Abstract
Objective
To assess whether high ribonucleotide reductase subunit M1 (RRM1) expression in patients with resected, muscle-invasive (T2–4NxM0) urothelial carcinoma (UC) correlated with longer overall survival (OS). RRM1 is the primary cellular target of gemcitabine and previous studies in resected early-stage lung cancer have shown a survival benefit for patients with high expression.
Patients and methods
In all, 84 radical cystectomy specimens with muscle-invasive UC were identified from existing tissue microarrays. The patients’ medical records were retrospectively reviewed to confirm pathology and stage. Specimens were analysed for RRM1 expression using automated quantitative analysis. The median value of RRM1 was established a priori as the threshold for high and low expression.
Results
The median age of the patients was 69 years. Stages were nearly equally distributed: 30%, 38%, and 32% for stage II, III, and IV, respectively. Most were high grade (99%) with no nodal involvement (69%). The median (range) OS was 2.0 (0–13.1) years. Tumoral RRM1 levels did not correlate with OS for the entire cohort, but when adjusted for age, high tumoral RRM1 expression in younger patients (aged < 70 years) correlated with increased survival. Younger patients with high RRM1 expression had a median OS of 10.6 years compared with 1.6 years in older patients (P = 0.001). There was no difference in OS among low RRM1 expressors: 2.3 vs 1.6 years in younger and older patients, respectively (P = 0.22).
Conclusions
Our results suggest that high RRM1 expression may be prognostic for improved survival in patients with muscle-invasive UC aged < 70 years.
Keywords: urothelial carcinoma, transitional cell carcinoma of the bladder, ribonucleotide reductase, RRM1, age, prognostic marker, survival
Introduction
Using genomic biomarkers to assess prognosis and chemosensitivity is considered an important next step in advancing the treatment of muscle-invasive bladder cancer. Although radical cystectomy (RC) will cure a substantial number of patients with early stage urothelial carcinoma (UC), many patients with muscle-invasive disease will recur after definitive surgery and die from the disease [1]. The most reliable prognostic factors for localized disease are pathological stage and histological grade, while prognosis of metastatic disease is driven by histology and clinical factors such as performance status, serum levels of haemoglobin and alkaline phosphatase, and presence of visceral metastases [1–7].
As genomic alterations are enumerated in the pathogenesis of UC, clarifying which genes contribute to the natural history of the disease and which confer chemoresistance is vital to achieving maximal response and avoiding toxicity. p53 and excision repair cross-complementation group 1 (ERCC1) genes, have already shown promise as prognostic and predictive markers, respectively, but have not yet been validated [8–10]. Ribonucleotide reductase subunit M1 (RRM1) is the regulatory subunit of ribonucleotide reductase and the primary cellular target of gemcitabine [11]. From past work elucidating its role in DNA repair, in gemcitabine metabolism, and in regulation of metastasis formation, we hypothesize that RRM1 is important in UC both for survival independent of treatment (prognosis) and in predicting response to chemotherapy [11–15].
The utility of RRM1 as a biomarker has been best assessed in non-small cell lung cancer (NSCLC) where it has been found to be both prognostic of survival and predictive of response to gemcitabine-based therapy. Increased mRNA expression of RRM1 was associated with increased survival of patients with NSCLC undergoing definitive surgical resection of localized disease and with decreased response to gemcitabine-platinum chemotherapy for locally advanced disease [12,15]. To our knowledge, only one other study has investigated the role of RRM1 in UC [8]. In that small series, patients with surgically incurable bladder cancer, who received gemcitabine-cisplatin with or without paclitaxel, showed a trend toward longer time to disease progression if their tumours expressed low RRM1 levels.
We retrospectively analyzed whether RRM1 was an independent prognosticator for survival in patients with UC who had undergone RC as primary treatment. Given previous studies showing a survival benefit to higher tumoral RRM1 expression in resected early stage lung cancer, as well as the trend toward longer time to disease progression with low expression in advanced bladder cancers, we hypothesized that patients with resectable, muscle-invasive UC with high tumoral RRM1 expression would have longer overall survival (OS) compared with patients with low tumoral RRM1 expression. Given the lack of subsequent treatment and response data for most of the patients, the present study did not address the predictive aspect of RRM1 expression (i.e. the interaction between RRM1 expression and treatment with gemcitabine or other systemic chemotherapy regimen).
Patients and methods
We identified 84 RC specimens with muscle-invasive UC on existing tissue microarrays (TMAs). The TMAs contained 340 specimens of various human tissues obtained from patients at Stanford University Medical Center from 1995 to 2002. Two separate specimen cores per patient were arrayed in the same recipient block with a total of two blocks to encompass all 340 patients. Tissue core punches were from near 100% tumour enriched regions.
