A panoramic review of the development, maturation, and future of concepts surrounding cardiac pacemaking.
Abstract
Physiological processes governing the heart beat have been under investigation for several hundred years. Major advances have been made in the recent past. A review of the present paradigm is presented here, including a look back at important steps that led us to where we are today, alongside a glimpse into the exciting future of pacemaker research.
The mechanisms initiating and perpetuating the spontaneous heartbeat in living organisms have occupied scientists' thoughts for several hundred years. Many mysteries remain, despite major advances in the last 20 years. The cells comprising the small, localized region of right atrium from which the cardiac impulse originates was discovered in 1907 by English medical student Martin Flack and his mentor Sir Arthur Keith during studies in small mammal hearts (63). They termed this heterogeneous yet distinctive collection of cells the “sino-auricular node” [now the “sino-atrial node” (SAN)] and sparked the deluge of scientific papers investigating why sino-atrial nodal cells (SANC) should behave in such a marvelous way, spontaneously depolarizing 2.8 billion times in the average human lifetime. This review presents an overview of modern concepts of the origin of the heart beat and provides a glimpse into the exciting future of pacemaker research.
Diastolic Depolarization is the Essence of Pacemaking
Spontaneous electrical depolarization during the diastolic phase [“phase 4,” diastolic depolarization (DD); see FIGURE 1] following an action potential is the hallmark of cells comprising pacemaking tissues of the heart (sino-atrial node, atrioventricular node, bundle of His, bundle branches, and His-Purkinje system). Diastolic depolarization causes a slow increase in membrane potential toward an excitation “threshold” (see FIGURE 1), at which point a stereotyped, all-or-nothing action potential fires. The production of repetitive, rhythmical action potentials is the primary responsibility of sino-atrial nodal cells, because their intrinsic rate of diastolic depolarization is (normally) the most rapid. Recent work in the atrioventricular node and Purkinje cells suggests that the mechanisms governing sino-atrial node automaticity are likely to be similar in all pacemaking tissues.
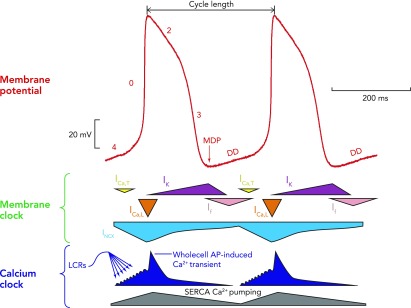
FIGURE 1.
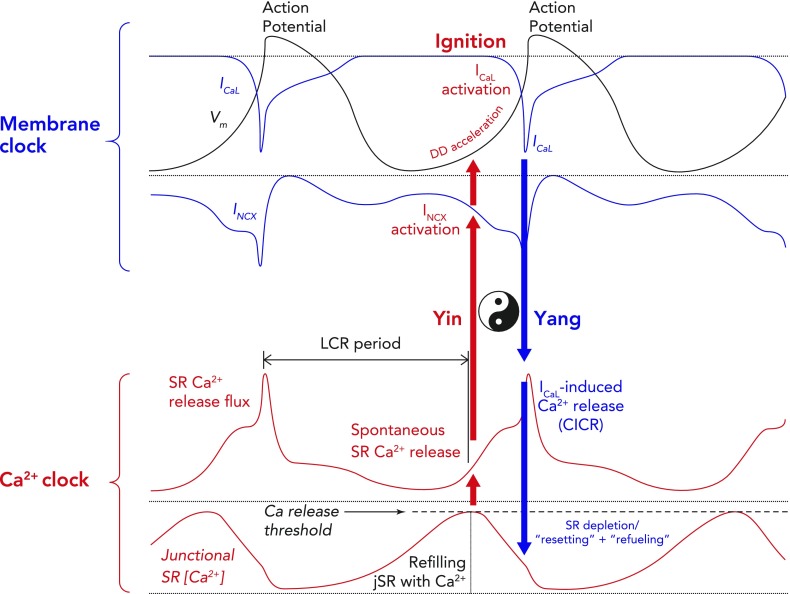
A schematic interplay of different membrane clock and calcium clock mechanisms
An example of a typical action potential of spontaneously beating rabbit SANC (red trace; top). The different phases of the AP are labelled. Special note should be taken of phase 4, representing diastolic depolarization, which is the defining feature of cells capable of pacemaking. Schematic representation of the timing and magnitude of the different components of the “membrane clock” is shown in the middle, whereas the timing and magnitude of the different components of the “Ca2+ clock” are shown at the bottom. It can be seen from the bottom (dark blue) that, during phase 4, total cytosolic [Ca2+] gradually builds due to accumulation of Ca2+ in the form of spontaneous local Ca2+ releases emanating from the sarcoplasmic reticulum. Toward the end of diastole, activation of L-type Ca2+ channels causes Ca2+-induced Ca2+ release from the sarcoplasmic reticulum via ryanodine receptors, resulting in the whole cell Ca2+ transient. Cytoplasmic Ca2+ is then removed by both the SR Ca2+ pump, SERCA, and the sarcolemmal sodium-calcium exchanger. MDP, maximum diastolic potential; DD, diastolic depolarization; ICa,T, T-type voltage-dependent Ca2+ current; ICa,L, L-type voltage-dependent Ca2+ current; INCX, sodium-calcium exchange current; IK, delayed rectifier potassium current; If, funny current; SERCA, sarco-endoplasmic reticulum ATPase; LCRs, local Ca2+ releases.
Action potentials generated by pacemaking tissues at appropriate times capture excitable cells of the working atrial and ventricular myocardium, which in turn perform their duty cycle, inducing timely and proportionate expulsion of blood from the heart. Individual sino-atrial nodal cells must continually respond to complex signals generated intrinsically at the subcellular, cellular, and tissue levels, as well as extrinsically from the autonomic nervous system, other hormonal systems, and tissue stretch, and transduce these signals into a beating rate that reflects the integral of the myriad inputs and satisfies the body's demands.
Ion Channels and the Membrane Clock
Since diastolic depolarization is electric in nature (discharging the membrane capacitor), the ensemble of ion channels spanning the sino-atrial nodal cell plasma membrane (sarcolemma) drew attention first in the search for mechanisms underlying spontaneous pacemaking (see FIGURES 1, 2, AND 4). These proteins facilitate selective passage of specific charged species through aqueous pores down concentration gradients created by diverse mechanisms, including exchangers and pumps (50). Ion channels can produce voltage-sensitive membrane currents, which vary in direction and amplitude depending on the membrane potential at a given moment, as well as the ionic constitution of the intra- and extracellular milieu. The currents are activated and then undergo time-dependent decay (see FIGURE 1) due to channel inactivation, with variable times to recovery before they may function once more.
FIGURE 2.
Schematic figure illustrating the cross talk between the membrane clock (right side of schematic SANC) and the Ca2+ clock (lying in the center of the schematic SANC)
Online animation reveals schematic behavior of individual ion channels, including the time-dependent nature of the currents that they produce and the coupling of this time- and voltage-dependent behavior to the intracellular Ca2+ clock (see animation at Physiology's website). The movie was created by Victor Maltsev.
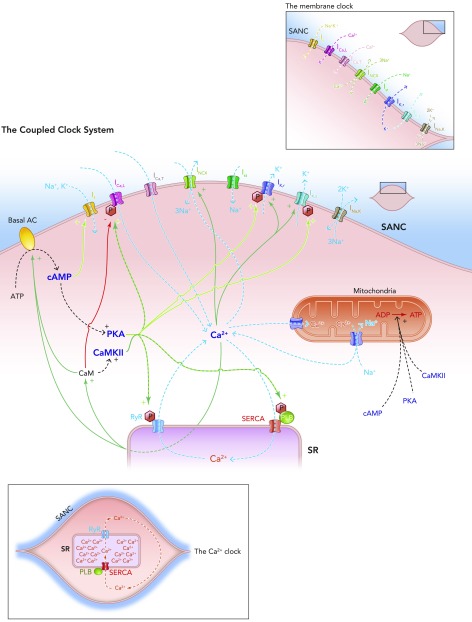
FIGURE 4.
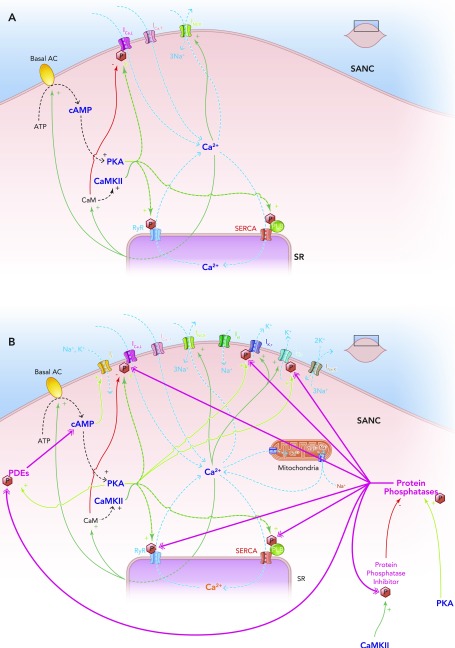
Schematic diagram of the individual elements of the membrane clock (top right) and the Ca2+ clock (bottom left) and how they come together to make up the coupled clock system (middle)
The situation under baseline conditions is shown, whereby constitutively active Ca2+-activated ACs produce relatively high levels of cAMP, which go on to activate PKA, in turn leading to relatively high levels of phosphorylation of critical mediators of automaticity belonging to both membrane (ICa,L, IK,r, IK,s) and Ca2+ clocks (RyR, PKA), with the overall result of higher levels of intracellular Ca2+. This elevated Ca2+ then further activates the ACs, and also calmodulin, leading to activation of CAMKII, and so further phosphorylation of critical mediators of automaticity, with the ultimate result of a further increase in intracellular Ca2+; Ca2+ release begets Ca2+ release. This is only maintained at mid-range by constitutively highly active phosphodiesterases and protein phosphatases (see FIGURE 5B).
Several ionic currents have important roles in spontaneous pacemaking, and because of their time-dependent behavior and sarcolemmal localization, their ensemble behavior has been termed “the membrane clock.”
