This review highlights recent advances in the field of nutrient sensing and describes emerging principles of the regulation of cell growth by metabolic cues in yeast and mammalian cells.
Abstract
Although nutrient availability is a major driver of cell growth, and continuous adaptation to nutrient supply is critical for the development and survival of all organisms, the molecular mechanisms of nutrient sensing are only beginning to emerge. Here, we highlight recent advances in the field of nutrient sensing and discuss arising principles governing how metabolism might regulate growth-promoting pathways. In addition, we discuss signaling functions of metabolic enzymes not directly related to their metabolic activity.
Although first only recognized during tumor formation, it has become widely accepted that enhanced cell growth is generally associated with specific metabolic alterations, characterized by higher glycolytic flux and increased fermentation rates (production of lactate or ethanol). In cancers, this phenomenon is often referred to as the Warburg effect and is widely exploited for detection of tumors using PET scans, but it has also been identified as a promising new avenue for cancer treatment (3, 8, 132, 136, 139). However, also in single cellular organisms such as yeast, in which cell growth is exclusively regulated by nutrient availability, increased cell growth is accompanied by reduced respiration dependence and higher glycolytic flux (37, 136). Yet, it is still rather unclear how these changes contribute to enhanced cell growth or whether the changes are mainly a consequence of cell growth.
Nutrient availability is sensed by highly conserved signaling cascades, including the target of rapamycin (TOR) kinase, cAMP-dependent kinase protein kinase A (PKA), and AMP-activated kinase (AMPK; encoded by Snf1 in yeast), which have widespread roles to adjust cellular physiology to nutrient supply (16, 34, 155). In higher eukaryotes, these nutritional signals are integrated with growth factor signaling to coordinate cell growth at the level of tissues and organisms (47, 88, 112).
Continuous adaptation to changes in nutrient supply is crucial for the development and survival of all organisms. For example, recent studies in mice established a critical requirement for nutrient sensing and induction of autophagy in newborn mice to survive the period of starvation before the onset of lactation (44, 87). Genetic defects in nutrient-sensitive signaling pathways are tightly linked to the development of cancers (92, 158). Moreover, defective glucose sensing in pancreatic beta-cells leads to impaired insulin secretion and, consequently, development of diabetes (7, 131). Thus identification of molecular mechanisms of nutrient sensing will be key to understanding a variety of diseases and may also help to develop novel intervention strategies.
Interestingly, growth factor stimulation and oncogenic transformation induce similar metabolic alterations as increased nutrient supply. These changes include enhanced nutrient uptake and glycolytic efficiency, but also redirecting cellular metabolism toward increased biomass production (23, 31, 49, 95, 136, 140, 151), contributing to enhanced cell growth, and coping with the increased need for cellular building blocks. Consequently, regulation of cellular metabolism by signaling networks cannot be considered unidirectional but as part of a highly interconnected cellular network linking nutrient availability, cellular metabolism, and signaling (88, 142). Indeed, recent publications demonstrated extensive regulation of metabolic enzymes by phosphorylation, which contributes to the regulation of cell growth by signaling pathways (14, 46, 113, 147, 150). In this review, we will exclusively focus on some recent advances in the field of nutrient sensing and discuss emerging concepts for how they activate cellular signaling pathways. In addition, we highlight novel cellular functions of metabolic enzymes that are distinct from their metabolic activity but might significantly contribute to the (de)regulation of cell growth under physiological and pathophysiological conditions.
Molecular Mechanisms of Nutrient Sensing
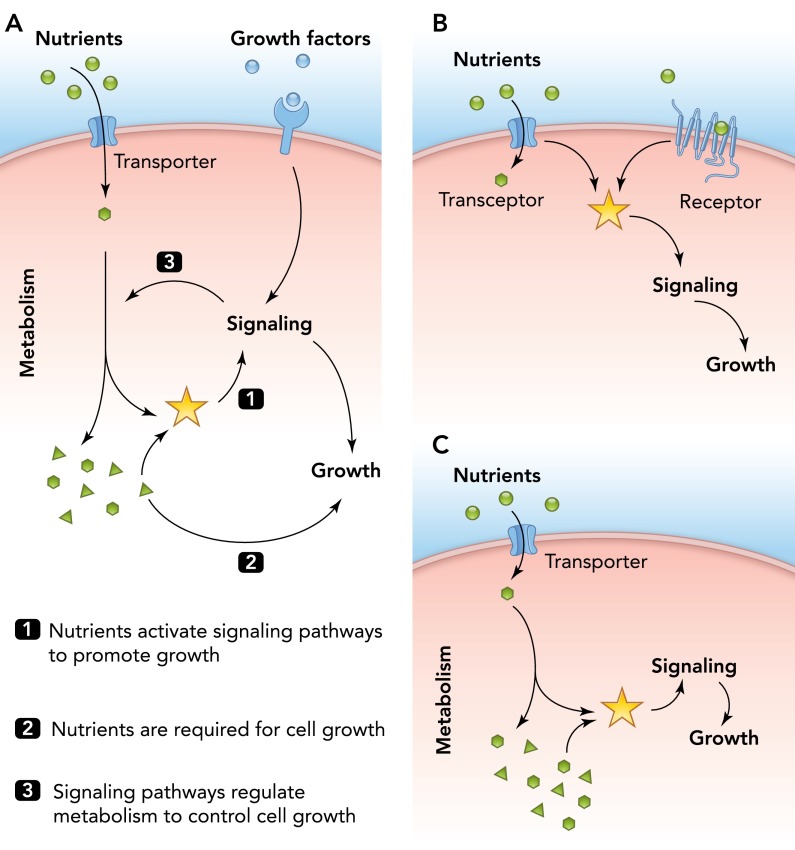
Despite its significance for understanding the regulation of cell growth by environmental signals, the molecular mechanisms that underlie nutrient sensing are still poorly characterized. Research in this field has focused primarily on the sensing of carbon (mostly glucose) and nitrogen sources (mostly amino acids), but similar mechanisms must exist for other nutrients as well (16). Importantly, nutrients cannot simply be considered signaling molecules but are likewise a prerequisite for cell growth by providing cellular energy and building blocks for biomass production (FIGURE 1A).
FIGURE 1.
Nutrient availability and growth factor signaling coordinately regulate cell growth
A: nutrient availability elicits metabolic signals that are sensed by and activate cellular signaling pathways. Schematic representation depicting the dual role of nutrients in the regulation of cell growth as cellular signals (1) and source of cellular building blocks (2) as well as the reciprocal interaction between cellular signaling and cellular metabolism (3) to coordinate cell growth. B and C: alternative mechanisms for how nutrients promote cell growth through activation of cellular signaling pathways. B: in yeast, nutrient availability can be sensed by receptors in the plasma membrane independently of or tightly coupled to nutrient uptake (transceptors). C: in all eukaryotes, nutrients are sensed indirectly through metabolic cues derived from cellular metabolism.
Due to its excellent genetics and well characterized metabolism, yeast is arguably the best model organism for identifying basic principles of metabolic processes, but studies using yeast also have made important contributions to understanding regulation of cell growth by nutrients through highly conserved signaling pathways present in yeast to humans (16, 30). In yeast, two different mechanisms for nutrient sensing exist (125). On the one hand, nutrients can be sensed by receptors in the plasma membrane, which bind to nutrients outside the cell and activate cellular signaling pathways (FIGURE 1B). Such extracellular mechanisms allow for a fast response to varying nutrient concentrations and help to coordinate nutrient signaling with cell growth (152). Yet, these mechanisms do not easily allow direct coordination of nutrient sensing with nutrient uptake. Interestingly, certain nutrient sensors in the plasma membrane have recently been found to combine activities for nutrient sensing and uptake. These so-called transceptors sense various nutrients, including glucose, nitrogen, or phosphate sources (54, 55, 71, 120, 126, 135), but the molecular mechanisms behind how they activate downstream signaling cascades remain to be identified.
On the other hand, nutrient sensing in yeast and mammals critically relies on metabolic signals that require nutrient uptake and metabolism (FIGURE 1, A AND C). For example, activation of PKA in yeast and pancreatic beta-cells requires glucose uptake and phosphorylation, demonstrating that a glucose metabolite or a closely associated property is mediating glucose sensing in these systems (32, 64, 85, 124). Intuitively, intracellular sensing mechanisms provide certain advantages over extracellular mechanisms using receptors. In particular, such mechanisms have the potential to directly link the availability of building blocks derived from nutrients with cellular signaling (FIGURE 1C), thereby reporting on the effective, intracellular concentration of the nutrient available for growth rather than on the concentration outside of the cell. Moreover, since all nutrients are funneled into the same cellular core metabolism, such mechanisms could also readily explain the sensing of structurally different nutrients, such as different carbon sources (C-sources) or amino acids. Nevertheless, the molecular nature of these metabolic cues and their sensing mechanisms are still poorly understood.
