This review describes recent studies demonstrating how fruit flies can be used to identify and characterize the mechanisms underlying obesity and to establish models of obesityassociated disorders.
Abstract
In recent years, obesity has been recognized as a major public health problem due to its increased prevalence in both children and adults and its association with numerous life-threatening complications including diabetes, heart disease, hypertension, and cancer. Obesity is a complex disorder that is the result of the interaction between predisposing genetic and environmental factors. However, the precise nature of these gene-gene and gene-environment interactions remains unclear. Here, we will describe recent studies demonstrating how fruit flies can be used to identify and characterize the mechanisms underlying obesity and to establish models of obesity-associated disorders.
In the past decade, the prevalence of obesity has reached alarming levels, resulting in the World Health Organization (WHO) recognizing it as a global epidemic, affecting significant portions of the population in both developed and developing countries. The WHO has estimated that by 2015 there will be 2.3 billion overweight adults worldwide, with 700 million of them classified as obese (72). Obesity is a metabolic disorder that can involve hyperglycemia, hypertension, and hyperlipidemia (69, 71) and is strongly associated with a high incidence of diabetes, arteriosclerosis, and cardiovascular disease (20, 32). In addition, obesity exacerbates many health problems including, but not limited to, sleep apnea, osteoarthritis, gallstones, and certain types of cancers (15, 65). Clinically and physiologically, obesity is a very heterogeneous disease. In addition to environmental factors such as high-fat diets and sedentary lifestyles, genetic predisposition is also believed to contribute to the imbalance between energy intake and expenditure that ultimately results in obesity and its associated morbidities (34). Despite intensive analysis, the etiological mechanisms that lead to energy imbalance and obesity are not fully understood.
Drosophila Models of Human Obesity
Recently, non-mammalian genetic model organisms including nematodes, zebrafish, and fruit flies have emerged as excellent paradigms for studying a wide variety of human diseases. Drosophila melanogaster is a particularly powerful model due to its wide array of available genetic tools, short generation time, and ability to perform large-scale, unbiased forward genetic screens. Moreover, analysis of the Drosophila genome has shown that ∼75% of all known human disease-related genes are conserved in flies (56).
Recently, Drosophila have been used in studies of metabolic disorders, since flies have many of the same basic metabolic functions as mammals, including the ability to maintain sugar homeostasis, storing and mobilizing energy stores, and modulating food intake in response to nutritional cues, and many of the molecular mechanisms that regulate these metabolic processes are conserved. Furthermore, many of the metabolic organs and tissues in mammals have functionally analogous counterparts in flies, including the liver, pancreas, and adipose tissue (see Table 1). For example, the fly fat body serves as the site of energy storage in the form of lipids and glycogen, similar to mammalian white adipose tissue and liver (12). A notable exception is the absence of a fly equivalent of brown adipose tissue, which is involved in energy expenditure in mammals. In this review, we will compare the mechanisms used by flies to maintain energy balance with those used in mammals and discuss the utility of Drosophila models to study obesity and its associated disorders.
Table 1.
Conservation of metabolic tissues between mammals and Drosophila
| Function | Mammals | Drosophila |
|---|---|---|
| Digestion and nutrient absorption | Stomach, small intestine | Midgut |
| Lipid storage | Adipose tissue | Fat body |
| Lipid mobilization | Adipose tissue, liver | Fat body, oenocytes |
| Glycogen storage | Liver | Fat body |
| Carbohydrate homeostasis | Pancreatic α and β cells | Neurosecretory neurons, corpora cardiaca |
Regulation of Lipid Metabolism
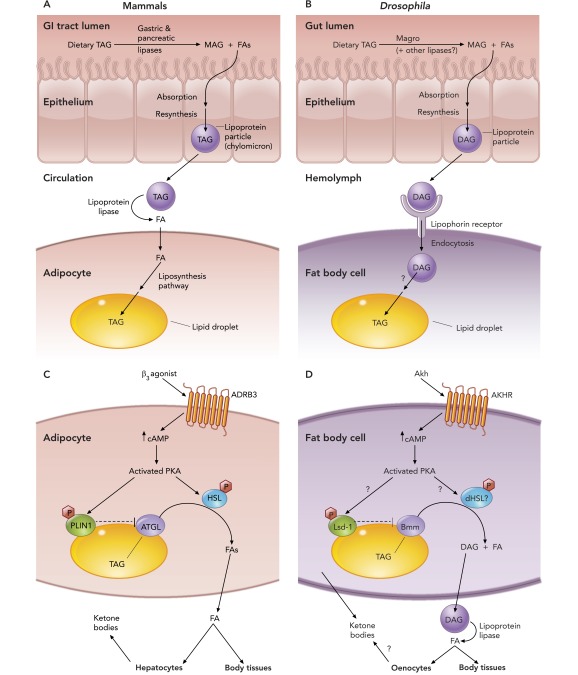
Lipids perform many crucial functions within cells. They are essential structural components of cell membranes, are important signaling molecules, and also serve as a source of energy during periods of prolonged nutrient deprivation. As such, the control of lipid metabolism is crucial for normal development and physiology (25, 38). These lipid metabolic processes employ a complex set of mechanisms that regulate lipid uptake, transport, storage, and mobilization, which are conserved between mammals and flies (see FIGURE 1), and their dysregulation can result in excess lipid storage and obesity.
