particles whereby the growth units diffuse from the higher concentration in the vicinity of small particles to the areas with a lower concentration around bigger particles.55 Local supersaturation in the solution surrounding larger particles leads to precipitation of these units on their surface, inducing their growth. In turn, undersaturated “islands” in the solution simultaneously appear around the surface of the smaller particles, leading to their further dissolution.
Now, whenever particles are preserved as dry powders, the air surrounding them will not present a medium that effectively mediates this transfer of matter. In general, the more inert the medium, the less good of a solvent it is for molecules comprising the particles, and the greater the barrier for Ostwald ripening will be. However, many applications demand the particles to be applied in a suspended form, and IV administration of pharmaceutical nanopowders is an example.56 In those cases, aggregation presents a most critical issue that leads to undesired modification of the particle properties over time.
The size of nanoparticles furthermore often determines the mechanism of their transport across the cell membrane and the metabolic fate.57 By knowing that the cellular uptake and overall response oftentimes depend on the particle size and largely differ for aggregates and singlets of the same particulate composition,58 controlling particle aggregation clearly appears as a vital issue. An example is given by 10-nm sized gold nanoparticles designed to aggregate in mildly acidic intracellular environments through their hydrolysis-susceptible citraconicamide-functionalized surface.59 After they enter the cell as individual nanoparticles, they begin to aggregate in endosomes,60 contributing to the destruction of the targeted cell. Conversely, it was found out that certain nanoparticles can bind to the cell membrane only insofar as they are aggregated, but can play their intracellular delivery role only as individual entities.61 Unlike bigger, circa 100-nm sized D-penicillamine-coated CdSe/ZnS core–shell nanoparticles that were readily internalized by HeLa cells, smaller, 8-nm sized particles thus merely anchored to the cell membrane and only at higher dosages, when their clustering became significant and when they were able to trigger endocytosis by reacting with a sufficient number of receptors, they began to penetrate the cells.62 Local sedimentation of nanoparticles on the cell membrane is then required to enable cell entry and perfectly stable colloidal dispersions could thus often turn out to be not so perfect owing to their promotion of excellent electrostatic repulsion of the individual particles. In those cases, aggregation state is the favorable one in the extracellular environment, while the effectiveness of their application may still critically depend on their ability to re-disperse in the intracellular milieu. Namely, if large enough, particle aggregates are bound to be trapped in the endocytic vesicles of cells following their internalization, while the endosomal escape of theirs or of the active transfection agents that they may carry could be limited to nanosized singlets only.63 A similar case is observed for magnetic nanoparticles for hyperthermia therapies and other magnetism-based drug delivery applications (Fig. 12). Namely, these particles can be attracted by an external magnetic field only insofar as they form aggregates with sufficiently large magnetic moments, while their smart hyperthermic performance in the target area depends on their superparamagnetism, which is conditioned by the sufficient spacing between individual nanoparticles.64 Temperature was shown to be an effective parameter for achieving controllable aggregation/re-dispersion of iron-oxide-based polymeric micelle-like particles.65 For the purpose of guiding the particles along relatively wide channels in the body by means of an external field, they could be preserved in an aggregated form, and then re-dispersed before their entering the cell or other comparatively small focus area in the body.
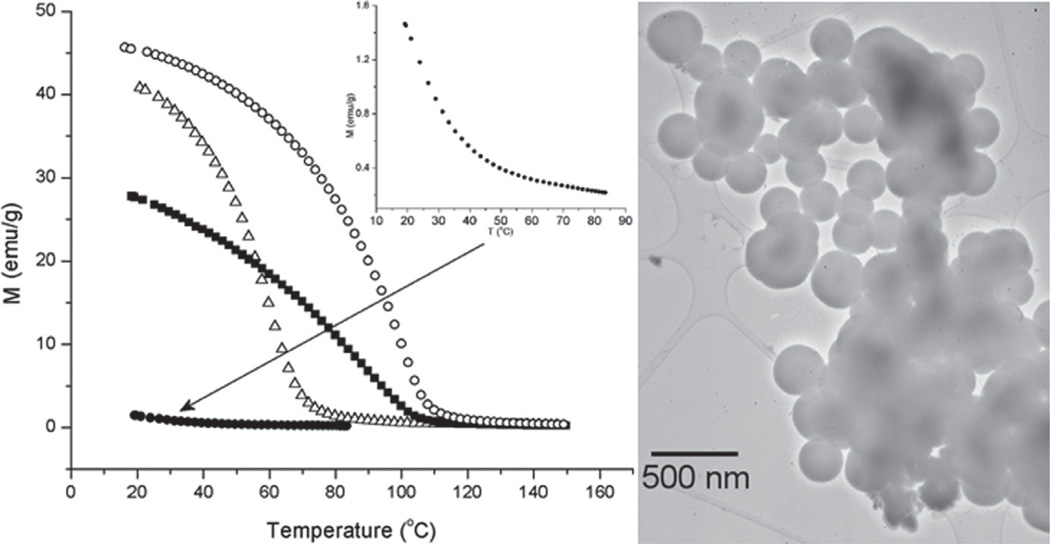
Figure 12.
An example of biocompatible magnetic nanoparticles with biomedical application: hyperthermia cancer therapy. By controlling the stochiometry of the given manganite compound (La1-xSrxMnO3+δ(0.16 < x < 0.5)), its Neel point, equivalent to the maximal temperature achievable due to relaxation energy losses in alternate magnetic field, could be varied and optimized for the given therapy (mild hyperthermic or more intensive thermoblastic, e.g.).
Still, agglomeration in formulations of intravenously administrable nanosized powders in pharmaceutics is considered undesirable from the safety viewpoint and principles of Stokes’ law. As in accordance with the latter, (a) reductions in the particle size, (b) increases in the medium viscosity and (c) bringing the density of suspended particles and the dispersion medium closer to each other have been traditionally used to ensure greater stability of many commercial suspensions, including the pharmaceutical ones.66 Namely, the formation of particles larger than 5 microns from nanosols is feared to be prone to result in capillary obstruction and embolism.67 Typically, when it comes to nanosized particles, the addition of (a) ionic compounds so as to affect the thickness and density of the double charge layer around the suspended particles, and (b) surfactants so as to promote steric repulsion of the particles, are basic strategies used to prevent particle agglomeration.68
Whether stabilization of sols using the former, electrostatic or the latter, steric effect presents a more optimal approach greatly depends on the nature of the solution medium. Thus, charge stabilization is of little effect in media with low dielectric constant, which includes most organic solvents, in which cases steric stabilization by the addition of surfactants or polymers is required to maintain the dispersed state and prevent flocculation. Furthermore, the amount of surfactant has to be optimized in order to provide the right conditions for stably dispersing the particles by its means. Namely, too little of it may leave enough space on the particle surface for particles to approach each other and clump, while too much of it may exceed the critical micelle concentration, separate the surfactant molecules from the particle surface or simply reduce their solvation, inducing their collapse and allowing flocculation to occur, something that the addition of surfactant was meant to prevent in the first place. The need to optimize the concentration of surfactant to prevent nanoparticles from irreversibly agglomerating is illustrated in Figure 13. Shown in it are polylactide-coglycolide spheres encapsulating a vinyl sulfone cysteine proteinase inhibitor, thoroughly meshed without polyvinyl alcohol (PVA) as a dispersant (Fig. 13(a)) and at its concentration of 1 wt% (Fig. 13(c)), and narrowly dispersed at [PVA] = 0.5 wt% (Fig. 13(b)). The outcome of application of surfactants as dispersing agents also often turns out to be uncontrollable tangling of surfactant molecules when expulsion of the solvent molecules from the area where the surfactant chains have begun to interlace during particle– particle collisions is thermodynamically favored. There is no way yet to predict the free energy of surfactant-medium interaction that is responsible for this effect and the process of finding the optimal surfactant/medium pairs for a given particle composition and morphology can be burdensome due to its being based on empirical trial-and-error approach.69 Since aggregation of nanopowders is difficult to control in a finite time span, more attempts may be expected to be made in the future with regard to controllable aggregation and re-dispersion of particles.
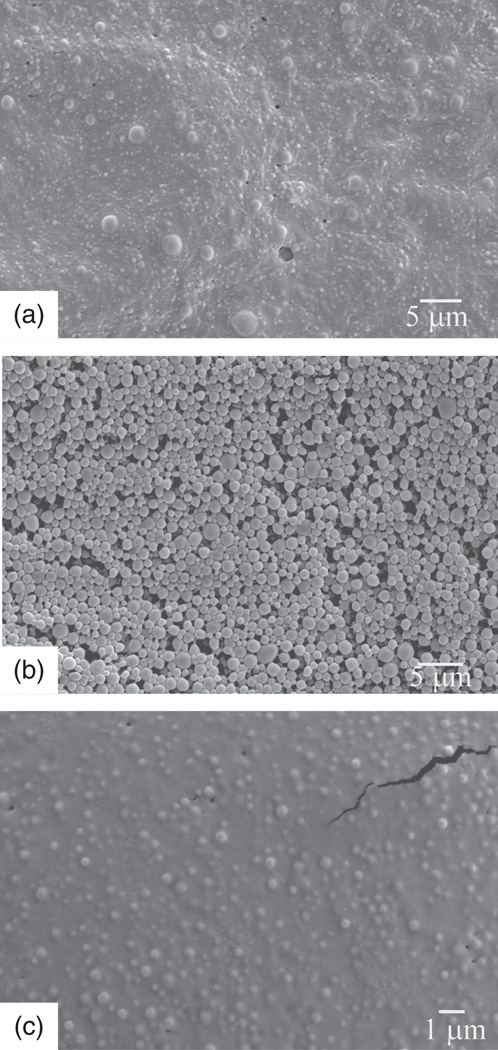
Figure 13.
SEM images of polylactide-co-glycolide spheres encapsulating a vinyl sulfone cysteine proteinase inhibitor, aggregated and partially coalesced without PVA as a dispersant (a) and at [PVA] = 1 wt% (c), and narrowly dispersed at [PVA] = 0.5 wt% (b).
Particle Size
Differently sized particles have routinely been shown to produce different effects when applied as drug delivery agents.70–72 For example, if intravenously deliverable magnetic particles guidable by means of an external magnetic field are too small, their magnetic moments might be negligible and they would be uncontrollably carried away by the bloodstream. If they are too big, on the other hand, they might not be capable of maneuvering effectively across the blood vessels and the intercellular space. For this reason, it is often stated that different targets in the body require differently sized particles for the most optimal penetration and drug delivery. In support of this, invoked could be the fact that different cell types find differently sized topographic clues of otherwise chemically identical compounds most optimal for their attachment and growth. For example, while mesenchymal stem cells thrive best on arrays of TiO2 nanotubes with diameters of less than 30 nm,73 the metabolic activity of osteoblasts is highest when the diameter of said nanotubes is larger than 30 nm.74 Then, MC3T3-E1 fibroblasts found polymeric substrates with high contents of alginate most optimal for their spreading and proliferation, whereas L929 fibroblasts grew best on those with low alginate contents.75 On a side note, cytotoxicity of certain materials has been shown to be greatly dependent on the cell type. For example, silver nanoparticles were more toxic to macrophages than to lung epithelial cells,76 while cobalt-doped hydroxyapatite was toxic to osteoblastic cells and nontoxic to cells of the intestinal epithelium.77 Moreover, biodistribution profiles of certain types of particles are known to sometimes undergo a sharp change in the range of 1–5 nm, while any bigger particles, be they of nano- or micro-sized nature, show highly similar accumulation in vital organs.78
The size distribution is also a crucial parameter and a special emphasis is placed on monodisperse particles (PDI < 0.1). One reason is that uniform sizes tend to result in uniform properties. The issues of inconsistent dosing consequently often result due to wide distribution of particle sizes (PDI > 0.5), an effect that also favors Ostwald ripening and destabilization of dispersions. Because Ostwald ripening is caused by the difference in solubility limit for small and large particles, the propensity of the system for it is inversely proportional to the narrowness of the particle size distribution. Hence, a rule of the thumb says that the more uniform the particles, the greater their stability with respect to Ostwald ripening. For the same reason, monodisperse nanopowders are preferred over polydisperse ones as precursors for the sintering of nonporous materials.79 To prevent the grain growth and retain the nanoparticulate nature of the material, two-step annealing is typically performed in those cases:80,81 the short one at higher temperatures, at which pores become thermodynamically unstable against shrinkage, so as to block the grain boundary migration, and then the prolonged one at lower temperatures to reduce the pores without grain growth.82
On the other hand, living organisms are far-fromequilibrium systems, which may suggest that tailoring the structure, properties and functionality of materials that are meant to be interfaced with them should follow the route of greater reliance on similarly metastable nanoscale architectures. In that sense, examples of successful usage of various multimodal particle or pore size distributions could be mentioned. One of them pertains to hydroxyapatite cylinders shown to possess higher drug release rates and bone ingrowth potential when pore size distribution in them was bimodal rather than monodisperse.83 Also, polydisperse, not monodisperse particles were those that, following a long series of attempts, successfully self-assembled into virus-like superlattices.84 Likewise, randomly patterned PMMA substrates, without any osteogenic supplements, were shown to be effective in inducing differentiation of mesenchymal stem cells to osteoblasts, unlike the chemically identical substrates with a perfect translational symmetry,85 suggesting that too much order in the cellular environment can be detrimental for the cell growth. In fact, when it comes to fabrication of scaffolds for bone tissue engineering, combination of (a) larger pores that are to home osteoblasts and (b) smaller pores through which nutrients and metabolites would transverse presents a better choice compared to scaffolds composed of uniformly distributed pores of equal dimensions.86 After all, since biological systems are all about an entwinement of symmetry and asymmetry, perfection and imperfection, replicating such blends rather than focusing solely on perfect symmetries achievable presents a task whose accomplishment will bring great benefits to future materials scientists and engineers. To understand that diversification rather than fosterage of monotonous sameness is the path to be followed is a vital challenge to both experimentalists who unquestionably overvalue structural symmetries and science authorities that erroneously deem the uniformity of opinions and methodologies to be a merit and not a sign of a dead end in the progression of our creativity.
