Abstract
Background
Obesity contributes much to the development of knee osteoarthritis. However, the association between obesity and outcome after knee replacement is controversial. We investigated whether there was an association between the preoperative body mass index (BMI) of patients who underwent total knee arthroplasty (TKA) and their quality of life (QoL) and physical function 3–5 years after surgery.
Methods
197 patients who had undergone primary TKA participated in a 3–5 year follow-up study. The outcome measures were the patient-reported Short Form 36 (SF-36) and the American Knee Society score (KSS).
Results
Ordinal logistic regression analysis (adjusted for age, sex, disease, and surgical approach) revealed a statistically significant correlation between BMI and 9 of the 14 outcome measures. For all outcome measures, we found an odds ratio (OR) of < 1. A difference in BMI of 1 kg/m2 increased the risk of a lower score from a minimum of 2% (OR = 0.98 (0.93–1.03); p = 0.5) (Mental Component score) to a maximum of 13% (OR = 0.87 (0.82–0.93); p < 0.001) (KSS function score).
Interpretation
Our findings indicate that TKA patients’ preoperative BMI is a predictor of the clinical effect and patients’ quality of life 3–5 years postoperatively. A high BMI increases the risk of poor QoL (SF-36) and physical function (KSS).
The correlation between knee osteoarthritis and obesity has been recognized for several years (Felson et al. 1988, Niu et al. 2009). However, the association between obesity and outcome after total knee arthroplasty (TKA) is ambiguous. Some studies have shown that overweight and obesity have no effect on pain and mobility after TKA (Amin et al. 2006, Hamoui et al. 2006, Krushell and Fingeroth 2007). Other studies have shown that obese patients with a body mass index (BMI) of > 30 have worse quality of life (QoL) (Stickles et al. 2001, Nunez et al. 2011a), poorer mobility (Mulhall et al. 2007), and less range of motion (ROM) after surgery than patients with a BMI of < 30 (Gadinsky et al. 2011). They also have more pain 6 and 12 months after surgery than patients with a BMI of < 30 (Naylor et al. 2011). In another study, overweight patients were less satisfied with their treatment than non-overweight patients 2 years after TKA (Merle-Vincent et al. 2011).
3 studies that found no correlation between obesity and the effect of TKA found that the hospital costs were higher in patients with a BMI of > 30 than in those with a BMI of < 30 (Vincent et al. 2007, Malinzak et al. 2009, Dowsey et al. 2011). Another study showed a correlation between BMI and early complications after TKA in terms of the duration of surgery, the use of more analgesics, and wound problems (Patel and Albrizio 2008). Other studies have shown that obese patients are less active and tend to gain weight after TKA, and the authors have recommended that obesity should be treated as an independent disease (McClung et al. 2000, Heisel et al. 2005, Lachiewicz and Lachiewicz 2008). Moreover, a poorer prosthetic survival has been reported for obese TKA patients than for non-obese TKA patients (Foran et al. 2004).
Surgery in overweight and obese patients is associated with several problems: practical problems with operation tables and instruments, increased operative time, and increased morbidity and mortality (Cheah and Kam 2005). Increased use of analgesics, problems with scarring (Patel and Albrizio 2008, Nunez et al. 2011a), and a correlation between obesity and deep infection after TKA (Chesney et al. 2008, Malinzak et al. 2009) have been reported. In contrast, Suleiman et al. (2012) found no difference in perioperative complication rates in patients undergoing TKA or total hip arthroplasty, based on BMI.
We investigated whether there was a correlation between preoperative BMI in primary TKA patients and the patients’ QoL and physical function 5 years after surgery. Our hypothesis was that higher BMI increases the risk of poor physical function and poor QoL following TKA relative to the risk in lean TKA patients.
Patients and methods
All patients who had undergone primary TKA at the Hospital of Southern Jutland during 2005 and 2006 were invited to participate in the study and to come to a postoperative follow-up after mean 4 (3–5) years.
