Abstract
Induced pluripotent stem (iPS) cells can be derived from human somatic cells by cellular reprogramming. This technology provides a potential source of non-controversial therapeutic cells for tissue repair, drug discovery, and opportunities for studying the molecular basis of human disease. Normally, mouse embryonic fibroblasts (MEFs) are used as feeder layers in the initial derivation of iPS lines. The purpose of this study was to determine whether SNL fibroblasts can be used to support the growth of human iPS cells reprogrammed from somatic cells using lentiviral expressed reprogramming factors. In our study, iPS cells expressed common pluripotency markers, displayed human embryonic stem cells (hESCs) morphology and unmethylated promoters of NANOG and OCT4. These data demonstrate that SNL feeder cells can support the derivation and maintenance of human iPS cells.
Keywords: induced pluripotent stem cells, derivation, maintenance, SNL, feeder cells
Introduction
Human embryonic stem cells (hESCs) are regarded as pluripotent cells since they have the ability to differentiate into virtually all cell types. hESCs hold great promise for regenerative medicine and have become a powerful tool for basic research (Thomson et al., 1998; Reubinoff et al., 2000). There are, however, several factors which limit the clinical applications of hESCs. One challenge is immunological incompatibility causing rejection and necessitating the use of immune-suppressing agents (Cabrera et al., 2006). In addition, the generation and use of hESCs is restricted in several countries due to legal and ethical considerations.
To overcome these limitations, a new research field of cell reprogramming has emerged based on the over-expression of selected transcription factors relevant to the pluripotent phenotype. This technology represents a breakthrough in regenerative medicine. One benefit of these induced pluripotent stem (iPS) cells is that since they can be derived from the patient, the problem of immunological incompatibility can be eliminated. Another benefit is that they largely remove the ethical concerns that restrict the use of hESCs.
iPS research is now in its infancy, and there are still many questions that need to be clarified. The process of derivation and maintenance of iPS cells needs to become more efficient and less complicated. Since the presence of feeder cells is required for self-renewal of hESCs and iPS cells (Nichols et al., 1998), investigators that have established such lines have generally used mouse embryonic fibroblasts (MEFs) as feeder cells (Thomson et al., 1998; Yu et al., 2007). As proliferation of primary MEFs is limited, it is necessary to isolate MEFs from mouse fetuses repeatedly to supply feeders for ES cells or iPS cells culture. MEFs also tend to lose the capacity to support proliferation of ES cells or iPS cells with increasing passages.
The SNL cell line is an immortalized subclone of the STO line manipulated to stably express the neomycin resistance and leukaemia inhibitory factor (LIF) genes (McMahon and Bradley, 1990). The STO cell line itself was derived from mouse SIM embryonic fibroblasts, and is resistant to 6-thioguanine and ouabain, sensitive to HAT selection (hypoxanthine, aminoprotein and thymidine) and negative for HPRT (hypoxanthine guanine phosphoribosyl transferase). As SNL cells are immortalized, they are easier to maintain than MEFs for the preparation of feeder layers. However, few groups have used it as a feeder cell line for iPS cell derivation. Shinya Yamanaka and his group utilized SNL feeder cells for the induction of mouse and human iPS cells by retrovirally delivered factors (OCT4, SOX2, KLF4 and C-MYC) (Takahashi and Yamanaka, 2006; Takahashi et al., 2007). In this study, we provide evidence that SNL cell line can be used for the establishment and maintenance of human iPS cells induced by lentiviral reprogramming factors (LIN28, NANOG, OCT4 and SOX2).