The medical records of patients with UC were retrospectively reviewed to confirm pathology and stage. Presence of muscle invasion on the RC specimen was mandatory. Inclusion criteria required patients who had undergone RC for muscle-invasive carcinoma of the bladder with confirmation of pathological T2–T4; N0, N+ disease. Patients with superficial bladder cancer, distant metastases, and non-TCC histologies were excluded. The study was approved by the Institutional Review Boards of Stanford University and H. Lee Moffitt Cancer Center and Research Institute.
Known prognostic factors such as pathological grade, stage, and histology were evaluated. Clinical factors such as performance status, haemoglobin, serum lactate dehydrogenase (LDH), and alkaline phosphatase were to be evaluated but missing data on most of patients prevented analysis. Pathological variables such as presence of nodal involvement or angiolymphatic invasion were also catalogued. As many patients did not receive follow-up care or adjuvant therapy at our institution, data on subsequent treatment and presence of disease progression were not available for most of the patients.
In situ detection and quantification of RRM1 protein expression was performed by immunofluorescence combined with automated quantitative analysis (AQUA). Antigens were retrieved by microwave oven treatment for 15 min in 0.01 mol/L of Na-citrate, Tris-HCl, or Tris-EDTA buffer at an optimized pH. The slides were blocked for 30 min with 0.3% BSA and then incubated overnight at 4 °C in appropriately diluted primary antibody/antiserum (R1AS-6b, 1 : 800). For identification of carcinomatous cells, antibody to cytokeratin was used (monoclonal mouse anti-human pancytokeratin AE1/AE3, 1 : 200, #M3515, Dako Cytomation, Carpinteria, CA, USA). Slides were washed and incubated with two different secondary antibodies for 1 h. (Envision® labelled polymer-horseradish peroxidase anti-rabbit, # K4011, specific to the RRM1 antiserum, 1 : 200; Alexa 555 goat anti-mouse, #A21424, specific to mouse keratin, Dako Cytomation). For fluorescence amplification for RRM1, slides were exposed to Cy5-Tyramide (1 : 50) for 10 min at room temperature. They were then mounted with Prolong Gold anti-fade reagent with 4′-6-diamidino-2-phenylindole (DAPI) mound solution. The final TMA slides were scanned with SpotGrabber, and image data were analysed with AQUA (Version 1.6, HistoRx, New Haven, Connecticut). Further details on the technical aspects of AQUA including signal acquisition and background signal adjustment can be found in the original publication by Camp et al. [16] The lowest possible AQUA score is 0 and the highest is 33 333. The value for RRM1 positivity was established a priori as the median threshold between high and low expression.
OS was defined as the time from RC to the date of death. The vital status of the patient was verified using the publically available National Social Security Death Index (SSDI). If not found, patients were censored as ‘alive’ as of 15 September 2008, which was approximately 3 months before this analysis, or as of a later date if supported by their medical record.
The primary endpoint of this analysis was OS based on high vs low RRM1 expression The mean values for the AQUA scores from duplicate readings of RRM1 nuclear expression were calculated and dichotomized into high and low expressors based on the median. A Cox proportional hazards model was fit to assess the significance of gene expression along with age, gender, tumour stage, and nodal status. Model covariates were selected by a backwards stepwise hierarchical procedure with first and second order interactions by the Bayesian Information Criterion. Significance was assessed via the log-rank test. Recent studies, such as that of Eisen et al. [17] a retrospective review of the impact of age in the TARGET trial, which evaluated sorafenib in cytokine-refractory metastatic RCC, have used a higher age limit of 70 years to define older and younger subsets. Their consult with geriatric clinicians indicated that this was the age beyond which individuals tended to be classified as elderly. Similarly, in a retrospective review of all studies published since 2000 that evaluated 5-year OS rates after RC for bladder cancer in elderly patients, no study classified the older subset as aged < 70 years [18]. Given these studies and the ageing population, we chose 70 years as a rationale threshold between younger and older patients.
Results
In all, 84 RC specimens were evaluable. Baseline patient and tumour characteristics were catalogued (Table 1). Most patients were men (75%) and the median (range) age of the patients was 69.3 (49.6–87.6) years. Most tumours were high grade (99%) with no nodal involvement (69%). Using the American Joint Committee on Cancer (AJCC) staging system, 33%, 43%, and 24% of the patients were T2, T3, and T4 respectively. There was a near equal distribution of stage: 30%, 38%, and 32% for stage II, III, and IV, respectively. Given missing data on most patients, the following variables could not be assessed: performance status, levels of serum LDH and alkaline phosphatase, details of adjuvant therapy administration, subsequent treatments, or time to progression.
Table 1.
