Abstract
Background and purpose
For prosthetic joint-associated infection (PJI), a regimen of debridement, antibiotics, irrigation, and retention of the prosthesis (DAIR) is generally accepted for acute infections. Various risk factors associated with treatment success have been described. The use of local antibiotic carriers (beads and sponges) is relatively unknown. We retrospectively analyzed risk factors in a cohort of patients from 3 hospitals, treated with DAIR for PJI.
Patients and methods
91 patients treated with DAIR for hip or knee PJI in 3 Dutch centers between 2004 and 2009 were retrospectively evaluated. The mean follow-up was 3 years. Treatment success was defined as absence of infection after 2 years, with retention of the prosthesis and without the use of suppressive antibiotics.
Results
60 patients (66%) were free of infection at follow-up. Factors associated with treatment failure were: a history of rheumatoid arthritis, late infection (> 2 years after arthroplasty), ESR at presentation above 60 mm/h, and infection caused by coagulase-negative Staphylococcus. Symptom duration of less than 1 week was associated with treatment success. The use of gentamicin sponges was statistically significantly higher in the success group, and the use of beads was higher in the failure group in the univariate analysis, but these differences did not reach significance in the logistic regression analysis. Less surgical procedures were performed in the group treated with sponges than in the group treated with beads.
Interpretation
In the presence of rheumatoid arthritis, duration of symptoms of more than 1 week, ESR above 60 mm/h, late infection (> 2 years after arthroplasty), and coagulase-negative Staphylococcus PJI, the chances of successful DAIR treatment decrease, and other treatment methods should be considered.
Prosthetic joint-associated infection (PJI) occurs in around 1–2% of primary total hip arthroplasties (THAs) and total knee arthroplasties (TKAs) (Trampuz and Zimmerli 2005, Kurtz et al. 2008, Del Pozo and Patel 2009). Infected artificial joints are often unresponsive to antibiotic treatment, due to poor vascular supply and biofilm formation. Generally, PJIs are classified in 3 groups, based on duration of symptoms and time after surgery: (I) early postoperative: symptoms less than 4 weeks after surgery; (II) late chronic: a gradual, indolent onset of symptoms; or (III) acute hematogenous: acute onset in a previously well-functioning prosthesis (Tsukayama et al. 1996, Toms et al. 2006). A similar classification describes early (3 months), delayed/low-grade (3–24 months), and late infection (> 24 months) (Trampuz and Zimmerli 2005).
Various risk factors have been described that are associated with occurrence of PJI, such as rheumatoid arthritis, diabetes mellitus, malignancy, obesity, and use of immunosuppressant drugs (Choong et al. 2007, Bongartz et al. 2008, Jamsen et al. 2009, Azzam et al. 2010, Lora-Tamayo et al. 2013). Revision surgery also increases the risk of PJI (Bongartz et al. 2008, Byren et al. 2009, Jamsen et al. 2009). Factors that have been associated with a worse outcome of PJI treatment include: infections caused by Staphylococcus spp. (Azzam et al. 2010), and more specifically by Staphylococcus aureus (Marculescu et al. 2006, Soriano et al. 2006, Byren et al. 2009, Cobo et al. 2011), polymicrobial PJI (Lora-Tamayo et al. 2013), intra-articular purulence (Azzam et al. 2010), retention of exchangeable components (Lora-Tamayo et al. 2013), and longer time between initial arthroplasty and PJI diagnosis (Brandt et al. 1997, Barberan et al. 2006, Marculescu et al. 2006, Lora-Tamayo et al. 2013).
Most PJIs are caused by coagulase-negative Staphylococcus (30–41%) and S. aureus (12–47%). Streptococcus spp. and Enterococcus spp. are less common causes, both at around 10% of the total, as are gram-negative bacteria such as Escherichia coli (< 5%) (Moran et al. 2007, Sharma et al. 2008, Byren et al. 2009). A prevalence of 5–39% has been described for polymicrobial infections (Moran et al. 2007, Azzam et al. 2010, Cobo et al. 2011, Westberg et al. 2012, Lora-Tamayo et al. 2013).