Those patients who had undergone primary TKA in both knees during the study period participated in the study only once, with data taken from the first knee surgery. The preoperative data were collected from the patient records. Variables collected to control for confounding were: sex; date of birth; smoking (yes/no) and alcohol status (> 14 units per week for females (yes/no), > 21 units per week for males (yes/no) where 1 unit = 1 beer or 1 glass of wine); operating surgeon (data not shown); deep infection (yes/no) and revision surgery within the first postoperative year (yes/no); and the exposure variable BMI. Data were obtained from the Danish Knee Replacement Register (DKR) concerning preoperative American Knee Society score (KSS), potential confounders, primary disease (OA, RA, or secondary OA (developed following joint surgery, trauma, or joint fracture)), and surgical procedure (curved incision with the medial parapatellar approach or midline incision with the medial parapatellar approach).
During the study period, 297 TKAs were performed in 255 patients. The 42 patients who had undergone primary TKA in both knees during the study period participated in the study only once. 21 patients died before the follow-up and 37 patients did not wish to participate in the study. Thus, 197 participants completed the follow-up (Table 1).
Table 1.
Demographics and dropout analysis
| Study population (n = 197) | Dead a | p-value (n = 21) a | Lost to follow-up (n = 37) a | p-value | |
|---|---|---|---|---|---|
| Sex, n (%) | 0.3 f | 0.3 f | |||
| Female | 144 (73) | 13 (62) | 24 (65) | ||
| Male | 53 (27) | 8 (38) | 13 (35) | ||
| Mean age (range), years | 67 (37–86) | 72 (54–84) | 0.03 i | 62 (35–80) | 0.02 i |
| Age groups, years (%) | |||||
| ≤ 49 | 13 (7) | 0 | 7 (19) | ||
| 50–64 | 68 (35) | 5 (24) | 18 (49) | ||
| 65–79 | 100 (51) | 9 (43) | 10 (27) | ||
| ≥ 80 | 16 (8) | 7 (33) | 2 (5) | ||
| Mean BMI (range), kg/m2 | 30 (20–47) | 28 (20–45) | 0.1 i | 29 (21–38) | 0.6 i |
| BMI, kg/m2; n (%) | |||||
| Normal (18.5–24.9) | 32 (15) | 6 (30) | 4 (11) | ||
| Overweight (25.0–29.9) | 76 (39) | 7 (35) | 18 (49) | ||
| Obese (30.0–34.9) | 58 (30) | 4 (20) | 12 (32) | ||
| Morbid obese (≥ 35.0) | 31 (16) | 3 (15) | 3 (8) | ||
| Primary disease, n (%) | 0.1 h | 0.3 h | |||
| Osteoarthritis | 169 (86) | 17 (81) | 31 (84) | ||
| Rheumatoid arthritis | 8 (4) | 3 (14) | 0 | ||
| Injuries | 20 (10) | 1 (4) | 6 (16) | ||
| Deep infection b , n (%) | 3 (2) | 0 | 1 h | 0 | 1 h |
| Revision, n (%) | 7 (4) | 0 | 1 h | 0 | 0.6 h |
| Mean knee score d (range) | 42 (0–69) | 35 (0–61) | 0.3 i | 39 (0–61) | 0.3 j |
| Mean function score d (range) | 52 (0–90) | 37 (0–70) | 0.01 i | 52 (30–70) | 0.8 j |
| Smoking, n (%) | 18 (9) | 5 (25) | 0.03 g | 11 (30) | 0.001 g |
| Alcohol e , n (%) | 6 (3) | 0 | 0.8 g | 1 (3) | 0.7 g |
a In a population of 255 TKA patients, 197 agreed to participate in the project, 21 patients died before 5 years of follow-up, and 37 patients did not want to participate in the study.
b Deep infection occurred within one year postoperatively.
c Revision surgery performed one year postoperatively.
d Preoperative KSS score.
e Females: > 14 units per week; males: > 21 units per week (1 unit = 1 beer or 1 glass of wine).
f p-values for comparisons between the study population and the group of dead, and p-values for comparisons between the study population and the lost to fallow-up group.
g Chi-square test.
h Fisher’s exact test.
i Mann-Whitney rank-sum test.
j t-test.
The outpatient control was carried out at 3–5 years in January and February 2010. The clinical examinations were performed by AL. The patients completed the SF-36 questionnaire (version 1) and knee stability, ROM, pain, and functional ability were assessed and the KSS scores were calculated. To control for confounding, the following parameters were recorded: the patient’s weight at follow-up, work status (yes/no), and whether he/she was living alone or had a partner (yes/no) (data not shown).