Patient and tumour characteristics
| Variable | Value |
|---|---|
| Median (range) age, years | 69.3 (49.6–87.6) |
| N (%): | |
| Gender: male/female | 63/21 (75/25) |
| Grade: high/low | 83/1 (99/1) |
| Nodal involvement: | |
| negative | 58 (69) |
| positive | 26 (31) |
| Stage | |
| II | 25 (30) |
| III | 32 (38) |
| IV | 27 (32) |
| TNM stage (AJCC) | |
| T2 | 28 (33) |
| T3 | 36 (43) |
| T4 | 20 (24) |
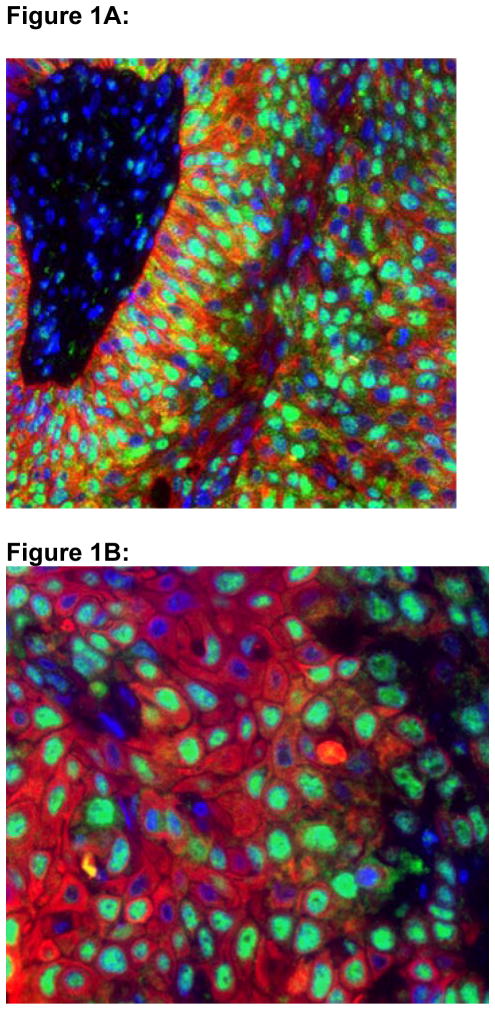
In all, 84 RC specimens with invasive UC and the other 256 ‘controls’ of various other human tissues, RRM1 expression showed a granular nuclear pattern (Fig. 1A,B). As RRM1 is required for nucleotide synthesis and other cellular functions, essentially all cells express RRM1. However, the levels of expression in various tumour types and noncancerous tissue vary over a wide range and overlap [15]. For the UC specimens (84), the gene-expression scores ranged from 305.4 to 3138.1 (Table 2). The median RRM1 expression, the threshold between high and low expression, was 1493.3.
Figure 1.
Figures 1A and 1B: Confocal Microscopy of RRM1 Expression in a Radical Cystectomy Specimen.
RRM1 displays a coarse granular staining pattern with predominant nuclear localization. DAPI staining of nuclei is shown in blue, immunofluorescent staining of cytokeratin is shown in red, and immunofluorescent staining of RRM1 is shown in green. Figure 1A is at 400x magnification; Figure 1B is at 600x magnification.
Table 2.
Descriptive summary of RRM1 tumour expression in all specimens, the levels of expression between the two specimens from each patient were quite similar
| RRM1 expression | Specimen 1 | Specimen 2 | Average |
|---|---|---|---|
| Minimum | 305.4 | 525.2 | 468.8 |
| Maximum | 3138.1 | 2825.4 | 2825.4 |
| Median | 1488.7 | 1479.6 | 1493.3 |
| Mean | 1507.3 | 1511.3 | 1512.4 |
| S D | 535.8 | 523.0 | 481.0 |
| Count | 80 | 81 | 84 |
At the time of this analysis, 63 patients had died (75%) and 21 patients were censored as alive (25%). Only one 70-year-old patient, whose tumour had high RRM1 expression (mean RRM1 was 1614.0), could not be evaluated for survival by the SSDI as he was not a USA citizen. He was censored with a survival of zero days as his last medical record was before surgery, which probably resulted in under-reporting of survival in his case.
