Abstract
The urothelium, which lines the inner surface of the renal pelvis, the ureters, and the urinary bladder, not only forms a high-resistance barrier to ion, solute and water flux, and pathogens, but also functions as an integral part of a sensory web which receives, amplifies, and transmits information about its external milieu. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical and thermal stimuli by releasing various factors such as ATP, nitric oxide, and acetylcholine. They express a variety of receptors and ion channels, including P2X3 purinergic receptors, nicotinic and muscarinic receptors, and TRP channels, which all have been implicated in urothelial-neuronal interactions, and involved in signals that via components in the underlying lamina propria, such as interstitial cells, can be amplified and conveyed to nerves, detrusor muscle cells, and ultimately the central nervous system. The specialized anatomy of the urothelium and underlying structures, and the possible communication mechanisms from urothelial cells to various cell types within the bladder wall are described. Changes in the urothelium/lamina propria (“mucosa”) produced by different bladder disorders are discussed, as well as the mucosa as a target for therapeutic interventions.
I. INTRODUCTION
A prerequisite for conscious bladder control is adequate sensory input to the central nervous system (CNS), and it is well established that changes in sensory mechanisms may give rise to disturbances in bladder function. For example, pelvic nerves are thought to convey sensations relating to the desire to void in contrast to sensations of bladder fullness, which are mediated by pudendal nerves. The urethra is very likely to be important in mediating the sense of “imminent” micturition (210). However, where the afferent impulses for bladder sensation and bladder activation are generated, and by what mechanisms, have not been fully established. However, at least two afferent signaling systems can be defined: the myogenic and mucosal pathways (11, 12). Bladder filling increases activity in in-series-coupled low-threshold mechanoreceptive afferents, thereby initiating activation of the micturition reflex. Studies have identified several classes of functionally distinct bladder sensory neurons, which include muscle-mucosal and mucosal mechanoreceptors as well as chemoreceptors. (138) Those in close proximity to the urothelium are sensitive to urothelially derived mediators resulting in increased afferent signaling (153). Changes in these afferent mechanisms may be associated with lower urinary tract symptoms (LUTS) for example detrusor overactivity (DO) and urinary incontinence (UI). New information on bladder sensory mechanisms is continuously added. Here the afferent mechanisms related to the bladder mucosa (urothelium and lamina propria) involved in bladder activation and sensation and their roles in normal bladder function and in some dysfunctional state are reviewed. In addition, the mucosa as a target for therapeutic interventions is discussed.
II. FUNCTIONAL ANATOMY
A. Mucosa
The bladder wall has three well-defined layers: the mucosa (innermost portion), the muscularis propria, and the adventitia/serosa (FIGURE 1). The mucosa (urothelium, basement membrane, lamina propria) also contains some smooth muscle cells, muscularis mucosae. Since this structure is not very well defined in the human bladder (and sometimes seems to be absent, see Refs. 88, 228), it may be questioned whether the human bladder, unlike the gut, has a true “submucosal” layer. However, the term is sometimes used to denote the part of the lamina propria closest to the muscularis propria.
Figure 1.
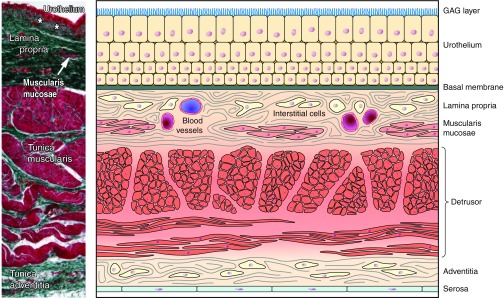
Components of the bladder wall. Left: counterstained transverse section through normal human urinary bladder. [Modified from Neuhaus et al. (215), with permission from John Wiley and Sons, Inc.] Right: cartoon depicting bladder wall components (Modified from Andersson 2012.)
B. Urothelium
The uroepithelium, or urothelium, lines the renal pelvis, ureters, bladder, upper urethra, and glandular ducts of the prostate and forms the interface between the urinary space and the underlying vasculature, connective, nervous, and muscular tissues (158). The urothelium is a transitional epithelial tissue, composed of at least three layers (FIGURE 2A): a basal cell layer attached to a basement membrane, an intermediate layer, and a superficial or apical layer composed of large hexagonal cells (diameters of 25–250 μm) known as “umbrella cells” (20, 158). The apical surface of umbrella cells possesses a unique asymmetric unit membrane (AUM), whose protein components (uroplakins, FIGURE 2B) have been well studied. Tight junctions, localized between the superficial umbrella cells, are composed of multiple proteins such as the occludins and claudins. These proteins, along with uroplakins, which are crystalline proteins that assemble into hexagonal plaques (131, 181, 266), contribute to the urothelial barrier function. In contrast, the region of the bladder neck and stratified epithelium of the urethra do not express these types of urothelial differentiation markers. Some have suggested that urothelial cells have cytoplasmic projections that anchor them to the basement membrane (20). A urothelial glycosaminoglycan (GAG) layer (FIGURE 1) covers the umbrella cells and has been suggested to contribute to urothelial barrier function.
Figure 2.
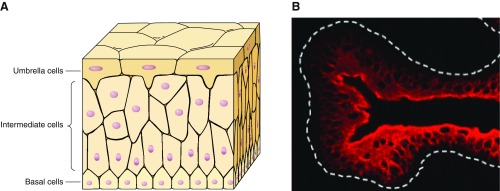
Urinary bladder urothelium and associated tight junctions. A: cartoon depicting multiple epithelial cell layers within the urothelium. B: immunohistochemical staining of mouse urothelium with an antibody to uroplakin III showing staining of superficial umbrella cells. [From Sun (266).]
The major part of the urinary tract shows similarity between a number of species and is lined with a fully differentiated urothelium (266). In contrast to the proximal urethra, there appears to be little difference between the urothelium of the trigone and the detrusor. Here the urothelium transitions to a stratified or columnar epithelium accompanied by a lack of urothelial-specific differentiation markers (266, 275). Similar to the lung epithelium, urethral epithelial cells express microvilli (FIGURE 3) on the apical surface. The presence of cilia or microvilli may have a number of functions including ability to increase the cell surface area, as well as affect bacterial adherence and fluid transport. There are at least three urothelial lineages consisting of the ureter/renal pelvis, detrusor/trigone, and bladder neck/proximal urethra (180). The functional significance of these findings has yet to be determined.
Figure 3.
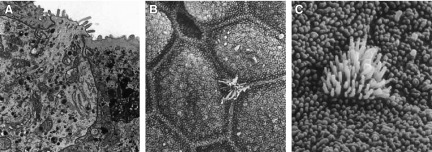
Ultrastructural features of urethral epithelium. Right: images depict “open type” paraneurons within the dog urethra. A: paraneuron reaching the lumen. B and C: scanning EM identifies (arrows) microvillous cells among the epithelial cells. A: ×14,000. B: ×4,600. C: ×16,000. [From Hashimoto et al. (126).]
Our understanding of urothelial function and identification/expression of a number of receptors and ion channels have been performed in vivo using anesthetized and awake animals and in vitro using isolated tissue and cell culture preparations (278). For example, flat sheet or cross-sectional preparations have allowed the mapping of receptive fields and localized application of various stimuli directly to the urothelium. To evaluate the involvement of urothelial-derived factors in bladder function, a common technique is to study the surgically removed “urothelium.” However, since the urothelium consists of only three to seven cell layers, it seems difficult, if not impossible, to remove it surgically without including some parts of the underlying lamina propria. This has to be considered when analyzing obtained results. Furthermore, while the use of isolated or cultured urothelial cells has been essential to our understanding of urothelial signaling, the extreme variation in culture conditions is very likely to influence the expression and function of receptors and ion channels, thus contributing to variability in results. While a “standard” isolation and culture protocol is difficult to achieve, a greater understanding is needed as to how culture conditions may influence urothelial cell growth and function.
C. Lamina Propria
A focus of current LUT research has been afferent mechanisms and the processes of how afferent information is generated and conveyed to the CNS in the control of micturition (11, 13, 153). One of the pathways defined involves the bladder mucosa, but attention has been given mainly to the urothelium (38). However, the urothelium may be regarded as one part of a signaling system involving also the lamina propria (LP) (12). The LP lies between the basement membrane of the mucosa and the muscularis propria (detrusor muscle, FIGURE 1) and is composed of an extracellular matrix containing several types of cells, including fibroblasts, adipocytes, interstitial cells, and sensory nerve endings. In addition, LP contains a rich vascular network, lymphatic channels, elastic fibers, and smooth muscle fascicles (muscularis mucosae) (4, 88, 129, 228, 229). Notably, the thickness of the LP varies within the bladder (228). The morphological characteristics of the LP, muscularis mucosae, and the detrusor muscle are important for pathological tumor staging of bladder cancer (228). However, LP is not only a landmark, but also a functionally active structure essential for, e.g., afferent signaling (see below).
1. Interstitial cells/myofibroblasts
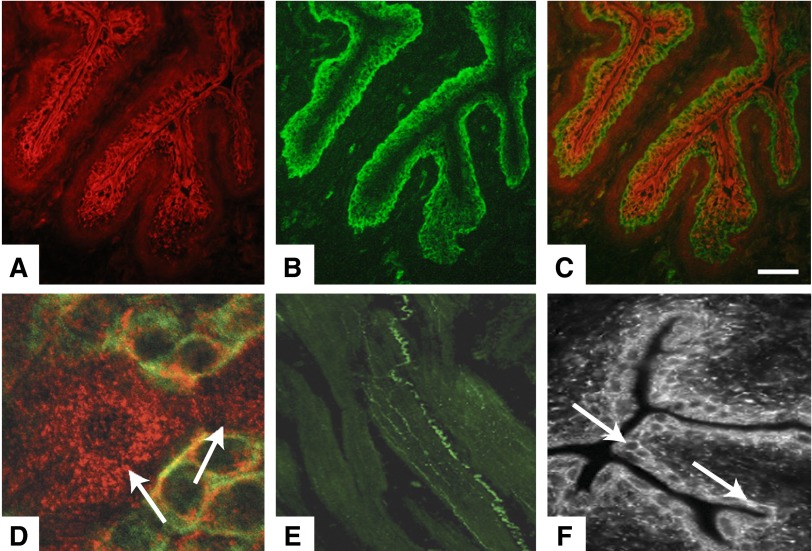
A dense layer of spindle-shaped cells has been described in bladder upper lamina propria in both humans (110, 111, 204, 205, 229, 265, 297) and animals (204, 205) (FIGURE 4). In the human bladder, close to the urothelium, Wiseman et al. (297) described a layer of such cells with the ultrastructural characteristics of myofibroblasts. However, there has been no consensus on the nomenclature. These spindle-shaped bladder cells have been categorized heterogeneously as interstitial cells (ICs), interstitial cells of Cajal (ICC), interstitial Cajal-like cells (ICLC) cells, myofibroblasts, or telocytes. The cells described by Wiseman et al. (297) had close contacts with nerves containing small clear vesicles with and without dense cores, implying that they had an efferent and afferent nerve supply. It has been questioned whether or not the normal human bladder contains myofibroblasts (98), which are generally considered to be smooth-muscle-like fibroblasts found in many tissues of the body, where they generally have functions in growth, repair, and wound healing. Significantly, myofibroblasts are contractile and immunopositive for filaments such as actin, vimentin, desmin, and myosin, and they contain fibronexus junctions (90). However, bladder ICs, even if they are excitable and show spontaneous electrical activity, do not seem to have any contractile properties (204). The cells have been identified morphologically and by use of different cellular markers (including c-Kit). As mentioned, they have a stellate-shaped morphology with several branches emanating from a central soma. Double-labeling with nerve antibodies showed that the ICs are located close to nerves, and structural connections between the lamina propria ICs and a cholinergic nerve plexus have been demonstrated (149). Sui et al. (264) used vimentin antibodies to label suburothelial cells with branched morphology. These cells formed a network apparently connected by connexin43 gap junctions, as shown by immunohistochemistry and transmission electron microscopy, and they exhibited spontaneous oscillations in membrane potential (265). Exposed to ATP, these cells responded by firing Ca2+ transients (71), but application of the cholinergic receptor agonist carbachol had no effect. Johnston et al. (149) found that the abundant microvessels in the lamina propria were associated with branched, elongated c-Kit positive ICs along the vessel axis, and they suggested the existence of an IC-vascular coupling (149). Mukerji et al. (211) observed muscarinic (M2 and M3) receptor immunoreactivity on cells in the lamina propria resembling ICs, suggesting that these cells could respond to cholinergic signaling, a finding confirmed by Grol et al. (123) who demonstrated M3 receptor immunoreactivity on a dense network of vimentin-positive cells lying immediately below the urothelium. Nile et al. (218) found stretch-independent regulation of prostaglandin (PG) E2 production within the isolated guinea pig lamina propria, and Rahnama'i et al. (239) demonstrated expression of PGE2 receptors (EP1 and EP2), indicating that the ICs can respond to PGE2.
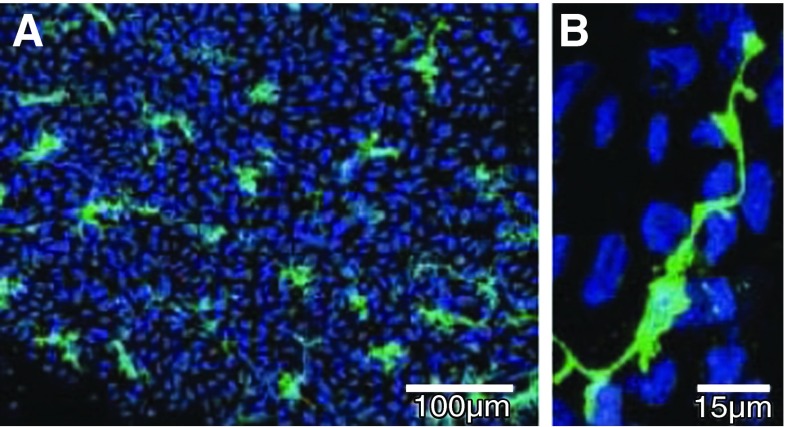
Figure 4.
c-Kit-labeled interstitial cells (ICs) in the lamina propria. A: whole-mount preparation of guinea pig bladder mucosa labeled with anti-c-Kit (green) and nuclei counterstained with DAPI (blue). B: high magnification showing an IC with lateral branches. [From McCloskey (204), with permission from John Wiley and Sons, Inc.]
In the murine bladder, Koh et al. (161) demonstrated subpopulation of ICs identified using antibodies against platelet-derived growth factor receptor-α (PDGFRα), and distinct from “conventional” ICs. PDGFRα(+) cells had a spindle-shaped or stellate morphology and often possessed multiple processes that contacted one another forming a loose network. PDGFRα+ cells were distributed as a densely packed network in the LP, and these cells were also distributed throughout the detrusor muscle, being located within and around the periphery of smooth muscle bundles. Many of the PDGFRα+ cells in the LP as well as the detrusor muscle colabeled with vimentin antibodies. PDGFRα+ cells were found to be present also in human and guinea pig bladder (207). In guinea pig bladder, PDGFRα+ cells had a branched stellate morphology and formed cellular networks in the lamina propria. PDGFRα+ cells were located close to nerves labeled by PGP9.5. Double labeling showed that PDGFRα+ cells were a subgroup of the vimentin+ population; moreover, a significant proportion of PDGFRα+ cells were also KIT+. The function of PDGFRα+cells in the urinary bladder is unknown, but a possible neuromodulatory role in bladder function cannot be excluded.
The role of bladder ICs, including those in the LP, has not been established. The ICs in the LP and within the detrusor may serve different functions. Available evidence suggests that the LP ICs may constitute a structural and functional link between urothelial cells and sensory nerves and/or between urothelial cells and detrusor smooth muscle cells (106, 142, 204). Moreover, these cells might be involved in the pathophysiology of urinary tract disorders (see below).
2. Afferent nerves
Different types of nerves have been described in the LP. Thus acetylcholinesterase-positive nerves were described by El-Badawi and Schenk (92), and further studied by Gosling and Dixon (120), who found marked regional variation in the distribution of such nerves. In the fundus and adjacent part of the body, nerves were rarely encountered, but in the lower part of the bladder body and bladder neck, there was a gradual increase in the number of nerves in the LP. Gosling and Dixon (120) proposed that these nerves might have a sensory function. Throughout the bladder neck itself, the nerves formed an extensive plexus adjacent to the urothelial lining (possibly making synaptic connections within urothelial cells). Given the location in close proximity to the urothelium, it is not surprising that changes in urothelial structure and function can occur with either pelvic nerve stimulation or neural activation following spinal cord injury (22).
The general distribution of LP nerves was confirmed by Gabella and Davis (108), who studied whole-mount preparations of the rat bladder. In the caudal regions of the bladder, calcitonin gene-related-immunoreactive (CGRP-IR) axons formed an elaborate meshwork (referred to as subepithelial plexus). The plexus lay very close to the network of capillaries in the LP, but the two patterns seemed unrelated. In the equatorial regions of the mucosa, the CGRP-IR axons were less abundant than in the caudal region, but had a similar distribution. In the cranial regions, the suburothelial plexus was absent and the mucosa did not contain CGRP-IR axons except for a few perivascular axons. Thus the highest density of mucosal innervation was found in the neck and the initial part of the urethra. The axons of the suburothelial plexus were very close to the urothelium; the finest of them, which were also the most distinctly varicose, ran parallel to and in apparent contact with the urothelium. These axons had thin side branches, which penetrated the urothelium. Some of these axons penetrated almost the full thickness of the urothelium, supporting the view of “synaptic connections” with urothelial cells.
Gosling and Dixon (120) found no nerve cell bodies in any part of the LP in any of the species investigated (guinea pigs, rats, rabbits, and cats). In human bladder, however, intramural ganglion cells were demonstrated both in the LP or embedded among the detrusor muscle bundles. The majority of the ganglia were small in size and contained from one to six neurons. These ganglion cells possessed fine structural characteristics of parasympathetic nerve cells. The existence of human intramural ganglion cells was confirmed by Smet et al. (256). They showed that in the human bladder, peptidergic (CGRP; tachykinin) nerves are localized mainly within the subepithelium, surrounding the vasculature as well as intramural ganglia. While these nerves have not been detected within the detrusor smooth muscle, vasoactive intestinal polypeptide (VIP)-containing nerves have been localized within both the suburothelial plexus as well as the detrusor muscle bundles. Ganglion cells, positive for choline acetyltransferase (ChAT), were demonstrated in the guinea pig LP (118).
The information on noradrenergic nerves in the LP is sparse. Noradrenergic nerves in the LP, considered to have vasomotor function, were demonstrated by Jen, Gosling and Dixon (147), and functional support for noradrenergic innervation of suburothelial microvessels has been presented (127).
