Abstract
Introduction: The quest for formalin substitutes has long been going on due to its health hazards. Honey has been proven as a safe alternative to formalin. However, we explored more economical, eco-friendly & readily available substances like sugar & jaggery as natural substitutes for formalin. The aim of this study was to compare the tissue fixation abilities of honey, sugar syrup & jaggery syrup with that of formalin using H & E stain and to determine the best fixative among the three.
Materials and Methods: Commercially available fresh goat meat (buccal mucosa) was cut into five bits & each bit was placed in five different containers containing 10% buffered formalin, distilled water, 20% honey, 20% sugar syrup & 30% jaggery syrup with formalin as positive control & distilled water as negative control. 24 hours tissue fixation was attained at room temperature followed by conventional processing and staining. The tissue sections were assessed for cytoplasmic, nuclear details & staining quality under light microscopy. Each criteria was rated on a scale of 1- 4 (1 for poor & 4 for excellent) & the whole procedure was blinded. Results were analysed by Kruskal Wallis ANOVA test. Inter-observer variability was determined by Kappa statistics.
Results: The preservation of tissue by honey, sugar & jaggery syrup was comparable to that of formalin. Among the three natural fixatives, jaggery syrup excelled.
Conclusion: Our effort of using sugar and jaggery for tissue fixation is first of its kind and yielded good results. Hence they can be successfully adopted in routine histopathology laboratories in place of formalin.
Clinical relevance: Natural fixatives can be used in place of the hazardous formalin with equal efficiency. Also, jaggery being highly economical and universally available can be employed in large scale as in screening camps.
How to cite this article: Patil S, Premalatha B R, Rao R S, Ganavi B S. Revelation in the Field of Tissue Preservation – A Preliminary Study on Natural Formalin Substitutes. J Int Oral Health 2013; 5(1):31-38.
Keywords: : Formalin substitutes, Natural tissue fixatives, Non- formalin fixatives, Honey, jaggery syrup, Sugar syrup
Introduction
Formaldehyde was first discovered in 1859 by the Russian chemist Alexander M. Butlerov. Later it was Ferdinand Blum in 19th century who while working on formaldehyde for disinfection accidentally found that it can "fix" the tissue and "the rest is history"; formalin became the fixative of choice in just a few years.1 Since then, the, formalin-fixed paraffin-embedded tissue (FFPET) stained with hematoxylin and eosin (H&E) is the "gold standard" and it has been said that, there is no other histopathology technique that provides so much information so quickly and for such little cost.2,3
Since last few decades, there is a quest for formaldehyde substitutes, motivated by two fundamental developments: The OSHA (Occupational Safety and Health Administration) regulation standard declaring it hazardous and advocating its substitution with less dangerous chemicals and the fact that formalin does not assure a complete DNA and messenger RNA (mRNA) recovery, essential to many tests of molecular biology which are now under continuous development.4,5 Since then a number of chemicals are tried & tested, but none of them could meet the standards set by formalin.
Bee honey has been shown to preserve tissue morphology similar to that by formalin.6 Since sugar & jaggery share similar composition with honey, they may also preserve the tissues. Thereby we explored the eco-friendly, economical & readily available substances like sugar & jaggery as substitutes for formalin. The aim of our study was to compare the tissue fixation abilities of honey, sugar syrup & jaggery syrup with that of formalin using H&E stain and to determine the best fixative among the three.
Materials and Methods:
Commercially available fresh goat meat (buccal mucosa) was bought and cut into five bits and each bit was placed in five different containers containing 10 % buffered formalin, distilled water, 20% honey, 20% sugar syrup & 30% jaggery syrup. Formalin was taken as the positive control & distilled water as negative control. 24 hours tissue fixation was attained at room temperature, followed by conventional processing and staining with H and E. The tissue sections were assessed by two examiners under light microscope & the whole procedure was blinded. The histomorphological criteria examined are elaborated in Table 1. The values obtained were compiled and analysed using Kruskal Wallis ANOVA test. Inter-observer variability was determined by Kappa statistics.