Materials and methods
Cell culture
Human fibroblasts cells (IMR90: CCL-186, ATCC, USA) were cultured in Dulbecco's Modified Eagle Medium (DMEM) (HyClone Laboratories, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, HyClone Laboratories), 2 mmol/L L-glutamine, 50 U/mL penicillin and 50 mg/mL streptomycin (HyClone Laboratories). Approximately 2 × 105 IMR90 cells were seeded in 6-well gelatin-coated plates the day before transduction. The cells were transduced using all four reprogramming factors in the presence of polybrene at 6.0 μg/mL final concentration in complete culture medium. Two rounds of overnight infection were performed. The medium was changed daily for 2 days. Cells were split 1 to 2 onto a layer of inactivated SNL cells and the media changed to serum-free human ES culture medium (DMEM/F12 supplemented with 20% KnockOut Serum Replacement (Invitrogen, Carlsbad, CA, USA), 0.1 mmol/L MEM non-essential amino acids (Sigma, USA), 1 mmol/L L-glutamine (HyClone Laboratories), 0.1 mmol/L β-mercaptoethanol (Sigma) and 5 ng/mL basic recombinant human fibroblast growth factor (bFGF; Invitrogen, Camarillo, CA, USA)).
At approximately 3 weeks, colonies with hESCs morphology (iPS colonies) were isolated mechanically for expansion. After the first five passages, an enzymatic method using collagenase IV (1 mg/mL) was used. Human iPS colonies were passaged every 4 to 6 days.
SNL cells were cultured in DMEM containing 2 mmol/L L-glutamine, 50 U/mL penicillin and 50 mg/mL streptomycin (HyClone Laboratories), 0.1 mmol/L MEM non-essential amino acids (Sigma), 0.1 mmol/L β-mercaptoethanol (Sigma) and 10% FBS (HyClone laboratories).
Human ES cell line H1 (WA01) and human iPS cells derived from foreskin (iPS(Foreskin)-1-DL-1) (purchased from WiCell, USA) were cultured on MEFs in serum-free human ES culture medium.
Mitotic inactivation of SNL cells
Cells were treated with 10 mg/mL Mitomycin C (Sigma) for 1.5 h. The Mitomycin C treated SNL cells were washed extensively in PBS and replated on 0.1% gelatin coated tissue culture dishes at a density of 3.5 × 104 cells/cm2. Feeder cell dishes were used within a week of plating. The original culture medium was changed to stem cell medium just before iPS cells were added.
Lentiviral production
Expression lentiviral vectors of human OCT4, SOX2, NANOG and LIN28 genes were obtained from Addgene (Yu et al., 2007). Viral particles were produced in the 293FT cell line using the psPAX2 packaging plasmid, pMD2.G envelope plasmid and FuGENE HD transfection reagent (Roche, USA). Virus was harvested at 36 h post-transfection for 3 days and concentrated 30-fold by ultracentrifugation. Viral stocks were stored at –80°C.
Alkaline phosphatase staining and immunocytochemical analysis
Direct alkaline phosphatase (AP) activity of human iPS cells was analyzed using an alkaline phosphatase red membrane substrate solution kit (Napthol AS-MX phosphate alkaline solution, Sigma 855; Fast Violet B salt, Sigma 201596-5G), according to the manufacturer's guidelines.
For immunocytochemical analysis, iPS colonies were fixed in 4% (v/v) paraformaldehyde (PFA) in PBS for 30 min then permeabilized with 0.1% Triton X-100 for 3 min at room temperature. To reduce nonspecific antibody binding, cells were incubated with DAKO protein block solution for 1 h at room temperature. After washing, the cells were incubated with the primary antibodies NANOG (Cat. #ab21603, Abcam Inc., MA, USA), OCT-4 (Cat. #Sc-5279, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and TRA-1-60 (Cat. #Sc-21705, Santa Cruz Biotechnology).
FITC-conjugated secondary antibodies (Jackson Immunoresearch, USA) were used for detection and nuclei were visualized by DAPI mounting medium (Vector Laboratories, USA). The immunoreactions were observed on fluorescence microscope (ZEISS, USA).