Changes in cellular metabolism can directly affect the activities of enzymatic functions by modulating the availability of their cofactors. For example, gene expression can be directly coupled to metabolic activity through availability of Acetyl-CoA and the NAD+/NADH ratio, which regulate acetylation and deacetylation of histones, respectively (142). Indeed, Acetyl-CoA has been suggested to be responsible for the regulation of cell growth and, consequently, cell cycle progression in yeast (17). Similarly, accumulation of α-ketoglutarate leads to increased prolyl-hydroxylation and destabilization of the transcriptional co-activator HIF1, which links expression of glycolytic enzymes in response to changing environmental conditions (80). Excellent recent reviews are available that address these direct modifications of cellular components by metabolic alterations (18, 79, 105, 142) and will therefore not be discussed further in this review.
Cell growth commonly refers to the accumulation of biomass but can manifest in increased cell size (hypertrophy) or increased cell number (hyperplasia). In yeast, cell growth and cell proliferation are tightly coupled through the cell size checkpoint, which halts progression through the G1/S transition of the cell cycle until cells reach a critical size. However, since nutrients not only stimulate the growth rate but also set the critical cell size through poorly understood mechanisms, increased nutrient availability leads to larger and faster proliferating cells (10, 36, 75, 76). In contrast, although evidence suggests the existence of a similar cell size checkpoint in certain mammalian cell types (39, 81), differentiated mammalian cells appear to lack such a strict connection between growth and proliferation (26, 27). Moreover, growth signals are interpreted in a cell-type-specific manner to evoke the appropriate physiological response. For example, the adaptive increase in beta-cell mass on over-nutrition is mostly due to enhanced proliferation of beta-cells (hyperplasia) (5, 13, 42), whereas the concomitant increase in heart size is caused by hypertrophy (122, 130). Despite these apparent cell-type-specific responses, the basic mechanisms of nutrient sensing discussed in this review are, unless specifically stated, common to all cell types.
Mechanisms of the Regulation of Cell Growth by Amino Acid Availability
One of the most important signaling pathways promoting cell growth in response to nutrient availability involves the TOR complex, a highly conserved S/T directed kinase present in all eukaryotes (93). TOR was first identified in yeast as the target of the naturally occurring macrolide rapamycin that has immunosuppressive and growth inhibitory functions (66). TOR is part of two separate multi-subunit complexes, termed TOR complex 1 and 2 (TORC1 and TORC2), respectively (84, 93, 94, 158). TORC1 promotes growth by activating a variety of anabolic processes, including ribosome biogenesis, translation, and nutrient uptake, and by suppressing catabolic processes, such as autophagy and the general stress response through phosphorylation of different direct downstream targets (16, 70, 72, 88, 93, 99, 106, 154). TORC1 activity is highly sensitive to nutrient availability but also integrates signals from various stresses and growth factors (47, 68, 134). In contrast, TORC2 activity has so far not been linked to nutrient availability but might rather be involved in spatial regulation of cell growth by controlling lipid homeostasis and the actin cytoskeleton (4, 9, 93) and will therefore not be considered further this review.
In yeast and mammals, TORC1 is activated by amino acid availability (84, 93, 158). In contrast to most other amino acids, withdrawal of branched-chain amino acids, in particular leucine, leads to a strong reduction of TORC1 activity, implying that leucine is the most potent activator of TORC1 (12, 60). In yeast, TORC1 activity can be stimulated by addition of any amino acid or ammonium ions, albeit with different efficiencies (12, 38, 134). Interestingly, depletion of intracellular glutamine levels mimics inactivation of TORC1 (29), suggesting a direct role for glutamine in the activation of TORC1. However, such experiments are difficult to interpret since amino acids can be interconverted by transamination, suggesting that understanding the molecular nature of the nitrogen signal is more likely to emerge from the genetic or biochemical identification of upstream regulators of the TORC1 complex.
In yeast and mammals, various direct activators of TORC1 have been identified. For example, the small GTPase Rheb is required for the activation of TORC1 in response to growth factors but has also been suggested to be essential for the activation of TORC1 by amino acids (47, 57, 109, 128). Rheb is activated by the tuberous sclerosis complex 1 and 2 (TSC1/2), which serves as a GTPase-activating protein (GAP) for Rheb and is regulated through direct phosphorylation by Akt in response to growth factor stimulation (47).
Interestingly, the yeast Rheb homolog Rhb1 does not seem to play a role in the activation of TORC1 in this organism (34, 133). However, two other GTPases, Gtr1 and Gtr2, which are part of the EGO complex, have been identified as activators of TORC1 in response to amino acids (12, 43). Gtr1 and Gtr2 form heterodimers and physically interact with TORC1 (12, 56). Loss of Gtr1 and Gtr2 function leads to a defect in activation of TORC1 toward its substrate Sch9, the yeast homolog of S6K (12, 134). In mammals, the family of RAG GTPases has been identified as direct homologs of Gtr1 and Gtr2 and also activates TORC1 (128). Somewhat surprisingly, active heterodimers of the EGO or Rag GTPases consist of one GTPase bound to GTP (Gtr1 or RagA/B) and one bound to GDP (Gtr2 and RagC/D) (12, 128), suggesting that multiple GAPs and GEFs should exist that may regulate GTPase and, consequently, TORC1 activity. Indeed, yeast Vam6 has been demonstrated to act as a GEF that activates TORC1 via Gtr1 (12), but the mechanisms regulating Vam6 remain to be identified.
In mammals, Rag GTPase activity is regulated by the multisubunit Ragulator complex, which tethers RAG GTPases to lysosomal membranes and acts as a GEF for RagA and RagB (6, 127, 157), thus activating the TORC1 complex in response to leucine levels. Surprisingly, Rag activation by the Ragulator complex has been shown to require its interaction with vacuolar ATPase (V-ATPase), a proton pump required for luminal acidification of the endocytic pathway as well as lysosomes (vacuoles in yeast) (48, 157). Although amino acids do not directly affect V-ATPase assembly, amino acid starvation enhanced the interaction of Ragulator with V-ATPase, thereby possibly preventing the activation of Rag (157). Interestingly, the proton gradient across the lysosomal membrane established by V-ATPase activity is required for storage of amino acids, including leucine, in the lysosomes, suggesting that accumulation of amino acids inside the organelle is required to activate Rag activity. Consistently, overexpression of a lysosomal amino acid transporter mediating the export of amino acids out of the lysosome prevented TORC1 activation by amino acids in vitro, but the exact mechanism underlying this regulation is still unknown.
