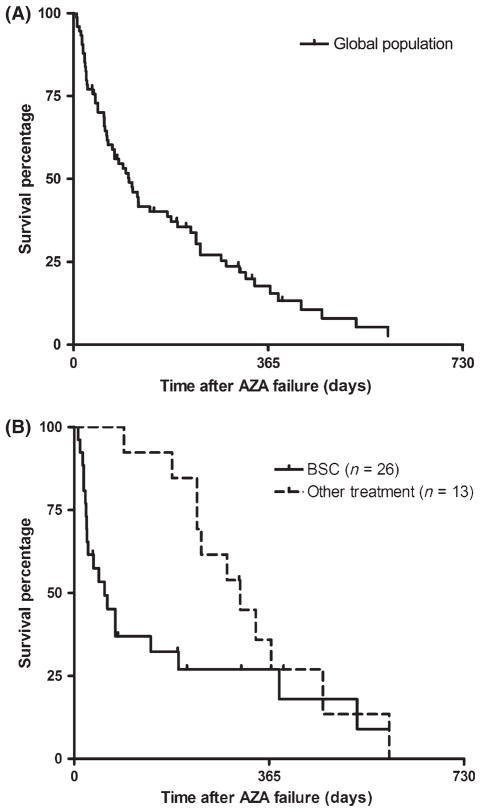
Between 30% and 40% of myelodysplastic syndrome (MDS) patients progress to acute myeloid leukaemia (AML). The prognosis of these secondary AML (sAML) cases is poor with frequent resistance to conventional chemotherapy and an overall survival of <1 year (Nimer, 2008). Epigenetic deregulation has recently been demonstrated as one of the driving phenomena during disease progression (Jiang et al, 2009). Demethylating agents lead to significant clinical benefit for both MDS and AML patients (Fenaux et al, 2009) and represent an attractive option for AML arising after MDS. Nevertheless, a majority of patients fail to respond to azacitidine (AZA) and most responders progress within 2 years (Fenaux et al, 2010). There is no standard of care for salvage therapy after AZA failure and investigational agents have been extensively studied. We recently showed that the outcome of high risk MDS after AZA failure was poor (Prebet et al, 2011), with a median overall survival (OS) of 5·6 months but, to date, the outcome of sAML after AZA failure has not been described, representing a potential limitation for the design and interpretation of clinical studies. We therefore analysed the outcome of 74 patients with sAML after AZA treatment failure, collected from two Johns Hopkins University trials (J9950 and J0443, n = 14) (Gore et al, 2006; Fandy et al, 2009) and the French AZA compassionate programme (n = 60) (Itzykson et al, 2011). Inclusion criteria were: (i) diagnosis of AML arising from MDS according to the French-American-British classification; (ii) 30% or more bone marrow blasts before AZA onset; (iii) having received at least one cycle of AZA. Details of the methodology and AZA regimens have been previously described (Prebet et al, 2011). A minimum of four cycles was planned for each patient. Cytogenetic risk was assessed according to the European Leukaemia Net classification. Response had been assessed with the International Working Group 2003 criteria (Cheson et al, 2003) and haematological improvements (HI) were also classified as response. Survival was calculated from the date of AZA failure. Disease status at the end of AZA was categorized as primary failure in the absence of any response to AZA (including HI), secondary failure if disease progressed after a first response (loss of HI or bone marrow progression) or AZA intolerance (if AZA was stopped because of adverse events regardless of clinical response). Statistical methods were as previously described (Prebet et al, 2011) and statistical analysis was performed using the R.2.3.0. software (R Development Core Team, Vienna, Austria). Patient characteristics are presented in Table I. Overall, patients had advanced disease, with 70% of the cases having a prior duration of MDS of more than 1 year and high-risk cytogenetics in 27% of the patients (including 14 complex cytogenetics). Fifty-two percent of the patients had received prior therapy before AZA: chemotherapy in 37 patients (low dose cytarabine n = 7, AML-like induction chemotherapy n = 30), and immunomodulatory drugs (n = 2). Thirty-two patients had received another drug in addition to AZA [histone deacetylase (HDAC)] inhibitors (n = 25, valproic acid, phenylbutyrate or entinostat) and chemotherapy (n = 7, anthracyclines and hydroxycarbamide given for cytoreduction on cycle 1). The median number of cycles of AZA was 4; 11 patients (15%) achieved a significant response (including six complete responses) and 12 patients (16%) achieved an HI without significant marrow response. At last follow-up, 66 patients had died, and eight remained alive. Median OS was 3·4 months with a 1-year survival probability of 8% [95% confidence interval (2·3–12·1%), Fig 1A]. There was no impact of age, cytogenetic stratification, bone marrow blast count or AZA treatment-related variables (schedule or duration of exposure). There was a trend for a better OS for patients that were not treated with intensive chemotherapy