A regimen of debridement, antibiotics, irrigation, and retention of the prosthesis (DAIR) is generally accepted for acute infections without complicating factors such as significant comorbidity or loosening of the prosthesis. DAIR has shown varying success rates: as low as 14% (Crockarell et al. 1998) and as high as 100% (Zimmerli et al. 1998). Success can be achieved in over 70% of the cases when patients with favorable factors are selected, such as those with short duration of symptoms (less than 3–4 weeks), a stable implant, and healthy soft tissues surrounding the prosthesis (Soriano et al. 2006, Byren et al. 2009, Vilchez et al. 2011, Sukeik et al. 2012, Osmon et al. 2013). In the case of chronic infections, implant retention is rarely successful. Implant removal leaves the patient disabled for weeks or even months (Osmon et al. 2013).
Local antibiotic treatment, with aminoglycosides in beads or sponges, could theoretically reach high local concentrations without exposing the patient to toxic serum levels. Beads have a prolonged release compared to sponges but do not reach such high concentrations (Diefenbeck et al. 2006). These can also act as foreign bodies, to which bacteria might adhere (Barth et al. 2011). We evaluated the outcome of DAIR for total hip and knee PJI in 3 Dutch hospitals, to study factors associated with successful outcome and to study the outcomes of the use of local antibiotic carriers.
Patients and methods
Study design
This was a retrospective cohort study, with a follow-up of at least 2 years or until the patient died. Prosthetic joint-associated infection was defined according to Crockarell et al. (1998), and required 1 or more of the following criteria:
(I) growth of the same microorganism in at least 2 culture specimens (preoperative joint aspiration and/or intraoperative, intracapsular specimen); (II) 1 positive culture, and intracapsular purulence during debridement procedure, acute inflammation on histopathological examination of intraoperative specimen, and/or an actively draining sinus tract; (III) culture-negative infection: negative culture results and at least 2 of intracapsular purulence during debridement procedure, acute inflammation on histopathological examination of intraoperative specimen, and an actively draining sinus tract.
The study population consisted of 91 patients who were treated with DAIR for PJI of total hip arthroplasty (THA) or total knee arthroplasty (TKA) at 3 Dutch hospitals between January 2004 and December 2009. 34 of the patients with PJI of the hip have already been described (Kuiper et al. 2013) and they were also included.
Treatment
The decision for or against DAIR treatment was made by the treating surgeon, in consultation with the orthopedic team. It was based on clinical signs and symptoms, type of infection, and absence of radiographic loosening. DAIR was repeated after 2 weeks if clinical symptoms and laboratory signs did not improve. The decision to remove the implant was made individually by the surgeon in consultation with the patient.
The decision to use local antibiotic carriers was made by the treating surgeon. Carriers were either gentamicin beads (Septopal; gentamicin sulfate in polymethylmethacrylate, 225 mg per chain; Biomet, Germany) or gentamicin sponges (Garacol; gentamicin sulfate in equine collagen, 130 mg per sponge; EusaPharma, UK). Antibiotic therapy, based on bacterial susceptibility and in consultation with either an infectious diseases specialist or a medical microbiologist, was administered for at least 6 weeks.
The joint was opened through the old scar or wound, and after tissue collection for multiple cultures (at least 3) from synovium, capsule, and interfaces, was thoroughly debrided—including synovial resection. Exchangeable components were replaced in most cases, but this was not standard procedure. After debridement, the joint and wound were meticulously irrigated with saline using pulsed lavage, and primarily closed. No drains or vacuum systems were used. Removal of gentamicin beads was always combined with debridement, and was therefore counted as a procedure. Postoperatively, antibiotic treatment was started, either with a broad range agent such as vancomycin (Vancomycin; Xellia Pharmaceuticals ApS, Denmark), or—when the causative species was known—an agent based on susceptibility. A thromboprophylactic agent (Nadroparine) was administered during the hospital stay.
Outcome
A successful treatment outcome was defined as the absence of clinical and laboratory signs of inflammation (C-reactive protein blood serum levels of < 10 mg/L) at a follow-up of 2 years. Patients who required chronic, suppressive antibiotic treatment, who underwent prosthesis removal, or who died within this 2-year period were considered to be cases of treatment failure.
Statistics
The assumption of normality was checked by visual inspection of the histograms, q-q plots, and box plots of the data. A Kolmogorov-Smirnov test was also performed on the data. For continuous variables with a normal distribution, mean and standard deviation (SD) are given, whereas variables that were not normally distributed are given as median and interquartile range (IQR). To determine whether patients with successful treatment differed significantly from patients with unsuccessful treatment, independent t-tests were performed for continuous variables with normal distribution and the non-parametric Mann-Whitney U-test was used for continuous variables without normal distribution. For categorical variables, chi-square tests were performed for large groups and Fisher’s exact test was used for small groups. Variables that were statistically significantly different between success and failure groups were subsequently analyzed with logistic regression to correct for confounding. Kaplan-Meier analysis was used to describe the infection-free survival (with treatment failure as endpoint).