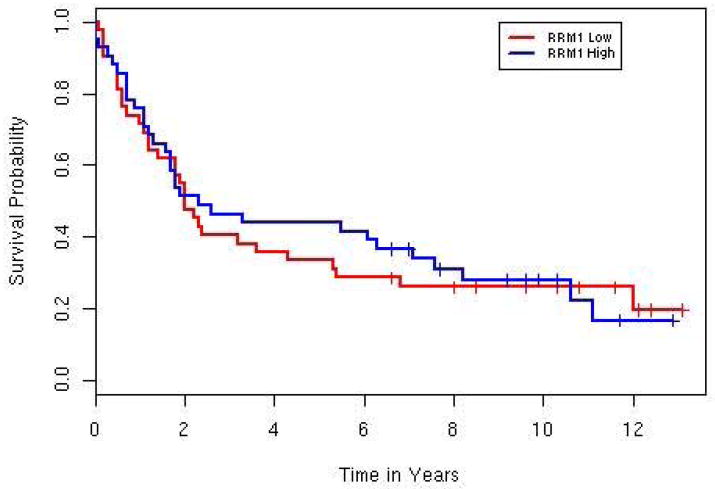
The median (range) OS for the 84 patients with UC was 2.0 (0–13.1) years. There was no difference in survival between high (> median) and low RRM1 expressors in all 84 patients (P = 0.81, Fig. 2). A Cox Proportional Hazards model was fit to the data with the following predictors: RRM1 expression, age, gender, T stage, nodal involvement (positive, negative), and stage group. Only age was prognostic of survival on univariate analysis (Table 3). On multivariate analysis, the model selected included RRM1 expression, age, and nodal status with interactions and is summarized in Table 4. Given nodal status, age, and RRM1 expression, T stage did not add anything to the multivariate model. In patients with high RRM1 expression, younger patients (17 aged <70 years) had significantly increased OS at 10.6 years vs 1.6 years in older patients (25 aged ≥ 70 years; P = 0.001; Fig. 3). However, in patients with low RRM1 expression there was no significant difference in survival based on age: 2.3 vs 1.6 years in patients aged < 70 and ≥ 70 years, respectively (P = 0.22, Fig. 3). There was an interaction between age and nodal status (hazard ratio 6.9, 95% CI 1.67–28.6, P = 0.008). As expected patients with negative nodal status had increased survival, but the difference was more pronounced in younger patients (5.5 vs 3.8 years) than in older patients (1.7 vs 0.9 years). Older patients had slightly less nodal involvement in this population, 29% vs 33%. Of the 43 younger patients, there were 17 high and 26 low expressors (Table 5). Fewer younger high expressors had nodal involvement (three patients) and stage T4 disease (two) compared with the younger low expressors (11 and 12 patients, respectively). While the patient numbers are too few for significance testing, the younger high expressors had increased survival compared with their respective T4 and node-positive low expressor cohorts. The median OS of T4 high expressors was 2.2 months compared with 1.0 month in the younger low expressors. The young, node-positive, high expressors had a median OS of 10.3 months compared with 2.3 months in their low expressing cohort.
Figure 2. Overall survival curves for RRM1 expression, high versus low, for the entire cohort.
No significant difference in survival between high and low RRM1 expressors was observed (2.1 vs. 2.0 years, p=0.81). The median OS was calculated with censored observations, which are denoted as “pluses” in the figure.
Table 3.
Univariate analysis evaluating baseline patient characteristics
| Characteristic | No. of patients | Median OS, years | Hazard ratio (95% CI) | P |
|---|---|---|---|---|
| RRM1 expression: | ||||
| high | 42 | 2.1 | 0.94 | 0.81 |
| low | 42 | 2.0 | (0.57, 1.54) | |
| Age, years: | ||||
| < 70 | 43 | 5.4 | 2.19 | 0.002 |
| ≥ 70 | 41 | 1.3 | (1.32, 3.63) | |
| Gender: | ||||
| male | 63 | 2.0 | 0.95 | 0.86 |
| female | 21 | 2.0 | (0.55, 1.66) | |
| T stage: | ||||
| T2 | 28 | 4.0 | 0.68 (0.40, 1.15) |
0.15 |
| T3 | 36 | 1.4 | 1.11 (0.67, 1.84) |
0.68 |
| T4 | 20 | 1.2 | 1.46 (0.82, 2.57) |
0.2 |
| Nodal involvement: | ||||
| positive | 26 | 1.8 | 0.89 | 0.66 |
| negative | 58 | 2.3 | (0.52, 1.51) | |
| Stage group | ||||
| II | 25 | 4.3 | 0.62 (0.36, 1.08) |
0.091 |
| III | 32 | 1.2 | 1.34 (0.80, 2.23) |
0.26 |
| IV | 27 | 1.8 | 1.20 (0.71, 2.02) |
0.51 |
Table 4.
