Abstract
Background
Peritoneal carcinomatosis of colonic origin (PCC) is a life-threatening diagnosis. Cytoreductive surgery (CS) with hyperthermic intraperitoneal chemotherapy (HIPEC) offers patients the prospect of long-term survival with alleviation of symptoms.
Methods
Patients underwent HIPEC for PCC and completed questionnaires preoperatively (T1) and after surgery at 3 (T2), 6 (T3), and 12 (T4) months. Questionnaires included the Functional Assessment of Cancer Therapy—Colon (FACT-C), Brief Pain Inventory (BPI), SF-36 Medical Outcomes Study Survey (SF-36), Center for Epidemiologic Studies—Depression Scale (CES-D), and the ECOG Performance Status Rating.
Results
A total of 62 patients were assessed before surgery. Median overall survival was 18 months, with 71.3 ± 6.3% survival at 1 year. Emotional well-being (P = .0007) improved after HIPEC. Social/family well-being (P = .065) and the colon subscale (P = .061) of the FACT worsened at T2, but recovered by T3. One-third to one-half of patients reported depressive symptoms over the course of the study. Pain scores increased above BL at T2, but decreased below BL at T3 and T4.
Conclusions
Emotional well being is improved after CS + HIPEC despite complications that may affect short-term recovery. Most patients remaining in the study recover to preoperative levels of functioning between 3 and 6 months after surgery. For some, survival can be attained without major decrement in QOL at 1 year. QOL concerns must be a key component in the evaluation for patients with PCC for CS and HIPEC.
Patients diagnosed with peritoneal carcinomatosis of colonic origin (PCC) or peritoneal surface malignancy (PSM) must choose whether to receive palliative care or undergo the more effective cytoreductive surgery (CS) with hyperthermic intraperitoneal chemotherapy (HIPEC). This is a high-risk treatment that offers the prospect of extending survival at the risk of considerable morbidity, and even mortality.1 Complications from surgery and chemotherapy may necessitate a prolonged hospital stay.2 CS with HIPEC offers a real hope of extended survival and the prospect of tumor control to patients who otherwise would rapidly descend to functional decline and certain death.3–7
CS plus HIPEC has also been primarily used for treatment of patients with PSM originating in the appendix, as well as the stomach, small bowel, ovary, and mesothelioma.8 However, outcomes for patients with PCC have been shown to be improved by CS and HIPEC. A prospective randomized trial revealed a doubling of survival with systemic chemotherapy with CS and HIPEC, compared with systemic chemotherapy alone.8 Long-term survival with acceptable quality of life (QOL) is possible among these patients who undergo CS plus HIPEC for peritoneal carcinomatosis.9
There is limited research describing patient-rated outcomes and QOL among patients with PCC who undergo CS and HIPEC. Health-related QOL can be reliably measured and is very meaningful and important to patients. However, there is a paucity of prospective data evaluating QOL in this setting. The purpose of this study was to investigate the impact of CS plus HIPEC on QOL in patients with PCC.
METHODS
Patients diagnosed and treated with CS and HIPEC for PCC at the Wake Forest University between September 1, 2001 and August 1, 2009 were studied. The study was approved by the Institutional Review Board. Inclusion criteria included disease confined to the peritoneal area and previously resected or at least resectable primary tumor site. Patients were >18 years of age, had normal organ function, not pregnant, and without major psychiatric problems limiting the ability to give informed consent. Exclusion criteria were uncontrolled diabetes mellitus, active infections, active peptic ulcer disease, recent (<3 months) myocardial infarction, heart failure, angina, uncontrolled hypertension, or severe pulmonary disease.
Procedure
Patients were evaluated and consented in the surgical oncology clinics. They then completed the QOL questionnaires at baseline, before surgery and at 3, 6, and 12 months after surgery. Remaining questionnaires were mailed at the appropriate due date and returned in a self-addressed stamped envelope.
Surgery and HIPEC Procedure
Details of operative protocols for CS and HIPEC are described elsewhere.2 The goal of CS is removal of all visible tumor; HIPEC addresses the remaining microscopic residual. All procedures were performed via midline incisions, and patients were supported with patient controlled analgesia pumps after HIPEC discharge. Routine follow-up for tumor recurrence is a 3- to 6-month interval.
