Abstract
Background: Proton pump inhibitors are used in endodontic disinfection of root canals for elimination of enterococcus faecalis. This invivo study on Wistner Rats is carried out to determine antimicrobial efficacy of proton pump inhibitor in combination with sodium hypochlorite & Mixture of Isomer of tetracycline, acid and detergent (MTAD) against E. Faecalis
Materials & Methods: Periapical lesions were induced on the 30 Incisor teeth of 30 male Wistner rats (10per group). After 28 days, root canals of each tooth were instrumented to 35# k file, during the process of instrumentation canals were irrigated with their respective irrigation solutions. Group-1: 2% CHX + 5.2% NaOCL, Group-2: MTAD(Dentsply Tulsa Dental, Tulsa, OK) + 5.2% NaOCL, Group-3: 8.5% Omeprazole (Dr Reddy's labs private limited – Hyderabad) + 5.2% NaOCL. Microbiological samples were collected by using #35 sterilized paper points after 28 days of inducing periapical lesions, Sample (S1) was collected before instrumentation and Sample (S2) was collected after instrumentation and Irrigation data were subjected to analysis of variance, followed by Newman Keuls Post Hoc test.
Results: Microbiological Analysis revealed significant decrease of colony forming units from S1 to S2 Samples in all the 3 groups.
Conclusion: Our data showed that association of Omeprazole with NaOCL showed a superior antibacterial efficacy against Enterococcus faecalis in comparision with other irrigants.
Key words: : Sodium Hypochlorite, Chlorhexidine, Gluconate, MTAD, Omeprazole
Introduction:
It is well established that pulp and periapical disease as well as failed root canal therapy (RCT) are due to the presence of microbes in the root canal system1. Reduction and elimination of bacteria and their byproducts should be given the outmost importance towards achieving a successful endodontic therapy. Although instrumentation of the root canal is the primary method of canal debridement, irrigation is a critical adjunct. Nevertheless, because of the anatomical complexities of many root canals, organic residues and bacteria located in the dentinal tubules cannot be sufficiently cleaned, even after meticulous mechanical procedures. Therefore, various substances have been used during and immediately after root canal preparation to remove debris and necrotic pulp tissue and to eliminate microorganisms from the root canal2.
Irrigation serves as a physical flush to remove debris as well as serves as a antimicrobial agent, tissue solvent and lubricant. Root canal irrigation plays a key role in the success of endodontic treatment, because it helps in the progressive removal of the smear layer and neutralizes the root canal microbial flora3. There is no single irrigating solution that alone sufficiently covers all of the functions required from an irrigant. Optimal irrigation is based on the combined use of 2 or several irrigating solutions, in a specific sequence, to predictably obtain the goals of safe and effective irrigation4
Several antimicrobial rinses are used in Endodontics including Sodium Hypochlorite, Chlorhexidine and Biopure MTAD. Various concentrations of sodium Hypochlorite (NaOCl) have been used as root canal irrigants for many decades. The main advantages of NaOCl are its ability to dissolve necrotic tissues and its antibacterial properties against most microorganisms5,6. Chlorhexidine is a cationic bisbiguanide, it is bacteriostatic at lower concentrations and bactericidal at higher concentrations (2%Chlorhexidine) and shows the property of substantivity7.Despite its usefulness as a root canal irrigant, it cannot be advocated as the main irrigant in standard endodontic procedures as it lacks the property to dissolve necrotic tissue remnants and fails to remove smear layer, so it can be advocated only as a final irrigant. Biopure MTAD (Dentsply, Tulsa OK) is a mixture of tetracycline isomer (doxycycline), an acid (citric acid), and a detergent (Tween 80). When used as a root canal irrigant, MTAD has been reported to safely remove the smear layer8 and effectively eliminate Enterococcus faecalis9. Studies have found E. faecalis to be a commonly recovered microbe in failing root canals10-12
Enterococcus faecalis is often found in a high percentage of root canal failures. It plays a major role in the etiology of persistent periradicular lesions after root canal treatment. Gomes et al13 showed that E.faecalis is frequently found in primary necrotic areas. Evans et al14 showed that it is resistant to calcium hydroxide because of its proton pump. E.faecalis can also survive by genetic polymorphisms and its ability to bind to dentin, invade dentinal tubules, and survive starvation15.