Morphology
In general, by controlling the shape of the particles, their contact surface could be tailored for a given application. Hence, for particles meant to have a high retention time in the bloodstream, spherical morphologies, having the lowest contact surface, would be the natural choice. In contrast, elongated, planar particles would present a logical choice for drug delivery carriers intended to adhere onto biological surfaces. Irregularly shaped, quasi-planar particles were thus shown to act as more effective drug carriers for delivery across the epithelium, owing to their larger surface area in contact with the epithelial layer of cells and, consequently, more efficient adherence thereto.87 Implicitly, these results challenge the widespread contemporary acceptance of the direct correlativity between the level of morphological symmetry of nano- and microparticles and their scientific appeal. This instance of more effective performance of asymmetric particles than their narrowly dispersed, spherical counterparts presents an excellent occasion for the correctness of the common beliefs that sphericity and symmetry directly translate to utility in the design of micro- and nanoparticles for biomedical or any other applications to be brought into question.
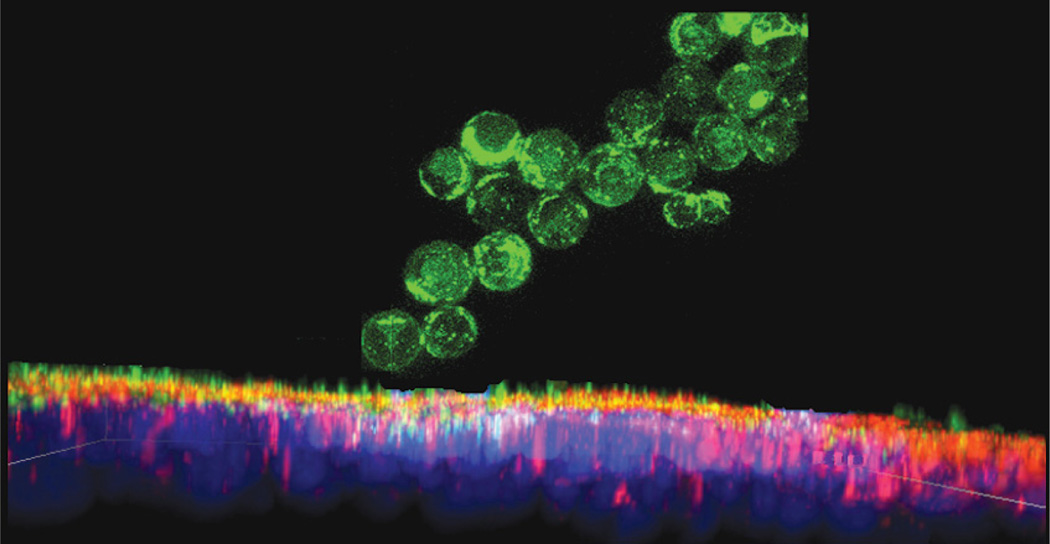
Highly irregular, plate-shaped and cylindroid particles were also shown to adhere to tumor vasculature and be sequestered by the liver, spleen and lung to a much greater extent than spherical particles did.88 On the other hand, although a spherical particle has to be smaller than 200 nm in diameter to bypass the asymmetric filtering units in spleen, so-called slits, disk-shaped red blood cells with diameters of about 10 microns easily pass through it,89 indicating that not only size of the particle, but its shape as well as stiffness are equally important properties thereof. Shape and texture of the particles can thus be optimized to promote adhesion by means of simple steric effects, and an additional example comes from the synthesis of silica beads with silicon nanowires outgrown from their surface (Fig. 14). These composite particles effectively adhered to microvilli of the epithelial cells of the gastrointestinal tract,90,91 prolonging the drug retention time and promoting the paracellular transport of small and medium sized drug molecules across the epithelium (Fig. 15).39
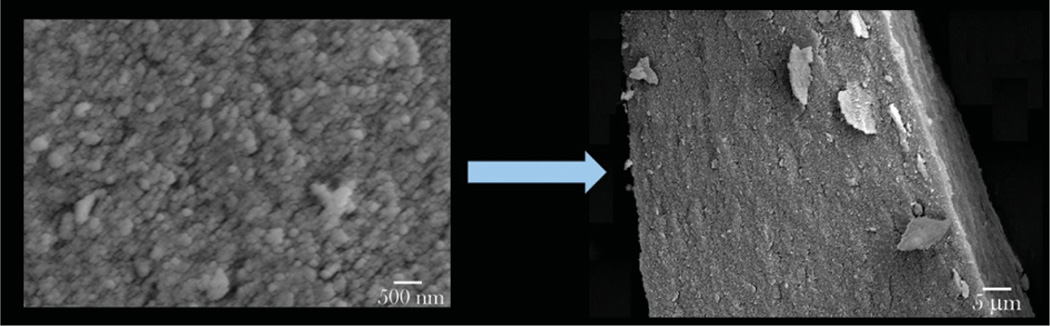
Figure 14.
SEM images of silicon-nanowire-coated silicon microbeads applicable as oral drug delivery carriers due to their ability to (a) adhere onto epithelial surface of the intestine by entwining with microvilli on the cell surface and (b) open the tight junction spacing in-between individual cells and enhance paracellular transport of the drug the particles are loaded with via the capillary effect.
Figure 15.
FITC-tagged silicon-nanowire-coated silica beads adhering onto the epithelial monolayer of Caco-2 cells; yellow color appears only where red-stained ZO-1 molecules of the tight junction and green-stained drug overlap, indicating the paracellular transport of the drug from the apical to the basolateral side of the epithelium. The cell nucleus is stained in blue.
As of recently, gold nanorods have also been favored over gold nanospheres, owing to the fact that the former exhibit a larger plasmon shift per refractive index unit and thus a greater sensitivity to changes in local environments.92 At the same time, the degree of cellular internalization of gold nanoparticles was shown to be directly proportional to their aspect ratio.93 Elongated particles also favor surface appearance of particular crystal faces, the slowest growing ones, which can have a tremendous effect on the interfacial energy and binding chemistry, as can be exemplified by the morphological modifications of Pt particles so that either hydrophobic (533) faces or wetted (553) ones, ostensibly similar in geometry, are exposed on the surface.94 Surface patterning has thus been applied to promote desired hydrophilicity and optimal peptide and cell adherence, demonstrating how morphology of a material can decisively affect its chemical interactivity.95 On the other hand, planar particles have a greater tendency to aggregate on blood vessel walls and block vital passageways, as exemplified by the typically plate- or needle-shaped cholesterol and apatite crystals that comprise atherosclerotic deposits.96 To avoid such asbestos-like apoptotic effects related to high aspect ratio morphologies,97,98 it is vital to carry out toxicity studies prior to biological application of such materials or show their solubility in relatively short periods of time, as is the case with silicon nanowires shown in Figure 14 or maghemite nanowires usable for cell manipulation and microrheology.99
Crystallinity
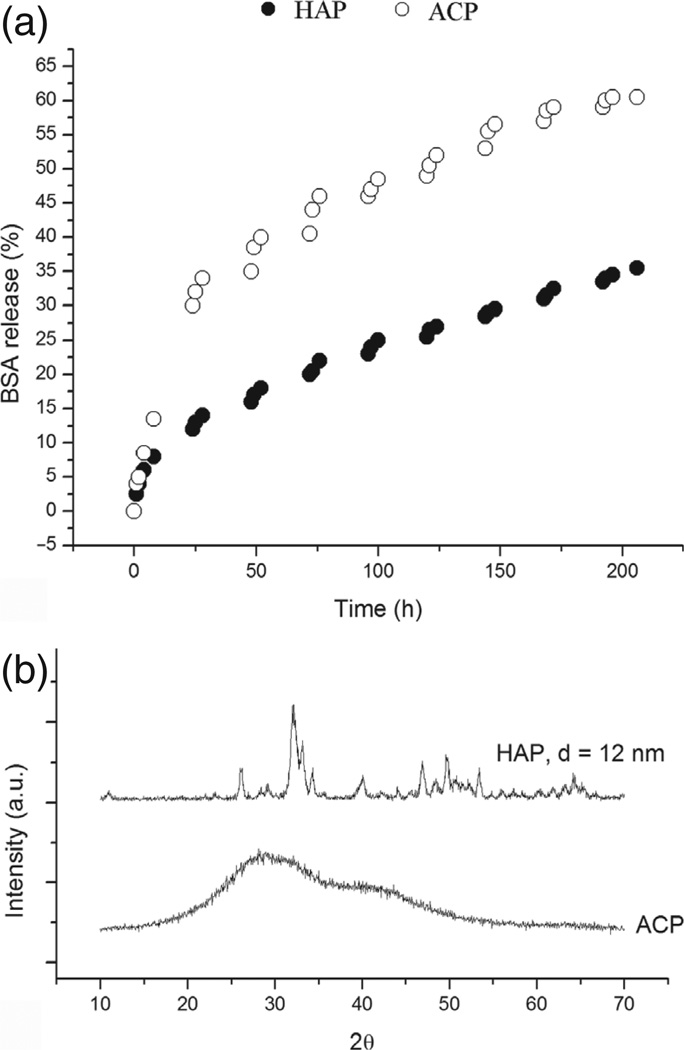
Kinetics of the formation of nanopowders, be it via precipitation or by top-down deposition and assembly, affects their crystallinity. In cases when the drug release is contingent upon the dissolution of the particles encapsulating it, their crystallinity can dramatically affect the drug elution profiles. One example could be found in calcium phosphate particles whose stoichiometry, that is, phase composition was used to tune their dissolution behavior and, hence, the release kinetics of small organic molecules, antibiotics and large polypeptides, e.g., bovine serum albumin, that the particles were loaded with by means of physisorption.100 As shown in Figure 16, the drug elution rate, directly proportionate to the solubility rate of the compound, is markedly higher for amorphous calcium phosphate than for its nanocrystalline phase, hydroxyapatite, with the crystallite size of 12 nm.
Figure 16.
(a) The release of bovine serum albumin (BSA) as a function of time for amorphous (ACP) and nanocrystalline calcium phosphate (HAP); (b) XRD patterns confirming the amorphous and nanocrystalline nature of the two powders.
In general, the dissolution of amorphous or less crystalline polymorphs is entropically favored over more crystalline phases with an identical stoichiometry. In this case, again, the distribution of crystallinity throughout the sample greatly matters. Namely, a minor amount of a crystalline phase in an amorphous sample is sometimes enough to induce the transition of the amorphous phase to a more stable, crystalline one. Just like Ostwald ripening is enhanced at wide size distributions, so do broad distributions in crystallinity favor the transition of an amorphous material to crystalline state. Owing to greater structural similarity of amorphous particles to the solution from which they were precipitated, the free energy of their formation is lower compared to that of well structured, crystalline particles.101 With the free energy relatable to supersaturation ratio, this translates to greater solubility limit for amorphous particles, as opposed to their crystalline counterparts, resulting in a similar gradient of concentration as that consequential to Ostwald ripening, and leading to dissolution of the amorphous phase and recrystallization of its atomic ingredients on the surface of the crystalline phase. Introducing a crystal into a sol composed of solely amorphous particles can thus sometimes induce a rapid transition of the particles into a more crystalline state. Surfactants, viscosity enhancers, ionic strength modifiers, the choice of dispersion media, sol density and temperature can all be used to somewhat control the crystallinity of the powders and its evolution in the course of aging following the synthesis step.