Table 1: Histomorphological criteria.
| HISTOMORPHOLOGIC CRITERIA | RATING |
| a) Cellular outline | Each histomorphologic criteria was rated on a scale of 1- 4 |
| c) Nuclear detail | 1. Poor |
| d) Staining quality | 2. Satisfactory |
| 3. Good | |
| e) Overall morphology | 4. Excellent |
Results:
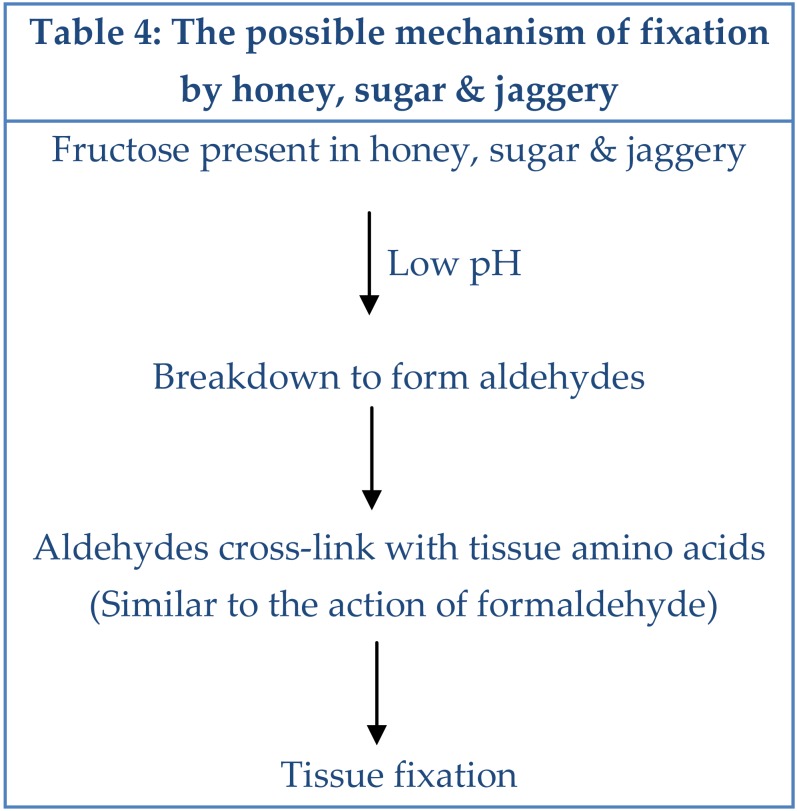
By Kruskal Wallis ANOVA test, the lowest mean score was obtained for tissues fixed with distilled water and the highest for formalin. The mean values for tissue sections fixed with honey, sugar and jaggery syrup were similar to each other and also closer to formalin. Among the three natural fixatives, the mean value of jaggery was superior (Graph 1). A Kappa value of 0.815 suggested high agreement between the observers.
Graph 1: Mean values of different fixatives.
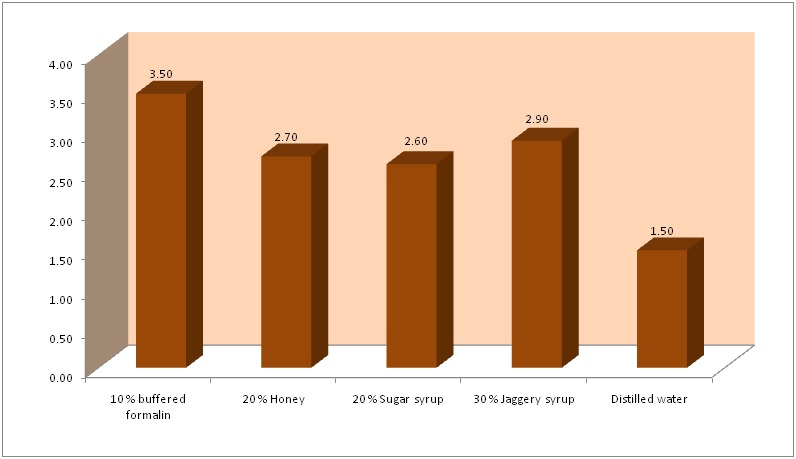
The tissue fixed in 10% buffered formalin gave the ideal results which acted as the positive control for the study (Figure 1 A). On the other hand, tissue in distilled water showed significant cellular swelling & poor staining with H & E indicating tissue autolysis (Figure 1 E). All the three natural fixatives were able to preserve the tissue over a period of 24 hours. In honey fixed tissue sections, the cytoplasmic and nuclear details were satisfactory but showed areas of uneven staining (Figure 1 B). Similarly, tissue fixed with sugar syrup showed fair cytoplasmic and nuclear details with uneven staining. Also, difficulty was encountered while sectioning the tissue which resulted in fold artefacts. (Figure 1 C). With jaggery fixation, the tissue sections had good overall morphology and also good nuclear, cytoplasmic details and staining quality. In addition, the cellular outlines were clearly discernible. (Figure 1 D). Hence all three natural substances were able to preserve the tissue over a period of 24 hours; with jaggery giving the best results. To sum up the overall results, the tissue fixation ability was in the following order: Formalin > Jaggery > Honey> Sugar > distilled water.