All statistical analyses were performed with IBM SPSS Statistics 20.0 and p-values < 0.05 were considered statistically significant.
Results
Population and patient characteristics
91 patients with prosthetic joint-associated infection (62 hips, 29 knees) were treated with DAIR, 60 of whom were free of infection without resection arthroplasty or use of suppressive antibiotics at follow-up: a 66% success rate. Factors analyzed for the success and failure groups are summarized in Table.
| Variable | Success (n = 60) |
Failure (n = 31) |
p-value (univariate) |
p-value (adjusted) |
Odds ratio |
|---|---|---|---|---|---|
| Demographics | |||||
| Mean age in years (SD a) | 70 (11) | 69 (13) | 0.7 | ||
| Female sex | 37 | 17 | 0.5 | ||
| Hip joint | 38 | 24 | 0.2 | ||
| Knee joint | 22 | 7 | 0.2 | ||
| Infection after revision surgery | 12 | 8 | 0.5 | ||
| Cemented arthroplasty | 22 | 20 | 0.01 | 0.7 | 0.3–7 |
| Comorbidities | |||||
| Cardiac | 31 | 16 | 1.0 | ||
| Pulmonary | 5 | 6 | 0.2 | ||
| Renal insufficiency | 6 | 0 | 0.09 | ||
| Diabetes | 7 | 6 | 0.4 | ||
| Rheumatoid arthritis | 3 | 7 | 0.03 | 0.03 | 1.2–84 |
| Malignancy | 4 | 3 | 0.7 | ||
| Clinical presentation | |||||
| Median duration of symptoms in days (IQR b) | 3 (5) | 6 (7) | 0.02 | ||
| Symptoms < 1 week | 44 | 16 | 0.02 | 0.05 | 1.0–18 |
| Symptoms < 3 weeks | 54 | 25 | 0.1 | ||
| Median time from arthroplasty to presentation | |||||
| in days (IQR) | 21 (51) | 42.5 (266) | 0.05 | ||
| Early infection (< 3 months) | 48 | 19 | 0.06 | ||
| Delayed infection (3–24 months) | 10 | 7 | 0.5 | ||
| Late infection (> 2 years) | 2 | 5 | 0.04 | 0.04 | 1.1–366 |
| Type 1 (early postoperative) | 38 | 14 | 0.2 | ||
| Type 2 (chronic) | 3 | 4 | 0.2 | ||
| Type 3 (acute hematogenous) | 19 | 13 | 0.3 | ||
| Mean CRP c at presentation, in mg/L (SD) | 164 (119) | 155 (118) | 0.7 | ||
| Mean ESR d at presentation, in mm/h (SD) | 54 (26) | 76 (27) | 0.002 | ||
| ESR > 60 mm/h | 19/47 | 20/24 | 0.001 | 0.005 f | 2.2–98 |
| Surgical treatment | |||||
| Mean number of procedures (SD) | 2.0 (0.8) | 2.4 (1.5) | 0.1 | ||
| Single DAIR procedure | 21 | 11 | 1.0 | ||
| Multiple DAIR procedures | 39 | 20 | 1.0 | ||
| Gentamicin sponges used | 48 | 16 | 0.005 | 0.4 | 0.05–3 |
| Gentamicin beads used | 4 | 8 | 0.02 | 1.0 | 0.06–18 |
| Sponges and beads used | 2 | 4 | 0.2 | ||
| No local antibiotic use | 6 | 3 | 1.0 | ||
| Microbiology (including polymicrobial infections) | |||||
| CNS e | 4 | 9 | 0.009 | 0.02 | 1.8–309 |
| Staphylococcus aureus | 34 | 16 | 0.6 | ||
| Streptococcus spp. | 10 | 1 | 0.09 | ||
| Escherichia coli | 3 | 1 | 1.0 | ||
| Enterobacter cloacae | 2 | 2 | 0.6 | ||
| Enterococcus faecalis | 5 | 0 | 0.2 | ||
| Other | 7 | 2 | 0.7 |
a SD: standard deviation.
b IQR: interquartile range.
c CRP: C-reactive protein.
d ESR: erythrocyte sedimentation rate.
e CNS: coagulase-negative Staphylococcus.
f ESR as a continuous value and ESR as a dichotomous value are the same variable: only the clinically more useful dichotomous ESR with a cutoff of 60 mm/h was analyzed by logistic regression.