The SF-36 includes 8 health domains: physical functioning (PF), role-physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role-emotional (RE), and mental health (MH). 2 component scores aggregate the 8 sub-domains into 2 component scales: physical component (PCS) and mental component (MCS).
The KSS consists of an objective knee score and a function score. The knee score measures knee pain (50%), range of motion (25%), and alignment and stability of the knee (25%). The function score examines walking distance (50%) and ability to walk up and down stairs (50%), and points are subtracted for use of walking aids. Each score is transformed into a 0–100 scale with higher scores indicating better status. The preoperative KSS scores were drawn from the DKR and the postoperative KSS values were recorded and transformed by AL.
Statistics
A dropout analysis was performed in which the 197 TKA patients who completed the follow-up period were compared with those 21 patients who had died within the 5-year follow-up period and with those 37 patients who declined the invitation to participate. The differences between the groups were assessed using Student’s t-test for continuous variables. Before the t-test, the assumptions of the model were tested. Distribution of the data was assessed by a histogram and a Bartlett test was performed for homogeneity of variances. The Mann-Whitney rank-sum test was used when data were not normally distributed or when there was no homogeneity of variances. The chi-square test was performed for the categorical variables and Fisher’s exact test was used for the variables that had few observations. For the main results, the ordinal logistic regression (proportional odds model (POM)) was applied. All the response variables (SF-36, KSS) and the exposure variable (BMI) were continuous, but a linear regression analysis could not be performed since there were ceiling effects for several of the response variables or because they were not consistent with a normal distribution of residuals. The POM gives a little more information than the binary logistic regression method, which applies when we have a categorical response of the simplest possible form—dichotomous. In our POM, all continuous response variables (SF-36, KSS) were generated to 4 ordered categorical variables (Table 2). Because of extreme ceiling effect for the SF-36 variables RP, SF, RE, and the KSS knee score, the variables were only generated to 2 or 3 ordered categorical variables (Table 2). In the POM, logistic regressions were made corresponding to the internal cut-points made for the response variables. The estimates from the regression models then were pooled to provide just one set of estimates. The POM assumption, that the relationship between any 2 pairs of response variable groups is statistically the same, was tested using a log likelihood test. Normal distribution was checked with a histogram and a probability plot. All the observations in the sample (n) were independent, had the same probability of events, and the sample sizes (n) were determined in advance. For the statistical analysis, the Stata 10 software was used. All p-values < 0.05 were considered statistically significant.
Table 2.
The SF-36 variables and the KSS variables used to generate ordered categorical variables were generated using the statistical software Stata
| Ordered categories | n | Score | |
|---|---|---|---|
| SHORT FORM 36 (SF-36) | |||
| Physical component score (PCS) | 1 | 49 | 14–44 |
| 2 | 49 | 45–50 | |
| 3 | 49 | 51–56 | |
| 4 | 50 | 57–67 | |
| Mental component score (MCS) | 1 | 49 | 22–55 |
| 2 | 49 | 56–59 | |
| 3 | 49 | 60–62 | |
| 4 | 50 | 63–69 | |
| Physical functioning (PF) | 1 | 37 | 10–55 |
| 2 | 50 | 60–80 | |
| 3 | 60 | 85–90 | |
| 4 | 50 | 95–100 | |
| Role limitation, physical (RP) | 1 | 32 | 0–75 |
| 2 | 165 | 100 | |
| Bodily pain (BP) | 1 | 45 | 0–42 |
| 2 | 53 | 51–72 | |
| 3 | 33 | 74–84 | |
| 4 | 66 | 100 | |
| General health (GH) | 1 | 47 | 10–62 |
| 2 | 49 | 65–80 | |
| 3 | 50 | 82–90 | |
| 4 | 51 | 92–100 | |
| Vitality (VT) | 1 | 43 | 10–50 |
| 2 | 47 | 55–75 | |
| 3 | 44 | 80–85 | |
| 4 | 63 | 90–100 | |
| Social functioning (SF) | 1 | 35 | 12–88 |
| 2 | 162 | 100 | |
| Role limitation, emotional (RE) | 1 | 22 | 0–67 |
| 2 | 175 | 100 | |
| Mental health (MH) | 1 | 44 | 4–76 |
| 2 | 50 | 80–88 | |
| 3 | 52 | 92–96 | |
| 4 | 51 | 100 | |
| KNEE SOCIETY SCORE (KSS) | |||
| Knee score | 1 | 45 | 35–90 |
| 2 | 53 | 93–98 | |
| 3 | 99 | 100 | |
| Knee score improvement | 1 | 44 | 0–59 |
| 2 | 43 | 60–80 | |
| 3 | 18 | 90–95 | |
| 4 | 92 | 100 | |
| Function score | 1 | 41 | –60 to 5 |
| 2 | 32 | 10–25 | |
| 3 | 41 | 30–35 | |
| 4 | 83 | 40–85 | |
| Function score improvement | 1 | 48 | –12 to 39 |
| 2 | 49 | 40–48 | |
| 3 | 46 | 49–61 | |
| 4 | 54 | 62–98 | |
Results
Dropout analysis
Patients who died during the study period had a higher mean age than those who completed the follow-up period, and those who declined the invitation to participate had a lower mean age. There were statistically significantly more smokers among those who died (25%) and among those who declined participation (30%) than among those who completed the follow-up (9%) (Table 1).