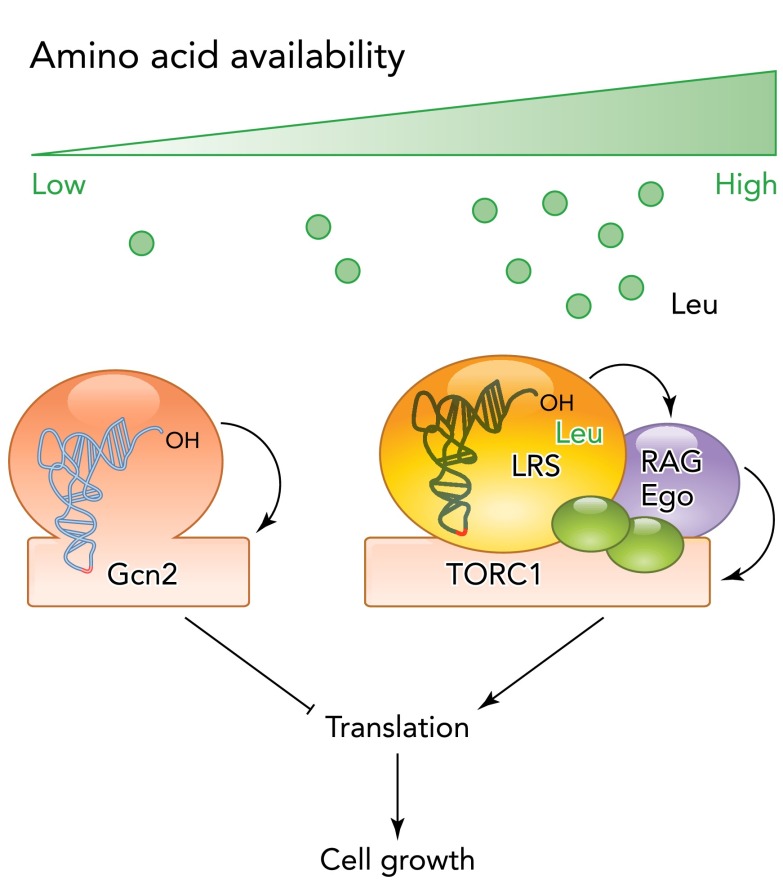
However, recent studies have identified another activator of Rag GTPases that might more directly link leucine availability to TORC1 activity (FIGURE 2). Interestingly, the leucine tRNA synthase (LRS), responsible for the charging of leucine onto its appropriate tRNA, was identified as a conserved interactor of TORC1 (15, 59). Depletion of LRS, but not of any other tRNA synthase, leads to a strong defect in TORC1 activity (59), suggesting that LRS acts as a sensor for leucine levels in the TORC1 pathway. Binding of leucine to LRS, but not catalytic activity, is required for TORC1 activity. Moreover, LRS interacts with RagD in a leucine-dependent manner and stimulates its GTPase activity. Surprisingly, although yeast LRS is likewise required for the activation of TORC1 (15), the residue responsible for GAP activity in mammalian LRS is not conserved in yeast (59), suggesting mechanistic differences in the activation of TORC1 in these two systems. Moreover, the exact property of LRS required for the activation of TORC1 remains to be established. In particular, it will be important to clarify whether the formation of a ternary complex of LRS, leucine, and the tRNA, or maybe of LRS, leucine, and ATP is required for Rag activation in vivo. In any case, these studies offer exiting new insights into mechanisms of nutrient sensing.
FIGURE 2.
Amino acid availability regulates cell growth at least in part through tRNA-related activities
Leu-tRNA (LRS) synthase forms a ternary complex with leucine and acts as a GAP for Rag-GTPases to promote TORC1 activity and, therefore, efficient protein translation and cell growth under nutrient-rich conditions. Note that tRNA binding may not be required for the activation of TORC1 by LRS (see text for details). During starvation, uncharged tRNAs accumulate, bind, and activate the protein kinase Gcn2 to reduce translation efficiency and cell growth.
An involvement of tRNA synthase is also reminiscent of the regulation of Gcn2 (84), a protein kinase involved the cellular response to amino acid starvation (FIGURE 2). Interestingly, Gcn2 possesses an amino acyl tRNA synthase-like domain that directly binds to accumulating uncharged tRNAs during starvation, thereby leading to the activation of kinase activity. In turn, Gcn2 phosphorylates eIF2α (encoded by SUI2 in yeast) to decrease general protein translation and promote the expression of amino acid biosynthesis genes by inducing a group of transcription factors such as ATF-4 (Gcn4 in yeast) (41, 155).
Thus it is tempting to speculate that sensing of amino acid availability and starvation is linked to cellular production of the effective metabolite of amino acids, acylated tRNAs, which ultimately determines whether sufficient amino acids are present for efficient protein synthesis and, thus, accumulation of biomass. In this model, acylation of tRNAs might directly link the signaling function of amino acids to activate TORC1 as well as their function as cellular building blocks.
Moreover, Gcn2 and TORC1 are part of a regulatory network and cross inhibit each other (84). For example, inhibition of TORC1 by Rapamycin leads to the TAP42-dependent dephosphorylation of Gcn2 at Ser577, thus enhancing tRNA binding (20). Similarly, activation of Gcn2 induces expression of the ATF4 target GADD34, which might contribute to inactivation of TORC1 through dephosphorylation of TSC2 (111, 141). Thus these processes might allow for a very sensitive response to even small changes in amino acid availability required for tight control of cell growth in changing environments.
Signals Derived from Central Carbon Metabolism: Glucose Sensing
Similar to amino acids, sensing the availability of a C-source involves a complex network of different nutrient-sensitive pathways. Most eukaryotic cells prefer glucose to other C-sources but can utilize alternative C-sources to cover the need for cellular energy and for production of building blocks for cell growth derived from central carbon metabolism. In yeast, the major glucose signaling pathways are the Ras/PKA and TORC1 pathways (16, 134), which are both activated by glucose availability, but the molecular mechanisms of their activation are still poorly understood.
In mammals, glucose sensing is best understood in pancreatic beta-cells, which measure blood glucose levels and secrete insulin to maintain overall glucose homeostasis (100). Importantly, these cells express a low-affinity isoform of hexokinase, glucokinase, which directly couples blood glucose levels to glycolytic flux and, therefore, ATP production. Increasing levels of ATP are then sensed by ATP-sensitive K+ channels, eventually leading to membrane depolarization and insulin secretion. Similar to yeast, PKA is activated by glucose and contributes to regulation of cell growth and insulin secretion in this cell type (28, 32, 64, 82). It should be noted, however, that PKA is activated through hormonal signals in response to starvation in other tissues, such as the liver (19, 74). In contrast, TORC1 is generally regulated by glucose levels in mammalian cells (47, 65), suggesting that mechanisms of glucose sensing might be conserved in these systems (FIGURE 3).
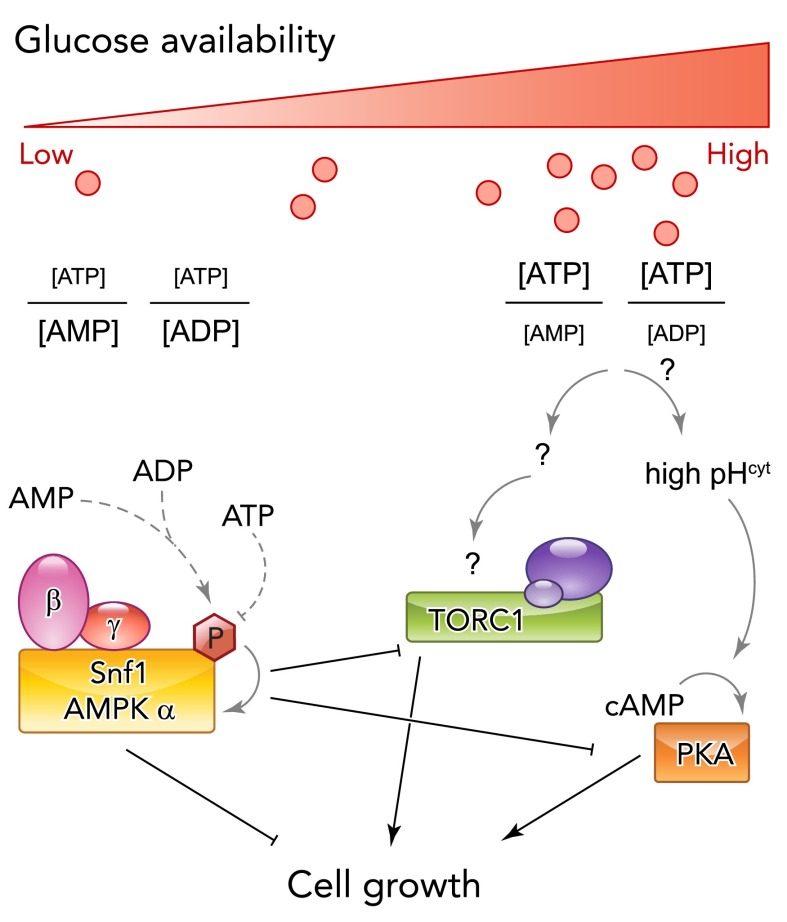
FIGURE 3.
Glucose (C-source) availability regulates cell growth through multiple signaling pathways
During glucose starvation, decreasing production of cellular energy leads to the activation of AMP-activated kinase (AMPK, yeast Snf1). Direct binding of AMP or ADP, but not of ATP, protects AMPK/Snf1 from dephosphorylation of the activation loop and therefore inactivation (dotted lines). Active AMPK/Snf1 regulates cellular metabolism and cell growth by direct phosphorylation of downstream targets required for cell growth as well as inhibition of glucose-activated pathways (TORC1, PKA). Mechanisms leading to the activation of growth-promoting pathway are less well understood but may likewise be linked to the energy status of the cells. In yeast, glucose sensing has been linked to the regulation of cytosolic pH, which is required to activate the Ras/PKA pathway and promote growth and might thus link energy metabolism and cellular signaling under nutrient-rich conditions.