D. Barrier Function
The urothelium plays a critical role as a permeability barrier to urine, and an intact barrier is a prerequisite for normal afferent signaling from the bladder. Several features of the superficial or umbrella cell layer aid the bladder in maintaining normal barrier function as the bladder fills and empties. These include a number of tight-junction proteins in addition to specialized lipids and uroplakin proteins in the umbrella cell apical membrane. The uroplakins have important functions including maintaining the urothelial barrier in part by preventing proteins as well as ionic and nonionic substances from gaining access (3, 20). In addition, a layer of polysaccharide GAG or mucin covers the superficial urothelium and may have a number of roles including a defense mechanism against microorganisms, carcinogens, and toxic substances in the urine (230). The lipid composition of the apical membrane is unusual and is rich in cholesterol, phosphatidylcholine, phosphatidylethanolamine, and cerebroside (132). Recent studies suggest that liposomes, consisting of an aqueous core enclosed in one or more phospholipid bilayers, may help to restore urothelial-barrier function (FIGURE 5). Liposomes have typically been used to transport drug molecules in a variety of cells. Urothelial cells appear to take up liposomes via an endocytotic process, providing evidence for a possible mechanism by which liposomes act as a drug delivery system (220). In addition, empty liposomes have shown promise to repair and enhance the barrier function of a dysfunctional urothelium (154, 237, 280, 281).
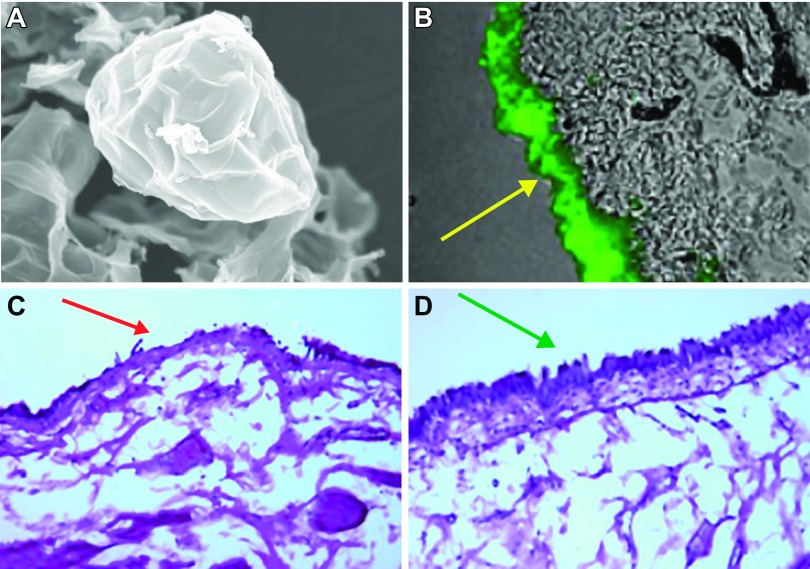
Figure 5.
Images following application of liposomes within the urinary bladder. A: representative image showing a typical liposome (×4,000; Lipella, Inc.). B: liposomes instilled in a rat bladder forming a coating (in green) on urothelial surface. C: intravesical instillation of protamine sulfate (PS) in rat urinary bladder exhibit damaged urothelium. D: intravesical instillation of PS followed by liposomes in rat urinary bladder exhibit an intact urothelium, demonstrating a protective effect of liposomes on urothelial barrier. [From Kaufman et al. (154).]
Distension of the bladder during filling is accompanied by a change in shape of the superficial epithelium. In addition, there is also an increase in vesicular traffic (i.e., exocytosis/endocytosis) which adds membrane to the apical or superficial cell surface, thereby permitting increases in bladder volume without loss of barrier function (32, 131, 293). Studies have revealed that stretch-induced exocytosis is likely to involve a number of signaling molecules including epidermal growth factor receptor (EGFR) (32, 70). These processes allow the bladder to accommodate increasing volumes of urine during filling without compromising barrier function. There is some evidence that superficial urothelial cells exhibit a lower level of endocytotic activity, which may be a protective mechanism against internalization of toxic substances excreted in the urine (164). Changes in the urothelial lipid environment (i.e., diet) may alter membrane traffic, which could result in internalization of toxic substances present in the urine (122). Studies have also suggested that vesicular traffic plays a role in regulating expression levels of receptor/channel proteins and mediators in urothelial cells (21, 38).
Epithelial integrity is maintained through a complex process of migration and proliferation (to restore cell numbers) and differentiation (to restore function) (243). Basal epithelial cells, which have been suggested to have stem-cell-like properties, typically exhibit very slow (3–6 mo) proliferative rates (132, 199). There is some suggestion that urothelial cell turnover and differentiation (in vitro) may not be influenced by cyclic mechanical changes. However, differentiation of urothelial cells (in culture) can be influenced by exogenous factors, including EGF, platelet-derived growth factor (PDGF), peroxisome proliferator-activated receptor γ (PPARγ), and small molecules such as retinoic acid (266, 288, 289), and accelerated proliferation can occur in various bladder pathologies. For example, when using agents (protamine sulfate; cyclophosphamide) that damage the umbrella cell layer (FIGURE 6), it has been shown that the urothelium rapidly undergoes both functional and structural changes to restore the barrier in response to injury (163, 164, 174). In the early stages of regeneration following disruption of the barrier, the superficial cells may appear smaller in size and often covered with microvilli (163). In some pathologies, a deficiency or defect in maturation or terminal differentiation of superficial umbrella cells have been reported, although the factors that may be involved are not yet known (136).
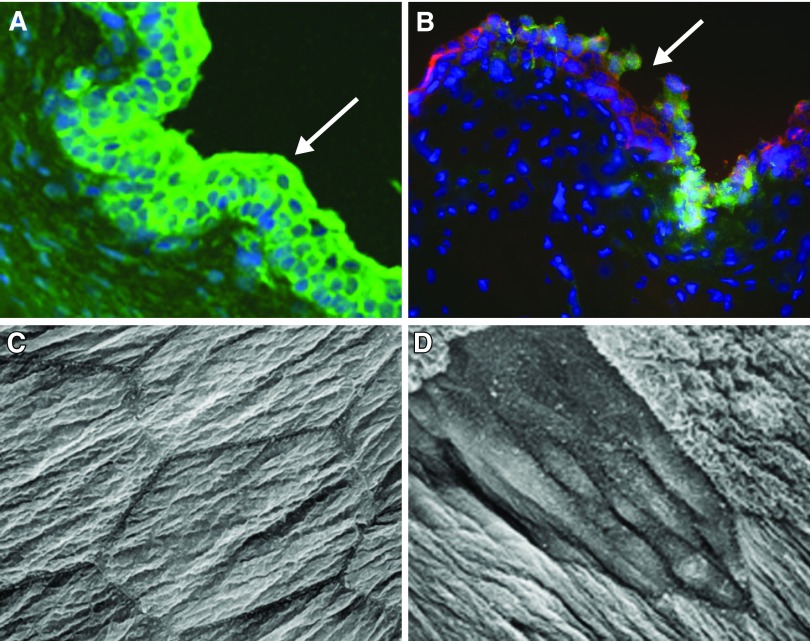
Figure 6.
Urothelial alterations following injury or inflammation. A: intact urothelium in rat urinary bladder (green pan-cytokeratin labeling epithelium; blue, DAP-I nuclear marker). B: area of damage (shown at arrow) to the rat urothelium following cyclophosphamide treatment in a rat. (Modified from Birder 2012.) C and D: scanning electron micrograph of apical surface of umbrella cell layer from normal rat (C) and 2 h after spinal cord injury (D). [Modified from Apodaca et al. (22).]
The processes underlying urothelial repair are complex, involving several structural elements, signaling pathways, trophic factors, and the cellular environment. Furthermore, the interaction between these biochemical signals and mechanical forces in the bladder during the course of urothelial repair is not well understood. For example, the initiation of urothelial proliferation or differentiation of intermediate cells is thought to involve upregulation of growth factors such as fibroblast growth factor (33). In addition, members of the PPARγ and EGFR signaling pathways may contribute to urothelial “re-epithelialization” in wound repair (289). There is also evidence that Hedgehog/Wnt signaling, acting across the basal urothelial cell-stromal cell boundary, contributes to increases in urothelial proliferation in response to injury (254). Although the urothelium maintains a tight barrier to ion and solute flux, a number of local factors or stressors, such as tissue pH, mechanical or chemical trauma, hormonal changes, or bacterial infection can modulate its barrier function (21, 38, 158). Stress-mediated activation of the hypothalamic-pituitary-adrenal axis can result in increased production of corticotrophin releasing factor (CRF), which can regulate neuroendocrine and autonomic responses to stress. The net effect can include disruption of the epithelial barrier and increased prevalence of infection. In addition, altered levels of circulating estrogens have been associated with changes to the urothelial structure including epithelial shedding or mucosal atrophy (241). Other conditions, such as bladder pain syndrome/interstitial cystitis (BPS/IC), senescence, or spinal cord injury are also associated with changes in the urothelial barrier (22, 175, 234) (FIGURES 6 AND 7). There is evidence that in many types of epithelium (including urothelium) adhesion molecules, such as members of the cadherin family, play important roles in establishing and maintaining epithelial-cell contacts (109). Altered urothelial-cadherin expression has been reported in patients with BPS/IC. Disruption of urothelial barrier integrity has also been linked to the expression of substances such as antiproliferative factor (APF), which also slows urothelial cell growth (80, 155). APF, a frizzled 8 protein detected in the urine of patients with BPS/IC (156), is secreted by bladder urothelial cells obtained from these patients. Treatment of urothelial cells from normal patients with purified APF decreased the expression of adhesion and tight junction proteins.
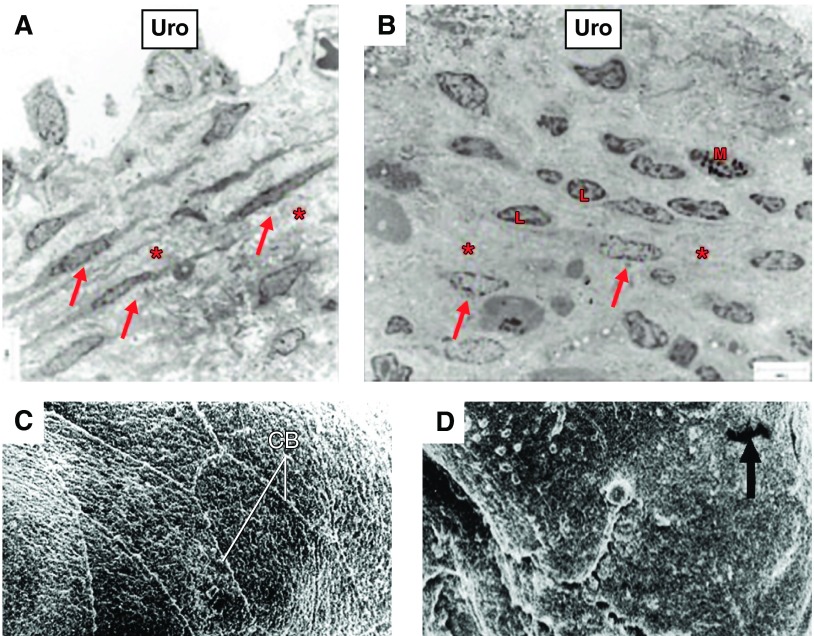
Figure 7.
Urothelial alterations associated with bladder pain syndrome or senescence. Electron micrograph in control (A) and BPS (B) bladders. In A, parallel layers of upper lamina propria interstitial cells (ULP ICs, arrows) within a dense extracellular matrix. In B, arrows indicate fragmented layers of UPL ICs and presence of lymphocytes (L) and mast cells (M). [A and B from Gevaert et al. (110), with permission from John Wiley and Sons.] C and D: scanning electron micrographs of the urinary bladder of adult (C) and aging (D) female rats showing the luminal surface. In aging rats, the polygonal cell appearance and well-defined cell boundries (CB) appeared to be lost with focal areas of denuded urothelium (arrow). [C and D from Mahmoud et al. (196a).]
Both physiological and psychological stress can result in a failure of urothelial and suburothelial “defensive” systems and thereby promote changes in both urothelial barrier and signaling function. For example, alterations in proteins including proteoglycans and bacterial defense molecules may lead to distinctive changes in urothelial structure and play a role in bacterial adherence (246). In this regard, urinary tract infections produced by uropathogenic Escherichia coli (UPEC) are initiated by bacterial adherence to uroplakin proteins on the apical surface of umbrella cells (10, 250). The UPEC express filamentous adhesive organelles (type 1 pili) that mediate bacterial attachment, invasion, and apoptosis of the urothelial cells. It has been suggested that urothelial differentiation (and increased uroplakin III expression) plays a pivotal role in sensitizing urothelial cells to UPEC-induced infection and possible cell death (276). Even acute contact (within hours) of the mucosal surface by bacteria may result in altered urothelial barrier function (298). UPEC can also internalize within umbrella cells forming intracellular colonies (biofilm-like pods) of UPEC that has been implicated in the mechanism of chronic urinary tract infections. By residing inside fusiform vesicles and commandeering the endocytic/exocytic machinery of urothelial cells, UPEC is able to escape elimination during voiding (47). During bladder distension, bacteria are excreted into the urine (which is likely to contain factors supporting bacterial survival). Evidence supports a role for endotoxin (lipopolysaccharide, LPS) on the bacterial cell wall in mediating the pain associated with UPEC infection (247).
Although the urothelium maintains a tight barrier, a number of factors (e.g., mechanical or chemical trauma, infection) can modulate the barrier function. When the barrier is compromised, the urothelium is unable to maintain the integrity of the bladder-urine interface. The result can be changes in the function of underlying cells within the bladder wall and sensory symptoms of urgency, frequency, and pain during bladder filling and voiding. Thus a complex chemical information transfer exists between the urothelium and cells within the bladder wall and disruption in this “sensory web” may be involved in bladder dysfunction.
III. UROTHELIUM-LAMINA PROPRIA INTERACTIONS
A. Normal Bladder Filling/the “Sensory Web”
It is likely that a cascade of urothelial inhibitory and stimulatory transmitter/mediators are involved in the transduction mechanisms underlying the activation of afferent fibers during bladder filling (12). The mucosal activation pathway (the sensory web) includes the urothelium, the afferent (and efferent) nerves, the ICs of the lamina propria, and possibly the muscularis mucosae (FIGURE 8). As mentioned, the suburothelial ICs are extensively linked by gap junctions and may serve as intermediaries between the urothelium and afferent nerves, and possibly between nerves and detrusor muscle. Whether or not the muscle cells of the muscularis mucosae will contribute is still unclear. However, it is clear that communication between these different structures ensures normal function of the organ and may explain how the effect of various neurotransmitters/mediators when given intravesically can modify bladder function by changing neurotransmission, the spontaneous activity of the detrusor smooth muscle, and thereby bladder function.
Figure 8.
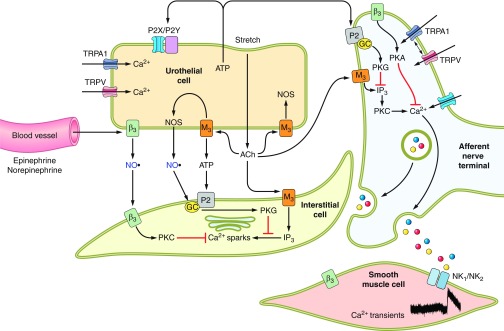
Hypothetical model depicting possible interactions between bladder nerves, urothelial cells, smooth muscle, interstitial cells, and blood vessels. Urothelial cells can also be targets for transmitters released from nerves or other cell types. Urothelial cells can be activated by either autocrine (i.e., autoregulation) or paracrine (release from nearby nerves or other cells) mechanisms. [Modified from Kanai et al. (153a), with permission from John Wiley and Sons, Inc.]
There is substantive evidence that urothelial cells are able to respond to a number of stimuli (physical as well as chemical). In turn, the urothelium can signal (via substances they release, often termed volume regulation) to cells in the bladder wall. In this manner, the urothelium is likely to play an important role in the complex transfer of information to and from the nervous system. The urothelium is able to respond to a wide variety of mechanical stresses during bladder filling and emptying by activating a number of possible transducer proteins. Possibilities of mechanical signals include bladder pressure, tension in the urothelium or bladder wall, torsion, geometrical tension, and movement of visceral organs. Recent studies have demonstrated that urothelial cells release transmitters (such as ATP) during changes in hydrostatic pressure (in ranges that normally trigger micturition) (222). Activation of transducer proteins by a variety of mechanical stimuli could also involve indirect mechanisms such as changes in the cytoskeleton, cell surface molecules such as integrins, or coactivation of urothelial channel proteins. The urothelium can also respond to changes in urine tonicity. Alterations in the composition of urine are a type of stress; the urine contents can vary in both their rate of delivery as well as the particular constituents.
Additional lines of evidence suggest that urothelial cells participate in the detection of both physical and chemical stimuli. As mentioned, bladder nerves (afferent and efferent) are localized in close proximity to, and some within, the urothelium (40, 41, 147). In addition, urothelial cells express numerous receptors/ion channels similar to what is found in both nociceptors and mechanoreceptors elsewhere in the body, and these cells secrete a number of transmitters or mediators capable of modulating, activating, or inhibiting sensory neurons. Examples of neuronal “sensor molecules” (receptors/ion channels) that have been identified in urothelium (TABLE 1) include receptors for purines, adenosine, norepinephrine, ACh, protease-activated receptors (PARs), amiloride- and mechanosensitive epithelial sodium channels (ENaC), bradykinin, neurotrophins, CRF, estrogens, endothelins, and various TRP channels (34, 35, 37, 42, 54, 58, 72, 75, 76, 97, 223). The expression of these various receptors enables the urothelium to respond to a number of “sensory inputs” from a variety of sources. These inputs include increased stretch during bladder filling, soluble factors (many found in the urine), or chemical mediators/peptides/transmitters released from nerves, inflammatory cells, and even blood vessels (20, 38, 125, 173). Thus various stimuli can lead to secretion of chemical substances capable of modulating the activity of underlying smooth muscle (141, 271), as well as nearby sensory neurons. For example, urothelial-specific overexpression of nerve growth factor (NGF) results in increased bladder nerve “sprouting” and increased voiding frequency (119, 251). It has been shown that urothelial-derived NO can be released in response to mechanical as well as chemical stimulation and may either facilitate or inhibit the activity of bladder afferent nerves conveying bladder sensation (18, 42). In this regard, activation of urothelial receptors and release of inhibitory mediators may explain, in part, the mechanism of action for therapies (e.g., β3-AR agonists) in treatment of bladder disorders such as the overactive bladder (OAB) (137, 283, 301).
Table 1.
Properties of ionic channels/receptors expressed within the urinary bladder urothelium
| Channel/Receptor | Activator(s) |
|---|---|
| TRPV1 | Heat (>43°C), low pH, anandamide, vanilloids |
| TRPV2 | Noxious heat (>53°C), mechanical |
| TRPV4 | Moderate heat (>24°C), cell swelling, 4α-PDD, 5′6′−EET |
| TRPM8 | Cold (8–28°C), menthol, icilin |
| TRPA1 | Mechanical, cinnamaldehyde, isothiocyanate |
| P2X (1–7) | ATP |
| P2Y (P2Y2/P2Y4) | Nucleotides (ATP; UTP; ADP) |
| P1 | Adenosine |
| Adrenergic alpha (–1, –2) | Endogenous catecholamines (norepinephrine; epinephrine) |
| Adrenergic beta (–1, –2, –3) | Endogenous catecholamines (norepinephrine; epinephrine), isoproterenol |
| Cholinergic muscarinic (M1–M5) | Acetylcholine, muscarine |
| Cholinergic nicotinic (α3, α5, α7, α3, β4) | Acetylcholine, nicotine, choline, cystisine |
| Estrogen Receptor (alpha; beta) | Estradiol, estrogen derivatives, selective estrogen-receptor modulators (SERM) |
| Degenerin/ENaC (DEG/ENaC) family (ENaC; ASIC) | Low pH, serum proteases, amiloride, mechanical? |
| Neurotrophins (trkA; p75; trkB) | NGF, BDNF |
| Bradykinin (B1; B2) | Bradykinin |
| Tachykinin (NK1; NK2) | Substance P, neurokinin A |
4α-PDD, 4alpha-phorbol 12,13-didecanoate; 5′6′-EET, 5,6-epoxyeicosatrienoic acid; NGF, nerve growth factor; BDNF, brain-derived nerve factor.