Unadjusted results
BMI correlated statistically significantly with 7 of the 14 endpoints, and odds ratios (ORs) of < 1 were found for all 14 endpoints. Patients with a high BMI achieved a lower effect of the operation comared to patients with a low BMI (Table 3).
Table 3.
The association between preoperative BMI and effect 3–5 years after TKA. The results are presented as odds ratios (ORs) and their 95% confidence intervals (CIs), calculated using the proportional odds analysis, unadjusted and with adjustment for age, sex, primary disease, and surgical approach
| n=197 | OR unadjusted | 95 % CI | p-value | OR adjusted | 95 % CI | p-value |
|---|---|---|---|---|---|---|
| SHORT FORM 36 (SF-36) | ||||||
| Physical component score (PCS) | 0.94 | (0.90–0.99) | 0.01 | 0.92 | (0.88–0.97) | 0.002 |
| Mental component score (MCS) | 0.98 | (0.94–1.03) | 0.5 | 0.98 | (0.93–1.03) | 0.5 |
| Physical functioning (PF) | 0.93 | (0.89–0.98) | 0.007 | 0.90 | (0.85–0.95) | < 0.001 |
| Role limitation, physical (RP) | 096 | (0.89–1.03) | 0.3 | 0.96 | (0.89–1.03) | 0.2 |
| Bodily pain (BP) | 0.96 | (0.92–1.01) | 0.1 | 0.96 | (0.91–1.01) | 0.1 |
| General health (GH) | 0.95 | (0.91–1.00) | 0.06 | 0.94 | (0.90–0.99) | 0.03 |
| Vitality (VT) | 0.93 | (0.89–0.98) | 0.006 | 0.92 | (0.87–0.97) | 0.002 |
| Social functioning (SF) | 094 | (0.88–1.01) | 0.09 | 0.92 | (0.86–0.99) | 0.03 |
| Role limitation, emotional (RE) | 0.95 | (0.88–1.03) | 0.2 | 0.95 | (0.87–1.04) | 0.3 |
| Mental health (MH) | 0.95 | (0.91–1.00) | 0.04 | 0.95 | (0.90–1.00) | 0.04 |
| KNEE SOCIETY SCORE (KSS) | ||||||
| Knee score | 0.95 | (0.90–1.00) | 0.04 | 0.94 | (0.90–0.99) | 0.02 |
| Knee score improvement | 0.98 | (0.94–1.03) | 0.5 | 0.97 | (0.92–1.02) | 0.3 |
| Function score | 0.92 | (0.88–0.97) | 0.003 | 0.87 | (0.82–0.93) | < 0.001 |
| Function score improvement | 0.93 | (0.88–0.98) | 0.005 | 0.90 | (0.86–0.95) | < 0.001 |
For the patient-reported physical component score (PCS), the risk of having a poorer score increased statistically significantly by 6% with a difference in BMI of 1. For the patient-reported mental component score (MCS), the risk of having a poorer score increased by 2% for a difference in BMI of 1, i.e. the association was not statistically significant. For the 8 SF-36 subdomains, the risk of a poorer score increased by 4–7% with a difference in BMI of 1. This was statistically significant for the variables PF, VT, and MH but it was not for the variables RP, BP, GH, SF, and RE. For the KSS, the risk of a poorer score increased by 2–8% with a difference in BMI of 1.