In yeast and mammalian cells, activation of PKA requires glucose uptake and phosphorylation (64, 124), suggesting that a glucose-derived metabolite or some closely associated property is required to mediate glucose sensing in both systems. Interestingly, glucose sensing and regulation of cell growth are at least partially mediated by cytosolic pH (32, 114, 115, 153). In yeast, cytosolic pH is highly sensitive to glucose availability. Cytosolic pH is regulated by P-ATPase, an ATP-dependent proton pump located in the plasma membrane (121). P-ATPase uses as much as 15% of the total cellular ATP production to maintain a high and stable cytosolic pH in the presence of glucose (50, 129), suggesting that cytosolic pH may be directly regulated by the energy status of the cell. Indeed, similar to glucose starvation, inhibition of glycolytic activity through a mutation in pyruvate kinase or through addition of the non-metabolizable glucose analog 2-deoxy-glucose leads to a rapid decrease in cytosolic pH and arrest of cell growth (32). Changes in cytosolic pH are sensed by V-ATPase, which in turn is required for full activation of PKA by an unknown mechanism (32, 33).
Although direct evidence for the regulation of cell growth by cytosolic pH in mammals is lacking, several lines of evidence also suggest a signaling function for cytosolic pH in this system. Most notably, cellular transformation is generally associated with an increased cytosolic pH (118, 119). This effect might be caused in part by altered cellular metabolism but has also been linked to MAPK-dependent phosphorylation of NHE-1, a H+/Na+ exchanger, which may contribute to increased cell growth upon growth factor stimulation (11, 21, 24, 73, 137). Finally, cytosolic pH increases in pancreatic beta-cells upon glucose stimulation (58, 77, 91, 101). Interestingly, beta-cells also require V-ATPase activity for full activation of PKA (32), suggesting conserved mechanisms in yeast and mammalian cells.
Similarly, the increase in ATP concentration upon glucose stimulation of pancreatic beta-cells triggers insulin secretion but might also contribute to the activation of PKA (85, 100). It is therefore tempting to speculate that glucose sensing is more generally linked to the energy status of the cell. In support of this, TORC1 activity was shown to be sensitive to ATP levels in vitro (35), but the significance of this finding in vivo has not been established. However, this simple model is challenged by the fact that glucose stimulation does not lead to a significant increase in ATP levels in yeast. Rather, ATP levels rapidly drop upon glucose addition, possibly due to the ATP demand in the first steps of glycolysis, but then subsequently stabilize at levels similar to those before glucose addition (86, 144). However, a significant simultaneous drop in ADP levels might overcompensate the lack of an increase of ATP levels, leading to an increased ATP/ADP ratio upon glucose addition (138, 143, 144). Eventually, this increased ATP/ADP ratio might trigger glucose sensing. Although highly speculative, such an energy-centric mechanism could easily explain how signals from different qualities and quantities of C-sources could be integrated simply by their different efficiency to sustain ATP generation.
Although further studies are required to test whether activation of PKA and TORC1 by glucose is indeed mediated by the energy status of the cell, it is well established that a drop in ATP/ADP ratios, or an accumulation of AMP, is responsible for the activation of AMP-activated kinase (AMPK) during glucose starvation (FIGURE 3) (53, 62, 63). AMPK is a ubiquitously expressed kinase consisting of a catalytic (α) subunit and two regulatory subunits (β and γ), which have different binding pockets for AXPs and mediate activation of AMPK by allosteric regulation. More specifically, the γ-subunit is able to bind all AXPs under physiological conditions (146). When ATP levels are low during glucose starvation, binding of ADP or AMP to AMPK allows phosphorylation of the activation loop of the catalytic subunit by upstream kinases, leading to drastically increased AMPK activity. In contrast, under rich conditions, ATP effectively competes with ADP or AMP for AMPK binding and promotes dephosphorylation of AMPK (145, 146).
A similar regulation of the activity by direct nucleotide binding has been demonstrated for the yeast AMPK homolog Snf1 in vitro. Likewise, Snf1 activation requires phosphorylation by upstream kinases, and ADP binding protects Snf1 from dephosphorylation by the Glc7 phosphatase (102), suggesting that Snf1 activity is also regulated by the energy status of the cell in vivo.
Once activated, both AMPK and Snf1 phosphorylate multiple targets in metabolism to promote gluconeogenesis or usage of alternative C-sources (53). Importantly, AMPK also mediates, at least in part, the inactivation of TORC1 by direct phosphorylation of TSC2 (47), thus contributing to the regulation of cell growth during glucose starvation. Similarly, deletion of Snf1 leads to increased phosphorylation of PKA targets in yeast (14), suggesting that activation of Snf1 suppresses PKA activity.
We therefore speculate that, rather than measuring the abundance of a glucose metabolite, glucose-sensing mechanisms might be linked to the energy status of the cell, which triggers positive signals in the presence of glucose but also a negative signal during glucose-limiting conditions. However, further understanding of glucose sensing will require careful genetic dissection of the upstream regulators of signaling pathways and their impact on the dynamics of nutrient signaling, as well as a more systematic analysis of metabolic control circuits under different nutrient conditions.
Alternative Functions of Metabolic Enzymes in Regulating Cell Growth
We have thus far only considered signals derived from metabolites that regulate cell growth, in which metabolic enzymes participate in the sensing process only through their metabolic function. For example, glucokinase in beta-cells limits glycolytic flux and therefore generation of the actual metabolic signal ATP (100). However, certain metabolic enzymes have acquired additional roles, which are mechanistically distinct from their metabolic function (FIGURE 4). Interestingly, these additional functions have all been linked to the regulation of transcription, therefore physically separating metabolic functions (mostly) in the cytosol and transcriptional roles in the nucleus. So far, such functions have been described for three key glycolytic enzymes, hexokinase (45, 116, 117, 123), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (25, 61, 156), and pyruvate kinase (51, 89, 96, 148, 149). Importantly, these transcriptional roles are tightly controlled by nutritional cues and contribute to the regulation of cell growth under normal conditions but also mediate cellular changes underlying cellular transformation.
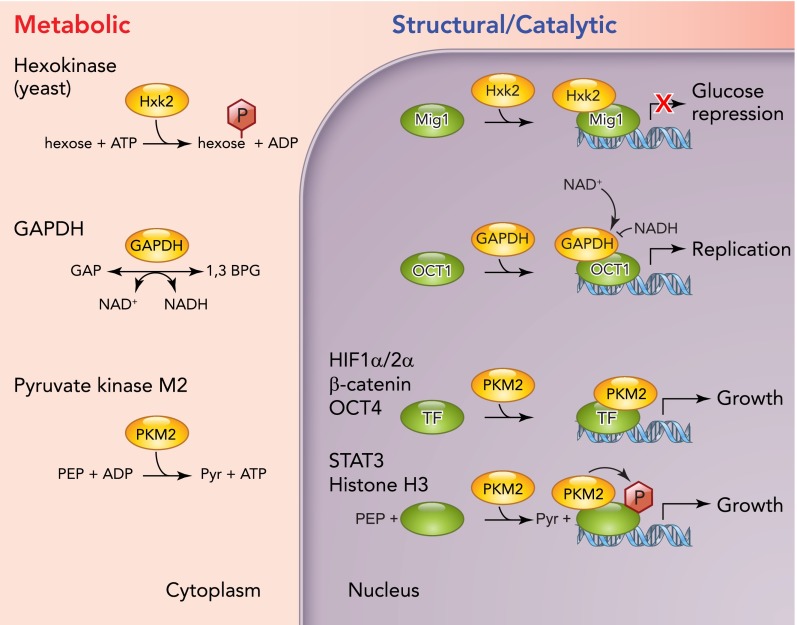
FIGURE 4.
Schematic representation of metabolic and nonmetabolic functions of glycolytic enzymes regulating cell growth
Three examples of metabolic enzymes with dual functions are shown. These enzymes catalyze key metabolic reactions in the cytoplasm (left) but also act in the nucleus to participate in transcriptional regulation of target genes (right). This function can be independent of their catalytic function (Hxk2 and PKM2) or regulated by binding to their cognate cofactors (GAPDH). In addition, PKM2 also acts as a protein kinase phosphorylating STAT-3 and histone H3 to regulate their activity at promoters and therefore gene expression.