There is insufficient information as to the mechanism underlying release of urothelial-derived mediators. Much of the information to date in terms of the dynamics and identity of substances contained in membrane-bound cytoplasmic vesicles is derived from membrane capacitance measurement and microscopy of fixed tissues and cells. For example, once released, mediators (such as acetylcholine or ATP) can elicit both paracrine as well as autocrine actions (293). These actions may include stimulation of membrane turnover (resulting in increased apical surface area), altering the sensitivity and localization of receptors/ion channels (that can sense bladder filling and urine contents) and augmenting the release of substances. Thus release of chemical factors from the urothelium could amplify signaling between and within urothelial cells as well as nearby bladder nerves.
There is evidence that epithelial cells in different organ systems may express similar receptor subtypes (100, 169, 194). Accordingly, epithelial cells could use multiple signaling pathways, whose intracellular mechanisms differ according to location and environmental stimuli. This would permit a greater flexibility for the cell to regulate function and respond to complex changes in their surrounding microenvironment. Whether urothelial-sensor molecules all feed into a diverse array of signaling pathways or share similarities with systems such as olfaction, whereby hundreds of receptors share identical transduction cascades (50), is yet to be uncovered.
1. Purinergic mechanisms
Since the first report of distension-evoked ATP release from the urothelium, abundant evidence has accumulated supporting a role for urothelially derived release of ATP in autocrine and paracrine signaling within the lower urinary tract. ATP is abundant in the cell cytoplasm and can be released extracellularly by several mechanisms including vesicular exocytosis, transporters such as a member of the ATP-binding cassette (ABC) transporter superfamily, or anion-selective channels such as the maxi-anion channel (54, 55, 160). ATP is released from both the apical and basolateral urothelial surfaces in response to bladder stretch and can act on purinergic urothelial receptors to stimulate stretch-induced exocytosis (293). A number of channels such as the amiloride-sensitive apical sodium channel, ENaC, may be involved in mechanotransduction by controlling basolateral release of ATP (91). In addition, urothelial release of ATP and stimulation of urothelial-P2Y receptors has also been suggested to contribute to basal levels of a variety of mediators. The resulting signal depends on a number of factors including the subtype of purinergic receptors expressed as well as expression of ATPases and other ecto-nucleotidases. These ecto-enzymes can be secreted or membrane-bound and act to degrade (ATP and UTP) to respective nucleotides, including conversion into adenosine, which activates its own class of P1 receptors also expressed within the urothelium. Studies in the mouse urinary bladder have shown a cell-specific expression pattern within smooth muscle, blood vessels, lamina propria, and the urothelium (304). The forms and expression patterns of these enzymes in health and disease are likely to affect the response profile of a given cell.
The expression of both P2X and P2Y receptors in nerve fibers and myofibroblasts in close proximity to the bladder lumen, and the sensitivity of these cells to ATP, suggests that basolateral ATP release from the urothelium may also influence function of myofibroblasts and bladder nerves (264). In addition, intercellular communication mediated by gap junctions in myofibroblasts could provide a mechanism for long-distance spread of signals from the urothelium to the detrusor muscle (141). Interestingly, this type of nucleotide-mediated wave of cell-cell communication may also play a role in the response to injury (107). While evidence supports a role for ATP and purinergic receptors in modulating symptoms in several urological diseases, the mechanisms underlying activation of the micturition pathway at lower bladder volumes (during urgency) and mediators (amount; type) involved are not understood. In addition, the directionality of transmitter release, the mixture of receptor subtypes in the apical and basolateral domains, and interactions between multiple-transmitters are likely to affect the nature of the output in both health and disease.
2. TRP channels
The first (indirect) evidence supporting urothelial-TRPV1 channel expression comes from a study that showed the TRPV1 agonist capsaicin could evoke release of the transmitter nitric oxide (NO). These and subsequent studies suggested that urothelial cells and afferent nerves may exhibit a number of similarities (36, 41) (FIGURE 9). Studies in a number of cell types have demonstrated that TRPV1 can be activated by vanilloids (capsaicin and resiniferatoxin or RTX), in addition to protons, moderate heat, nitro-fatty acids, lipid metabolites, and a number of inflammatory mediators (30, 60, 63, 130, 269). Activation of urothelial cells with capsaicin or RTX can increase intracellular calcium, evoke transmitter (NO and ATP) release, and elicit transient currents (41, 167). Studies (including in TRPV1 null mice) have shown that this channel is important in cystitis-induced mechanical hyperactivity (294) and may play a role in amplifying the response to chemical and mechanical stimuli. In the bladder, metabolic stress and inflammatory conditions can lead to increased production of numerous substances (such as nitro-fatty acids and NGF), which can activate TRP channels thereby increasing bladder reflex activity. NGF can alter cellular sensitivity by a number of mechanisms including increased translocation or trafficking of TRPV1 to the plasma membrane (119, 148, 261, 262). It has been well described that NGF is involved in a large number of bladder pathologies and is likely to be involved in altering sensation, urgency, and bladder overactivity. In this regard, recent evidence has shown increased TRPV1 expression and function (measured electrophysiologically) detected in bladder urothelial cells isolated from patients diagnosed with overactive bladder (45, 179). Thus it seems likely that urothelial TRPV1 might participate in a manner similar to that in neurons, in the detection of irritant stimuli following bladder inflammation or infection.
Figure 9.
Expression of TRPs within the urinary bladder. A: cytokeratin-staining for bladder urothelium. B: TRPV4-positive immunoreactivity in mouse urothelium. C: a merged image of both cytokeratin staining and TRPV4 in mouse urothelium. D: confocal image of bladder urothelium in bladder whole mount stained for TRPV1 (cy3, red) and cytokeratin 17 (FITC, green), a marker for basal urothelial cells. Diffuse cytoplasmic pattern of TRPV1 staining can be seen in the apical and underlying urothelial layers. E: immunohistochemical localization of TRPA1 within the urinary bladder wall (scale, 100 μm). F: immunohistochemical localization of TRPM8 within the urinary bladder urothelium. [Modified from Chopra and Birder, unpublished observations; Birder et al. (39); Streng et al. (263); and Yamada et al. (300).]
TRPV1−/− mice exhibit a number of alterations in bladder function which include an increase in nonvoiding contractions and a significant decrease in stretch-evoked addition of apical membrane and release of ATP (41). The altered neural responses to distension detected in TRPV1−/− mice demonstrate a role of TRPV1 in excitability of lower threshold bladder afferents (83). These changes in bladder function are likely to be complex and could be due to expression of TRPV1 at multiple sites. Taken together, these findings support a wider role for TRPV1 in the bladder that includes involvement in normal voiding function in addition to pain sensation.
3. Additional TRP channels
Much less is known about the involvement of other TRPs in bladder function or disease (17, 96). TRPV4, which is a nonselective cation channel activated by a number of stimuli, including heat, shear stress, changes in osmolarity, and lipid ligands, is expressed mainly within the urothelium (182) (FIGURE 9). Recent evidence has shown that this expression occurs in association with adherence junctions where they may be preferentially activated by stretch and lead to the release of ATP (300). While a definitive role for TRPV4 in bladder function has not been established, there is evidence that null mice exhibit impaired voiding responses, and intravesical instillation of a TRPV4 agonist in the rat triggers a novel voiding reflex that could regulate the late phase of micturition (40, 112). Additional studies suggest that activation of urothelial-TRPV4 facilitates bladder reflexes via activation of mechanosensitive, capsaicin-(insensitive) C fibers (7). In addition, in the awake ewe, TRPV4 was shown to be involved in a urethra to bladder reflex, proposed to facilitate bladder emptying (79). Additional members of the TRP family include TRPM8 (cold-menthol sensory receptor) and TRPA1 (characterized as a thermoreceptor activated by noxious cold) (FIGURE 9). Both of these channels are expressed in C-fiber afferents as well as urothelium, and there is speculation that they may play a role in symptoms associated with overactive and painful bladder disorders (91, 263). Of interest is the finding that hydrogen sulfide, which may be formed during infection/inflammation, is an activator of TRPA1(19, 263).
4. NO
Several lines of evidence indicate that urothelial NO has a role in the micturition reflex pathway (18, 42). Birder et al. (42), using reverse transcription-PCR, showed that inducible NO synthase (iNOS) and endothelial NOS (eNOS), but not neuronal NOS (nNOS) genes, were expressed in cultured rat urothelial cells. In the porcine urothelium, Persson et al. (235) found a strong NADPH diaphorase reaction, and by immunohistochemistry they demonstrated thin NOS-IR fibers in the lamina propria, mostly in the vicinity of vessels. However, no nNOS immunolabeling was observed in the urothelium. Gillespie et al. (116, 117) demonstrated that interstitial cells, lying immediately below the guinea pig urothelium, respond to NO with a rise in cGMP. They found a high degree of complexity in nNOS distribution and NO target cells in the lamina propria and suggested that this may reflect distinct physiological functions that have yet to be identified.
The release of NO from the urothelium or other cells is thought to either facilitate or inhibit the activity of bladder afferent nerves. Reduction in NO levels adjacent to the urothelium (caused by pathology or intravesical administration of oxyhemoglobin) resulted in bladder hyperactivity, suggesting an inhibitory role of NO in the control of bladder function (227). Munoz et al. (212) found that while electrical stimulation-evoked release of some urothelial mediators (ATP) was attenuated by disruption of the urothelium, release of NO was maintained which may suggest that under these conditions NO is derived from suburothelial structures. Interestingly, NO release triggered by cholinoreceptor stimulation was lost after urothelial disruption, consistent with NO derived from multiple sources including the urothelium.
The expression of the enzyme NOS can be altered in a number of conditions which suggests that the neurotransmitter function of NO is also able to be altered in pathology (84). For example, studies have reported changes in NOS expression and/or NO levels in patients with BPS/IC as well as in IC cats (46, 134). Excess NO levels have been linked to changes in epithelial barrier function in a number of pathologies including BPS/IC (175, 231). Studies have shown that both aminoguanidine and TGF-β1 inhibit expression of iNOS and have been shown to prevent changes in urinary bladder following cyclophosphamide treatment in rats (1, 162).
A study by Aizawa et al. (6) examined the effect of NO on sensory signaling by directly recording afferent activity arising from the bladder in vivo. Release of NO can be inhibited using nonmetabolizable analogs that compete with l-arginine as substrate for NOS. One such inhibitor, l-NAME, increased the afferent response to bladder filling by ∼50%, which was reversed by activation of NO pathways with l-arginine. Thus NO seems to be able to inhibit afferent activity, an observation consistent with earlier cystometric analysis of the effect of activating and inhibiting the NO pathway (59, 225, 236). Taken together, these findings suggest that alterations in NO levels may have a role in the modulation of urothelial signaling. NO mechanisms may also be involved in the loss of membrane barrier integrity in many disease states.
5. Cholinergic receptors
A) MUSCARINIC RECEPTORS.
Muscarinic receptors have been demonstrated on the urothelium and in the suburothelium (lamina propria) by several investigators (29, 51, 124, 128, 198, 209). In some of these studies not only the urothelium, but also part of the lamina propria were included in the tissues investigated. The porcine urothelium (+ lamina propria?) was found to express a high density of muscarinic receptors ([3H]QNB binding), even higher than the bladder smooth muscle (128). In the rat and human urothelium, the receptor proteins and mRNAs, respectively, for all muscarinic receptor subtypes (M1–M5) were demonstrated (284). In the human bladder mucosa, the expression was not age or gender dependent (29). The expression pattern of the different subtypes in the human urothelium was reported to differ: the M1 receptors on basal cells, M2 receptors on umbrella cells, M3 and M4 receptors homogeneously, and M5 receptors with a decreasing gradient from luminal to basal cells (51). Mansfield et al. (198) found, using RT-PCR analysis, an abundant expression of muscarinic M2 receptors in the human bladder mucosa. Some of these receptors may occur at other locations than the urothelium proper, e.g., on suburothelial ICs (123, 209). Using a well-characterized antibody, Grol et al. (123) showed that the muscarinic receptors in the lamina propria were located specifically on the ICs. The physiological significance of what appears to be a cholinergic signaling system in the mucosa is unclear. Ikeda and Kanai (140, 142) suggested that muscarinic receptors within the mucosa were involved in urotheliogenic signaling, enhancing intrinsic detrusor contractions. Isolated strips of porcine urothelium with lamina propria were shown to exhibit spontaneous contractile activity that was increased by stretch. The mechanism appeared to involve endogenous ACh release acting on M3 receptors (209).
It has also been suggested that cholinergic mechanisms may be involved in the urothelial release of an unknown inhibitory factor (72, 128). Nile and Gillespie (219) demonstrated that complex signal interactions may occur within the urothelium involving ACh, ATP, NO, and PGE2. They suggested a role for M2 receptors and NO in the cholinergic regulation of PGE2 production in the bladder wall. Muscarinic receptors also seem to play a role also for suburothelial afferent nerve activity (305). The complex role of urothelial muscarinic receptors were further illustrated by the finding that carbachol stimulated cell proliferation of a human urothelial cell line (28).
B) NICOTINIC RECEPTORS.
The nicotinic ACh receptor family is currently known to consist of at least 17 different subunits (α1–10, β1–4, γ, δ, and ε) (184, 197). Functional nicotinic ACh receptors (nAChRs) are composed of homo- or heteropentamers of either α-subunits alone or α- and β-subunits. Subtype composition determines receptor characteristics such as preferred ligand affinity and cation permeability. Beckel et al. (35) studied the expression of nAChRs in the rat urothelium, and whether activation of these receptors can alter voiding reflexes. They found that mRNA for the α3, α5, α7, β3, and β4 nicotinic subunits could be identified using RT-PCR. Western blotting also confirmed urothelial expression of the α3- and α7-subunits. In the mouse urothelium, Zarghooni et al. (306), also using RT-PCR, detected mRNAs coding for the nicotinic receptor subunits α2, α4, α5, α6, α7, α9, and 10, whereas the α3-subunit was not expressed. By immunohistochemistry, they localized subunit α9 exclusively in the umbrella cells, whereas the subunits α4, α7, and α10 were also detected in the intermediate and basal cell layers. The α-subunit was localized only in the basal cell layer. Beckel et al. (35) showed that the nAChRs mediated inhibition of bladder reflexes through α7 receptors and excitation of bladder reflexes through α3-containing (α3*) receptors. They also examined the effects of intravesical nicotine before and after disrupting the urothelial barrier with protamine sulfate that may allow intravesically administered agents to pass into the underlying tissue. Before protamine sulfate treatment, nicotine had an inhibitory effect, increasing the interval between voiding contractions. When administered after protamine sulfate treatment, nicotine had the opposite effect, exciting the bladder reflex and decreasing the voiding intervals. Beckel et al. (35) suggested that after breaking the urothelial barrier, nicotine acted on suburothelial targets, such as afferent nerve terminals, while nicotine infusion without protamine sulfate treatment activated receptors in the urothelium.
Further studies by Beckel and Birder (34) showed that stimulation of different nicotinic receptors on the rat urothelium modulated ATP release in a reciprocal manner, with α7 stimulation inhibiting ATP release and α3* stimulation decreasing or increasing basal ATP release, depending on agonist concentration. The mechanism for these effects was suggested to involve distinct calcium-dependent intracellular signaling pathways: α7 through a ryanodine-sensitive intracellular mechanism and α3* through extracellular influx.
6. Adrenergic receptors
A) α-ADRENOCEPTORS.
Expression of α1-AR mRNAs in the rat urothelium has been reported (144, 302), and the protein was demonstrated by immunohistochemistry. Interestingly, Walden et al. (292) found that the bladder urothelium contained high levels of α1A-AR mRNA, but undetectable levels of α1A-AR protein. Thus the α1A-AR mRNA appeared to be transcribed, but not translated. Functionally, Ishihama et al. (144) demonstrated the presence of α1D-ARs in the rat urothelium and suggested that activation of these ARs by endogenous catecholamines can facilitate the micturition reflex. If catecholamines are released from nerves close to the urothelium or from perivascular nerves, such facilitation may be achieved through release of mediators such as ATP (38). Kurizaki et al. (171) investigated the possible relationship between the bladder mucosal expression of α1-ARs mRNAs, storage symptoms, and urodynamic findings in patients with LUTS and benign prostatic obstruction (BPO). They found a relationship between the expression of α1D-AR mRNA in the bladder mucosa and storage-phase urodynamics in LUTS/BPO patients, suggesting a role of α1D-ARs in bladder sensation. Whether or not such effects contribute to the clinical efficacy of α1-AR antagonists remains to be established.
B) β-ADRENOCEPTORS.
Tyagi et al. (283) demonstrated the presence of mRNA for β1-, β2-, and β3-ARs in the human urothelium and showed that the β3-AR protein was functional. Other studies have shown the expression of β3-ARs in the urothelium of different species including human (183, 224), rat (165, 166), and pig (201). In the human bladder wall, immunostainings for all three β-AR subtypes were found not only in the urothelium and detrusor, but also in suburothelial myofibroblast-like cells, intramural ganglion cells, and Schwann cells of intramural nerves. Stainings for all receptor subtypes were more prominent in the urothelium than in the detrusor (183).
Birder et al. (42) showed that β-AR stimulation (isoproterenol, dobutamine, terbutaline) activated the adenylyl cyclase pathway in bladder epithelial cells and initiated an increase in intracellular Ca2+ that triggered NO production and release. It has been suggested that β-AR agonists, possibly via urothelial β3-ARs (201), might stimulate the urothelial release of an unidentified factor that inhibits detrusor contractility directly or indirectly (213). In addition, systemic activation of β3-ARs using the selective agonist CL316,243 inhibited the mechanosensitive Aδ afferent fiber activity and PGE2-induced C-fiber hyperactivity (5). Kurizaki et al. (172) determined the bladder mucosal expression level of β3-AR mRNA in BPO patients with LUTS and correlated it with urodynamic parameters, such as bladder capacity, DO, and the degree of BOO. The expression of bladder mucosal β3-AR mRNA was significantly decreased in patients with severe BOO, suggesting that β3-ARs might be affected by the degree of BOO. However, the functional importance of urothelial β3-ARs remains to be established.
8. Estrogen receptors
The effects of estrogen hormones are mediated primarily through binding to the two major estrogen receptors (ER) subtypes, ERα and ERβ, which are considered to be ligand-activated transcription factors. These receptors are expressed in the human urothelium (48, 273, 277), but details on their mechanisms of action are incomplete. Ligand-bound receptors translocate to the nucleus to act as transcription factors regulating gene expression. Rapid, non-genomic effects of estrogen have been reported. Such effects may be mediated via ERs or unrelated estrogen binding sites in or near the plasma membrane through activation of signaling pathways (e.g., mitogen-activated protein kinase) or through interaction with various growth factors (274).