Data Collection
The instruments we used to measure QOL were: Functional Assessment of Cancer Therapy—Colon Scale (FACT-C), Medical Outcomes Study Health Survey, Short Form (SF-36), Center for Epidemiologic Studies—Depression Scale (CES-D), Eastern Cooperative Oncology Group (ECOG) Performance Rating Scale, and Brief Pain Inventory. The presurgery questionnaire included soci-odemographic information, current symptoms, and perceptions concerning personal physical appearance.
The ECOG Scale assesses patients’ current performance status on the following scale: 0 = normal; 1 = ambulatory with some symptoms; 2 = requires bed rest <50% of daytime hours; 3 = requires bed rest >50% of daytime hours; 4 = completely bedridden.10 The FACT-C includes: the FACT-G plus a 9-item subscale that addresses symptoms specifically related to the colon.11 The FACT-G is a 27-item questionnaire that assesses QOL issues of cancer patients across the domains of physical (PWB), social/ family (SFWB), emotional (EWB), and functional well-being (FWB). Within the FACT-G, a treatment outcome index (TOI) that combines FWB, PWB, and the colon subscale can be calculated.12–14 Higher scores indicate better functioning and QOL. The SF-36 is a 36-item questionnaire that assesses 8 areas of perceived health: physical functioning, role physical, role emotional, bodily pain, general health, vitality, social functioning, and mental health.15,16 The CES-D is a 20-item questionnaire that assesses depressive symptoms during the past week.17 We use the CES-D to screen for “possible, probable, and case” depression with scores of ≥16, ≥23, and ≥28, respectively. Patients with scores ≥16 were contacted and evaluated for significant depressive symptoms by phone; psychosocial or psychiatric consultations were offered when appropriate. The BPI is a 10-item questionnaire that asks patients to rate their pain at its worst and least over the past week on a scale from 1 (no pain) to 10 (as bad as you can imagine).18 It measures the extent to which pain has interfered with general activity, mood, walking, normal work, relationships, sleep, and enjoyment of life over the past week.
Surgical Data
The completeness of cytoreduction classification system is derived from the American Joint Committee on Cancer staging manual and includes: R0, no gross disease with negative microscopic margins; R1, no gross disease with positive microscopic margins; R2a, residual tumor of up to 5 mm; R2b, 5–20 mm; and R2c > 20 mm residual disease. Patients undergoing the procedure for R2c had malignant ascites and were treated for palliation. Surgical information on complications (within 30 days from surgery), creation of ileostomy or colostomy, recurrence, and months alive following CS plus HIPEC were recorded. The extent to which patients returned to normal activities following surgery was assessed. The scale was from 10–100% with possible responses listed in increments of 10 percentage points.
Statistical Analysis
In addition to descriptive statistics, survival estimates were calculated by the Kaplan–Meier method. To assess the effect of time on the outcome measures, repeated measures by mixed models (SAS PROC MIXED) were fit for the dependent measures. The mixed approach allows for analyses of incomplete or missing data while also accounting for correlation within individuals. Changes in outcome scores between visits were assessed using pairwise comparisons of least squares means in PROC MIXED. Repeated measures analysis of covariance was used to assess the effect of income on QOL measures. Due to small sample sizes, colostomy and ileostomy comparisons were done using the Wilcoxon Rank Sum Test. P values <.05 were considered to be statistically significant.
RESULTS
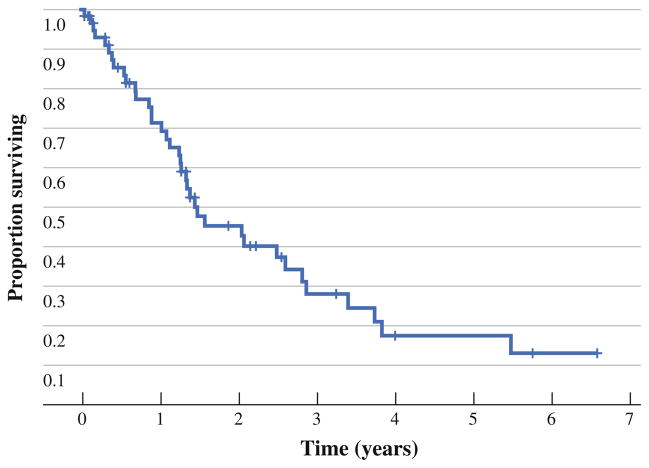
A total of 61 patients completed the presurgery questionnaire. Patient characteristics are reported in Table 1. Also, 19 patients completed QOL packets at 1 year, 12 had not yet reached the 1-year follow-up, and 31 did not complete the questionnaires due to death (n = 15), unknown reasons (n = 10), hospitalization/too sick (n = 4), and refusal (n = 2). Median survival was 18 months, with 71.3 ± 6.3% survival at 1 year and 45.4 ± 7.3% at 2 years. Median follow-up time was 3.2 years. Figure 1 shows the survival curve.