Adjusted results
After adjustment for confounders (age, sex, primary disease, and surgical approach), 9 of the 14 endpoints were significantly correlated with BMI; for all 14 endpoints, the ORs decreased or were unchanged and the 95% confidence intervals (CIs) were slightly narrower or were unchanged (Table 3). Also the p-values were lower or unchanged after adjustment (Table 3). The adjusted model increased the precision of the estimates. The analytical model was tested for other potential confounders (smoking, weight at follow-up, work status, living alone or with a partner, alcohol status, surgeon, deep infection, and revision surgery within the first postoperative year), but these adjustments did not affect the results of the analyses (data not shown).
After model adjustment (Table 3), the SF-36 PCS showed that for 2 people of the same age, the same sex, the same primary disease, and operated with the same surgical approach, a difference in BMI of 1 was associated with an 8% increased risk of a poorer score for the person with the higher BMI. After adjustment for these variables, the MCS remained unchanged, but the risk of having a poorer score rose by 4–12% for the 8 SF-36 subdomains. The change of 2 additional variables, GH and SF, was statistically significant after adjustment. This indicates that a person with high BMI had poorer general health, and that physical and emotional problems to some extent interfered with the social activities of those with a high BMI. There were no significant correlations between BMI and the variables RP, BP, and RE. After adjustment of the model, the risk of having a poor KSS increased for those with a high BMI. The increase was in the order of 3–14% for a difference in BMI of 1.
Adjusted results with a difference in BMI of 5 and 10
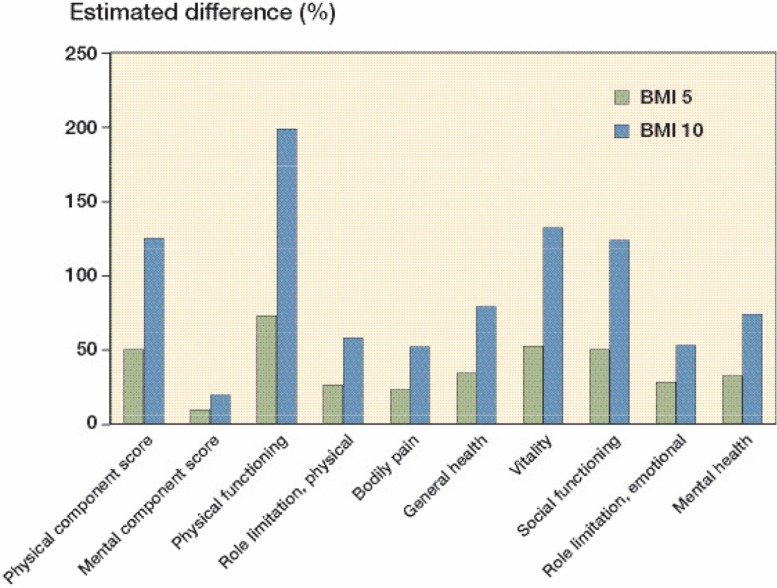
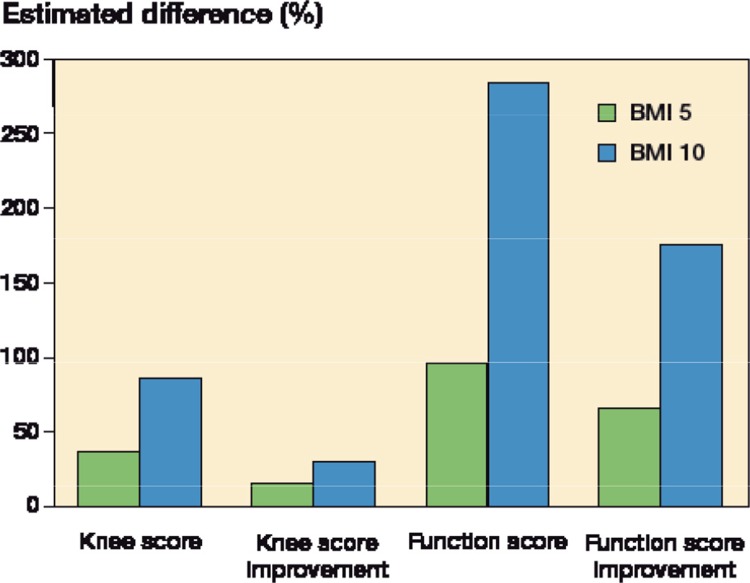
In 2 people of the same age and sex and with the same primary disease and surgical approach, a difference in BMI of 5 would involve a 50% increase in the risk of a poorer SF-36 PCS in the person with the higher BMI. With a difference in BMI of 10, the risk increased by 125%. For the SF-36 MCS, a difference in BMI of 5 would involve a 9% higher risk of a poorer score for the person with the higher BMI. With a difference in BMI of 10, the risk would be increased by 19%. For the 8 subdomains, the risk of having a poorer score rose by 23–73% with a difference in BMI of 5, and by 52–199% with a difference in BMI of 10 (Figure 1). For the KSS, the risk of having a poorer score increased by 15–96% for a difference in BMI of 5, and by 31–284% for a difference in BMI of 10 (Figure 2).