Figure 1: Photomicrograph of the tissues fixed in: A. Formalin, B. Honey, C. Sugar syrup, D. Molasses syrup, E. Distilled water (H & E, 40X).
Discussion:
Formalin is the universal fixative in routine histopathology. The fundamental advantage stems from its continuous and almost universal use for over 100 years and all the accumulated scientific knowledge on it.2 Also, formalin is readily available, economical, fairly convenient to store, allows long-term storage, preserves lipids well, and has been accepted as the closest thing there is to the perfect fixative, with no clear "all-purpose" alternative found to date.7, 3
On the other hand, formalin has two well known disadvantages. Firstly, formalin is highly toxic. The International Agency for Research on Cancer (IARC) classifies formaldehyde as a human carcinogen that can cause nasopharyngeal cancer.8 Lu et al found strong evidence that can support a genotoxic and cytotoxic mode of action for the carcinogenesis of inhaled formaldehyde in respiratory nasal epithelium.9 The various health hazards of formalin are collated in Table 2. Guidelines for ambient formaldehyde levels in living spaces have been set in several countries in the range of 0.05 to 0.4 ppm, with a preference to 0.1 ppm.10 Secondly, the chemical action of formalin binds severely to DNA, RNA and proteins, which makes them difficult or impossible to extract in a useful form for molecular tests.11,12
Table 2: Health hazards of formalin13,14,15.
| ORGAN SYSTEM | ADVERSE EFFECTS |
| Skin & mucous membrane | Irritation of the mucous membranes of the mouth and upper respiratory tract, allergic contact dermatitis |
| Respiratory system | Sneezing, coughing, laryngospasm, pulmonary edema, temporary reversible decrease in lung function, degenerative diseases, inflammatory and hyperplastic changes of the nasal mucosa, asthma, chronic rhinitis, loss of olfactory functioning |
| Eye | Irritation, lacrimation, conjunctivitis |
| Gastrointestinal tract | Irritation, nausea, vomiting, diarrhoea, loss of appetite, burns and ulceration, abdominal pain, gastrointestinal hemorrhage, pharyngeal congestion, chronic pharyngitis |
| Cardiovascular system | Tachypnoea, nodal tachycardia |
| Central nervous system | Dizziness, depression, headaches, sleep disorders, memory loss, convulsions and coma |
| Haematopoietic system | High serum alanine-amino transferase (ALT), Lower RBC, WBC, platelet and hemoglobin counts |
| Renal system | Renal failure |
| Reproductive system | Menstrual disorder, dysmenorrhea and spontaneous abortion |
For centuries, honey has been shown to be a successful antibacterial agent having the potential to preserve compounds without any harmful effects on users. In 2006, Rahma Al-Maaini and Philip Bryant showed that tissues fixed in low concentrations of honey at room temperature gave results comparable to formalin fixed control tissues.16 Properties of honey such as high osmolarity, low pH and the presence of components such as hydrogen peroxide and phenol inhibine, all contribute to its anti-oxidative and antibacterial effects.17-21 Later, several studies proved that honey can be a safe alternative to formalin in conventional histochemical and immunohistochemical staining methods.6,22,23
Sugar and jaggery are derivatives of sugarcane juice and are well known for their preservative properties.24,25,26 The use of sugar for wound healing is one of the earliest known methods. According to Herszage and associates, treatment with sugar destroys bacteria non-specifically by creating an environment of low water activity which inhibits bacterial growth.27
Jaggery is a widely used traditional Indian sweetener. Of the total world production, more than 70% of jaggery is produced in India.28 A study by M.A. Harish Nayaka et al, proved the presence of cytoprotective and antioxidant activity in jaggery.25 On the other hand, molasses which is also a cane derivative; is used widely in US, UK, Denmark, Russia, Africa and Asia.29 Table 3 elaborates the properties of molasses and jaggery and Figure 2 depicts the two products.