In a recent study by Claudia Wagner et al16 showed that association of Omeprazole with Ca(OH)2 favoured a superior repair of rat periapical lesions and seemed to display different selective activity over endodontic microbiota in comparision with conventional Ca(OH)2 dressing. So we decided to use Omeprazole the Proton Pump Inhibitor in combination with 5.2% Sodium Hypochlorite (NaOCL) and to compare its antimicrobial efficacy with other combination of irrigants such as MTAD with 5.2% NAOCL and 2% Chlorhexidine Gluconate with 5.2% NAOCL
RATIONALE FOR CHECKING E.FAECALIS
Studies indicate that the prevalence of E. faecalis is low in primary endodontic infections and high in persistent infections. E. faecalis is also more commonly associated with asymptomatic cases than with symptomatic ones. Although E. faecalis possesses several virulence factors, its ability to cause periradicular disease seems from its ability to survive the effects of root canal treatment and persist as a pathogen in the root canals and dentinal tubules of teeth. Our challenge as endodontic specialists is to implement methods to effectively eliminate this microorganism during and after root canal treatment. There are currently 23 Enterococci species and these are divided into five groups based on their interaction with mannitol, sorbose, and arginine. E. faecalis belongs to the same group as E. faecium, E.casseliflavus, E. mundtii, and E. gallinarum. These five species form acid in mannitol broth and hydrolyze arginine, however they fail to form acid in sorbose broth. After establishing that the gram-positive coccus is a member of one of the five groups in the Enterococcus genus, several conventional tests are used to identify the specific species. E. faecalis can normally be identified by further testing with arabinose, tellurite, and pyruvate. E. faecalis is arabinose negative and except for some atypical variants, is the only member of the group to utilize pyruvate and to tolerate tellurite.
MATERIALS AND METHODS
30 Incisor teeth of 30 male Wistner rats (180-200g body weight ,10 animals per group) were used (figure 1). Experimental protocols were approved by local Animal Ethic committee and animals were maintained following guide to management and use of experimental animals.
Figure 1:
Study Animals
On day 1each Rat was anesthesized by an intra peritoneal injection of xylazine (10mg/kg) combined with ketamine (100mg/kg) (figure 2). Pulp exposure was performed on incisor tooth by using ½ no. round bur to depth of bur diameter (figure 3) followed by instrumentation of canal with #10 no. k file ( Dentsply maillifer, SA) endodontic instrument (figure 4) , under irrigation with saline solution. Exposed pulps were left open to oral environment to allow formation of periapical lesions for 28 days.
Figure 2:
Intra Peritoneal Injection of Xylazine (10mg/kg) combined with Ketamine (100mg/kg)
Figure 3:
Access cavity preparation
Figure 4:
Initial instrumentation with 10# k file
Irrigating Solutions Used:
5.2% Sodium Hpochlorite(NAOCL, Vishal dentocare, pvt limited, India), 2% Chlorhexidine Gluconate CHX, Dentachlor, Ammdent, India), MTAD(Dentsply Tulsa Dental, Tulsa,OK) and 8.5% OMEPRAZOLE (Dr Reddy's labs private limited,Hyderabad) all irrigation solutions were irrigated using 30 gauze side vented needle & Syringe( Nirlife, Nirma limited, India)
Group-1: 2% CHX + 5.2% NAOCL (Irrigated separately for 30 sec each)
Group-2: MTAD(Dentsply Tulsa Dental, Tulsa,OK) + 5.2% NAOCL (Irrigated separately for 30 sec each)
Group-3: 8.5% OMEPRAZOLE(omeprazole 8.5%, Dr Reddy's labs private limited – Hyderabad) + 5.2% NAOCL (Both are combined and irrigated for 30 sec)
Procedure for Root Canal Preparation:
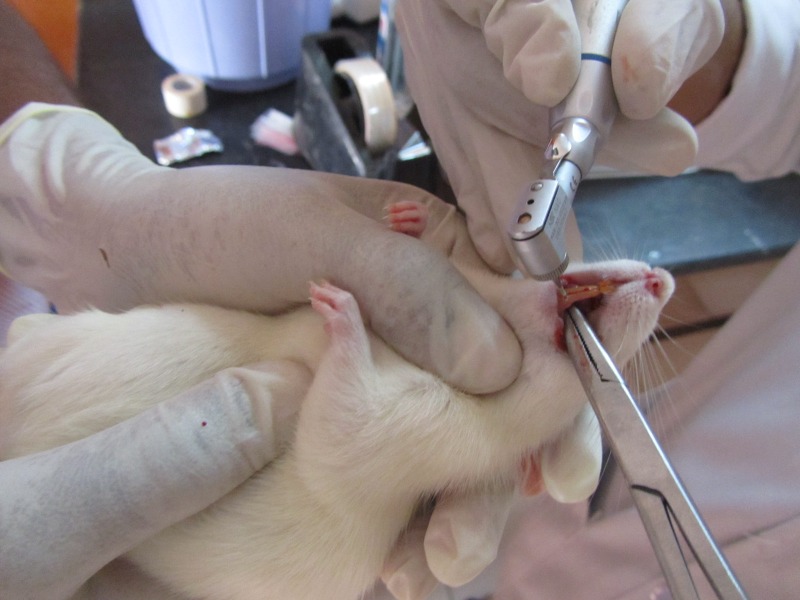
On day 28 Rats were divided into 3 Groups reanesthetized and root canals were instrumented to 35# k file Dentsply maillefer, SA) during the process of instrumentation canals were irrigated with their respective irrigation solutions (figure 5).