Tincello et al. (277) reported that nuclear ERα immunoreactivity was present in squamous epithelium, but absent from transitional epithelium. ERβ immunoreactivity was expressed in both squamous and transitional cell epithelium. They suggested that ERα and ERβ genes are transcribed in human urothelial tissue, but only ERβ is translated into protein, implying that the urothelium responds to endogenous estrogen via ERβ. Tincello et al. (277) also suggested that estrogen (endogenous and exogenous) acts on the bladder by modulating the urothelial sensory functions, influencing sensations of bladder filling, with secondary indirect effects on the bladder motor functions, and that ERβ receptor is the mediator of this effect. It is well established that ERs mediate proliferation of epithelia, including human urothelial cells (31, 272, 274). This effect seems to involve stimulation of NGF synthesis, and thus the proliferative effect of estrogen may, at least partially, be mediated by NGF (272).
9. Transporters
Recent interest has developed in terms of membrane transporters expressed in a number of cell types including that of the epithelium. In the urinary bladder, active transporters for substances, such as urea, would prevent access of this urinary solute to the underlying tissues. Urothelial cells also express transporters for glucose (i.e., Glut1) (240), which is observed in the majority of urothelial carcinomas, and alterations in expression or function could contribute to bladder injury and dysfunction in conditions such as diabetes. The release of transmitters such as ATP from urothelial cells with physical or chemical stimuli occurs when ATP-containing vesicles traffic and fuse to the plasma membrane. It has been hypothesized that ATP channels and transporters (including a vesicular transporter termed VNUT) can uptake nucleotides and store/secrete ATP (145). In contrast, the expression of vesicular ACh transporter (VAChT) and corresponding release of ACh via vesicular exocytosis has not been demonstrated in many types of cholinergic epithelial cell types. Instead, these cells (including the uroepithelium) express polyspecific organic cation transporters (OCT1–3) which perform a number of functions including bidirectional ACh transport (185). OCTs also perform additional functions including translocation of choline (necessary for synthesis of ACh as well as membrane components) across the plasma membrane as well as elimination of endogenous amines, and are involved in active transport of a number of drugs including antimuscarinics, used to treat overactive bladder symptoms. Thus many membrane transporters (such as the ATP binding cassette and solute carrier superfamilies) can perform key roles in the therapeutic efficacy of drugs as well as in adverse drug reactions. These transporters can work together with drug-metabolizing enzymes in terms of absorption and elimination of drugs. Although there is a need for further research in this area, one might speculate that defects in expression or function of these membrane proteins in the urinary bladder could play a role in a number of disorders impacting sensory function.
IV. CHANGES IN BLADDER DISORDERS
A. Bladder Pain Syndrome
A hallmark of chronic visceral pain syndromes, including BPS/IC, is pain excluding other demonstrable visceral pathology. At present, there is no known etiology nor treatments that can effectively diminish or abolish the symptoms. The diagnosis of BPS/IC is based on symptom criteria which include suprapubic pain associated with bladder filling often accompanied by a strong desire to void and increased frequency of urination (52, 62, 200). In addition, overlapping or chronic conditions (such as OAB) often share a number of similar features (such as urgency and suprapubic pressure) (195).
Investigators have used a wide array of animal models including administration of an irritant or immune stimulant to healthy rodents (295) to study mechanisms or pathways that could plan a role in the pathogenesis or symptom progression in BPS/IC. For example, ERβ (female) null mice exhibit urinary bladder alterations that have also been associated with BPS/IC in humans(143). There is also a naturally occurring disease in cats termed feline interstitial cystitis (FIC), which shares nearly all the characteristics of the nonulcerative form of BPS/IC found in humans (53, 295). Although such animal models are widely used and provide necessary and valuable mechanistic information, there are a number of limitations. This may include the inability of many “subchronic” models of bladder injury or inflammation to model the symptoms of this chronic syndrome that involve significant plasticity in receptors, ion channels, mediators, and cell types (53, 296).
BPS/IC has often been described as a disease of the urothelium (121). Ultrastructurally, an altered vascular supply is observed in its ulcerative form with locations of moderate-to-severe redness, interspersed among a whitish discoloration. There is also evidence that the urothelium in BPS/IC is associated with altered synthesis of a number of proteins including those involved in cellular differentiation, barrier function, and bacterial defense mechanisms. In this regard, studies have shown in patients diagnosed with the ulcerative form of IC, that laser removal of damaged urothelium is associated with reduction of symptoms of bladder or pelvic pain (242). This treatment stimulates a rapid urothelial turnover, and patients undergoing this treatment report a prolonged period without pain (6–12 mo) after therapy. In terms of barrier “repair,” instillation of liposomes composed of phospholipids has been shown to support repair of the urothelial barrier in animals following bladder irritation (154, 282). Although the mechanism is not well defined, by forming a protective coating on the urothelium, liposomes may act as a mucosal protective agent and thereby decrease irritation of underlying afferent nerves (FIGURE 5). In this regard, use of intravesical liposomes on a single subject with ulcerative BPS/IC has shown promise to repair and enhance the barrier function of a dysfunctional urothelium, although further trials are needed to fully assess this type of treatment (237).
As mentioned previously, the urothelium is likely to play an important role by actively communicating with bladder nerves, smooth muscle cells, or even cells belonging to the immune and inflammatory systems. Altered expression or sensitivity of molecular targets such as TRPV1, acid-sensing channels, and muscarinic receptors have been reported in BPS/IC patients as well as in animal models for the syndrome (82, 124, 140, 249). In addition, augmented release of transmitters, most notably ATP, from the urothelium can lead to painful sensations by excitation of purinergic receptors on sensory fibers both peripherally and centrally (43, 56, 267). Thus inhibition of purinergic P2X3 receptors has been shown to be effective in suppressing afferent excitation in various animal models and may be effective in clinical conditions associated with pain such as BPS/IC (101, 113). Onabotulinum toxin A (BoNT-A) has been used in the treatment of lower urinary tract disorders including BPS/IC and appears to have a positive therapeutic effect (64). By inhibiting SNARE-dependent exocytotic processes, BoNT-A can prevent the release of transmitters (such as ATP) as well as normalizing the expression of various receptors, channels, and trophic factors (188). Preliminary studies using immunoblotting indicate expression of the SNARE proteins SNAP23 and SNAP25, as well as the high-affinity binding site SV2, in both rodent and human mucosa (114). These and other studies suggest that the urothelium may be a target for this treatment and that urothelial-released mediators may contribute to sensory urgency/pain.
Studies support a role for stress in the exacerbation and very likely the development of a number of LUT disorders including OAB and BPS/IC. For example, rats exposed to various types of stress (water avoidance, intruder stress) exhibit symptoms of bladder dysfunction including increased micturition frequency as well as anxiety-like behavior (257, 299). Furthermore, an exaggerated acoustic startle response has been demonstrated in both cats diagnosed with FIC as well as in BPS/IC patients (279, 296). This response is a brain stem reflex responding to unexpected loud stimuli and parallels that of autonomic control. The underlying assumption in most persistent pain syndromes is that changes occur in both the target organ as well as central and peripheral pain processing pathways. Recent studies have focussed on the biological basis for mind-body interactions and have identified both structural and functional changes in the brain of chronic pain patients that could influence the body and cause disease (202, 203).
There is a great deal of interest in identifying a “biomarker” that could be of value in the diagnosis (and not just predictive of symptom progression) for BPS/IC. A range of factors have been studied including APF, EGF, insulin-like growth factor I (IGF-I), insulin-like growth factor binding protein 3 (IGFBP3), as well as urinary chemokines and have been shown to be correlated to BPS/IC. While some reports suggest urinary markers (e.g., APF, HB-EGF; EGF) may be useful to discriminate BPS/IC and asymptomatic controls (156, 307), these may not correlate with findings using bladder biopsies (94). The reason in part may be due to variability in biopsy location, depth of sample (containing different cell types), as well as other technical variations. There is some suggestion that there seems to be more of a proinflammatory state with increased infiltration of mast cells in BPS/IC patients compared with controls. In addition, increased levels of NGF in urine and tissue have been linked with bladder pathologies including patients with idiopathic “sensory” urgency (urgency without incontinence), overactivity, and BPS/IC (170, 188). Studies in cats diagnosed with FIC have reported increased NGF levels in bladder urothelium (44), and a major source of NGF has been shown to come from urothelium, which may contribute to increased neural excitability and emergence of bladder pain in BPS/IC (206).
2. Neurogenic injury
LUT dysfunction and complications are a major concern in the management of spinal cord injured (SCI) patients. While C-fiber afferents have a role in development of hyperactivity in SCI-neurogenic and idiopathic detrusor overactivity (NDO, IDO), there is support for the involvement of the urothelium in the development and symptoms of NDO and IDO. For example, antimuscarinics, intravesical vanilloids and also botulinum toxin have been shown to restore urothelial-muscarinic receptor expression to control levels, and these findings also correlated with improvement of patient symptoms (81). There is also evidence in SCI rats that NGF (both mRNA and protein levels) are increased in bladder (261). NGF, which is elevated in patients with DO (187), mediates a number of processes (via activation of multiple signal transduction pathways), including epithelial signaling, cell growth, proliferation, and differentiation (9, 177, 260). In patients with NDO, there is a corresponding increase in NGF protein that is decreased or normalized with treatment with BoNT-A (115). As NGF has been shown to increase expression of a number of ion channels including TRPV1, it is not surprising that patients with neurogenic bladders exhibit increased urothelial-TRPV1 expression (82). Changes in TRPV1 expression were found to be normalized in patients responding favorably to intravesical vanilloid therapy (23). Increases in TRPV1 expression and/or sensitization may play a role in mechanical changes including allodynia as reported in models for peripheral nerve injury.
Another characteristic change observed in many types of epithelia following injury, and in the urothelium following SCI, is an early degeneration followed by regeneration. There is limited understanding of tight junction assembly or turnover, however, when the urothelium is injured by chemical/mechanical denuding, transection of the spinal cord, or exposure to uropathogenic Escherichia coli, it undergoes rapid regeneration. Epithelial integrity is maintained through a complex process of proliferation and differentiation. During a regenerative process, studies suggest that proliferation takes precedence over differentiation. In rodents, it has been shown that 2 h after experimental transection of the spinal cord, there is a significant disruption of the urothelial barrier with accompanying permeability and ultrastructural changes (23). These alterations are prevented by pretreatment with ganglionic blockers, suggesting that bladder nerves play a role in acute response of urothelial cells to injury. In SCI patients there is evidence that impairment of voluntary voiding may correlate with increased metaplasia and/or diminished urothelial differentiation (286). Changes in epithelial proliferation or metaplasia may be due to altered expression of molecules such as parathyroid hormone-related protein (PTHrP), which is a secretory factor that can act in autocrine, paracrine, as well as intracrine manners in a number of tissues.(178) In SCI patients, PTHrP is significantly elevated in mucosal biopsies, suggesting a link between its growth-promoting properties and changes within the urothelium in SCI (287).
Histological changes to the bladder mucosa have been also linked with chronic indwelling and/or suprapubic catheters in SCI patients (285). This may not be surprising if one compares these findings to those observed in SCI patients, who can achieve more complete, low pressure emptying of the bladder (regular intermittent catheterization or other means). The changes reported include inflammatory changes within the bladder mucosa as well as within the urethral epithelium. There seems to be no apparent difference between the urothelium of the trigone compared with the detrusor, in contrast to cells from the proximal urethra. In this region, there is a transition from urothelium to a stratified or columnar epithelium with evidence for peptide/amine-producing endocrine cells distributed within the urethral epithelium (126), and it has been suggested that both epithelial and endocrine cells in the urethra may serve as sensors for chemical as well as mechanical signals. It is possible that interactions between nonneuronal cells and afferents, including that of the recently identified flow responsive afferents within the urethra, may be important in functional and structural changes occurring following spinal cord injury (157, 259).
Spinal cord injury has been associated with a number of changes to the urothelium including both structural changes in addition to defects in urothelial signaling. Studies in SCI animals have revealed that factors released from the mucosal layer can influence spontaneous detrusor contractions (107, 142). This activity may involve a number of factors including release of ATP from urothelium acting on underlying cells within the suburothelial space. In this regard, enhanced release of ATP from the urothelium of biopsies taken from patients with DO (neurogenic as well as idiopathic) may play a role in changes in cell signaling within the bladder wall (168). In addition, chronic SCI animals (and in patients with urge symptoms) show an increase in connexin-hemichannel expression and increased intercellular communication within LUT compared with normal controls (141, 215). These findings suggest that altered LUT-junctional proteins, perhaps by contributing to amplification of chemical signaling between and within LUT cells, may play a role in the development of DO following SCI. Urothelial communication is not unidirectional, as input from bladder nerves or underlying cells can influence LUT structure and function.
3. Bladder overactivity
Several factors may contribute to the genesis of detrusor overactivity (DO) and associated storage LUTS, including the OAB syndrome; these clearly include myogenic and neurogenic factors, but a contribution of the urothelium is also likely (14, 245). Three theories, the myogenic, neurogenic, and urotheliogenic, each of which probably contributes in varying proportion to the complex mechanisms underlying the genesis of DO and the OAB syndrome, have been put forward and are discussed in detail elsewhere (245).
According to the urothelium-based hypothesis, changes in urothelial receptor function and neurotransmitter release, as well as changes in the sensitivity and coupling of the suburothelial ICs, may lead to enhancement of involuntary bladder contractions (12, 38). The suburothelial afferent nerve plexus that lies immediately beneath the urothelium is particularly dense in the bladder neck and the trigone. The urothelium interacts closely with underlying structures, e.g., the ICs and afferent nerves, and together these structures can be regarded to work as a functional unit. It is likely that a cascade of inhibitory and stimulatory transmitters/mediators are involved in the transduction mechanisms activating afferent fibers during bladder filling (12).
The urothelium seems to be a site with abundant expression of both NGF and its receptors, and NGF has been found to be elevated in the bladders and urine of patients with various types of storage LUTS, including BOO due to benign prostate hyperplasia (BPH), diabetic cystopathy, NDO (often caused by spinal cord injury), and IDO (with no apparent underlying pathology), BPS/IC, and also in models of bladder inflammation in animals (187, 221). Bladder NGF lowers the threshold for bladder TRPV1 signaling, and TRPV1 is essential for NGF-driven bladder dysfunction (105). NGF may well be involved in the pathogenesis of OAB syndrome/DO, although its precise role appears to be dependent on the specific underlying condition (216).
There seem to be other, thus far unidentified, factors in the urothelium that could influence bladder function (103, 128, 271). Thus muscarinic receptor stimulation was found to release a previously unrecognized nonadrenergic, nonprostanoid inhibitory factor from the rat urinary bladder (103). It could not be determined whether the factor was released from the urothelium or the detrusor or from both tissues (103). However, other investigators reported that a diffusible, nonidentified inhibitory factor appearing to be neither NO, a cyclooxygenase product, a catecholamine, adenosine, GABA, nor any substance sensitive to apamin, was released from the urothelium (128). Whether or not the release and/or effects of the unidentified factor(s) involves TRP channels remains to be established.
Ikeda and Kanai (142), using optical mapping approaches, examined the modulation of intrinsic (i.e., spontaneous) detrusor contractions by the mucosa in normal adult and spinal cord transected (SCT) rat bladders. Normal bladders showed low-amplitude, high-frequency contractions with disorganized patterns of activity, whereas the contractions in bladders from SCT animals had high amplitude and low frequency and displayed an organized spread of membrane potential and intracellular Ca2+. An urotheliogenic origin for intrinsic activity was suggested, where structures within the mucosal layer organize and thereby enhance intrinsic detrusor contractions (142).
It is obvious that mechanisms that can influence the generation and release of factors from the urothelium can affect afferent nerve activity, and also may be interesting targets for drugs aiming to control sensory and motor activity of the bladder. The TRP superfamily of channels may be involved in such mechanisms, and there is good evidence, both morphological and functional, for such an involvement, but information is still incomplete.
V. TREATMENTS TARGETING THE MUCOSA
The GAG layer has been suggested to be impaired, disrupted, or abnormal in some patients with BPS/IC. When damaged, the urothelium may become permeable to urine components, which may result in local inflammation, neural sensitization, and subsequent pain, frequency, and urgency (208). Important components of the GAG layer include chondroitin sulfate, hyaluronic acid, heparin sulfate, dermatan sulfate, and keratin sulfate (135), and some agents with GAG-like properties, such as sodium hyaluronate, heparin, and pentosan polysulfate, have been administered intravesically to IC/BPS patients with variable results (87, 208, 233). In rats, hyaluronic acid was shown to reduce NGF production and bladder overactivity in a cyclophosphamide (CYP)-induced cystitis rat model (133).
A. Pentosan Polysulfate/Chondroitin Sulfate
Pentosan polysulfate is a semi-synthetic, sulfated polysaccharide similar to heparin and resembles the GAGs covering the urothelium. The drug has anticoagulant and fibrinolytic properties. However, its exact mechanism of action has not been established. It has been assumed that pentosan polysulfate could replace damaged parts of the urothelial GAG layer, thereby preventing solutes from coming into contact with the underlying structures (232). In rats, pretreatment with pentosan polysulfate was shown to reduce the damage done by the acrolein, the metabolite of cyclophosphamide, on urothelial cells in the bladder of female rats (152). The drug has also been reported to reduce bladder epithelial permeability in humans. On the basis of results of the potassium sensitivity test (233), it was suggested that pentosan polysulfate can reduce urothelial permeability in humans. The drug may also have cytoprotective effects, resulting in a reduction in inflammation of the bladder mucosa. An in vitro study of human uroepithelial cells (248) showed that the nuclear factor-κB, a nuclear transcription factor that mediates the inflammatory response and which is activated in chronic inflammatory diseases, was inhibited by pentosan polysulfate. In vitro, the drug appeared to dose-dependently inhibit stimulation of connective tissue and mucosal mast cells (73, 74).
Chondroitin sulfate is a structural component particularly important for bladder mucosal integrity. The effects of this agent were investigated and compared with placebo in two prospective, randomized, double-blind studies in patients with BPS/IC receiving weekly instillations of active drug or placebo (216, 217). In the first study, which included 65 patients (33 treated with chondroitin sulfate), the difference in treatment effect between placebo and active agent was not statistically significant. However, twice as many patients reported a clinically significant benefit with intravesical chondroitin sulfate compared with placebo. It was recognized that the study was underpowered, but still considered to provide data required to design a well-powered randomized vehicle-controlled trial. In the second study, minor improvements in BPS /IC-related symptom and pain were observed, but compared with placebo, there were no significant differences. The authors concluded that the magnitude of benefit in the study did not support the use of chondroitin sulfate as monotherapy for BPS/IC.
B. Liposomes
As mentioned previously, liposomes are spherical vesicles consisting of an aqueous core enclosed in one or more phospholipid layers, that can be loaded with a great variety of factors, such as small drug molecules, proteins, nucleotides, and even plasmids. However, even empty liposomes have shown promise to repair and enhance the barrier function of a dysfunctional urothelium (154, 237, 281). The effect of empty liposomes was assessed using a rat model for urothelial damage/injury (protamine sulfate) followed by intravesical instillation with KCl that resulted in bladder hyperactivity. Treatment with empty liposomes was found to partially reverse the irritation-induced increase in bladder contractions (104). These findings provide evidence that liposomes may restore the damaged or disrupted urothelial layer that may occur following infection or inflammation.