The first enzyme that was discovered to possess such an additional function was yeast hexokinase (Hxk2), which is one of three proteins catalyzing the first and irreversible reaction of glycolysis, the phosphorylation of glucose at the C6 position. In the presence of glucose, Hxk2 is highly abundant and suppresses the expression of the other two isoforms, Hxk1 and Glk1 (123), which are more prevalent under nonfermentative conditions (67, 107). In yeast, glucose triggers a widespread transcriptional program that suppresses the expression of genes not required during growth in glucose, commonly referred to as glucose repression. Deletion of HXK2 leads to a relief of glucose repression (98). Surprisingly, this defect was not rescued by overexpression of Glk1 but was rescued by expression of a catalytically inactive form of Hxk2, demonstrating that glucose repression does not require the metabolic function of Hxk2 (107). Indeed, Hxk2 directly binds to the transcriptional coactivator Mig1, a central player in yeast glucose repression (1, 16). In complex with Mig1, Hxk2 binds to the promoters of its target genes (1), thus leading to the establishment of a stable repressor complex, which inhibits the expression of these glucose responsive genes.
Although glucose levels tightly regulate nuclear localization of Hxk2, the exact molecular mechanisms of this regulation remain to be established. In the absence of glucose, Hxk2 is phosphorylated by Snf1 at Ser14, which contributes to its nuclear export, possibly by interfering with the nuclear localization signal (NLS) located within the extreme NH2 terminus of Hxk2 (45, 116, 117). Phosphorylation of Ser14 is counteracted by the PP2A phosphatase Glc7, which dephosphorylates this site under nutrient-rich conditions (2). However, nuclear localization is still subject to glucose regulation in snf1Δ mutants or in cells expressing non-phosphorylatable Ser14Ala substitutions, demonstrating that additional mechanisms must exist to regulate nuclear translocation of Hxk2 (45). Interestingly, overexpression of Mig1 strongly enhances the nuclear localization of Hxk2 (1), suggesting that Hxk2 binding to Mig1 may sequester Hxk2 in the nucleus. In addition, several other phosphorylation sites have been identified in Hxk2 (46), but their significance for the nuclear accumulation of Hxk2 or in glucose repression has not been established yet.
Similarly, in mammals, the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which converts glyceraldehyde-3-phosphate and NAD+ to 1,3-bisphophoglycerate and NADH, was identified as an essential component of the multisubunit complex OCA-S responsible for transcriptional regulation of histone expression during S-phase (156). Interestingly, the transcriptional coactivation of GAPDH was stimulated by NADH and inhibited by NAD+, suggesting that GAPDH serves to link the metabolic state of the cell to S-phase progression via regulation of histone levels. Moreover, GAPDH has also been identified as a factor regulating cellular survival. Interestingly, this function has also been linked to nuclear translocation and transcriptional regulation, but the mechanisms of regulation are less clear (25, 69).
Another glycolytic enzyme that received significant attention as a transcriptional regulator is pyruvate kinase. This enzyme catalyzes the last step of glycolysis mediating the transfer of a phosphate group from phosphoenolpyruvate (PEP) to ADP, thereby producing ATP and pyruvate. In mammals, four different isoforms of pyruvate kinase exist, which vary in their regulation and specific activity (103). PKM1 is the predominant isoform expressed in brain and muscle, whereas PKM2 is found in all proliferating tissues, including embryonic cells and adult stem cells. PKL is found in gluconeogenetic organs such as the liver and also the kidney. Finally, PKR is expressed mainly in erythrocytes.
Interestingly, PKM1 and PKM2 are expressed from the same gene by differential splicing (110), and the switch toward the expression of PKM2 is an important molecular feature that contributes to the metabolic reprogramming of cancer cells (22, 97, 103, 136). Alternative exon usage in PKM2 renders the enzyme sensitive to allosteric regulation by fructose 1,6-bisphosphate (FBP) (40, 78) and SAICAR (83), an intermediate metabolite of de novo purine nucleotide synthesis, and regulation by binding to phosphotyrosine-containing peptides (23). This suggests that tight regulation of pyruvate kinase activity is required to promote cell growth under certain conditions. Indeed, partial inhibition of pyruvate kinase activity is required to redirect metabolism toward higher pentosephosphate pathway activity, which is essential to produce NADPH and riboses for biomass production (136).
However, PKM2 has been shown to have various signaling functions, in which the enzyme itself, rather than its metabolic activity, contributes to the regulation of cell growth and physiology. For example, PKM2 directly binds to the transcription factor OCT4, a transcription factor that is essential to maintain pluripotency of embryonic stem cells (90, 108). Moreover, overexpression of PKM2 significantly enhanced expression of OCT4-dependent transcripts, suggesting that PKM2 also contributes to the regulation of stem cell fate (90). However, the underlying regulation of this interaction has not been established yet.
Similarly, PKM2 binds to hypoxia-inducible factor-1 (HIF1), a transcriptional regulator required for upregulation of glycolytic activity under hypoxic conditions (79, 80, 96). Interestingly, cooperation of PKM2 and HIF1 in transcriptional induction of HIF1 targets did not require the catalytic activity of PKM2 but required its hydroxylation at Pro403 and Pro408 by proline hydroxylase 3 (PDH3) (96), thereby further linking induction of HIF1 targets to the metabolic status of the cell. Transcriptional targets include PKM2 itself, the glucose transporter GLUT1, as well as lactate dehydrogenase A (LDHA), and the pyruvate dehydrogenase kinase 1 (PDK1), which increases production of lactate from pyruvate and decreases the flux of pyruvate to the mitochondria, respectively. Thus the PKM2-HIF1 interaction induces a positive feedback loop to further promote glycolytic activity and contributes to the establishment of the Warburg effect.
Another surprising twist in the analysis of transcriptional functions of PKM2 was the discovery that PKM2 can also act as a protein kinase to directly phosphorylate transcriptional regulators, such as STAT3 (52). Interestingly, similar to its metabolic function, the protein kinase function toward STAT3 required PEP but not ATP as the phosphate donor in the kinase reaction and promotes expression of STAT3 target genes, such as the MEK5, a MAP kinase kinase functioning upstream of ERK5.
Likewise, PKM2 contributes to the regulation of cell proliferation and tumorigenesis by regulation of β-catenin-dependent transcription. In this pathway, PKM2 binds to Tyr-phosphorylated β-catenin at the CCND1 promoter to induce cyclin D1 expression (149). Interestingly, when bound to β-catenin, PKM2 also phosphorylates histone H3 at Thr11 (148), thus replacing the histone deacetylase HDAC3 from the promoter and allowing histone H3 acetylation and cyclin D1 expression. Nuclear localization of PKM2 is triggered by its phosphorylation at Ser37 through ERK2 (150), thereby establishing an unconventional ERK2-dependent yet WNT/Wingless-independent β-catenin pathway.
Although in most cases the mechanism of regulation of the transcriptional activity of PKM2 is still not completely understood, available evidence suggests that its transcriptional activity is mostly regulated at the level of subcellular localization. As mentioned earlier, nuclear localization is regulated by direct phosphorylation by ERK2 (150). In addition, pyruvate kinase has been shown to exist in equilibrium between dimeric and tetrameric complexes (52, 103, 104). Interestingly, only the tetrameric form of pyruvate kinase has full metabolic activity and is predominantly cytoplasmic, whereas the dimeric form is preferentially located in the nucleus. Thus regulation of the oligomerization state of the enzyme, possibly through phosphorylation (103), might directly trigger nuclear translocation and promote its transcriptional activity.
Taken together, these studies of nuclear functions of PKM2 and other metabolic enzymes demonstrate the importance of signaling functions of metabolic enzymes in normal and disease states. With examples found in yeast and mammals, further understanding of these processes should certainly reveal further fascinating biology but might also underscore the importance of metabolic enzymes as drug targets to treat cancer.
Conclusions and Perspectives
All living organisms are continuously challenged to adapt their physiology to changes in environmental conditions, such as nutrient availability or stresses. In this review, we have summarized recent progress in our understanding of the regulation of cell growth by nutrients through metabolic signals but have also described signaling functions of metabolic enzymes that are distinct from their enzymatic activity. Remarkably, these processes are highly conserved from yeast to man, and progress in the field has indeed been stimulated by these different model systems.
Nevertheless, even in the humble yeast, it has become clear that regulation of cell growth by nutrient availability is mediated by a complex network of interconnected signaling pathways that respond to different metabolic signals. This complexity is further increased in multicellular organisms, in which nutritional signals are integrated with growth factor signaling to balance cell growth within an organism and interpreted in a cell-type-specific context to ensure the appropriate physiological response. To untangle these complex relationships, ongoing research will need to further identify the molecular mechanisms governing integration of metabolism and cellular signaling. Molecular details should clarify the multi-facetted influence of nutrition on cellular physiology, identify the underpinnings of the associated disease states, and might ultimately open new strategies for therapeutic intervention.