16 patients died during follow-up. 9 had a follow-up of at least 2 years, 8 of whom were treated successfully and 1 of whom died 32 months after revision surgery. 7 patients died within 2 years of follow-up and they were considered treatment failures. 2 of these patients were free of symptoms when they died. 2 deaths were infection-related: 1 patient died of sepsis and 1 patient refused further treatment, both within 3 months of the start of symptoms (Figure 1). No other permanent complications were seen. 7 patients developed high creatinine levels during treatment, but renal function normalized in all 7 in the months that followed.
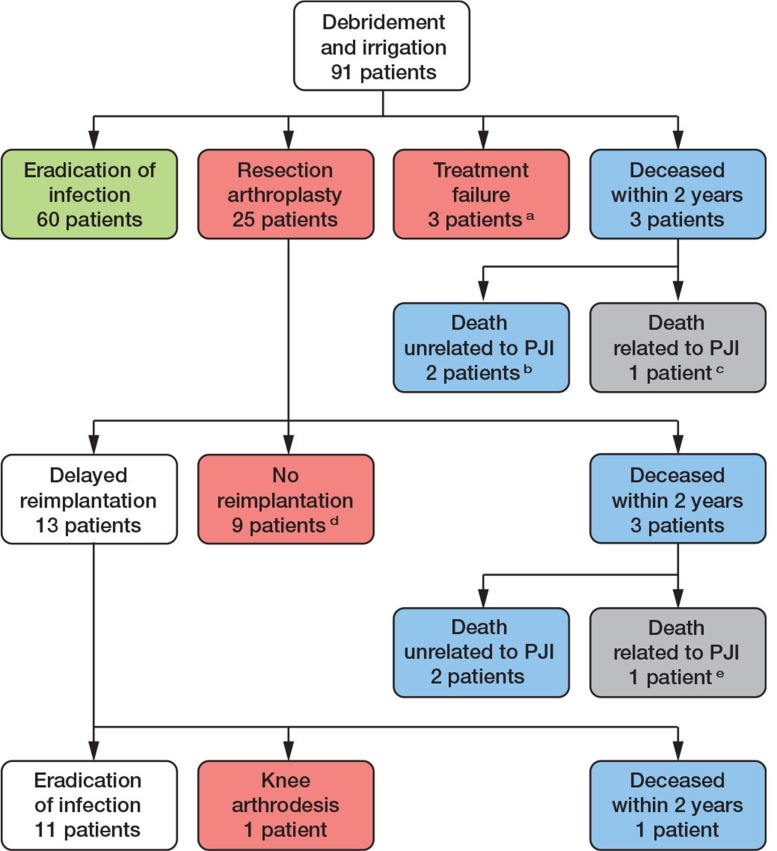
Figure 1.
Flow chart of surgical treatment of patients included in the study.
a 3 patients chronic lowgrade infection, of which 1 chronic use of antibiotics.
b 2 patients had no infectious symptoms (but were assigned to ‘failure’ as adequate follow-up was not possible).
c 1 patient refused further surgical and antibiotic treatment and died 19 days after DAIR procedure.
d 2 arthrodesis knee; 1 above knee amputation after failed arthrodesis; in 6 patients the resection situation was accepted.
e 1 patient died 14 days after prosthesis removal due to sepsis.
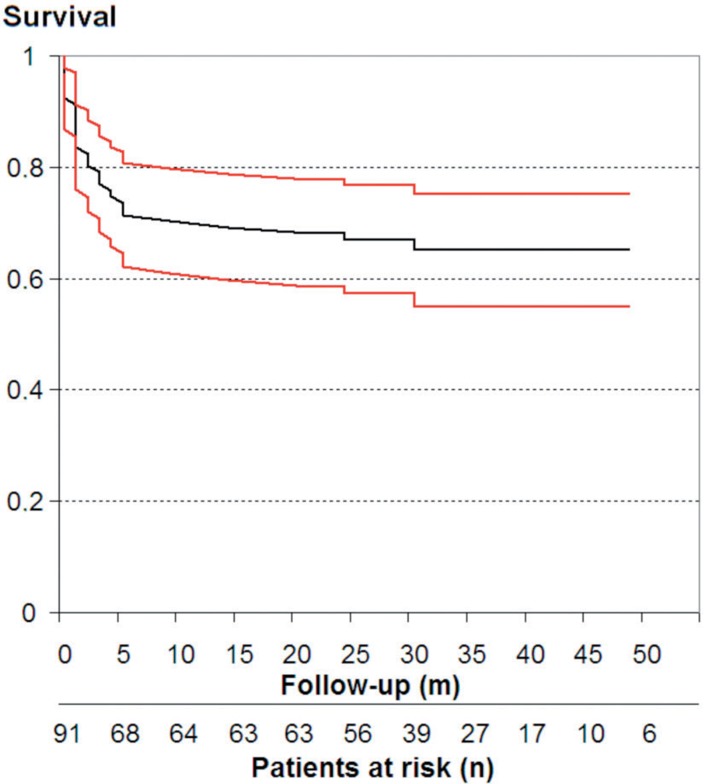
Mean duration of follow-up was 35 (0–79) months. See Figure 1 for a flow chart of surgical treatment in this study, and Figure 2 for infection-free survival.