TABLE 1.
Patient characteristics and demographic information
| Characteristic | Value |
|---|---|
| Total patients, n (%) | 62 (100) |
| Age (years) | 53.4 ± 12.2 |
| Range | 29.0–80.0 |
| Sex, n (%) | |
| Female | 29 (47) |
| Male | 33 (53) |
| Race, n (%) | |
| African American | 6 (10) |
| White | 56 (90) |
| Education (years), n (%) | |
| 1–8 | 1 (2) |
| 9–11 | 4 (7) |
| High school | 14 (25) |
| Some college/junior college | 18 (31) |
| College/postgraduate | 14 (25) |
| Advanced degree | 6 (10) |
| Unknown | (missing 5) |
| Marital status, n (%) | |
| Married | 52 (85) |
| Single | 3 (5) |
| Separated/divorced | 5 (8) |
| Widowed | 1 (2) |
| Unknown | (missing 1) |
| Occupation, n (%) | |
| Homemaker | 5 (8) |
| Disabled | 21 (34) |
| Unemployed | 4 (7) |
| Retired | 13 (21) |
| Full-time | 13 (21) |
| Part-time | 5 (8) |
| Unknown | (missing 1) |
| Income, n (%) | |
| $0–$30,000 | 11 (21) |
| $30,000–$50,000 | 14 (27) |
| $50,000–$70,000 | 10 (19) |
| Over $70,000 | 17 (33) |
| Unknown | (missing 10) |
FIG. 1.
Kaplan–Meier overall survival curve after CS with HIPEC for PCC
Postoperative complications were neutropenia (n = 8), infection (n = 13), diarrhea (n = 3), anastomotic leak (n = 2), anemia (n = 2), and persistent nausea (n = 2). There were single occurrences of jaundice, pneumothorax, fever, hematoma, wound dehiscence, respiratory failure, atrial fibrillation, and small bowel perforation. Also, 32 (52%) patients did not experience any major postoperative complications.
QOL scores for patients who received a colostomy (n = 9) or an ileostomy (n = 14) at the time of surgery were compared with QOL scores for patients on the study who did not. There were no significant differences in overall QOL score on the FACT for the following comparisons at baseline and 3 months: colostomy versus ileostomy, ileostomy versus all others, and colostomy versus all others.
Patients who reported annual income <$30,000 were compared with patients who reported annual income of >$30,000 at baseline across QOL measures. Those who earned less scored higher (P = .026) on the CES-D indicating greater incidence of depressive symptoms. Further, lower income patients reported significantly more pain interference, (P = .025), but improved general health (P = .025), worsened physical function (P = .003), functional well being (P = .023), and treatment outcome index (P = .047), from baseline to T4.
FACT-C QOL
Significant changes were observed for EWB with scores improving at 3 months and staying above baseline at 6 and 12 months (Table 2). Trends were seen for SFWB (P = .065) and the Colon Subscale (P = .061). Although not significant, the observed mean for PWB, FWB, FACT-G, FACT-C, and all dropped below baseline levels at 3 months but rebounded to above or near baseline at 6 and 12 months. Analysis of EWB for the 33 patients with complete data sets show means (± SD) at T1, T2, T3, and T4: 15.9 (±4.3), 18.6 (±3.6), 18.1 (±4.7), and 17.4 (±2.8), with a P value of .0004. This would seem to indicate that the intervention helped increase EWB over the time course of the study.
TABLE 2.