Acknowledgments
We thank Sung Sik Lee, Frank van Drogen, Peter Mirtschink, and Alicia Smith for helpful discussions and critical reading of the manuscript.
Footnotes
Work in the Peter laboratory is supported by the European Research Council (ERC), the Swiss National Science Foundation (SNF), and the ETH Zürich.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: S.S. and R.D. drafted manuscript; S.S., M.P., and R.D. edited and revised manuscript; S.S., M.P., and R.D. approved final version of manuscript; R.D. prepared figures.
References
- 1.Ahuatzi D, Herrero P, de la Cera T, Moreno F. The glucose-regulated nuclear localization of hexokinase 2 in Saccharomyces cerevisiae is Mig1-dependent. J Biol Chem 279: 14440–14446, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Alms GR, Sanz P, Carlson M, Haystead TA. Reg1p targets protein phosphatase 1 to dephosphorylate hexokinase II in Saccharomyces cerevisiae: characterizing the effects of a phosphatase subunit on the yeast proteome. EMBO J 18: 4157–4168, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A, Yang H, Mattaini KR, Metallo CM, Fiske BP, Courtney KD, Malstrom S, Khan TM, Kung C, Skoumbourdis AP, Veith H, Southall N, Walsh MJ, Brimacombe KR, Leister W, Lunt SY, Johnson ZR, Yen KE, Kunii K, Davidson SM, Christofk HR, Austin CP, Inglese J, Harris MH, Asara JM, Stephanopoulos G, Salituro FG, Jin S, Dang L, Auld DS, Park HW, Cantley LC, Thomas CJ, Vander Heiden MG. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol 8: 839–847, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronova S, Wedaman K, Aronov PA, Fontes K, Ramos K, Hammock BD, Powers T. Regulation of ceramide biosynthesis by TOR complex 2. Cell Metab 7: 148–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balcazar N, Sathyamurthy A, Elghazi L, Gould A, Weiss A, Shiojima I, Walsh K, Bernal-Mizrachi E. mTORC1 activation regulates beta-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. J Biol Chem 284: 7832–7842, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150: 1196–1208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell GI, Polonsky KS. Diabetes mellitus and genetically programmed defects in beta-cell function. Nature 414: 788–791, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Bensinger SJ, Christofk HR. New aspects of the Warburg effect in cancer cell biology. Semin Cell Dev Biol 23: 352–361, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Berchtold D, Piccolis M, Chiaruttini N, Riezman I, Riezman H, Roux A, Walther TC, Loewith R. Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat Cell Biol 14: 542–547, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Bernstein KA, Bleichert F, Bean JM, Cross FR, Baserga SJ. Ribosome biogenesis is sensed at the Start cell cycle checkpoint. Mol Biol Cell 18: 953–964, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchini L, L'Allemain G, Pouyssegur J. The p42/p44 mitogen-activated protein kinase cascade is determinant in mediating activation of the Na+/H+ exchanger (NHE1 isoform) in response to growth factors. J Biol Chem 272: 271–279, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Binda M, Peli-Gulli MP, Bonfils G, Panchaud N, Urban J, Sturgill TW, Loewith R, De Virgilio C. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol Cell 35: 563–573, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Blandino-Rosano M, Chen AY, Scheys JO, Alejandro EU, Gould AP, Taranukha T, Elghazi L, Cras-Meneur C, Bernal-Mizrachi E. mTORC1 signaling and regulation of pancreatic beta-cell mass. Cell Cycle 11: 1892–1902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodenmiller B, Wanka S, Kraft C, Urban J, Campbell D, Pedrioli PG, Gerrits B, Picotti P, Lam H, Vitek O, Brusniak MY, Roschitzki B, Zhang C, Shokat KM, Schlapbach R, Colman-Lerner A, Nolan GP, Nesvizhskii AI, Peter M, Loewith R, von Mering C, Aebersold R. Phosphoproteomic analysis reveals interconnected system-wide responses to perturbations of kinases and phosphatases in yeast. Sci Signal 3: rs4, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell 46: 105–110, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Broach JR. Nutritional control of growth and development in yeast. Genetics 192: 73–105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai L, Sutter BM, Li B, Tu BP. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol Cell 42: 426–437, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai L, Tu BP. Driving the cell cycle through metabolism. Ann Rev Cell Dev Biol 28: 59–87, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Carmen GY, Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal 18: 401–408, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Cherkasova VA, Hinnebusch AG. Translational control by TOR and TAP42 through dephosphorylation of eIF2alpha kinase GCN2. Genes Dev 17: 859–872, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang Y, Chou CY, Hsu KF, Huang YF, Shen MR. EGF upregulates Na+/H+ exchanger NHE1 by post-translational regulation that is important for cervical cancer cell invasiveness. J Cell Physiol 214: 810–819, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452: 230–233, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452: 181–186, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Coaxum SD, Garnovskaya MN, Gooz M, Baldys A, Raymond JR. Epidermal growth factor activates Na+/H+ exchanger in podocytes through a mechanism that involves Janus kinase and calmodulin. Biochim Biophys Acta 1793: 1174–1181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colell A, Green DR, Ricci JE. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Diff 16: 1573–1581, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Conlon I, Raff M. Differences in the way a mammalian cell and yeast cells coordinate cell growth and cell-cycle progression. J Biol 2: 7, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conlon IJ, Dunn GA, Mudge AW, Raff MC. Extracellular control of cell size. Nat Cell Biol 3: 918–921, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Costes S, Longuet C, Broca C, Faruque O, Hani EH, Bataille D, Dalle S. Cooperative effects between protein kinase A and p44/p42 mitogen-activated protein kinase to promote cAMP-responsive element binding protein activation after beta cell stimulation by glucose and its alteration due to glucotoxicity. Ann NY Acad Sci 1030: 230–242, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Crespo JL, Powers T, Fowler B, Hall MN. The TOR-controlled transcription activators GLN3, RTG1, and RTG3 are regulated in response to intracellular levels of glutamine. Proc Natl Acad Sci USA 99: 6784–6789, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Virgilio C, Loewith R. Cell growth control: little eukaryotes make big contributions. Oncogene 25: 6392–6415, 2006 [DOI] [PubMed] [Google Scholar]
- 31.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7: 11–20, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Dechant R, Binda M, Lee SS, Pelet S, Winderickx J, Peter M. Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V-ATPase. EMBO J 29: 2515–2526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dechant R, Peter M. The N-terminal domain of the V-ATPase subunit ‘a’ is regulated by pH in vitro and in vivo. Channels (Austin) 5: 4–8, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Dechant R, Peter M. Nutrient signals driving cell growth. Curr Opin Cell Biol 20: 678–687, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Sci Signal 294: 1102, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Di Talia S, Wang H, Skotheim JM, Rosebrock AP, Futcher B, Cross FR. Daughter-specific transcription factors regulate cell size control in budding yeast. PLoS Biol 7: e1000221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz-Ruiz R, Rigoulet M, Devin A. The Warburg and Crabtree effects: on the origin of cancer cell energy metabolism and of yeast glucose repression. Biochim Biophys Acta 1807: 568–576, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Dilova I, Aronova S, Chen JCY, Powers T. Tor signaling and nutrient-based signals converge on Mks1p phosphorylation to regulate expression of Rtg1. Rtg3p-dependent target genes. J Biol Chem 279: 46527–46535, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Dolznig H, Grebien F, Sauer T, Beug H, Mullner EW. Evidence for a size-sensing mechanism in animal cells. Nat Cell Biol 6: 899–905, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Dombrauckas JD, Santarsiero BD, Mesecar AD. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry 44: 9417–9429, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Dong J, Qiu H, Garcia-Barrio M, Anderson J, Hinnebusch AG. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol Cell 6: 269–279, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Dubouloz F, Deloche O, Wanke V, Cameroni E, De Virgilio C. The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol Cell 19: 15–26, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Efeyan A, Zoncu R, Chang S, Gumper I, Snitkin H, Wolfson RL, Kirak O, Sabatini DD, Sabatini DM. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature 493: 679–683, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez-Garcia P, Peláez R, Herrero P, Moreno F. Phosphorylation of yeast hexokinase 2 regulates its nucleocytoplasmic shuttling. J Biol Chem 287: 42151–42164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, Ross MM, Shabanowitz J, Hunt DF, White FM. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat Biotech 20: 301–305, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23: 3151–3171, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8: 917–929, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Therap 121: 29–40, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Gancedo JM, Serrano R. Energy yielding metabolism. In: The Yeasts (2nd ed.), edited by Rose AH, Harrison JS. New York: Academic, 1988, p. 205 [Google Scholar]
