This review focuses on the regulation of V-ATPase-dependent luminal acidification in renal intercalated cells and epididymal clear cells.
Abstract
Specialized cells in the body express high levels of V-ATPase in their plasma membrane and respond to hormonal and nonhormonal cues to regulate extracellular acidification. Mutations in or loss of some V-ATPase subunits cause several disorders, including renal distal tubular acidosis and male infertility. This review focuses on the regulation of V-ATPase-dependent luminal acidification in renal intercalated cells and epididymal clear cells, which are key players in these physiological processes.
Luminal or extracellular acidification is a crucial process for the normal physiological function of several organs, including kidney, epididymis, bone, ear, and nose (6, 39, 48, 55, 69, 78, 115). In the kidney, intercalated cells (ICs) are involved in the maintenance of systemic acid/base balance (13, 67, 150, 151). Type A ICs (A-ICs) secrete protons into the tubule lumen and, thus, remove acid from the body (e.g., to correct systemic acidosis), whereas type B ICs transport protons across their basolateral membrane and secrete bicarbonate into the lumen, thereby retaining acid to correct systemic alkalosis. In the epididymis, a small organ located downstream of the testis, clear cells (CCs) acidify the luminal compartment, a process that is essential for the proper maturation and storage of spermatozoa (97, 123). In bone, osteoclasts create a local acidic environment that is essential for bone resorption (109). In the inner ear, luminal acidification by interdental and other cells contributes to establishing a high K concentration in the endolymph (48), whereas in the nasal epithelium, acidification is involved in the sense of olfaction (92, 99, 101). All these specialized acidifying cells have in common a high level of expression of the proton pump V-ATPase in their plasma membrane. V-ATPase-dependent luminal acidification in these cells is under tight physiological regulation, and dysfunction of the V-ATPase has been associated with several pathological conditions, including renal distal tubular acidosis, deafness, formation of kidney stones, proteinuria, impairment of the sense of smell, osteopetrosis, and osteoporosis (50, 67–69, 92, 101, 109, 128, 132, 141, 149). A role of the V-ATPase in cancer has recently emerged, and its expression at the plasma membrane correlates with the invasive characteristics of various malignant cells (reviewed in Refs. 39, 52, 104, 146, 155). This now positions the V-ATPase as a potential target for the treatment of cancers, including those of the prostate, breast, skin, and brain (43, 56, 83, 91, 120, 159). In addition to mediating proton transport across the plasma membrane of specialized cells in some organs, the V-ATPase is involved in the acidification of intracellular organelles in all eukaryotic cells (6, 39, 55, 115). This review focuses on the role of the V-ATPase in luminal acidification and discusses how this process is regulated in renal A-ICs and epididymal CCs.
Structure of the V-ATPase
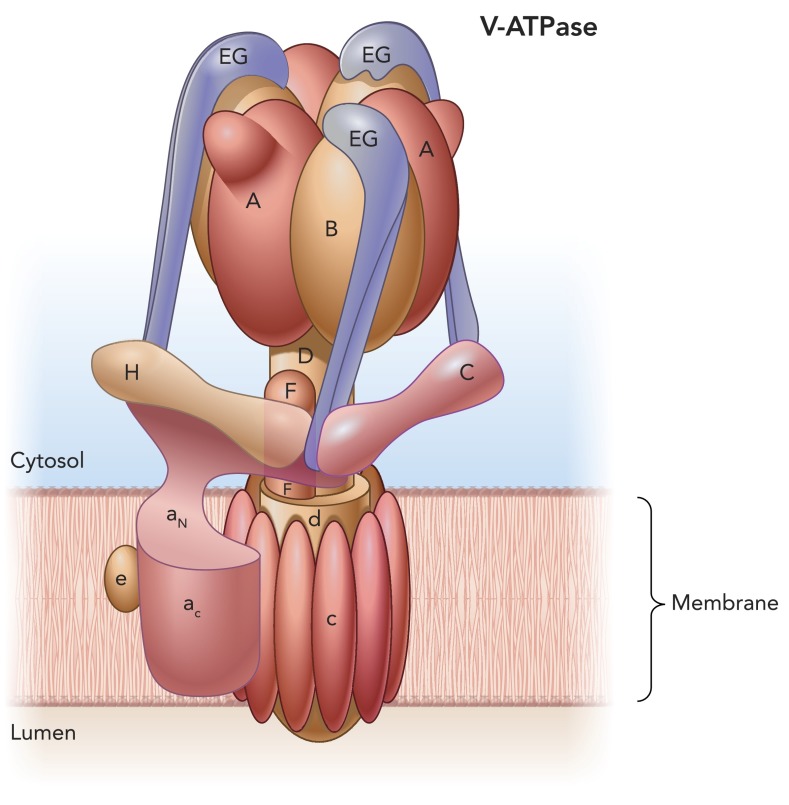
The V-ATPase is a complex enzyme that is composed of several subunits that are assembled into two domains: a transmembrane V0 domain and a cytosolic V1 domain (27, 39, 78, 127, 151). Most of our knowledge of V-ATPase structure has been acquired from studies on the yeast enzyme (38, 39, 65, 66, 88, 115). The V1 domain (ATP6V1) is formed by eight subunits that are designated by capital letters, each having a specific stoichiometry (A3B3CDE3FG3H1–2). Subunits of the V0 domain (ATP6V0) are designated by lower case letters (a, c, c′, c′′, d, e). The V-ATPase is a rotary pump that is energized by ATP hydrolysis, which occurs in a catalytic hexamer formed by three A and three B subunits (FIGURE 1). A central axle composed of subunits D and F connects the A3B3 complex to a proteolipid ring formed by the c, c′, and c′′ subunits (in mammals, the c′ isoform is absent). ATP hydrolysis drives rotation of the D-F axle, which in turns induces rotation of the proteolipid c-ring. Proton translocation occurs at the interface between the rotating ring and the larger transmembrane subunit a. The complex is stabilized by three peripheral stators formed by subunits C, E, G, and H (53, 88). Various “knockdown” studies, mostly in yeast, have shown the requirement of all of the V0 and V1 sector subunits for normal assembly and function of the holoenzyme (45, 66).
FIGURE 1.
V-ATPase subunit composition and organization
Subunits A, B, C, D, E, F, G, and H form the V1 cytosolic domain, and subunits a, c, d, and e form the V0 transmembrane domain. ATP catalytic sites are located at the B/A subunit interfaces. Protons are transported between subunit a and the proteolipid c-ring. The V-ATPase rotor is formed by subunits D, F, and d and the c-ring. Three EG complexes together with subunits C and H form a stator, which is connected to the AB hexamer and the large transmembrane subunit a. The function of subunit e is still unknown. Image is based on data from Refs. 39 and 88.
Some of the V-ATPase subunits have more than one isoform, and several putative splice variants have also been identified (85). For example, four “a” V-ATPase subunit isoforms are present in the human genome (127). Subunit a1 is expressed ubiquitously; subunit a2 is present in the kidney, lung, spleen, and epididymis; subunit a3 was first localized in osteoclasts; and subunit a4 is present in the kidney, inner ear, and epididymis. Subunit B has two isoforms. B1 was originally labeled the “kidney” isoform but was later found in other tissues including the epididymis, whereas B2 was initially called the “brain” isoform and was later found to be ubiquitously expressed on intracellular organelles (18, 90, 108). The different subcellular localizations of the V-ATPase holoenzyme are established through the assembly of a specific set of V-ATPase subunit isoforms. For example, a4 and B1 are mainly present in the plasma membrane and recycling vesicles of epididymal CCs (FIGURE 2) and renal ICs (30, 102, 105). Mutations in the a4 and B1 subunits lead to distal renal tubular acidosis and deafness in humans, illustrating their important role in luminal acidification in the kidney and inner ear (68, 128). No information on the fertility status of the affected male patients is yet available. The involvement of the V-ATPase in kidney and male reproductive physiology was further confirmed in mice that lack the transcription factor Foxi1, which is a master regulator of some V-ATPase subunits in ICs and CCs as well as in interdental cells in the inner ear (7, 8, 143). Foxi1 KO mice develop distal tubular acidosis and are deaf. Furthermore, the males are infertile due to the inability of their sperm to move up the female tract and fertilize an egg. However, these mice also lack expression of other important acid-base regulatory proteins, including the anion exchangers AE1 and pendrin, which also could contribute to their various phenotypic manifestations.
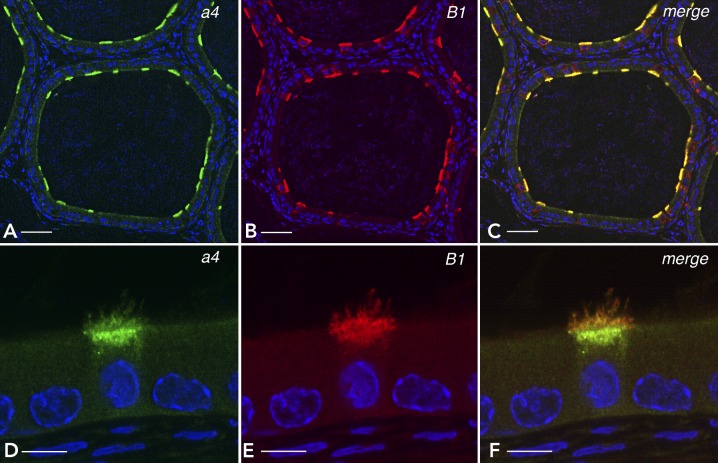
FIGURE 2.