Functional Assessment of Cancer Therapy—Colon (FACT-C) mean scores at 4 time points
| Test | Baseline (n = 61) | 3 months (n = 33) | 6 months (n = 27) | 12 months (n = 19) | P value |
|---|---|---|---|---|---|
| PWB | 21.1 ± 5.2 | 19.7 ± 5.3 | 21.6 ± 5.5 | 21.4 ± 5.1 | .27 |
| SFWB | 24.2 ± 3.7 | 22.6 ± 3.6 | 24.4 ± 2.9 | 23.4 ± 4.1 | .065 |
| EWB | 16.2 ± 4.4 | 18.6 ± 3.6 | 17.6 ± 4.5 | 17.2 ± 3.6 | .0007 |
| FWB | 18.1 ± 5.9 | 16.9 ± 5.2 | 19.1 ± 5.9 | 18.8 ± 5.8 | .31 |
| Colon subscale | 18.8 ± 3.2 | 17.1 ± 4.5 | 18.7 ± 4.5 | 18.9 ± 3.1 | .061 |
| FACT-G | 79.5 ± 13.7 | 77.8 ± 13.4 | 82.6 ± 15.0 | 80.8 ± 12.8 | .23 |
| FACT-C | 98.3 ± 15.3 | 94.8 ± 16.6 | 101.7 ± 19.0 | 99.7 ± 14.2 | .19 |
| TOI | 57.9 ± 12.0 | 53.6 ± 12.8 | 59.6 ± 14.5 | 59.2 ± 10.9 | .10 |
P value from repeated measures analysis of variance
Data reported as mean ± standard deviation
PWB physical well-being, SFWB social/family well-being, EWB emotional well-being, FWB functional well-being, FACT-C FACT-G plus the colon subscale, TOI Treatment Outcome Index
SF-36 Health Survey
Significant changes were seen for role physical at baseline and 3 months (P = .002) and between 3 and 6 months (P = .007) (see Fig. 2). Bodily pain decreased from 3 to 6 months (P = .005) and 3 to 12 month post-surgery (P = .022). Social functioning decreased between baseline and 6 months (P = .006) and baseline and 12 months (P = .011).
FIG. 2.
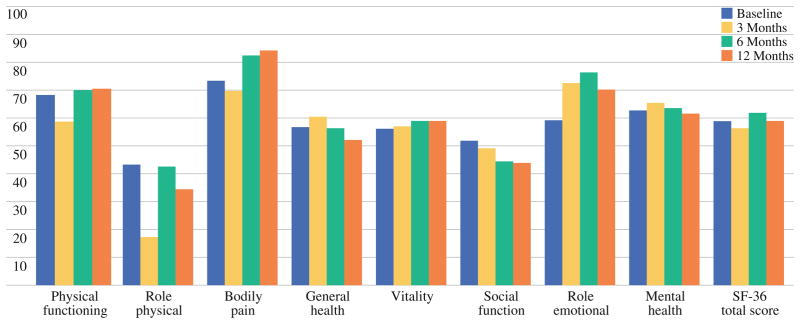
Medical Outcomes Study Health Survey, Short Form (SF-36), mean scores at baseline, and at 3, 6, and 12 months
Depressive Symptoms/CES-D
The mean score for CES-D at baseline, 3, 6, and 12 months was 13.7 ± 9.9, 11.4 ± 7.9, 12.8 ± 10.1, and 13 ± 9.9, respectively (P = .31). The incidence of possible, probable, and case depression trended downward over a 1-year period. A score of ≥ 16 placed patients in a category of “possible depression.” At baseline, 41% scored ≥ 16 alerting researchers to follow-up with an interventional phone call.
Brief Pain Inventory
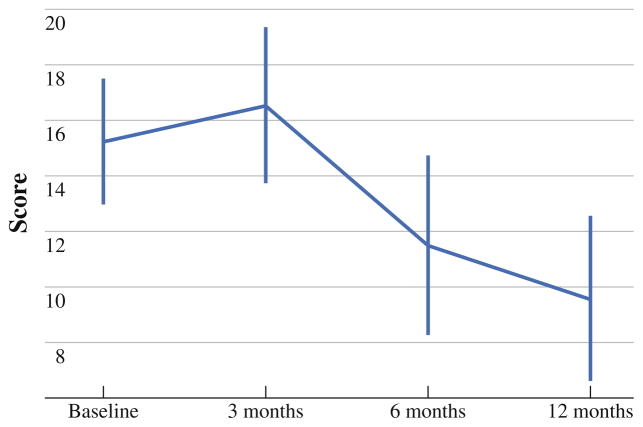
Pain interference with walking around was statistically significant between baseline and 12 months (P = .04). At baseline and 3 months, 54 and 26% reported no pain interference, respectively. Scores rebounded at 6 months and 1 year with 56 and 63% of patients reporting no pain interference with walking around, respectively. Pain scores increased above baseline at 3 months, but decreased below baseline at 6 and 12 months post CS and HIPEC (Fig. 3).
FIG. 3.