Table 3: Properties of Molasses and Jaggery 30,31.
| FEATURES | MOLASSES | JAGGERY |
| Description | Thick, dark brown, uncrystallized juice obtained from raw sugar during the refining process. | If pure clarified sugarcane juice is boiled, what is left as solid is jaggery. |
| Total sucrose content | >46 % | 65- 85 % |
| Water content | 20 % | 10- 12% |
| Usage | Livestock and poultry feeds, fertilizer, fuel | Traditional Indian sweetener |
| Availability | US, UK, Denmark, Russia, Africa, Asia | India, Pakistan, Mexico, South America, Burma, African countries, Srilanka, Thailand |
Figure 2: A. Jaggery, B. Molasses.
Since honey is not universally available and it is impractical to use honey in large scale due to its high cost, we went further in the quest for exploring substances that can overcome these major drawbacks. The decision of choosing sugar and jaggery was made as their composition is similar to that of honey. This attempt of ours is the first of its kind with no existing literature on the usage of jaggery and sugar as formalin substitutes.
To begin with, we tried to standardize the dilution of sugar and jaggery by using different concentrations (10 %, 20 %, 30 %, and so on). Higher concentrations were found to cause tissue shrinkage & loss of tissue architecture. A concentration of 20 % for sugar & 30 % for jaggery syrup gave the optimum results. Previous studies on honey has shown that low concentrations of 10% and 20% can fix the tissues.16 Hence we considered 20 % honey, 20% sugar syrup and 30% jaggery syrup for our study.
The possible mechanism of fixation by sugar & jaggery may be similar to that of honey.22,30,31 This requires an acidic pH. At concentrations that we used, the pH of all the three substances ranged between 4.5 to 5.5 which was in favour of this concept (Table 4).
Table 4: The possible mechanism of fixation by honey, sugar & jaggery.
All the three natural substances: honey, sugar & jaggery gave promising results. But jaggery exceeded our expectations, even surpassing the proven honey. There are several advantages of using honey, sugar & jaggery for tissue fixation: they are non- hazardous, compatible with routine processing, staining and do not require additional equipments. Jaggery, in addition is easily available and highly economical when compared to honey. It costs about 1/6th the price of honey. The natural substitutes can be used where formalin may not be available on time of biopsy and also in large scale, as in screening camps.
All these natural substances are liable to develop molds over time; hence it is advisable to use thymol crystals as an antimicrobial agent. In addition, jaggery fixed specimen showed brownish discoloration. (Figure 3) Nevertheless, there was no interference with subsequent staining. Some of the problems faced with usage of these natural fixatives during tissue handling and their remedy are enlisted in Table 5.
Figure 3: Photograph of gross tissue specimens fixed in: A. Formalin, B. Honey, C. Sugar syrup, D. Jaggery syrup, E. Distilled water .