Figure 1.
The estimated difference in Short Form 36 (SF-36) score 3–5 years postoperatively between patients with a difference in BMI of 5 kg/m2 and between patients with a difference in BMI of 10 kg/m2, adjusted for age, sex, primary disease, and surgical approach.
Figure 2.
The estimated difference in Knee Society score (KSS) 3–5 years postoperatively between patients with a difference in BMI of 5 kg/m2 and between patients with a difference in BMI of 10 kg/m2, adjusted for age, sex, primary disease and surgical approach.
Discussion
Quality of life
Our findings indicate that TKA patients’ preoperative BMI is a predictor of the clinical effect and the quality of life of a patient 3–5 years postoperatively. After adjustment for confounders, 5 of the 8 SF-36 subdomains, and also the physical component score (PCS), were significantly associated with BMI. There is therefore an inverse correlation between BMI and performing physical activity (PF) such as climbing stairs and walking. Patients with a high BMI are more limited than patients with a lower BMI in performing all kinds of physical activities without limitations. The higher the BMI, the more nervous and sad (MH) the patient was, and the more difficult it was for the patient to maintain social functioning (SF). These patients also felt more tired and worn out (VT), and they assessed their general health (GH) to be worse than that of the patients with lower BMI. The association between BMI and the mental component score (MSC) was not statistically significant. There was no significant association between BMI and problems with work or other activities as a result of physical problems (RP). Also, no correlation between BMI and pain (BP) was found, and the data do not suggest that the BMI had an impact on problems at work or in performing other daily activities because of emotional problems (RE).
Knee Society Score
We found a correlation between the KSS score and BMI. The follow-up function score and the improvement of function score showed a clear correlation. Patients with increased BMI did not achieve the same functional capacity as TKA patients with lower BMI. They were not able to walk such long distances and to climb stairs to the same degree as patients with normal BMI. The KSS knee score was also inversely correlated with BMI, whereas the improvement of the knee score from baseline to follow-up showed no significant correlation.
Other studies
Our results are consistent with other studies that have used SF-36 (Stickles et al. 2001, Naylor et al. 2008) and KSS (Foran et al. 2004, Krushell and Fingeroth 2007). A prospective study of 1,011 primary TKA patients found negative linear correlation between BMI and PCS score 1-year postoperative (Stickles et al. 2001). Naylor et al. (2008) found obesity to be associated with several SF-36 domains at 12 months of follow-up. This indicates that obesity influences the patient’s generic (overall bodily) QoL after TKA. There is disagreement about the effect of obesity on health-related outcomes after TKA. Stickles et al. (2001) found significant differences in the absolute Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score at the12-month follow-up between obese patients and non-obese patients undergoing TKA. There were no differences in improvement in WOMAC score between obese and non-obese patients. A case-control study by Nunez et al. (2011b) found no differences in WOMAC score at 12-month follow-up between obese patients and non-obese patients. Another case-control study by Krushell and Fingeroth (2007), with 5–14 years of follow-up, found that patients with a BMI of > 40 had lower KSS knee and function scores postoperatively than controls with a BMI of < 30. Likewise, Foran et al. (2004) found that a BMI of > 30 had a negative influence on KSS score 7 years after TKA, and that obese patients had a lower rate of improvement than non-obese patients.