RNA isolation and TaqMan Human Stem Cell Pluripotency Array
Total RNA was extracted from human embryonic stem cell line H1, iPS cells derived from foreskin, iPS cells derived from IMR90 and IMR90 cells using a miRNeasy Mini Kit (Cat. #217004, Qiagen, CA, USA) according to the manufacturer's instructions and concentration was assessed using a NanoDrop 1,000 Spectrophotometer (Thermo Fisher Scientific Inc., DE, USA). Total RNA (2 μg) from each sample was used for the first-strand cDNA synthesis using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc., CA, USA) following the manufacturer's protocol. The cDNA from each sample was used as a PCR template for the TaqMan® Stem Cell Pluripotency Array Micro Fluidic Cards (Cat. #4385225, Applied Biosystems Inc.), using an Applied Biosystems 7900HT Real-Time PCR System. The TaqMan Human Stem Cell Pluripotency Array results were analyzed using the SDS software version 2.2 RQ Manager program (Applied Biosystems Inc.).
Bisulfite sequencing
Genomic DNA was isolated with the DNeasy Tissue Kit (Qiagen, USA). Bisulfite treatment was carried out using the MethylCode™ Bisulfite Conversion Kit (Invitrogen) following the manufacturer's instructions. The promoter regions of the human NANOG and OCT4 genes were amplified by PCR using primers NANOG-2 and OCT4-4 as described (Freberg et al., 2007) (Table 1). The resulting amplified PCR products were gel-purified, sub-cloned into the pCR4TOPO vector (Invitrogen) and sequenced.
Table 1.
Bisulfite sequencing primers used in this study
| Gene | Sequence (5′→3′) | Seq. coverage relative to TSS |
|---|---|---|
| NANOG | Sense: GAGTTAAAGAGTTTTGTTTTTAAAAATTAT | –1203 to –911 |
| Antisense: TCCCAAATCTAATAATTTATCATATCTTTC | ||
| OCT4 | Sense: GGATGTTATTAAGATGAAGATAGTTGG | –2136 to –1721 |
| Antisense: CCTAAACTCCCCTTCAAAATCTATT |
Adipogenic differentiation
For embryoid body (EB) formation, the hanging drop method was used. iPS cells were incubated in hanging drops for 2 days in ES medium without bFGF. Drops were then transferred to 6-well ultra-low-attachment dishes (Corning NC, USA) and the medium changed every other day for 14 days. EBs were trypsinized, transferred to 0.1% gelatin-coated 6-well tissue culture dish and allowed to reach 100% confluence. They were then cultured in adipogenic differentiation medium consisting of DMEM/F12 culture medium, 20% KnockOut Serum Replacement, and an adipogenic cocktail (1 μmol/L dexamethasone, 5 μg/mL insulin, 100 μmol/L indomethacin and 500 μmol/L IBMX). Medium was changed twice a week and differentiation was evaluated by measuring the lipid vacuoles by Oil Red O staining after 3 weeks induction of differentiation.
Oil Red O staining
Cells were washed with PBS twice, fixed in 4 % formaldehyde for 30 min and then stained with 0.3% (w/v) Oil Red O solution in isopropanol (Cat. #s1849-160z, Poly Scientific, NY, USA) for 50 min at room temperature. The 0.3% (w/v) Oil Red O solution was prepared from the dilution of 0.5% Oil Red O solution with water and filtered before use. The cells were then washed 3 times with water to remove unbound dye. Nuclei were visualized by the staining with hematoxylin solution for 10 min. Adipocytes containing lipid droplets were stained red by the Oil Red O solution while the nucleuses were stained black/blue with the hematoxylin. Oil Red O staining of undifferentiated iPS cells and IMR90 cells grown in parallel culture served as the control sample for this assay.