Decay of IK conductance (gK decay)
Spontaneous diastolic depolarization was initially attributed to the decay of IK2, a theoretical outward current that had been activated by the action potential (see FIGURE 1, purple segments). This gradual decrease in delayed rectifier K+ current due to time- and voltage-dependent inactivation of K+ channels following an action potential was said to “uncover” theoretical inward (“background”) currents after repolarization (53). The inward current was believed to be mediated by intracellular movement of Na+ ions and referred to as Ib,Na, although its molecular basis remains unclear.
The Funny Current
The most notorious ionic current contributing to the membrane clock is the hyperpolarization-activated cation current, or “funny” current (If), carried by HCN channels (mainly HCN4 in the human sino-atrial node) (see FIGURE 1, pink segments) (3). This current is in fact a mixed inward Na+ and K+ current, activated with slow kinetics on hyperpolarization at membrane potential −50 to −65 mV (4, 8). It was originally labeled as funny because ionic currents in cardiac cells are usually activated by depolarization rather than by hyperpolarization.
The role of If in pacemaking was initially speculated to be mainly protective (78), shielding central sino-atrial nodal cells from the hyperpolarizing, bradycardic effect of surrounding atrial myocardium. Later, the focus shifted to If being “the pacemaker current” (53), controlling basal normal automaticity and contributing to the response of the sino-atrial node to autonomic inputs. Specifically, If was proposed to be primarily responsible for the process of diastolic depolarization, moving membrane potential from its nadir following repolarization [the maximum diastolic potential (MDP)] to potentials at which voltage-gated Ca2+ channels activate (approximately −40 mV) to complete diastolic depolarization and achieve the “threshold” potential (see FIGURE 1) (40). This purported central role of If as a general pacemaking mechanism was questioned when it was shown that some species do not express If at all in cardiac regions leading pacemaking [e.g., the bullfrog sinus venosus (61)]. In those species that do express If, in vitro blockade of If variably increases cycle length between 5 and 20% (11, 46, 76) but never stops pacemaking completely.
If-carrying HCN channels are exclusively expressed in pacemaking regions of the heart. The presence of the Hcn4 gene is often utilized as a marker of pacemaking tissue (9). Geographical colocalization of HCN channels with pacemaking regions is taken at times as evidence of If's central importance in automaticity (3), although the concept that the mere presence of a channel guarantees its importance in the physiological function of that region is debatable (34).
HCN channel activity has been shown to be modulated by direct interaction with cyclic adenosine monophosphate (cAMP), produced at the sino-atrial nodal cell sarcolemma from adenosine triphosphate (ATP) by both Ca2+-activated and Ca2+-inhibited adenylate cyclases (ACs; see FIGURE 4). Activation or inhibition of these ACs is a critical pathway by which the sympathetic and parasympathetic arms of the autonomic nervous system control pacemaking rate (see FIGURE 6) (12). cAMP produced as a consequence of β-adrenergic stimulation causes a depolarizing shift in the voltage dependence of the If activation curve (13), meaning that more If current is available at diastolic potentials, so causing a steeper slope to diastolic depolarization and shorter time between maximum diastolic potential and threshold, i.e., a faster beating rate (so long as the duration of the action potential is assumed to remain constant) (12). Muscarinic receptor activation acts on If in the opposite manner (12, 14). The physiological relevance of this rate modulation in vivo has recently been questioned by data demonstrating that a full range of responses to autonomic agonists (affecting both β-adrenoceptors and muscarinic receptors) is possible even in the complete absence of If or when If function is deficient (1, 19, 21, 22, 37, 60).
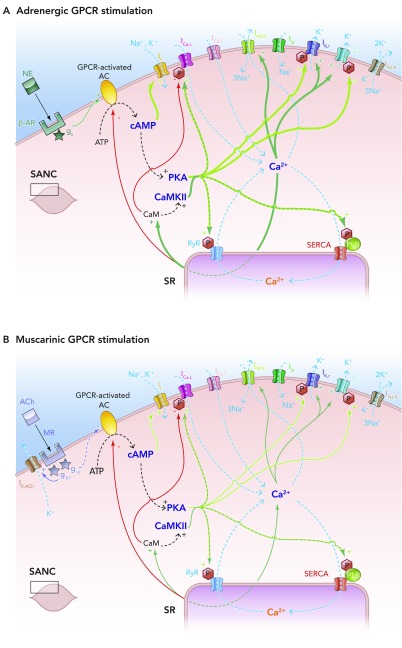
FIGURE 6.
Effects of autonomic nervous system activity on the coupled clock system of pacemaking
A: schematic illustration of the effects of adrenergic stimulation on the coupled clock system of pacemaking. In vivo, epinephrine produced from the adrenal glands and norepinephrine from sympathetic nerve terminals binds to β-adrenoceptors, also known as G-protein-coupled receptors. This leads to the Gs subunit activating G-protien-coupled receptor-activated, Ca2+-inhibited adenylate cylases (ACs), which produce cAMP from ATP in additional quantities to that being made by constitutively active, Ca2+-stimulated ACs. This additional cAMP further activates PKA (and If) to lead to an increase in levels of phosphorylation of critical mediators of automaticity belonging to both the membrane and Ca2+ clocks. This in turn leads to the overall levels of intracellular Ca2+ being higher, with subsequently higher levels of activation of calmodulin and thence CAMKII, with more phosphorylation. The ultimate effect is more rapid cycling of greater amounts of Ca2+, and hence an increase in the ticking speed of the coupled clock system. B: schematic illustration of the effects of muscarinic stimulation on the coupled clock system of pacemaking. Acetylcholine (ACh) produced from parasympathetic nerve terminals binds to muscarinic receptors (MR) on SANC (MR also being a form of G-protein-coupled receptors), leading to two predominant effects. The first of these is the direct interaction between the gβγ subunit and IK,ACh, which leads to an outward, repolarizing K+ current. The second effect is that the giα subunit interacts directly with G-protein-coupled receptor-coupled ACs to inhibit these enzymes, causing a decrease in the amount of cAMP produced, with less activation of PKA (and If). Less activation of PKA means that levels of phosphorylation fall, with levels of intracellular Ca2+ falling too. Less intracellular Ca2+ means less activation of CAMKII, so even less phosphorylation. The combined effects of IK,ACh activation and AC inhibition means a slower rate of ticking of the coupled clock system.
Thus the views on If's exact role in the generation of automaticity have shifted with time. If has now come full circle and is once more generally viewed as a safety net (provider of “depolarization reserve,” preventing excessive hyperpolarization) (21) for the prevention of excessive bradycardias (78). The more bradycardic a sino-atrial nodal cell, the more important If is likely to be, due to its slow activation kinetics. The effects of If may be greatest of all in cells with more negative maximum diastolic potentials than sino-atrial nodal cells (e.g., Purkinje cells), since its activation voltage is less than −65 mV, lower than that typically seen in sino-atrial nodal cells.
L-Type Voltage-Dependent Ca2+ Channels
The L-type Ca2+ current, ICa,L, generates the upstroke of the action potential in central sino-atrial nodal cells, where the fast sodium current (usually responsible for this part of the action potential in atria and ventricles) is absent (see FIGURE 1, orange segments). ICa,L activates at voltages more negative than the threshold potential (see FIGURE 1), i.e., −50 to −40 mV (18), suggesting additional involvement in later diastolic depolarization as well as in the action potential upstroke. It has been suggested from mouse experiments that mainly L-type Ca2+ channels containing the pore-forming α1-subunit Cav1.3 are involved in diastolic depolarization, whereas Cav1.2-containing channels are preferentially involved in the depolarizing upstroke of the sino-atrial nodal cell action potential (45).
T-Type Voltage-Dependent Ca2+ Channels
The T-type Ca2+ current, ICa,T, activates at more negative potentials (approximately −60 mV) than its L-type cousin (see FIGURE 1, yellow segments) (47). It has a postulated role in bringing about early diastolic depolarization, based on evidence that loss of ICa,T slows spontaneous pacemaking (18).
INa,K
This sarcolemmal ATPase pump (see FIGURE 4), extruding three Na+ ions and transporting two K+ ions intracellularly, generates a net outward, hyperpolarizing current (INa,K or Ip) that influences pacemaking by slowing the rate of automaticity (46). In sino-atrial nodal cells, INa,K contributes to setting the maximum diastolic potential (55), and If-derived Na+ ions are considered important in determining the magnitude of INa,K due to the exquisite sensitivity of the sodium-potassium pump to fluctuating concentrations of Na+ (58).
The Integrated Ion Current Membrane Clock
Using reductionist experimental techniques, it has been possible to define time- and voltage-dependent behaviors of individual ionic currents. Harnessing the resultant ion channel biophysical data, diverse in silico theoretical models of sino-atrial nodal cell behavior have been produced, capable of recapitulating the shapes of spontaneous action potentials. Since the ensemble of surface membrane ion channels can generate spontaneous and self-perpetuating action potentials in silico (76), it was dubbed a “membrane clock” (44). Thus the membrane clock theory of pacemaking proposes that dynamic time- and voltage-dependent interaction of the above membrane-delimited electrogenic ion channels leads diastolic depolarization from maximum diastolic potential to threshold potential, so activating L-type voltage-dependent Ca2+ channels to effect the rapid upstroke of the action potential. Repolarization to the maximum diastolic potential is then effected by the array of voltage-dependent K+ channels that yield transient outward (Ito), fast delayed rectifier (IK,r), and slow delayed rectifier (IK,s) K+ currents, after which point the whole process starts again (see FIGURES 1, 2, AND 4).
Intracellular Ca2+ Cycling and the Ca2+ Clock
The existence of a second independent (yet entrainable) clock generating diastolic intracellular Ca2+ releases from a major intracellular store [the sarcoplasmic reticulum (SR); see FIGURE 4, BOTTOM LEFT] proved to be an intriguing hypothesis. Intracellular Ca2+ releases in cardiac cells were, for many years, considered to represent pathological phenomena, since they could only be recreated experimentally under conditions of abnormal electrolyte concentrations or toxic doses of digitalis (52).