FIGURE 1.
Lipid metabolism pathways in mammals and Drosophila
A and B: dietary fat digestion, transport, and storage. Dietary fats in the form of triglycerides (TAG) are broken down in the gut lumen by lipases to facilitate absorption into the epithelium, where the monoglyceride (MAG) and fatty acids (FAs) are used to reform TAG [or diglyceride (DAG) in flies] and packaged into lipoprotein particles. These particles are released into circulation and travel to the site of lipid storage (adipose tissue in mammals and fat body in flies) if the fats are not immediately required for energy production. In mammals, the TAG is broken down by lipoprotein lipase into glycerol and FAs that can then be taken up into the adipocytes and used to generate TAG, which is then stored in the intracellular lipid droplets. In flies, the lipoprotein particles are endocytosed by fat body cells via a lipophorin receptor-mediated mechanism, and the DAG is extracted and used to synthesize TAG, which is deposited into the lipid droplets by an unknown mechanism. C and D: lipid mobilization. In times when sugars are not readily available for energy production, a signal is sent to the lipid storage tissues to mobilize fats. β3-Agonists/adipokinetic hormone (Akh) bind to their respective G-protein-coupled receptors that activate PKA via adenyl cylase and increase in cAMP levels. In mammals, PKA phosphorylates perilipin 1 (PLIN1), relieving its inhibition of adipose triglyceride lipase (ATGL). PKA also phosphorylates hormone-sensitive lipase (HSL), thereby activating it, and, together with ATGL, the two proteins catalyze the breakdown of TAG from the lipid droplets to FAs. In Drosophila, PKA can phosphorylate the PLIN1 homolog lipid storage droplet-1 (Lsd-1), but whether this has functional significance is unknown. Flies also have a putative HSL homolog (CG11055), but its role in this process has not been elucidated. Akh receptor (AKHR) signaling stimulates the ATGL homolog Brummer (Bmm), which catalyzes the breakdown of lipid droplet TAG to DAG and FAs that are released into the circulation. In mammals, the FAs released from the adipocytes are taken up by the body tissues for energy production. Hepatocytes also use the FAs to produce ketone bodies that are used by the brain and muscle for energy production. In flies, the released DAG is taken up into lipoprotein particles and travels to the body tissues where it is further broken down to FAs by lipoprotein lipase. The fly fat body can also produce ketone bodies, but it is not known whether the hepatocyte-like oenocytes are also capable of ketogenesis. ADRB3, β3-adrenergic receptor.
Lipid Uptake and Transport
In terms of energy metabolism, a crucial role is played by the fatty acid (FA) branch of lipid metabolism, which includes FA synthesis and uptake, storage of FAs as triacylglycerides (TAG), and conversion of FAs to produce energy. Similar to vertebrates, Drosophila can obtain FAs from their diets, normally in the form of TAG that gets broken down to free FAs (FFAs) and monoacylglceride (MAG) by lipases including magro, which is homologous to mammalian gastric lipase (63). Magro expression is directly regulated by the orphan nuclear receptor DHR96 (63), a homolog of the liver X receptors that are required to regulate triglyceride levels in mice (60). DHR96 mutant flies are lean, resistant to high-fat diet-induced obesity, and insensitive to treatment with a lipase inhibitor, suggesting that a defect in breakdown of dietary lipids is affecting fat accumulation in these mutants (63).
The FFAs and MAG in the gut lumen are then able to be absorbed by the enterocytes and converted to diacylglceride (DAG) that is exported to the hemolymph (the fly equivalent of blood) as lipoprotein (also called lipophorin) particles. Unlike vertebrates, these lipoprotein particles are present in the hemolymph and do not require de novo synthesis (12). Moreover, insect lipoprotein particles are thought to be a reusable shuttle system since they can deliver their lipid cargo to target tissues without internalization and degradation of the particle itself (12). Another point of divergence between Drosophila and mammals is the predominant neutral lipid in the circulation: DAG in flies vs. TAG in mammals. It is not known whether either acylglyceride provides any distinct advantage. However, in the insect system at least, lipophorins that carry DAG seem to function more efficiently as reusable shuttles compared with lipophorins in species that carry TAG (51).