Drug Release Profiles
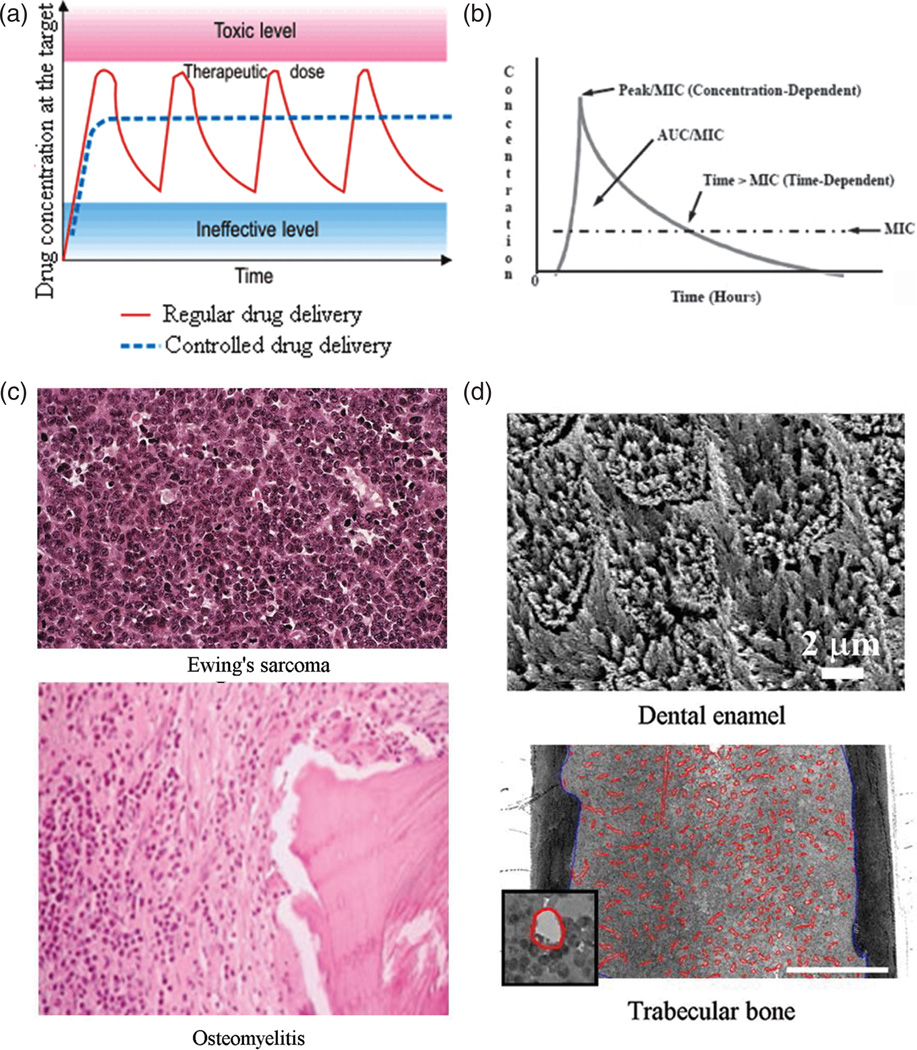
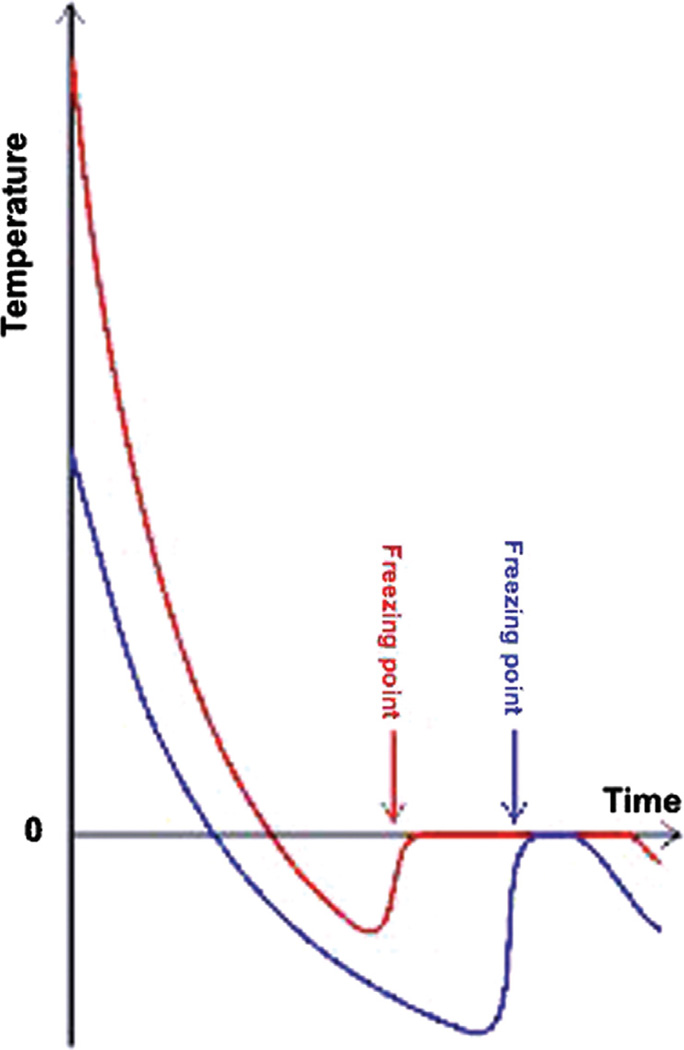
Achieving constant rates of delivery by means of slowly degrading and drug-releasing carriers implanted in the body is often presented as an ideal alternative to the traditional drug therapies based on repetitive administration and consequently inconsistent ups-and-downs in concentration of the active ingredient in the target area (Fig. 17(a)); however, more complex and specially designed concentration gradients of the released drug are expected to result from the future research in this field. Although burst releases have been traditionally considered as undesirable in the drug delivery field, sometimes there are clear advantages of theirs, as in the cases when drugs are antibiotics administered so as to eradicate a microbial source of infection. Namely, as shown in Figure 17(b), the ability of the carrier to release the drug in the amount that exceeds the minimal inhibitory concentration (MIC) for the given combination of the antibiotic and the pathological agent is crucial in prevention of the rise of resistance of the bacterial population to the administered antibiotic.
Figure 17.
(a) A curve showing the typical drug concentration at the target site as a function of time during repetitive drug administration in high dosages (red line) in comparison with the ideal therapeutic dose achievable through sustained release from drug-loaded carriers (blue intercepted line); (b) The drug concentration peak and period during which it stays above the MIC, two parameters important to optimize depending on the nature of the antibiotic drug: concentration- or time-dependent; (c) Histological slides showing greater pervasiveness of abnormal tissue in Ewing’s sarcoma, a form of bone cancer, compared to osteomyelitis where infection is more localized, advances as a front and is demarcated by the white line composed of leukocytes; (d) SEM image of dental enamel, an example of a nonvascular hard tissue, 98 wt% of which is composed of calcium phosphate, and an optical micrograph of trabecular bone, the most vascular hard tissue, with blood vessels outlined in red (the scale bar represents 0.5 mm). Reprinted with permission from [121], R. Quintilliani, Pharmacokinetics/pharmacodynamics for critical care clinicians. Crit. Care Clin. 24, 335 (2008). © 2008, Elsevier; From [122], S. L. Ellis, et al., The relationship between bone, hemopoietic stem cells, and vasculature, Blood 118, 1516 (2011). © 2011, American Society of Hematology.
Multiple-stage release profiles can be considered to hold potentially far greater benefits compared to monotonous drug elution kinetics. For example, the rapid initial release of paclitaxel from polymeric microparticles was effective in halting proliferation of ovarian cancer cells, while its more sustained, second phase of release allowed for gradual eradication of the tumor.102 The drug release profile considered desirable in targeting diseases that involve pain or hormonal disorders is a pulsating one, whereby periods of instantaneous release are followed by periods of minimal or no release, an effect that could be potentially achieved either by (a) making the carrier sensitive to environmental stimuli, be it pH,103 the presence of a disease marker,104 temperature,105,106 ultrasound107 or external electromagnetic fields,108,109 or by (b) the design of multishell particles, such as the triple-shell calcium-phosphate-DNA nanoparticles, for example.110
In that sense, there can be no perfect drug release carrier, but only a perfect carrier for a given target area and pathology in the body. Not a single quality could be assessed nor defined without reference to both the system and its overall environment;111 consequently, no two drug delivery treatments should be the same. The ideal release properties depend on: (a) the drug that is to be delivered; (b) the nature of the illness treated for; and (c) the targeted area in the body. For example, some antibiotics, such as beta-lactam antimicrobials, are time-dependent, while others, including quinolones and amino-glycosides, are concentration-dependent (Fig. 17(b)).112 As for the nature of the pathology treated, suppression of cancer requires high therapeutic specificity, unlike bacterial or fungal infections. Cancer cells are notorious for developing resistance to drugs; therefore, prompter and more potent treatments are required when conceiving cancer chemotherapies. The target is then also ideally not only the cancer cell per se but the surrounding niche too, which may be healthy but is still producing growth factors to support the cancer cells.113 Unlike cancer, which is more pervasive and harder to eliminate, osteomyelitis tends to be more localized and spread with a well outlined front, with white blood cells clearly bordering the area of infection (Fig. 17(c)).
Another example comes from the fact that the expression of osteogenic markers suggestive of bone formation greatly depends not only on the biomaterial used to augment the osteogenic response, but on the cell type and their immediate environment; thus, the same calcium phosphate materials were shown to induce only osteoconductive behavior of adherent MC3T3-E1114 fibroblasts, the pre-myogenic C2C12 cell line115 and SaOS-2 cells,116 and markedly increased levels of osteogenic differentiation of human mesenchymal stem cells.117 Materials with strong anticancer properties may thus be expected to have a more suppressive or less metabolically augmentative effect on immortal cell lines than on stem-cell-derived or primary cells of the same type. The concept of intrinsic osteoinduc-tivity of specific calcium phosphate ceramics, meant to be definable with no reference to cellular types and milieus, can be therefore seen as fundamentally deficient.118,119 Moreover, infections in the hardly approachable interior of bone and on the surface of teeth are quite different and, logically, call for different types of drug delivery. Finally, vascularization and other features of the target tissue largely predetermine the optimal drug release profiles, often dependent on the clearance period for the given particles. In that sense, it does make a difference whether particles are being injected to cancellous (i.e., trabecular/spongy) bone, the most vascular hard tissue, or to less vascular cortical bone or even one of hard tissues in teeth, such as enamel that is deprived of any vascularization at all120 (Fig. 17(d)). The delivery carrier and the target area in the body are thus entwined within a feedback loop so that the former defines the scope of its potential application in the latter, while the latter calls for specific particle properties to be effectively treated.
Biocompatibility
As we see, the quality of a material applied to restore damaged tissues could not be defined without referring both to the area of its application in the body and the finest characteristics of the material. Differently sized particles would be required for drug-delivery treatments of different tissues,123 whereby different mechanical and chemical properties of the biomaterial are employed to tackle pathologies occurring at different scales. Finally, an essential feature of a biomaterial, defined as “a material intended to interface with biological systems to evaluate, treat, augment or replace any tissue, organ or function in the body,” 124 is its biocompatibility, which could not be measured without an interaction with the patient. Even though it is known that pathological states of an organism need to be explained in terms of both the effect of foreign factors and the susceptibility of the host organism, it is still puzzling why a specific material becomes refused by one and accepted by other patients, serving as a reminder of the subjective nature of every medical treatment. Needless to say, the effective application of each biomaterial critically depends on a favorable feedback interaction between the living system and the material, during which both are most often subject to change. A perfect hard tissue substitute is, thus, meant to be thoroughly absorbed by the body in the course of the regeneration of the treated tissue, which, though, still stands as an unattained ideal.
Biodegradability
Certain drug delivery agents possess artificial reservoirs from which the drug is released to the physiological environment125 or have the drug entrapped in surface pores;126 in those cases, degradability is in no way related to the drug release kinetics and the implanted material often has to be surgically removed at the end of its therapeutic lifetime. On the other side of the spectrum are particles that release encapsulated drugs only inasmuch as their structure degrades.127–130 It goes without saying that degradation properties of the material in question should be carefully analyzed so as to avoid their potentially toxic effect on the body. Some molecules, such as CTAB, are, for example, biologically inert when they are surface-bound; detached from the particle, they become highly cytotoxic.131 As science of nutrition can teach us132 and propositions of holism confirm,133 it is the synergy of different chemical components that is responsible for the effect each one of them individually will have on the organism.
In the same light, we could conclude that all the variables in a physical system are tightly interconnected, and each one of the abovementioned parameters can be shown to depend on all the other ones. Moreover, most of these effects stand in an antagonistic relationship with each other; that is, modifying the synthesis conditions in the direction of promoting one set of desired properties most often has an unfavorable effect on other product properties. For example, formation of complex particle morphologies, e.g., platelets, star-shaped or uniaxially grown crystals, is conditioned by (a) a comparatively slow attainment of the metastable state from which the phase transition would occur, and (b) a slow corresponding crystal growth. In turn, this tardiness tends to have a detrimental effect on the particle size, provided one seeks to stay in the nanosized range. Namely, low nucleation rate that results under these conditions directly translates to low density of crystallization centers and, consequently, bigger particles. Conversely, the immediate downside of the mainstream methods for fabrication of ultrafine particles is the low level of control of particle morphology. By controlling the latter, however, many of the mentioned properties of biomedical relevance could be tuned. Drug release profiles thus often depend on the texture of the material; e.g., when drug molecules are stored in pores whose size is bigger than the molecular size, the drug is expected to be released by diffusion, whereas when the pore size is comparable to that of the drug molecules, zero order rate of release, constant over time, may be observed.134 Materials with rougher surfaces may also attract anchoring cells to them quicker than smooth materials can, which speeds up their degradation in the body and, hence, the drug release. Small molecules too tend to be best adsorbed on rough surfaces, as could be demonstrated by the absence of adsorption of carbon monoxide on atomically smooth gold.135 Titanium implants with naturally smooth surfaces have thus been shown as ineffective in binding cells; to prevent their rejection by the body, they are either coated with a more bio-compatible layer or subjected to sandblasting and etching procedures prior to their application in vivo.136 The necessity for a biomaterial to possess a rough surface, for only as such can the conditions for an optimal cell attachment be achieved, brings us over to the general question that pertains to intrinsic imperfections that all biomaterials are to ideally exhibit. Note that the same argument applies to adsorption of small molecules; namely, as it could be expected from the fact that most adsorption mechanisms are driven by the Gibbs isotherm, which dictates that the greater the surface energy, the greater the adsorption, hydrogen adsorption capacity becomes markedly enhanced as one shifts from using perfectly ordered carbon crystals on the atomic scale to those containing topological defects.137
One of the crucial questions that the materials science applications in biomedicine will need to answer is how to define the optimal imperfections that each material applicable in biological systems, each one of which could be defined as a system composed of “unreliable components that achieve reliable outcomes,” 138 is to possess. Namely, in medicine of restorative hard tissues, for many years it has been considered that the stronger the material used as an in vivo substitute for the damaged tissue, the better. However, applying metals as substitutes for the damaged bone turned out to be unfavorable exactly because of their superior mechanical properties. Once implanted in the body, metals would absorb most of the mechanical stimuli that the surrounding tissue is subjected to and just as one’s living in a perfectly sterile environment slowly puts one’s immune system to sleep and makes one less resilient to intruding species, this stress-shielding effect caused the surrounding bone to become weaker and eventually completely resorbed, leading to the collapse of the local biomechanical structure.139 Similarly, if a biomaterial with identical elastic properties as natural bone is applied, the implanted cells would not do any work to “instill life” therein; eventually the probability for its rejection by the body would be high. Therefore, a biomaterial has to remain somewhat imperfect in order for the host cells to create a milieu capable of cell proliferation, tissue re-growth and full integration of the bioresorbable material. When the aforementioned titanium implants are subjected to etching to endow them with surface roughness, the processing makes them seemingly less perfect at the first sight, although it provides a more optimal integration with the organism in the long run. Likewise, since it is known that most cells prefer stiff surfaces to grow on,140,141 one may be allured to seed them on plastics, stiffer than glass; under those conditions, however, they would often tend to adhere intensely indeed, but only owing to pathological disruption of the spindle morphology, which interferes with the cell replication cycles and metabolic pathways and suppresses the natural cell response to stimuli. How to be imperfectly perfect is one of the vital questions that the mindsets and methods of materials scientists need to embrace in their search for ever greater bridging of the gaps that separate them from the life science coast of knowledge.