Figure 5:
Irrigation with combined Omeprazole and 5.2 % NaOCL
Microbiologcal Analysis:
Microbiological samples were collected by using #35 sterilized paper points 28 days after inducing periapical lesions, S1: Sample (S1) was collected before Instrumentation and Irrigation S2: Sample (S2) was collected after Instrumentation and Irrigation
In all cases absorbent paper points were aseptically transferred to 1.5ml microcentrifuge tubes (aliquots) containing brain heart infusion broth. Then solution is agitated by vortexing to suspend attached bacteria into solution. To estimate the number of colony forming units per ml, serial decimal dilutions (upto 10-3) were prepared. Aliquots of 100μl of each dilution and original suspension were spread onto surface of blood agar and incubated aerobically at 370 c for 24hrs.
Statistical Analysis:
Result were analysed as mean ± standard error of mean of 10 animals in each experimental group. Data were subjected to analysis of variance, followed by Newman Keuls Post Hoc test.
Results
Results of this investigation showed that Group 3 had the maximum zones of inhibition against the E.faecalis. Comparatively Group 2 showed maximum zones of inhibition than Group 1( Table 1).
Table 1 - Colony forming units.
| Groups | S 1 Sample( CFU/ml) Average mean value of 10 rats | S 2 sample (CFU/ml) Average mean value of 10 rats |
|---|---|---|
| Group –I | 50341±2476 | 24,815±1226 |
| Group –II | 48321±1718 | 9,443±921 |
| Group –III | 47216±1345 | 4291±634 |
Microbiologcal Analysis After placement of respective intra canal medicaments in all the 3 groups in
Group 1 - There was a mean reduction of 51% in colony forming units of E.faecalis
Group 2 - There was a mean reduction of 79% in colony forming units of E.faecalis.
Group 3 - There was a mean reduction of 82% in colony forming units of E.faecalis.
Discussion
In present study we decided to use Omeprazole the Proton Pump Inhibitor in combination with 5.2% Sodium Hypochlorite (NaOCL) and to compare its antimicrobial efficacy with other combination of irrigants such as MTAD with 5.2% NAOCL and 2% Chlorhexidine Gluconate with 5.2% NAOCL in rat model of periapical lesions. Usefulness of rat models to emulate human endodontic lesions has been well established.
Several in vitro studies have shown Sodium Hypochlorite solution lethal to E. faecalis, yet when E. faecalis-infected dentine is exposed to sodium hypochlorite solution, some cells of E. faecalis may survive17-19. The recovery of E. faecalis in the root canals of failed cases after endodontic retreatment 20 also implies an ability of E. faecalis to survive.
The results of this invivo study showed that the combination of 8.5% Omeprazole and 5.2% NaOCL (Group-III) is more effective against E.faecalis than combinations of MTAD and 5.2% NAOCL (Group-II) and 2% Chlorhexidine (CHX) and 5.2% NaOCL (Group-I). In this study CHX Group did not perform any better than MTAD and Omeprazole Groups but it is important to note that this study did not address the property of substantivity of the medicament.