Lipid Storage and Mobilization
Lipophorin travels with its DAG cargo to body tissues for use in energy production or to the fat body for storage. There, it binds lipophorin receptors similar to the low-density lipoprotein receptor (12, 18) and unloads the DAG, which gets converted to TAG and stored in intracellular lipid droplets (LDs). There have been many studies of the LD components that have demonstrated that these proteins are responsible for the regulation of TAG storage and mobilization and that the LD proteome is conserved between flies and mammals. These studies have been described in several recent reviews (35–37, 48). For example, when either flies or humans require the mobilization of lipids for energy production, lipolytic signals act on LD components to allow lipases to access the stored TAG in the droplet (see FIGURE 1). In mammals, the FAs released from TAG breakdown can enter the blood and be taken up by the body tissues. In flies, the TAG breakdown products are delivered as DAG by lipophorins, which travel to the tissues where lipoprotein lipase catalyzes the release of FAs from the DAG. The fatty acids can then be taken up by the cell to undergo β-oxidation and energy production.
Hepatocyte-like Functions in Drosophila FA Metabolism
The mammalian liver can take up FAs from the circulation in proportion to their blood concentration, and excessive uptake (e.g., during high levels of lipolysis) results in accumulation of lipid droplets in the hepatocytes. Increased FA uptake also enhances ketogenesis, which uses β-oxidation products to produce soluble ketone bodies that are released into the blood and can be used as an energy source for many tissues, including the brain and heart. This process is thought to aid in the distribution of metabolic fuel to the different tissues during periods of negative energy balance.
In flies, most of the hepatocyte functions are performed by the fat body. In addition, specialized cells, called oenocytes, underlying the fat body accumulate lipid droplets under starvation conditions similar to hepatocytes (26). However, it is not known whether these cells are capable of ketogenesis (a function that may reside solely in the fat body). In addition, the function of oenocytes has only been studied in larvae, so it is unknown whether they serve other or additional functions in adults where the metabolic programs may be different.
Regulation of Carbohydrate Metabolism
Carbohydrates are a primary energy source for living organisms; as such, the maintenance of carbohydrate homeostasis plays an important role in energy balance in flies and humans. As previously mentioned, Drosophila have functionally analogous pancreatic β- and α-cells that secrete insulin-like petides and adipokinetic hormone, which are the fly versions of insulin and glucagon, respectively. In addition, many of the signaling pathways and mechanisms are conserved between flies and mammals. These studies have been described by several reviews (3, 4, 27, 66) but will not be discussed here.
CNS Regulation of Energy Metabolism
The central nervous system (CNS) has been shown to play a pivotal role in regulating energy homeostasis. Consistent with this role, ∼25% of all the genes in the last Human Obesity Gene Map, and nearly all the genes implicated in monogenic obesity are known to be expressed in the brain (55). Furthermore, recent genome-wide association studies in humans have implicated single-nucleotide polymorphisms in several neuronal genes with predisposition to high BMI (40, 67, 73).
The CNS has also recently been shown to play a role in regulating energy metabolism in Drosophila. In a genome-wide RNAi screen (53), one-third of the ∼500 genes found to affect stored fat levels when ubiquitously knocked down were also able to alter fat levels if their expression was specifically reduced in neurons. In addition, Al-Anzi et al. (1) performed a screen searching for neuronal populations involved in the regulation of lipid stores uncovering two distinct populations. Altering the neuronal activity of these neuronal populations resulted in changes in food consumption, lipid metabolism, and macromolecule metabolism that could be reversed by restoring normal neuronal activity. However, although these screens implicate the CNS in fly energy metabolism and despite the importance of the CNS in mammalian energy balance, the molecular mechanisms are still largely unknown and have not been the focus for many Drosophila metabolic studies.
Inputs to the CNS
The CNS is thought to act as the central coordinator that monitors the body's energy status and initiates the appropriate outputs to correct any perturbations by modulating feeding behavior and metabolism. These signals conveying the body's energy status are in the form of satiation signals from the gastrointestinal tract, signals from the metabolic tissues, and signals from nutrients themselves such as fatty acids and glucose (33, 59, 62).
There is some evidence that suggests that the Drosophila brain can directly sense nutrients. Alteration in the activity of the nutrient-sensing target of rapamycin (TOR) pathway in the brain can affect feeding behavior, potentially in response to nutritional status (57). In addition, certain gustatory receptors are expressed on some nonsensory neurons in brain regions implicated in metabolism and feeding behavior, suggesting a potential function of these receptors in nutrient sensing in these neurons (68).