Localization of the V-ATPase B1 and a4 subunit isoforms in epididymal clear cells
The distal region (cauda) of rat epididymis was immunofluorescently labeled with antibodies against a4 (A and D: green) and B1 (B and E: red). C and F: merged images from A and B, and D and E, respectively. B1 and a4 colocalize in the apical membrane and subapical vesicles in clear cells exclusively. Nuclei and sperm are labeled in blue with DAPI. Bars = 50 μm (A, B, C) and 5 μm (D, E, F).
Regulation of the V-ATPase
Targeting and Recycling
Proton secretion by renal A-ICs and epididymal CCs is regulated largely via V-ATPase recycling between subapical vesicles and the apical membrane (1, 13, 19, 118, 119, 123, 151), although other mechanisms can also regulate V-ATPase function and proton secretion, including association/dissociation of the V1 and V0 domains (see below), and efficiency of coupling between ATP hydrolysis and proton pumping. Regulation of V-ATPase activity via coupling efficiency has been described mainly in yeast and will not be discussed here, but readers are referred to previous reviews on this subject (10, 39, 65, 78, 135). In ICs and CCs, an increase in proton secretion correlates with an increase in the amount of V-ATPase present in the plasma membrane. Apical membrane accumulation of the V-ATPase leads to an amplification of the cell surface via formation and extension of apical microvilli or microplicae (76), which contain a high density of V-ATPase molecules (FIGURE 3). V-ATPase recycling from a pool of subapical vesicles is reflected in part by the very high endocytic activity of CCs and A-ICs (1, 14, 15, 51, 76, 86, 97). Disruption of the microtubule network by the pharmacological agent colchicine or by cold induces an almost complete redistribution of V-ATPase from the plasma membrane to intracellular vesicles in ICs and CCs (9, 12, 20), an indication that the V-ATPase constitutively recycles between the cell surface and cytoplasmic vesicles. V-ATPase recycling is also regulated via modulation of the actin cytoskeleton. Indeed, subunits B1, B2, and C are reported to be actin-binding proteins (24, 57, 144). Depolymerization of the cortical actin skeleton via inhibition of RhoA and its effector ROCKII induces the apical membrane accumulation of V-ATPase and extension of V-ATPase-labeled microvilli in epididymal CCs (122).
FIGURE 3.
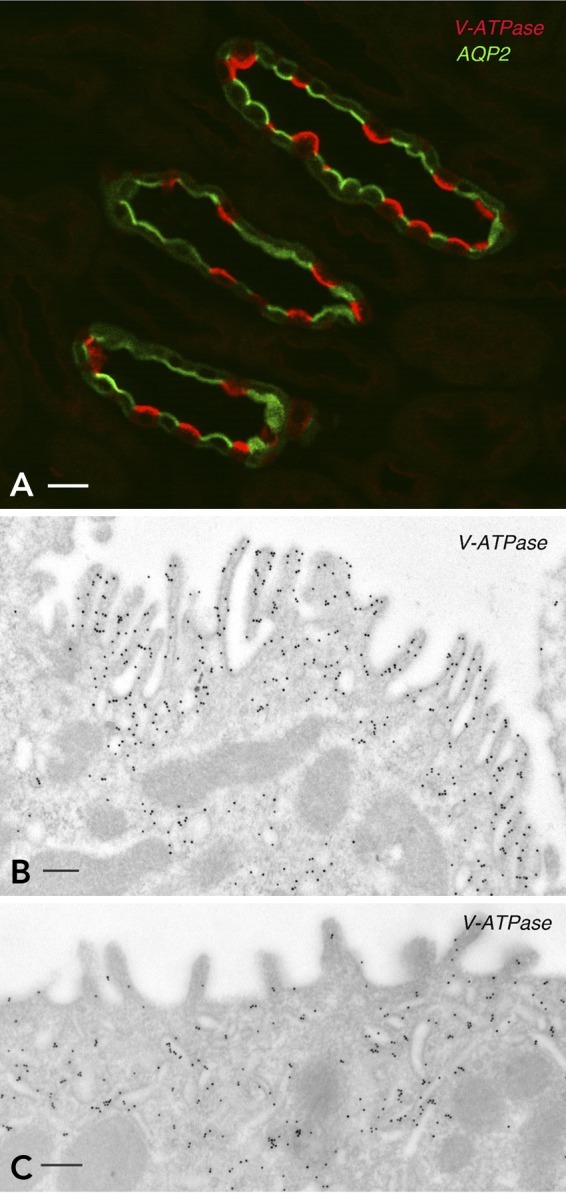
Expression of the V-ATPase in kidney intercalated cells
A: the renal inner stripe of the outer medulla was immunofluorescently labeled for the V-ATPase B1 subunit (red) and AQP2 (green). The V-ATPase and AQP2 are located apically in intercalated cells and principal cells, respectively. B and C: immunogold electron microscopy labeling of the apical pole of two intercalated cells for the V-ATPase A subunit. B: an activated IC with numerous apical microvilli that contain a high density of V-ATPase-associated gold particles. C: a resting IC with fewer and less developed microvilli. Most V-ATPase-gold particles are located in subapical tubulo-vesicles.
Although the V-ATPase is significantly accumulated on the plasma membrane by stimuli that increase proton secretion, the relative contribution of increased exocytosis and/or decreased endocytosis remains to be quantified. Previous studies have shown that CO2 exposure increases the exocytosis of vesicles containing the V-ATPase in acid-secreting cells in the turtle bladder, in parallel with an increase in proton secretion by this epithelium (140). CO2 also stimulates proton secretion in renal proximal tubules and collecting ducts (117). In epididymal CCs, the V-ATPase is present only at low levels in subapical endocytotic vesicles labeled with a fluid phase marker during stimulation of proton secretion (FIGURE 4). This suggests that specific inhibition of V-ATPase endocytosis (but not endocytosis in general) also plays a role in its membrane accumulation. Endocytosis of another recycling protein, the AQP2 water channel, is inhibited during vasopressin stimulation of epithelial cell water permeability (13, 75), suggesting that this may be a more general cell biological mechanism to acutely regulate the surface expression of membrane proteins and, thereby, modulate epithelial transport processes.
FIGURE 4.

Immunofluorescence labeling of CCs from the cauda epididymis
Immunofluorescence labeling of CCs from the cauda epididymis after in vivo luminal perfusion with a control solution adjusted to the resting pH of 6.6 (A) or with an activating solution containing bicarbonate and the cAMP permeant analog cpt-cAMP (B). Horseradish peroxidase (HRP) was added to both solutions to label endosomes (red). Epididymis sections were labeled for the V-ATPase B1 subunit (green). Arrows indicate the frontier between the apical cytoplasm and the base of microvilli. Short microvilli labeled for the V-ATPase are detected in the resting CC shown in A. The yellow color in A shows colocalization of V-ATPase in HRP-containing endosomes located in the subapical pole of clear cells. In the activated CC shown in B, the V-ATPase is mainly located in longer and more numerous microvilli (green), and it is absent from the apical endosomes that contain the internalized HRP (red). Scale bars = 5 μm. Figure reproduced from Ref. 125 with permission from the American Society of Andrology.
V-ATPase interacts with components of the SNARE complex during its exocytosis (3), and V-ATPase-dependent proton secretion in CCs requires the participation of the v-SNARE cellubrevin (12). The mechanism by which plasma membrane V-ATPase is recycled back into the cell by endocytosis is unclear. It was shown many years ago that the endocytotic vesicles involved in this process are not clathrin coated (22), nor do they contain any of the other recognized coat proteins that regulate vesicle trafficking such as caveolin (11) and COP proteins (Breton S, Brown D, unpublished observations). Instead, V-ATPase-rich recycling vesicles are coated with the V-ATPase itself (17), leading to the hypothesis that the holoenzyme is a vesicle coating material that might direct trafficking of acidifying vesicles (16). Any one of the numerous V-ATPase subunits could interact with different components of the intracellular trafficking machinery. Dissecting which, if any of them, are involved in this process is an important goal for the understanding of physiological regulation in different cell types that acidify the extracellular environment, as well as for our general understanding of the role of the V-ATPase in vesicle trafficking in all cells.