- 51.Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell 45: 598–609, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell 45: 598–609, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghillebert R, Swinnen E, Wen J, Vandesteene L, Ramon M, Norga K, Rolland F, Winderickx J. The AMPK/SNF1/SnRK1 fuel gauge and energy regulator: structure, function and regulation. FEBS J 278: 3978–3990, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Giots F, Donaton MCV, Thevelein JM. Inorganic phosphate is sensed by specific phosphate carriers and acts in concert with glucose as a nutrient signal for activation of the protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol 47: 1163–1181, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Gojon A, Krouk G, Perrine-Walker F, Laugier E. Nitrate transceptor(s) in plants. J Exp Bot 62: 2299–2308, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Gong R, Li L, Liu Y, Wang P, Yang H, Wang L, Cheng J, Guan KL, Xu Y. Crystal structure of the Gtr1p-Gtr2p complex reveals new insights into the amino acid-induced TORC1 activation. Genes Dev 25: 1668–1673, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab 7: 456–465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gunawardana SC, Rocheleau JV, Head WS, Piston DW. Nutrient-stimulated insulin secretion in mouse islets is critically dependent on intracellular pH. BMC Endocr Disord 4: 1, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 149: 410–424, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem 273: 14484–14494, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Harada N, Yasunaga R, Higashimura Y, Yamaji R, Fujimoto K, Moss J, Inui H, Nakano Y. Glyceraldehyde-3-phosphate dehydrogenase enhances transcriptional activity of androgen receptor in prostate cancer cells. J Biol Chem 282: 22651–22661, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8: 774–785, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem 67: 821–855, 1998 [DOI] [PubMed] [Google Scholar]
- 64.Hatakeyama H, Kishimoto T, Nemoto T, Kasai H, Takahashi N. Rapid glucose sensing by protein kinase A for insulin exocytosis in mouse pancreatic islets. J Physiol 570: 271–282, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253: 905–909, 1991 [DOI] [PubMed] [Google Scholar]
- 67.Herrero P, Galíndez J, Ruiz N, Martínez-Campa C, Moreno F. Transcriptional regulation of the Saccharomyces cerevisiae HXK1, HXK2 and GLK1 genes. Yeast 11: 137–144, 1995 [DOI] [PubMed] [Google Scholar]
- 68.Hosiner D, Lempiäinen H, Reiter W, Urban J, Loewith R, Ammerer G, Schweyen R, Shore D, Schüller C. Arsenic toxicity to Saccharomyces cerevisiae is a consequence of inhibition of the TORC1 kinase combined with a chronic stress response. Mol Biol Cell 20: 1048–1057, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang Q, Lan F, Zheng Z, Xie F, Han J, Dong L, Xie Y, Zheng F. Akt2 kinase suppresses glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-mediated apoptosis in ovarian cancer cells via phosphorylating GAPDH at threonine 237 and decreasing its nuclear translocation. J Biol Chem 286: 42211–42220, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huber A, Bodenmiller B, Uotila A, Stahl M, Wanka S, Gerrits B, Aebersold R, Loewith R. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes Dev 23: 1929–1943, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hyde R, Cwiklinski EL, MacAulay K, Taylor PM, Hundal HS. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability. J Biol Chem 282: 19788–19798, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev 69: 79–100, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jenkins EC, Jr, Debnath S, Gundry S, Uyar U, Fata JE. Intracellular pH regulation by Na+/H+ exchanger-1 (NHE1) is required for growth factor-induced mammary branching morphogenesis. Dev Biol 365: 71–81, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab 284: E671–E678, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Jorgensen P, Nishikawa JL, Breitkreutz BJ, Tyers M. Systematic identification of pathways that couple cell growth and division in yeast. Science 297: 395–400, 2002 [DOI] [PubMed] [Google Scholar]
- 76.Jorgensen P, Tyers M. How cells coordinate growth and division. Curr Biol 14: 1014–1027, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Juntti-Berggren L, Arkhammar P, Nilsson T, Rorsman P, Berggren PO. Glucose-induced increase in cytoplasmic pH in pancreatic beta-cells is mediated by Na+/H+ exchange, an effect not dependent on protein kinase C. J Biol Chem 266: 23537–23541, 1991 [PubMed] [Google Scholar]
- 78.Jurica MS, Mesecar A, Heath PJ, Shi W, Nowak T, Stoddard BL. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure 6: 195–210, 1998 [DOI] [PubMed] [Google Scholar]
- 79.Kaelin WG., Jr Cancer and altered metabolism: potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harbor Symp Quant Biol 76: 335–345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30: 393–402, 2008 [DOI] [PubMed] [Google Scholar]
- 81.Kafri R, Levy J, Ginzberg MB, Oh S, Lahav G, Kirschner MW. Dynamics extracted from fixed cells reveal feedback linking cell growth to cell cycle. Nature 494: 480–483, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kasai H, Suzuki T, Liu TT, Kishimoto T, Takahashi N. Fast and cAMP-sensitive mode of Ca2+-dependent exocytosis in pancreatic beta-cells. Diabetes 51, Suppl 1: S19–S24, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Keller KE, Tan IS, Lee YS. SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Science 338: 1069–1072, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim J, Guan KL. Amino acid signaling in TOR activation. Annu Rev Biochem 80: 1001–1032, 2011 [DOI] [PubMed] [Google Scholar]
- 85.Kitaguchi T, Oya M, Wada Y, Tsuboi T, Miyawaki A. Extracellular calcium influx activates adenylate cyclase 1 and potentiates insulin secretion in MIN6 cells. Biochem J 450: 365–373, 2013 [DOI] [PubMed] [Google Scholar]
- 86.Kresnowati MT, van Winden WA, Almering MJ, ten Pierick A, Ras C, Knijnenburg TA, Daran-Lapujade P, Pronk JT, Heijnen JJ, Daran JM. When transcriptome meets metabolome: fast cellular responses of yeast to sudden relief of glucose limitation. Mol Syst Biol 2: 49, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature 432: 1032–1036, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 149: 274–293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee J, Kim HK, Han YM, Kim J. Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int J Biochem Cell Biol 40: 1043–1054, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Lee J, Kim HK, Han YM, Kim J. Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int J Biochem Cell Biol 40: 1043–1054, 2008 [DOI] [PubMed] [Google Scholar]
- 91.Lindstrom P, Sehlin J. Effect of glucose on the intracellular pH of pancreatic islet cells. Biochem J 218: 887–892, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol 7: 277–285, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 189: 1177–1201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10: 457–468, 2002 [DOI] [PubMed] [Google Scholar]
- 95.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27: 441–464, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145: 732–744, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo W, Semenza GL. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol Metab 23: 560–566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ma H, Botstein D. Effects of null mutations in the hexokinase genes of Saccharomyces cerevisiae on catabolite repression. Mol Cell Biol 6: 4046–4052, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318, 2009 [DOI] [PubMed] [Google Scholar]
- 100.MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci 360: 2211–2225, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martínez-Zaguilán R, Gurulé MW, Lynch RM. Simultaneous measurement of intracellular pH and Ca2+ in insulin-secreting cells by spectral imaging microscopy. Am J Physiol Cell Physiol 270: C1438–C1446, 1996 [DOI] [PubMed] [Google Scholar]
- 102.Mayer FV, Heath R, Underwood E, Sanders MJ, Carmena D, McCartney RR, Leiper FC, Xiao B, Jing C, Walker PA, Haire LF, Ogrodowicz R, Martin SR, Schmidt MC, Gamblin SJ, Carling D. ADP regulates SNF1, the Saccharomyces cerevisiae homolog of AMP-activated protein kinase. Cell Metab 14: 707–714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 43: 969–980, 2011 [DOI] [PubMed] [Google Scholar]
- 104.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol 15: 300–308, 2005 [DOI] [PubMed] [Google Scholar]
- 105.McCarthy N. Metabolism: unmasking an oncometabolite. Nat Rev Cancer 12: 229, 2012 [DOI] [PubMed] [Google Scholar]