Some of the most studied signals between the CNS and the periphery in the regulation of metabolism are the so-called “adiposity factors” leptin and insulin that are secreted by adipose tissue and pancreas, respectively, and circulate in proportion to body fat mass. These hormones bind to receptors on neurons in the hypothalamus that are part of a complex neuronal network whose purpose is to regulate feeding behavior and nutrient metabolism to promote energy homeostasis (reviewed in Refs. 29, 33, 59, 62). Although there are several Drosophila insulin-like peptides (Ilps), it was previously thought that flies did not have a leptin homolog, although it was known that the fat body secretes humoral factors that signal to the brain in response to nutritional status (9, 17, 24). This issue has recently been resolved with the discovery of a functional homolog of leptin called Unpaired-2 (Upd-2) (54). Similar to leptin, Upd-2 is a cytokine secreted by fat tissue and corresponds to positive energy balance and activates Jak/STAT signaling in the brain. In flies, the Upd-2 signaling cascade regulates the secretion of Ilps from neurosecretory neurons [also called insulin-producing cells (IPCs)], which mediate the metabolic functions of Upd-2. The identification of a functional leptin homolog that shares little sequence homology demonstrates the potential for the discovery of homologs for other neuronal factors that play important roles in mammalian energy homeostasis but have no clear counterpart in flies (notably Agouti-related protein and the melanocortins). Given the conservation of other metabolic genes and pathways between flies and mammals, it is possible that additional neuronal factors and mechanisms are functionally conserved as well. However, uncovering these homologs will require functional assays rather than relying on sequence analysis. To this end, the hits from the RNAi screen mentioned previously (53) could serve as a starting point for the identification of these molecules.
Neuronal Signaling Network and Outputs from the CNS
In flies, the neuronal circuitry mediating energy balance has yet to be fully characterized, but it appears that some of the known circuits converge on the endocrine system, brain regions that innervate the muscles required for feeding, and the Ilp-producing cells (47). Much of the focus has been on the interneuronal signaling to the IPCs or signaling within the IPCs to regulate the production and/or secretion of the insulin-like peptides, identifying many factors in these processes, including sNPF (31, 39), ERK (39), S6K (74), JNK (70), serotonin (43), Cbl (75), tachykinins (6), and Minibrain (Mnb) (28).
The IPCs send processes to several tissues including the aorta, the ring gland (the fly endocrine gland), and parts of the digestive tract (13, 16), suggesting that these are the target tissues for CNS outputs, although this area requires further study. The fat body is one of the targets for the Ilps that are released into the circulation, and activation of insulin receptor signaling results in effects on lipid and carbohydrate metabolism (66). For example, signals from the IPCs control the sugar stores in the fat body through regulation of tobi (target of brain insulin) expression, which encodes an α-glucosidase that can catalyze the breakdown of glycogen (11).
The regulation of feeding behaviors is another important function of the CNS in energy homeostasis. Several mutants with changes in food preference and consumption have been identified in flies, including neuropeptide F and hugin, whose mammalian homologs neuropeptide Y and neuromedin U play roles in energy homeostasis (reviewed in Ref. 46). The IPCs also secrete Drosulfakinins (DSKs), which function in feeding behavior and, more specifically, in food intake and preference (64). Interestingly, DSKs are related to vertebrate cholecystokinin (CCK) and play a similar satiation function as CCK.
Although there is still much to learn about role of the CNS in energy metabolism in flies, there is great potential for these models since fly geneticists have many tools to study neuronal function, including methods to target expression of genes and RNAi constructs to specific neuronal populations, tools for measuring and manipulating neuronal activity, and well developed behavioral tests, which altogether should aid in the functional dissection of these circuits.
Genetic Analyses of Obesity
Genetics of Human Obesity
Estimates indicate that up to 70% of weight variation is determined by genetic factors (8, 14, 30, 41). Some of these factors have been identified by studying rare monogenic forms of obesity, which have identified mutations in several genes including leptin, the leptin receptor, and the melanocortin 4-receptor genes. Although these monogenic forms account for <1% of all cases of obesity (21, 22), knowledge of these genes and their functions has significantly increased our understanding of obesity.