Limitations of the study
Firstly, causes of death were not recorded. However, based on data collected at baseline, it is reasonable to assume that the patients who died before follow-up was completed were not systematically better or worse than the study population. The study population had a higher mean age at baseline and had more smokers than the group that died before follow-up. The deceased group also had a lower mean baseline KSS function score, perhaps because they were older and therefore had a lower level of functioning. The average BMI of the deceased group was slightly—but not significantly—lower than the average BMI of the study population. We do not believe that this difference was caused by a selection bias. Of those who declined the invitation to participate in the project, two-thirds were less than 65 years of age, which meant that they were probably still in active employment and therefore unable to take time off work to participate in the study. In comparison, less than half of the group who completed the follow-up were younger than 65 years. It is possibly that those who declined participation were actually doing well and were therefore uninterested in participating in a 5-year clinical follow-up. This might give rise to selection bias, but there was no difference in baseline BMI or baseline KSS scores between the groups. Secondly, in the statistical analysis model, several potential confounders were controlled for, but we did not control for patients’ postoperative pain and training efforts, although these factors are important for patient rehabilitation. Thirdly, we did not account for any injuries that the patient might have incurred during the follow-up period, such as fracture (independent of knee prosthesis). Moreover, no consideration was paid to other disorders such as comorbidities (Cheah et al. 2005, Nunez et al. 2011b, Jones et al. 2012) or other implant surgery in the hip or contralateral knee. These factors may have influenced the patient outcomes and may therefore have been potential confounders.
In conclusion, our results suggest that obesity increases the risk of poor QoL (SF-36) and the risk of low health-related outcome (KSS) 3–5 years after TKA. There is a need for further studies to determine whether preoperative weight loss would improve a patient’s QoL and functional capacity postoperatively.
Acknowledgments
All authors contributed to the conception and design of the study, interpretation of the data, and revision of the final manuscript. AL and JOL planned the study. AL wrote the protocol, performed the clinical examinations, collected data, and wrote the manuscript.
No competing interests declared.
References
- Amin AK, Patton JT, Cook RE, Brenkel IJ. Does obesity influence the clinical outcome at five years following total knee replacement for osteoarthritis? J Bone Joint Surg (Br) 2006;88(3):335–40. doi: 10.1302/0301-620X.88B3.16488. [DOI] [PubMed] [Google Scholar]
- Cheah MH, Kam P CA. Obesity: Basic science and medical aspects relevant to anaesthetists. Anaesthesia. 2005;60(10):1009–21. doi: 10.1111/j.1365-2044.2005.04229.x. [DOI] [PubMed] [Google Scholar]
- Chesney D, Sales J, Elton R, Brenkel IJ. Infection after knee arthroplasty a prospective study of 1509 cases. J Arthroplasty. 2008;23(3):355–9. doi: 10.1016/j.arth.2007.05.052. [DOI] [PubMed] [Google Scholar]
- Dowsey MM, Liew D, Choong P FM. Economic burden of obesity in primary total knee arthroplasty. Arthritis Care and Research. 2011;63(10):1375–81. doi: 10.1002/acr.20563. [DOI] [PubMed] [Google Scholar]
- Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109(1):18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- Foran JR, Mont MA, Rajadhyaksha AD, Jones LC, Etienne G, Hungerford DS. Total knee arthroplasty in obese patients: a comparison with a matched control group. J Arthroplasty. 2004;19(7):817–24. doi: 10.1016/j.arth.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Gadinsky NE, Ehrhardt JK, Urband C, Westrich GH. Effect of body mass index on range of motion and manipulation after total knee arthroplasty. Arthroplasty. 2011;8(26):1194–7. doi: 10.1016/j.arth.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Hamoui N, Kantor S, Vince K, Crookes PF. Long-term outcome of total knee replacement: does obesity matter? Obes Surg. 2006;16(1):35–8. doi: 10.1381/096089206775222140. [DOI] [PubMed] [Google Scholar]