INCREASING EXPERIMENTATION SENSITIVITY
As we continue to chart the nano realm, the windows within which the optimal conditions for fabrication of nanostructures with desired properties are found will become narrower and narrower, resulting in ever greater bodies of irreproducible results and, therefore, a greater level of frustration among the experimentalists, including, quite possibly, an exacerbation of the trend toward culling and filtering of experimental data that already assumes epidemic proportions.142 My earlier works contained more in-breadth discourses on the limits in precision with which we will be able to control physical processes as we keep on proceeding along the line of the constant increase in the fineness of scientific manipulation of material structures.143 Imperfectly repeatable synthesis protocols, producing large variations of the resulting material properties, will be particularly critical for products which cannot be tested and potentially discarded prior to application, as is the case with drug delivery implants or other therapeutic nanodevices. On the characterization side, one of the first signs that critical limits in our capacity to control the physical processes on the ultrafine scale have been reached will have come from an inability to decouple the effects of the measurement systems on the qualities of the measured systems from their qualities per se. For example, during a recent ultrastructural characterization of graphene, it was impossible to tell whether the detected moving of a line of point defects was naturally occurring or it was due to the conversion of the electron beam energy to the thermal lattice movement.144 Similarly, widely dispersed, irregular and flat PbS nanoparticles—relatively robust to start with—were seen transforming to uniform spherical particles after 11 min of residing under the TEM beam,145 yielding a dramatic example of the fact well known from the basic tenet of the theory of measurements: both the observer and the observed are involved in defining the observed system’s qualities.
On the synthesis side, the first crucial signs of reaching these limits will be associated with persistently and pervasively irreproducible experimental outcomes. This could be compared to magnifying any imaginable signal up to the level when we start discerning merely fluctuations describable as white noise. Yet, an incessant challenge is to establish methods to convert this apparent randomness into more sophisticated signals. Dynamic light scattering methods for probing the diffusion and size properties of colloidal systems were, for example, designed to extract meaningful information from the seemingly random, Brownian motion, reminding us that everything that appears random could be actually seen as ordered and governed by certain laws when understood on a sufficiently small scale. Whether we have the effect of air bubbles in water146 (which have been invoked as the reason why attempts to confirm one such simple yet fundamental phenomenon such as Mpemba effect, depicted in Figure 18, have failed147), seasonal atmospheric variations, stirring rates148–150 or the Earth’s magnetic field,151,152 these minor effects will be increasingly paid attention to. The reaction volume is, for example, now already established as a crucial parameter of a synthesis procedure. Many attempts to scale-up a preparation procedure for its transfer from the lab bench to industry have failed exactly owing to the sensitivity of the given process to the reaction vessel size and oftentimes geometry and chemical composition of its walls as well.153,154 Overly tall reaction volumes, for example, increased the probability of precipitation of aragonite or vaterite—the two of the less stable CaCO3 phases—from a solution in the presence of an organic matrix, while flattened volumes were more prone to yield calcite, the most thermodynamically stable and the least soluble of CaCO3 phases.155 Recently it was demonstrated that osteoblasts cultured in star-shaped microwells differentiated to a significantly higher degree compared to the cells cultured in the microwells of pentagonal or square geometries,156 while the mechanoreciprocity principle dictates that there is a direct correspondence between the mechanical cues of the environment and cytoskeletal forces on which many signaling pathways within the cell and its phenotype crucially depend.157 Not a single reaction system is, after all, perfectly isolated from its environment and quite often it is the interface between the system and its environment (that is, a reaction and its container) that presents a crucial factor that drives the reaction towards its desired outcome. The only place where a liquid reaction system could exist virtually free from an interface with a physical environment would be in vacuum, the conditions under which the liquid would, however, swiftly evaporate.
Figure 18.
Mpemba effect states that even though the temperature of cold water reaches the freezing point faster than the warm water when cooled under the same conditions, warm water will be the one to freeze faster.
One example of the effect of reaction volume comes from the recently proposed evolutionary tree of the morphology of gold nanorods prepared by hydrolysis of HAuCl4 in the presence of CTAB at mild to highly acidic pHs.158 It was found out that shifting only the reaction volume from 0.9 to 45 ml changed the size and aspect ratio of gold nanoparticles by more than a single order of magnitude. Reaction systems that are subject to heating tend to be particularly sensitive to variations in volume, due to gradations in the heat flux. The purity of common reaction systems also presents a critical parameter of synthesis; as shown in Figure 19, introducing seemingly negligible amounts of impurities sometimes results in drastic changes in morphology and other properties of the material. Typical distilled water, the epitome of purity in the lab, contains a few solutes at c > 0.01 mM (a few ppm), a few dozens of solutes at c > 1 nM, and hundreds of solutes at c > 10 pM,159 alongside dissolved air and other gases (circa 5 mM) collected during aging under atmospheric conditions. These gaseous impurities are expected to exert critical influences on reaction pathways, as many reactions are now known to be catalyzed by the dissolved gas,160 as well as that its presence can be comparable to defects in a solid body.161 Furthermore, from the particle growth kinetics standpoint, a tradeoff naturally exists: the slower the process, the greater the level of the control of fabricated properties, but at the same time the greater the chance that the internal or external impurities will be introduced that will drive the reaction in an undesired direction and prevent its repeatability. In general, as the fineness of the chemical reactions and physicochemical processes increases and as windows of conditions that lead to desirable products are narrowed, impurities previously disregarded will have to be taken into account. The effects of impurities in as minor concentrations as in the aforementioned picomolar range will thus prove as increasingly important in attaining ideals of sophisticated sensitivity and precise localizations of ultrafine physicochemical processes.
Figure 19.
Cholesterol particles prepared using 2-propanol of laboratory grade (a) and technical grade (b) as the solvent.
Then, as biomolecules will become increasingly utilized in fabrication of ultrafine structures, sensitivity of their folding and assembly into proper configurations will introduce uncontrollable energy landscapes into the game. For example, single post-translational modifications can drastically change the protein functionality,162 while recombinant proteins may occasionally exhibit completely different properties compared to their native counterparts despite having identical primary structures, which is often caused by different folding.163 Elimination of a disulfide bond in dodecapeptides isolated by phage or cell surface display systems has thus been shown to induce a 3-fold increase in the size of calcium phosphate particles precipitated in the presence of these peptides.164 Many of these effects have been so regularly neglected in the lab practice that questioning them, even though it is well justified from the point of view of experimental rigorousness, might be seen as malevolent interference with the bench work. One example comes from the insufficiently investigated effects of fluorescent tags. Cells have been, for example, shown to react significantly differently when a fluorescent molecule such as FITC, used merely to visualize it in immunohistochemical analyses, is tagged onto the analyzed macromolecule or particle in question.165 The effects of the cell circadian rhythm on nanoparticle-cell interactions are also still largely unexplored, even though it is known that the expression of almost 50% of genes in certain cell types shows variations depending on it.166 Another example may come from the often ignored effect buffers exert on the reactions run in their presence. That buffers can drastically affect the experimental outcomes can be illustrated by precipitation reactions whose products were modified with respect to phase composition or polymorphic identity depending on the nature of the buffer. For example, whether organic Tris or inorganic phosphates are used as buffers can lead to precipitation of thoroughly different calcium phosphate phases.167 This is partly a consequence of the ability of Tris and particularly Bis-Tris to sequester free calcium ions from the solution and form metallo-organic complexes, the effect that was both theoretically predicted168 and experimentally confirmed.169 Whether one precipitates calcium phosphates from simulated body fluid, normally buffered using Tris/Bis-Tris, or from phosphate buffer saline or a non-buffered aqueous solution, can thus have a drastic effect on the nature of the precipitated phase.170 Also, since Tris has been shown to exhibit a toxic effect in mammalian cell cultures,171–173 the biological response to solids formed in the presence of Tris can also significantly differ from that observed for materials precipitated in the presence of a different buffer.
That seemingly minor changes in atmosphere can have a crucial effect on some of the most routine phase transformations induced at high annealing temperatures is demonstrated by the proposed seasonal variation in humidity as a factor that obstructed the degradation of hydroxyapatite upon heating and limited the reproducibility of its calcinations.174 Then, although routine errors of pH measurements typically prevent experimentalists to report values precise to the second decimal digit, pH variations on exactly the second decimal digit during a preparation of zinc oxide led to significant changes in the morphology and photoluminescent spectra of the particles.175 Thus we arrive once more at the crucial challenge in attempts to further our ability to control physicochemical transformations at the nanoscale: when will the limits in experimental sensitivity be reached and what will we do then?
CRISSCROSSING THEORY AND EXPERIMENTS
Most materials scientists would readily describe their expertise as falling in the realm of either theoretical or experimental knowledge, but not both. Although the term “tunable” has been extensively used to describe size-dependent material properties, strictly speaking it cannot be associated with the attribute “designable” since the connections between lab notebook blueprints and the final experimental outcomes in terms of desirable material properties are not readily establishable. Although it has ever since been the dream of materials scientists to devise a perfectly precise algorithm that connects synthesis parameters, material structure and properties, its function and performance, it is still permeated with numerous missing links. For example, although quantum dots do exhibit size-dependent optoelectronic properties, Fermi levels of quantum dots can still not be designed prior to fabricating them. Only after the material is prepared and characterized for its properties, one would go back and mark the experimental conditions that have yielded the desired properties as worth reporting or passing on to further stages in the chain of technological development (Fig. 20). However, the synthesis method sometimes modifies material properties far beyond those predictable from their chemical nature. A tricalcium phosphate ceramic obtained in a mechanochemical-hydrothermal process thus paradoxically exhibited greater resistance to degradation than hydroxyapatite, demonstrating that the synthesis method and the structural features it produces may have a more decisive effect on the powder properties than its bare chemical composition.176 That experimental settings can counter-intuitively lead to surprising properties has been also evidenced by the case wherein the capacity of magnesium to absorb hydrogen doubled upon the addition of 50 wt% of fully inert, hydrogen-saturated titanium hydrate to it, owing to the nucleation-promoting effect of the latter, showing how sometimes an intentional introduction of impurities or functionless components in order to make the material less pure and perfect results in its greater reactivity and an intriguing functional upgrade.177 A similar example comes from the field of mixed zinc ferrites:178 namely, with the addition of diamagnetic Zn2+ to nickel ferrite lattice, comprising magnetic Ni2+ and Fe3+, the magnetic moment of the compound increases and reaches a maximum at approximately 1:1 molar ratio of zinc-to-nickel.179 This peculiar effect is explained by the ability of Zn2+ ions to replace Fe3+ ions from the tetrahedral, A sites of the inverse spinel structure of NiFe2O4 and thus diminish the compensation of the superexchange, antifer-romagnetic coupling of the magnetic moments of Fe3+ ions positioned on the A sites and Fe3+ and Ni2+ ions resting on the octahedral, B sites of the crystal lattice.180
Figure 20.
A “nano” train showing how synthesis of new materials, evaluation of their properties, their processing and integration into components and then assembly into consumer products with a specific performance are all interconnected.
The same observation applies to the field of drug discovery, where despite the extensive initial screening steps, the final candidates for a drug undergo randomized, trial-and-error studies in vitro, on animals and then on humans, eventually unexplainably failing or living up to their promises, or even yielding an unforeseen therapeutic effect. Sildenafil citrate, a.k.a. Viagra, which had been designed to be a hypertension-relieving drug, but failed in that and was only later associated with the side effects for which it is currently demanded on the market, offers a particularly popular example. As pointed out by Arthur Kornberg, “Discoveries are commonly serendipitous. The best plan over many decades has been no plan.”181 Moreover, the Nobel Laureate went on to claim that “medical research is still more a game of pool than billiards; you score points regardless of which pocket the ball goes into,” quoting the example of Harold Varmus who received the Nobel Prize in 1989 together with Michael Bishop for their studies on breast cancer, despite the fact that “actually, Varmus’ studies yielded no insights into human breast cancer, but they did provide an important advance in understanding brain development. At the same time, a research program headed by Robert Weinberg at MIT, directed toward a rat brain tumor, did make a major contribution to understanding human breast cancer. Of the several genes now known to be involved in human breast cancer, all but one were discovered by researchers working on something other than breast cancer.” For example, after 80% of patients with glaucoma had unexpectedly experienced growth and thickening of eyelashes upon applying bimatoprost, a drug used to treat glaucoma, the FDA decided to approve the drug as a pharmaceutical product for eyelash growth.182 In fact, the discovery of most other chemicals in contemporary usage, from cisplatin to Teflon to polio vaccine and penicillin, is owed to relentless and serendipitous trial-and-error methodology rather than to well-planned assault on particular research targets.183,184
Reinforcing links between the theory and the experiment, in all contexts of nanoscience, from particle syntheses to the design of nanoscale drug delivery platforms, would bring us closer to the ideal of predicting experimental outcomes with a greater certainty. And with our ability to estimate the material properties based on the known experimental settings of synthesis, the first chain in the “nano” train depicted in Figure 20 will be made much stronger and stable than it is today.
CONJOINING TOP-DOWN AND BOTTOM-UP SYNTHETIC APPROACHES
Many subfields of materials science have traditionally relied on classical, top-down ways of synthesizing new materials. During the last decade or so, most of those have, however, undergone the trend of adoption of soft and more elegant, colloidal chemistry approaches, as opposed to the robust top-down methods.185 However, whereas the former may be said to exhibit drawbacks associated with the lack of facileness to process the self-assembled systems into technologically functional units, the main downside of the latter is the lack of ability to simultaneously organize a multitude of entities in a time- and cost-effective manner. Still, by applying these two mainstream synthetic approaches in parallel, so that they complement each other’s strengths and weaknesses, there is a chance that more superior bases for fabrication of new materials will be established. Top-down controllability would play a role in component integration stages, while soft, simple and inexpensive methods often replicable on a kitchen stove would render materials science know-how easily transferable from hi-tech hot spots to countries at the technological standstill, able to scientifically contribute to bridging the gap between rich and poor, on which sustainability of the world as we know it pivotally depends.186,187 In an effort to build such synergetic instrumentation, the techniques for (a) controlled, lithographic deposition and (b) spontaneous growth and assembly have been combined, enabling facile fabrication of different nanoscale symmetries via varying the ratio between the deposition flux and the diffusion rate.188 An overall impression is thus that the most effective routes for fabrication of functional nanostructures in the future will greatly depend on the ability of experimentalists to utilize top-down and bottom-up techniques in tandem.189
UNDERSTANDING A VARIETY OF CONTEXTS THAT CO-DEFINE THE EXPERIMENTATION OUTCOMES
The boundary that separates the potentials of these two synthetic approaches was summed up nicely by the thought of Richard Smalley: “Much like you can’t make a boy and a girl fall in love with each other simply by pushing them together, you cannot make precise chemistry occur as desired between two molecular objects with simple mechanical motion along a few degrees of freedom. Chemistry, like love, is more subtle than that. You need to guide the reactants down a particular reaction coordinate, and this coordinate treads through a many-dimensional hyperspace… Near the center of the typical chemical reaction, the particular atoms that are going to form the new bonds are not the only ones that jiggle around: so do all the atoms they are connected to and the ones connected to these in turn. All these atoms must move in a precise way to ensure that the result of the reaction is the one intended.”190,191 These words shed light on the contextual nature of all chemical phenomena, an effect unknowingly utilized by practically any scientist in search for the right conditions that would yield a desired product. Examples of this effect that implicitly underlies the all-pervading holistic nature of physical phenomena are countless, ranging from modifications of functionality of cells autogenically transplanted from one tissue to another to oncogenes causing cancer or not depending on whether they are inserted into grown animal cells or embryos192 to pancreatic cells able to release insulin only when forming islets of specific sizes,193,194 neither too big nor too small.