The more common forms of obesity likely involve both gene-gene and gene-environment interactions. However, unlike the monogenic forms of obesity, identifying specific susceptibility genes has proven to be very difficult. Previously, searches for genes that predispose to obesity were mainly based on candidate gene approaches and genome-wide linkage studies. Although this led to the identification of over 430 genes or chromosomal regions, only a small percentage of these have been replicated by multiple studies (52, 55). More recently, genome-wide association studies (GWAS) have been used to uncover common single nucleotide polymorphisms (SNPs) associated with various obesity measures and have greatly increased the speed of gene discovery. The most promising finding from these GWAS studies was the identification of variants in FTO (the fat mass and obesity-associated gene), which were unequivocally associated with body mass index (BMI) and obesity in white Europeans (23, 61). Although these studies have broadened our understanding of the genetic basis of common obesity, individual susceptibility genes only produce modest effects on obesity measures, which account for a small portion of the genetic component to obesity (7, 19).
Genetic Screens in Drosophila
It is clear that the present methods used in human genetic studies are insufficient to account for the variation in BMI and that we need to find alternative strategies. Drosophila may be able to help in this aspect as screens are one of the major strengths of flies as genetic models, and researchers have access to a large number of resources, including multiple online databases and libraries of transgenic lines (some of which cover most of the fly genome) (45). Using one of these libraries, Pospisilik et al. (53) screened over 11,500 transgenic RNAi lines corresponding to ∼10,500 genes for changes in fat levels in adult flies. Of the 500 hits, 60% have human orthologs, including genes involved in insulin signaling, sugar and lipid homeostasis, nutrient transport, and adipocyte development and function. In addition, this screen identified a large number of potential lipid storage regulators that were not previously associated with obesity. Although these candidate genes have not been further characterized, there is potential here to find novel susceptibility genes or to uncover molecular mechanisms of disease that could be relevant to human obesity. Interestingly, gene ontology analysis of the hits from this screen found hedgehog signaling to be the most highly enriched signaling pathway and that this pathway acts in the fat body. Subsequently, it was shown that hedgehog signaling in mice regulates early adipogenic factors that function in white vs. brown adipocyte specification, thus exemplifying how genetic work in flies can impact our knowledge of mammalian physiology (53).
Genome-Wide Association Studies in Flies
It has become possible to perform GWAS studies in Drosophila with the introduction of a panel of ∼200 fully sequenced, inbred strains from the Drosophila Genetic Reference Panel (DGRP) intended for use in analysis of population genomics and quantitative traits (44). Although 200 lines are far less than the number of subjects in human GWAS studies, there are still many distinct advantages to performing these studies in flies. For instance, it is possible to test many individuals with identical genotypes, allowing for more accurate measures of specific quantitative traits. In addition, these studies offer much greater control over the environment, allowing researchers to either reduce environmental variables or to study the effect of specific environmental influences. This is of particular importance in obesity studies where environment is known to affect genetic susceptibility.
A disadvantage of human GWAS is the limitation on how many SNPs can be tested (i.e., how many probes can fit on a chip). Consequently, the most common SNPs are predominantly selected for testing, and therefore these studies overlook low-frequency variants. This limitation does not apply when using the DGRP lines where all SNPs are known, and, in fact, the majority of SNPs in these lines are low-frequency variants (44). In addition, there are also many mapped polymorphic microsatellite loci that can be used for analysis. Ayroles et al. (2) have previously used these lines to look for genetic variants involved in resistance to fitness-related quantitative traits. Interestingly, most of the variants implicated in these traits are low-frequency variants, and the lower the frequency the greater the effect. If this is also true in human populations, then it could explain why variants identified in human GWAS studies (that mainly look at high-frequency SNPs) have such modest effects on weight and BMI. Given the many advantages of fly GWAS studies and the hundreds of novel candidate genes identified in previous studies, our understanding of human obesity could greatly benefit from Drosophila models.
Modeling Obesity-Related Disorders in Flies
The observation that many of the metabolic functions found in mammals are also conserved in Drosophila suggests that flies might also provide a useful model to study obesity-associated disorders, including diabetes and cardiovascular disease. In fact, studies have shown that ablation of insulin-producing cells in Drosophila larvae gives rise to phenotypes that recapitulate many symptoms of Type 1 diabetes, including high carbohydrate levels and poor growth (10, 58). More recently, several groups have developed models of Type 2 diabetes by feeding Drosophila larvae a high-sugar diet. These studies found that the resulting larvae were smaller and developed phenotypes that resemble those observed in patients with Type 2 diabetes, including hyperglycemia, insulin resistance, and accumulation of fat (49, 50). Of note, the growth retardation observed in these larvae appears to be a direct consequence of peripheral insulin resistance. In addition to the phenotypes described above, the expression of several genes involved in lipogenesis, gluconeogenesis, and β-oxidation were also upregulated in high-sugar-fed larvae, consistent with what is observed in humans with Type 2 diabetes. Interestingly, adult flies fed a high-sugar diet exhibit cardiac dysfunction and arrhythmias as well as a shortened lifespan, although it is not clear whether these phenotypes are associated with defects in metabolic dysfunction.