V-ATPase Regulation by Subunit Assembly and Disassembly
The V-ATPase is unusual among membrane-associated transport proteins in that it consists of two functionally distinct domains, the V0 and V1 sectors, which may be dissociated under various experimental and physiological conditions to regulate pump activity (also reviewed in Refs. 39, 64, 78, 135). In the absence of the V1 sector, which contains the ATPase catalytic site, the transmembrane V0 domain remains within the lipid bilayer but cannot function as a proton pump. Pump function is, therefore, disabled by either complete or partial dissociation of V1. Regulation of the V-ATPase by V0–V1 dissociation has been examined most extensively in nonmammalian systems, such as in the insect midgut during molting (133), the blowfly salivary gland (162), and the yeast vacuole (63). Assembly/disassembly has also been reported in a limited number of mammalian systems, including LLC-PK1 cells (116) and dendritic cells (137). More recently, disassembly of the V-ATPase was proposed in alveolar type II epithelial cells, although no direct proof that this process occurs was provided in that study (26). The extent to which this mechanism plays a role in regulating protein secretion in the two cell types discussed here (intercalated cells and clear cells) is unknown.
The factors that physiologically regulate dissociation of the V-ATPase complex are generally not well understood, but this process has been induced experimentally by low temperature in V-ATPase reconstituted from chromaffin granules (87) and low glucose or starvation in yeast (reviewed in Ref. 64). In contrast, high extracellular pH stabilizes the V0/V1 complex in yeast (32, 94). Most of the V1 domain remains intact after dissociation, except for subunit C, which dissociates from both domains (44, 63, 148). In the blowfly salivary gland, assembly of the V1 domain into a functional membrane holoenzyme has been linked to serotonin stimulation, leading to cAMP elevation and PKA activity. The V1 subunit is a target for PKA, and it was proposed that subunit C phosphorylation may be a switch that is involved in assembly of the V0/V1 complex (147, 148).
One intracellular component that plays a central role in the assembly/disassembly process in yeast is the so-called RAVE (regulator of V-ATPase in vacuoles and endosomes) complex. RAVE interacts with subunit C of the V1 sector during the assembly process (126). The RAVE complex has not yet been identified in mammalian cells, although proteins called rabconnectins, which are partially homologous to one component of the RAVE complex (Rav1), are required to support V-ATPase activity in Drosophila and mammalian cell lines (121, 160), as well as in zebrafish synaptic vesicles (34).
It is likely that assembly/disassembly and vesicle trafficking make different quantitative contributions to the regulation of proton transport across plasma membranes vs. intracellular membranes such as endosomes, Golgi and TGN cisternae, and lysosomes. More work is needed to dissect their relative roles in these functionally distinct membrane subdomains. It is, however, probable that even disassembled portions of the V-ATPase may be trafficked to and from the plasma membrane by vesicle transport, adding a degree of complexity to efforts aimed at distinguishing these two distinct regulatory processes.
Nonhormonal Regulation
In addition to being an essential player in the establishment of male fertility, the epididymis also represents a powerful model system in which the regulation of V-ATPase-dependent luminal acidification in general can be examined. Indeed, the lumen of the epididymis is much larger than that of the collecting duct, which allows for the manipulation of the luminal environment without affecting the basolateral side, e.g., by perfusion of the intact tissue in vivo (13). An important question is: How do these proton-secreting cells sense and respond to variations in the acid/base status of the extracellular environment? Both CCs and A-ICs express the “bicarbonate” sensor, soluble adenylate cyclase (sAC). In the epididymis, alkalinization of the normally acidic luminal fluid leads to compensatory activation of proton secretion by CCs. This restores the acidic luminal environment that sperm require to mature and remain quiescent during storage (97, 125). A series of studies revealed that the sAC is a key player in this homeostatic sensing and regulatory system. Increased luminal bicarbonate activates sAC in CCs, which in turn generates cAMP, activates PKA, and results in apical proton pump accumulation and increased proton secretion (95, 96). In the kidney, sAC is also highly expressed in ICs, and it co-immunoprecipitates in a complex with the V-ATPase (98). Following impairment of bicarbonate reabsorption in proximal tubules (e.g., resulting from a Fanconi syndrome) and a subsequent increase in the distal delivery of bicarbonate into the lumen of the collecting duct, we hypothesize that entry of HCO3− into A-ICs also leads to activation of sAC and, as in the epididymis, an increase in apical V-ATPase recruitment (95, 98). PKA, but not EPAC, participates in V-ATPase apical accumulation (2, 96). This series of events would provide a feedback mechanism by which A-ICs sense deviations in urinary bicarbonate concentration and regulate their rate of proton secretion accordingly to correct acid/base imbalance. The mechanisms by which tubular epithelial cells detect apical and/or basolateral cues to regulate luminal proton secretion and, in the kidney, acid-base balance are complex and have been explored in recent reviews (21, 136).
But how does PKA activation result in plasma membrane V-ATPase accumulation in these cells? The V-ATPase contains several phosphorylation sites on different subunits, some of which are putative PKA target sites (100). In cultured cells, phosphorylation of the A subunit by PKA is necessary for plasma membrane accumulation of the V-ATPase (2, 47). PKA activity has also been linked to V-ATPase phosphorylation and/or its membrane recruitment or assembly in other tissues, including Manduca sexta midgut (148), blowfly salivary gland (31, 111, 147), and the ciliary body epithelium in the eye (154). In addition, the V-ATPase C subunit of the plant Arabidopsis (AtBHA-C) is phosphorylated at multiple sites (59). By analogy with other systems, notably AQP2 trafficking in renal collecting duct principal cells (75), phosphorylation of the V-ATPase may lead to membrane accumulation by inhibition of endocytosis, although this remains to be demonstrated. Furthermore, the presence of other kinase sites on V-ATPase subunits suggests complex regulatory functions of phosphorylation in the trafficking, assembly, and activity of the holoenzyme that have not yet been explored in any detail.
Early studies on the turtle bladder showed that the CO2-stimulated exocytosis of proton-pump-containing vesicles is Ca2+ dependent (140). Similarly, pH- and cAMP-induced V-ATPase apical recruitment in the epididymis requires calcium (4, 156). Interestingly, an increase in intracellular calcium is required to induce maximal activation of sAC by bicarbonate, thereby maximizing cAMP generation within the cell (25). A potential link between bicarbonate activation of CCs and the requirement for intracellular calcium was provided by a study showing the participation of purinergic receptors in the regulation of V-ATPase in CCs (5). Extracellular ATP activates either purinergic P2X receptors, which are ligand-gated ion channels, or the G-protein-coupled P2Y receptors, leading to an increase in intracellular calcium (106). Importantly, some purinergic receptors are regulated by extracellular pH (58). ATP and its metabolite adenosine both induce V-ATPase apical accumulation in CCs, providing a potential connection between luminal pH and the role of calcium in V-ATPase regulation (5). In addition, extracellular ATP stimulates bone resorption by osteoclasts (40, 70). Several purinergic receptors are present in the kidney (54, 107, 138, 139, 145), but the regulation of acid/base transport in this organ via purinergic signaling has not been characterized.
Hormonal Regulation
Luminal acidification in the kidney is regulated by the renin-angiotensin-aldosterone system (reviewed in Refs. 150, 151). Angiotensinogen, rennin, and the angiotensin I-converting enzyme (ACE) are present along the nephron, forming a local paracrine regulatory system (110, 112). Consequently, the concentration of angiotensin II (ANGII) is higher in the lumen of the nephron compared with blood. ANGII induces the translocation of the V-ATPase from an intracellular pool to the apical membrane and stimulates proton secretion in type A-ICs (103, 113, 153). This effect is mediated by the ANGII type 1 receptor (AT1), which upon binding to ANGII triggers the PLC/PKC pathway (113).
In the epididymis, luminal ANGII also stimulates V-ATPase-proton secretion in CCs, but this effect is mediated by the ANGII type II receptor (AGTR2) and requires the contribution of adjacent basal cells (124). Components of the renin-angiotensin system are also present in the lumen of the epididymis, including ANGI and ANGII (35, 114, 129, 158). ACE exists in two forms, a somatic form and a testicular form (t-ACE) expressed in spermatozoa. Absence of t-ACE in knockout mice leads to male infertility due to a defect in sperm function, indicating a potential epididymal dysfunction (35, 46, 71). t-ACE is released from sperm as they mature in the epididymal lumen, and it contributes to the production of ANGII (42, 82, 134). ANGII binds to the ANGII type II receptor (AGTR2) in basal cells, which detect ANGII via a narrow body extension that probes the luminal content (124). Activation of AGTR2 triggers the production of nitric oxide, which then diffuses out of basal cells to enter CCs, where it activates the cGMP pathway. cGMP elevation induces the apical accumulation of the V-ATPase followed by stimulation of V-ATPase-dependent proton secretion in CCs (124). Thus, in the epididymis, CCs respond differently to ANGII compared with renal ICs, in which the ANGII type I receptor, followed by activation of the PKC pathway, is responsible for V-ATPase stimulation (113). ANGII has also been reported to increase apical V-ATPase expression and function in the renal proximal tubule (152) by an effect involving tyrosine kinase, p38 MAPK, and PI3K activation (23).