C. Capsaicin and RTX
Demonstration of the TPRV1 receptor in the urinary tract was initially demonstrated using radioactive RTX (270), and its expression in urothelial cells and afferent nerves was confirmed by immunohistochemistry (39). Despite extensive information on its morphology and function in animal models, the role of the TRPV1 channel in normal human bladder function is still controversial. On the other hand, its role in the pathophysiology and treatment of particularly neurogenic DO (NDO) is well established (17, 96).
The clinical application of TRPV1 agonists has been extensively reviewed elsewhere (17, 96). Maggi et al. (196), instilling capsaicin intravesically to patients with bladder hypersensitivity disorders, reported the drug to produce a concentration-related reduction of the first desire to void, bladder capacity, and pressure threshold for micturition, suggesting the occurrence of capsaicin-sensitive structures in the human bladder.
The C-fiber initiated micturition reflex is usually inactive, but can be enhanced in patients with chronic spinal cord lesions above sacral segments (84), in those with chronic bladder outlet obstruction (61), and in those with IDO (255). In NDO patients, the enhancement of the micturition reflex was accompanied by an increase in the number of suburothelial C-fibers expressing TRPV1 (49). The bladder lamina propria of NDO patients, who responded to intravesical RTX, exhibited a significant decrease in the density of TRPV1 immunoreactive fibers, compared with nonresponders (49). A decrease in TRPV1 expression in urothelial cells of NDO patients was also demonstrated after intravesical application of RTX (23, 24).
Changes in suburothelial C-fibers expressing neuropeptides (256) or TRPV1 (189) were also reported in patients with “sensory” urgency (urgency without incontinence or DO). In IDO patients, the response to intravesical RTX was closely associated with overexpression of TRPV1 in the bladder mucosa (189). Urothelial TRPV1 expression and sensitivity were increased in patients with IDO (45). In women with sensory urgency, TRPV1 mRNA expressed in trigonal mucosa was not only increased, but also inversely correlated with the bladder volume at first sensation of filling during cystometry, further indicating that TRPV1 plays a role in premature bladder sensation (191).
D. Botulinum Toxin
BoNT-A has been shown to inhibit contraction of both smooth and skeletal muscle by blocking neurotransmitter release. Thus animal studies have shown BoNT-A can decrease transmitter release from the urothelium (258) and neuropeptide release (193) from bladder afferents. BoNT-A does not affect the spontaneous intrinsic contractions of the detrusor (245). There is evidence that the toxin can inhibit bladder sensory pathways contributing to storage symptoms. Studies in patients with IDO revealed that BoNT-A injections were not associated with any inflammatory or fibrotic changes within the urothelium or suburothelium (25). Thus the effects on suburothelial afferent nerves may contribute to the beneficial aspect of BoNT-A treatment even in NDO patients, where the main goal is to reduce the contractility of the bladder.
E. NGF Antibodies
The urothelium is a prominent site of NGF expression in several species (221). Abundant NGF staining was reported in the urothelium of normal rats with a weaker signal in detrusor muscle. A similar pattern was observed in mice. In humans, a strong urothelial NGF expression was demonstrated in women with idiopathic sensory urgency and IC (192). Bladder biopsies from patients without LUTS expressed NGF in the apical urothelium (188). Also the receptors for NGF (trkA and p75 NTR) have been demonstrated in the urothelium from animals and humans (221). In the rat, Dmitrieva and McMahon (88) reported that acute intravesical NGF application induced rapid and enduring sensitization of pelvic C and Aδ afferents to mechanical stimuli. Thus the urothelium seems to be a site with abundant expression of both NGF and its receptors and could be expected to be a reasonable target for treatment of bladder pain and hyperactivity. Biologic antibodies (tanezumab) to block the NGF effect have been tried in a proof of concept study in patients with BPS/IC with some success (95). However, because of adverse effects reported after tanezumab treatment in other type studies (pain treatment), further testing has not been continued.
Although it has been suggested that analyses of NGF in urine might be useful for diagnosis and monitoring of treatment effects in patients with the OAB syndrome (170, 190, 192), this is an open question. OAB is a clinical diagnosis, which can be made with or without NGF analysis, and responses to treatment are based on a clinical assessment. Not until the urine levels of NGF can be linked to specific pathophysiological changes (which does not seem likely), such analyses may give useful information beyond what can be obtained by clinical evaluation.
F. Muscarinic Receptor Antagonists
In the mouse and human urothelium, ACh was shown to be present in a nanomolar range per gram of wet weight, and RT-PCR and immunohistochemistry data indicated the presence of the ACh generating enzyme carnitine acetyltransferase, but not choline acetyltransferase (185). The vesicular ACh transporter, used by neurons to transport ACh into synaptic vesicles, was detected in suburothelial cholinergic nerve fibers, but not in the urothelium, and it was concluded that the urothelial nonneuronal cholinergic system differs from that of neurons with respect to molecular components of ACh synthesis and release machinery (125, 185). Nevertheless, the urothelium is a source of ACh, and muscarinic receptors within the urothelium and underlying structures can be targets for antimuscarinic drugs.
All the muscarinic receptor subtypes are found in the urothelium and suburothelium, with predominant expression of M2, M3 receptors (11, 15, 51). The density of M2 and M3 receptors in the LP were found to be increased in the bladders of patient with IDO and BPS/IC, and there was a correlation between suburothelial muscarinic receptor density and urgency scores (211).
In a rat model, antimuscarinics have been shown to decrease the afferent activity in both Aδ and C-fibers initiated by filling of the bladder (85, 86, 139). The muscarinic receptors in the mucosa can be affected by antimuscarinics via the bloodstream, but some antimuscarinics and their active metabolites are excreted in urine in amounts that may affect the mucosal muscarinic receptors from the luminal side (16). However, it has not been established to what extent effects on the mucosal signaling pathway contribute to the therapeutic efficacy of the clinically used antimuscarinics.
G. P2X3-Receptor Antagonists
Sensory nerve fibers expressing P2X3 immunoreactivity have been found projecting into the LP, urothelium, and detrusor smooth muscle (78, 93, 176, 186, 291, 293, 303), suggesting a role for P2X3-containing receptors in regulating bladder sensory functions. As discussed previously, ATP is released from the urothelium in response to bladder distension (99, 291, 293), and these findings can be mimicked in isolated urothelial cell cultures (37, 268). Studies using isolated bladder-pelvic nerve preparations from rats (214) or mice (244, 291) have shown that distension also leads to increased afferent nerve activity. Such activity can be mimicked by ATP and/or α,β-MeATP, and intravesical instillation of these drugs can also produce bladder overactivity in conscious rats, sensitive to blockade with TNP-ATP (226). When given intravesically, purinergic receptor antagonists, such as suramin or PPADS, can inhibit nonvoiding bladder contractions in BOO rats, and increase bladder capacity in normal conscious animals (290).
The importance of homomultimeric P2X3 and heteromultimeric P2X2/3 receptors in regulating bladder reflex excitability has been demonstrated in P2X3- and P2X2-deficient mice (77). In anesthetized P2X3, P2X2, and P2X2/P2X3 double knockout animals, the reflexes in response to bladder filling were shown to be reduced and the volume threshold for activation increased, despite normal levels of distension-evoked ATP release from the urothelium. These data suggest that P2X3 and P2X2/3 receptors are important in sensing volume changes during normal bladder filling. They may also participate in lowering the threshold for C-fiber activation under pathophysiological conditions, making them promising targets for therapeutic interventions. In several animal models, selective dual P2X3, P2X2/3 antagonists have been shown to be effective at reducing nocifensive responses, hyperalgesia, and allodynia (150, 151). New drugs are in development (101, 102, 113), but so far no clinical experiences have been published.
H. EP1-Receptor Antagonists
There is evidence to suggest that PGE2 through afferent pathway signaling is involved in the control of bladder function in both the normal physiological state and under pathological conditions (15). In humans, intravesical injection of PGE2 produces DO, urgency, and a decrease in bladder capacity (253). Urinary levels of PGE2 have also been shown to be increased in patients with OAB compared with controls (2, 159). PGE2 acts via stimulation of EP receptors (EP-1 to -4), and the EP-1 receptor is expressed in both urothelium and suburothelial cells. Using EP1−/− mice, Schroder et al. (252) showed that the EP1 receptor appeared to have a role in the development of DO caused by PGE2 and BOO (252). In addition, in BOO rats, the EP1 receptor antagonist PF-2907617-02 was shown to increase bladder capacity, micturition volume, and the micturition interval (177a). Antagonism of the EP1 receptor was therefore assumed to provide symptomatic relief in OAB patients, and the principle was investigated as a potential treatment of these patients. In a study in cynomolgus monkeys, the selective EP-1 receptor antagonist ONO-8539 was found to significantly increase bladder capacity without increasing residual volume. However, when this drug was evaluated in a phase 2 clinical trial in OAB patients, no beneficial effects were found (65).
I. TRP Channel Antagonists
As mentioned previously, several TRP channels are expressed in the bladder and may act as sensors of stretch and/or chemical irritation (17, 26, 27, 96). TRPV1 is present and active both in the urothelium and in the nerve fibers of several species including humans (69). TRPV4 was initially described in the urothelium of rodents and humans (146). Coexpression of the two receptors was observed in 20% of rat urothelial cells (167). TRPV4 may also be expressed in bladder afferents; ∼30% of L6 dorsal root ganglia neurons that project to the urinary bladder coexpressed TRPV1 and TRPV4 (57, 66).
Several antagonists of TRP channels have been tested in animal models of bladder overactivity. The TRPV1 antagonists (GRC 6211) did not show any relevant effect on bladder activity of intact rodents (68), but were effective in animals with induced cystitis. Cystometric studies showed that TRPV4−/− mice had a marked increase in the intercontraction interval compared with wild-type (WT) littermates, and were shown to be incontinent, most probably due to incomplete bladder emptying (112). The TRPV4 antagonists (HC-067047) decreased the frequency of bladder contractions and increased the intercontraction interval (97). These observations indicated that TRPV4 may have a role in the control of normal micturition. TRPV1 or TRPV4 seems to have a role in the increase of micturition frequency associated with cystitis (67, 97). Blockade of TRPV1 and TRPV4 with selective antagonists confirms the observations carried out in knockout animals. A problem with some of these antagonists (238) is their adverse effect profile, TRPV1 antagonists producing hyperthermia and increased risk of cardiac ischemia, and TRPV4 antagonists precipitating urinary retention and overflow incontinence (112).
The roles of the various members of the TRP superfamily in normal LUT function and in various types of LUT disease need to be further studied. Based on preclinical results, use of TRP channel antagonists for treatment of LUTS seems to be a promising approach, but positive proof of concept studies in humans is still lacking.
VI. SUMMARY AND FUTURE RESEARCH DIRECTIONS
The bladder mucosa, consisting of the urothelium, basement membrane, and underlying lamina propria (the sensory web), works in concert with other components in the bladder wall. The urothelium not only forms as a highly efficient barrier to potentially harmful urine components, but also exhibits properties similar to those of nociceptive and mechanoceptive afferent neurons. Activation of the urothelial cells by chemical, thermal, or mechanical stimuli can evoke the release of various mediators or neurotransmitters that through the sensory web can influence nerve activity, detrusor cell contraction, and ultimately bladder function. The lamina propria with its several types of interstitial cells and afferent nerves may act as a coordination center for bladder activity both during bladder filling (spontaneous activity) and for initiation of the micturition reflex, thus having an important integrative role in, e.g., signal transduction to the central nervous system (nociception, mechanosensation). The mucosa undergoes important changes in many bladder diseases, e.g., bladder pain syndrome, neurological injuries, and bladder overactivity, and is an important target for treatment.
Even if the information on mucosal signaling mechanisms has accumulated during the last decade, there are several areas that need to be further explored. The role of the various types of interstitial cells of the lamina propria have not yet been established, and further research would be rewarding. We need to determine how representative for specific bladder disorders are acquired samples of normal and diseased human bladder and factors collected from urine. With such knowledge, factors produced by the bladder mucosa can be used as biomarkers for specific diseases and may be useful both for both diagnostic purposes and for monitoring treatment effects. Several aspects of urothelial properties, including gender differences, influence of age, hormone status, and common diseases, have not been fully explored. Pharmacological interventions aimed at targeting urothelial receptor/ion channel expression or release mechanisms may provide new strategies for the clinical management of bladder disorders. For example, intravesical administration of liposomes may prove useful to improve barrier function in bladder pathologies such as BPS/IC and as carriers of active compounds. TRP channels as well as their role in different bladders dysfunctions and as targets for treatment deserve further attention.
We have begun to understand the fundamental properties of the bladder mucosa. However, bladder mucosal biology, including signaling mechanisms, is a rapidly expanding research field with exciting translational possibilities, and considerable progress in this area can be anticipated.
GRANTS
This work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grants R01 DK57284, R37 DK54824, and P50 DK64539.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: L. A. Birder, Univ. of Pittsburgh School of Medicine, A 1217 Scaife Hall, 3550 Terrace St., Pittsburgh, PA 15261 (e-mail: lbirder@pitt.edu).
REFERENCES
- 1. Abraham P, Rabi S, Selvakumar D. Protective effect of aminoguanidine against oxidative stress and bladder injury in cyclophosphamide-induced hemorrhagic cystitis in rat. Cell Biochem Funct 27: 56–62, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Abrams P, Amarenco G, Haab F. Urinary prostaglandin E2 levels are elevated in patients with overactive bladder and painful bladder syndrome and correlate with bladder diary symptoms. EAU Abstr 783: 2010 [Google Scholar]
- 3. Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol 287: F305–F318, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Aitken KJ, Bagli DJ. The bladder extracellular matrix. Part I: architecture, development and disease. Nat Rev Urol 6: 596–611, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Aizawa N, Igawa Y, Nishizawa O, Wyndaele JJ. Effects of CL316,243, a beta 3 adrenoceptor agonist, and intravesical prostaglandin E2 on the primary bladder afferent activity of the rat. Neurourol Urodyn 29: 771–776, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Aizawa N, Igawa Y, Nishizawa O, Wyndaele JJ. Effects of nitric oxide on the primary bladder afferent activities of the rat with and without intravesicle acrolein treatment. Eur Urol 59: 264–271, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Aizawa N, Wyndaele JJ, Homma Y, Igawa Y. Effects of TRPV4 cation channel activation on the primary bladder afferent activities of the rat. Neurourol Urodyn 31: 148–155, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Al-Motabagani MA. Age-related changes in the urinary bladder of the female albino rats. Int J Morphol 23: 309–316, 2005 [Google Scholar]
- 9. Aloe L, Tirassa P, Lambiase A. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers. Pharmacol Res 57: 2008 [DOI] [PubMed] [Google Scholar]
- 10. Anderson G, Palermo J, Schilling J, Roth R, Heuser J, Hultgren S. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301: 105–107, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Andersson KE. Antimuscarinic mechanisms and the overactive detrusor: an update. Eur Urol 59: 377–386, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Andersson KE. Bladder activation: afferent mechanisms. Urology 59: 43–50, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Andersson KE. Detrusor myocyte activity and afferent signaling. Neurourol Urodyn 29: 97–106, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Andersson KE. Overactive bladder–pharmacological aspects. Scand J Urol Nephrol Suppl 210: 72–81, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56: 581–631, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Andersson KE, Fullhase C, Soler R. Urothelial effects of oral agents for overactive bladder. Curr Urol Rep 9: 459–464, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Andersson KE, Gratzke C, Hedlund P. The role of the transient receptor potential (TRP) superfamily of cation-selective channels in the management of the overactive bladder. BJU Int 106: 1114–1127, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Andersson KE, Persson K. Nitric oxide synthase and the lower urinary tract: possible implications for physiology and pathophysiology. Scand J Urol Nephrol 175: 43–53, 1995 [PubMed] [Google Scholar]