Multivariate analysis: Cox proportional hazard model
| Variable | Hazard ratio (95% CI) | P |
|---|---|---|
| Age | 0.02 (0.001, 0.70) | 0.031 |
| RRM1 expression | 108.98 (4.57, 2600.98) | 0.004 |
| Nodal Status | 1.05 (0.43, 2.55) | 0.92 |
| RRM1 expression and nodal status | 0.08 (0.017, 0.35) | <0.001 |
| Age and RRM1 status | 3.66 (1.06, 12.61) | 0.04 |
| Age and nodal status | 6.90 (1.67, 28.60) | 0.008 |
Figure 3. Overall survival curves for RRM1 expression based on age (<70 yrs or >70 yrs).
Age makes a significant difference in estimated survival for patients with high tumoral RRM1 expression (left, 10.6 vs. 1.6 years, p=0.0013), but no difference for patients with low tumoral RRM1 expression (right, 2.3 vs. 1.6 years, p=0.22).
Table 5.
RRM1 expression by stage and age: of the younger patients with T4 disease, high RRM1 expressors had a median OS of 2.2 years compared with 1.0 year in the low-expressor cohort. Of the younger patients with node-positive disease, high expressors had a median OS of 10.3 years compared with 2.3 years in low expressors
| T stage | Age < 70 years
|
Age ≥ 70 years
|
||||||
|---|---|---|---|---|---|---|---|---|
| RRM1 high (n = 17) | Median OS, years | RRM1 low (n = 26) | Median OS, years | RRM1 high (n = 25) | Median OS, years | RRM1 low (n = 16) | Median OS, years | |
| N (%): | ||||||||
| T2 | 7 | 8 | 15 (35) | 6 | 7 | 13 (32) | ||
| T2N0 | 7 | 7 | 14 | 5 | 6 | 11 | ||
| T2N1 | 0 | 1 | 1 | 1 | 1 | 2 | ||
| T3 | 8 | 6 | 14 (33) | 15 | 7 | 22 (54) | ||
| T3N0 | 5 | 1 | 6 | 12 | 3 | 15 | ||
| T3N1 | 3 | 5 | 8 | 3 | 4 | 7 | ||
| T4 | 2 | 2.2 | 12 | 1.0 | 14 (33) | 4 | 2 | 6 (15) |
| T4N0 | 2 | 7 | 9 | 3 | 0 | 3 | ||
| T4N1 | 0 | 5 | 5 | 1 | 2 | 3 | ||
| Total nodal+ | 3 | 10.3 | 11 | 2.3 | 14/43 (33) | 5 | 7 | 12/41 (29) |
Discussion
In 2008, 68 810 new cases and 14 100 deaths from bladder cancer were estimated to occur [19]. If patients have not received neoadjuvant platinum-based chemotherapy, the current standard of care for muscle-invasive disease after RC and no adverse features, is no subsequent systemic therapy. Conversely, if patients have adverse clinical and pathological features after RC, such as nodal involvement or angiolymphatic invasion, most physicians advocate adjuvant treatment with platinum-based regimens due to the improved time to recurrence reported in the small, often underpowered studies that have been completed [20–24].
The most reliable prognostic factors for survival in resectable, muscle-invasive bladder cancer are stage and histological grade, but factors such as angiolymphatic invasion, extracapsular nodal extension, and gene expression may also play a role [1,4,25]. In contrast, prognostic factors for survival in patients with metastatic disease are driven mostly by clinical factors. Poor performance status, the presence of visceral metastases (e.g. bone, liver, lung), non-TCC histology, high LDH, low haemoglobin, and high alkaline phosphatase suggestive of liver or bone metastases have all correlated with poorer survival in clinical trials [2,3,5–7]. More recently, the contribution of certain genes, such as p53 and ERCC1, to the prognosis and response to chemotherapy in UC has been recognized but not validated [8–10].
RRM1 is the regulatory subunit of ribonucleotide reductase. Ribonucleotide reductase is an enzyme that converts ribonucleotides to deoxynucleotides, which are essential for DNA synthesis and repair [11,26]. Tumoral RRM1 expression has correlated with survival in patients with early stage resected NSCLC and with chemotherapy resistance in patients with locally advanced inoperable NSCLC. Zheng et al. [15] evaluated RRM1 and ERCC1 gene expression in 187 patients with resected early stage NSCLC who were not treated with adjuvant chemotherapy. The median disease-free survival and OS were significantly greater in patients with high RRM1 expression than in those with low expression (> 120 vs 54.5 months, P = 0.004). OS was also significantly increased in patients with high RRM1 expression (> 120 vs 60.2 months, P = 0.02). RRM1 expression highly correlated with ERCC1 expression. The survival advantage was limited to the 30% of patients with NSCLC with tumours that had high expression of both RRM1 and ERCC1. Conversely, NSCLC cell lines genetically engineered for high RRM1 expression and patients with locally advanced NSCLC with high tumoral RRM1 expression had increased resistance to gemcitabine-platinum chemotherapy [12].