Initial suspicions regarding the importance of Ca2+ signals in sino-atrial nodal cells developed from observations that blocking intracellular SR Ca2+ release with the plant alkaloid ryanodine in cat latent atrial pacemaker cells led to a progressive decrease in the rate of the latter part of diastolic depolarization (57). This suggested occurrence of a late diastolic phase of Ca2+ release from the SR. Confocal microscopy and Ca2+-sensitive fluorescent probes allowed the identification of spontaneous, roughly periodic, diastolic Ca2+ releases localized to the subsarcolemmal region of pacemaking cells. These were dubbed “local Ca2+ releases” (LCRs), and the timing of their occurrence relative to the prior peak of the action potential-induced whole cell Ca2+ transient was referred to as “local Ca2+ release period” (see FIGURE 3).
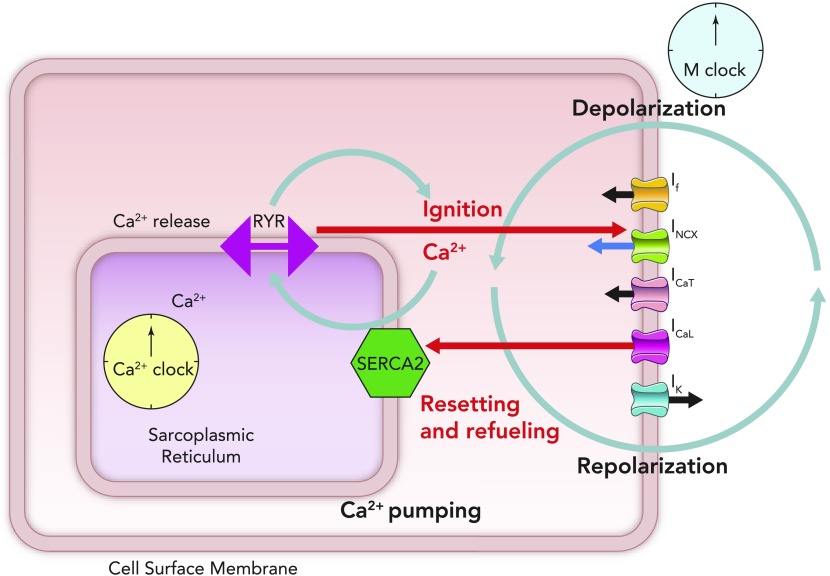
FIGURE 3.
Schematic of computer modeling simulations of the intricate relationship between critical components of the membrane and Ca2+ clocks, with respect to phases of the action potential
Two consecutive whole action potentials are illustrated in black at the top. The critical components of the membrane clock are illustrated in blue at the top. ICa,L is depicted as an inward current because its waveform is shown as occurring below the isoelectric line. It can be seen that, during the latter parts of diastolic depolarization, ICa,L activates and rapidly increases to cause the rapid upstroke of the SANC action potential. ICa,L then gradually fades during repolarization until it becomes negligible at the maximum diastolic potential. INCX is also depicted as a blue waveform below the isoelectric line, clarifying that it is an inward current. INCX functions throughout the cardiac cycle, gradually increasing as diastolic depolarization proceeds and peaking in response to the large increase in ICa,L that occurs concomitant with Ca2+-induced Ca2+ release from the SR. The critical components of the Ca2+ clock are illustrated in red at the bottom. SR Ca2+ release flux increases during diastolic depolarization as the signal mass of diastolic LCRs grows. This then peaks as there is en masse activation of RyRs due to their interaction with cytosolic Ca2+ derived from L-type Ca2+ channels, leading to Ca2+-induced Ca2+ release. SR Ca2+ release then falls during repolarization, reaching a nadir at a time similar to (but not the same as) the maximum diastolic potential (Ca2+ nadir usually lagging behind the maximum diastolic potential). Ca2+ release flux then grows once more as the SR is refilled with Ca2+ by SERCA and the threshold for RyR Ca2+ release in the form of LCRs is reached. The concept of the “LCR period” is illustrated as the time taken from the peak of the prior action potential-induced Ca2+ transient to the timing of first release of LCRs (corresponding to the timing of maximal junctional SR Ca2+ content). “Ignition” is the term used to describe the effect of diastolic LCRs that interact with NCX to cause the membrane potential to increase toward the threshold for activation of the L-type Ca2+ current, which “ignites” an action potential. Finally, Ca2+ concentration within the “junctional” SR is depicted in the lowermost red waveform. Junctional refers to the part of the SR that is closest to the sarcolemma (so-called jSR). jSR Ca2+ concentration is at its greatest just before RyRs begin to release diastolic LCRs in early diastole. As the signal mass of LCRs produced increases, so the jSR Ca2+ concentration decreases, reaching a nadir when L-type Ca2+ channel-derived Ca2+ prompts Ca2+-induced Ca2+ release. Following this, the SR gradually refills, courtesy of the action of SERCA, until it once more reaches a peak in early diastole just before the resumption of production of LCRs. Of greater importance than any of the individual component waveforms in this model is the sum of the complex interaction between them. There is a yin-yang relationship between behaviors of the two clocks. For example, as jSR Ca2+ concentration peaks, RyR Ca2+ release flux in the form of LCRs also begins to increase, leading to an increase in INCX and a quickening of the rate of diastolic depolarization (red arrow). As ICa,L reaches its peak just before the peak of the action potential, INCX is also reaching its maximum, as is RyR Ca2+ release flux (CICR), whereas jSR Ca2+ concentration is about to hit rock bottom (blue arrow). These relationships rock back and forth on a beat-to-beat basis to ensure continued automaticity in the coupled clock model.
Local Ca2+ releases emerge spontaneously in sino-atrial nodal cells under basal conditions in the form of sparks from SR-based Ca2+ release channels, ryanodine receptors (RyRs) (23, 27) (see FIGURES 1 AND 3). The presence of Ca2+ overload or sympathetic stimulation is not required for their production. Confocal and two-dimensional fluorescence imaging techniques demonstrate that local Ca2+ releases begin to tentatively emerge from the SR following the dissipation of the global, action potential-induced Ca2+ transient, as a hypothetical intra-SR Ca2+ threshold for spontaneous RyR activation is achieved. During the ensuing diastole, the integrated local Ca2+ release Ca2+ signal increases at a variable rate, reaching a crescendo immediately before the onrushing action potential. This, in turn, triggers en masse emptying of the SR of its Ca2+ (see FIGURE 1), completely engulfing the Ca2+ being produced in the form of local Ca2+ releases. The SR is then refilled by its ATPase pump SERCA, and the cycle begins once more.
RyRs are critical to the generation of cardiac automaticity; knockout of RyR2 is embryonically lethal in mice (66). Inducible, cardiac-specific knockout of RyR in the more developmentally advanced mouse decreases RyR protein expression by ∼50% and leads not only to features of cardiomyopathy but also to bradycardia and severe arrhythmias (7).
To appreciate the intrinsic behavior of the Ca2+ clock, it was necessary to once more adopt a reductionist approach, disengaging it from the membrane clock, preventing ionic/chemical or electrical cross-contamination. Studying the Ca2+ clock in isolation, it has become clear that the phenomenon of spontaneous diastolic Ca2+ release is an almost rhythmic (1–5 Hz) process, not dependent on the functioning of membrane-bound ion channels or even the presence of the sarcolemma for its perpetuation. Specifically, local Ca2+ releases continue in chemically “skinned” sino-atrial nodal cells in which the membrane has been permeabilized, and effectively “removed.” Local Ca2+ releases are also capable of continuing to occur in sino-atrial nodal cells with intact sarcolemmal functioning in the presence of voltage clamp at voltages preventing loss of Ca2+ from the cell (−10 mV), i.e., voltages where the sodium-calcium exchange current (INCX) is negligible (41, 44, 74).
INCX
Clarification of the relevance of local Ca2+ releases to automaticity came with a critical discovery: inhibiting SR Ca2+ release with ryanodine led to a decrease in INCX (36), suggesting that local Ca2+ release interaction with this membrane-bounded exchanger could affect membrane potential–the SR could “talk to” the sarcolemma (see FIGURE 3)!
INCX is generated by the sarcolemmal sodium-calcium exchanger (NCX), a high-capacity, low-Ca2+ affinity, voltage-dependent, time-independent electrogenic protein that functions throughout the cardiac cycle (62). NCX's importance to pacemaking was largely overlooked for many years because of its very nature, being an exchanger and not an ion channel. “Forward mode” NCX exchanges one intracellular Ca2+ for three extracellular Na+ ions (see FIGURE 4), generating net inward current, estimated during diastole at between 9 and 48 pA for a “standard” 30-pF sino-atrial nodal cell (35). This is similar or larger than the magnitude of If, suggesting that both mechanisms are important for sino-atrial nodal cell automaticity.
NCX has important features distinguishing it from ion channels. NCX has no specific time-dependent gating or clocking mechanism, its time-varying behavior being derived from the rhythmicity of local Ca2+ releases from the SR. This means that, in contrast to If, for example, which takes a significant amount of time to activate upon sino-atrial nodal cell repolarization, NCX is instantaneously activated by exposure to intracellular Ca2+. As diastolic depolarization proceeds, INCX grows as the signal mass of local Ca2+ released from the SR increases, whereas nonselective If becomes smaller in magnitude as it approaches its reversal potential of approximately −30 mV.
The central tenet of the Ca2+ clock is that diastolic local Ca2+ releases interact with sarcolemmal NCX to yield an inward current, progressively increasing the rate of diastolic depolarization toward threshold for the firing of an action potential via activation of L-type Ca2+ channels (5). The process is aided by tight geographical colocalization of RyR and NCX across the subsarcolemmal gap within sino-atrial nodal cells (38). As previously noted, if INCX is rendered negligible by clamping it at its reversal potential (−10 mV), rhythmical local Ca2+ releases persist indefinitely because Ca2+ loss from the cell is prevented (74). Contrastingly, if sino-atrial nodal cell action potentials are prevented from occurring by voltage clamping the cell at its maximum diastolic potential, rhythmical local Ca2+ releases occur only transiently before dying out after a few cycles due to cell Ca2+ depletion due to ongoing functioning of NCX (74).