Figure 2.
Kaplan-Meier survival analysis (patients free of infection) of 91 patients treated with debridement, antibiotics, irrigation, and retention (DAIR). Red lines show 95% confidence intervals.
Factors associated with outcome
In univariate analysis, 8 factors were statistically significantly associated with treatment failure (Table). After logistic regression analysis, 5 factors were associated with failure: rheumatoid arthritis, late infection, ESR at presentation above 60 mm/h, symptom duration of more than 7 days before the start of treatment, and PJI caused by coagulase-negative Staphylococcus (Table). Revision arthroplasty was not associated with either treatment failure or success.
Surgical treatment
The mean number of procedures was similar in successfully and unsuccessfully treated patients (Table). No difference in success rate was seen between patients who underwent 1 DAIR procedure and patients who underwent multiple procedures.
The use of gentamicin sponges was statistically significantly higher in the success group, and the use of beads was significantly higher in the failure group. These differences were not significant in the logistic regression analysis. The mean number of procedures was lower when sponges were used (2.0, SD 0.8) than when beads were used (2.8, SD 1.4) (p = 0.006).
Microbiology
Preoperative aspiration was performed in 65 patients, and a causative microorganism was found in 60 of them. Intraoperative samples were collected from all 91 cases, which yielded at least 1 positive result in 87 patients. For the other 4 patients, aspiration fluid yielded positive cultures. No culture-negative infections were seen.
Most infections were caused by S. aureus (Table). MRSA was responsible for only 2 cases of PJI, both of which were treated successfully. PJI caused by coagulase-negative Staphylococcus was associated with a low success rate and streptococcal infections were associated with a high success rate. All streptococcal infections were treated within 1 week of onset of symptoms. 5 patients had a polymicrobial prosthetic joint-associated infection; all were treated successfully.
Discussion
Demographics and comorbidity
Of the 91 patients included in this study, 60 were treated successfully with DAIR. Revision arthroplasty has been described by others as a risk factor for PJI (Bongartz et al. 2008, Byren et al. 2009, Jamsen et al. 2009), but it was not associated with treatment failure in this cohort. Of all comorbidities described, only rheumatoid arthritis was found to be associated with PJI; this was also found in 1 other study (Bongartz et al. 2008). Only 2 patients with rheumatoid arthritis were using immunosuppressive drugs (1 in the success group and 1 in the failure group).
Clinical presentation
In most cases (60/91), treatment was started within 1 week of onset of infectious symptoms, which had a better outcome. The treatment success with early infections and infections of short duration of symptoms is commonly attributed to lack of biofilm formation (Del Pozo and Patel 2009), and it is strongly recommended (Osmon et al. 2013) that DAIR should only be used for patients with a short duration of symptoms (less than 3 weeks) or time after initial arthroplasty of less than 30 days (Osmon et al. 2013). We found that having symptoms for less than 1 week was associated with treatment success.
Although usually discouraged, 7 patients with a duration of symptoms of more than 4 weeks were treated with DAIR. In all cases, the decision to use DAIR was made by the treating surgeon because of early PJI occurring within 3 months after initial surgery (Trampuz and Zimmerli 2005). We included these 7 patients nonetheless, because this might further identify factors associated with outcome. Duration of symptoms of more than 4 weeks was not a factor associated with treatment outcome.
An ESR at presentation of more than 60 mm/h was associated with treatment failure. A low ESR at presentation could indicate a shorter duration of infection, and might therefore be predictive of a higher chance of success. Other studies have focused on the ability of these blood infection markers (CRP and ESR) to establish a diagnosis of PJI (Greidanus et al. 2007, Muller et al. 2008), and 1 study found that high CRP was predictive of failure (Lora-Tamayo et al. 2013), but to our knowledge ESR has never been described as a factor associated with treatment outcome.