Results
Generation of iPS cells over SNL
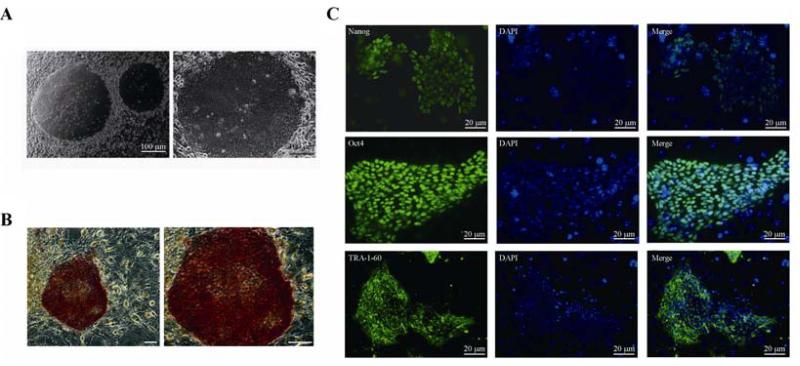
iPS colonies with human ES cell morphology became visible around 3 weeks post viral-transduction. Under high magnification (100 ×), these iPS cells were found to have a clear edge, a high ratio of nucleus to cytoplasm and prominent nucleoli (Fig. 1A). Moreover, these iPS cells maintained the undifferentiated state when cultured in the presence of the SNL feeder layer with bFGF for at least 25 passages (data not shown). These data indicated that iPS cells can generated from IMR90 fibroblasts by lentiviral-reprogramming fators over SNL feeder cells.
Fig. 1.
Generation of lentivirus-induced hiPS on SNL feeders. A: morphology of iPS-IMR90 when grown over SNL feeders. Scale bar = 100 μm. B: human iPS cells colonies cultured on SNL feeders stain positive for alkaline phosphatase, an indicator of pluripotency (brightfield: 50 × (left), 100 × (right); scale bar = 100 μm). C: immunofluorescent staining of Nanog, Oct4 and TRA-1-60 in iPS cells derived from IMR90 cells using SNL as feeders. Scale bar = 20 μm.
iPS cells express pluripotent related genes
In common with pluripotent stem cells, our iPS cells showed a high level of AP activity (Fig. 1B). Further characterization of theses human iPS colonies showed that they were positive for standard pluripotency markers such as NANOG, OCT4 and TRA-1-60 as determined by immunofluorescence. OCT4 and NANOG were strongly stained in the undifferentiated iPS cells, and located in the nucleus. TRA-1-60 was localized to the cell surface (Fig. 1C).
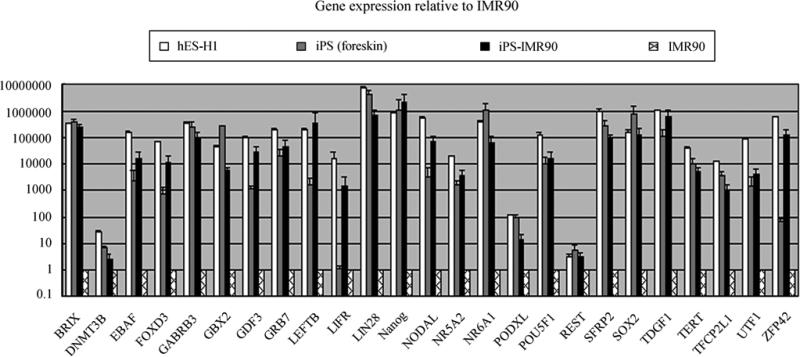
Next, to determine whether the pluripotency genes in iPS cells were reactivated or not, gene expression in iPS-IMR90 cells, hES-H1 cells, iPS (foreskin) cells and IMR90 fibroblasts were conducted using pre-poured Human pluripotency microfluidic plate assays (Applied Biosystems). All the pluripotenty genes were detectable in iPS-IMR90 cells and the expression level was similar to that seen in the hES-H1 cell line and iPS (foreskin) cells. The comparison of pluripotency genes among these 4 cell lines indicated that iPS-IMR90 cells had a reactivated embryonic program of transcription. This confirmed most of the genes correlated to pluripotency were activated in iPS-IMR90 cells maintained on SNL feeder cells (Fig. 2).
Fig. 2.
Genes’ expressions analyzed by the TaqMan Human Stem Cell Pluripotency Array. Relative gene expression level of 25 pluripotent marker genes of 4 cell lines represents fold changes relative to that of IMR90 cells normalized to GAPDH expression level.