prior to AZA (median OS 4 months vs. 2·8 months, P = 0·12). Only complex cytogenetics (n = 14) were associated with significantly poorer outcome (median OS 1·9 months vs. 3·9 months for non complex cytogenetics (P = 0·05)). Survival of the present sAML patients following AZA failure was significantly shorter than that of higher risk MDS (median OS = 7·5 months, P = 0·001), but similar to that of refractory anaemia with excess blasts in transformation (i.e. bone marrow blast count between 20% and 29% median OS: 4·1 months, P = NS) we previously reported (Prebet et al, 2011). Information regarding treatment given after AZA failure was available in 39 patients (Fig 1B). Most of the patients were treated with best supportive care (BSC, n = 20) or palliative chemotherapy (n = 6, hydroxycarbamide, 6-mercaptopurine or low dose melphalan). Median survival was 2 months and was not influenced by the need for palliative chemotherapy (data not shown). Thirteen patients (33%) were treated with ‘active’ therapies: none of four patients treated with intensive AML-like chemotherapy responded or could bridge to transplantation (i.e. bone marrow blast count between 20% and 29% median OS 7·7 months). This is in line with a recent report regarding intensive chemotherapy for sAML (Bello et al, 2011), which showed that treatment with hypomethylating agents prior to induction chemotherapy was associated with a poorer outcome. Six patients were treated with other epigenetic agents (decitabine (n = 4) or HDAC inhibitors (n = 2)) but none responded (median survival 11 months). Finally, three patients were treated with allogeneic SCT. None of them received salvage regimens before transplantation. Two of them died of progressive disease after 10 and 11 months, respectively, and one patient was still alive after 14 months. There was a trend for improved survival in the group who received ‘active’ treatments as compared to palliative care (10 months vs. 2 months respectively, P = 0·08, Fig 1B). These results may possibly reflect patient selection bias but also stress the need for clinical trials in that subgroup of patients.
Table I.
Characteristics of the secondary AML patients who failed AZA treatment.
| Secondary AML (n = 74) | |
|---|---|
| Median age (years) | 70 |
| Male/female | 1·6 (46/28) |
| Therapy-related (Unknown = 8) | 4/66 (6%) |
| Disease duration before AZA | |
| <1 year | 22 (30%) |
| 1–2 years | 21 (29%) |
| More than 2 years | 31 (41%) |
| Median bone marrow blast before AZA (%) | 48% (30–74%) |
| Cytogenetics (AML stratification) | |
| Intermediate risk | 47 (64%) |
| High risk | 27 (36%) |
| Frontline AZA treatment | 36 (49%) |
| AZA-based combination | 27 (36%) |
| Cycle1 dose of AZA > 500 mg/m2 | 59 (79%) |
| Median duration of AZA cycles | 29 days |
| Number of AZA cycles | |
| 6 or less | 54 (73%) |
| 7–12 | 13 (18%) |
| More than 12 | 7 (9%) |
| Type of failure | |
| Primary failure with SD | 30 (42%) |
| Primary Failure with PD | 21 (28%) |
| Secondary failure (after prior response) | 21 (28%) |
| AZA intolerance | 2 (2%) |
AML, acute myeloid leukaemia; AZA, azacitidine; SD, stable disease; PD, progressive disease.
AZA courses are described with the total dose per cycle; 500 mg/m2/cycle (corresponding to 95% of the registered 75 mg/m2/day for 7 days schedule) was defined as the reference dose.
Fig 1.
(A) Kaplan Meier estimates of the overall survival after azacitidine (AZA) failure for secondary acute myeloid leukaemia. (B) Survival analysis according to the salvage treatment regimen. The curves represent the survival estimates for the cohort of patients. Each tick mark represents a censored patient. Overall response rate for each treatment group is presented with the number of patients evaluable for response in each cohort. Best supportive care (BSC) included palliative chemotherapy; other treatments included allogeneic transplantation, epigenetic-targeted agents and intensive ‘acute myeloid leukaemia-like’ chemotherapy regimens.