As an extension of this effect, the hypothesis was outlined stating that each self-assembly process is, strictly speaking, a co-assembly since the spontaneous reorganization of a system may take place only in parallel with a well coordinated reorganization of its immediate environment.195 Such a perspective where qualities of a system are inextricably related to the state of its environment has been established in agreement with the previously proposed co-creational thesis and its cognitive and epistemological connotations.196,197 The idea of co-assembly was supported by studies on reverse micelles198 where it was noticed that these molecular aggregates do not act as passive templates for the formation of nanocrystals within their cores, but undergo mutual transformations with the nucleating phase.199 Hence, different compounds may be synthesized by following the same preparation procedure, depending on whether reverse micelles are present in the reaction system or not. They also play an active role in catalyzing the transformations between intermediaries which may have a crucial effect on the final products of precipitation reactions and other phase transitions that take place in their presence.200 Also, as shown in Figure 21(a)–(b), folding of proteins in reverse micelles is greatly affected by the space limitation effect, demonstrating the extent to which the physical environment affects the structure and, therefore, the function of these basic biomolecular units. Figure 21(c)–(d) concordantly show that even the structure of water inside of reverse micelles is different from the bulk water and subject to change depending on the size of the micelle. Similarly, whoever has designed an experiment to assess the controlled release rate of a molecule encapsulated within a nanocarrier must have become familiar with how modifications of the parameters that define the environment into which the eluted drug molecules will diffuse, including its volume, temperature, agitation level, salt content, frequency of replenishment, etc., can be used to tune the release kinetics profiles to a desired one. Figure 22(a) correspondingly shows a hypothetic example of an identical drug delivery device yielding two kinetically distinct release profiles depending on the release measurement methodology applied: zero-order upon daily replacements of a comparatively small volume of the solvent and first-order one upon the usage of larger solvent volume and no daily replenishments thereof. Namely, whereas the drug is released in burst fashion when a sufficient volume of the solvent is provided, limited amounts of the solvent place a cap on the maximal amount that could be released before the saturation is reached, leaving space for the eventual fabrication of a desired release profile. None of these two methods, however, mimic the biological conditions under which fast clearance of the released drug is typically observed, nor they account for the effects of the complex interface between the device and numerous macromolecules and cells of the host organism on drug release. And today we know that although size, shape and elasticity of nanoparticles in biological milieus do influence their biodistribution profiles, the route of uptake and the mechanism of interaction with the cell are mainly determined by the protein corona adsorbed on the particles and the surface propensities that it endows them with.201,202 How to devise in vitro drug release testing procedures that would be able to replicate in vivo conditions better, typically characterized by (a) a more dynamic flow of fluids; (b) specific local pH profiles that are often disease-dependent; and (c) much more complex and selective media, is another challenge for the drug delivery field. The natural consequence of this stance is that same molecules can exhibit dual and quite often antipodal effects on specific physicochemical processes, as epitomized by polypeptides that can, depending on their concentration and other experimental conditions, either promote or inhibit growth of crystals in interaction with them.203–205
Figure 21.
(a) A protein inside of a reverse micelle; (b) an empirically derived plot of the adiabatic compressibility of cytochrome c, lysozyme and lactoglobulin, indicative of the dimensions and tertiary structure of the protein, as a function of parameterwthe molar ratio between the amount of water and the amount of surfactant in the microemulsion, directly proportional to the size of reverse micelle; (c) a blue shift in the absorbance of water in the microwave range induced by a decrease in the size of nanoscopic water pools of reverse micelles, along with (d) the number of water molecules in reverse micelles of the given size. Reprinted with permission from [206], D. Valdez, et al., Hydration and protein folding in water and in reverse micelles: compressibility and volume changes. Biophys. J. 80, 2751 (2001). © 2001, Elsevier; From [207], D. E. Moilanen, et al., Confinement or properties of the interface? dynamics of nanoscopic water in reverse micelles, J. Am. Chem. Soc. 129, 14311 (2007). © 2007, American Chemical Society.
Figure 22.
(a) Hypothetic release curves for a drug delivery device under two different measurement regimens: daily replacements of a comparatively small volume of the solvent (-●-), and the usage of a considerably larger solvent volume, with no daily replenishments thereof (-▲-); (b) A disparity between the prediction of global market share of nanotechnologies in 2009 made by the National Science Foundation five years earlier and the true value estimated in 2011. Source: BCC Research Reports 2005 and 2011.
If an important advice lurks underneath this observation too, it is the one urging careless and pretentious researchers to think twice before they indulge in irrational and premature expansion of the relevance of their findings beyond the scope of experimental conditions utilized in the lab. In fact, if the history of science teaches us something, it is that the greatest threats for its proper understanding and progress come not from those who nondeliberately commit mistakes in their research, but from those who pump up their conclusions, derived from a limited set of experimental conditions, well beyond the scopes of their validity. There is no doubt that the burst of the nanotechnology bubble in the years following the peak in the estimated asset value observed at around 2005, inflated as the result of unrealistically proximal prospects of nanoscience portrayed by scientists in hunts for funding, can illustrate the extent to which pretentiousness in the scientific domain can threaten the projected lines of progress. Hence, as shown in Figure 22(b), while the National Science Foundation estimated the global market share of nan-otechnologies in 2009 at $1 trillion five years earlier, the true value ended up being only $11.7 billion. Nourishing the spirit of impartiality and humbleness that naturally springs from the ideals of smallness attributed to the field of nanoscience and that has ensued the tradition of empiricism since the times of Renaissance will certainly present a vital challenge in the times of increasingly entrepreneurial approach to scientific management and thriving collaborations between academia and industry.
CONCLUSIONS
“‘It is hard to be brave,’ said Piglet, sniffing slightly, ‘when you’re only a Very Small Animal.’ Rabbit, who had begun to write very busily, looked up and said: ‘It is because you are a very small animal that you will be Useful in the adventure before us.”208
This critical review began with the proposition stating that the interest in physical chemistry of nanoscale phenomena is a natural consequence of the spatial refinement of the human ability to controllably manipulate the material substratum of our physical reality. It ended with humble acceptance of the limited nature of our experimentally derivable knowledge, accentuating searching rather than finding as the key to fruitfully performed scientific research. In its course, examples were given to illustrate an immense sensitivity of material properties to grain size on the nanoscale, a phenomenon that directly contributed to the rise of nanoscience as a special field of scientific inquiry. Alongside this thesis, main systemic challenges faced by the present and future scientists in this field were outlined. For a while, we stood on the constantly expanding seashore of the coast of nanoscience and nanoengineering and envisioned the parts of the island where the most significant advances may be expected to occur and where most of the attention of scientist in the field is to be directed: crossing the gap between life science and materials science; increasing experimentation sensitivity; crisscrossing theoretical and experimental knowledge; and conjoining top-down and bottom-up synthetic approaches. As for materials and their application areas discussed, a special emphasis was placed on calcium phosphate nanoparticles and their usage in controlled drug delivery and other applications of biomedical relevance. Finally, the rise and prominence of nanoscience is a reminder of the necessity to reawaken the humble wonder that has been intrinsic to the advancement of scientific thought ever since its inception. For, as suggested by the standard theory of measurements and illustrated by the weak interpretation of the uncertainty principle, to observe the small details of physical reality and interact with them, we, as observers and causal agents, need to be equally small. Hence, Time to Be Small as the maxim derived naturally from the herein elaborated idea that the most valuable scientific insights will increasingly originate from ever finer details of Nature. Expansion of thoughts that would develop this correlation into humanistic, ethical and aesthetical directions, however, presents the beginnings of some other, far more interdisciplinary stories than this one.
Acknowledgments
Writing of this review was supported by the National Institute of Health grant K99-DE021416: Osteogenic calcium phosphate nanoparticles with designable drug release kinetics.
Biography

Vuk Uskoković, a native of Belgrade, Serbia, is a principal investigator in the Department of Bioengineering and Therapeutic Science of University of California, San Francisco. He holds a B.Sc. degree in Physical Chemistry, an M.Sc. degree in Materials Science and Engineering, and a Ph.D. degree in Nanosciences and Nanotechnologies. He is a former research member of Jožef Stefan Institute in Ljubljana where he developed methods for the synthesis of magnetic nanoparticles applicable in electrical engineering and biomedicine. At the Center for Advanced Materials Processing of Clarkson University, Potsdam, NY, he explored the mechanisms of precipitation of compounds implicated in the development of atherosclerotic vascular disease. His appointment with the Division of Biomaterials and Bioengineering at the Dental School of University of California, San Francisco involved research of the biomimetic formation of biomineralized tissues. Currently he devises controlled drug delivery platforms using nanotechnologies for a variety of medical and pharmaceutical applications. Beside his dedication to scientific research, he has published studies from the fields of cognitive science, ecoscience, philosophy, theosophy and social science.
REFERENCES
- 1.Uskoković V. Nanomaterials and nanotechnologies: Approaching the crest of this big wave. Curr. Nanosci. 2008;4:119. [Google Scholar]
- 2.Chokski AH, Rosen A, Karch J, Gleiter H. On the validity of the Hall-Petch relationship in nanocrystalline materials. Scripta Metall. 1989;23:1679. [Google Scholar]
- 3.Andrievski RA. Fundamentals of nanostructured materials: Possibilities and problems. Moscow: BINOM; 2012. [Google Scholar]
- 4.Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf AM, McCullough J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomed. Nanotech. Bio. Med. 2013;9:1. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pande CS, Cooper KP. Nanomechanics of Hall-Petch relationship in nanocrystalline materials. Progress Mat. Sci. 2009;54:689. [Google Scholar]
- 6.Liang LH, Liu D, Jiang Q. Size-dependent continuous binary solution phase diagram. Nanotech. 2003;14:438. [Google Scholar]
- 7.Lu K, Jin ZH. Melting and superheating of low-dimensional materials. Curr. Op. Solid State Mat. Sci. 2001;5:39. [Google Scholar]
- 8.Sutter EA, Sutter PW. Size-dependent phase diagram of nanoscale alloy drops used in vapor-liquid-solid growth of semiconductor nanowires. ACS Nano. 2010;4:4943. doi: 10.1021/nn101366w. [DOI] [PubMed] [Google Scholar]
- 9.Bose S, Banerjee R, Genc A, Raychaudhuri P, Fraser HL, Ayyub P. Size induced metal insulator transition in nanostructured Niobium thin films: Intragranular and intergranular contributions. J. Phys.: Condens. Matt. 2006;18:4553. [Google Scholar]
- 10.Tuller HL. Ionic conduction in nanocrystalline materials. Solid State Ionics. 2000;131:143. [Google Scholar]
- 11.Tschöpe A, Sommer E, Biringer R. Grain size-dependent electrical conductivity of polycrystalline cerium oxide I: Experiments. Solid State Ionics. 2001;139:255. [Google Scholar]
- 12.Guo X, Zhang X. Grain size dependent grain boundary defect structure: Case of doped Zirconia. Acta Mater. 2003;51:2539. [Google Scholar]
- 13.Demetry C, Shi X. Grain size-dependent electrical properties of Rutile (TiO2) Solid State Ionics. 1999;118:271. [Google Scholar]
- 14.de R, Rica la, Stevens MM. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the Naked eye. Nature Nanotech. 2012;7:821. doi: 10.1038/nnano.2012.186. [DOI] [PubMed] [Google Scholar]
- 15.Mitsakakis K, Sekula-Neuner S, Lenhert S, Fuchs H, Gizeli E. Convergence of dip-pen nanolithography and acoustic biosensors towards a rapid-analysis multi-sample microsystem. Analyst. 2012;137:3076. doi: 10.1039/c2an35156k. [DOI] [PubMed] [Google Scholar]
- 16.Uskoković V, Drofenik M. Synthesis of Lanthanum-strontium manganites by oxalate-precursor co-precipitation methods in solution and in reverse micellar microemulsion. J. Magn. Magn. Mater. 2006;303:214. [Google Scholar]
- 17.Bohra M, Prasad S, Kumar N, Misra DS, Sahoo SC, Venkataramani N, Krishnan R. Large room temperature magnetization in nanocrystalline zinc ferrite thin films. Appl. Phys. Lett. 2006;88:262506. [Google Scholar]
- 18.Yamazoe N. New approaches for improving semiconductor gas sensors. Sensors Actuators B: Chem. 1991;5:7. [Google Scholar]
- 19.Gurlo A, Ivanovskaya M, Bârsan N, Schweizer-Berberich M, Weimar U, Göpel W, Diéguez A. Grain size control in nanocrystalline In2O3 semiconductor gas sensors. Sensors Actuators B: Chem. 1997;44:327. [Google Scholar]
- 20.2012 Retrieved from http://www.discovernano.northwestern.edu/whatis/index_html/sizematters_html.