There has been little work assessing the affect of RRM1 in UC. One series of 57 patients investigated the relationship between expression of RRM1, ERCC1, BRCA1, caveolin-1 and outcome in metastatic or locally advanced, surgically incurable (T4b, N0–1) patients with bladder cancer treated with gemcitabine-cisplatin or gemcitabine-cisplatin-paclitaxel [8]. There was a trend toward longer time to progression with advanced bladder tumours that expressed low levels of all markers. RRM1 failed to predict survival or response to chemotherapy. Given the small sample size of the advanced bladder cancer study, their findings of a positive trend toward time to progression with low RRM1 expression, and the prior NSCLC data showing increased survival with high RRM1 expression in patients with resected lung cancer and no subsequent chemotherapy, the prognostic potential of RRM1 in resectable UC deserved investigation.
While the present retrospective analysis did not find a difference in OS based on degree of RRM1 expression, there was an interesting interaction between age and RRM1 expression. In patients with higher RRM1 expression, younger patients (aged < 70 years) had higher OS than older patients (Fig. 3). While pathological factors are known to be prognostic for survival, whether advanced age correlates with poorer survival after RC remains controversial. Several series have shown that RC and aggressive treatment may improve survival even in octagenerians, but other studies [18,27–29] such as the large retrospective review of 888 patients by Nielsen et al. [29] have concluded that higher chronological age is associated with poorer survival in this setting. Selection bias, comorbidities such as renal insufficiency and cardiovascular disease, and the under use of perioperative chemotherapy are certainly potential contributors to poorer survival in older patients.
Furthermore, the difference in survival based on RRM1 expression in the younger vs older cohorts cannot simply be attributed to a higher percentage of earlier stage cancers in the younger subset at the time of RC (Table 5). The two groups were roughly equal with 33% of younger patients having nodal involvement compared with 29% of the older subset. In terms of T stage, 33% of younger patients had T3 disease compared with 54% of older patients, and 33% of younger patients had T4 disease compared to only 15% of older patients. This novel finding suggests that the biology of bladder cancer in ‘younger’ patients is inherently different from that in the older cohort, such that RRM1 gene expression should be the target of more comprehensive investigation in this subset of patients.
The question of whether the difference in OS amongst the younger patients can be attributed to the low expressors having a greater amount of higher stage disease was examined. Of the younger patient high- and low-expressor subsets, 47% and 23% had T3 disease, 12% and 47% had T4 disease, and 18% and 42% had nodal involvement, respectively (Table 5). Given the small cohort sizes, we are unable to exclude this possibility. However, if true, one would expect the median survival of both T4 groups or both nodal groups to be fairly similar. Any conclusions are limited by the small number of patients in this subset analysis, but the median OS in the comparative groups was not as similar as one might expect if it was due to T stage or nodal status alone. The younger T4 high expressors (two patients) had a median OS of 2.2 months compared with 1 month in the low expressors (12). Likewise, the younger node-positive high expressors (three patients), had a median OS of 10.3 months compared with 2.3 months among their low RRM1-expressing cohort (11).
The observation of more low expressors with higher stage disease lends further support to the theory that RRM1 may act as a tumour suppressor gene, and that high expression may result in less aggressive or slower progressing tumours. This relationship between RRM1 expression and survival was reported in natural survival experiments where transgenic mice with high RRM1 levels developed fewer tumours, repaired chemically induced DNA damage more efficiently, and lived longer than mice with low RRM1 levels after identical exposure to a carcinogen [30]. If true in humans, this finding would be most relevant in the population that stands to live the longest if their disease is treated, i.e. those patients aged < 70 years. We postulate that high expression of the likely tumour suppressor gene, RRM1, is associated with better resistance to genome damage caused by carcinogens [30]. As a population ages, one would expect to see a higher percentage of people with higher RRM1 levels. The results of the present study support this postulation: there were a higher percentage of older patients with high RRM1-expressing tumours (25) compared with low expressing tumours (16).