- 19. Andrade EL, Ferreira J, Andre E, Calixto JB. Contractile mechanisms coupled to TRPA1 receptor activation in rat urinary bladder. Biochem Pharm 72: 104–114, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Apodaca G. The uroepithelium: not just a passive barrier. Traffic 5: 117–128, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 72: 1057–1064, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Apodaca G, Kiss S, Ruiz W, Meyers S, Zeidel M, Birder L. Disruption of bladder epithelium barrier function after spinal cord injury. Am J Physiol Renal Physiol 284: F966–F976, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Apostolidis A, Brady CM, Yoangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology 65: 400–405, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Apostolidis A, Gonzales GE, Fowler CJ. Effect of intravesical resiniferatoxin (RTX) on lower urinary tract symptoms, urodynamic parameters, and quality of life of patients with urodynamic increased bladder sensation. Eur Urol 50: 1299–1305, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Apostolidis A, Jacques TS, Freeman A, Kalsi V, Popat R, Gonzales G, Datta SN, Ghazi-Noori S, Elneil S, Dasgupta P, Fowler CJ. Histological changes in the urothelium and suburothelium of human overactive bladder following intradetrusor injections of botulinum neurotoxin type A for the treatment of neurogenic or idiopathic detrusor overactivity. Eur Urol 53: 1245–1253, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Araki I. TRP channels in urinary bladder mechanosensation. Adv Exp Med Biol 704: 861–879, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Araki I, Du S, Kobayashi H, Sawada N, Mochizuki T, Zakoji H, Takeda M. Roles of mechanosensitive ion channels in bladder sensory transduction and overactive bladder. Int J Urol 15: 681–687, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Arrighi N, Bodei S, Lucente A, Michel MC, Zani D, Simeone C, Cunico SC, Spano P, Sigala S. Muscarinic receptors stimulate cell proliferation in the human urothelium-derived cell line UROtsa. Pharmacol Res 64: 420–425, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Arrighi N, Bodei S, Peroni A, Mirabella G, Zani D, Simeone C, Cunico SC, Spano P, Signala S. Detection of muscarinic receptor subtypes in human urinary bladder mucosa: age and gender-dependent modifications. Neurourol Urodyn 27: 421–428, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Artim DE, Bazely F, Daugherty SL, Sculptoreanu A, Koronowski KB, Schopfer FJ, Woodcock SR, Freeman BA, de Groat WC. Nitro-oleic acid targets transient receptor potential (TRP) channels in capsaicin sensitive afferent nerves of rat urinary bladder. Exp Neurol 232: 90–99, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bai W, Oliveros-Saunders B, Wang Q, Acevedo-Duncan ME, Nicosia SV. Estrogen stimulation of ovarian surface epithelial cell proliferation. In Vitro Cell Dev Biol Anim 36: 657–666, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Balestreire EM, Apodaca G. Apical EGF receptor signaling: regulation of stretch-dependent exocytosis in bladder umbrella cells. Mol Biol Cell 13: 830–843, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bassuk JA, Cockrane K, Mitchell ME. Induction of urothelial cell proliferation by fibroblast growth factor-7 in RAG1-deficient mice. Adv Exp Med Biol 539: 623–633, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Beckel JM, Birder LA. Differential expression and function of nicotinic acetylcholine receptors in the urinary bladder epithelium of the rat. J Physiol PMID: 22250215: 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beckel JM, Kanai AJ, Lee SJ, de Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol 290: F103–F110, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Birder LA, Apodaca G, de Groat WC, Kanai AJ. Adrenergic and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol Renal Physiol 275: F226–F229, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CAT. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol 285: F423–F429, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract 4: 46–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA 98: 13396–13401, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Birder LA, Kullmann FA, Lee H, Barrick S, de Groat WC, Kanai A, Caterina M. Activation of urothelial transient receptor potential vanilloid 4 by 4alpha phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharm Exp Ther 323: 227–235, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick SR, Kanai AJ, Wang E, Ruiz WG, de Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci 22: 8063–8070, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington Roppolo CA, JR, Ford AP, de Groat WC, Burnstock G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats witn interstitial cystitis. Am J Physiol Renal Physiol 287: F1084–F1091, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Birder LA, Wolf-Johnston AS, Chib MK. Beyond neurons: involvement of urothelial and glial cells in bladder function. Neurourol Urodyn 29: 88–96, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Birder LA, Wolf-Johnston AS, Sun Y, Chai TC. Alteration in TRPV1 and muscarinic (M3) receptor expression and function in idiopathic overactive bladder urothelial cells. Acta Physiol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Birder LA, Wolf-Johnston A, Buffington CAT, Roppolo JR, de Groat WC, Kanai AJ. Altered inducible nitric oxide synthase expression and nitric oxide production in the bladder of cats with feline interstitial cystitis. J Urol 173: 625–629, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med 13: 625–630, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Blakeman PJ, Hilton P, Bulmer JN. Oestrogen and progesterone receptor expression in the female lower urinary tract, with reference to estrogen status. BJU Int 86: 32–38, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Brady CM, Apostolidis A, Yiangou Y, Baecker PA, Ford AP, Freeman A, Jacques Fowler TS, CJ, Anand P. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol 46: 247–253, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron 17: 681–683, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Bschleipfer T, Schukowski K, Weidner W, Grando SA, Schwantes U, Kummer W, Lips KS. Expression and distribution of cholinergic receptors in the human urothelium. Life Sci 80: 2303–2307, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Buffington CA. A watershed year for interstitial cystitis. J Urol 183: 854–855, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Buffington CA. Bladder pain syndrome/interstitial cystitis. In: Urogenital Pain in Clinical Practice, edited by Baranowski AP, Abrams P, Fall M. New York: Informa Healthcare USA, 2008, p. 169–183 [Google Scholar]
- 54. Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmcol Sci 22: 182–188, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat 194: 335–342, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Burnstock G. Targeting the visceral purinergic system for pain control. Curr Opin Pharmacol 12: 80–86, 2012 [DOI] [PubMed] [Google Scholar]
- 57. Cao DS, Yu SQ, Premkumar LS. Modulation of transient receptor potential vanilloid 4-mediated membrane currents and synaptic transmission by protein kinase C. Mol Pain 10: 50–55, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carattino MD, Sheng S, Kleyman TR. Mutations in the pore region modify epithelial sodium channel gating by shear stress. J Biol Chem 280: 4393–4401, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Caremel R, Oger-Roussel S, Behr-Roussel D, Grise P, Giuliano FA. Nitric oxide/cyclic guanosine monophosphate signalling mediates an inhibitory action on sensory pathways of the micturition reflex in the rat. Eur Urol 2010 [DOI] [PubMed] [Google Scholar]
- 60. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Chai TC, Gray ML, Steer WD. The incidence of a positive ice water test in bladder outlet obstructed patients: evidence for bladder neural plasticity. J Urol 160: 34–38, 1998 [PubMed] [Google Scholar]
- 62. Chai TC, Keay S. New theories in interstitial cystitis. Nat Clin Pract Urol 1: 85–89, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J Urol 162: 3–11, 1999 [DOI] [PubMed] [Google Scholar]
- 64. Chancellor MB, Fowler CJ, Apostolidis A, de Groat WC, Smith CP, Somogyi GT, Aoki R. Drug insight: biological effects of botulinum toxin A in the lower urinary tract. Nat Clin Prac Urol 5: 319–328, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chapple C, Abrams P, Andersson KE. A randomised, double-blind, placebo-controlled phase II study to investigate the efficacy and safety of the EP-1 receptor antagonist, ONO-8539, in overactive bladder syndrome. AUA Abstr 2011 [DOI] [PubMed] [Google Scholar]
- 66. Charrua A, Boudes M, de Ridder D, Cruz CD, Cruz F. TRPV1 and TRPV4 expression in bladder neurons during normal condition and during cystitis. Eur Urol. In press [Google Scholar]
- 67. Charrua A, Cruz CD, Cruz F, Avelino A. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol 177: 1537–1541, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Charrua A, Cruz CD, Narayanan S. CRC-6211, a new oral specific TRPV1 antagonist, decreases bladder overactivity and noxious bladder input in cystitis animal models. J Urol 181: 379–386, 2009 [DOI] [PubMed] [Google Scholar]
- 69. Charrua A, Reguenga C, Cordeiro JM. Functional transient receptor potential vanilloid 1 is expressed in human urothelial cells. J Urol 182: 2944–2950, 2009a [DOI] [PubMed] [Google Scholar]
- 70. Cheng J, Huang H, Zhang ZT. Overexpression of epidermal growth factor receptor in urothelium elicits urothelial hyperplasia and promotes bladder tumore growth. Cancer Res 62: 4157–4163, 2002 [PubMed] [Google Scholar]
- 71. Cheng S, Scigalla FP, Speroni di Fenizio P, Zhang ZG, Stolzenberg JY, Neuhaus J. ATP enhances spontaneous calcium activity in cultured suburothelial myofibroblasts of the human bladder. PLoS One 6: e2569, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chess-Williams R. Muscarinic receptors of the urinary bladder: detrusor, urothelial and prejunctional. Auton Autocoid Pharmacol 22: 133–145, 2002 [DOI] [PubMed] [Google Scholar]
- 73. Chiang G, Patra P, Letourneau E. Pentosanpolysulfate (Elmiron) is a potent inhibitor of mast cell histamine secretion. Adv Exp Med Biol 539: 713–739, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Chiang G, Patra P, Letourneau R. Pentosanpolysulfate inhibits mast cell histamine secretion and intracellular calcium ion levels: an alternative explanation of its beneficial effect in interstitial cystitis. J Urol 164: 2119–2125, 2000 [PubMed] [Google Scholar]
- 75. Chopra B, Barrick SR, Meyers S, Beckel JM, Zeidel ML, Ford AP, de Groat WC, Birder LA. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol 562: 859–871, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chopra B, Gever J, Barrick SR, Hanna-Mitchell AT, Beckel JM, Ford AP, Birder LA. Expression and function of rat urothelial P2Y receptors. Am J Physiol Renal Physiol 294: F821–F829, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X3 receptor subunit in mediating multiple sensory effects of ATP. J Physiol 567: 621–639, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hypotreflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407: 1011–1015, 2000 [DOI] [PubMed] [Google Scholar]
- 79. Combrisson H, Allix S, Robain G. Influence of temperature on urethra to bladder micturition reflex in the awake ewe. Neurourol Urodyn 26: 290–295, 2007 [DOI] [PubMed] [Google Scholar]
- 80. Conrads TP, Tocci GM, Hood BL, Zhang CO, Guo L, Koch KR, Michejda CJ, Veenstra TD, Keay SK. CKAP4/p63 is a receptor for the frizzled-8 protein-related antiproliferative factor from interstitial cystitis patients. J Biol Chem 281: 37836–37843, 2006 [DOI] [PubMed] [Google Scholar]
- 81. Cruz C, Cruz F. Spinal cord injury and bladder dysfunction: new ideas about an old problem. Scientific World J 11: 214–234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cruz F, Dinis P. Resiniferatoxin and botulinum toxin type A for treatment of lower urinary tract symptoms. Neurourol Urodyn 26: 920–927, 2007 [DOI] [PubMed] [Google Scholar]
- 83. Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent activity in wild-type and TRPV1 knockout mice. J Physiol 583: 663–674, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. De Groat WC, Kruse MN, Vizzard MA, Cheng CL, Araki I, Yoshimura N. Modification of urinary bladder function after spinal cord injury. Adv Neurol 72: 347–364, 1997 [PubMed] [Google Scholar]
- 85. De Laet K, de Wachter S, Wyndaele JJ. Systemic oxybutynin decreases afferent activity of the pelvic nerve of the rat: new insights into the working mechanism of antimuscarinics. Neurourol Urodyn 25: 156–161, 2006 [DOI] [PubMed] [Google Scholar]
- 86. De Wachter S, Wyndaele JJ. Intravesical oxybutynin: a local anesthetic effect on bladder C afferents. J Urol 169:1892–1895, 2003 [DOI] [PubMed] [Google Scholar]
- 87. Dimitrakov J, Kroenke K, Steers WD. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med 167: 1922–1929, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dixon JS, Gosling JA. Histology and fine structure of the muscularis mucosae of the human urinary bladder. J Anat 136: 265–271, 1983 [PMC free article] [PubMed] [Google Scholar]
- 89. Dmitrieva N, McMahon SB. Sensitization of visceral afferents by nerve growth factor in the adult rat. Pain 66: 87–97, 1996 [DOI] [PubMed] [Google Scholar]
- 90. Drake MJ, Fry CH, Eyden B. Structural characterization of myofibroblasts in the bladder. BJU Int 97: 29–32, 2006 [DOI] [PubMed] [Google Scholar]
- 91. Du S, Araki I, Mikami Y, Zakoji H, Beppu M, Yoshiyama M, Takeda M. Amiloride-sensitive ion channels in urinary bladder epithelium involved in mechanosensory transduction by modulating stretch-evoked adenosine triphosphate release. Urology 69: 590–595, 2007 [DOI] [PubMed] [Google Scholar]
- 92. El-Badawi A, Schenk EA. Dual innervation of the mammalian urinary bladder. A histochemical study of the distribution of cholinergic and adrenergic nerves. Am J Anat 119: 405–428, 1966 [DOI] [PubMed] [Google Scholar]
- 93. Elneil S, Skepper JN, Kidd EJ, Williamson JG, Ferguson DR. Distribution of PX2(1) and P2X(3) receptors in the rat and human urinary bladder. Pharmacology 63: 120–128, 2001 [DOI] [PubMed] [Google Scholar]
- 94. Erickson DR, Tomaszewski JE, Kunselman AR. Urine markers do not predict biopsy findings or presence of bladder ulcers in interstitial cystitis/painful bladder syndrome. J Urol 179: 1850–1856, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Evans RJ, Moldwin RM, Cossons N, Darekar A, Mills IW, Scholfield D. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol 185: 1716–1721, 2011 [DOI] [PubMed] [Google Scholar]
- 96. Everaerts W, Gevaert T, Nilius B, de Ridder D. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol Urodyn 27: 264–273, 2008 [DOI] [PubMed] [Google Scholar]
- 97. Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owslanik G, Vennekens R, de Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA 107: 19084–19089, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Eyden B. Are there myofibroblasts in normal bladder? Eur Urol 56: 427–429, 2009 [DOI] [PubMed] [Google Scholar]
- 99. Ferguson DR, Kennedy I, Burton TJ. ATP is released from rat urinary bladder epithelial cells by hydrostatic pressure changes-a possible sensory mechanism? J Physiol 505: 503–511, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Folkerts G, Nijkamp FP. Airway epithelium: more than just a barrier. Trends Pharmcol Sci 19: 334–241, 1998 [DOI] [PubMed] [Google Scholar]
- 101. Ford AP. In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signalling 8: S3–S26, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ford AP, Cockayne DA. ATP and P2X purinoceptors in urinary tract disorders. Handb Exp Pharmacol 202: 485–526, 2011 [DOI] [PubMed] [Google Scholar]
- 103. Fovaeus M, Fujiwara M, Hogestatt ED, Persson K, Andersson KE. A non-nitergic smooth muscle relaxant factor released from rat urinary bladder by muscarinic receptor stimulation. J Urol 161: 649–653, 1999 [PubMed] [Google Scholar]
- 104. Fraser MO, Chuang YC, Tyagi P, Yokoyama T, Yoshimura N, Huang L, de Groat WC, Chancellor MB. Intravesical liposome administration: a novel treatment for hyperactive bladder in the rat. Urology 61: 656–663, 2003 [DOI] [PubMed] [Google Scholar]
- 105. Frias B, Charrua A, Avelino A, Michel MC, Cruz F, Cruz CD. Transient receptor potential vanilloid 1 mediates nerve growth factor-induced bladder hyperactivity and noxious input. BJU Int 110: E422–429, 2012 [DOI] [PubMed] [Google Scholar]
- 106. Fry CH, Sui GP, Kanai AJ, Wu C. The function of suburothelial myofibroblasts in the bladder. Neurourol Urodyn 26: 914–919, 2007 [DOI] [PubMed] [Google Scholar]
- 107. Fry CH, Young JS, Jabr RI, McCarthy C, Ikeda Y, Kanai AJ. Modulation of spontaneous activity in the overactive bladder: the role of P2Y agonists. Am J Physiol Renal Physiol 302: F1447–F1454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol 27: 141–155, 1998 [DOI] [PubMed] [Google Scholar]
- 109. Georgopoulos NT, Kirkwood LA, Walder DC, Southgate J. Differential regulation of growth-promoting signalling pathways by E-cadherin. PLoS One 5: e13621, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gevaert T, De Vos R, Everaerts W, Libbrecht L, Van Der Aa F, van den Oord J, Roskams T, De Ridder D. Characterization of upper lamina propria interstitial cells in bladders from patients with neurogenic detrusor overactivity and bladder pain syndrome. J Cell Mol Med 15: 2586–2593, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gevaert T, de Vos R, van der Aa F, Joniau S, van den Oord J, Roskams T, de Ridder D. Identification of telocytes in the upper lamina propria of the human urinary tract. J Cell Mol Med 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gevaert T, Vriens J, Segal A, Evaraerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, De Wachter I, Van Leuven F, Voets T, De Ridder D, Nilius B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117: 3453–3462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gever JR, Soto R, Henningsen RA, Martin RS, Hackos DH, Panicker S, Rubas W, Oglesby IB, Dillon MP, Milla ME, Burnstock G, Ford AP. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol 160: 1387098, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Giannantoni A, Amantini C, Proietti S, Farfariello V, Vianello A, Santoni G, Porena M. Normal human urothelial cell lines express onabotulinumtoxinA SV2 high affinity receptors. EAU Abstr 2012 [Google Scholar]
- 115. Giannantoni A, Di Stasi SM, Nardicchi V, Zucchi A, Macchioni L, Bini V, Goracci G, Porena M. Botulinum-A toxin injections into the detrusor muscle decrease nerve growth factor bladder tissue levels in patients with neurogenic detrusor overactivity. J Urol 175: 2341–2344, 2006 [DOI] [PubMed] [Google Scholar]
- 116. Gillespie JI, Marerink-van Ittersum M, de Vente J. Expression of neuronal nitric oxide synthase (nNOS) and nitric-oxide-induced changes in cGMP in the urothelial layer of the guinea pig bladder. Cell Tissue Res 321: 341–351, 2005 [DOI] [PubMed] [Google Scholar]
- 117. Gillespie JI, Markerink-van Ittersum M, de Vente J. Endogenous nitric oxide/cGMP signalling in the guinea pig bladder: evidence for distinct populations of sub-urothelial interstitial cells. Cell Tissue Res 325: 325–332, 2006 [DOI] [PubMed] [Google Scholar]
- 118. Gillespie JI, Markerink-van Ittersum M, de Vente J. Sensory collaterals, intramural ganglia and motor nerves in the guinea-pig bladder: evidence for intramural neural circuits. Cell Tissue Res 325: 33–45, 2006 [DOI] [PubMed] [Google Scholar]
- 119. Girard BM, Malley SE, Vizzard MA. Neurotrophin/receptor expression in urinary bladder of mice with overexpression of NGF in urothelium. Am J Physiol Renal Physiol 300: F345–F355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gosling JA, Dixon JS. Sensory nerves in the mammalian urinary tract. An evaluation using light and electron microscopy. J Anat 117: 133–144, 1974 [PMC free article] [PubMed] [Google Scholar]
- 121. Graham E, Chai TC. Dysfunction of bladder urothelium and bladder urothelial cells in interstitial cystitis. Curr Urol Rep 7: 440–446, 2006 [DOI] [PubMed] [Google Scholar]
- 122. Grasso EJ, Scalambro MB, Calderon RO. Differential response of the urothelial V-ATPase activity to the lipid environment. Cell Biochem Biophys 61: 157–168, 2011 [DOI] [PubMed] [Google Scholar]