Overall pain interference score from the Brief Pain Inventory
Self-Rated Performance Status General Health and Returning to Normal
Performance status is reported in Table 3. By year 1, 47% reported normal activity, 47% reported having some symptoms, and 5% reported needing some extra time in bed. Patients assessed their current health as much/somewhat better at 3, 6, and 12 months, respectively (n = 15, 45%; n = 14, 52%; n = 11, 61%, respectively) or somewhat worse/much worse (n = 13, 39%; n = 10, 37%; n = 3, 17%, respectively). Postsurgical questionnaires asked patients to rate the extent to which they have resumed their normal activities on a scale from 0–100%. At 3, 6, and 12 months, respectively, patients had returned to 56, 70, and 73% of their normal activity.
TABLE 3.
Patient performance status rating
| Performance status rating scorea | Time
|
|||
|---|---|---|---|---|
| Baseline n = 62 (n (%)) | 3 months n = 32 (n (%)) | 6 months n = 26 (n (%)) | 12 months n = 19 (n (%)) | |
| 0 | 30 (48) | 6 (19) | 11 (42) | 9 (47) |
| 1 | 20 (32) | 19 (59) | 9 (35) | 9 (47) |
| 2 | 12 (19) | 5 (16) | 4 (15) | 1 (5) |
| 3 | 0 | 2 (6) | 1 (4) | 0 |
| 4 | 0 | 0 | 1 (4) | 0 |
Scores were self-reported by patient
DISCUSSION
Cytoreductive surgery + HIPEC is a formidable intervention. Although long-term disease-free survival is possible, most patients do recur and may experience significant morbidity. This makes systematic, patient assessment of their QOL imperative. This study represents the first evaluation of patients undergoing this procedure for peritoneal carcinomatosis exclusively of colonic origin (PCC). Trends measured by the FACT and SF-36 suggest an impairment of QOL posttreatment up to 3 months across the majority of subscales but recovery to near or above baseline at 6 and 12 months postsurgery. EWB improved significantly (P = .0007) at 3 months and remained above baseline at 6 and 12 months. This suggests that most survivors of the procedure experience improved EWB throughout the postoperative period despite experiencing a decline in physical functioning measures for the first 3 months after HIPEC.
We have previously reported QOL measurements from various histologies treated with CS and HIPEC.19 Morbidity and mortality are significant; however, long-term survival with good QOL is possible. We previously reported 3–8 years following treatment for PSM, approximately 63% of patients reported good QOL. Physical and functional well-being declined immediately postsurgery, but increased above baseline at 3, 6, and 12 months.9 Thus, the experience with HIPEC for colorectal primary is similar to our experience with other primary sites.
Although we are aware of no other study evaluating QOL for patients with PCC undergoing CS and HIPEC, there are several other studies that are relevant. A study of 67 patients (7 with colorectal primary) suggested that depression is the likely reason for major complaints reported from patients with PSM in long-term survivors following HIPEC.20 Data from the European Organization for Research and treatment of Cancer QOL questionnaire, collected 4 years (range, 1–8 years) following surgery, suggested impaired QOL during the first 6–12 months following surgery and a return to satisfactory QOL thereafter.20
Tuttle et al. measured toxicity and QOL in 35 (7 of colonic origin) patients before and after CS plus HIPEC.2 They reported that QOL returned to baseline at 4 months and was greatly improved at 8 and 12 months. A Danish study observed similar trends among patients with pseudomyxoma peritonei who underwent CS with intraperitoneal chemotherapy.21 These studies support the findings in this trial, though our study is composed solely of patients with nonappendiceal colorectal primary lesions. It is important to evaluate QOL in patients with PCC as their prognosis predicts likelihood of survival less than half of that found for patient undergoing CS and HIPEC for appendiceal cancer.1,4,8,22 The resection status of the subjects is associated with length of survival; these data, stratified across resection status, are shown in Table 4.
TABLE 4.