Limitations of the present study include its retrospective nature and small sample size. Inherent in any retrospective study is missing data. RRM1 is considered a primary cellular target of gemcitabine and studies in NSCLC have shown improved response to gemcitabine in patients with low tumoral expression [11,12]. For median OS among the four cohorts in the present study, the outlier was the younger, high RRM1-expressing group at > 10 years. If RRM1 is a principle determinant of gemcitabine efficacy in bladder cancer as suggested by the previously described work in in vivo studies, murine models, and in human NSCLC and other solid tumours, perioperative chemotherapy with gemcitabine should not be as effective in patients with high RRM1. If chemotherapy were the primary reason for the younger patients’ better outcome and assuming most patients received gemcitabine-platinum combinations as was common practice at Stanford and the surrounding community since 2000, the expectation would be that either patients with low RRM1 would do better than those with high expression or that patients with low RRM1 would do at least as well as those with high expression. Given its frequent use in the adjuvant setting for UC, correlating survival with RRM1 expression and gemcitabine administration should be examined. Additionally, as nearly half of patients were aged ≥ 70 years, death from nonmalignant causes was certainly a possibility. Information on disease-specific mortality was not available for most patients. But, the risk of recurrence in this patient population is upwards of 30–50% [1]. Based on actuarial life expectancy tables, one would expect an average patient to have approximately 13 life-years remaining if aged 70 years, 10 years if aged 75 years, and 8 years if aged 80 years [31]. Thus, it is likely that with a median OS of approximately 2 years in the present study population that many patients might have died from recurrent bladder cancer. However, without the actual disease-specific survival data, we cannot draw any firm conclusions. A future prospective study involving analysis of disease-specific survival is planned. The impact of other genes such as ERCC1 and p53 and their relationship to RRM1 should also be evaluated.
Accepting these limitations, the discovery of a significant interaction between the degree of RRM1 expression and age in this exploratory analysis is certainly hypothesis generating. Future plans include prospective analysis in a larger cohort with assessment of other pretreatment clinical characteristics, the role of perioperative chemotherapy, and the type of therapy received (e.g. gemcitabine, type of platinum).
In conclusion, the age of personalized medicine is imminent. Using genetic markers to prognosticate and to eventually select patients most likely to benefit from gemcitabine- or toxic platinum-containing regimens is the next logical step in improving the treatment of resectable, muscle-invasive bladder cancer. To our knowledge, the present study is the first to evaluate the impact of RRM1 expression on survival in patients with resectable UC. The present results suggest that high RRM1 expression may be a prognostic factor for improved survival in patients with resectable, muscle-invasive UC who are aged < 70 years. These results deserve further study in a larger prospective analysis with disease-specific survival assessment and correlation with other possible prognostic genes such as ERCC1.
Acknowledgments
We greatly appreciate the contribution of Kelli Montgomery in the Stanford Department of Pathology for the organization of the TMA database and for administrative support.
L.C.H. was funded by NCI/NIH T32 CA009287. G.B. is funded by R01-CA102726 and R01-CA129343. G.B. has a licensing agreement with Genzyme and receives royalties. For the present study, his laboratory received de-identified specimens with no link to outcome. No other authors had disclosures to report.
Abbreviations
- RRM1
ribonucleotide reductase subunit M1
- ERCC1
excision repair cross-complementation group 1
- RC
radical cystectomy
- UC
urothelial carcinoma
- OS
overall survival
- TMA
tissue microarray
- LDH
lactate dehydrogenase
- NSCLC
non-small cell lung cancer
- AQUA
automated quantitative analysis
- DAPI
4′-6-diamidino-2-phenylindole
- SSDI
National Social Security Death Index
- AJCC
American Joint Committee on Cancer
References
- 1.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–81. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 3.Bellmunt J, Albanell J, Paz-Ares L, et al. Pretreatment prognostic factors for survival in patients with advanced urothelial tumors treated in a phase I/II trial with paclitaxel, cisplatin, and gemcitabine. Cancer. 2002;95:751–7. doi: 10.1002/cncr.10762. [DOI] [PubMed] [Google Scholar]
- 4.Bochner BH, Kattan MW, Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol. 2006;24:3967–72. doi: 10.1200/JCO.2005.05.3884. [DOI] [PubMed] [Google Scholar]
- 5.Geller NL, Sternberg CN, Penenberg D, Scher H, Yagoda A. Prognostic factors for survival of patients with advanced urothelial tumors treated with methotrexate, vinblastine, doxorubicin, and cisplatin chemotherapy. Cancer. 1991;67:1525–31. doi: 10.1002/1097-0142(19910315)67:6<1525::aid-cncr2820670611>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Loehrer PJ, Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1992;10:1066–73. doi: 10.1200/JCO.1992.10.7.1066. [DOI] [PubMed] [Google Scholar]