In addition to ANGII, aldosterone is also a key regulator of luminal acidification in the kidney (150, 151). A defect in aldosterone signaling causes type IV distal renal tubular acidosis (type IV dRTA) (33), an effect that is at least partially mediated via modulation of V-ATPase-dependent luminal acidification in the kidney (33, 41, 49, 89, 131). In addition to a long-term genomic effect of aldosterone, more recent studies have identified nongenomic actions of aldosterone (reviewed in Refs. 36, 74, 130). Short-term stimulation of collecting ducts isolated from the outer medulla of mouse and human kidneys increases V-ATPase activity in ICs (156, 157). Aldosterone stimulation in these cells is independent of transcription or translation and is not inhibited by the mineralocorticoid receptor blocker spironolactone. It is mediated by Gαq proteins and requires PLC and PKC activity, as well as ERK1/2 MAPK kinase activity. Parallel activation of the cAMP/PKA pathway was also observed. However, activation of PKA with 8-Br-cAMP together with inhibition of PKC failed to induce V-ATPase activation, whereas activation of PKC with DOG stimulated V-ATPase even in the presence of the PKA inhibitor H89. It was, therefore, proposed that the PLC/PKC is the main pathway for aldosterone stimulation and that PKA may play a modulatory role. Aldosterone treatment of rats in vivo induced the translocation of V-ATPase from intracellular vesicles to the apical membrane in A-ICs of outer medullary collecting ducts (156). Thus aldosterone stimulates proton secretion in A-ICs via a rapid nongenomic action mediated by a Gαq protein-coupled receptor. This indicates the possibility that similar epididymal CCs might also be regulated by circulating aldosterone, and ongoing studies are currently being performed in our laboratory to determine whether this is the case.
Animal Models for the Study of the V-ATPase
Several mouse models have been generated to study the function of the V-ATPase. Mice lacking the B1 subunit (B1 KO mice) were produced as a model for human distal tubular acidosis caused by mutations in the B1 gene (37). Surprisingly, normal systemic acid/base balance was observed in these mice. In addition, the males were fertile, and a normal acidic pH was measured in the lumen of the epididymis (30). Importantly, an increased amount of V-ATPase containing the B2 subunit is inserted into the plasma membrane of ICs and CCs, showing a compensatory function of this “ubiquitous” isoform in B1-null mice (30, 102). In wild-type (WT) mice, only a very small amount of the B2 isoform can be detected at the surface of these cells, where it colocalizes with B1. Although the increase in plasma membrane delivery of the B2 subunit is sufficient to maintain a normal acid/base status under baseline conditions, severe acidosis was observed in B1 KO mice exposed to an acid load in vivo (NH4Cl in the drinking water) (37). This suggests that the B2 isoform does not fully compensate for the absence of B1 when these mice are stress tested. In addition, a reduced rate of V-ATPase-dependent proton extrusion was observed in ICs from collecting ducts isolated in vitro (102), and ANGII failed to stimulate V-ATPase-dependent luminal acidification (113).
How can we explain the finding that B1-null mice show no overt phenotype under baseline conditions, whereas humans with B1 mutations develop severe dRTA? Although the role of dietary differences has not been completely excluded, cell culture studies shed some light on this phenomenon at a mechanistic level. In IMCD (inner medullary collecting duct) cells, transfected B1 subunits containing some of these point mutations do not assemble into functional holoenzymes, and, furthermore, they competitively inhibit trafficking of the endogenous B1-containing V-ATPase to the plasma membrane (161). Based on these results, it was proposed that B1 contains a targeting sequence allowing plasma membrane expression of the holoenzyme. However, data from the B1-null mice clearly show that the holoenzyme can be located at the cell surface even in the absence of the B1 subunit in mice, implying that B1 is not an absolute requirement for plasma membrane targeting of the V-ATPase. This is also supported by the fact that surface expression of the V-ATPase is abundant in cells that express predominantly the B2 isoform, such as proximal tubules (151) and osteoclasts (72). It is possible, therefore, that the presence of mutated B1 subunits in humans inhibits the formation and cell surface trafficking of B2-containing V-ATPases. More proton secretory function would then be retained in cells that express no B1 at all compared with cells expressing mutated B1 subunits. A detailed analysis to correlate the nature of the human mutations to the severity of their phenotype would be informative in this respect.
In another mouse model, the B1 subunit promoter was used to drive cell-specific expression of enhanced green fluorescent protein (EGFP) (84). Specific expression of EGFP was achieved in ICs, epididymal CCs, and clara cells of the lung, providing a powerful model for the characterization of B1-expressing cells. These mice allowed the isolation of renal ICs and epididymal CCs by fluorescence-activated cell sorting (FACS). Their proteome was compared with the respective EGFP-negative cell population by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (29). Out of the 2,297 and 1,564 proteins that were detected in the IC and CC populations, respectively, 202 and 178 were enriched compared with EGFP-negative cells. A few proteins were commonly enriched in ICs and CCs, including the V-ATPase B1, A, and a4 subunits, as well as proteins involved in PKA binding-endosomal transport (Lrba), the progesterone receptor (Pgrmc1), and proteins involved in cytoskeleton dynamics. This proteomic analysis provides a framework for the future characterization of the common and distinct functions of B1-expressing renal ICs and epididymal CCs. The complete database of proteins in these V-ATPase-rich cells is publicly available at http://dir.nhlbi.nih.gov/papers/lkem/kevcpd/KvcpData.aspx.
In addition, the B1-EGFP mice were crossed with B1-KO mice to generate a novel EGFP-B1 KO mouse model (142). This allowed for the isolation of renal ICs that were lacking the B1 subunit by FACS. Western blot analysis showed a markedly increased expression of the “compensatory” B2 subunit in B1-KO ICs vs. WT-ICs. However, significant decreases in the expression of the ubiquitous subunits A, E, and H were also detected, which would account for the overall reduction in V-ATPase activity that was reported in collecting ducts isolated from B1-KO mice compared with WT mice (102).
Mice lacking the a4 subunit were recently engineered and showed profound dRTA and hearing loss (50, 92). Proximal tubule function was also impaired in these mice and was manifested by proteinuria, phosphaturia, reduced endocytosis, and accumulation of material in the lysosomes of proximal tubule cells (50). It was proposed that the severe acidosis observed in patients harboring mutations in the ATP6V0A4 gene might be the result of a complex phenotype that involves proximal tubule function in addition to collecting duct ICs. Interestingly, V-ATPase subunits A, B1, and E1 were also markedly downregulated in the a4-null mice. The downregulation of several (but not all) components of the holoenzyme in V-ATPase a4 and B1 subunit-deficient mice suggests that their expression is regulated in parallel, perhaps at the transcriptional or translational level. Alternatively, the assembly of intact holoenzymes could be disrupted in these mice, leading to a less stable holoenzyme complex. Interestingly, ICs from the a4-null mice have a greatly reduced number of characteristic V-ATPase transporting “tubulovesicles” in their cytoplasm (50) (these structures are visible in FIGURE 3), suggesting that the expression and perhaps the assembly of the V-ATPase are closely related to the biogenesis of this specialized organelle.
Foxi1 is a forkhead transcription factor that has been proposed as a regulator of V-ATPase expression and as a determinant of the cellular composition of the collecting duct and the epididymis (143). Mice lacking Foxi1 have an essentially normal kidney, but their collecting ducts do not contain cells that express the anion exchangers AE1 (typically in A-ICs), pendrin (characteristic of type B ICs), or several V-ATPase subunits. Cells with the specific morphology of ICs are absent, and most collecting duct cells express AQP2 in addition to carbonic anhydrase II, usually an IC marker (15). Using a luciferase-based promoter assay, Foxi1 was shown to be a direct regulator of a4 (as well as pendrin and AE1), but the level of expression of B1 subunit mRNA was unchanged in Foxi1-null mice, despite the fact that B1 protein was undetectable (7, 143). This result implies that, although Foxi1 may directly regulate transcription of some V-ATPase subunit genes, others such as B1 are regulated indirectly, perhaps as a result of reduced a4 subunit levels. This would, therefore, be consistent with the reduction in some subunit levels that was reported in a4-null mice.
The V-ATPase as a pH Sensor
Marshansky and colleagues showed that the V-ATPase is not only involved in net proton secretion and acidification of intracellular organelles but that it acts as a pH sensor that regulates key enzymes and modulates its own function (61, 77, 80, 81). The a2 isoform of the V-ATPase recruits “coat” proteins to endosomal membranes in response to vesicle acidification (79), a process that directs intracellular trafficking and targeting events (6, 16, 39, 55, 78, 115). The V-ATPase itself was proposed to be a member of the “coat” protein family along with clathrin, COP proteins, and caveolin, which all produce morphologically detectable protein coats on vesicles and, in some cases, plasma membranes (16). In addition to recruiting proteins to the endosomal membrane, it has been shown recently that association of GTPase regulatory proteins such as cytohesin 2 with the a4 NH2 terminus actually modulates the enzymatic GEF activity of the cytohesin and, thereby, of its cognate small GTPase, Arf6 (60). This positions the V-ATPase as a pH sensor that no only recruits endosomal coat proteins in an acidification-dependent manner but can also modulate the enzymatic activity of GTPases that are involved in various aspects of cellular function. The V-ATPase has, therefore, now transitioned from being only a proton pump to being recognized as a protein complex that is a central part of a cellular signal transduction cascade on endosomes and, possibly, on other membrane domains also. Other data relating the V-ATPase to notch (160) and Wnt (28) signaling pathways also position this enzyme as a central player in a variety of regulatory pathways, and some V-ATPase subunits have even been linked to the regulation of apoptosis (73, 93). Importantly, activation of notch signaling results in greatly increased numbers of ICs in the mouse collecting duct, along with the development of nephrogenic diabetes insipidus due to the loss of vasopressin-sensitive principal cells (62). Future studies are required to determine whether a notch-V-ATPase interaction is involved in the development of the collecting duct and how it may govern the relative number of intercalated cells and principal cells.