Loss of NCX is devastating; targeted inactivation is embryonically lethal in mice (31). Atrial (including sino-atrial node)-specific NCX knockout in mice causes failure of normal sino-atrial node pacemaking, although these animals survive to adulthood due to the presence of a bradycardic junctional escape rhythm (16, 17).
Coupling of the Clocks → Mutual Entrainment
Recent studies suggest that there are myriad points where the function of the two clocks overlap; these so-called nodes are emphasized in the coupled clock system illustrated in FIGURE 4 (35). These points have assumed increasing importance in pacemaking fidelity as new in silico models of pacemaker function have emerged (42) (see FIGURE 3). It is now accepted by most in the pacemaker field that the two clocks function synergistically in a coupled system involving their mutual entrainment, ensuring robustness alongside reliability of function through multiple functional redundancies. The important coupling nodes are described below.
Interaction of Local Ca2+ Release-Derived Ca2+ and NCX
NCX has a dual affiliation as a member of both membrane and Ca2+ clocks. The link between SR-derived, RyR-generated local Ca2+ releases and membrane-bounded NCX is a critical “node” between the SR and sarcolemma (see FIGURES 3 AND 4). If forward mode NCX is blocked (e.g., by acute removal of extracellular Na+), sino-atrial nodal cell automaticity abruptly ceases, because the rate of diastolic depolarization is insufficient to activate sarcolemmal ICa,L, even though local Ca2+ releases persist (5, 59). Similarly, buffering intracellular Ca2+ impairs the ability of local Ca2+ releases to occur and interact with NCX and can lead to abolition of spontaneous action potential firing rate (38). Crucially, blockade of INCX causes failure of pacemaking even when other critical mechanisms of pacemaking, including ICa,L, are intact (5) or even enhanced [as illustrated in cases of simulated ischaemia (39)].
L-Type Channel-Derived Ca2+ and Ca2+-Induced Ca2+ Release from the SR
L-type Ca2+ channels are also dually affiliated members of both clocks. The Ca2+ influx from these ion channels induces en masse Ca2+ release from the SR via RyRs (termed Ca2+-induced Ca2+ release; see FIGURE 4). This resets the SR every cycle in a Ca2+-depleted state, preventing SR Ca2+ overload and synchronizing (in a depleted state) SR Ca2+ content across sino-atrial nodal cells. In time, with SERCA-mediated refilling, SR Ca2+ content reaches a threshold level when RyRs will begin to produce local Ca2+ releases once more, with subsequent activation of NCX and perpetuation of the next cycle.
The en masse Ca2+ depletion of SR caused by Ca2+ induced Ca2+ release ensures that subsequent timing of local spontaneous RyR activation to generate local Ca2+ releases is precise and occurs at a time when interaction with NCX will yield an optimal depolarizing effect to drive membrane potential toward threshold. Through ensuring robust Ca2+ influx every cycle, the membrane clock (via both L- and T-type Ca2+ channels) guarantees that there will be sufficient Ca2+ for SERCA to replenish the SR store, even in the presence of ongoing NCX functioning, which would otherwise deplete the cell's Ca2+ stores.
Voltage-Dependent K+ Channels and Intracellular Ca2+
Sarcolemmal voltage-dependent K+ channels that are activated upon depolarization and subsequently effect sino-atrial nodal cell repolarization also affect cell Ca2+ balance indirectly. They do this via their hyperpolarizing effect, which activates NCX and inactivates ICa,L (35), thereby affecting the amount of Ca2+ available for pumping back into the SR, thus forming another node point between membrane and Ca2+ clocks. In turn, these K+ channels can themselves be affected by levels of cytosolic Ca2+ (20, 67), thus illustrating the complexity of cross talk between the two coupled clocks.
The Function of the Two Clocks is Modulated by the Same Biochemical Mechanisms: cAMP, PKA, and CaMKII
A major step forward supporting the coupling of the clocks came with the demonstration that, under baseline conditions, there exists high levels of cAMP in sino-atrial nodal cells, and, further, high levels of phosphorylation of critical proteins belonging to both membrane and Ca2+ clocks (high relative to working myocardial cells) (70). ACs, responsible for cAMP generation in sino-atrial nodal cells, are constitutively highly active (FIGURE 4). Some are Ca2+ inhibited (77) (AC5 and AC6, also found in ventricular myocytes), whereas others are Ca2+ activated (48) (AC1 and AC8). Others (AC2) are Ca2+ independent. Ca2+ itself also binds to calmodulin, which activates ACs even further (FIGURE 4).
One of the major roles of cAMP in sino-atrial nodal cells is protein kinase A (PKA) activation. PKA, alongside calmodulin-activated Ca2+-calmodulin kinase II (CaMKII), phosphorylates L-type Ca2+ channels, RyRs, phospholamban, and voltage-dependent K+ channels (together referred to as “key protein mediators of automaticity”; see FIGURE 4) (70, 75). Phospholamban is the molecular “brake” on SERCA, and its phosphorylation disengages its inhibitory action. Such high basal levels of phosphorylation of the aforementioned proteins appear critical for pacemaker function: inhibition of either PKA or CAMKII leads to cessation of automaticity (70, 73). The phosphorylation state of phospholamban has been used as an indicator of global sino-atrial nodal cell phosphorylation status (35, 68), since the mechanisms by which it is phosphorylated share a commonality with the mechanisms responsible for the phosphorylation of other key protein mediators of automaticity, i.e., via PKA, CAMKII.
PKA- and CAMKII-mediated phosphorylation promote Ca2+ influx via ICa,L and lead to more rapid kinetics of SERCA Ca2+ pumping, and possibly a lower threshold for release of Ca2+ by RyRs, with significant amplification of local Ca2+ release signal mass (70). Phosphorylation synchronizes RyR activation (65) and also increases IK kinetics to speed up repolarization. Higher cytoplasmic levels of Ca2+ during Ca2+ transients and more substantial diastolic releases further activate Ca2+-sensitive adenylate cyclases, which produce more cAMP to further activate PKA, calmodulin, and CAMKII.
This positive feedback system, whereby Ca2+ release begets more Ca2+ release (FIGURE 5A), is critically kept in check by constitutively high levels of phosphodiesterase (PDE) (71) and protein phosphatase (PP) (84) activity. Together, these constantly antagonize the phosphorylating effects of PKA/CAMKII (FIGURE 5B) (72). The net result is that the level of phosphorylation of proteins of the coupled-clock system in the basal state is naturally set to a level that keeps the action potential firing rate at about the midpoint of its functional range. This allows regulation of the action potential firing rate by gradations in the phosphorylation status (see below). The fact that processes involved in both clocks are regulated by the same systems of phosphorylation and dephosphorylation strongly suggests that the ticking speed of the two clocks will be inextricably coupled in vivo and represents another node point.
FIGURE 5.
The coupled clock system
A: Ca2+ release begets Ca2+ release. A stripped down schematic figure illustrating how, without highly constitutively active phosphodiesterases and protein phosphatases, the coupled clock system would inexorably accelerate itself toward its fastest rate. B: schematic demonstration of the sites of action of the highly constitutively active phosphodiesterases (PDEs) and protein phosphatases (PPs) that keep the coupled clock system in check, allowing it to operate approximately in the mid-range of its capabilities. Note that protein kinase A phosphorylates and activates PDEs, increasing their activity; this is antagonized by the activity of PPs. Protein kinase A also activates PPs directly. Ca2+-calmodulin kinase II phosphorylates and activates protein phosphatase inhibitors, which go on to inhibit PPs, which in turn antagonize the phosphorylating effect of CAMKII on PP inhibitors.
Accelerator and Brake: The Response to the Autonomic Nervous System and the Importance of Timing of Local Ca2+ Releases
The phosphorylation status of phospholamban (and by deduction the phosphorylation status of all targets of PKA and CAMKII) can be pharmacologically increased or decreased by substances that mimic the effects of G-protein-coupled receptor signaling (via β-adrenergic receptors of the sympathetic nervous system and muscarinic receptors of the parasympathetic nervous system) to decrease or increase cycle length, respectively, via an increase or decrease in phosphorylation of the critical nodes of the coupled clock system described above in the basal state (35) (FIGURE 6). Such signaling has the remarkable ability to facilitate the wide range of pacing frequencies necessary in vivo in humans, yielding heart rates ranging from 30 to 240 beats/min. The ability of small modifications in phosphorylation status of key mediators of automaticity (as indexed by SR Ca2+ pumping rate, reflecting the degree of phosphorylation of phospholamban controlling SERCA behavior) has been modeled in biophysically detailed in silico models of sino-atrial nodal cells, demonstrating that such variability in phosphorylation levels are capable of producing the whole gamut of beating rates seen in vivo (see FIGURE 7).
FIGURE 7.
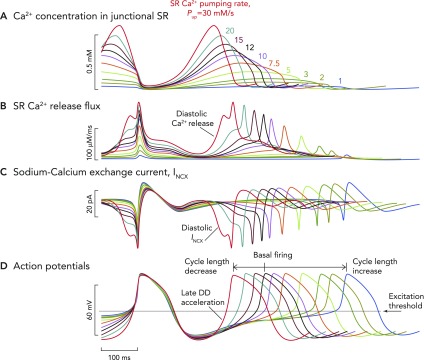
Computer model simulation of a biophysically detailed SANC
Computer model simulation of a biophysically detailed SANC incorporating coupled membrane and Ca2+ clock mechanisms of automaticity, illustrating the wide variety of pacing rates that can be achieved simply by varying the pumping rate (Pup) of Ca2+ back into the SR (in vivo, this occurs via the action of the SR ATPase SERCA). Variable SR Ca2+ pumping rates are an index of the degree of phosphorylation of the regulatory molecule phospholamban, which acts as a molecular brake on the pumping rate of SERCA. With increasing degrees of phospholamban phosphorylation, the brake on SERCA functioning is released, and Ca2+ pumping proceeds at a greater rate. The inverse is true when levels of phosphorylation decrease: rate of Ca2+ pumping by SERCA back into the SR decreases. It is likely that the level of phospholamban phosphorylation also reflects the degree of phosphorylation (and therefore activation) of other key mediators of automaticity, including L-type Ca2+ channels, RyRs, and delayed rectifier K+ channels. The maximum rates of SERCA Ca2+ pumping are shown in A. Baseline rates of pumping are shown by the black waveform and are set at rates of 12 mM/s, although they can be varied in the model between 1 and 30 mM/s. A modeled increase in the rate of SERCA Ca2+ pumping causes earlier and larger magnitude Ca2+ release flux from RyRs (B), meaning that, at faster pumping rates, INCX is activated earlier and with a greater magnitude (C). This means that the time to achieve the threshold potential from maximum diastolic potential for the firing of an action potential is progressively shorter. Hence, greater SR Ca2+ pumping rates cause progressively shorter cycle lengths (D). The opposite is true for slower rates of SR Ca2+ pumping: later occurring, smaller RyR Ca2+ release flux, with later occurring, smaller INCX, leading to longer periods of time to achieve threshold potential for firing of an action potential, leading to slower beating rates. Such a mechanism would allow a wide variability in heart rate sufficient to satisfy the majority of demands placed on the system by the organism in vivo.