- 7.Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1997;15:2564–9. doi: 10.1200/JCO.1997.15.7.2564. [DOI] [PubMed] [Google Scholar]
- 8.Bellmunt J, Paz-Ares L, Cuello M, et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann Oncol. 2007;18:522–8. doi: 10.1093/annonc/mdl435. [DOI] [PubMed] [Google Scholar]
- 9.Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994;331:1259–64. doi: 10.1056/NEJM199411103311903. [DOI] [PubMed] [Google Scholar]
- 10.George B, Datar RH, Wu L, et al. p53 gene and protein status: the role of p53 alterations in predicting outcome in patients with bladder cancer. J Clin Oncol. 2007;25:5352–8. doi: 10.1200/JCO.2006.10.4125. [DOI] [PubMed] [Google Scholar]
- 11.Danesi R, Altavilla G, Giovannetti E, Rosell R. Pharmacogenomics of gemcitabine in non-small-cell lung cancer and other solid tumors. Pharmacogenomics. 2009;10:69–80. doi: 10.2217/14622416.10.1.69. [DOI] [PubMed] [Google Scholar]
- 12.Bepler G, Kusmartseva I, Sharma S, et al. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol. 2006;24:4731–7. doi: 10.1200/JCO.2006.06.1101. [DOI] [PubMed] [Google Scholar]
- 13.Bergman AM, Eijk PP, Ruiz van Haperen VW, et al. In vivo induction of resistance to gemcitabine results in increased expression of ribonucleotide reductase subunit M1 as the major determinant. Cancer Res. 2005;65:9510–6. doi: 10.1158/0008-5472.CAN-05-0989. [DOI] [PubMed] [Google Scholar]
- 14.Gautam A, Li ZR, Bepler G. RRM1-induced metastasis suppression through PTEN-regulated pathways. Oncogene. 2003;22:2135–42. doi: 10.1038/sj.onc.1206232. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med. 2007;356:800–8. doi: 10.1056/NEJMoa065411. [DOI] [PubMed] [Google Scholar]
- 16.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323–7. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 17.Eisen T, Oudard S, Szczylik C, et al. Sorafenib for older patients with renal cell carcinoma: subset analysis from a randomized trial. J Natl Cancer Inst. 2008;100:1454–63. doi: 10.1093/jnci/djn319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Froehner M, Brausi MA, Herr HW, Muto G, Studer UE. Complications following radical cystectomy for bladder cancer in the elderly. Eur Urol. 2009;56:443–54. doi: 10.1016/j.eururo.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 20.Skinner DG, Daniels JR, Russell CA, et al. The role of adjuvant chemotherapy following cystectomy for invasive bladder cancer: a prospective comparative trial. J Urol. 1991;145:459–67. doi: 10.1016/s0022-5347(17)38368-4. [DOI] [PubMed] [Google Scholar]
- 21.Stockle M, Meyenburg W, Wellek S, et al. Advanced bladder cancer (stages pT3b, pT4a, pN1 and pN2): improved survival after radical cystectomy and 3 adjuvant cycles of chemotherapy. Results of a controlled prospective study. J Urol. 1992;148:302–7. doi: 10.1016/s0022-5347(17)36578-3. [DOI] [PubMed] [Google Scholar]
- 22.Stockle M, Meyenburg W, Wellek S, et al. Adjuvant polychemotherapy of nonorgan-confined bladder cancer after radical cystectomy revisited: long-term results of a controlled prospective study and further clinical experience. J Urol. 1995;153:47–52. doi: 10.1097/00005392-199501000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Advanced Bladder Cancer meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003;361:1927–34. doi: 10.1016/s0140-6736(03)13580-5. [DOI] [PubMed] [Google Scholar]
- 24.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 25.Fleischmann A, Thalmann GN, Markwalder R, Studer UE. Extracapsular extension of pelvic lymph node metastases from urothelial carcinoma of the bladder is an independent prognostic factor. J Clin Oncol. 2005;23:2358–65. doi: 10.1200/JCO.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 26.Reichard P. From RNA to DNA, why so many ribonucleotide reductases? Science. 1993;260:1773–7. doi: 10.1126/science.8511586. [DOI] [PubMed] [Google Scholar]
- 27.Clark PE, Stein JP, Groshen SG, et al. Radical cystectomy in the elderly: comparison of clincal outcomes between younger and older patients. Cancer. 2005;104:36–43. doi: 10.1002/cncr.21126. [DOI] [PubMed] [Google Scholar]
- 28.Hollenbeck BK, Miller DC, Taub D, et al. Aggressive treatment for bladder cancer is associated with improved overall survival among patients 80 years old or older. Urology. 2004;64:292–7. doi: 10.1016/j.urology.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen ME, Shariat SF, Karakiewicz PI, et al. Advanced age is associated with poorer bladder cancer-specific survival in patients treated with radical cystectomy. Eur Urol. 2007;51:699–708. doi: 10.1016/j.eururo.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Gautam A, Bepler G. Suppression of lung tumor formation by the regulatory subunit of ribonucleotide reductase. Cancer Res. 2006;66:6497–502. doi: 10.1158/0008-5472.CAN-05-4462. [DOI] [PubMed] [Google Scholar]
- 31.Social Security Online. [Accessed February 2010];Period Life Table. Available at: http://www.ssa.gov/OACT/STATS/table4c6.html.