Summary and Conclusion
Acidification of the extracellular milieu via the V-ATPase plays a crucial role in maintaining healthy organ function and is perturbed in several pathological conditions. The V-ATPase is involved in numerous physiological processes, including systemic acid/base balance, male fertility, bone remodeling, hearing, and olfaction. In addition, its role in cancer has recently emerged, particularly as a key mediator of the invasive ability of malignant cells. The V-ATPase is composed of many subunits, several of which exist as more than one isoform. Different specialized cells, in which the V-ATPase is highly expressed, often contain holoenzymes of distinct isoform composition. This heterogeneity plays a role in determining the subcellular localization of the V-ATPase and is important for cellular function. Plasma membrane accumulation of the V-ATPase is mediated via vesicle recycling and is increased by the cAMP, cGMP, and PLC/PKC pathways in response to various nonhormonal (e.g., pH, bicarbonate, CO2) and hormonal (e.g., ANGII, aldosterone) stimuli. Recycling of V-ATPase-rich vesicles requires intact microtubules and the actin cytoskeleton. Phosphorylation of some V-ATPase subunits by PKA plays a role in its plasma membrane accumulation, but the exact mechanisms involved in this process remain unknown. V-ATPase activity in yeast and insects is also regulated via association/dissociation of the cytosolic V1 domain with the transmembrane V0 domain. However, whether this process is a general regulatory mechanism in intact mammalian cells still remains to be determined. Finally, the V-ATPase is now considered a coat protein that recruits proteins to endosomal membranes in a pH-dependent manner and modulates the activity of key GTPases and signaling molecules. The V-ATPase is, therefore, a multifaceted enzyme that plays fundamental roles in the response of specialized cells to extracellular cues that regulate acidification of the extracellular environment in various organs and tissues.
Acknowledgments
We thank Dr. Yechun Ruan for preparing the illustration shown in FIGURE 1, and Dr. Marija Ljubojevic for the picture shown in FIGURE 2. Finally, we thank our many colleagues and collaborators who have made significant contributions to our V-ATPase studies over many years.
Footnotes
This work was supported by National Institutes of Health grants HD-040793 and DK-097124 (to S. Breton), DK-038452 (to S. Breton and D. Brown), and DK-042956 (to D. Brown). The imaging work was performed in the Microscopy Core Facility of the Massachusetts General Hospital Program in Membrane Biology, which was partially supported by Center for the Study of Inflammatory Bowel Disease Grant DK43351 and Boston Area Diabetes and Endocrinology Research Center award DK57521. S. Breton is a recipient of the Charles and Ann Sanders MGH Scholars Award.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: S.B. and D.B. conception and design of research; S.B. and D.B. analyzed data; S.B. and D.B. interpreted results of experiments; S.B. and D.B. prepared figures; S.B. and D.B. drafted manuscript; S.B. and D.B. edited and revised manuscript; S.B. and D.B. approved final version of manuscript.
References
- 1.Al-Awqati Q. Plasticity in epithelial polarity of renal intercalated cells: targeting of the H+-ATPase and band 3. Am J Physiol Cell Physiol 270: C1571–C1580, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Alzamora R, Thali RF, Gong F, Smolak C, Li H, Baty CJ, Bertrand CA, Auchli Y, Brunisholz RA, Neumann D, Hallows KR, Pastor-Soler NM. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J Biol Chem 285: 24676–24685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee A, Shih T, Alexander EA, Schwartz JH. SNARE proteins regulate H+-ATPase redistribution to the apical membrane in rat renal inner medullary collecting duct cells. J Biol Chem 274: 26518–26522, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+ATPase (V-ATPase) recycling. J Biol Chem 280: 8452–8463, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Belleannee C, Da Silva N, Shum WW, Brown D, Breton S. Role of purinergic signaling pathways in V-ATPase recruitment to apical membrane of acidifying epididymal clear cells. Am J Physiol Cell Physiol 298: C817–C830, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyenbach KW, Wieczorek H. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol 209: 577–589, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Blomqvist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, Persson AE, Bergstrom GG, Enerback S. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest 113: 1560–1570, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomqvist SR, Vidarsson H, Soder O, Enerback S. Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. EMBO J 25: 4131–4141, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breton S, Brown D. Cold-induced microtubule disruption and relocalization of membrane proteins in kidney epithelial cells. J Am Soc Nephrol 9: 155–166, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Breton S, Brown D. New insights into the regulation of V-ATPase-dependent proton secretion. Am J Physiol Renal Physiol 292: F1–F10, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Breton S, Lisanti MP, Tyszkowski R, McLaughlin M, Brown D. Basolateral distribution of caveolin-1 in the kidney. Absence from H+-atpase-coated endocytic vesicles in intercalated cells. J Histochem Cytochem 46: 205–214, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Breton S, Nsumu NN, Galli T, Sabolic I, Smith PJ, Brown D. Tetanus toxin-mediated cleavage of cellubrevin inhibits proton secretion in the male reproductive tract. Am J Physiol Renal Physiol 278: F717–F725, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Brown D, Bouley R, Paunescu TG, Breton S, Lu HAJ. New insights into the dynamic regulation of water and acid-base balance by renal epithelial cells. Am J Physiol Cell Physiol 302: C1421–C1433, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown D, Breton S. H+V-ATPase-dependent luminal acidification in the kidney collecting duct and the epididymis/vas deferens: vesicle recycling and transcytotic pathways. J Exp Biol 203: 137–145, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Brown D, Breton S. Mitochondria-rich, proton-secreting epithelial cells. J Exp Biol 199: 2345–2358, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Brown D, Breton S, Ausiello DA, Marshansky V. Sensing, signaling and sorting events in kidney epithelial cell physiology. Traffic 10: 275–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown D, Gluck S, Hartwig J. Structure of the novel membrane-coating material in proton-secreting epithelial cells and identification as an H+ATPase. J Cell Biol 105: 1637–1648, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown D, Lui B, Gluck S, Sabolic I. A plasma membrane proton ATPase in specialized cells of rat epididymis. Am J Physiol Cell Physiol 263: C913–C916, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Brown D, Paunescu TG, Breton S, Marshansky V. Regulation of the V-ATPase in kidney epithelial cells: dual role in acid/base homeostasis and vesicle trafficking. J Exp Biol 212: 1762–1772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown D, Sabolic I, Gluck S. Colchicine-induced redistribution of proton pumps in kidney epithelial cells. Kidney Int Suppl 33: S79–S83, 1991 [PubMed] [Google Scholar]
- 21.Brown D, Wagner CA. Molecular mechanisms of acid-base sensing by the kidney. J Am Soc Nephrol 23: 774–780, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown D, Weyer P, Orci L. Nonclathrin-coated vesicles are involved in endocytosis in kidney collecting duct intercalated cells. Anat Rec 218: 237–242, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Carraro-Lacroix LR, Girardi AC, Malnic G. Long-term regulation of vacuolar H+-ATPase by angiotensin II in proximal tubule cells. Pflügers Arch 458: 969–979, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Chen SH, Bubb MR, Yarmola EG, Zuo J, Jiang J, Lee BS, Lu M, Gluck SL, Hurst IR, Holliday LS. Vacuolar H+-ATPase binding to microfilaments: regulation in response to phosphatidylinositol 3-kinase activity and detailed characterization of the actin-binding site in subunit B. J Biol Chem 279: 7988–7998, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Chintagari NR, Mishra A, Su L, Wang Y, Ayalew S, Hartson SD, Liu L. Vacuolar ATPase regulates surfactant secretion in rat alveolar type II cells by modulating lamellar body calcium. PLos One 5: e9228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cipriano DJ, Wang Y, Bond S, Hinton A, Jefferies KC, Qi J, Forgac M. Structure and regulation of the vacuolar ATPases. Biochim Biophys Acta 1777: 599–604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327: 459–463, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Da Silva N, Pisitkun T, Miller RL, Nelson RD, Knepper MA, Brown D, Breton S. Proteomic analysis of proton-transporting epididymal celar cells and renal intercalated cells. Am J Physiol Cell Physiol 298: C1326–C1342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Da Silva N, Shum WWC, El-Annan J, Paunescu TG, McKee M, Smith PJS, Brown D, Breton S. Relocalization of the V-ATPase B2 subunit to the apical membrane of epididymal clear cells of mice deficient in the B1 subunit. Am J Physiol Cell Physiol 293: C199–C210, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Dames P, Zimmermann B, Schmidt R, Rein J, Voss M, Schewe B, Walz B, Baumann O. cAMP regulates plasma membrane vacuolar-type H+-ATPase assembly and activity in blowfly salivary glands. Proc Natl Acad Sci USA 103: 3926–3931, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diakov TT, Kane PM. Regulation of vacuolar proton-translocating ATPase activity and assembly by extracellular pH. J Biol Chem 285: 23771–23778, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DuBose TD, Jr, Caflisch CR. Effect of selective aldosterone deficiency on acidification in nephron segments of the rat inner medulla. J Clin Invest 82: 1624–1632, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Einhorn Z, Trapani JG, Liu Q, Nicolson T. Rabconnectin3alpha promotes stable activity of the H+ pump on synaptic vesicles in hair cells. J Neurosci 32: 11144–11156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esther CR, Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest 74: 953–965, 1996 [PubMed] [Google Scholar]