This report is the first to specifically address the issue of the outcome of sAML after primary or secondary AZA failure. The median OS of 3·6 months was even poorer than the survival of higher risk MDS in this situation, with a 1-year survival of only 8%. This even worse prognosis also indicates the need for closer monitoring of sAML during AZA treatment. Conventional treatment, such as BSC or cytotoxic drugs, appeared of little benefit for such patients. Besides cytogenetics, no significant pretreatment variable was associated with OS, possibly due to the limited number of patients and/or the dismal outcome in all sub-groups. Our study gives the expected baseline for patients treated with conventional care and will be useful for the design of future clinical trials.
Acknowledgments
We would like to thank all the centres from the Groupe Francophone des Myelodysplasies and the Division of Hematologic Malignancies, Sidney Kimmel Cancer Center at Johns Hopkins University. TP is supported by a grant from Fondation Monahan, Fullbright fundation, Paris, France. SG is supported by NIH grant 2K24CA111717-06A1.
Footnotes
Authors Contribution
TP treated patients, conceived research, collected data, analysed the data and wrote the manuscript. NV PF SG FD treated patients, analysed the data and participated in the writing of the manuscript. BQ, CG, ST, and OBR treated patients and collected data. BE analysed the data and participated in the writing of the manuscript.
Disclosures of conflicts of interest
TP, NV, ST, CG, PF and SG received research support from Celgene. SG owns stock in Celgene.
References
- Bello C, Yu D, Komrokji RS, Zhu W, Wetzstein GA, List AF, Lancet JE. Outcomes after induction chemotherapy in patients with acute myeloid leukemia arising from myelodysplastic syndrome. Cancer. 2011;117:1463– 1469. doi: 10.1002/cncr.25598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of Clinical Oncology. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- Fandy TE, Herman JG, Kerns P, Jiemjit A, Sugar EA, Choi SH, Yang AS, Aucott T, Dauses T, Odchimar-Reissig R, Licht J, McConnell MJ, Nasrallah C, Kim MK, Zhang W, Sun Y, Murgo A, Espinoza-Delgado I, Oteiza K, Owoeye I, Silverman LR, Gore SD, Carraway HE. Early epigenetic changes and DNA damage do not predict clinical response in an overlapping schedule of 5-azacytidine and entinostat in patients with myeloid malignancies. Blood. 2009;114:2764–2773. doi: 10.1182/blood-2009-02-203547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach C, Silverman LR. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF, Dombret H, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. Journal of Clinical Oncology. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci M, Grever M, Galm O, Dauses T, Karp JE, Rudek MA, Zhao M, Smith BD, Manning J, Jiemjit A, Dover G, Mays A, Zwiebel J, Murgo A, Weng LJ, Herman JG. Combined DNA methyl-transferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Research. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- Itzykson R, Thepot S, Quesnel B, Dreyfus F, Beyne-Rauzy O, Turlure P, Vey N, Recher C, Dartigeas C, Legros L, Delaunay J, Salanoubat C, Visanica S, Stamatoullas A, Isnard F, Marfaing-Koka A, de BS, Chelghoum Y, Taksin AL, Plantier I, Ame S, Boehrer S, Gardin C, Beach CL, Ades L, Fenaux P. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–411. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Dunbar A, Gondek LP, Mohan S, Rataul M, O’Keefe C, Sekeres M, Saunthararajah Y, Maciejewski JP. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood. 2009;113:1315–1325. doi: 10.1182/blood-2008-06-163246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimer SD. Myelodysplastic syndromes. Blood. 2008;111:4841–4851. doi: 10.1182/blood-2007-08-078139. [DOI] [PubMed] [Google Scholar]
- Prebet T, Gore SD, Esterni B, Gardin C, Itzykson RR, Thepot S, Dreyfus F, Beyne-Rauzy O, Recher C, Ades L, Quesnel B, Beach CL, Fenaux P, Vey N. Outcome of high risk myelodysplastic syndrome after azacitidine treatment failure. Journal of Clinical Oncology. 2011;29:3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]