Surgical treatment
In staged revision, gentamicin-loaded beads are often used to fill the dead space after arthroplasty removal, but evidence on their effectiveness is limited (Diefenbeck et al. 2006, Barth et al. 2011). To our knowledge, their use in DAIR has never been studied. The use of gentamicin sponges in treatment of PJI has only been described in a few studies (Swieringa and Tulp 2005, Diefenbeck et al. 2006, Swieringa et al. 2008, Kuiper et al. 2013). The report by Kuiper et al. (2013) includes 34 of the patients included in the present study.
A higher success rate for sponges and a lower success rate for beads was found in univariate analysis, but this was not confirmed in multivariate analysis. The use of gentamicin sponges was associated with fewer procedures.
The collagen-based gentamicin sponges used are biodegradable and do not need removal surgery, as opposed to beads. Furthermore, sponges reach higher local antibiotic concentrations than beads (Diefenbeck et al. 2006), and it has been suggested that beads are themselves foreign bodies and therefore maintain the infection (Barth et al. 2011). Our cohort may have been too small to allow us to find statistically significant differences between the 2 antibiotic carriers, but selection bias must be considered as well: one might argue that beads were used in cases of severe infection, where additional debridement procedures were anticipated and needed.
Microbiology
We found that CNS infection was associated with treatment failure. Other authors have also described staphylococcal or, more specifically, S. aureus infection to have a higher risk of failure (Marculescu et al. 2006, Soriano et al. 2006, Byren et al. 2009, Azzam et al. 2010). Possible explanations for this phenomenon are the ability of the species to form a biofilm, and the virulence of the causative microorganism: coagulase-negative Staphylococcus is known to be of low virulence, which may delay and impede the diagnosis.
Treatment of streptococcal infections had a high success rate, and they were all treated within 1 week after symptoms became apparent. That this was not a significant factor may be explained by sample size, but the high success rate might also be explained by the short duration of symptoms. 1 study also found a correlation between streptococcal infections and good outcome (Meehan et al. 2003).
Only 5 of 91 patients had an infection caused by multiple microorganisms, and all were free of infection at follow-up. Some authors have also found relatively few polymicrobial infections, between 5 and 10% (Aboltins et al. 2007, Azzam et al. 2010), but others have described much higher rates of multi-organism PJI: between 19 and 39% (Moran et al. 2007, Cobo et al. 2011, Vilchez et al. 2011, Westberg et al. 2012, Lora-Tamayo et al. 2013). All of these studies used culture to identify microorganisms, and no DNA techniques. Whether this difference is a matter of culture method or whether some other (regional) factor might be involved remains uncertain. Only 1 study found higher failure for polymicrobial infection, with a hazard ratio of 1.8 (Lora-Tamayo et al. 2013).
Limitations
This was a retrospective study, with its inherent caveats for interpretation. The sample size may have been too small for identification of any weaker risk factors, and selection bias cannot be ruled out. Also, although comparable, the irrigation and debridement procedures and culture methods were not standardized in the different hospitals. The possible bias in the use of gentamicin beads and sponges has already been mentioned.
Conclusion
Several factors were associated with treatment failure: a history of rheumatoid arthritis, duration of symptoms of more than 1 week, late infection (more than 2 years after arthroplasty), ESR at presentation above 60 mm/h, and the presence of coagulase-negative Staphylococcus. When 1 or more of these factors is present in a patient, one should realize that the chances of successful DAIR treatment decrease. Furthermore, when local antibiotic carriers were used, gentamicin-loaded sponges—which do not require additional removal surgery—showed outcome results comparable to those with beads, but with fewer procedures. Prospective studies will be needed to evaluate their effect on PJI and biofilm formation.
Acknowledgments
All the authors contributed to interpretation of the data and to revision of the final manuscript. JK, SV, RS, HG, DV, and PN contributed to the conception and design of the study and to provision of the study patients. JK, SV, and RS collected data. JK wrote the protocol, analyzed data, and wrote the manuscript.
We thank Dr M.J.M. Hoozemans for his help with the statistical analysis.
No competing interests declared.