- 21.Kipling R. Trafalgar Square. London, UK: 1891. The english flag, Rudyard Kipling: The Complete Verse. [Google Scholar]
- 22.Uskoković V, Matijević E. Uniform particles of pure and silica coated Cholesterol. J. Coll. Interface Sci. 2007;315:500. doi: 10.1016/j.jcis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Uskoković V, Kim M-K, Li W, Habelitz S. Enzymatic processing of amelogenin during continuous crystallization of apatite. J. Mater. Res. 2008;32:3184. doi: 10.1557/JMR.2008.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uskoković V, Drofenik M. Four novel co-precipitation procedures for the synthesis of Lanthanum-Strontium manganites. Mater. Des. 2007;28:667. [Google Scholar]
- 25.Dai H, Choe WS, Thai CK, Sarikaya M, Traxler BA, Baneyx F, Schwartz D. Nonequilibrium synthesis and assembly of hybrid inorganic-protein nanostructures using an engineered DNA binding protein. J Am. Chem. Soc. 2005;127:15637. doi: 10.1021/ja055499h. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad G, Dickerson MB, Church BC, Cai Y, Jones SE, Naik RR, King JS, Summers CJ, Kröger N, Sand-hage Rapid KH. room-temperature formation of crystalline calcium molybdate phosphor microparticles via peptide-induced precipitation. Adv. Mater. 2006;18:1759. [Google Scholar]
- 27.Dickerson MB, Naik RN, Stone MO, Cai Y, Sandhage KH. Identification of peptides that promote the rapid precipitation of germania nanoparticle networks via use of a peptide display library. Chem. Commun. (Camb.) 2004;15:1776. doi: 10.1039/b402480j. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson HL, Gustafsson J, Cronholm P, Moller L. Size-dependent toxicity of metal oxide particles—A comparison between nano- and micrometer size. Toxicol. Lett. 2009;188:112. doi: 10.1016/j.toxlet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Uskoković V. Nanotechnologies: What we do not know. Techn. Soc. 2007;29:43. [Google Scholar]
- 30.Service RF. Nanomaterials show signs of toxicity. Science. 2003;300:243. doi: 10.1126/science.300.5617.243a. [DOI] [PubMed] [Google Scholar]
- 31.Limbach LK, Li Y, Grass RN, Brunner TJ, Hintermann MA, Muller M, Gunther D, Stark WJ. Oxide nanoparticles uptake in human lung fibroblasts: Effects of particle size, agglomeration, and diffusion at low concentrations. Environ. Sci. Techn. 2005;39:9370. doi: 10.1021/es051043o. [DOI] [PubMed] [Google Scholar]
- 32.Devadasu VR, Bhardwaj V, Kumar MNVR. Can controversial nanotechnology promise drug delivery. Chem. Rev. 2013 doi: 10.1021/cr300047q. in press. [DOI] [PubMed] [Google Scholar]
- 33.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 34.Canton I, Battaglia G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012;41:2718. doi: 10.1039/c2cs15309b. [DOI] [PubMed] [Google Scholar]
- 35.Zhao F, Zhao Y, Liu Y, Chang C, Chen C, Zhao Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7:1322. doi: 10.1002/smll.201100001. [DOI] [PubMed] [Google Scholar]
- 36.Win KY, Feng S-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26:2713. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 37.Georgantzopoulou A, Balachandran YL, Rosenkranz P, Dusinska M, Lankoff A, Wojewodzka M, Kruszewski M, Guignard C, Audinot JN, Girija S, Hoffmann L, Gutleb AC. Ag nanoparticles: size- and surface-dependent effects on model aquatic organisms and uptake evaluation with NanoSIMS. Nanotoxicol. 2013 doi: 10.3109/17435390.2012.715312. in press, PMID: 22834480. [DOI] [PubMed] [Google Scholar]
- 38.Uskoković V. Of sustainability elephants and prefab sprouts. Int. J. Sustain. Soc. 2008;1:85. [Google Scholar]
- 39.Zhao J, Castranova V. Toxicology of nanomaterials used in nanomedicine. J. Toxicol. Environ. Health. B Crit. Rev. 2011;14:593. doi: 10.1080/10937404.2011.615113. [DOI] [PubMed] [Google Scholar]
- 40.Uskoković V, Lee PP, Walsh L, Fischer KE, Desai TA. Silicon nanowire coated microparticles as epithelial drug delivery devices. The effect of PEGylation on particle-epithelium interactions. Biomater. 2012;33:1663. doi: 10.1016/j.biomaterials.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nefzger M, Kreuter J, Voges R, Liehl E, Czok R. Distribution and elimination of polymethyl methacrylate nanoparticles after peroral administration to rats. J. Pharm. Sci. 1984;73:1309. doi: 10.1002/jps.2600730934. [DOI] [PubMed] [Google Scholar]
- 42.Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials: Developing the next generation of vaccines. Trends Immunol. 2006;27:573. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Slütter B, Bal SM, Que I, Kaijzel E, Löwik C, Bouwstra J, Jiskoot W. Antigen-adjuvant nanoconjugates for nasal vaccination: An improvement over the use of nanoparticles? Mol. Pharm. 2010;7:2207. doi: 10.1021/mp100210g. [DOI] [PubMed] [Google Scholar]
- 44.Uskoković V. Surface charge effects involved in the control of stability of sols comprising uniform Cholesterol particles. Mater. Manufact Proces. 2008;23:620. [Google Scholar]
- 45.Uskoković V. Theoretical and practical aspects of colloid science and self-assembly phenomena revisited. Rev. Chem. Eng. 2007;23:301. [Google Scholar]
- 46.Uskoković V, Drofenik M. Synthesis of nanocrystalline Nickel-Zinc ferrites within reverse micelles. Mater. Tech. 2003;37:129. [Google Scholar]
- 47.Pileni MP. Supra- and nanocrystallinities: A new scientific adventure. J. Phys. Condens. Matter. 2011;23:503102. doi: 10.1088/0953-8984/23/50/503102. [DOI] [PubMed] [Google Scholar]
- 48.Ma H, Hao J. Ordered patterns structures via interfa-cial self-assembly: Superlattices, honeycomb structures and coffee rings. Chem. Soc. Rev. 2011;40:5457. doi: 10.1039/c1cs15059f. [DOI] [PubMed] [Google Scholar]
- 49.Vanmaekelbergh D. Self-assembly of colloidal nanocrystals as route to novel classes of nanostructured materials. Nano Today. 2011;6:419. [Google Scholar]
- 50.Uskoković V, Desai TA. Phase composition control of calcium phosphate nanoparticles for tunable drug delivery kinetics and treatment of osteomyelitis. Part 1: Preparation and drug release. J. Biomed. Mater. Res. A. 2013 doi: 10.1002/jbm.a.34426. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs-A review of drug nanocrystal technology and lipid nanoparticles. J. Biotech. 2004;112:151. doi: 10.1016/j.jbiotec.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Mout R, Moyano DF, Rana S, Rotello VM. Surface func-tionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012;41:2539. doi: 10.1039/c2cs15294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fahr A, Liu X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin. Drug Deliv. 2007;4:403. doi: 10.1517/17425247.4.4.403. [DOI] [PubMed] [Google Scholar]
- 54.Uskoković V. Nova Science. Hauppauge, NY: 2009. Trends in Practical Colloid Science. [Google Scholar]
- 55.Carstensen JT. In: Solubility, Advanced Pharmaceutical Solids. Carstensen JT, editor. New York: Marcel Dekker; 2001. pp. 27–50. [Google Scholar]
- 56.Wong J, Brugger A, Khare A, Chaubal M, Papadopoulos P, Rabinow B, Kipp J, Ning J. Suspensions for intravenous (IV) injection: A review of development, preclinical and clinical aspects. Adv. Drug Deliv. Rev. 2008;60:939. doi: 10.1016/j.addr.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Singh N, Manshian B, Jenkins GJS, Griffiths SM, Williams PM, Maffeis TGG, Wright CJ, Doak SH. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomater. 2009;30:3891. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Albanese A, Chan WC. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano. 2011;5:5478. doi: 10.1021/nn2007496. [DOI] [PubMed] [Google Scholar]
- 59.Nam J, Won N, Jin H, Chung H, Kim S. pH-induced aggregation of gold nanoparticles for photothermal cancer therapy. J. Am. Chem. Soc. 2009;131:13639. doi: 10.1021/ja902062j. [DOI] [PubMed] [Google Scholar]
- 60.Kneipp J, Kneipp H, McLaughlin M, Brown D, Kneipp K. In vivo molecular probing of cellular compartments with gold nanoparticles and nanoaggregates. Nano Lett. 2006;6:2225. doi: 10.1021/nl061517x. [DOI] [PubMed] [Google Scholar]
- 61.Koh AL, Shachaf CM, Elchuri S, Nolan GP, Sinclair R. Electron microscopy localization and characterization of functional-ized composite organic-inorganic SERS nanoparticles on leukemia cells. Ultramicroscopy. 2008;109:111. doi: 10.1016/j.ultramic.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang X, Röcker C, Hafner M, Brandholt S, Dörlich RM, Nienhaus GU. Endo- and exocytosis of zwitterionic quantum dot nanoparticles by live HeLa cells. ACS Nano. 2010;4:6787. doi: 10.1021/nn101277w. [DOI] [PubMed] [Google Scholar]
- 63.Cebrian V, Martin-Saavedra F, Yague C, Arruebo M, Santamaria J, Vilaboa N. Size-dependent transfection efficiency of PEI-coated gold nanoparticles. Acta Biomater. 2011;7:3645. doi: 10.1016/j.actbio.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 64.Uskoković V, Košak A, Drofenik M. Silica-coated Lanthanum-Strontium manganites for hyperthermia treatments. Mater. Lett. 2006;60:2620. [Google Scholar]
- 65.Talelli M, Rijcken CJ, Lammers T, Seevinck PR, Storm G, van Nostrum CF, Hennink WE. Superparamagnetic iron oxide nanoparticles encapsulated in biodegradable thermosensitive polymeric micelles: Toward a targeted nanomedicine suitable for image-guided drug delivery. Langmuir. 2009;25:2060. doi: 10.1021/la8036499. [DOI] [PubMed] [Google Scholar]
- 66.Nutan MTH, Reddy IK. General Principles of Suspensions. In: Kulshrestha AK, Singh ON, Wall GM, editors. Pharmaceutical Suspensions: From Formulation Development to Manufacturing. New York: Springer; 2009. pp. 39–66. [Google Scholar]
- 67.Patravale VB, Date AA, Kulkarni RM. Nanosuspensions as a promising drug delivery strategy. J. Pharmaceut. Pharmacol. 2004;56:827. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 68.Uskoković V. Dynamic light scattering and microelectrophore-sis: Main prospects and limitations. J. Disp. Sci. Techn. 2012;33:1762. doi: 10.1080/01932691.2011.625523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu L, Zhang J, Watanabe W. physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011;63:456. doi: 10.1016/j.addr.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Y, Sun X, Zhang G, Trewyn BG, Slowing II, Lin VS. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: Size and surface effects. ACS Nano. 2011;5:1366. doi: 10.1021/nn103077k. [DOI] [PubMed] [Google Scholar]
- 71.Oh WK, Kim S, Choi M, Kim C, Jeong YS, Cho BR, Hahn JS, Jang J, Cellular uptake cytotoxicity. and innate immune response of silica-titania hollow nanoparticles based on size and surface functionality. ACS Nano. 2010;4:5301. doi: 10.1021/nn100561e. [DOI] [PubMed] [Google Scholar]
- 72.Caldorera-Moore M, Guimard N, Shi L, Roy K. Designer nanoparticles: Incorporating size shape and triggered release into nanoscale drug carriers. Expert Opin. Drug Deliv. 2010;7:479. doi: 10.1517/17425240903579971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park J, Bauer S, van der Mark K, Schmuki P. Nanosize and vitality:? TiO2 nanotube diameter directs cell fate. Nano Lett. 2007;7:1686. doi: 10.1021/nl070678d. [DOI] [PubMed] [Google Scholar]
- 74.Brammer KS, Oh S, Cobb CJ, Bjursten LM, van der Heyde H, Jin S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009;5:3215. doi: 10.1016/j.actbio.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 75.Salgado CL, Oliveera MB, Mano JF. Combinatorial cell-3D biomaterials cytocompatibility screening for tissue engineering using bioinspired superhydrophobic substrates. Integr. Biol. (Camb.) 2012;4:318. doi: 10.1039/c2ib00170e. [DOI] [PubMed] [Google Scholar]
- 76.Suresh AK, Pelletier DA, Wang W, Morrell-Falvey JL, Gu B, Doktycz MJ. Cytotoxicity induced by engineered silver nanocrystallites is dependent on surface coatings and cell types. Langmuir. 2012;28:2727. doi: 10.1021/la2042058. [DOI] [PubMed] [Google Scholar]
- 77.Ignjatović N, Ajduković Z, Savić V, Najman S, Mihailović D, Vasiljević P, Stojanović Z, Uskoković V, Uskoković D. Nanoparticles of cobalt-substituted hydroxyapatite in regeneration of mandibular osteoporotic bones. J. Mater. Sci. Mater. Med. doi: 10.1007/s10856-012-4793-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirn S, Semmler-Behnke M, Schleh C, Wenk A, Lipka J, Schäffler M, Takenaka S, Möller W, Schmid G, Simon U, Kreyling WG. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm. 2011;77:407. doi: 10.1016/j.ejpb.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lukić MJ, Stojanović Z, Škapin SD, Macek-Kržmanc M, Mitric M, Marković S, Uskoković D. Dense fine-grained biphasic calcium phosphate (BCP) bioceramics designed by two-step sintering. J. Eur. Ceram. Soc. 2011;31:19. [Google Scholar]
- 80.Lukić MJ, Škapin SD, Markovic´ S, Uskokovic´ D. Processing route to fully dense nanostructured HAp Bioceramics: From powder synthesis to sintering. J. Am. Ceram. Soc. 2012;95:3394. [Google Scholar]
- 81.Wang X-H, Deng X-Y, Bai H-L, Zhou H, Qu W-G, Li L-T. Two-step sintering of ceramics with constant grain-size II: BaTiO3 and Ni-Cu-Zn ferrite. J. Am. Ceram. Soc. 2006;89:438. [Google Scholar]