Shabahang and Torabinejad21 showed the efficacy of BioPure MTAD against E.faecalis. Within the parameters of this study, Group-II that is combination of MTAD and 5.2% NaOCL had an observable effectiveness against E.faecalis similar to Group-III when compared to Group-I. Its effectiveness is attributed to its anticollagenase activity, low pH, and ability to be released gradually over time22. The effects of MTAD are enhanced when sodium hypochlorite is used as an irrigant during instrumentation. Use of NaOCl as an initial rinse followed by the use of MTAD as a final rinse result in a 30% reduction in the antimicrobial substantivity of MTAD23. So in the present study MTAD is used as an initial rinse followed by the use of 5.2% NaOCl as final rinse.
According to Booth2 , in acid or alkaline environments, most bacterial cells maintain pH homeostasis in which the internal pH is kept within a narrow range so that enzymes and proteins maintain normal function. The pH homeostasis is based on two principal components: a passive function consisting of a low cell membrane permeability to ions and a buffering ability of the cytoplasm; and an active mechanism that functions mainly through controlled transport of cations (potassium, sodium and protons) across the cell membrane24-26. In acidic environments, a cation antiport system is thought to raise the internal pH by expelling protons across the cell wall. In an alkaline medium, cations/protons are pumped into the cell to lower the internal pH. Extensive work with Enterococcus hirae (formerly Streptococcus faecalis) has confirmed a fundamental role for a potassium/proton antiport system in maintaining cytoplasmic pH in an alkaline environment27-28.
Kinoshita et al29 determined the role of the proton pump in maintaining survival of E. faecalis in a high pH environment, in this study CCCP was used to shut down the pump, and there was a 20-fold reduction in cell survival after 30 min exposure to high pH compared to cells that were not exposed to CCCP. These results show that a functioning proton pump, which drives protons into the cell to acidify the cytoplasm, is critical for the survival of E. faecalis in a highly alkaline environment. Presumably, when the environmental alkalinity reaches pH 11.5 or above, this life-saving mechanism is overwhelmed.
Results of this study support the findings of Claudia Wagner et al16. The improved results of Group III may be due to presence of Omeprazole the Proton Pump Inhibitor which is known to block proton movement across cell membrane15 and hence homeostasis of bacteria is not maintained and further due to the presence of NaOCL (pH 11.0) which has antibacterial and oxidative properties destroys the cytoplasam and inhibits dehydrogenases in microorganisms24 might have played a key role in further reduction of the bacterial count.
Conclusion
Our data showed that association of Omeprazole with NaOCL showed a superior antibacterial efficacy against E.faecalis in comparision with irrigants. In the changing face of dental care, continued research on E. faecalis and its elimination from the dental apparatus may well define the future of the endodontic specialty.
Footnotes
Source of Support: Nil
Conflict of Interest: None Declared
Contributor Information
Padma Gandi, Department of Conservative Dentistry & Endodontics, SVS Institute of Dental Sciences, Andhra Pradesh, India.
Sravanthi Rajah Vasireddi, Department of Conservative Dentistry & Endodontics, SVS Institute of Dental Sciences, Andhra Pradesh, India.
Sindhura Reddy Gurram, Department of Conservative Dentistry & Endodontics, SVS Institute of Dental Sciences, Andhra Pradesh, India.
Kishore Darasani1, Department of Conservative Dentistry & Endodontics, SVS Institute of Dental Sciences, Andhra Pradesh, India.