- 36.Falkenstein E, Christ M, Feuring M, Wehling M. Specific nongenomic actions of aldosterone. Kidney Int 57: 1390–1394, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Finberg KE, Wagner CA, Bailey MA, Paunescu TG, Breton S, Brown D, Giebisch G, Geibel JP, Lifton RP. The B1-subunit of the H+ ATPase is required for maximal urinary acidification. Proc Natl Acad Sci USA 102: 13616–13621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finnigan GC, Hanson-Smith V, Houser BD, Park HJ, Stevens TH. The reconstructed ancestral subunit a functions as both V-ATPase isoforms Vph1p and Stv1p in Saccharomyces cerevisiae. Mol Biol Cell 22: 3176–3191, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nature Rev Mol Cell Biol 8: 917–929, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Gallagher JA. ATP P2 receptors and regulation of bone effector cells. J Musculoskelet Neuronal Interact 4: 125–127, 2004 [PubMed] [Google Scholar]
- 41.Garg LC, Narang N. Effects of aldosterone on NEM-sensitive ATPase in rabbit nephron segments. Kidney Int 34: 13–17, 1988 [DOI] [PubMed] [Google Scholar]
- 42.Gatti JL, Druart X, Guerin Y, Dacheux F, Dacheux JL. A 105- to 94-kilodalton protein in the epididymal fluids of domestic mammals is angiotensin I-converting enzyme (ACE); evidence that sperm are the source of this ACE. Biol Reprod 60: 937–945, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Gleize V, Boisselier B, Marie Y, Poea-Guyon S, Sanson M, Morel N. The renal v-ATPase a4 subunit is expressed in specific subtypes of human gliomas. Glia 60: 1004–1012, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Graf R, Harvey WR, Wieczorek H. Purification and properties of a cytosolic V1-ATPase. J Biol Chem 271: 20908–20913, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Graham LA, Powell B, Stevens TH. Composition and assembly of the yeast vacuolar H+-ATPase complex. J Exp Biol 203: 61–70, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Hagaman JR, Moyer JS, Bachman ES, Sibony M, Magyar PL, Welch JE, Smithies O, Krege JH, O'Brien DA. Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci USA 95: 2552–2557, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hallows KR, Alzamora R, Li H, Gong F, Smolak C, Neumann D, Pastor-Soler NM. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol 296: C672–C681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harvey WR, Xiang MA. K+ pump: from caterpillar midgut to human cochlea. J Insect Physiol 58: 590–598, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Hays SR. Mineralocorticoid modulation of apical and basolateral membrane H+/OH−/HCO3− transport processes in the rabbit inner stripe of outer medullary collecting duct. J Clin Invest 90: 180–187, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hennings JC, Picard N, Huebner AK, Stauber T, Maier H, Brown D, Jentsch TJ, Vargas-Poussou R, Eladari D, Hubner CA. A mouse model for distal renal tubular acidosis reveals a previously unrecognized role of the V-ATPase a4 subunit in the proximal tubule. EMBO Mol Med 4: 1057–1071, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hermo L, Dworkin J, Oko R. Role of epithelial clear cells of the rat epididymis in the disposal of the contents of cytoplasmic droplets detached from spermatozoa. Am J Anat 183: 107–124, 1988 [DOI] [PubMed] [Google Scholar]
- 52.Hernandez A, Serrano-Bueno G, Perez-Castineira JR, Serrano A. Intracellular proton pumps as targets in chemotherapy: V-ATPases and cancer. Curr Pharm Design 18: 1383–1394, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Hildenbrand ZL, Molugu SK, Stock D, Bernal RA. The C-H peripheral stalk base: a novel component in V1-ATPase assembly. PLos One 5: e12588, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hillman KA, Burnstock G, Unwin RJ. The P2X7 ATP receptor in the kidney: a matter of life or death? Nephron Exp Nephrol 101: e24–e30, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Hinton A, Bond S, Forgac M. V-ATPase functions in normal and disease processes. Pflügers Arch 457: 589–598, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Hinton A, Sennoune SR, Bond S, Fang M, Reuveni M, Sahagian GG, Jay D, Martinez-Zaguilan R, Forgac M. Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J Biol Chem 284: 16400–16408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holliday LS, Lu M, Lee BS, Nelson RD, Solivan S, Zhang L, Gluck SL. The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J Biol Chem 275: 32331–32337, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Holzer P. Acid sensing by visceral afferent neurones. Acta Physiol (Oxf) 201: 63–75, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong-Hermesdorf A, Brux A, Gruber A, Gruber G, Schumacher K. A WNK kinase binds and phosphorylates V-ATPase subunit C. FEBS Lett 580: 932–939, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Hosokawa H, Dip PV, Merkulova M, Bakulina A, Zhuang Z, Khatri A, Jian X, Keating SM, Bueler SA, Rubinstein JL, Randazzo PA, Ausiello DA, Gruber G, Marshansky V. The N-terminus of a-subunit isoforms is involved in signaling between V-ATPase and cytohesin-2. J Biol Chem 288: 5896–5913, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8: 124–136, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Jeong HW, Jeon US, Koo BK, Kim WY, Im SK, Shin J, Cho Y, Kim J, Kong YY. Inactivation of Notch signaling in the renal collecting duct causes nephrogenic diabetes insipidus in mice. J Clin Invest 119: 3290–3300, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kane PM. Disassembly and reassembly of the yeast vacuolar H+-ATPase in vivo. J Biol Chem 270: 17025–17032, 1995 [PubMed] [Google Scholar]
- 64.Kane PM. Targeting reversible disassembly as a mechanism of controlling V-ATPase activity. Curr Protein Peptide Sci 13: 117–123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kane PM. The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol Mol Biol Rev 70: 177–191, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kane PM, Parra KJ. Assembly and regulation of the yeast vacuolar H+-ATPase. J Exp Biol 203: 81–87, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Karet FE. Physiological and metabolic implications of V-ATPase isoforms in the kidney. J Bioenerg Biomembr 37: 425–429, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skvorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 21: 84–90, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Kartner N, Manolson MF. V-ATPase subunit interactions: the long road to therapeutic targeting. Curr Protein Peptide Sci 13: 164–179, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Kaunitz JD, Yamaguchi DT. TNAP, TrAP, ecto-purinergic signaling, and bone remodeling. J Cell Biochem 105: 655–662, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O'Brien DA, Smithies O. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature 375: 146–148, 1995 [DOI] [PubMed] [Google Scholar]
- 72.Lee BS, Holliday LS, Ojikutu B, Krits I, Gluck SL. Osteoclasts express the B2 isoform of vacuolar H+-ATPase intracellularly and on their plasma membranes. Am J Physiol Cell Physiol 270: C382–C388, 1996 [DOI] [PubMed] [Google Scholar]
- 73.Li G, Yang Q, Krishnan S, Alexander EA, Borkan SC, Schwartz JH. A novel cellular survival factor–the B2 subunit of vacuolar H+-ATPase inhibits apoptosis. Cell Death Differ 13: 2109–2117, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, Wehling M. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev 83: 965–1016, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Lu HA, Sun TX, Matsuzaki T, Yi XH, Eswara J, Bouley R, McKee M, Brown D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem 282: 28721–28732, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Madsen KM, Tisher CC. Response of intercalated cells of rat outer medullary collecting duct to chronic metabolic acidosis. Lab Invest 51: 268–276, 1984 [PubMed] [Google Scholar]