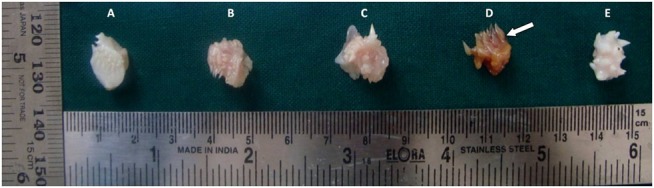
Table 5: Problems encountered with different fixatives and their remedies.
| PROBLEM | FIXATIVES | REMEDY |
| Breach in continuity of sections |
|
|
| Intense staining with eosin |
|
|
| Folding of the tissue sections |
|
|
It is heartening to know that some of the commonly available, day to day substances give pleasant surprises! Fixation of tissue by sugar syrup & jaggery syrup is an innovative attempt. Among the three natural fixatives investigated, the humble jaggery has all the novel qualities to be an excellent substitute for formalin in tissue fixation. Natural fixative is the arena which requires further exploration and large scale implementation.
Conclusion:
Formalin has taken over the field of fixation since 19th century. Natural substitutes like honey, sugar & jaggery are a boon when health hazards of formalin are considered. In our study, the preservation of tissue by honey, sugar & jaggery was comparable to that of formalin. Surprisingly, among the natural fixatives, jaggery syrup showed better preservation over a period of 24 hours when compared to the proven honey. Thus, we conclude that the eco-friendly natural fixatives have all the novel qualities to replace formalin. And, jaggery syrup as a substitute for formalin is a breakthrough in the field of tissue preservation. Here we are introducing the new HEALTHY FIXATIVES!!!
Footnotes
Source of Support: Nil
Conflict of Interest: None Declared
Contributor Information
Shankargouda Patil, Department of Oral Pathology, M S Ramaiah Dental College, Bangalore, India.
BR Premalatha, Department of Oral Pathology, M S Ramaiah Dental College, Bangalore, India.
Roopa S Rao, Department of Oral Pathology, M S Ramaiah Dental College, Bangalore, India.
BS Ganavi, Department of Oral Pathology, M S Ramaiah Dental College, Bangalore, India.
References:
- 1.Cecil H Fox, Frank B Johnson, John Whiting, Peter P Roller. Formaldehyde Fixation. J Histochem Cytochem. 1985;33(8):845–853. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- 2.Rosai J. Why microscopy will remain a cornerstone of surgical pathology. Lab Invest. 2007;80:403–408. doi: 10.1038/labinvest.3700551. [DOI] [PubMed] [Google Scholar]
- 3.Rene J Buesa. Methods in Pathology Histology without formalin? Annals of Diagnostic Pathology. 2008;12:387–396. doi: 10.1016/j.anndiagpath.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Formaldehyde. OSHA [Google Scholar]
- 5.Kok LP, Boon ME. Coulomb Press; Leyden: 2003. Microwaves for the Art of Microscopy; p. 368. [Google Scholar]
- 6.Ozkan N, Salva E, Cakalagaoglu F, Tuzuner B. Honey as a substitute for formalin? Biotechnic & Histochemistry. 2012;87(2):148–153. doi: 10.3109/10520295.2011.590155. [DOI] [PubMed] [Google Scholar]
- 7.Hopwood D. Fixatives and fixation: A review. Histochem J. 1996;1:323–360. doi: 10.1007/BF01003278. [DOI] [PubMed] [Google Scholar]
- 8.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol. http://monographs.iarc.fr/ENG/Monographs/vol88/index.php. IARC. 2006;88 [PMC free article] [PubMed] [Google Scholar]
- 9.Lu K, Collins LB, Ru H, Bermudez E, Swenberg JA. Distribution of DNA adducts caused by inhaled formaldehyde is consistent with induction of nasal carcinoma but not leukemia. Toxicol Sci. 2010;116(2):441–451. doi: 10.1093/toxsci/kfq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Documentation of the TLVs® and BEIs® with Other Worldwide Occupational Exposure Values—2003. American Conference of Government Industrial Hygienists ®Worldwide. 2003 [Google Scholar]