References
- Aboltins CA, Page MA, Buising KL, Jenney A WJ, Daffy JR, Choong P FM, Stanley PA. Treatment of staphylococcal prosthetic joint infections with debridement, prosthesis retention and oral rifampicin and fusidic acid . Clin Microbiol Infect. 2007;13(6):586–91. doi: 10.1111/j.1469-0691.2007.01691.x. [DOI] [PubMed] [Google Scholar]
- Azzam KA, Seeley M, Ghanem E, Austin MS, Purtill JJ, Parvizi J. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited . J Arthroplasty. 2010;25(7):1022–7. doi: 10.1016/j.arth.2010.01.104. [DOI] [PubMed] [Google Scholar]
- Barberan J, Aguilar L, Carroquino G, Gimenez MJ, Sanchez B, Martinez D, Prieto J. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients . Am J Med. 2006;119(11):993–1010. doi: 10.1016/j.amjmed.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Barth RE, Vogely HC, Hoepelman AI, Peters EJ. ‘To bead or not to bead?’ Treatment of osteomyelitis and prosthetic joint-associated infections with gentamicin bead chains . Int J Antimicrob Agents. 2011;38(5):371–5. doi: 10.1016/j.ijantimicag.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Bongartz T, Halligan CS, Osmon DR, Reinalda MS, Bamlet WR, Crowson CS, Hanssen AD, Matteson EL. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis . Arthritis Rheum. 2008;59(12):1713–20. doi: 10.1002/art.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt CM, Sistrunk WW, Duffy MC, Hanssen AD, Steckelberg JM, Ilstrup DM, Osmon DR. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention . Clin Infect Dis. 1997;24(5):914–9. doi: 10.1093/clinids/24.5.914. [DOI] [PubMed] [Google Scholar]
- Byren I, Bejon P, Atkins BL, Angus B, Masters S, McLardy-Smith P, Gundle R, Berendt A. One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome . J Antimicrob Chemother. 2009;63(6):1264–71. doi: 10.1093/jac/dkp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong P FM, Dowsey MM, Carr D, Daffy J, Stanley P. Risk factors associated with acute hip prosthetic joint infections and outcome of treatment with a rifampinbased regimen . Acta Orthop. 2007;78(6):755–65. doi: 10.1080/17453670710014527. [DOI] [PubMed] [Google Scholar]
- Cobo J, Miguel LG, Euba G, Rodriguez D, Garcia-Lechuz JM, Riera M, Falgueras L, Palomino J, Benito N, del Toro MD, Pigrau C, Ariza J. Early prosthetic joint infection: outcomes with debridement and implant retention followed by antibiotic therapy . Clin Microbiol Infect. 2011;17(11):1632–7. doi: 10.1111/j.1469-0691.2010.03333.x. [DOI] [PubMed] [Google Scholar]
- Crockarell JR, Hanssen AD, Osmon DR, Morrey BF. Treatment of infection with debridement and retention of the components following hip arthroplasty . J Bone Joint Surg (Am) 1998;80:9, 1306–13. doi: 10.2106/00004623-199809000-00009. [DOI] [PubMed] [Google Scholar]
- Del Pozo JL, Patel R. Infection Associated with Prosthetic Joints . N Engl J Med. 2009;361(8):787–94. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbeck M, Mückley T, Hofmann GO. Prophylaxis and treatment of implant-related infections by local application of antibiotics . Injury (Suppl 2) 2006;37:S95–104. doi: 10.1016/j.injury.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Greidanus NV, Masri BA, Garbuz DS, Wilson SD, McAlinden MG, Xu M, Duncan CP. Use of erythrocyte sedimentation rate and C-reactive protein level to diagnose infection before revision total knee arthroplasty. A prospective evaluation . J Bone Joint Surg (Am) 2007;89(7):1409–16. doi: 10.2106/JBJS.D.02602. [DOI] [PubMed] [Google Scholar]
- Jamsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases . J Bone Joint Surg (Am) 2009;91(1):38–47. doi: 10.2106/JBJS.G.01686. [DOI] [PubMed] [Google Scholar]
- Kuiper J WP, Brohet RM, Wassink S, van den Bekerom M PJ, Nolte PA, Vergroesen DA. Implantation of resorbable gentamicin sponges in addition to irrigation and debridement in 34 patients with infection complicating total hip arthroplasty . Hip Int. 2013;2(23):173–80. doi: 10.5301/HIP.2013.10612. [DOI] [PubMed] [Google Scholar]
- Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States . J Arthroplasty. 2008;23(7):984–91. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Lora-Tamayo J, Murillo O, Iribarren JA, Soriano A, Sanchez-Somolinos M, Baraia-Etxaburu JM, Rico A, Palomino J, Rodriguez-Pardo D, Horcajada JP, Benito N, Bahamonde A, Granados A, del Toro MD, Cobo J, Riera M, Ramos A, Jover-Saenz A, Ariza J. A. Large multicenter study of methicillin-susceptible and methicillin-resistant staphylococcus aureus prosthetic joint infections managed with implant retention . Clin Infect Dis. 2013;56(2):182–94. doi: 10.1093/cid/cis746. [DOI] [PubMed] [Google Scholar]
- Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, Osmon DR. Outcome of prosthetic joint infections treated with debridement and retention of components . Clin Infect Dis. 2006;42(4):471–8. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- Meehan AM, Osmon DR, Duffy M CT, Hanssen AD, Keating MR. Outcome of penicillin-susceptible streptococcal prosthetic joint infection treated with debridement and retention of the prosthesis . Clin Infect Dis. 2003;36(7):845–9. doi: 10.1086/368182. [DOI] [PubMed] [Google Scholar]
- Moran E, Masters S, Berendt AR, McLardy-Smith P, Byren I, Atkins BL. Guiding empirical antibiotic therapy in orthopaedics: The microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention . J Infect. 2007;55(1):1–7. doi: 10.1016/j.jinf.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Muller M, Morawietz L, Hasart O, Strube P, Perka C, Tohtz S. Diagnosis of periprosthetic infection following total hip arthroplasty–evaluation of the diagnostic values of pre- and intraoperative parameters and the associated strategy to preoperatively select patients with a high probability of joint infection . J Orthop Surg Res. 2008;3:31. doi: 10.1186/1749-799X-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the infectious diseases society of america . Clin Infect Dis. 2013;56(1):e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- Sharma D, Douglas J, Coulter C, Weinrauch P, Crawford R. Microbiology of infected arthroplasty: implications for empiric peri-operative antibiotics . J Orthop Surg (Hong Kong) 2008;16(3):339–42. doi: 10.1177/230949900801600314. [DOI] [PubMed] [Google Scholar]
- Soriano A, Garcia S, Bori G, Almela M, Gallart X, Macule F, Sierra J, Martinez JA, Suso S, Mensa J. Treatment of acute post-surgical infection of joint arthroplasty . Clin Microbiol Infect. 2006;12(9):930–3. doi: 10.1111/j.1469-0691.2006.01463.x. [DOI] [PubMed] [Google Scholar]
- Sukeik M, Patel S, Haddad FS. Aggressive early debridement for treatment of acutely infected cemented total hip arthroplasty . Clin Orthop. 2012;11(470):3164–70. doi: 10.1007/s11999-012-2500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swieringa AJ, Tulp NJ. Toxic serum gentamicin levels after the use of gentamicin-loaded sponges in infected total hip arthroplasty . Acta Orthop. 2005;76(1):75–7. doi: 10.1080/00016470510030355. [DOI] [PubMed] [Google Scholar]
- Swieringa AJ, Goosen J HM, Jansman F GA, Tulp N JA. In vivo pharmacokinetics of a gentamicin-loaded collagen sponge in acute periprosthetic infection: serum values in 19 patients . Acta Orthop. 2008;79(5):637–42. doi: 10.1080/17453670810016650. [DOI] [PubMed] [Google Scholar]
- Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty . J Bone Joint Surg (Br) 2006;88(2):149–55.. doi: 10.1302/0301-620X.88B2.17058. [DOI] [PubMed] [Google Scholar]
- Trampuz A, Zimmerli W. Prosthetic joint infections: update in diagnosis and treatment . Swiss Med Wkly. 2005;135(17-18):243–51. doi: 10.4414/smw.2005.10934. [DOI] [PubMed] [Google Scholar]
- Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections . J Bone Joint Surg (Am) 1996;78(4):512–23. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- Vilchez F, Martinez-Pastor JC, Garcia-Ramiro S, Bori G, Macule F, Sierra J, Font L, Mensa J, Soriano A. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement . Clin Microbiol Infect. 2011;17(3):439–44. doi: 10.1111/j.1469-0691.2010.03244.x. [DOI] [PubMed] [Google Scholar]
- Westberg M, Grogaard B, Snorrason F. Early prosthetic joint infections treated with debridement and implant retention: 38 primary hip arthroplasties prospectively recorded and followed for median 4 years . Acta Orthop. 2012;83(3):227–32. doi: 10.3109/17453674.2012.678801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group . JAMA. 1998;279(19):1537–41. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]