- 82.Chen I-W, Wang X-H. Sintering dense nanocrystalline ceramics without final-stage grain growth. Nature. 2000;404:168. doi: 10.1038/35004548. [DOI] [PubMed] [Google Scholar]
- 83.Hasegawa M, Sudo A, Komlev VS, Barinov SM, Uchida A. High release of antibiotic from a novel hydroxyapatite with bimodal pore size distribution. J. Biomed. Mater. Res. B Appl. Biomater. 2004;70:332. doi: 10.1002/jbm.b.30047. [DOI] [PubMed] [Google Scholar]
- 84.Xia Y, Nguyen TD, Yang M, Lee B, Santos A, Podsiadlo P, Tang Z, Glotzer SC, Kotov NA. Self-assembly of self-limiting monodisperse supraparticles from polydisperse nanoparti-cles. Nature Nanotech. 2011;6:580. doi: 10.1038/nnano.2011.121. [DOI] [PubMed] [Google Scholar]
- 85.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CDW, Oreffo ROC. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature Mater. 2007;6:997. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 86.Sosnowski S, Woźniak P, Lewandowska-Szumiel M. Polyester scaffolds with bimodal pore size distribution for tissue engineering. Macromol. Biosci. 2006;6:425. doi: 10.1002/mabi.200600003. [DOI] [PubMed] [Google Scholar]
- 87.Uskoković V, Lee K, Lee PP, Fischer KE, Desai TA. Shape effect in the design of nanowire-coated microparticles as epithelial drug delivery devices. ACS Nano. 2012;6:7832. doi: 10.1021/nn3019865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van de, Ven AL, Kim P, Haley O, Fakhoury JR, Adriani G, Schmulen J, Moloney P, Hussain F, Ferrari M, Liu X, Yun SH, Decuzzi P. Rapid tumoritropic accumulation of system-ically injected plateloid particles and their biodistribution. J. Control. Release. 2012;58:148. doi: 10.1016/j.jconrel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Champion JA, Katare YK, Mitragotri S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release. 2007;121:3. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fischer KE, Nagaraj G, Daniels RH, Li E, Cowles VE, Miller JL, Bunger MD, Desai T. Hierarchical nanoengineered surfaces for enchanced CytoAdhesion and drug delivery. Biomaterials. 2011;32:3499. doi: 10.1016/j.biomaterials.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fischer KE, Jayagopal A, Nagaraj G G, Daniels R, Li E, Silvestrini M, Desai T. Nanoengineered surfaces enhance drug loading and adhesion. Nano Lett. 2011;11:1076. doi: 10.1021/nl103951e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu X, Gao X. Multilayer coating of gold nanorods for combined stability and biocompatibility. Phys. Chem. Chem. Phys. 2011;13:10028. doi: 10.1039/c0cp02434a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA. 2008;105:11613. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van der Niet MJTC, den Dunnen A, Koper MTM, Juurlink LBF. Tuning hydrophobicity of platinum by small changes in surface morphology. Phys. Rev. Lett. 2011;107:146103. doi: 10.1103/PhysRevLett.107.146103. [DOI] [PubMed] [Google Scholar]
- 95.Checco A, Hoffmann T, DiMasi E, Black CT, Ocko BM. Morphology of air nanobubbles trapped at hydrophobic nanopat-terned surfaces. Nano Lett. 2010;10:1354. doi: 10.1021/nl9042246. [DOI] [PubMed] [Google Scholar]
- 96.Uskoković V. Insights into morphological nature of precipitation of cholesterol. Steroids. 2008;73:356. doi: 10.1016/j.steroids.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 97.Sera Y, Kang KY. Asbestos and cancer in the Sennan district of Osaka. Tohoku J. Exp Med. 1981;133:313. doi: 10.1620/tjem.133.313. [DOI] [PubMed] [Google Scholar]
- 98.Okada S, Ito H, Nagai A, Komotori J, Imai H. Adhesion of osteoblast-like cells on nanostructured hydroxyapatite. Acta Biomater. 2010;6:591. doi: 10.1016/j.actbio.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 99.Safi M, Yan M, Guedeau-Boudeville MA, Conjeaud H, Garnier-Thibaud V, Boggetto N, Baeza-Squiban A, Niedergang F, Averbeck D, Berret JF. Interactions between magnetic nanowires and living cells: Uptake, toxicity, and degradation. ACS Nano. 2011;5:5354. doi: 10.1021/nn201121e. [DOI] [PubMed] [Google Scholar]
- 100.Uskoković V, Desai TA. Phase composition control of calcium phosphate nanoparticles for tunable drug delivery kinetics and treatment of osteomyelitis. Part 2: Antibacterial and osteoblastic response. J. Biomed. Mater. Res. A. 2013;24:343. doi: 10.1002/jbm.a.34437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gower LB. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 2008;108:4551. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu Z, Tsai M, Lu D, Wang J, Wientjes MG, Au JL. Tumor-penetrating microparticles for intraperitoneal therapy of ovarian cancer. J. Pharmacol. Exp. Ther. 2008;327:673. doi: 10.1124/jpet.108.140095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lim E, Huh Y, Yang J, Lee K, Suh J, Haam S. pH-triggered drug-releasing magnetic nanoparticles for cancer therapy guided by molecular imaging by MRI. Adv. Mater. 2011;23:2436. doi: 10.1002/adma.201100351. [DOI] [PubMed] [Google Scholar]
- 104.He L, Zuo Q, Xie S, Huang Y, Xue W. Intelligent hydrogels for drug delivery system. Recent Pat. Drug Deliv. Formul. 2011;5:265. doi: 10.2174/187221111797200533. [DOI] [PubMed] [Google Scholar]
- 105.Liu T, Liu K, Liu D, Chen S, Chen I. Temperature-sensitive nanocapsules for controlled drug release caused by magnetically triggered structural disruption. Adv. Function. Mater. 2009;19:616. [Google Scholar]
- 106.Kojima C. Design of stimuli-responsive dendrimers. Expert Opin. Drug Deliv. 2010;7:307. doi: 10.1517/17425240903530651. [DOI] [PubMed] [Google Scholar]
- 107.Tinkov S, Bekeredjian R, Winter G, Coester C. Microbubbles as ultrasound triggered drug carriers of pharmaceutical. Science. 2009;98:1935. doi: 10.1002/jps.21571. [DOI] [PubMed] [Google Scholar]
- 108.Volodkin DV, Madaboosi N, Blacklock J, Skirtach AG, Mohwald H. Surface-supported multilayers decorated with bio-active materials aimed at light-triggered drug delivery. Langmuir. 2009;25:14037. doi: 10.1021/la9015433. [DOI] [PubMed] [Google Scholar]
- 109.Wells LA, Lasowski F, Fitzpatrick SD, Sheardown H. Responding to change: Thermo- and photo-responsive polymers as unique biomaterials. Crit. Rev. Biomed. Eng. 2010;38:487. doi: 10.1615/critrevbiomedeng.v38.i6.10. [DOI] [PubMed] [Google Scholar]
- 110.Sokolova V, Radtke I, Heumann R, Epple M. Effective trans-fection of cells with multi-shell calcium phosphate-DNA nanopar-ticles. Biomater. 2006;27:3147. doi: 10.1016/j.biomaterials.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 111.Uskoković V. Co-creation of experiential qualities. Pragmat. Cogn. 2011;19:562. [Google Scholar]
- 112.Aarts HJM, Guerra B, Malorny B. Molecular methods for detection of antibiotic resistance. In: Aarestrup FM, editor. Antimicrobial Resistance in Bacteria of Animal Origin. Washington, DC: American Society for Microbiology Press; 2006. p. 55. [Google Scholar]
- 113.Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin. Cancer Biol. 2008;18:311. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Popp JR, Laflin KE, Love BJ, Goldstein AS. In vitro evaluation of osteoblastic differentiation on amorphous calcium phosphate-decorated poly(lactic-co-glycolic acid) scaffolds. J. Tissue Eng. Regen. Med. 2011;5:780. doi: 10.1002/term.376. [DOI] [PubMed] [Google Scholar]
- 115.Both SK, Yuan H, van Blitterswijk CA, de Boer J. Positive effect of calcium phosphate ceramics on osteogenic differentiation of embryonic stem cells by influencing the culture medium composition. Acta Biomater. 2012 under review. [Google Scholar]
- 116.Wang C, Duan Y, Marković B, Barbara J, Howlett CR, Zhang X, Zreigat H. Phenotypic expression of bone-related genes in osteoblasts grown on calcium phosphate ceramics with different phase compositions. Biomater. 2004;25:2507. doi: 10.1016/j.biomaterials.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 117.Yuan H, Fernands H, Habibovic P, de Boer J, Barradas AMC, de Ruiter A, Walsh WR, van Blitterswijk CA, de Bruijn JD. Osteoinductive ceramics as a synthetic alternative to autol-ogous bone grafting. PNAS. 2010;107:13614. doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ripamonti U. Smart biomaterials with intrinsic osteoinductivity: Geometric control of bone differentiation. In: Davies JE, editor. Bone Engineering. Toronto: EM2 Corporation; 2000. pp. 215–222. [Google Scholar]
- 119.Ripamonti U, Crooks J, Kirkbride AN. Sintered porous hydroxyapatites with intrinsic osteoinductive activity: Geometric induction of bone formation. S. Afr. J. Sci. 1999;95:335. [Google Scholar]
- 120.Uskoković V. Prospects and pits on the path of biomimetics: The case of tooth enamel. J. Biomim. Biomater. Tissue Eng. 2010;8:45. doi: 10.4028/www.scientific.net/JBBTE.8.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Quintilliani R. Pharmacokinetics/pharmacodynamics for critical care clinicians. Crit. Care Clin. 2008;24:335. doi: 10.1016/j.ccc.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 122.Ellis SL, Grassinger J, Jones A, Borg J, Camenisch T, Haylock D, Bertoncello I, Nilsson SK. The relationship between bone, hemopoietic stem cells, and vasculature. Blood. 2011;118:1516. doi: 10.1182/blood-2010-08-303800. [DOI] [PubMed] [Google Scholar]
- 123.Uskoković V, Košak A, Drofenik M, Preparation of silica-coated Lanthanum-Strontium manganite particles with designable curie point. for application in hyperthermia treatments. Int. J. Appl. Ceram. Tech. 2006;3:134. [Google Scholar]
- 124.Williams DF. The Williams Dictionary of Biomaterials. Liverpool: Liverpool University Press; 1999. [Google Scholar]
- 125.Ahmed A, Bonner C, Desai TA. Bioadhesive microdevices with multiple reservoirs: A new platform for oral drug delivery. J. Control. Release. 2002;81:291. doi: 10.1016/s0168-3659(02)00074-3. [DOI] [PubMed] [Google Scholar]
- 126.Tao SL, Desai TA. Microfabricated drug delivery systems: From particles to pores. Adv. Drug Deliv. Rev. 2003;55:315. doi: 10.1016/s0169-409x(02)00227-2. [DOI] [PubMed] [Google Scholar]
- 127.Vukomanović M, Šarcev I, Petronijević B, Škapin SD, Ignjatović N, Uskoković D. Poly(D,L-lactide-co-glycolide)/hydroxyapatite core-shell nanospheres, Part 4: A change of the surface properties during degradation process and the corresponding in vitro cellular response. Coll. Surf. B: Biointer. 2012;91:144. doi: 10.1016/j.colsurfb.2011.10.049. [DOI] [PubMed] [Google Scholar]
- 128.Mitranić I, Stevanović M, Nedeljković B, Ignjatović N, Uskoković D. Controllable synthesis of horseradish peroxidase loaded Poly(D,L-lactide) nanospheres. J. Bionanosci. 2009;3:22. [Google Scholar]
- 129.Liu Y, Sun J, Zhang P, He Z. Amphiphilic polysaccharide-hydrophobicized graft polymeric micelles for drug delivery nanosystems. Curr. Med. Chem. 2011;18:2638. doi: 10.2174/092986711795933696. [DOI] [PubMed] [Google Scholar]
- 130.Sinico C, Fadda AM. Vesicular carriers for dermal drug delivery. Expert Opin. Drug Deliv. 2009;6:813. doi: 10.1517/17425240903071029. [DOI] [PubMed] [Google Scholar]
- 131.Connor E, Mwamuka J, Gole A, Murphy C, Wyatt M. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 132.Jacobs DR, Gross MD, Tapsell LC., Jr Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009;89:1543S. doi: 10.3945/ajcn.2009.26736B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Uskoković V. On holism and the contextual character of natural qualities. World Futures: J. Gen. Evol. 2012;68:406. [Google Scholar]
- 134.Bernards DA, Desai TA. Nanotemplating of biodegradable polymer membranes for constant-rate drug delivery. Adv. Mater. 2010;22:2358. doi: 10.1002/adma.200903439. [DOI] [PubMed] [Google Scholar]
- 135.Guczi L, Horvath D, Paszti Z, Peto G. Effect of treatments on gold nanoparticles: Relation between morphology, electron structure and catalytic activity in CO oxidation. Catalysis Today. 2002;72:101. [Google Scholar]
- 136.Müller B. Tailoring biocompatibility: Benefitting patients. Mater. Today. 2010;13:58. [Google Scholar]
- 137.Guo J, Morris JR, Ihm Y, Contescu CI, Gallego NC, Duscher G, Pennycook SJ, Chisholm MF. Topological defects: Origin of nanopores and enhanced adsorption performance in nanoporous carbon. Small. 2012;8:3283. doi: 10.1002/smll.201200894. [DOI] [PubMed] [Google Scholar]
- 138.Beer S. On the nature of models: Let us now praise famous men and women too (from Warren McCulloch to Candace Pert) Inform. Sci. 1999;2:69. [Google Scholar]
- 139.Mistry AS, Mikos AG. Tissue engineering strategies for bone regeneration. Adv. Biochem. Engin./Biotech. 2005;94:1. doi: 10.1007/b99997. [DOI] [PubMed] [Google Scholar]
- 140.Walcott S, Sun SX. A mechanical model of actin stress fiber formation and substrate elasticity sensing in adherent cells. Proc. Natl. Acad. Sci. USA. 2010;107:7757. doi: 10.1073/pnas.0912739107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guo WH, Frey MT, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J. 2006;90:2213. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Noorden RV. Science publishing: The trouble with retractions. Nature. 2011;478:26. doi: 10.1038/478026a. [DOI] [PubMed] [Google Scholar]