Survival stratified by resection status
| Resection status | N (%) | Survival
|
||
|---|---|---|---|---|
| Median (months) | 1 year | 2 year | ||
| R0/R1 | 34 (56) | 34 | 96.4% (±3.5%) | 72.4% (±8.9%) |
| R2a | 18 (29) | 15.8 | 66.6% (±12.4%) | 22.2% (±11.3%) |
| R2b | 6 (10) | 7.3 | 16.7% (±15.2%) | 0% |
| R2c | 3 (5) | 3.9 | 0% | 0% |
A systematic review of literature (37 articles) of the efficacy of CS plus HIPEC for patients diagnosed with PSM from appendiceal carcinoma found only two studies reporting QOL data.22 This prompted a consensus panel to suggest guidelines recommending monitoring QOL as standard of care for patients undergoing CS and HIPEC.23
There has been much published on the morbidity, mortality, and efficacy of CS plus HIPEC for patients with PSM.24–26 One goal of recording and disseminating information about the survival and QOL risks and benefits of CS plus HIPEC for PCC is to better inform patients and clinicians during the decision-making process. An important finding in this study is that despite short-term functional decline following the procedure, emotional well-being improved over baseline throughout the post-operative period. This may be the result of improved outlook for patients who successfully manage all the stressors that accompany CS + HIPEC. Once the initial treatment procedure is completed and physical recovery is underway, hope for extended life is restored and emotional well-being can improve.
The selection process for this substantial operative undertaking is crucial since the procedure and follow-up care is taxing. Even patients who meet all anatomic and oncologic criteria are not guaranteed longevity and/or disease-free survival. Preoperative depressive symptoms and the extent of social support available to the patient should be included in the decision-making process for HIPEC. A positive attitude and strong motivation will be crucial to help the rehabilitation process.
Randomized trials for PCC have proven difficult to complete. Patients may perceive that other therapies would place them into a less than maximally aggressive treatment protocol.22,27 Despite the risk of morbidity and mortality, patients undergoing CS with HIPEC for PCC have the opportunity to achieve survival unprecedented with any other therapy. The QOL data for patients with PCC is quite similar to our previous data on patients with appendiceal primary, suggesting that QOL is more closely related to the procedure than the source of the PSM.8
There are several important limitations of this study. First, the QOL data must be interpreted with caution as there was a significant attrition rate in this study. Clearly, for those who were physically impaired, QOL would likely be poor and would not be reflected within the data captured and reported herein, which could suggest a poorer result in overall QOL for the cohort. Significant efforts were expended to improve data capture, and the rate of non-participation may be related to functional decline in patients who have recurred. Second, the total number of patients surveyed is small, homogenous, and confined to one treatment center.
In summary, parameters of QOL show that full recovery may require 3–6 months. However, despite short-term functional decline, EWB improves throughout the postoperative period. Patients and clinicians considering CS + HIPEC as a treatment option must weigh the risks of short-term physical impairment of QOL against benefits of improved emotional QOL and extended survival. Systematic evaluation of QOL at several time points is imperative in the postoperative period, particularly for signs of treatable symptoms such as depression. Further work is needed to seek interventions that can improve QOL. Additionally, QOL measures should be part of future trials with CS + HIPEC, hopefully as part of a multicenter trial.
References
- 1.Shen P, Hawksworth J, Lovato J, Loggie BW, Geisinger KR, Fleming RA, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11:178–86. doi: 10.1245/aso.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Tuttle TM, Zhang Y, Greeno E, Knutsen A. Toxicity and quality of life after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2006;13:1627–32. doi: 10.1245/s10434-006-9186-6. [DOI] [PubMed] [Google Scholar]
- 3.Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63:364–7. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Levine EA, Stewart JH, Russell GB, Geisinger KR, Loggie BL, Shen P. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007;204:943–53. doi: 10.1016/j.jamcollsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 5.Beaujard AC, Glehen O, Caillot JL, Francois Y, Bienvenu J, Panteix G, et al. Intraperitoneal chemohyperthermia with mitomycin C for digestive tract cancer patients with peritoneal carcinomatosis. Cancer. 2000;88:2512–9. doi: 10.1002/1097-0142(20000601)88:11<2512::aid-cncr12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Witkamp AJ, deBree E, Kaag MM, Boot H, Beijnen JH, van Slooten GW, et al. Extensive cytoreductive surgery followed by intraoperative hyperthermic intraperitoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur J Cancer. 2001;37:979–84. doi: 10.1016/s0959-8049(01)00058-2. [DOI] [PubMed] [Google Scholar]