The change in cycle length that occurs as a result of phosphorylation or dephosphorylation of key mediators of automaticity is linearly related to the shortening or lengthening of local Ca2+ release period (see FIGURE 8) (35), generating the flexibility required by the sino-atrial node to operate at heart rates across the physiological spectrum and facilitating rapid response to the demands of the body translated through the opposing arms of the autonomic nervous system. The basal behavior of a sino-atrial nodal cell can be conceptualized to be like a car's engine idling at cruising speed, right in the middle of the range of possible velocities it is capable of producing. A small push on the accelerator increases levels of phosphorylation from baseline, ultimately shortening local Ca2+ release period and cycle length. A small push on the brake has the opposite effect. The system is tuned to idle in the middle of its operational capabilities, facilitating maximum efficiency and rapidity of responsiveness.
FIGURE 8.
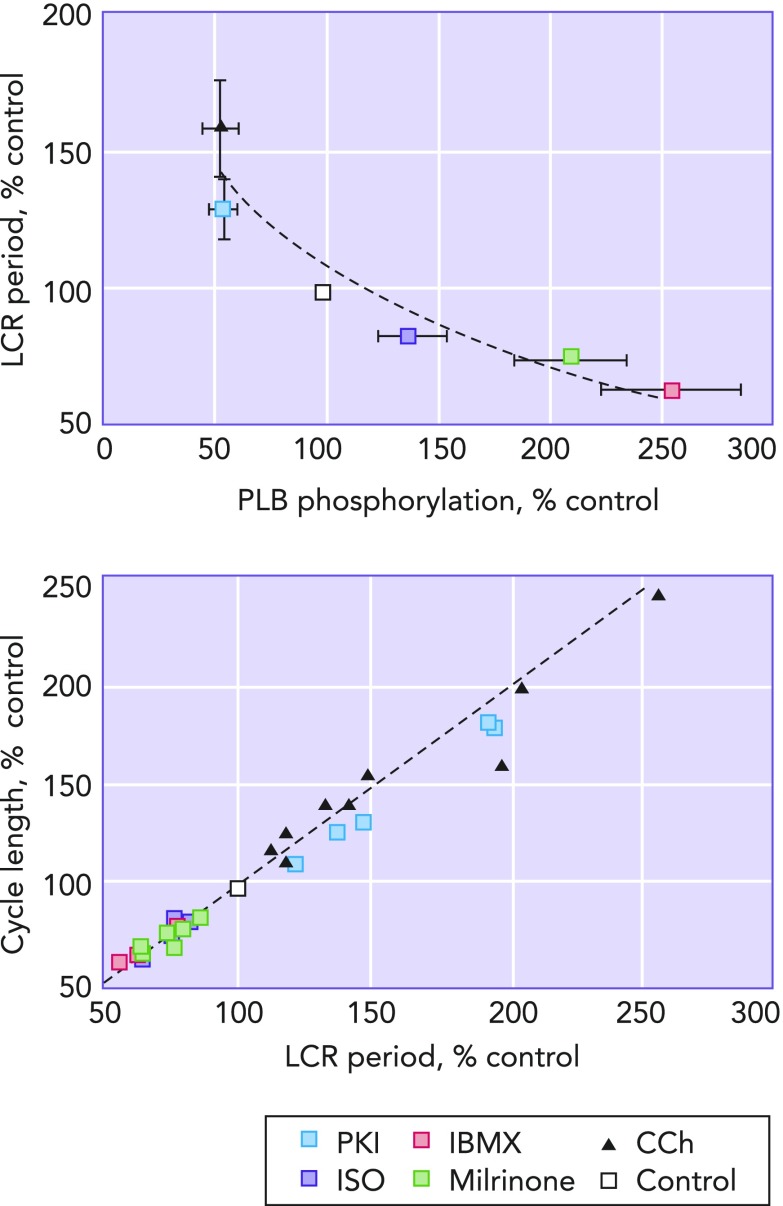
LCR period as the master integrated function of the coupled clock system
Left: the relationship between the amount of phospholamban phosphorylation compared to control and LCR period. It can clearly be seen that decreasing the amount of phospholamban phophorylation relative to that present at control (white square) increases LCR period, and, vice-versa, increasing phospholamban phosphorylation relative to control decreases LCR period. Right: a clear and linear relationship passing through the origin between LCR period and cycle length is shown. Image is adapted from Ref. 35 and used per Circulation Research's permissions policy.
The local Ca2+ release period has a unique position at the center of the axes of action of both membrane and Ca2+ clocks, and rather than simply describing the behavior of the Ca2+ clock in isolation, because of the intricate interrelation of the two clocks through the above-described node points, local Ca2+ release period actually summarizes the combined behavior of the membrane and Ca2+ clocks working synergistically. Because of this, local Ca2+ release period has been described as “the master integrated function” of the coupled clock system (see FIGURE 8) (35).
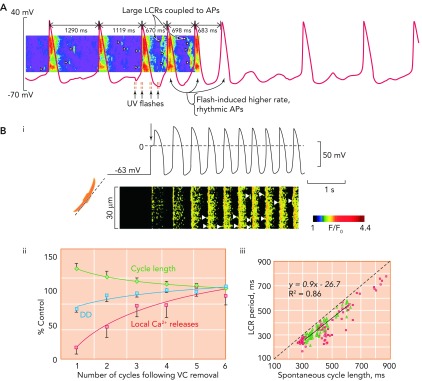
The local Ca2+ release period is not only related to cycle length changes between baseline and following transition to different steady states but also is closely related to cycle length changes on a beat-to-beat basis. Data from rabbit sino-atrial nodal cells (see FIGURE 9, A AND B) have demonstrated that there is beat-to-beat regulation of cycle length that is affected by intracellular Ca2+ cycling and reported by local Ca2+ release period (82). In these experiments, baseline local Ca2+ release period was proportionately related to cycle length on a beat-to-beat basis. When sino-atrial nodal cells are loaded with a Ca2+ buffer (FIGURE 9A), the kinetics of cytosolic Ca2+ removal markedly slowed, leading to prolongation and irregularity of local Ca2+ release period associated with prolongation and irregularity of cycle length (FIGURE 9A, first two cycles exhibiting markedly different duration), and the relationship between local Ca2+ release period and cycle length markedly diminished. However, following flash photolysis of the buffer, which causes Ca2+ to be released, beating rate increased and became more regular, and the beat-to-beat relationship between local Ca2+ release period and cycle length was restored (FIGURE 9A, green arrows).
FIGURE 9.
The critical relationship between local Ca2+ release period and cycle length
A: exposure of rabbit SANC to a caged Ca2+ buffer causes progressive uncoupling of the tight relationship between LCR period and CL. Note the two long cycles at the start of the trace where LCRs (arrow heads) appear to be randomly scattered between action potentials, which occur at an abnormally slow rate. With UV flashes, Ca2+ is released from the caged buffer, and there is a temporary return to rhythmical, more rapidly occurring action potentials, with large and well organized LCRs occurring in the period befopre the upstroke of the action potential. Gradually, the effect of the flash fades, and the Ca2+ is once more taken up by the buffer, with a return to slower, more irregular action potentials. Image is from Ref. 77 and used with permission from Physiological Reviews. B: Bi shows simultaneous recordings of membrane potential (top) and confocal linescan measured Ca2+ fluorescence (bottom) in a SANC that is acutely released from voltage clamp at −65 mV. The arrow shows the exact moment of release of voltage clamp. It can crudely be seen that there is a progressive increase in beating rate, with a beat-to-beat recovery in LCR signal mass. Bii demonstrates the mean rate of recovery of local Ca2+ releases (red squares), cycle length (green diamonds), and diastolic depolarization rate (blue circles) in 5 SANC following acute release of voltage clamp. There is a high degree of correlation between recovery of LCRs and both increasing rate of diastolic depolarization rate (r2 = 0.78) and decrease in CL (r2 = 0.92). Biii demonstrates the close relationship between LCR period and CL following release of voltage clamp (green triangles). This data is compared with data concerning the relationship between LCR period and CL during spontaneous beating (red squares). Image is from Ref. 69 and used with permission from Biophysical Journal.
Additional evidence of the importance of local Ca2+ release period with respect to dictating cycle length during transition from one steady state to another is given in data relating to the effects of the release of sino-atrial nodal cells voltage clamped at −65 mV (FIGURE 9B) (74). The initially quiescent, voltage-clamped cell responds to release of voltage clamp by gradual beat-by-beat amplification of local Ca2+ release signal mass (the product of local Ca2+ release size, amplitude, and duration; FIGURE 9 Bi) (74). This effect is associated with a gradual increase in diastolic depolarization rate and a concomitant decrease in cycle length until pre-voltage clamp parameters are recapitulated (FIGURE 9 Bii AND Biii) (74).
Ivabradine: A Pure Membrane Clock Antagonist That Also Affects the Ca2+ Clock?