DNA demethylation of iPS cells
To assess whether iPS-IMR90 cells maintained on SNL feeder were molecularly similar to hES cells, we examined methylation status of the NANOG and OCT4 promoters of hES-H1 cells (passage 52), iPS-IMR90 cells (passage 15) and IMR90 firbroblasts (passage 10) by bisulfite sequencing. Both promoter elements, which were hypermethylated in IMR90 fibroblasts and hypomethylated in hES-H1 cells, showed hypomethylated in fibroblast-derived hiPS (Fig. 3). Thus, the examined OCT4 and NANOG regulatory regions of iPS-IMR90 cells displayed distinct methylation patterns to IMR90 cells while similar to hES cells. Since there is an inverse relationship between CpG methylation and transcriptional activity, the comparison of methylation status among these 3 cell lines indicated that OCT4 and NANOG were reactivated in iPS-IMR90 cells and silent in IMR90 fibroblasts, demonstrating proper epigenetic reprogramming of these two pluripotency genes in iPS-IMR90 cells.
Fig. 3.
The promoters of Nanog and Oct4 analyzed by bisulfite genomic sequencing for DNA methylation status in hES-H1 cells (passage 52), iPS-IMR90 cells (passage 15) and IMR90 fibroblasts (passage 10). Open and closed circles indicate unmethylated and methylated CpGs, respectively. Numbers represent the sequence coverage of examined CpG relative to the transcription start site (TSS).
iPS in vitro differentiated to adipocytes
To examine the developmental potential of iPS cells using SNL cell as feeders, we used the standard method of in vitro differentiation to generate EBs. iPS-IMR90 (passage 15) cells efficiently formed cystic EBs in suspension culture of ultra-low-attachment dishes without bFGF (Fig. 4A). Cystic EBs derived from iPS-IMR90 cells can further differentiate into adipocytes when induced by adipose culture medium as shown by Oil Red O staining (Fig. 4B). This demonstrates that iPS cells can maintain their differentiation potential on SNL feeder cells.
Fig. 4.
iPS in vitro differentiated to adipocytes. A: EBs formed from human iPS colonies which were expanded over SNL feeders for 15 passages and grown in suspension culture in ultra-low-attachment dishes without bFGF for about 2 weeks. Right and left panels represent EBs under higher magnification (100 ×) and lower magnification (50 ×), respectively. Scale bar = 50 μm (left) or 20 μm (right). B: Oil Red O staining of adipocytes derived from human iPS cells when induced by adipose culture medium for 3 weeks. Nuclei are stained black/blue with the hematoxylin. Right and left panels represent adipocytes by Oil Red O staining under higher magnification (400 ×) and lower magnification (200 ×), respectively. Scale bar = 20 μm (left) or 10 μm (right).
Discussion
Several types of feeder cells have been successfully used for hES cells culture. MEFs were used (Thomson et al., 1998; Reubinoff et al., 2000) followed by human fetal muscle, adult fallopian tube fibroblasts (Richards et al., 2002, 2003) and STO cells (Park et al., 2003). Fibroblasts differentiated from hESCs have been successfully used as feeder cells by several groups (Xu et al., 2004; Stojkovic et al., 2005; Saxena et al., 2008). Human placental fibroblasts from early pregnancy were also functional as feeder cells (Genbacev et al., 2005; Simon et al., 2005) and adult bone marrow cells were employed by Cheng and colleagues (Cheng et al., 2003).
However, placental fibroblasts are not easy to isolate, and human skin cells vary in quality and do not support optimal growth after 20 passages (Unger et al., 2008). It is difficult to justify the use of human adult marrow cells as feeder cells because human adult marrow cells can be directly used for cell therapy and research under guided differentiation. The derivation of MEFs is also rather labor intensive.
SNL is a ready-made immortal MEFs cell line and can be maintained as easily as other cell lines. It is extensively used in the culture of mouse ES cells for which it was designed, expressing LIF constitutively and being neomycin resistant. Since it is easier to handle and can be expanded repeatedly over long periods, SNL has advantages over MEF cells and hEF cells. Recently, Takahashi and colleagues used it to establish mouse and human induced pluripotent stem cell culture using retrovirally expressed transcription factors (OCT4, SOX2, KLF4 and C-MYC) to reprogram human dermal fibroblasts (Takahashi and Yamanaka, 2006; Takahashi et al., 2007).