Adult flies fed a high-fat diet also exhibited dysregulation of insulin signaling and glucose homeostasis as well as defects in cardiac function, including reduced cardiac contractibility and increased accumulation of cardiac lipids, similar to what is observed in diabetic cardiomyopathies (5). Interestingly, both the metabolic and heart defects could be reversed by blocking insulin signaling or by increasing lipase activity either in adipose tissue or in the heart itself. Finally, defects in heart function are not limited to flies with high-sugar or high-fat diets but have also been found in Drosophila that carry single gene mutations. For example, mutations that disrupt phospholipid metabolism give rise to flies that exhibit high levels of triglycerides both systemically and within cardiac tissue (42), develop tachycardia, and are prone to cardiac arrest. Altogether, these studies demonstrate that the link between obesity and its associated disorders can be studied in Drosophila.
In conclusion, obesity and its related disorders have become a major epidemic that affects the health of both children and adults. It is a complex disorder with many underlying genetic and environmental causes. Although insight into these causes has come from studying cases of monogenic obesity in humans, additional approaches are required to fully characterize the molecular mechanisms underlying this disease. Large-scale genetic screens in Drosophila have already begun to help identify novel factors that may contribute to obesity in flies and, more importantly, in humans. Furthermore, Drosophila have been used to model many aspects of obesity and its associated disorders. With the conservation of metabolic functions and molecular pathways between flies and mammals, combined with the suite of genetic tools available to researchers, Drosophila will continue to be a valuable resource for understanding the complex nature of obesity.
Acknowledgments
We thank Dr. David Knight and Julia Maeve Bonner for helpful discussions and critical comments on this manuscript.
Footnotes
This work is supported by a grant from the Canadian Institutes of Health Research (MOP 97871) to G. L. Boulianne. I. Trinh is the recipient of a University of Toronto Open Fellowship, and G. L. Boulianne holds a Tier 1 Canada Research Chair in Molecular and Developmental Neurobiology.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: I.T. prepared figures; I.T. and G.L.B. drafted manuscript; I.T. and G.L.B. edited and revised manuscript; I.T. and G.L.B. approved final version of manuscript.
References
- 1.Al-Anzi B, Sapin V, Waters C, Zinn K, Wyman RJ, Benzer S. Obesity-blocking neurons in Drosophila. Neuron 63: 329–341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayroles JF, Carbone MA, Stone EA, Jordan KW, Lyman RF, Magwire MM, Rollmann SM, Duncan LH, Lawrence F, Anholt RR, Mackay TF. Systems genetics of complex traits in Drosophila melanogaster. Nat Genet 41: 299–307, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab 6: 257–266, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha KN. The epicurean fly: using Drosophila melanogaster to study metabolism. Pediatr Res 65: 132–137, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, Ocorr K, Bodmer R, Oldham S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab 12: 533–544, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birse RT, Soderberg JA, Luo J, Winther AM, Nassel DR. Regulation of insulin-producing cells in the adult Drosophila brain via the tachykinin peptide receptor DTKR. J Exp Biol 214: 4201–4208, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Bogardus C. Missing heritability and GWAS utility. Obesity (Silver Spring) 17: 209–210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchard C. Current understanding of the etiology of obesity: genetic and nongenetic factors. Am J Clin Nutr 53: 1561S–1565S, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Britton JS, Edgar BA. Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development 125: 2149–2158, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA 102: 3105–3110, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab 7: 321–332, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA. Fat metabolism in insects. Annu Rev Nutr 21: 23–46, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Cao C, Brown MR. Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res 304: 317–321, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Carmelli D, Cardon LR, Fabsitz R. Clustering of hypertension, diabetes, and obesity in adult male twins: same genes or same environments? Am J Hum Genet 55: 566–573, 1994 [PMC free article] [PubMed] [Google Scholar]
- 15.Chow WH, Gridley G, Fraumeni JF, Jr, Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 343: 1305–1311, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab 13: 92–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell 114: 739–749, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Dantuma NP, Potters M, De Winther MP, Tensen CP, Kooiman FP, Bogerd J, Van der Horst DJ. An insect homolog of the vertebrate very low density lipoprotein receptor mediates endocytosis of lipophorins. J Lipid Res 40: 973–978, 1999 [PubMed] [Google Scholar]