The concept of robust coupling between clocks predicts that the bradycardic effects of ivabradine, a pure If blocker, will be mediated ultimately by an effect on the Ca2+ clock, because blocking If will be expected to decrease intracellular Ca2+ (a so-called reverse “Bowditch treppe” effect) as action potential firing rate slows, i.e., a direct membrane clock effect and an indirect Ca2+ clock effect. Work is presently underway to substantiate this hypothesis and to provide evidence of the inextricable coupling of the clocks (Lakatta et al., unpublished observations).
Novel Insights into Mechanisms of Automaticity
The Role of Regulators of G-Protein Signaling (RGS) Proteins: Giα, Giβγ
Regulator of G-protein signaling proteins (or RGS proteins) have recently been discovered in the heart and inactivate G-proteins, resulting in termination of signaling by both Gα and Gβγ proteins (80). They are believed to be critical for regulation of parasympathetic signaling within the heart. More than 20 such proteins exist. Work with RGS4 (10) and RGS6 (80) has confirmed that these proteins are essential for modification of the response to parasympathetic activity via the acetylcholine-activated potassium current IK,ACh (a critical hyperpolarizing effector in the response to muscarinic receptor activation; see FIGURE 6), and without them (for example, in knockout mouse models) the response to muscarinic G-protein-coupled receptor stimulation is exaggerated.
Adult rabbit sino-atrial nodal cells in culture beat ∼50% slower than freshly isolated sino-atrial nodal cells and show a longer action potential duration alongside lower levels of phospholamban and RyR phosphorylation (79). Cultured cells also have lower levels of RGS2. Adenovirus infection of sino-atrial nodal cells to increase RGS2 expression is capable of partially rescuing the beating rate of cultured sino-atrial nodal cells. Inactivation of Gi signaling by pertussis toxin leads to action potential firing rates close to levels seen in freshly isolated sino-atrial nodal cells. Cultured sino-atrial nodal cell beating rates can also be completely rescued by exposure to isoprenaline (i.e., stimulation of the β-adrenergic G-protein-coupled receptor). These findings suggest that the lower beating rate of cultured sino-atrial nodal cells compared with freshly isolated sino-atrial nodal cells is due to unregulated Gi signaling, with concomitant negative effects on downstream targets for phosphorylation.
The Influence of Sino-Atrial Nodal Cell Mitochondrial Ca2+ Buffering on Automaticity
Given the critical position that intracellular Ca2+ occupies at the center of the current paradigm of the coupled clock system of pacemaking, cellular functions that interfere with cell Ca2+ balance could be expected to affect pacemaking. Sino-atrial nodal cells have a high density of mitochondria (81). Yaniv et al. (83) recently showed that these have a significant role to play in cytosolic Ca2+ buffering in rabbit sino-atrial nodal cells. Blocking Ca2+ influx into mitochondria via its uniporter decreases levels of intra-mitochondrial Ca2+, while leading to an increase in SR Ca2+ content and local Ca2+ release size/duration/amplitude, and a shortening of local Ca2+ release period, with subsequent shortening of cycle length. Blocking mitochondrial Ca2+ efflux via mitochondrial NCX has the opposite effect. Notably, blocking If has no effect in preventing the effects of interfering with mitochondrial Ca2+ cycling on cycle length.
It is also noteworthy that cAMP, PKA, and CAMKII regulate the production of energy in the form of ATP in sino-atrial nodal cell mitochondria (see FIGURE 4), whereas it is Ca2+ that regulates ATP production in ventricular myocyte mitochondria (81).
Other cellular organelles that affect cell Ca2+ balance will likely affect automaticity in health and disease; further work is required in this area.
Biological Pacemakers
The quest to replace electronic pacemakers with a biological substitute continues to proceed down many diverse avenues. Based on the ideas discussed above, a successful biological pacemaker design would likely target the integral function of the entire system rather than one particular component (the natural robust pacemaker system has multiple redundant mechanisms; see FIGURES 1–3). Approaches that simplistically attempt to address only a single part of the system [such as targeted upregulation of If (49, 56) or downregulation of IK1 (49)] have turned out to be insufficient. HCN2 gene transfer to upregulate If, for example, leads only to unacceptably slow idioventricular rates and moderate autonomic responsiveness (56).
An approach more likely to succeed involves upregulating the whole Ca2+ clock in working cardiomyoytes, converting them into pacemaking cells by concomitantly activating the coupled membrane clock. Increasing expression of Ca2+-activated ACs is one strategy to achieve this. Indeed, such a strategy has already been used to generate automaticity in neonatal rat ventricular myocytes (32), embryonic stem cell-derived cardiomyocytes (85), and P19-derived pacemaker-like cells (43). Direct addition of cAMP to embryonic stem cell-derived stem cells has also demonstrated flexibility and rhythmicity in pacemaking (85). In these cells, blocking the effects of cAMP and therefore clock coupling with PKA inhibitors causes rhythmicity to disappear (85). Adenoviral introduction of Ca2+-activated AC1 into the left bundle branches of atrio-ventricular blocked canines has been shown to be capable of generating sino-atrial nodal cell-like automaticity (6). An alternative approach that also awakens the coupled clock system in non-pacemaking cells is the reactivation of the sino-atrial nodal cell-type gene programme within ventricular myocytes by transcription factors that have been shown to have a key role to play in the embryogenesis of the sino-atrial node: both tbx18 in neonatal rat ventricular myocyte (29, 30) and TBX3 in mature murine cardiomyocytes (2) have shown promise in this regard.
Future Focuses
Each step forward in pacemaker research over the years has subsequently generated more questions than it succeeded in answering. Therefore, despite a hitherto unprecedented degree of biophysical appreciation of pacemaking mechanisms, we continue to face challenging questions in need of intensive further investigation, the most pressing of which are summarized below. One important thing, however, becomes crystal clear: the cardiac pacemaker is a complex system of numerous interacting components localized both to the cell membrane and within the cell. How robust and flexible pacemaking within such a complex system emerges needs further detailed experimental studies, as well as development and application of new approaches of systems biology and complex systems.
Human aging is well known both in vivo and in vitro to be associated with dysfunction of the sinoatrial node (51), along with the rest of the cardiac conduction system, leading to the excessive requirement for implantation of permanent pacing systems in the elderly. The exact mechanisms by which age-related sino-atrial nodal dysfunction occurs, however, remain unclear, although many have been implicated. These include an age-related cell-to-cell disconnection due to decreased connexin 43 expression (26) and a downregulation of ICa,L (25). Aging in murine sino-atrial nodal cells has more recently been associated with a decrease in SR Ca2+ cycling and responsiveness to phosphodiesterase inhibition (64). Similar changes in Ca2+ handling mechanisms have been shown in rodent working cardiomyocytes (24), where there is an established age-related increase in myocyte size, decrease in myocyte number, and increase in matrix connective tissue. Future work to completely elucidate the electrophysiological basis of the age-related remodeling of the sino-atrial node that contributes ultimately to its dysfunction will be important in defining potential new therapeutic measures to retard or prevent such undesirable change, with the ultimate aim of subverting the need for any artificial pacemaker systems, biological or not.
The exact molecular mechanism of Ca2+ release by RyRs remains unclear: How does intra- and extra-SR Ca2+ influence RyR open probability? Do all RyRs have the same threshold for release of local Ca2+ releases, or is this heterogeneous? Is there local variability in Ca2+ concentration within the SR that makes certain RyRs more likely to open and release Ca2+? Is there local variation in extra-SR cAMP that makes certain RyRs more likely to open? Are certain areas of the cell more likely to exhibit local Ca2+ releases than others, and, if so, what is it that controls the generation of these “hotspots”? By what process does stochastic opening and closing of RyRs become synchronized to generate roughly periodic local Ca2+ releases? Is there local RyR cross talk, and, if so, by what mechanism?
Protein kinase C (PKC) is becoming recognized as an important factor that influences the phosphorylation status of critical mediators of automaticity in sino-atrial nodal cells, alongside the well characterized behaviors of PKA and CAMKII. Pilot studies have shown that blocking PKC activity leads to a 2.5-fold decrease in the beating rate of rabbit sino-atrial nodal cells (69), with effects on local Ca2+ release characteristics and timing that are likely to be mediated by the loss of the effect of PKC on the combination of L-type Ca2+ channels, SERCA, and RyR. Exactly how the behavior of PKC fits in with the much more established actions of PKA is the focus of ongoing work.
Newly discovered mechanisms of regulating sino-atrial nodal cell Ca2+ balance have generated much recent interest. Sarcolemmal store-operated Ca2+ channels (SOCCs) have the ability to affect cell Ca2+ balance through a poorly characterized ability to detect the level of Ca2+ present within intracellular stores (28), with low detected levels leading to an increase in Ca2+ flux intracellularly. Blocking store-operated Ca2+ channel activity slows pacemaking in isolated murine sino-atrial node by 65% without affecting L-type Ca2+ channels (28). Their contribution to pacemaking automaticity requires further elucidation.
A more complete definition of the role of INa,K in sino-atrial nodal cells and a complete characterization of the importance of the regulatory protein phospholemman is required. It is recognized that, in working cardiomyocytes, phospholemman is the major target for PKA- and PKC-mediated phosphorylation and that phosphorylation leads to removal of its inactivating effects on the sodium-potassium ATPase (15). Phospholemman inhibits NCX when phosphorylated (86) and has a similar effect on L-type Ca2+ channels (15). The implications of the behavior of INa,K and phospholemman in pacemaking cells requires clarification, as does the regulation of cellular Na+.
Summary
The present paradigm of sino-atrial nodal cell automaticity involves a coupled clock system, whereby sarcolemmal proteins of the electrical membrane clock interact with the intracellular Ca2+ cycling apparatus making up the chemical Ca2+ clock and vice versa, with the behavior of each individual clock's components constantly refining and responding to the behavior of the components of the other clock (mutual entrainment). By behaving in this way, one clock guarantees ongoing, accurate, and sufficient functioning of its counterpart and vice versa, and in doing so ensures that this most vital of life's foundation stones is optimally robust and reliable. The diverse nature of the clocks offers heterogeneous redundancy at the single sino-atrial nodal cell level, whereas the diverse characteristics of the sino-atrial nodal cell population comprising the sino-atrial node offers a further layer of heterogeneous redundancy, maximizing robustness. Previous reductionist approaches to pacemaker investigation unwittingly has divorced the clocks, treating them as “asymmetrically” important (54), i.e., feuding partners with one party more important than the other. Such approaches suggested that one clock might exist perfectly well without the other, when all along the happy marriage and symmetrical importance of individual clock functions is what makes the system behave in such an elegantly harmonious manner. Certain crucial mechanisms responsible for automaticity at baseline are simply scaled up or down by phosphorylation/dephosphorylation in response to the G-protein-coupled receptor signaling employed by the autonomic nervous system.