- 7.Verwaal VJ, van Tinteren H, Ruth SV, Zoetmulder FA. Toxicity of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2004;85:61–7. doi: 10.1002/jso.20013. [DOI] [PubMed] [Google Scholar]
- 8.McQuellon RP, Russell GB, Shen P, Stewart JH, 4th, Saunders W, Levine EA. Survival and health outcomes after cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of appendiceal origin. Ann Surg Oncol. 2007;15:125–33. doi: 10.1245/s10434-007-9678-z. [DOI] [PubMed] [Google Scholar]
- 9.McQuellon RP, Loggie BW, Lehman AB, Russell GB, Fleming RA, Shen P, et al. Long-term survivorship and quality of life after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2003;10:155–62. doi: 10.1245/aso.2003.03.067. [DOI] [PubMed] [Google Scholar]
- 10.Zubrod CG, Schneiderman M, Frei E, Brindley C, Gold GG, Shnider B, et al. Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramind. J Chronic Dis. 1960;11:7–33. [Google Scholar]
- 11.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 12.Eton D, Fairclough D, Cella D, Yount S, Bonomi P, Johnson D. Early change in patient-reported health during lung cancer chemotherapy predicts clinical outcomes beyond those predicted by baseline report: results from Eastern Cooperative Oncology Group Study 5592. J Clin Oncol. 2003;21:1536–43. doi: 10.1200/JCO.2003.07.128. [DOI] [PubMed] [Google Scholar]
- 13.McQuellon R, Thaler H, Cella D, Moore D. Quality of Life (QOL) outcomes from a randomized trial of cisplatin versus cisplatin plus paclitaxel in advanced cervical cancer: a gyneco-logic oncology group study. Gynecol Oncol. 2006;101:296–304. doi: 10.1016/j.ygyno.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–86. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 15.Ware JR, Jr, Snow KK, Kosinski M, Gandek B. Manual and Interpretation Guide. Boston: Nimrod Press; 1993. SF-36 health survey. [Google Scholar]
- 16.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 18.Daut RL, Cleeland CS, Flanery RC. Development of the Wis-consin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 19.McQuellon RP, Loggie BW, Fleming RA, Russel GB, Lehman AB, Rambo TD. Quality of life after intraperitoneal hyperthermic chemotherapy (HIPEC) for peritoneal carcinomatosis. Eur J Surg Oncol. 2001;27:65–73. doi: 10.1053/ejso.2000.1033. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt U, Dahlke MH, Klempnauer J, Schlitt HJ, Piso P. Per-ioperative morbidity and quality of life in long-term survivors following cytoreductive surgery and hyperthermic interperitoneal chemotherapy. Eur J Surg Oncol. 2005;31:53–8. doi: 10.1016/j.ejso.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Jess P, Iversen LH, Nielsen MB, Hansen F, Laurberg S, Rasmussen P. Quality of life after cytoreductive surgery plus early intraperi-toneal postoperative chemotherapy for pseudomyxoma peritonei: a prospective study. Dis Colon Rectum. 2008;51:868–74. doi: 10.1007/s10350-008-9223-6. [DOI] [PubMed] [Google Scholar]
- 22.Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2006;14:484–92. doi: 10.1245/s10434-006-9182-x. [DOI] [PubMed] [Google Scholar]
- 23.McQuellon R, Gavazzi C, Piso P, Swain D, Levine E. Quality of life and nutritional assessment in peritoneal surface malignancy (PSM): recommendations for care. J Surg Oncol. 2008;98:300–5. doi: 10.1002/jso.21050. [DOI] [PubMed] [Google Scholar]
- 24.Wilson TR, Alexander DJ. Clinical and non-clinical factors influencing postoperative health-related quality of life in patients with colorectal cancer. Br J Surg. 2008;95:1408–15. doi: 10.1002/bjs.6376. [DOI] [PubMed] [Google Scholar]
- 25.Ulander K, Jeppsson B, Grahn G. Quality of life and independence in activities of daily living preoperatively and at follow-up in patients with colorectal cancer. Support Care Cancer. 1997;5:402–9. doi: 10.1007/s005200050099. [DOI] [PubMed] [Google Scholar]
- 26.Anthony T, Long J, Hynan LS, Sarosi GA, Jr, Nwariaku F, Huth J, et al. Surgical complications exert a lasting effect on disease-specific health-related quality of life for patients with colorectal cancer. Surgery. 2003;134:119–25. doi: 10.1067/msy.2003.212. [DOI] [PubMed] [Google Scholar]
- 27.Elias D, Delperro JR, Sideris L, Benhamou E, Pocard M, Baton O, et al. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Ann Surg Oncol. 2004;11:518–21. doi: 10.1245/ASO.2004.09.008. [DOI] [PubMed] [Google Scholar]