- 19.Day FR, Loos RJ. Developments in obesity genetics in the era of genome-wide association studies. J Nutrigenet Nutrigenomics 4: 222–238, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 365: 1415–1428, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Farooqi IS, O'Rahilly S. Monogenic human obesity syndromes. Recent Prog Horm Res 59: 409–424, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Farooqi IS, O'Rahilly S. Monogenic obesity in humans. Annu Rev Med 56: 443–458, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316: 889–894, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab 10: 199–207, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Gibbons GF, Islam K, Pease RJ. Mobilisation of triacylglycerol stores. Biochim Biophys Acta 1483: 37–57, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Gutierrez E, Wiggins D, Fielding B, Gould AP. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 445: 275–280, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Haselton AT, Fridell YW. Adult Drosophila melanogaster as a model for the study of glucose homeostasis. Aging (Milano) 2: 523–526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong SH, Lee KS, Kwak SJ, Kim AK, Bai H, Jung MS, Kwon OY, Song WJ, Tatar M, Yu K. Minibrain/Dyrk1a regulates food intake through the Sir2-FOXO-sNPF/NPY pathway in Drosophila and mammals. PLoS Genet 8: e1002857, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horvath TL, Diano S. The floating blueprint of hypothalamic feeding circuits. Nat Rev Neurosci 5: 662–667, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Ichihara S, Yamada Y. Genetic factors for human obesity. Cell Mol Life Sci 65: 1086–1098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapan N, Lushchak OV, Luo J, Nassel DR. Identified peptidergic neurons in the Drosophilabrain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin. Cell Mol Life Sci. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med 347: 305–313, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Kleinridders A, Konner AC, Bruning JC. CNS-targets in control of energy and glucose homeostasis. Curr Opin Pharmacol 9: 794–804, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Kopelman PG. Obesity as a medical problem. Nature 404: 635–643, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Kuhnlein RP. The contribution of the Drosophila model to lipid droplet research. Prog Lipid Res 50: 348–356, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Kuhnlein RP. Energy homeostasis regulation in Drosophila: a lipocentric perspective. Results Probl Cell Differ 52: 159–173, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Kuhnlein RP. Thematic review series: Lipid droplet synthesis and metabolism: from yeast to man. Lipid droplet-based storage fat metabolism in Drosophila. J Lipid Res 53: 1430–1436, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CH, Olson P, Evans RM. Minireview: Lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 144: 2201–2207, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ, Jung SA, Kim AK, You KH, Tatar M, Yu K. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat Cell Biol 10: 468–475, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Lee KT, Byun MJ, Kang KS, Park EW, Lee SH, Cho S, Kim H, Kim KW, Lee T, Park JE, Park W, Shin D, Park HS, Jeon JT, Choi BH, Jang GW, Choi SH, Kim DW, Lim D, Park MR, Ott J, Schook LB, Kim TH. Neuronal genes for subcutaneous fat thickness in human and pig are identified by local genomic sequencing and combined SNP association study. PLos One 6: e16356, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li S, Loos RJ. Progress in the genetics of common obesity: size matters. Curr Opin Lipidol 19: 113–121, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Lim HY, Wang W, Wessells RJ, Ocorr K, Bodmer R. Phospholipid homeostasis regulates lipid metabolism and cardiac function through SREBP signaling in Drosophila. Genes Dev 25: 189–200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo J, Becnel J, Nichols CD, Nassel DR. Insulin-producing cells in the brain of adult Drosophila are regulated by the serotonin 5-HT1A receptor. Cell Mol Life Sci 69: 471–484, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackay TF, Richards S, Stone EA, Barbadilla A, Ayroles JF, Zhu D, Casillas S, Han Y, Magwire MM, Cridland JM, Richardson MF, Anholt RR, Barron M, Bess C, Blankenburg KP, Carbone MA, Castellano D, Chaboub L, Duncan L, Harris Z, Javaid M, Jayaseelan JC, Jhangiani SN, Jordan KW, Lara F, Lawrence F, Lee SL, Librado P, Linheiro RS, Lyman RF, Mackey AJ, Munidasa M, Muzny DM, Nazareth L, Newsham I, Perales L, Pu LL, Qu C, Ramia M, Reid JG, Rollmann SM, Rozas J, Saada N, Turlapati L, Worley KC, Wu YQ, Yamamoto A, Zhu Y, Bergman CM, Thornton KR, Mittelman D, Gibbs RA. The Drosophila melanogaster Genetic Reference Panel. Nature 482: 173–178, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthews KA, Kaufman TC, Gelbart WM. Research resources for Drosophila: the expanding universe. Nat Rev Genet 6: 179–193, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Melcher C, Bader R, Pankratz MJ. Amino acids, taste circuits, and feeding behavior in Drosophila: towards understanding the psychology of feeding in flies and man. J Endocrinol 192: 467–472, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Melcher C, Pankratz MJ. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol 3: e305, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy DJ. The dynamic roles of intracellular lipid droplets: from archaea to mammals. Protoplasma 249: 541–585, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, Cagan RL, Baranski TJ. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech 4: 842–849, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pasco MY, Leopold P. High sugar-induced insulin resistance in Drosophila relies on the lipocalin Neural Lazarillo. PLos One 7: e36583, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pennington JE, Nussenzveig RH, Van Heusden MC. Lipid transfer from insect fat body to lipophorin: comparison between a mosquito triacylglycerol-rich lipophorin and a sphinx moth diacylglycerol-rich lipophorin. J Lipid Res 37: 1144–1152, 1996 [PubMed] [Google Scholar]
- 52.Perusse L, Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Snyder EE, Bouchard C. The human obesity gene map: the 2004 update. Obes Res 13: 381–490, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, Bayer M, Haschemi A, Puviindran V, Tar K, Orthofer M, Neely GG, Dietzl G, Manoukian A, Funovics M, Prager G, Wagner O, Ferrandon D, Aberger F, Hui CC, Esterbauer H, Penninger JM. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell 140: 148–160, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 151: 123–137, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Perusse L, Bouchard C. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 14: 529–644, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Reiter LT, Potocki L, Chien S, Gribskov M, Bier E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res 11: 1114–1125, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol 20: 1000–1005, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296: 1118–1120, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Sanchez-Lasheras C, Konner AC, Bruning JC. Integrative neurobiology of energy homeostasis-neurocircuits, signals and mediators. Front Neuroendocrinol 31: 4–15, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev 14: 2831–2838, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orru M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 3: e115, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seeley RJ, Woods SC. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci 4: 901–909, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Sieber MH, Thummel CS. The DHR96 nuclear receptor controls triacylglycerol homeostasis in Drosophila. Cell Metab 10: 481–490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soderberg JA, Carlsson MA, Nassel DR. Insulin-producing cells in the drosophila brain also express satiety-inducing cholecystokinin-like peptide, drosulfakinin. Front Endocrinol (Lausanne) 3: 109, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab 89: 2522–2525, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Teleman AA. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem J 425: 13–26, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I, Jonsdottir T, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Jonsson F, Borch-Johnsen K, Hansen T, Andersen G, Jorgensen T, Lauritzen T, Aben KK, Verbeek AL, Roeleveld N, Kampman E, Yanek LR, Becker LC, Tryggvadottir L, Rafnar T, Becker DM, Gulcher J, Kiemeney LA, Pedersen O, Kong A, Thorsteinsdottir U, Stefansson K. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 41: 18–24, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Thorne N, Amrein H. Atypical expression of Drosophila gustatory receptor genes in sensory and central neurons. J Comp Neurol 506: 548–568, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21: 697–738, 2000 [DOI] [PubMed] [Google Scholar]
- 70.Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121: 115–125, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350: 2362–2374, 2004 [DOI] [PubMed] [Google Scholar]
- 72.WHO Obesity and Overweight: World Health Organization, 2006. World Health Organization, 2006 [Google Scholar]
- 73.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, Coin L, Collins FS, Connell JM, Cooper C, Smith GD, Dennison EM, Deodhar P, Elliott P, Erdos MR, Estrada K, Evans DM, Gianniny L, Gieger C, Gillson CJ, Guiducci C, Hackett R, Hadley D, Hall AS, Havulinna AS, Hebebrand J, Hofman A, Isomaa B, Jacobs KB, Johnson T, Jousilahti P, Jovanovic Z, Khaw KT, Kraft P, Kuokkanen M, Kuusisto J, Laitinen J, Lakatta EG, Luan J, Luben RN, Mangino M, McArdle WL, Meitinger T, Mulas A, Munroe PB, Narisu N, Ness AR, Northstone K, O'Rahilly S, Purmann C, Rees MG, Ridderstrale M, Ring SM, Rivadeneira F, Ruokonen A, Sandhu MS, Saramies J, Scott LJ, Scuteri A, Silander K, Sims MA, Song K, Stephens J, Stevens S, Stringham HM, Tung YC, Valle TT, Van Duijn CM, Vimaleswaran KS, Vollenweider P, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41: 25–34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu Q, Zhang Y, Xu J, Shen P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci USA 102: 13289–13294, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu Y, Sun Y, He S, Yan C, Rui L, Li W, Liu Y. Neuronal Cbl controls biosynthesis of insulin-like peptides in Drosophila melanogaster. Mol Cell Biol 32: 3610–3623, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]