A General Theory of Cardiac Function has Emerged
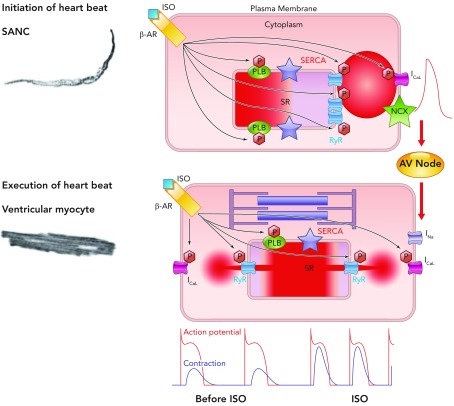
A general theory of the heart's function has emerged from the concurrent study of sino-atrial nodal cells alongside cells of the working myocardium (see FIGURE 10). It has long been recognized that, as the heart beats faster, it beats stronger [the so-called Bowditch effect (33)]. But, surprisingly, it was only recently realized that these diverse cell types employ similar apparatuses to achieve, first, increased chronotropy, and then increased inotropy. Specifically increased chronotropy is achieved via increased sino-atrial nodal cell Ca2+ cycling kinetics via a cross talk of SERCA (SR Ca2+ uptake) → RyR (Ca2+ release) → NCX (inward current during diastole and Ca2+ efflux) → L-type Ca2+ channels (action potential and Ca2+ influx). Increased inotropy is achieved also via increased Ca2+ cycling kinetics utilizing a cross talk of the very same molecules: L-type Ca2+ channels (Ca2+ influx) → RyR (Ca2+ release) → SERCA (SR Ca2+ uptake) → NCX (Ca2+ efflux). Through exploration of this general theory, our appreciation of the physiology and pathophysiology of cardiac automaticity might allow ongoing fascinating insights into this most critical cardiac function.
FIGURE 10.
A general theory of cardiac function, involving similar mechanisms in SANC and ventricular myocytes
Roughly periodic diastolic Ca2+ releases in the form of LCRs in SANC interact with sarcolemmal NCX to produce an inward current that increases the rate of diastolic depolarization toward the threshold potential for activation of L-type Ca2+ channels. Ca2+ influx through these channels depolarizes SANC and activates RyRs to produce en masse emptying of the SR in a process known as CICR and leads to the action potential-induced Ca2+ transient. Depolarization of SANC spreads via cell-to-cell contacts, leading to depolarization of atrial myocytes and, following conduction via the atrioventricular node, to depolarization of ventricular myocytes. This electrical process involves influx of Na+ ions into ventricular myocytes via fast sodium channels. Depolarization subsequently causes influx into ventricular myocytes of Ca2+ via L-type Ca2+ channels, which again activate SR-based RyRs, leading to en masse emptying of the SR of its Ca2+, which binds to the sarcomere to cause shortening and the ultimate goal of cardiac inotropism. In this schema, the chemical signal of Ca2+ release from the SR in SANC initiates an electrical signal, which is passed to ventricular myocytes. In ventricular myocytes, electrical depolarization then initiates a chemical signal (intracellular Ca2+ release) to produce the ultimate goal: a timely and efficient mechanical oscillation, i.e., a heart beat. Thus heart rate (chronotropy) controlled by SANC leads to execution of a heart beat by ventricular myocytes by utilizing an almost identical set of molecules behaving in the same, albeit differently ordered, manner, a general theory of cardiac function. Increasing or decreasing the rate of activity of SANC in turn increases or decreases the force of contraction of ventricular myocytes via phosphorylation/dephosphorylation of the same molecules (phospholamban, RyR, L-type Ca2+ channels, and delayed rectifier K+ channels) via the same mechanisms involving PKA and CAMKII in these two neighboring yet physiologically diverse cell types (see supplemental movie at Physiology's website; the movie was created by Victor Maltsev).
Supplementary Material
Acknowledgments
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: O.M., V.A.M., and E.G.L. performed experiments; O.M. and V.A.M. analyzed data; O.M. and V.A.M. interpreted results of experiments; O.M. and V.A.M. prepared figures; O.M., V.A.M., and E.G.L. drafted manuscript; O.M., V.A.M., and E.G.L. edited and revised manuscript; O.M., V.A.M., and E.G.L. approved final version of manuscript; V.A.M. and E.G.L. conception and design of research.
References
- 1.Alig J, Marger L, Mesirca P, Ehmke H, Mangoni ME, Isbrandt D. Control of heart rate by cAMP sensitivity of HCN channels. Proc Natl Acad Sci USA 106: 12189–12194, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakker ML, Boink GJ, Boukens BJ, Verkerk AO, van den Boogaard M, den Haan AD, et al. T-box transcription factor TBX3 reprogrammes mature cardiac myocytes into pacemaker-like cells. Cardiovasc Res 94: 439–449, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Baruscotti M, Bottelli G, Milanesi R, DiFrancesco JC, DiFrancesco D. HCN-related channelopathies. Pflügers Arch 460: 405–415, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Baruscotti M, Bucchi A, DiFrancesco D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol Ther 107: 59–79, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: molecular partners in pacemaker regulation. Circ Res 88: 1254–1258, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Boink GJ, Nearing BD, Shlapakova IN, Duan L, Kryukova Y, Bobkov Y, et al. Ca2+-stimulated adenylyl cyclase AC1 generates efficient biological pacing as single gene therapy and in combination with HCN2. Circulation 126: 528–536, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bround MJ, Asghari P, Wambolt RB, Bohunek L, Smits C, Philit M, et al. Cardiac ryanodine receptors control heart rate and rhythmicity in adult mice. Cardiovasc Res 96: 372–380, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown HF, DiFrancesco D, Noble SJ. How does adrenaline accelerate the heart? Nature 280: 235–236, 1979. [DOI] [PubMed] [Google Scholar]
- 9.Chandler NJ, Greener ID, Tellez JO, Inada S, Musa H, Molenaar P, et al. Molecular architecture of the human sinus node: insights into the function of the cardiac pacemaker. Circulation 119: 1562–1575, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Cifelli C, Rose RA, Zhang H, Voigtlaender-Bolz J, Bolz SS, Backx PH, et al. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ Res 103: 527–535, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Denyer JC, Brown HF. Pacemaking in rabbit isolated sino-atrial node cells during Cs+ block of the hyperpolarization-activated current If. J Physiol 429: 401–409, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiFrancesco D. Funny channels in the control of cardiac rhythm and mode of action of selective blockers. Pharmacol Res 53: 399–406, 2006. [DOI] [PubMed] [Google Scholar]
- 13.DiFrancesco D, Ferroni A, Mazzanti M, Tromba C. Properties of the hyperpolarizing-activated current (If) in cells isolated from the rabbit sino-atrial node. J Physiol 377: 61–88, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiFrancesco D, Tromba C. Acetylcholine inhibits activation of the cardiac hyperpolarizing-activated current, If. Pflügers Arch 410: 139–142, 1987. [DOI] [PubMed] [Google Scholar]
- 15.Fuller W, Tulloch L, Shattock M, Calaghan S, Howie J, Wypijewski K. Regulation of the cardiac sodium pump. Cell Mol Life Sci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groenke S, Larson Dl Nakano H, Nakano A, Proenza C, Philipson KD, et al. Atrial-specific NCX KO mice reveal dependence of sinoatrial node pacemaker activity on sodium-calcium exchange. Biophys J 102: 663a, 2012. [Google Scholar]
- 17.Groenke S, Larson ED, Zhang R, Nakano H, Jordan MC, Roos KP, et al. The Na+-Ca2+ exchanger is required for sinoatrial node pacemaker activity in murine myocardium. Biophys J 100: 247a, 2011. [Google Scholar]
- 18.Hagiwara N, Irisawa H, Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol 395: 233–253, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harzheim D, Pfeiffer KH, Fabritz L, Kremmer E, Buch T, Waisman A, et al. Cardiac pacemaker function of HCN4 channels in mice is confined to embryonic development and requires cyclic AMP. EMBO J 27: 692–703, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heath BM, Terrar DA. Protein kinase C enhances the rapidly activating delayed rectifier potassium current, IKr, through a reduction in C-type inactivation in guinea-pig ventricular myocytes. J Physiol 522: 391–402, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrmann S, Stieber J, Stockl G, Hofmann F, Ludwig A. HCN4 provides a ‘depolarization reserve’ and is not required for heart rate acceleration in mice. EMBO J 26: 4423–4432, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoesl E, Stieber J, Herrmann S, Feil S, Tybl E, Hofmann F, et al. Tamoxifen-inducible gene deletion in the cardiac conduction system. J Mol Cell Cardiol 45: 62–69, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol 524: 415–422, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janczewski AM, Lakatta EG. Modulation of sarcoplasmic reticulum Ca2+ cycling in systolic and diastolic heart failure associated with aging. Heart Fail Rev 15: 431–445, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones SA, Boyett MR, Lancaster MK. Declining into failure: the age-dependent loss of the L-type calcium channel within the sinoatrial node. Circulation 115: 1183–1190, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Jones SA, Lancaster MK, Boyett MR. Ageing-related changes of connexins and conduction within the sinoatrial node. J Physiol 560: 429–437, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju YK, Allen DG. The distribution of calcium in toad cardiac pacemaker cells during spontaneous firing. Pflügers Arch 441: 219–227, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Ju YK, Chu Y, Chaulet H, Lai D, Gervasio OL, Graham RM, et al. Store-operated Ca2+ influx and expression of TRPC genes in mouse sinoatrial node. Circ Res 100: 1605–1614, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Kapoor N, Marbán E, Cho HC. Lineage reprogramming from cardiomyocytes to pacemaker cells via a single transcription factor. Biophys J 102: 637a, 2012. [Google Scholar]
- 30.Kapoor N, Marbán E, Cho HC. Tbx18-reprogrammed cardiomyocytes exhibit pacemaker phenotype with upregulated calcium and voltage-dependent clock mechanisms. Circulation 124: A14024, 2011. [Google Scholar]