- Heisel C. Silva M, la Rosa MA, Schmalzried TP. The effects of lower-extremity total joint replacement for arthritis on obesity. Orthopedics. 2005;28(2):157–9. doi: 10.3928/0147-7447-20050201-18. [DOI] [PubMed] [Google Scholar]
- Jones CA, Cox V, Jhangri GS, Suarez-Almazor ME. Delineating the impact of obesity and its relationship on recovery after total joint arthroplasties. Osteoarthritis Cartilage. 2012;20(6):511–8. doi: 10.1016/j.joca.2012.02.637. [DOI] [PubMed] [Google Scholar]
- Krushell RJ, Fingeroth RJ. Primary total knee arthroplasty in morbidly obese patients: a 5- to 14-year follow-up study. J Arthroplasty (Suppl 2) 2007;22(6):77–80. [Google Scholar]
- Lachiewicz AM, Lachiewicz PF. Weight and activity change in overweight and obese patients after primary total knee arthroplasty. J Arthroplasty. 2008;23(1):33–40. doi: 10.1016/j.arth.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty (Suppl 1) 2009;24(6):84–8. doi: 10.1016/j.arth.2009.05.016. [DOI] [PubMed] [Google Scholar]
- McClung CD, Zahiri CA, Higa JK, Amstutz HC, Schmalzried TP. Relationship between body mass index and activity in hip or knee arthroplasty patients. J Orthop Res. 2000;18(1):35–9. doi: 10.1002/jor.1100180106. [DOI] [PubMed] [Google Scholar]
- Merle-Vincent F, Couris CM, Schott AM, Conrozier T, Piperno M, Mathieu P, Vignon E. Factors predicting patient satisfaction 2 years after total knee arthroplasty for osteoarthritis. Joint Bone Spine. 2011;78(4):383–6. doi: 10.1016/j.jbspin.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Mulhall KJ, Ghomrawi HM, Mihalko W, Cui Q, Saleh KJ. Adverse effects of increased body mass index and weight on survivorship of total knee arthroplasty and subsequent outcomes of revision TKA. J Knee Surg. 2007;20(3):199–204. doi: 10.1055/s-0030-1248043. [DOI] [PubMed] [Google Scholar]
- Naylor JM, Harmer AR, Heard RC. Severe other joint disease and obesity independently influence recovery after joint replacement surgery: an observational study. Aust J Physiother. 2008;54(1):57–64. doi: 10.1016/s0004-9514(08)70067-9. [DOI] [PubMed] [Google Scholar]
- Niu J, Zhang YQ, Torner J, Nevitt M, Lewis CE, Aliabadi P, Sack B, Clancy M, Sharma L, Felson DT. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61(3):329–35. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez M, Lozano L, Nunez E, Segur JM, Sastre S. Factors influencing health-related quality of life after TKA in patients who are obese. Clin Orthop. 2011a;469(4):1148–53. doi: 10.1007/s11999-010-1671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez M, Lozano L, Nunez E, Sastre S, Luis Del Val J. Suso,S. Good quality of life in severely obese total knee replacement patients: A case-control study. Obesity Surgery. 2011b;21(8):1203–8. doi: 10.1007/s11695-010-0197-9. [DOI] [PubMed] [Google Scholar]
- Patel AD, Albrizio M. Relationship of body mass index to early complications in knee replacement surgery. Arch Orthop Trauma Surg. 2008;128(1):5–9. doi: 10.1007/s00402-007-0364-1. [DOI] [PubMed] [Google Scholar]
- Stickles B, Phillips L, Brox WT, Owens B, Lanzer WL. Defining the relationship between obesity and total joint arthroplasty. Obes Res. 2001;9(3):219–23. doi: 10.1038/oby.2001.24. [DOI] [PubMed] [Google Scholar]
- Suleiman LI, Ortega G, Ong’Uti SK, Gonzalez DO, Tran DD, Onyike A, Turner PL, Fullum TM, Does BMI affect perioperative complications following total knee and hip arthroplasty? J Surg Res. 2012;174(1):7–11. doi: 10.1016/j.jss.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Vincent KR, Lee LW, Alfano AP. Effect of obesity on inpatient rehabilitation outcomes following total knee arthroplasty. Clin Rehabil. 2007;21(2):182–90. doi: 10.1177/0269215506069245. [DOI] [PubMed] [Google Scholar]