- 11.Gillespie JW, Best CJM, Bichsel VE, Cole KA, Greenhut SF, Hewitt SM. Evaluation of non-formalin tissue fixation for molecular profiling studies. Am. J. Pathol. 2002;160:449–452. doi: 10.1016/S0002-9440(10)64864-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox ML, Schray CL, Luster CN, Stewart ZS, Korytko PJ, Khan KNM. Assessment of fixatives, fixation and tissue processing on morphology and RNA integrity. Exp. Mol. Pathol. 2006;80:183–191. doi: 10.1016/j.yexmp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Ki-Hyun Kim, Jahan Shamin Ara, Lee Jong-Tae. Exposure to Formaldehyde and Its Potential Human Health Hazards. Journal of Environmental Science and Health, Part C: Environmental Carcinogenesis and Ecotoxicology. 2011;29(4):277–299. doi: 10.1080/10590501.2011.629972. [DOI] [PubMed] [Google Scholar]
- 14.Wakefield JC. Health Protection Agency. London: 2008. Formaldehyde–Toxicological. [Google Scholar]
- 15.Office of Environmental Health Hazard Assessment. Environmental Protection Agency; California: 2001. Prioritization of Toxic Air Contaminants - Children’s Environmental Health Protection. [Google Scholar]
- 16.AI-Maaini Rahma, Bryant Philip. The Effectiveness of Honey as a Substitute for Formalin in the Histological Fixation of Tissue. The Journal of Histotechnology. 2006;29(3):173–176. [Google Scholar]
- 17.White JW, Subers MH, Schepartz AI. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose oxidase system. Biochim Biophys Acta. 1963;73:57–70. doi: 10.1016/0006-3002(63)90359-7. [DOI] [PubMed] [Google Scholar]
- 18.Molan PC. The antibacterial activity of honey.1. The nature of the antibacterial activity. Bee world. 1992;73:15–28. [Google Scholar]
- 19.Molan PC. A brief review of the use of honey as a clinical dressing. Austr J Wound Manage. 1998;(6):148–158. [Google Scholar]
- 20.Molan PC. Selection of honey for use as a wound dressing. Primary Intention. 2000;8:87–92. [Google Scholar]
- 21.Al- Jabri AA, Nzeako B, Al Maurooqi Z, Al Naqdy A, Nsanze H. In vitro antibacterial activity of Omani and African honey. Br J Biomed Sci. 2003;60:1–4. doi: 10.1080/09674845.2003.11783668. [DOI] [PubMed] [Google Scholar]
- 22.AI-Maaini Rahma, Bryant Philip. Honey as an Alternative to Formalin in the demonstration of Connective Tissue Components. The Journal of Histotechnology. 2008;31(2):67–72. [Google Scholar]
- 23.Gunter Mandy, Bryant Philip. Immunohistochemical evaluation of ductal carcinoma in breast after preservation in honey. The J Histotechnol. 2009;32(2):54–59. [Google Scholar]
- 24.Karthikeyan, J, Samipillai S. Sankar. Sugarcane in therapeutics. Journal of Herbal Medicine and Toxicology. 2010;4(1):9–14. [Google Scholar]
- 25.Nayaka MA. Harish, Sathisha UV., Manohar MP., Chandrashekar KB., Dharmesh Shylaja M. Cytoprotective and antioxidant activity studies of jaggery sugar. Food Chemistry. 2009;115:113–118. [Google Scholar]
- 26.Mann Jim. Sugar revisited – again. Bulletin of the World Health Organization. 2003;81(8):552. [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas Atanu, Bharara Manish, Hurst Craig, Rainer Gruessner, Armstrong David, Rilo Horacio. Use of sugar on the healing of diabetic ulcers: A review. Journal of Diabetes Science and Technology. 2010;4(5):1139–1145. doi: 10.1177/193229681000400512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao PVK Jagannadha, Das Madhusweta, Das SK. Jaggery- A Traditional Indian sweetener. Indian Journal of Traditional Knowledge. 2007;6(1):95–102. [Google Scholar]
- 29.Leo V Curtin. National Feed Ingredients Association. West Des Moines, Iowa: 1983. Molasses - General Considerations. Molasses in Animal Nutrition. [Google Scholar]
- 30.White JW Jr, Doner LW. Agriculture Handbook. 1980. Honey composition and properties. Bee keeping in the United States; p. 335. [Google Scholar]
- 31.Henriques A, Burton NF, Cooper RA. Antibacterial activity of selected Portuguese honeys. J Apic Res. 2005;44:119–123. [Google Scholar]