- 31.Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, et al. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J 15: 1209–1211, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Kryukova YN, Protas L, Robinson RB. Ca2+-activated adenylyl cyclase 1 introduces Ca2+-dependence to beta-adrenergic stimulation of HCN2 current. J Mol Cell Cardiol 52: 1233–1239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakatta EG. Beyond bowditch: the convergence of cardiac chronotropy and inotropy. Cell Calcium 35: 629–642, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Lakatta EG, DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol 47: 157–170, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled system of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res 106: 659–673, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Qu J, Nathan RD. Ionic basis of ryanodine's negative chronotropic effect on pacemaker cells isolated from the sinoatrial node. Am J Physiol Heart Circ Physiol 273: H2481–H2489, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J 22: 216–224, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyashkov AE, Juhaszova M, Dobrzynski H, Vinogradova TM, Maltsev VA, Juhasz O, et al. Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size. Circ Res 100: 1723–1731, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Maltsev VA, Lakatta EG. Cardiac pacemaker cell failure with preserved If, ICaL, and IKr: a lesson about pacemaker function learned from ischemia-induced bradycardia. J Mol Cell Cardiol 42: 289–294, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maltsev VA, Lakatta EG. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res 77: 274–284, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Maltsev VA, Lakatta EG. Normal heart rhythm is initiated and regulated by an intracellular calcium clock within pacemaker cells. Heart Lung Circ 16: 335–348, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maltsev VA, Lakatta EG. Synergism of coupled subsarcolemmal Ca2+ clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am J Physiol Heart Circ Physiol 296: H594–H615, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maltsev VA, Lakatta EG, Zahanich I, Sirenko S. Engineered Biological Pacemakers International Patent Application. 2010. [Google Scholar]
- 44.Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharm Sci 100: 338–369, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, et al. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci USA 100: 5543–5548, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev 88: 919–982, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, et al. Bradycardia and slowing of the atrioventricular conduction in mice lacking Cav3.1/α1G T-type calcium channels. Circ Res 98: 1422–1430, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the If pacemaker current. J Physiol 582: 1195–1203, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miake J, Marban E, Nuss HB. Biological pacemaker created by gene transfer. Nature 419: 132–133, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Molleman A. Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology. Chichester, UK: John Wiley & Sons, 2003. [Google Scholar]
- 51.Monfredi O, Dobrzynski H, Mondal T, Boyett MR, Morris GM. The anatomy and physiology of the sinoatrial node: a contemporary review. Pacing Clin Electrophysiol 33: 1392–1406, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Noble D. Modeling the heart: from genes to cells to the whole organ. Science 295: 1678–1682, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol 353: 1–50, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noble D, Noble PJ, Fink M. Competing oscillators in cardiac pacemaking: historical background. Circ Res 106: 1791–1797, 2010. [DOI] [PubMed] [Google Scholar]
- 55.Noma A, Irisawa H. Contribution of an electrogenic sodium pump to the membrane potential in rabbit sinoatrial node cells. Pflügers Arch 358: 289–301, 1975. [DOI] [PubMed] [Google Scholar]
- 56.Rosen MR, Brink PR, Cohen IS, Robinson RB. Biological pacemakers based on If. Med Biol Eng Comput 45: 157–166, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Rubenstein DS, Lipsius SL. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ Res 64: 648–657, 1989. [DOI] [PubMed] [Google Scholar]
- 58.Sakai R, Hagiwara N, Matsuda N, Kassanuki H, Hosoda S. Sodium-potassium pump current in rabbit sino-atrial node cells. J Physiol 490: 51–62, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanders L, Rakovic S, Lowe M, Mattick PA, Terrar DA. Fundamental importance of Na+-Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J Physiol 571: 639–649, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schweizer PA, Duhme N, Thomas D, Becker R, Zehelein J, Draguhn A, et al. cAMP sensitivity of HCN pacemaker channels determines basal heart rate but is not critical for autonomic rate control. Circ Arrhythmia Electrophysiol 3: 542–552, 2010. [DOI] [PubMed] [Google Scholar]
- 61.Shibata EF, Giles WR. Ionic currents that generate the spontaneous diastolic depolarization in individual cardiac pacemaker cells. Proc Natl Acad Sci USA 82: 7796–7800, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shigekawa M, Iwamoto T. Cardiac Na+-Ca2+ exchange: molecular and pharmacological aspects. Circ Res 88: 864–876, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Silverman ME, Hollman A. Discovery of the sinus node by Keith and Flack: on the centennial of their 1907 publication. Heart 93: 1184–1187, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sirenko S, Shukla S, Liu J, Lakatta EG. Age associated decrease in intrinsic action potential (AP) firing rate in sinoatrial node cells is linked to deficient intrinsic cAMP-PKA-Ca2+ signaling. Biophys J 100: 434a, 2011. [Google Scholar]
- 65.Song LS, Wang SQ, Xiao RP, Spurgeon H, Lakatta EG, Cheng H. β-Adrenergic stimulation synchronizes intracellular Ca2+ release during excitation-contraction coupling in cardiac myocytes. Circ Res 88: 794–801, 2001. [DOI] [PubMed] [Google Scholar]
- 66.Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M. Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. EMBO J 17: 3309–3316, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tohse N. Calcium-sensitive delayed rectifier potassium current in guinea pig ventricular cells. Am J Physiol Heart Circ Physiol 258: H1200–H1207, 1990. [DOI] [PubMed] [Google Scholar]
- 68.Vinogradova TM, Lakatta EG. Regulation of basal and reserve cardiac pacemaker function by interactions of cAMP-mediated PKA-dependent Ca2+ cycling with surface membrane channels. J Mol Cell Cardiol 47: 456–474, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vinogradova TM, Lakatta EG. Spontaneous beating of rabbit sinoatrial node cells requires basal protein kinase C activity. Biophys J 98: 99a, 2010. [Google Scholar]
- 70.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res 98: 505–514, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Vinogradova TM, Lyashkov AE, Zhu W, Spurgeon H, Maltsev VA, Lakatta EG. Constitutive phosphodiesterase activity confers negative feedback on intrinsic cAMP-PKA regulation of local rhythmic subsarcolemmal calcium releases and spontaneous beating in rabbit sinoatrial nodal. Biophys J 88: 303a, 2005. [Google Scholar]
- 72.Vinogradova TM, Sirenko S, Lyashkov AE, Younes A, Li Y, Zhu W, et al. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circ Res 102: 761–769, 2008. [DOI] [PubMed] [Google Scholar]
- 73.Vinogradova TM, Zhou YY, Bogdanov KY, Yang D, Kuschel M, Cheng H, et al. Sinoatrial node pacemaker activity requires Ca2+/calmodulin-dependent protein kinase II activation. Circ Res 87: 760–767, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Vinogradova TM, Zhou YY, Maltsev V, Lyashkov A, Stern M, Lakatta EG. Rhythmic ryanodine receptor Ca2+ releases during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ Res 94: 802–809, 2004. [DOI] [PubMed] [Google Scholar]
- 75.Walsh KB, Kass RS. Regulation of a heart potassium channel by protein kinase A and C. Science 242: 67–69, 1988. [DOI] [PubMed] [Google Scholar]
- 76.Wilders R. Computer modelling of the sinoatrial node. Med Biol Eng Comput 45: 189–207, 2007. [DOI] [PubMed] [Google Scholar]
- 77.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87: 965–1010, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Yanagihara K, Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflügers Arch 385: 11–19, 1980. [DOI] [PubMed] [Google Scholar]
- 79.Yang D, Lyashkov AE, Li Y, Ziman BD, Lakatta EG. RGS2 overexpression or Gi inhibition rescues the impaired PKA signaling and slow AP firing of cultured adult rabbit pacemaker cells. J Mol Cell Cardiol 53: 687–694, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang J, Huang J, Maity B, Gao Z, Lorca RA, Gudmundsson H, et al. RGS6, a modulator of parasympathetic activation in heart. Circ Res 107: 1345–1349, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yaniv Y, Juhaszova M, Lyashkov AE, Spurgeon HA, Sollott SJ, Lakatta EG. Ca2+-regulated-cAMP/PKA signaling in cardiac pacemaker cells links ATP supply to demand. J Mol Cell Cardiol 51: 740–748, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yaniv Y, Maltsev VA, Escobar AL, Spurgeon HA, Ziman BD, Stern MD, et al. Beat-to-beat Ca2+-dependent regulation of sinoatrial nodal pacemaker cell rate and rhythm. J Mol Cell Cardiol, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yaniv Y, Spurgeon HA, Lyashkov AE, Yang D, Ziman BD, Maltsev VA, et al. Crosstalk between mitochondrial and sarcoplasmic reticulum Ca2+ cycling modulates cardiac pacemaker cell automaticity. PLos One 7: e37582, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zahanich I, Li Y, Lyashkov AE, Lukyanenko YO, Vinogradova TM, Younes A, et al. Protein phosphatase 1 regulates normal automaticity of the heart's pacemaker node cells by site-specific modulation of phospholamban phosphorylation that regulates spontaneous subsarcolemmal local Ca2+ releases. Circulation 122: A21546, 2010. [Google Scholar]
- 85.Zahanich I, Sirenko SG, Maltseva LA, Tarasova YS, Spurgeon HA, Boheler KR, et al. Rhythmic beating of stem cell-derived cardiac cells requires dynamic coupling of electrophysiology and Ca cycling. J Mol Cell Cardiol 50: 66–76, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang XQ, Ahlers BA, Tucker AL, Song J, Wang J, Moorman JR, et al. Phospholemman inhibition of the cardiac Na+/Ca2+ exchanger. Role of phosphorylation. J Biol Chem 281: 7784–7792, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.