- 143.Uskoković V. Challenges for the modern science in its descend towards nano scale. Curr. Nanosci. 2009;5:372. doi: 10.2174/157341309788921381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Meyer JC, Kisielowski C, Erni R, Rossell MD, Crommie MF, Zettl A. Direct imaging of lattice atoms and topo-logical defects in graphene membranes. Nano Lett. 2008;8:3582. doi: 10.1021/nl801386m. [DOI] [PubMed] [Google Scholar]
- 145.Dasgupta NP, Jung HJ, Trejo O, McDowell MT, Hryciw A, Brongersma M, Sinclair R, Prinz FB. Atomic layer deposition of lead sulfide quantum dots on nanowire surfaces. Nano Lett. 2011;11:934. doi: 10.1021/nl103001h. [DOI] [PubMed] [Google Scholar]
- 146.Melikhov IV, Lazic S, Vuković Ž. The effect of dissolved impurity on calcium phosphate nucleation in supersaturated medium. J. Coll. Interface Sci. 1989;127:317. [Google Scholar]
- 147.Ball P. Does hot water freeze first? Phys. World. 2006;19:19. [Google Scholar]
- 148.Malaiya A, Vyas SP. Preparation and characterization of indomethacin magnetic nanoparticles. J. Microencapsul. 1988;5:243. doi: 10.3109/02652048809064169. [DOI] [PubMed] [Google Scholar]
- 149.Menzinger M, Jankowski P. Heterogeneities and stirring effects in the Belusov-Zhabotinskii reaction. J. Phys. Chem. 1986;90:1217. [Google Scholar]
- 150.Debuigne F, Jeunieau L, Wiame M, Nagy JB. Synthesis of organic nanoparticles in different W/O microemulsions. Langmuir. 2000;16:7605. [Google Scholar]
- 151.Madsen HEL. Influence of magnetic field on the precipitation of some inorganic salts. J. Crystal Growth. 1995;152:94. [Google Scholar]
- 152.Reitas AMBF, Andgraf FJGL, Yvlt JN, Iuletti MG. Influence of magnetic field in the kinetics of crystallization of diamagnetic and paramagnetic inorganic salts. Crystal Res. Tech. 1999;34:1239. [Google Scholar]
- 153.Uskoković V. Composites comprising cholesterol and car-boxymethyl cellulose. Coll. Surf. B: Biointer. 2008;61:250. doi: 10.1016/j.colsurfb.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 154.Bunker CE, Harruff BA, Pathak P, Payzant A, Allard LF, Sun YP. Formation of cadmium sulfide nanoparticles in reverse micelles: Extreme sensitivity to preparation procedure. Langmuir. 2004;20:5642. doi: 10.1021/la049607+. [DOI] [PubMed] [Google Scholar]
- 155.Hou W-T, Feng Q-L. Morphologies and growth model of biomimetic fabricated calcite crystals using amino acids and insoluble matrix membranes of mytilus edulis. Crystal Growth Design. 2006;6:1086. [Google Scholar]
- 156.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA. 2010;107:4872. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Butcher DT, Alliston T, Weaver VM. A tense situation: Forcing tumour progression. Nature Rev. Cancer. 2009;9:108. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sohn K, Kim F, Pradel KC, Wu J, Peng Y, Zhou F, Huang J. Construction of evolutionary tree for morphological engineering of nanoparticles. ACS Nano. 2009;3:2191. doi: 10.1021/nn900521u. [DOI] [PubMed] [Google Scholar]
- 159.Kosmulski M. Surfactant Science Series. Vol. 145 Boca Raton: CRC Press, Francis & Taylor; 2009. Surface charging and points of zero charge. [Google Scholar]
- 160.Walsh D, Lebeau B, Mann S. Morphosynthesis of calcium carbonate (Vaterite) microsponges. Adv. Mater. 1999;11:324. [Google Scholar]
- 161.Ninham B. Self-Assembly—Some Thoughts. In: Robinson BH, editor. Self-Assembly. Amsterdam: IOS Press; 2003. [Google Scholar]
- 162.Wiedemann-Bidlack FB, Kwak SY, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. Effects of phosphorylation on the self-assembly of native full-length porcine amelogenin and its regulation of calcium phosphate formation in vitro . J. Struct. Biol. 2011;173:250. doi: 10.1016/j.jsb.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sahasrabuddhe AA, Gaikwad SM, Krishnasastry MV, Khan MI. Studies on recombinant single chain Jacalin lectin reveal reduced affinity for saccharides despite normal folding like native Jacalin. Protein Sci. 2004;13:3264. doi: 10.1110/ps.04968804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baneyx F, Chu D, Zhu W, Kitayaporn S, Schwartz D, Murali-Krishna K, Kavanagh T. Biomineralization and size control of calcium phosphate core protein shell nanoparticles: Potential for vaccine applications. Bioconj. Chem. 2012;23:610. doi: 10.1021/bc200654v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kitchens K, Kolhatkar R, Swaan P, Eddington N, Ghandehari H. Transport of poly(amidoamine) dendrimers across caco-2 cell monolayers: Influence of size, charge and fluorescent labeling. Pharm. Res. 2006;23:2818. doi: 10.1007/s11095-006-9122-2. [DOI] [PubMed] [Google Scholar]
- 166.Lacruz RS, Hacia JG, Bromage TG, Boyde A, Lei Y, Xu Y, Miller JD, Paine ML, Snead ML. The circadian clock modulates enamel development. J. Biol. Rhythms. 2012;27:237. doi: 10.1177/0748730412442830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Eiden-Assmann S, Viertelhaus M, Heiss A, Hoetzer KA, Felsche J. The influence of amino acids on the biomineralization of hydroxyapatite in gelatin. J. Inorg. Biochem. 2002;91:481. doi: 10.1016/s0162-0134(02)00481-6. [DOI] [PubMed] [Google Scholar]
- 168.Bastos IN, Platt GM, Andrade MC, Soares GD. Theoretical study of Tris and Bistris effects on simulated body fluids. J. Mol. Liq. 2008;139:121. [Google Scholar]
- 169.Serro AP, Saramago B. Influence of sterilization on the mineralization of Ti implants induced by incubation in various biological model fluids. Biomater. 2003;24:4749. doi: 10.1016/s0142-9612(03)00372-7. [DOI] [PubMed] [Google Scholar]
- 170.Nouri A, Castro R, Santos JL, Fernandes C, Rodrigues J, Tomás H. Calcium phosphate-mediated gene delivery using simulated body fluid (SBF) Int. J. Pharm. 2012;434:199. doi: 10.1016/j.ijpharm.2012.05.066. [DOI] [PubMed] [Google Scholar]
- 171.Eagle H. Buffer combinations for mammalian culture. Science. 1971;174:500. doi: 10.1126/science.174.4008.500. [DOI] [PubMed] [Google Scholar]
- 172.Good NE, Winget GO, Winter W, Connolly TN, Izawa S, Singh RMM. Hydrogen ion buffers for biological research. Biochem. 1966;5:467. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- 173.Swim HE, Parker RF. Non-bicarbonate buffers in cell culture media. Science. 1955;122:466. doi: 10.1126/science.122.3167.466. [DOI] [PubMed] [Google Scholar]
- 174.Muralithran G, Ramesh S. The effects of sintering temperature on the properties of hydroxyapatite. Ceram. Int. 2000;26:221. [Google Scholar]
- 175.Wu W-Y, Kung W-Y, Ting J-M. Effect of pH values on the morphology of zinc oxide nanostructures and their photolumines-cence spectra. J. Am. Ceram. Soc. 2011;94:699. [Google Scholar]
- 176.Wang H, Lee J-K, Moursi A, Lannutti JL. Ca/P ratio effects on the degradation of hydroxyapatite in vitro . J. Biomed. Mater. Res. 2003;67A:599. doi: 10.1002/jbm.a.10538. [DOI] [PubMed] [Google Scholar]
- 177.Konstanchuk I. Reactivity of nanocrystalline magnesium. In: Uskoković DP, editor. YUCO-MAT: Programme and the Book of Abstracts. Belgrade: Materials Research Society of Serbia; 2011. [Google Scholar]
- 178.Uskoković V, Drofenik M. Synthesis of nanocrystalline nickel-zinc ferrites via a microemulsion route. Mater. Sci. Forum. 2004;453-4:225. [Google Scholar]
- 179.Naughton BT, Clarke DR. Lattice expansion and saturation magnetization of nickel-zinc ferrite nanoparticles prepared by aqueous precipitation. J. Am. Ceram. Soc. 2007;90:3541. [Google Scholar]
- 180.Goldman A. Modern Ferrite Technology. 2nd. New York: Springer; 2006. p. 57. [Google Scholar]
- 181.Kornberg A. Of Serendipity and Science. 2009 Retrieved from http://www.rockefeller.edu/pubinfo/Pasteur/Kornberg_essay.html.
- 182.Banaszek A. Company profits from side effects of glaucoma treatment. CMAJ. 2011;183:E1058. doi: 10.1503/cmaj.109-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Roberts RM. Serendipity: Accidental Discoveries on Science. New York: Wiley; 1989. [Google Scholar]
- 184.Ball P. Chancing upon chemical wonders. Chem. World. 2006;3:32. [Google Scholar]
- 185.Uskoković V, Bertassoni LE. Nanotechnology in dental sciences: Moving towards a finer way of doing dentistry. Materials. 2010;3:1674. doi: 10.3390/ma3031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Uskoković V, Ševkušić M, Uskoković DP. Strategies for the scientific progress of the developing countries in the new millennium: The case of serbia in comparison with slovenia and South Korea. Sci. Techn. Innov. Studies. 2010;6:33. [Google Scholar]
- 187.Uskoković V, Uskoković DP. Extrapolating strategies for the scientific and technological development of underdeveloped societies from the examples of South Korea, Slovenia and Serbia. Int. J. Tech. Manag. Sustain. Dev. 2011;10:125. [Google Scholar]
- 188.Barth JV, Costantini G, Kern K. Engineering atomic and molecular nanostructures at surfaces. Nature. 2005;437:671. doi: 10.1038/nature04166. [DOI] [PubMed] [Google Scholar]
- 189.Hobbs RG, Petkov N, Holmes JD. Semiconductor nanowire fabrication by bottom-up and top-down paradigms. Chem. Mater. 2012;24:1975. [Google Scholar]
- 190.Baum R. Nanotechnology: Drexler and smalley make the case for and against ‘molecular assemblers,’. Chem. Engin. News. 2003;81:37. [Google Scholar]
- 191.Smalley RE. Of chemistry love and nanobots. Sci. Am. 2001;285:76. doi: 10.1038/scientificamerican0901-76. [DOI] [PubMed] [Google Scholar]
- 192.Dolberg DS, Bissell MJ. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309:552. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- 193.Meda P, Bosco D, Chanson M, Giordano E, Vallar L, Wollheim C, Orci L. Rapid and reversible secretion changes during uncoupling of rat insulin-producing cells. J. Clin. Invest. 1990;83:759. doi: 10.1172/JCI114772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Brereton HC, Carvell MJ, Asare-Anane H, Roberts G, Christie MR, Persaud SJ, Jones PM. Homotypic cell contact enhances insulin but not glucagon secretion. Biochem. Biophys. Res. Commun. 2006;344:995. doi: 10.1016/j.bbrc.2006.03.214. [DOI] [PubMed] [Google Scholar]
- 195.Uskoković V. Isn’t self-assembly a misnomer? multi-disciplinary arguments in favor of co-assembly. Adv. Colloid Interface Sci. 2008;141:37. doi: 10.1016/j.cis.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 196.Uskoković V. On the relational character of mind and nature. Res Cogitans: J. Phil. 2009;6:286. [Google Scholar]
- 197.Uskoković V. On the light doves and learning on mistakes. Axiomathes: Int. J. Ontol. Cogn. Syst. 2009;19:17. [Google Scholar]
- 198.Uskoković V, Drofenik M. Synthesis of materials within reverse micelles. Surf. Rev. Lett. 2005;12:239. [Google Scholar]
- 199.Uskoković V, Drofenik M. Reverse micelles: Inert nano-reactors or physico-chemically active guides of the capped reactions. Adv. Colloid Interface Sci. 2007;133:23. doi: 10.1016/j.cis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 200.Uskoković V, Drofenik M. A mechanism for theformation of nanostructured NiZn ferrites via a microemulsion-assisted precipitation method. Colloids Surf. A: Physicochem. Eng. Asp. 2005;266:168. [Google Scholar]
- 201.Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nature Nanotech. 2012;7:779. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 202.Prapainop K, Witter DP, Wentworth P., Jr A chemical approach for cell-specific targeting of nanomaterials: Small-molecule-initiated misfolding of nanoparticle corona proteins. J. Am. Chem. Soc. 2012;134:4100. doi: 10.1021/ja300537u. [DOI] [PubMed] [Google Scholar]
- 203.Tarasevich BJ, Howard CJ, Larson JL, Snead ML, Simmer JP, Paine M, Shaw WJ. The nucleation and growth of calcium phosphate by amelogenin. J. Crystal Growth. 2007;304:407. doi: 10.1016/j.jcrysgro.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.George A, Veis A. Phosphorylated proteins and control over apatite nucleation, crystal growth and inhibition. Chem. Rev. 2008;108:4670. doi: 10.1021/cr0782729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hunter GK, O’Young J, Grohe B, Karttunen M, Goldberg HA. The flexible polyelectrolyte hypothesis of protein-biomineral interaction. Langmuir. 2010;26:18639. doi: 10.1021/la100401r. [DOI] [PubMed] [Google Scholar]
- 206.Valdez D, Le Huerou JY, Gindre M, Urbach W, Waks M. Hydration and protein folding in water and in reverse micelles: Compressibility and volume changes. Biophys. J. 2001;80:2751. doi: 10.1016/S0006-3495(01)76243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Moilanen DE, Spry DB, Levinger NE, Fayer MD. Confinement or properties of the interface? dynamics of nanoscopic water in reverse micelles. J. Am. Chem. Soc. 2007;129:14311. doi: 10.1021/ja073977d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Milne AA. Winnie-the-Pooh. New York: Penguin; 1926. [Google Scholar]