- 123. Grol S, Essers PB, van Koeveringe GA, Martinez-Martinez P, de Vente J, Gillespie JI. M(3) muscarinic receptor expression on suburothelial interstitial cells. BJU Int 104: 398–405, 2009 [DOI] [PubMed] [Google Scholar]
- 124. Gupta GN, Lu SG, Gold MS, Chai TC. Bladder urothelial cells from patients with interstitial cystitis have an increased sensitivity to carbachol. Neurourol Urodyn 28: 1022–1027, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, DeGroat WC, Birder LA. Non-neuronal acetylcholine and urinary blader urothelium. Life Sci 80: 2298–2302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hashimoto Y, Ushiki T, Uchida T, Yamada J, Iwanaga T. Scanning electron microscopic observation of apical sites of open-type paraneurons in the stomach, intestine and urethra. Arch Histol Cytol 62: 181–189, 1999 [DOI] [PubMed] [Google Scholar]
- 127. Hashitani H, Takano H, Fujita K, Mitsui R, Suzuki H. Functional properties of suburothelial microvessels in the rat bladder. J Urol 185: 2382–2391, 2011 [DOI] [PubMed] [Google Scholar]
- 128. Hawthorn MH, Chapple CR, Cock M, Chess-Williams R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol 129: 416–419, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Heppner TJ, Layne JJ, Pearson JM, Sarkissian H, Nelson MT. Unique properties of muscularis mucosae smooth muscle in guinea pig urinary bladder. Am J Physiol Regul Integr Comp Physiol 301: R351–R362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hicks GA. TRP channels as therapeutic targets: hot property, or time to cool down? Neurogastroenterol Motil 18: 590–594, 2006 [DOI] [PubMed] [Google Scholar]
- 131. Hicks M. The mammalian urinary bladder: an accomodating organ. Biol Rev 50: 215–246, 1975 [DOI] [PubMed] [Google Scholar]
- 132. Hicks M, Ketterer B, Warren R. The ultrastructure and chemistry of the luminal plasma membrane of the mammalian urinary bladder: a structure with low permeability to water and ions. Phil Trans R Soc Lond 268: 23–38, 1974 [DOI] [PubMed] [Google Scholar]
- 133. Ho DR, Chen CS, Lin WY, Chang PJ, Huang YC. Effect of hyaluronic acid on urine nerve growth factor in cyclophosphamide-induced cystitis. Int J Urol 18: 525–531, 2011 [DOI] [PubMed] [Google Scholar]
- 134. Hosseini A, Ehren I, Wiklund NP. Nitric oxide as an objective marker for evaluation of treatment response in patients with classic interstitial cystitis. J Urol 172: 2261–2265, 2004 [DOI] [PubMed] [Google Scholar]
- 135. Hurst RE. Structure, function and pathology of proteoglycans and glycosaminoglycans in the urinary tract. World J Urol 12: 3–10, 1994 [DOI] [PubMed] [Google Scholar]
- 136. Hurst RE, Moldwin RM, Mulhulland SG. Bladder defense molecules, urothelial differentiation, urinary biomarkers, and interstitial cystitis. Urology 69: 17–23, 2007 [DOI] [PubMed] [Google Scholar]
- 137. Igawa Y, Aizawa N, Homma Y. Beta 3 adrenoceptor agonists: possible role in the treatment of overactive bladder. Korean J Urol 51: 811–818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol 128: 593–607, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Iijima K, de Wachter S, Wyndaele JJ. Effects of the M3 receptor selective muscarinic antagonist darifenacin on bladder afferent activity of the rat pelvic nerve. Eur Urol 52: 842–847, 2007 [DOI] [PubMed] [Google Scholar]
- 140. Ikeda Y, Birder L, Buffington C, Roppolo J, Kanai A. Mucosal muscarinic receptors enhance bladder activity in cats with feline interstitial cystitis. J Urol 181: 1415–1422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A. Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol 293: F1018–F1025, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ikeda Y, Kanai A. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: role of mucosal muscarinic receptors. Am J Physiol Renal Physiol 295: F454–F461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Imamov O, Yakimchuk K, Morani A. Estrogen receptor beta-deficient female mice develop a bladder phenotype resembling human interstitial cystitis. Proc Natl Acad Sci USA 104: 9806–9809, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ishihama H, Momota Y, Yanase H, Wang X, de Groat WC, Kawatani M. Activation of alpha 1D adrenergic receptors in the rat urothelium facilitates the micturition reflex. J Urol 175: 358–364, 2006 [DOI] [PubMed] [Google Scholar]
- 145. Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, Torii K, Uneyama H. Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophy Res Commun 388: 1–5, 2009 [DOI] [PubMed] [Google Scholar]
- 146. Janssen DA, Hoenderop JG, Jansen KC. The mechanoreceptor TRPV4 is localized in adherence junctions of the human bladder urothelium: a morphological study. J Urol 186: 1121–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 147. Jen PY, Dixon JS, Gosling JA. Immunohistochemical localization of neuromarkers and neuropeptides in human fetal and neonatal urinary bladder. Br J Urol 75: 230–235, 1995 [DOI] [PubMed] [Google Scholar]
- 148. Ji RR, Samad TA, Jin SX. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 26: 57–68, 2002 [DOI] [PubMed] [Google Scholar]
- 149. Johnston L, Woolsey S, Cunningham RM, O'Kane H, Duggan B, Keane P, McCloskey KD. Morphological expression of KIT positive interstitial cells of Cajal in human bladder. J Urol 184: 370–377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kaan TK, Yip PK, Grist J, Cefalu JS, Nunn PA, Ford AP, Zhong Y, McMahon SB. Endogenous purinergic control of bladder activity via presynaptic P2X3 and P2X2/3 receptors in the spinal cord. J Neurosci 30: 4503–4507, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kaan TK, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, Nunn PA, Dickenson AH, Ford AP, Zhong Y, Malcangio M, McMahon SB. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain 133: 2549–2564, 2010 [DOI] [PubMed] [Google Scholar]
- 152. Kalota SJ, Stain PC, Parsons CL. Prevention of acrolein-induced bladder injury by pentosanpolysulfate. J Urol 148: 163–166, 1992 [DOI] [PubMed] [Google Scholar]
- 153. Kanai AJ, Andersson KE. Bladder afferent signaling: recent findings. J Urol 183: 1288–1295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153a. Kanai A, Wyndaele JJ, Andersson KE, Fry C, Ikeda Y, Zabbarova I, de Wachter S. Researching bladder afferents determining the effects of β (3)-adrenergic receptor agonists and botulinum toxin type A. Neurol Urodyn 30: 684–691, 2011 [DOI] [PubMed] [Google Scholar]
- 154. Kaufman J, Tyagi P, Chancellor MB. Intravesical liposomal (LP09) instillation protects bladder urothelium from chemical irritation. Am Urol Assoc 1506: 2009 [Google Scholar]
- 155. Keay SK, Szekely Z, Conrads TP, Veenstra TD, Barchi JJ, Zhang CO, Koch KR, Michejda CJ. An antiproliferative factor from interstitial cystitis patients is a frizzled 8 protein-related sialoglycopeptide. Proc Natl Acad Sci USA 101: 11803–11808, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Keay SK, Zhang CO, Shoenfelt J. Sensitivity and specificity of antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor as urine markers for interstitial cystitis. Urology 57: 9–14, 2001 [DOI] [PubMed] [Google Scholar]
- 157. Kennelly MJ, Arena KC, Shaffer N, Bennett ME, Grill WM, Grill JH, Boggs JW. Electrical stimulation of the urethra evokes bladder contractions in a woman with spinal cord injury. J Spinal Cord Med 33: 261–265, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Khanderwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297: F1477–F1501, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kim JC, Park EY, Hong SH. Changes of urinary nerve growth factor and prostaglandins in male subjects with overactive bladder symptom. Int J Urol 12: 875–880, 2005 [DOI] [PubMed] [Google Scholar]
- 160. Knight GE, Bodin P, de Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol 282: F281–F288, 2002 [DOI] [PubMed] [Google Scholar]
- 161. Koh BH, Roy R, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP, Hatton WF, Ward SM, Sanders KM, Koh SD. PDGFRalpha cells in mouse urinary bladder: a new class of interstitial cells. J Cell Mol Med 16: 691–700, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Korkmaz A, Oter S, Sadir S, Coskun O, Topal T, Ozler M, Bilgic H. Peroxynitrite may be involved in bladder damage caused by cyclophosphamide in rats. J Urol 173: 1793–1796, 2005 [DOI] [PubMed] [Google Scholar]
- 163. Kreft ME, Jezernik K, Kreft M, Romih R. Apical plasma membrane traffic in superficial cells of bladder urothelium. Ann NY Acad Sci 1152: 18–29, 2009 [DOI] [PubMed] [Google Scholar]
- 164. Kreft ME, Romih R, Kreft M, Jezernik K. Endocytotic activity of bladder superficial urothelial cells is inversely related to their differentiation stage. Differentiation 77: 48–59, 2009 [DOI] [PubMed] [Google Scholar]
- 165. Kullmann FA, Downs TR, Artim DE, Limberg BJ, Shah M, Contract D, de Groat WC, Rosenbaum JS. Urothelial beta-3 adrenergic receptors in the rat bladder. Neurourol Urodyn 30: 144–150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kullmann FA, Limberg BJ, Artim DE, Shah M, Downs TR, Contract D, Wos J, Rosenbaum JS, de Groat WC. Effects of beta3-adrenergic receptor activation on rat urinary bladder hyperactivity induced by ovariectomy. J Pharmacol Exp Ther 330: 704–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Kullmann FA, Shah MA, Birder LA, de Groat WC. Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol 296: F892–F901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kumar V, Chapple CR, Rosario D, Tophill PR, Chess-WIlliams R. In vitro release of adenosine triphosphate from the urothelium of human bladders with detrusor overactivity, both neurogenic and idiopathic. Eur Urol 57: 1087–1092, 2010 [DOI] [PubMed] [Google Scholar]
- 169. Kummer W, Wiegand S, Akinci S, Schinkel AH, Wess J, Koepsell H, Habergerger RV, Lips KS. Role of acetylcholine and muscarinic receptors in serotonin-induced bronchoconstriction in the mouse. J Mol Neurosci 30: 67–68, 2006 [DOI] [PubMed] [Google Scholar]
- 170. Kuo HC, Liu HT, Chancellor MB. Can urinary nerve growth factor be a biomarker for overactive bladder. Rev Urol 12: e69–e77, 2010 [PMC free article] [PubMed] [Google Scholar]
- 171. Kurizaki Y, Ishizuka O, Imamura T, Midori I, Ogawa T, Igawa Y, Nishizawa O, Andersson KE. Relation between expression of alpha-adrenoceptor mRNAs in bladder mucosa and urodynamic findings in men with lower urinary tract symptoms. Scand J Urol Nephrol Suppl. In press [DOI] [PubMed] [Google Scholar]
- 172. Kurizaki Y, Ishizuka O, Imamura T, Nishizawa O, Andersson KE. Expression of beta-adrenoceptor mRNA in bladder mucosa and urodynamic findings in men with lower urinary tract symptoms. Neurourol Urodyn. In press [DOI] [PubMed] [Google Scholar]
- 173. LaBerge J, Malley SE, Zvarova K, Vizzard MA. Expression of corticotrophin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 291: R692–R703, 2006 [DOI] [PubMed] [Google Scholar]
- 174. Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, Apodaca G, Zeidel ML. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol 283: F242–F253, 2002 [DOI] [PubMed] [Google Scholar]
- 175. Lavelle JP, Meyers SA, Ruiz WG, Buffington CAT, Zeidel M, Apodaca G. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol 278: F540–F553, 2000 [DOI] [PubMed] [Google Scholar]
- 176. Lee HY, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol 163: 2002–2007, 2000 [PubMed] [Google Scholar]
- 177. Lee JH, Kim JW, Im YS, Seong GJ, Lee HK. Cyclosporine A induces nerve growth factor expression via activation of MAPK p38 and NFAT5. Cornea 1: 19–24, 2011 [DOI] [PubMed] [Google Scholar]
- 177a. Lee T, Hedlund P, Newgreen D. Urodynamic effects of a novel EP(1) receptor antagonist in normal rats and rats with bladder outlet obstruction. J Urol 177: 1562–1567, 2007 [DOI] [PubMed] [Google Scholar]
- 178. Li J, Karaplis AC, Huang DC, Siegel PM, Camirand A, Yang XF, Muller WJ, Kremer R. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. J Clin Invest 121: 4655–4669, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Li M, Sun Y, Simard JM, Chai TC. Increased transient receptor potential vanilloid type 1 (TRPV1) signaling in idiopathic overactive bladder urothelial cells. Neurourol Urodyn 30: 606–611, 2011 [DOI] [PubMed] [Google Scholar]
- 180. Liang FX, Bosland MC, Huang H, Romih R, Baptiste S, Deng FM, Wu XR, Shapiro E, Sun TT. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol 171: 835–844, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Liang FX, Riedel I, Deng FM, Zhou G, Wu C, Wu WR, Kong WP, Moll R, Sun TT. Organization of uroplakin subunits: transmembrane topology, pair formation and plaque composition. J Biochem 355: 13–18, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Liedtke W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. Pflügers Arch 451: 176–180, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Limberg BJ, Andersson KE, Kullmann AF, Burner G, de Groat WC, Rosenbaum JS. Beta-adrenergic receptor subtype expression in myocyte and non-myocyte cells in human female bladder. Cell Tissue Res 342: 295–306, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G. Structure and function of neuronal nicotinic acetylcholine receptors. Prog Br Res 109: 125–137, 1996 [DOI] [PubMed] [Google Scholar]
- 185. Lips KS, Wunsch J, Zarghooni S, Bschleipfer T, Schukowski K, Weidner W, Wessler I, Schwantes U, Koepsell H, Kummer W. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol 51: 1042–1053, 2007 [DOI] [PubMed] [Google Scholar]
- 186. Liu F, Takahashi N, Yamaguchi O. Expression of P2X3 purinoceptors in suburothelial myofibroblasts of the normal human urinary bladder. Int J Urol 16: 570–575, 2009 [DOI] [PubMed] [Google Scholar]
- 187. Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur Urol 56: 700–707, 2009 [DOI] [PubMed] [Google Scholar]
- 188. Liu HT, Kuo HC. Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology 70: 463–468, 2007 [DOI] [PubMed] [Google Scholar]
- 189. Liu HT, Kuo HC. Increased expression of transient receptor potential vanilloid subfamily 1 in the bladder predicts the response to intravesical instillations of resiniferatoxin in patients with refractory idiopathic detrusor overactivity. BJU Int 100: 1086–1090, 2007 [DOI] [PubMed] [Google Scholar]
- 190. Liu HT, Wang YS, Chancellor M. Nerve growth factor levels in urine correlate with expression in urothelium but not suburothelium in the bladder tissues of overactive bladder syndrome. J Urol 183: e644–645, 2010 [Google Scholar]
- 191. Liu L, Manseifld KJ, Kristiana I, Vaux KJ, Millard RJ, Burcher E. The molecular basis or urgency: regional difference of vanilloid receptor expression in the human bladder. Neurourol Urodyn 26: 433–438, 2007 [DOI] [PubMed] [Google Scholar]
- 192. Lowe EM, Anand P, Terenghi G. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 79: 572–577, 1997 [DOI] [PubMed] [Google Scholar]
- 193. Lucioni A, Bales GT, Lotan TL, McGehee DS, Cook SP, Rapp DE. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int 101: 366–370, 2008 [DOI] [PubMed] [Google Scholar]
- 194. Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature 445: 858–865, 2007 [DOI] [PubMed] [Google Scholar]
- 195. MacDiarmid SA, Sand PL. Diagnosis of interstitial cystitis/painful bladder syndrome in patients with overactive bladder symptoms. Rev Urol 184: 9–16, 2007 [PMC free article] [PubMed] [Google Scholar]
- 196. Maggi CA, Barbanti G, Santicioli P, Beneforti P, Misuri D, Meli A, Turini D. Cystometric evidence that capsaicin-sensitive nerves modulate the afferent branch of micturition reflex in humans. J Urol 142: 150–154, 1989 [DOI] [PubMed] [Google Scholar]
- 196a. Mahmoud HM, Refaat SH, Abdel-Aal IH, Saudy FZ. Age-Related Changes in the urinary bladder of female Albino Rats. Egypt J Histol 27: 402–416, 2004 [Google Scholar]
- 197. Mamalaki A, Tzartos SJ. Nicotinic acetylcholine receptor: structure, function and main immunogenic region. Adv Neurol 4: 339–354, 1994 [DOI] [PubMed] [Google Scholar]
- 198. Mansfield KJ, Liu L, Mitchelson FJ, Moore KH, Millard RJ, Burcher E. Muscarinic receptor subtypes in human bladder detrusor and mucosa, studied by radioligand binding and quantitative competitive RT-PCR: changes in aging. Br J Pharmacol 144: 1089–1099, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Martin BF. Cell replacement and differentiation in transitional epithelium: a histological and autoradiographic study of the guinea-pig bladder and ureter. J Anat 112: 433–455, 1972 [PMC free article] [PubMed] [Google Scholar]
- 200. Martinez-Bianchi V, Halstater BH. Urologic chronic pelvic pain syndrome. Prim Care 37: 527–546, 2010 [DOI] [PubMed] [Google Scholar]
- 201. Masunaga K, Chapple CR, McKay NG, Yoshida M, Sellers DJ. The beta 3-adrenoceptor mediates the inhibitory effects of beta-adrenoceptor agonists via the urothelium in pig bladder dome. Neurourol Urodyn 29: 2010 [DOI] [PubMed] [Google Scholar]
- 202. May A. Chronic pain may change the structure of the brain. Pain 137: 7–15, 2008 [DOI] [PubMed] [Google Scholar]
- 203. Mayer EA, Bushnell MC. Functional pain disorders: time for a paradigm shift? In: Functional Pain Syndromes: Presentation and Pathophysiology, edited by Mayer EA, Bushnell MC. Seattle, WA: IASP Press, 2009, p. 19–33 [Google Scholar]
- 204. McCloskey KD. Interstitial cells in the urinary bladder–localization and function. Neurourol Urodyn 29: 82–87, 2010 [DOI] [PubMed] [Google Scholar]
- 205. McCloskey KD. Interstitial cells of Cajal in the urinary tract. Handb Exp Pharmacol 202: 233–254, 2011 [DOI] [PubMed] [Google Scholar]
- 206. Micera A, Lambiase A, Stampachiacchiere B. Nerve growth factor and tissue repair remodeling: trkA (NGFR) and p75 (NTR), two receptors one fate. Cytokine Growth Factor Rev 18: 245–246, 2007 [DOI] [PubMed] [Google Scholar]
- 207. Monaghan KP, Johnston LL, McCloskey KD. Identification of PDGFRalpha-positive populations of interstitial cells in human and guinea-pig bladder. J Urol 188: 639–647, 2012 [DOI] [PubMed] [Google Scholar]
- 208. Morales A, Emerson L, Nickel JC, Lundie M. Intravesical hyaluronic acid in the treatment of refractory interstitial cystitis. J Urol 156: 45–48, 1996 [PubMed] [Google Scholar]
- 209. Moro C, Uchiyama J, Chess-Williams R. Urothelial/lamina propria spontaneous activity and the role of M3 muscarinic receptors in mediating rate responses to stretch and carbachol. Urology 78: 1442–1449, 2011 [DOI] [PubMed] [Google Scholar]
- 210. Morrison JFB. Sensations arising from the lower urinary tract. In: The Physiology of the Lower Urinary Tract, edited by Torrens M, Morrison JFB. Berlin: Springer-Verlag, 1987, p. 89–131 [Google Scholar]
- 211. Mukerji G, Yiangou Y, Grogono J, Underwood J, Agarwal SK, Khullar V, Anand P. Localization of M2 and M3 muscarinic receptors in human bladder disorders and their clinical correlations. J Urol 176: 367–373, 2006 [DOI] [PubMed] [Google Scholar]
- 212. Munoz A, Gangitano DA, Smith CP, Boone TB, Somogyi GT. Removal of urothelium affects bladder contractility and release of ATP but not release of NO in rat urinary bladder. BMC Urol 24: 10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Murakami S, Chapple CR, Akino H, Sellers DJ, Chess-Williams R. The role of the urothelium in mediating bladder responses to isoprenaline. BJU Int 99: 669–673, 2007 [DOI] [PubMed] [Google Scholar]
- 214. Namasivayam S, Eardley I, Morrison JF. Purinergic sensory neurotransmission in the urinary bladder: an in vitro study in the rat. BJU Int 84: 854–860, 1999 [DOI] [PubMed] [Google Scholar]