- 106.Meijer AJ, Codogno P. Autophagy: regulation by energy sensing. Curr Biol 21: 227–229, 2011 [DOI] [PubMed] [Google Scholar]
- 107.Moreno F, Herrero P. The hexokinase 2-dependent glucose signal transduction pathway of Saccharomyces cerevisiae. FEMS Microbiol Rev 26: 83–90, 2002 [DOI] [PubMed] [Google Scholar]
- 108.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Schöler H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95: 379–391, 1998 [DOI] [PubMed] [Google Scholar]
- 109.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA 102: 14238–14243, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem 261: 13807–13812, 1986 [PubMed] [Google Scholar]
- 111.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 153: 1011–1022, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol 13: 79–85, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Oliveira AP, Ludwig C, Picotti P, Kogadeeva M, Aebersold R, Sauer U. Regulation of yeast central metabolism by enzyme phosphorylation. Mol Sys Biol 8: 623, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Orij R, Brul S, Smits GJ. Intracellular pH is a tightly controlled signal in yeast. Biochim Biophys Acta 1810: 933–944, 2011 [DOI] [PubMed] [Google Scholar]
- 115.Orij R, Urbanus ML, Vizeacoumar FJ, Giaever G, Boone C, Nislow C, Brul S, Smits GJ. Genome-wide analysis of intracellular pH reveals quantitative control of cell division rate by pHc in Saccharomyces cerevisiae. Genome Biol 13: R80, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peláez R, Fernandez-Garcia P, Herrero P, Moreno F. Nuclear import of the yeast hexokinase 2 protein requires α/β-importin-dependent pathway. J Biol Chem 287: 3518–3529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peláez R, Herrero P, Moreno F. Nuclear export of the yeast hexokinase 2 protein requires the Xpo1 (Crm1)-dependent pathway. J Biol Chem 284: 20548–20555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Perona R, Portillo F, Giraldez F, Serrano R. Transformation and pH homeostasis of fibroblasts expressing yeast H+-ATPase containing site-directed mutations. Mol Cell Biol 10: 4110–4115, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perona R, Serrano R. Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nature 334: 438–440, 1988 [DOI] [PubMed] [Google Scholar]
- 120.Popova Y, Thayumanavan P, Lonati E, Agrochao M, Thevelein JM. Transport and signaling through the phosphate-binding site of the yeast Pho84 phosphate transceptor. Proc Natl Acad Sci USA 107: 2890–2895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Portillo F. Regulation of plasma membrane H+-ATPase in fungi and plants. Biochim Biophys Acta 1469: 31–42, 2000 [DOI] [PubMed] [Google Scholar]
- 122.Pulakat L, DeMarco VG, Ardhanari S, Chockalingam A, Gul R, Whaley-Connell A, Sowers JR. Adaptive mechanisms to compensate for overnutrition-induced cardiovascular abnormalities. Am J Physiol Regul Integr Comp Physiol 301: R885–R895, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rodriguez A, De La Cera T, Herrero P, Moreno F. The hexokinase 2 protein regulates the expression of the GLK1, HXK1 and HXK2 genes of Saccharomyces cerevisiae. Biochem J 355: 625–631, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rolland F, De Winde JH, Lemaire K, Boles E, Thevelein JM, Winderickx J. Glucose-induced cAMP signalling in yeast requires both a G-protein coupled receptor system for extracellular glucose detection and a separable hexose kinase-dependent sensing process. Mol Microbiol 38: 348–358, 2000 [DOI] [PubMed] [Google Scholar]
- 125.Rolland F, Winderickx J, Thevelein JM. Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem Sci 26: 310–317, 2001 [DOI] [PubMed] [Google Scholar]
- 126.Samyn DR, Ruiz-Pavon L, Andersson MR, Popova Y, Thevelein JM, Persson BL. Mutational analysis of putative phosphate- and proton-binding sites in the Saccharomyces cerevisiae Pho84 phosphate:H+ transceptor and its effect on signalling to the PKA and PHO pathways. Biochem J 445: 413–422, 2012 [DOI] [PubMed] [Google Scholar]
- 127.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Serrano R. Effect of ATPase inhibitors on the proton pump of respiratory-deficient yeast. FEBS J 105: 419–424, 1980 [DOI] [PubMed] [Google Scholar]
- 130.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev 20: 3347–3365, 2006 [DOI] [PubMed] [Google Scholar]
- 131.Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci USA 94: 13209–13214, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tennessen JM, Baker KD, Lam G, Evans J, Thummel CS. The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab 13: 139–148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Urano J, Tabancay AP, Yang W, Tamanoi F. The Saccharomyces cerevisiae Rheb G-protein is involved in regulating canavanine resistance and arginine uptake. J Biol Chem 275: 11198–11206, 2000 [DOI] [PubMed] [Google Scholar]
- 134.Urban J, Soulard A, Huber A, Lippman S, Mukhopadhyay D, Deloche O, Wanke V, Anrather D, Ammerer G, Riezman H, Broach JR, De Virgilio C, Hall MN, Loewith R. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell 26: 663–674, 2007 [DOI] [PubMed] [Google Scholar]
- 135.Van Zeebroeck G, Bonini BM, Versele M, Thevelein JM. Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor. Nat Chem Biol 5: 45–52, 2009 [DOI] [PubMed] [Google Scholar]
- 136.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wakabayashi S, Bertrand B, Shigekawa M, Fafournoux P, Pouyssegur J. Growth factor activation and “H+-sensing” of the Na+/H+ exchanger isoform 1 (NHE1). Evidence for an additional mechanism not requiring direct phosphorylation. J Biol Chem 269: 5583–5588, 1994 [PubMed] [Google Scholar]
- 138.Walther T, Novo M, Rossger K, Letisse F, Loret MO, Portais JC, Francois JM. Control of ATP homeostasis during the respiro-fermentative transition in yeast. Mol Sys Biol 6: 344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Warburg O. On the origin of cancer cells. Science 123: 309–314, 1956 [DOI] [PubMed] [Google Scholar]
- 140.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21: 297–308, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Watanabe R, Tambe Y, Inoue H, Isono T, Haneda M, Isobe KI, Kobayashi T, Hino O, Okabe H, Chano T. GADD34 inhibits mammalian target of rapamycin signaling via tuberous sclerosis complex and controls cell survival under bioenergetic stress. Intl J Mol Med 19: 475–483, 2007 [PubMed] [Google Scholar]
- 142.Wellen KE, Thompson CB. A two-way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol 13: 270–276, 2012 [DOI] [PubMed] [Google Scholar]
- 143.Wilson WA, Hawley SA, Hardie DG. Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Curr Biol 6: 1426–1434, 1996 [DOI] [PubMed] [Google Scholar]
- 144.Wu L, van Dam J, Schipper D, Kresnowati MT, Proell AM, Ras C, van Winden WA, van Gulik WM, Heijnen JJ. Short-term metabolome dynamics and carbon, electron, and ATP balances in chemostat-grown Saccharomyces cerevisiae CEN. PK 113–7D following a glucose pulse. Appl Environ Microbiol 72: 3566–3577, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, Martin SR, Carling D, Gamblin SJ. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature 449: 496–500, 2007 [DOI] [PubMed] [Google Scholar]
- 146.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ. Structure of mammalian AMPK and its regulation by ADP. Nature 472: 230–233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu YF, Zhao X, Glass DS, Absalan F, Perlman DH, Broach JR, Rabinowitz JD. Regulation of yeast pyruvate kinase by ultrasensitive allostery independent of phosphorylation. Mol Cell 48: 52–62, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, Aldape K, Hunter T, Alfred Yung WK, Lu Z. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell 150: 685–696, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, Gao X, Aldape K, Lu Z. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature 480: 118–122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, Lyssiotis CA, Aldape K, Cantley LC, Lu Z. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol 14: 1295–1304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ye J, Mancuso A, Tong X, Ward PS, Fan J, Rabinowitz JD, Thompson CB. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc Natl Acad Sci USA 109: 6904–6909, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Youk H, van Oudenaarden A. Growth landscape formed by perception and import of glucose in yeast. Nature 462: 875–879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Young BP, Shin JJ, Orij R, Chao JT, Li SC, Guan XL, Khong A, Jan E, Wenk MR, Prinz WA, Smits GJ, Loewen CJ. Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism. Science 329: 1085–1088, 2010 [DOI] [PubMed] [Google Scholar]
- 154.Zaman S, Lippman SI, Schneper L, Slonim N, Broach JR. Glucose regulates transcription in yeast through a network of signaling pathways. Mol Sys Biol 5: 245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zaman S, Lippman SI, Zhao X, Broach JR. How Saccharomyces responds to nutrients. Annu Rev Genetics 42: 27–81, 2008 [DOI] [PubMed] [Google Scholar]
- 156.Zheng L, Roeder RG, Luo Y. S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell 114: 255–266, 2003 [DOI] [PubMed] [Google Scholar]
- 157.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science 334: 678–683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]