References:
- 1.Siqueira JF Jr. Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. 2001;34(1):1–10. doi: 10.1046/j.1365-2591.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 2.Jhonson RB, Remeikins NA. Effective shelf-life of prepared sodium hypochlorite solution. J Endodon. 1993;19(1):40–43. doi: 10.1016/S0099-2399(06)81040-X. [DOI] [PubMed] [Google Scholar]
- 3.Torabinejad M, Cho Y, Khademi AA, Bakland LK, Shabahang S. The effect of various concentrations of sodium hypochlorite on the ability of MTAD to remove the smear layer. J Endod. 2003;29(4):233–239. doi: 10.1097/00004770-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Markus Haapasalo, Ya Shen, Wei Qian, DDS, Yuan Gao. Irrigation in Endodontics. Dent Clin N Am. 2010;54:291–312. doi: 10.1016/j.cden.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: part 3. J Endod. 1983;9(4):137–142. doi: 10.1016/S0099-2399(83)80032-6. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigation regimens. J Endod. 1987;13(4):147–157. doi: 10.1016/s0099-2399(87)80132-2. [DOI] [PubMed] [Google Scholar]
- 7.Wang CS, Arnold RR, Trope M, Teixeira FB. Clinical efficiency of 2%chlorhexidine gel in reducing intracanal bacteria . JOE. 2007 Nov;33(11):1283–1289. doi: 10.1016/j.joen.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, Kim J, Shabahang S. A new solution for the removal of the smear layer. J Endod. 2003;29(3):170–175. doi: 10.1097/00004770-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Torabinejad M, Shabahang S, Aprecio RM, Kettering JD. The antimicrobial effect of MTAD: an in vitro investigation. J Endod. 2003;29(6):400–403. doi: 10.1097/00004770-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Molander A, Reit C, Dahlen G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J. 1998;31(1):1–7. [PubMed] [Google Scholar]
- 11.Sundqvist G, Figdor D, Persson S, Sjogren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(1):86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 12.Hancock HH, Sigurdsson A, Trope M, Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North Am population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(5):579–586. doi: 10.1067/moe.2001.113587. [DOI] [PubMed] [Google Scholar]
- 13.Gomes BP, Pinheiro ET, Sousa EL, Jacinto RC, Zaia AA, Ferraz CC, De-Souza-Filho FJ. Enterococcus faecalis in dental root canals detected by culture and by polymerase chain reaction analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(2):247–253. doi: 10.1016/j.tripleo.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Evans M, Davies JK, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35(3):221–288. doi: 10.1046/j.1365-2591.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- 15.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32(2):93–98. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 16.Wagner C, Barth VC, de Olieviera, Campos MM. Effectiveness of the Proton Pump Inhibitor Omeprazole Associated with Calcium Hydroxide as Intracanal Medication: An In Vivo Study. J Endod. 2011;37(9):1253–1257. doi: 10.1016/j.joen.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Orstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endodontics and Dental Traumatology. 1990;6(4):142–149. doi: 10.1111/j.1600-9657.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 18.Vahdaty A, Pitt Ford TR, Wilson R. Efficacy of chlorhexidine in disinfecting dentinal tubules in vitro. Endodontics and Dental Traumatology. 1993;9(6):243–248. doi: 10.1111/j.1600-9657.1993.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 19.Heling I, Chandler N. Antimicrobial effect of irrigant combinations within dentinal tubules. International Endodontic Journal. 1998;31(1):8–14. [PubMed] [Google Scholar]
- 20.Sundqvist G, Figdor D, Persson S, Sjogren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surgery Oral Medicine Oral Pathology, Oral Radiology and Endodontics. 1998;85(1):86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 21.Shabahang S, Torabinejad M. Effect of MTAD on Enterococcus Faecalis-contaminated root canals of extracted human teeth. J Endod. 2003;29(9):576–579. doi: 10.1097/00004770-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Tay FR, Hiraishi N, Schuster GS, Pashley DH, Loushine RJ, Ounsi HF, Grandini S, Yat JY, Mazzoni A, Donnelly A, King NM. Reduction in antimicrobial substantivity of MTAD after initial Sodium Hypochlorite irrigation. JOE. 2006;32(10):970–975. doi: 10.1016/j.joen.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Kovac J, Kovac D. Effect of irrigating solutions in endodontic therapy. Brastisl Lek Listy. 2011;112(7):410–415. [PubMed] [Google Scholar]
- 24.Booth I. Regulation of cytoplasmic pH in bacteria. Microbiological Reviews. 49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakinuma Y. Inorganic cation transport and energy transduction in Enterococcus hirae and other streptococci. Microbiology and Molecular Biology Reviews. 1998;62(4):1021–1045. doi: 10.1128/mmbr.62.4.1021-1045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Booth I. The regulation of intracellular pH in bacteria. Novartis Foundation Symposium. 221:19–37. doi: 10.1002/9780470515631.ch3. [DOI] [PubMed] [Google Scholar]
- 27.Kakinuma Y, Igarashi K. Potassium/proton antiport system of growing Enterococcus hirae at high pH. Journal of Bacteriology. 1995;177(8):2227–2229. doi: 10.1128/jb.177.8.2227-2229.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakinuma Y, Igarashi K. Isolation and properties of Enterococcus hirae mutants defective in the potassium/proton antiport system. Journal of Bacteriology. 1999;181(13):4103–4105. doi: 10.1128/jb.181.13.4103-4105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinoshita N, Unemoto T, Kobayashi H, Tsutomu Unemoto. Proton Motive Force Is Not Obligatory for Growth of Escherichia coli. Journal of Bacteriology. 1984;160(3):1074–1077. doi: 10.1128/jb.160.3.1074-1077.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]