- 77.Marshansky V. The V-ATPase a2-subunit as a putative endosomal pH-sensor. Biochem Soc Trans 35: 1092–1099, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 20: 415–426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merkulova M, Bakulina A, Thaker YR, Gruber G, Marshansky V. Specific motifs of the V-ATPase a2-subunit isoform interact with catalytic and regulatory domains of ARNO. Biochim Biophys Acta 1797: 1398–1409, 2010 [DOI] [PubMed] [Google Scholar]
- 80.Merkulova M, Hurtado-Lorenzo A, Hosokawa H, Zhuang Z, Brown D, Ausiello DA, Marshansky V. Aldolase directly interacts with ARNO and modulates cell morphology and acidic vesicle distribution. Am J Physiol Cell Physiol 300: C1442–C1455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Merkulova M, McKee M, Dip PV, Gruber G, Marshansky V. N-terminal domain of the V-ATPase a2-subunit displays integral membrane protein properties. Protein Sci 19: 1850–1862, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Metayer S, Dacheux F, Dacheux JL, Gatti JL. Germinal angiotensin I-converting enzyme is totally shed from the rodent sperm membrane during epididymal maturation. Biol Reprod 67: 1763–1767, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Michel V, Licon-Munoz Y, Trujillo K, Bisoffi M, Parra KJ. Inhibitors of vacuolar ATPase proton pumps inhibit human prostate cancer cell invasion and prostate-specific antigen expression and secretion. Intl J Cancer 132: E1–E10, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, Breton S, Nelson RD. The V-ATPase B1 subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol 288: C1134–C1144, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Miranda KC, Karet FE, Brown D. An extended nomenclature for mammalian V-ATPase subunit genes and splice variants. PLos One 5: e9531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moore HD, Bedford JM. The differential absorptive activity of epithelial cells of the rat epididymus before and after castration. Anat Rec 193: 313–327, 1979 [DOI] [PubMed] [Google Scholar]
- 87.Moriyama Y, Nelson N. Cold inactivation of vacuolar proton-ATPases. J Biol Chem 264: 3577–3582, 1989 [PubMed] [Google Scholar]
- 88.Muench SP, Trinick J, Harrison MA. Structural divergence of the rotary ATPases. Q Rev Biophys 44: 311–356, 2011 [DOI] [PubMed] [Google Scholar]
- 89.Mujais SK. Effects of aldosterone on rat collecting tubule N-ethylmaleimide-sensitive adenosine triphosphatase. J Lab Clin Med 109: 34–39, 1987 [PubMed] [Google Scholar]
- 90.Nelson RD, Guo XL, Masood K, Brown D, Kalkbrenner M, Gluck S. Selectively amplified expression of an isoform of the vacuolar H+-ATPase 56-kilodalton subunit in renal intercalated cells. Proc Natl Acad Sci USA 89: 3541–3545, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishisho T, Hata K, Nakanishi M, Morita Y, Sun-Wada GH, Wada Y, Yasui N, Yoneda T. The a3 isoform vacuolar type H+-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res 9: 845–855, 2011 [DOI] [PubMed] [Google Scholar]
- 92.Norgett EE, Golder ZJ, Lorente-Canovas B, Ingham N, Steel KP, Frankl FE. Atp6v0a4 knockout mouse is a model of distal renal tubular acidosis with hearing loss, with additional extrarenal phenotype. Proc Natl Acad Sci USA 109: 13775–13780, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okahashi N, Nakamura I, Jimi E, Koide M, Suda T, Nishihara T. Specific inhibitors of vacuolar H+-ATPase trigger apoptotic cell death of osteoclasts. J Bone Miner Res 12: 1116–1123, 1997 [DOI] [PubMed] [Google Scholar]
- 94.Padilla-Lopez S, Pearce DA. Saccharomyces cerevisiae lacking Btn1p modulate vacuolar ATPase activity to regulate pH imbalance in the vacuole. J Biol Chem 281: 10273–10280, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem 278: 49523–49529, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pastor-Soler N, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294: C488–C494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pastor-Soler N, Pietrement C, Breton S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology 20: 417–428, 2005 [DOI] [PubMed] [Google Scholar]
- 98.Paunescu TG, Da Silva N, Russo LM, McKee M, Lu HAJ, Breton S, Brown D. Association of soluble adenylyl cyclase (sAC) with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol 294: F130–F138, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Paunescu TG, Jones AC, Tyszkowski R, Brown D. V-ATPase expression in the mouse olfactory epithelium. Am J Physiol Cell Physiol 295: C923–C930, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol 298: F643–F654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paunescu TG, Rodriguez S, Benz E, McKee M, Tyszkowski R, Albers MW, Brown D. Loss of the V-ATPase B1 subunit isoform expressed in non-neuronal cells of the mouse olfactory epithelium impairs olfactory function. PLos One 7: e45395, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paunescu TG, Russo LM, Da Silva N, Kovacikova J, Van Hoek AN, McKee M, Wagner CA, Breton S, Brown D. Compensatory membrane expression of the V-ATPase B2 subunit isoform in renal medullary intercalated cells of B1-deficient mice. Am J Physiol Renal Physiol 293: F1915–F1926, 2007 [DOI] [PubMed] [Google Scholar]
- 103.Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells. J Am Soc Nephrol 19: 84–91, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perez-Sayans M, Garcia-Garcia A, Scozzafava A, Supuran CT. Inhibition of V-ATPase and carbonic anhydrases as interference strategy with tumor acidification processes. Curr Pharm Design 18: 1407–1413, 2012 [DOI] [PubMed] [Google Scholar]
- 105.Pietrement C, Sun-Wada GH, Da Silva N, McKee M, Marshansky V, Brown D, Futai M, Breton S. Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol Reprod 74: 185–194, 2006 [DOI] [PubMed] [Google Scholar]
- 106.Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal 5: 433–446, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Praetorius HA, Leipziger J. Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol 72: 377–393, 2010 [DOI] [PubMed] [Google Scholar]
- 108.Puopolo K, Kumamoto C, Adachi I, Magner R, Forgac M. Differential expression of the “B” subunit of the vacuolar H+-ATPase in bovine tissues. J Biol Chem 267: 3696–3706, 1992 [PubMed] [Google Scholar]
- 109.Qin A, Cheng TS, Pavlos NJ, Lin Z, Dai KR, Zheng MH. V-ATPases in osteoclasts: structure, function and potential inhibitors of bone resorption. Intl J Biochem Cell Biol 44: 1422–1435, 2012 [DOI] [PubMed] [Google Scholar]
- 110.Quan A, Baum M. Endogenous production of angiotensin II modulates rat proximal tubule transport. J Clin Invest 97: 2878–2882, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rein J, Voss M, Blenau W, Walz B, Baumann O. Hormone-induced assembly and activation of V-ATPase in blowfly salivary glands is mediated by protein kinase A. Am J Physiol Cell Physiol 294: C56–C65, 2008 [DOI] [PubMed] [Google Scholar]
- 112.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension 34: 1265–1274, 1999 [DOI] [PubMed] [Google Scholar]
- 113.Rothenberger F, Velic A, Stehberger PA, Kovacikova J, Wagner CA. Angiotensin II stimulates vacuolar H+-ATPase activity in renal acid-secretory intercalated cells from the outer medullary collecting duct. J Am Soc Nephrol 18: 2085–2093, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Saez F, Legare C, Laflamme J, Sullivan R. Vasectomy-dependent dysregulation of a local renin-angiotensin system in the epididymis of the cynomolgus monkey (Macaca fascicularis). J Androl 25: 784–796, 2004 [DOI] [PubMed] [Google Scholar]
- 115.Saroussi S, Nelson N. The little we know on the structure and machinery of V-ATPase. J Exp Biol 212: 1604–1610, 2009 [DOI] [PubMed] [Google Scholar]
- 116.Sautin YY, Lu M, Gaugler A, Zhang L, Gluck SL. Phosphatidylinositol 3-kinase-mediated effects of glucose on vacuolar H+-ATPase assembly, translocation, and acidification of intracellular compartments in renal epithelial cells. Mol Cell Biol 25: 575–589, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwartz GJ, Al-Awqati Q. Carbon dioxide causes exocytosis of vesicles containing H+ pumps in isolated perfused proximal and collecting tubules. J Clin Invest 75: 1638–1644, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schwartz GJ, Al-Awqati Q. Regulation of transepithelial H+ transport by exocytosis and endocytosis. Annu Rev Physiol 48: 153–161, 1986 [DOI] [PubMed] [Google Scholar]
- 119.Schwartz GJ, Barasch J, Al-Awqati Q. Plasticity of functional epithelial polarity. Nature 318: 368–371, 1985 [DOI] [PubMed] [Google Scholar]
- 120.Sennoune SR, Bakunts K, Martinez GM, Chua-Tuan JL, Kebir Y, Attaya MN, Martinez-Zaguilan R. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol 286: C1443–C1452, 2004 [DOI] [PubMed] [Google Scholar]