- 215. Neuhaus J, Pfeiffer F, Wolburg H, Horn LC, Dorschner W. Alterations in connexin expression in the bladder of patients with urge symptoms. BJU Int 96: 854–860, 1999 [DOI] [PubMed] [Google Scholar]
- 216. Nickel JC, Egerdie RB, Steinhoff G, Palmer B, Hanno P. A multicenter, randomized, double-blind, parallel group pilot evaluation of the efficacy and safety of intravesical sodium chondroitin sulfate versus vehicle control in patients with interstitial cystitis/painful bladder syndrome. Urology 76: 804–809, 2010 [DOI] [PubMed] [Google Scholar]
- 217. Nickel JC, Hanno P, Kumar K, Thomas H. Second multicenter, randomized, double-blind, parallel-group evaluation of effectiveness and safety of intravesical sodium chondroitin sulfate compared with inactive vehicle control in subjects with interstitial cystitis/bladder pain syndrome. Urology 79: 1220–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 218. Nile CJ, de Vente J, Gillespie JI. Stretch independent regulation of prostaglandin E2 production within the isolated guinea-pig lamina propria. BJU Int 105: 540–548, 2010 [DOI] [PubMed] [Google Scholar]
- 219. Nile CJ, Gillespie JI. Interactions between cholinergic and prostaglandin signaling elements in the urothelium: role for muscarinic type 2 receptors. Urology 79: 217–223, 2012 [DOI] [PubMed] [Google Scholar]
- 220. Nirmal J, Tyagi P, Dang L, Hanna-Mitchell A, Wolf-Johnston A, Kaufman J, Birder L, Chancellor M. Endocytosis uptake of liposomes in urothelium cells detected by transmission electron microscopy. Am Urol Assoc 1202359: 2012 [Google Scholar]
- 221. Ochodnicky P, Cruz CD, Yoshimura N, Michel MC. Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol Urodyn 30: 1227–1241, 2011 [DOI] [PubMed] [Google Scholar]
- 222. Olsen SM, Stover JD, Nagatomi J. Examining the role of mechanosensitive ion channels in pressure mechanotransduction in rat bladder urothelial cells. Ann Biomed Eng 39: 688–697, 2011 [DOI] [PubMed] [Google Scholar]
- 223. Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev 84: 579–621, 2004 [DOI] [PubMed] [Google Scholar]
- 224. Otsuka A, Shinbo H, Matsumoto R, Kurita Y, Ozono S. Expression and functional role of beta-adrenoceptors in the human urinary bladder urothelium. Naunyn-Schmiedebergs Arch Pharmcol 377: 473–481, 2008 [DOI] [PubMed] [Google Scholar]
- 225. Ozawa H, Chancellor MB, Jung SY, Yokoyama T, Fraser MO, Yu Y, de Groat WC, Yoshimura N. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol 162: 2211–2216, 1999 [DOI] [PubMed] [Google Scholar]
- 226. Pandita RK, Andersson KE. Intravesical adenosine triphophate stimulates the micturition reflex in awake, freely moving rats. J Urol 168: 1230–1234, 2002 [DOI] [PubMed] [Google Scholar]
- 227. Pandita RK, Mizusawa H, Andersson KE. Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J Urol 164: 545–550, 2000 [PubMed] [Google Scholar]
- 228. Paner GP, Ro JY, Wojeik EM, Venkataraman G, Datta MW, Amin MB. Further characterization of the muscle layers and lamina propria of the urinary bladder by systematic histologic mapping: implications for pathologic staging of invasive urothelial carcinoma. Am J Surg Pathol 31: 1420–1429, 2007 [DOI] [PubMed] [Google Scholar]
- 229. Paner GP, Shen SS, Lapentino S, Venkataraman G, Barkan GA, Quek ML, Ro JY, Amin MB. Diagnostic utility of antibody to smoothelin in the distinction of muscularis propria from muscularis mucosae of the urinary bladder: a potential ancillary tool in the pathologic stages of invasive urothelial carcinoma. Am J Surg Pathol 33: 91–98, 2009 [DOI] [PubMed] [Google Scholar]
- 230. Parsons CL, Boychuk D, Jones S, Hurst R, Callahan H. Bladder surface glycosaminoglycans: an epithelial permeability barrier. J Urol 143: 139–142, 1990 [DOI] [PubMed] [Google Scholar]
- 231. Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 69: 9–16, 2007 [DOI] [PubMed] [Google Scholar]
- 232. Parsons CL. The therapeutic role of sulfated polysaccharides in the urinary bladder. Urol Clin North Am 21: 93–100, 1994 [PubMed] [Google Scholar]
- 233. Parsons CL, Forrest J, Nickel JC. Effect of pentosan polysulfate therapy on intravesical potassium sensitivity. Urology 59: 329–333, 2002 [DOI] [PubMed] [Google Scholar]
- 234. Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J Urol 145: 732–735, 1991 [DOI] [PubMed] [Google Scholar]
- 235. Persson K, Alm P, Johansson K, Larsson B, Andersson KE. Nitric oxide synthase in pig lower urinary tract: immunohistochemistry, NADPH diaphorase histochemistry and functional effects. Br J Pharmacol 110: 521–530, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Persson K, Igawa Y, Mattiasson A, Andersson KE. Effects of inhibition of the l-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br J Pharmacol 107: 178–184, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Peters KM, Hasenau DL, Anthony M, Kaufman J, Killinger KA, Chancellor MB. Novel therapy with intravesical liposomes for ulcerative interstitial cystitis/painful bladder syndrome. LUTS. In press [DOI] [PubMed] [Google Scholar]
- 238. Planells-Cases R, Valente P, Ferrer-Montiel A, Qin F, Szallasi A. Complex regulation of TRPV1 and related thermo-TRPs: implications for therapeutic intervention. Adv Exp Med Biol 704: 491–515, 2011 [DOI] [PubMed] [Google Scholar]
- 239. Rahnam'i MS, van Koeveringe GA, Essers PB. Prostaglandin receptor EP1 and EP2 site in guinea pig bladder urothelium and lamina propria. J Urol 183: 1241–1247, 2010 [DOI] [PubMed] [Google Scholar]
- 240. Reis H, Tschirdewahn S, Szarvas T, Rubben H, Schmid KW, Grabellus F. Expression of GLUT1 is associated with increasing grade of malignancy in non-invasive and invasive urothelial carcinomas of the bladder. Oncol Lett 2: 1149–1153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Robinson D, Cardozo L. Estrogens and the lower urinary tract. Neurourol Urodyn 30: 754–757, 2011 [DOI] [PubMed] [Google Scholar]
- 242. Rofeim O, Hom D, Freid RM. Use of the neodymium:YAG laser for interstitial cystitis: a prospective study. J Urol 166: 134–136, 2001 [PubMed] [Google Scholar]
- 243. Romih R, Korosec P, de Mello W, Jezernik K. Differentiation of epithelial cells in the urinary tract. Cell Tissue Res 320: 259–268, 2005 [DOI] [PubMed] [Google Scholar]
- 244. Rong W, Spyer KM, Burnstock G. Activation and sensitization of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol 541: 591–200, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Roosen A, Chapple CR, Dmochowski Fowler RR, CJ, Gratzke C, Roehrborn CG, Stief CG, Andersson KE. A refocus on the bladder as the originator of storage lower urinary tract symptoms: a systematic review of the latest literature. Eur Urol 56: 810–819, 2009 [DOI] [PubMed] [Google Scholar]
- 246. Rostand KS, Esko JD. Microbial adherence to and invasion through proteoglycans. Infect Immun 65: 1–8, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Rudick CN, Billips BK, Pavlov VI, Yaggie RE, Schaeffer AJ, Klumpp DJ. Host-pathogen interactions mediating pain of urinary tract infection. J Infect Dis 201: 1240–1249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Sadhukhan PC, Tchetgen MB, Rackley RR, Vasavada SP, Liou L, Bandyopadhyay SK. Sodium pentosan polysulfate reduces urothelial responses to inflammatory stimuli via an indirect mechanism. J Urol 168: 289–292, 2002 [PubMed] [Google Scholar]
- 249. Sanchez-Freire V, Blanchard MG, Burkhard FC, Kessler TM, Kellenberger S, Monastyrskaya K. Acid-sensing channels in human bladder: expression, function and alterations during bladder pain syndrome. J Urol 186: 1509–1516, 2011 [DOI] [PubMed] [Google Scholar]
- 250. Schilling J, Hultgren S. Recent advances into the pathogenesis of recurrent urinary tract infections: the bladder as a reservoir for uropathogenic Escherichia coli. Int J Antimicrob Agents 19: 457–560, 2002 [DOI] [PubMed] [Google Scholar]
- 251. Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534–R547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Schroder A, Newgreen D, Andersson KE. Detrusor responses to prostaglandin E2 and bladder outlet obstruction in wild-type and EP1 receptor knockout mice. J Urol 172: 1166–1170, 2004 [DOI] [PubMed] [Google Scholar]
- 253. Schussler B. Comparison of the mode of action of prostaglandin E2 (PGE2) and sulprostone, a PGE2-derivative, on the lower urinary tract in healthy women. Urol Res 18: 349–352, 1990 [DOI] [PubMed] [Google Scholar]
- 254. Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472: 110–116, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Silva C, Ribeiro MJ, Cruz F. The effect of intravesical resiniferatoxin in patients with idiopathic detrusor instability suggests that involuntary detrusor contractions are triggered by C-fiber input. J Urol 168: 575–579, 2002 [PubMed] [Google Scholar]
- 256. Smet PJ, Moore KH, Jonavicius J. Distribution and colocalization of calcitonin gene-related peptide, tachykinins, and vasoactive intestinal peptide in normal and idiopathic unstable human urinary bladder. Lab Invest 77: 37–49, 1997 [PubMed] [Google Scholar]
- 257. Smith AL, Leung J, Kun S, Zhang R, Karagiannides I, Raz S, Lee U, Glovatschka V, Pothoulakis C, Bradesi S, Mayer EA, Rodriguez LV. The effects of acute and chronic psychological stress on bladder function in a rodent model. Urology 78: 961–967, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Smith CP, Gangitano DA, Munoz A, Salas NA, Boone TB, Aoki KR, Francis J, Somogyi GT. Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem Int 52: 1068–1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Snellings AE, Yoo PB, Grill WM. Urethral flow-responsive afferents in the cat sacral dorsal root ganglia. Neurosci Lett 516: 34–38, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Sonar SS, Schwinge D, Kilic A, Yildirim AO, Conrad ML, Seidler K, Muller B, Renz H, Nockher WA. Nerve growth factor enhances Clara cell proliferation after lung injury. Eur Respir J 36: 105–115, 2010 [DOI] [PubMed] [Google Scholar]
- 261. Steers WD, Tuttle JB. Mechanisms of disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol 3: 101–110, 2006 [DOI] [PubMed] [Google Scholar]
- 262. Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinostide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol 128: 509–522, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, Andersson KE, Hogestatt ED, Zygmunt PM. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol 53: 391–399, 2008 [DOI] [PubMed] [Google Scholar]
- 264. Sui GP, Wu C, Fry CH. Characterization of the purinergic receptor subtype on guinea-pig suburothelial myofibroblasts. BJU Int 97: 1327–1331, 2006 [DOI] [PubMed] [Google Scholar]
- 265. Sui GP, Wu C, Fry CH. Electrical characteristics of suburothelial cells isolated from the human bladder. J Urol 171: 938–943, 2004 [DOI] [PubMed] [Google Scholar]
- 266. Sun TT. Altered phenotype of cultured urothelial and other stratified epithelial cells: implications for wound healing. Am J Physiol Renal Physiol 291: F9–F21, 2006 [DOI] [PubMed] [Google Scholar]
- 267. Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol 290: C27–C34, 2006 [DOI] [PubMed] [Google Scholar]
- 268. Sun Y, Chai TC. Effects of dimethyl sulphoxide and heparin on stretch-activated ATP release by bladder urothelial cells from patients with interstitial cystitis. BJU Int 90: 381–385, 2002 [DOI] [PubMed] [Google Scholar]
- 269. Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev 51: 159–212, 1999 [PubMed] [Google Scholar]
- 270. Szallasi A, Goso C, Blumberg PM, Manzini S. Competitive inhibition by capsazepine of [3H]resiniferatoxin binding to central (spinal cord and dorsal root ganglia) and peripheral (urinary bladder and airways) vanilloid (capsaicin) receptors in the rat. J Pharmacol Exp Ther 267: 728–733, 1993 [PubMed] [Google Scholar]
- 271. Templeman L, Chapple CR, Chess-Williams R. Urothelium derived inhibitory factor and cross-talk among receptors in the trigone of the bladder of the pig. J Urol 167: 742–745, 2002 [DOI] [PubMed] [Google Scholar]
- 272. Teng J, Wang Y, Bjorling DE. Estrogen-induced proliferation of urothelial cells is modulated by nerve growth factor. Am J Physiol Renal Physiol 282: F1075–F1083, 2002 [DOI] [PubMed] [Google Scholar]
- 273. Teng J, Wang ZY, Jarrard DF, Bjorling DE. Roles of estrogen receptor α and β in modulating urothelial cell proliferation. Endocr Relat Cancer 15: 351–364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Teng J, Wang ZY, Prossnitz ER, Bjorling DE. The G protein-coupled receptor GPR30 inhibits human urothelial cell proliferation. Endocrinology 149: 4024–4034, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Thomas JC, DeMarco RT, Pope JC. Molecular biology of ureteral bud and trigonal development. Curr Urol Rep 6: 146–151, 2005 [DOI] [PubMed] [Google Scholar]
- 276. Thumbikat P, Berry RE, Zhou G, Billips BK, Yaggie RE, Zaichuk T, Sun TT, Schaeffer AJ, Klumpp DJ. Bacteria-induced uroplakin signaling mediates bladder response to infection. PLOS Pathogens 5: 1–17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Tincello DG, Taylor AH, Spurling SM, Bell SC. Receptor isoforms that mediate estrogen and progestagen action in the female lower urinary tract. J Urol 181: 1474–1478, 2009 [DOI] [PubMed] [Google Scholar]
- 278. Truschel ST, Ruiz WG, Shulman T, Pilewski J, Sun TT, Zeidel ML, Apodaca G. Primary uroepithelial cultures. A model system to analyze umbrella cell barrier function. J Biol Chem 274: 15020–15029, 1999 [DOI] [PubMed] [Google Scholar]
- 279. Twiss C, Kilpatrick L, Craske M, Buffington CAT, Ornitz E, Rodriguez LV, Mayer EA, Naliboff BD. Increased startle responses in interstitial cystitis: evidence for central hyperresponsiveness to visceral related threat. J Urol 181: 2127–2133, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Tyagi P, Chancellor M, Yoshimura N, Huang L. Activity of different phospholipids in attenuating hyperactivity in bladder irritation. BJU Int 101: 627–632, 2008 [DOI] [PubMed] [Google Scholar]
- 281. Tyagi P, Chuang YC, Yoshimura N, Kaufmann J, Chancellor MB. Bladder instillation of liposomes for bladder coating and drug delivery platform. LUTS 1: S90–S93, 2009 [Google Scholar]
- 282. Tyagi P, Hsieh VC, Yoshimura N, Kaufman J, Chancellor MB. Instillation of liposomes vs dimethyl sulphoxide or pentosan polysulphate for reducing bladder hyperactivity. BJU Int 104: 1689–1692, 2009 [DOI] [PubMed] [Google Scholar]
- 283. Tyagi P, Thomas CA, Yoshimura N, Chancellor MB. Investigations into the presence of functional beta1, beta2 and beta3-adrenoceptors in urothelium and detrusor of human bladder. Int Braz J Urol 35: 76–83, 2009 [DOI] [PubMed] [Google Scholar]
- 284. Tyagi S, Tyagi P, Han-le S, Yoshimura N, Chancellor MB, de Miguel F. Qualitative and quantitative expression profile of muscarinic receptors in human urothelium and detrusor. J Urol 176: 1673–1678, 2006 [DOI] [PubMed] [Google Scholar]
- 285. Vaidyanathan S, Mansour P, Soni BM, Singh G, Sett P. The method of bladder drainage in spinal cord injury patients may influence the histological changes in the mucosa of neuropathic bladder: a hypothesis. BMC Urol 30: 2–5, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Vaidyanathan S, McDicken IW, Ikin AJ, Mansour P, Soni BM, Singh G, Sett P. A study of cytokeratin 20 immunostaining in the urothelium of neuropathic bladder of patients with spinal cord injury. BMC Urol 29: 2–7, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Vaidyanathan S, McDicken IW, Mansour P, Singh G, Soni BM, McCreavy DT, Wiodarski B, Carron JA, Frasser WD, Sett P, Gallagher JA. Parathyroid hormone-related protein (1–34) and urothelial redifferentiation in the neuropathic urinary bladder. Spinal Cord 38: 546–551, 2000 [DOI] [PubMed] [Google Scholar]
- 288. Varley CL, Stahlschmidt J, Lee WC, Holder J, Diggle C, Selby PJ, Trejdosiewicz LK, Southgate J. Role of PPAR gamma and EGFR signaling in the urothelial terminal differentiation process. J Cell Sci 117: 2029–2036, 2004 [DOI] [PubMed] [Google Scholar]
- 289. Varley CL, tahlschmidt J, Smith B, Stower M, Southgate J. Activation of peroxisome proliferator-activated receptor-gamma reverses squamous metaplasia and induces transitional differentiation in normal human urothelial cells. Am J Pathol 164: 1789–1798, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Velasco C, Guarneri L, Leonardi A, Testa R. Effects of intravenous and intravesical administration of suramin, terazosin and BMY 7378 on bladder instability in conscious rats with bladder outlet obstruction. BJU Int 92: 131–136, 2003 [DOI] [PubMed] [Google Scholar]
- 291. Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21: 5670–5677, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Walden PD, Durkin MM, Lepor H, Wetzel JM, Gluchowski C, Gustafson EL. Localization of mRNA and receptor binding sites for the alpha 1a-adrenoceptor subtype in the rat, monkey and human urinary bladder and prostate. J Urol 157: 1032–1038, 1997 [PubMed] [Google Scholar]
- 293. Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest 115: 2412–2422, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain 139: 158–167, 2008 [DOI] [PubMed] [Google Scholar]
- 295. Westropp JL, Buffington CA. In vivo models of interstitial cystitis. J Urol 167: 694–702, 2002 [DOI] [PubMed] [Google Scholar]
- 296. Westropp JL, Kass PH, Buffington CA. Evaluation of the effects of stress in cats with idiopathic cystitis. Am J Vet Res 67: 731–736, 2006 [DOI] [PubMed] [Google Scholar]
- 297. Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int 91: 89–93, 2003 [DOI] [PubMed] [Google Scholar]
- 298. Wood MW, Breitschwerdt EB, Nordone SK, Linder KE, Gookin JL. Uropathogenic E. coli promote a paracellular urothelial barrier defect characterized by altered tight junction integrity, epithelial cell sloughing and cytokine release. J Comp Pathol 5: 1–9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of coricotrophin-releasing factor. Am J Physiol Regul Integr Comp Physiol 296: R1671–R1678, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Yamada T, Ugawa S, Ueda T, Ishida Y, Kajita K, Shimada S. Differential localizations of the transient receptor potential channels TRPV4 and TRPV1 in the mouse urinary bladder. J Histochem Cytochem 57: 277–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Yamaguchi O, Chapple CR. Beta3 adrenoceptors in urinary bladder. Neurourol Urodyn 26: 752–756, 2007 [DOI] [PubMed] [Google Scholar]
- 302. Yanase H, Wang X, Momota Y, Nimura T, Kawatani M. The involvement of urothelial alpha1A adrenergic receptor in controlling the micturition reflex. Biomed Res 29: 239–244, 2008 [DOI] [PubMed] [Google Scholar]
- 303. Yiangou Y, Facer P, Ford A, Brady C, Wiseman O, Fowler CJ, Anand P. Capsaicin receptor VR1 and ATP-gated ion channel P2X3 in human urinary bladder. BJU Int 87: 774–789, 2001 [DOI] [PubMed] [Google Scholar]
- 304. Yu W, Robson SC, Hill WG. Expression and distribution of ectonucleotidases in mouse urinary bladder. PLoS One 6: e18704, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Yu Y, de Groat WC. Effects of stimulation of muscarinic receptors on bladder afferent nerves in the in vitro bladder-pelvic afferent nerve preparation of the rat. Brain Res 1361: 43–53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Zarghooni S, Wunsch J, Bodenbenner M, Bruggmann D, Grando SA, Schwantes U, Wess J, Kummer W, Lips KS. Expression of muscarinic and nicotinic acetylcholine receptors in the mouse urothelium. Life Sci 80: 2308–2313, 2007 [DOI] [PubMed] [Google Scholar]
- 307. Zhang CO, Li ZI, Kong CZ. APF, HB-EGF and EGF biomarkers in patients with ulcerative vs. non-ulcerative interstitial cystitis. BMC Urol 29: 5–7, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]