- 121.Sethi N, Yan Y, Quek D, Schupbach T, Kang Y. Rabconnectin-3 is a functional regulator of mammalian Notch signaling. J Biol Chem 285: 34757–34764, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shum WW, Da Silva N, Belleannee C, Brown D, Breton S. Regulation of V-ATPase recycling via a RhoA- and ROCKII-dependent pathway in epididymal clear cells. Am J Physiol Cell Physiol 301: C31–C43, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shum WW, Da Silva N, Brown D, Breton S. Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk. J Exp Biol 212: 1753–1761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shum WW, Da Silva N, McKee M, Smith PJS, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell 135: 1108–1117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shum WW, Ruan YC, Da Silva N, Breton S. Establishment of cell-cell crosstalk in the epididymis: control of luminal acidification. J Androl 32: 576–586, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smardon AM, Kane PM. RAVE is essential for the efficient assembly of the C subunit with the vacuolar H+-ATPase. J Biol Chem 282: 26185–26194, 2007 [DOI] [PubMed] [Google Scholar]
- 127.Smith AN, Lovering RC, Futai M, Takeda J, Brown D, Karet FE. Revised nomenclature for mammalian vacuolar-type H+-ATPase subunit genes. Mol Cell 12: 801–803, 2003 [DOI] [PubMed] [Google Scholar]
- 128.Smith AN, Skaug J, Choate KA, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Lifton RP, Scherer SW, Karet FE. Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 26: 71–75, 2000 [DOI] [PubMed] [Google Scholar]
- 129.Speth RC, Daubert DL, Grove KL. Angiotensin II: a reproductive hormone too? Regul Pept 79: 25–40, 1999 [DOI] [PubMed] [Google Scholar]
- 130.Stockand JD. New ideas about aldosterone signaling in epithelia. Am J Physiol Renal Physiol 282: F559–F576, 2002 [DOI] [PubMed] [Google Scholar]
- 131.Stone DK, Seldin DW, Kokko JP, Jacobson HR. Mineralocorticoid modulation of rabbit medullary collecting duct acidification. A sodium-independent effect. J Clin Invest 72: 77–83, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stover EH, Borthwick KJ, Bavalia C, Eady N, Fritz DM, Rungroj N, Giersch AB, Morton CC, Axon PR, Akil I, Al-Sabban EA, Baguley DM, Bianca S, Bakkaloglu A, Bircan Z, Chauveau D, Clermont MJ, Guala A, Hulton SA, Kroes H, Li Volti G, Mir S, Mocan H, Nayir A, Ozen S, Rodriguez Soriano J, Sanjad SA, Tasic V, Taylor CM, Topaloglu R, Smith AN, Karet FE. Novel ATP6V1B1 and ATP6V0A4 mutations in autosomal recessive distal renal tubular acidosis with new evidence for hearing loss. J Med Genetics 39: 796–803, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sumner JP, Dow JA, Earley FG, Klein U, Jager D, Wieczorek H. Regulation of plasma membrane V-ATPase activity by dissociation of peripheral subunits. J Biol Chem 270: 5649–5653, 1995 [DOI] [PubMed] [Google Scholar]
- 134.Thimon V, Frenette G, Saez F, Thabet M, Sullivan R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum Reprod 23: 1698–1707, 2008 [DOI] [PubMed] [Google Scholar]
- 135.Toei M, Saum R, Forgac M. Regulation and isoform function of the V-ATPases. Biochemistry 49: 4715–4723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tresguerres M, Buck J, Levin LR. Physiological carbon dioxide, bicarbonate, and pH sensing. Pflügers Arch 460: 953–964, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science 299: 1400–1403, 2003 [DOI] [PubMed] [Google Scholar]
- 138.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs 175: 105–117, 2003 [DOI] [PubMed] [Google Scholar]
- 139.Vallon V, Osswald H. Adenosine receptors and the kidney. Handb Exp Pharmacol: 443–470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.van Adelsberg J, Al-Awqati Q. Regulation of cell pH by Ca2+-mediated exocytotic insertion of H+-ATPases. J Cell Biol 102: 1638–1645, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vargas-Poussou R, Houillier P, Le Pottier N, Strompf L, Loirat C, Baudouin V, Macher MA, Dechaux M, Ulinski T, Nobili F, Eckart P, Novo R, Cailliez M, Salomon R, Nivet H, Cochat P, Tack I, Fargeot A, Bouissou F, Kesler GR, Lorotte S, Godefroid N, Layet V, Morin G, Jeunemaitre X, Blanchard A. Genetic investigation of autosomal recessive distal renal tubular acidosis: evidence for early sensorineural hearing loss associated with mutations in the ATP6V0A4 gene. J Am Soc Nephrol 17: 1437–1443, 2006 [DOI] [PubMed] [Google Scholar]
- 142.Vedovelli L, Rothermel JT, Finberg KE, Wagner CA, Azroyan A, Hill E, Breton S, Brown D, Paunescu TG. Altered V-ATPase expression in renal intercalated cells isolated from B1-subunit deficient mice by fluorescence activated cell sorting. Am J Physiol Renal Physiol 304: F522–F532, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vidarsson H, Westergren R, Heglind M, Blomqvist SR, Breton S, Enerback S. The forkhead transcription factor Foxi1 is a master regulator of vacuolar H-ATPase proton pump subunits in the inner ear, kidney and epididymis. PLos One 4: e4471, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vitavska O, Wieczorek H, Merzendorfer H. A novel role for subunit C in mediating binding of the H+-V-ATPase to the actin cytoskeleton. J Biol Chem 278: 18499–18505, 2003 [DOI] [PubMed] [Google Scholar]
- 145.Vitzthum H, Weiss B, Bachleitner W, Kramer BK, Kurtz A. Gene expression of adenosine receptors along the nephron. Kidney Int 65: 1180–1190, 2004 [DOI] [PubMed] [Google Scholar]
- 146.von Schwarzenberg K, Wiedmann RM, Oak P, Schulz S, Zischka H, Wanner G, Efferth T, Trauner D, Vollmar AM. Mode of cell death induction by pharmacological vacuolar H+-ATPase (V-ATPase) inhibition. J Biol Chem 288: 1385–1396, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Voss M, Blenau W, Walz B, Baumann O. V-ATPase deactivation in blowfly salivary glands is mediated by protein phosphatase 2C. Arch Insect Biochem Physiol 71: 130–138, 2009 [DOI] [PubMed] [Google Scholar]
- 148.Voss M, Vitavska O, Walz B, Wieczorek H, Baumann O. Stimulus-induced phosphorylation of vacuolar H+-ATPase by protein kinase A. J Biol Chem 282: 33735–33742, 2007 [DOI] [PubMed] [Google Scholar]
- 149.Wagner CA. When proton pumps go sour: urinary acidification and kidney stones. Kidney Int 73: 1103–1105, 2008 [DOI] [PubMed] [Google Scholar]
- 150.Wagner CA, Devuyst O, Bourgeois S, Mohebbi N. Regulated acid-base transport in the collecting duct. Pflügers Arch 458: 137–156, 2009 [DOI] [PubMed] [Google Scholar]
- 151.Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar-ATPase. Physiol Rev 84: 1263–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 152.Wagner CA, Giebisch G, Lang F, Geibel JP. Angiotensin II stimulates vesicular H+-ATPase in rat proximal tubular cells. Proc Natl Acad Sci USA 95: 9665–9668, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wagner CA, Mohebbi N, Uhlig U, Giebisch GH, Breton S, Brown D, Geibel JP. Angiotensin II stimulates H+-ATPase activity in intercalated cells from isolated mouse connecting tubules and cortical collecting ducts. Cell Physiol Biochem 28: 513–520, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wax MB, Saito I, Tenkova T, Krupin T, Becker B, Nelson N, Brown D, Gluck SL. Vacuolar H+-ATPase in ocular ciliary epithelium. Proc Natl Acad Sci USA 94: 6752–6757, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wiedmann RM, von Schwarzenberg K, Palamidessi A, Schreiner L, Kubisch R, Liebl J, Schempp C, Trauner D, Vereb G, Zahler S, Wagner E, Muller R, Scita G, Vollmar AM. The V-ATPase-inhibitor archazolid abrogates tumor metastasis via inhibition of endocytic activation of the Rho-GTPase Rac1. Cancer Res 72: 5976–5987, 2012 [DOI] [PubMed] [Google Scholar]
- 156.Winter C, Kampik NB, Vedovelli L, Rothenberger F, Paunescu TG, Stehberger PA, Brown D, John H, Wagner CA. Aldosterone stimulates vacuolar H+-ATPase activity in renal acid-secretory intercalated cells mainly via a protein kinase C-dependent pathway. Am J Physiol Cell Physiol 301: C1251–C1261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Winter C, Schulz N, Giebisch G, Geibel JP, Wagner CA. Nongenomic stimulation of vacuolar H+-ATPases in intercalated renal tubule cells by aldosterone. Proc Natl Acad Sci USA 101: 2636–2641, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wong PY, Uchendu CN. The role of angiotensin-converting enzyme in the rat epididymis. J Endocrinol 125: 457–465, 1990 [DOI] [PubMed] [Google Scholar]
- 159.Xu X, You J, Pei F. Silencing of a novel tumor metastasis suppressor gene LASS2/TMSG1 promotes invasion of prostate cancer cell in vitro through increase of vacuolar ATPase activity. J Cell Biochem 113: 2356–2363, 2012 [DOI] [PubMed] [Google Scholar]
- 160.Yan Y, Denef N, Schupbach T. The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev Cell 17: 387–402, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang Q, Li G, Singh SK, Alexander EA, Schwartz JH. Vacuolar H+-ATPase B1 subunit mutations that cause inherited distal renal tubular acidosis affect proton pump assembly and trafficking in inner medullary collecting duct cells. J Am Soc Nephrol 17: 1858–1866, 2006 [DOI] [PubMed] [Google Scholar]
- 162.Zimmermann B, Dames P, Walz B, Baumann O. Distribution and serotonin-induced activation of vacuolar-type H+-ATPase in the salivary glands of the blowfly Calliphora vicina. J Exp Biol 206: 1867–1876, 2003 [DOI] [PubMed] [Google Scholar]