In this study, we show it is possible to successfully derive and maintain hiPS cells on SNL cells after reprogramming with lentivirally expressed transcription factors (LIN28, NANOG, OCT4 and SOX2). Human iPS cells were derived from fibroblast IMR90 cells on SNL cells. These hiPS cells had features in common with human embryo-derived pluripotent stem cells, including similar morphology, expression of pluripotency markers (AP, NANOG, OCT4 and TRA-1-60) and the formation of EBs in suspension culture. Analysis using Taqman Human Stem Cell Pluripotency Array showed that hiPS reactived all the pluripotency genes normlly expressed in the hES H1 (WA01) cell line. The promoters of NANOG and OCT4 in hiPS cells were demethylated as detected by bisulfite sequencing. Oil Red O staining confirmed that when induced by adipose culture medium, the iPS cells can differentiate into adipocytes. These results suggest the hiPS cells established on SNL cells can maintain pluripotency.
Xeno-free feeders would be necessary for clinical tests and treatment (Unger et al., 2008). In the future, feeder cell may not be required to establish stable hiPS cell lines. At present, the use of feeder cells is still the most efficient and cost-effective option to derive and propagate stable hESCs and hiPS cells.
In conclusion, our study strongly suggests that SNL cells can be used as feeder cells for the establishment and maintenance of human iPS cells. Further studies are needed to determine whether the conditioned medium generated by SNL feeder cells can maintain human iPS pluripotency in feeder free culture conditions and improve reprogramming efficiency.
Acknowledgements
This work was supported by the National Institute of Health, USA (No. R21 RR025408). We are grateful to Zhan Wang and Shantaram Bharadwaj for their fruitful discussions. Reprogramming genes were supplied by Addgene. Chuanying Pan was supported by China Scholarships Council.
References
- Cabrera CM, Cobo F, Nieto A, Concha A. Strategies for preventing immunologic rejection of transplanted human embryonic stem cells. Cytotherapy. 2006;8:517–518. doi: 10.1080/14653240600944287. [DOI] [PubMed] [Google Scholar]
- Cheng L, Hammond H, Ye Z, Zhan X, Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131–142. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- Freberg CT, Dahl JA, Timoskainen S, Collas P. Epigenetic reprogramming of OCT4 and NANOG regulatory regions by embryonal carcinoma cell extract. Mol. Biol. Cell. 2007;18:1543–1553. doi: 10.1091/mbc.E07-01-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Krtolica A, Zdravkovic T, Brunette E, Powell S, Nath A, Caceres E, McMaster M, McDonagh S, Li Y, Mandalam R, Lebkowski J, Fisher SJ. Serum-free derivation of human embryonic stem cell lines on human placental fibroblast feeders. Fertil. Steril. 2005;83:1517–1529. doi: 10.1016/j.fertnstert.2005.01.086. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) protooncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Park JH, Kim SJ, Oh EJ, Moon SY, Roh SI, Kim CG, Yoon HS. Establishment and maintenance of human embryonic stem cells on STO, a permanently growing cell line. Biol. Reprod. 2003;69:2007–2014. doi: 10.1095/biolreprod.103.017467. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546–556. doi: 10.1634/stemcells.21-5-546. [DOI] [PubMed] [Google Scholar]
- Saxena S, Hanwate M, Deb K, Sharma V, Totey S. FGF2 secreting human fibroblast feeder cells: a novel culture system for human embryonic stem cells. Mol. Reprod. Dev. 2008;75:1523–1532. doi: 10.1002/mrd.20895. [DOI] [PubMed] [Google Scholar]
- Simon C, Escobedo C, Valbuena D, Genbacev O, Galan A, Krtolica A, Asensi A, Sanchez E, Esplugues J, Fisher S, Pellicer A. First derivation in Spain of human embryonic stem cell lines: use of long-term cryopreserved embryos and animal-free conditions. Fertil. Steril. 2005;83:246–249. doi: 10.1016/j.fertnstert.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Stojkovic P, Lako M, Stewart R, Przyborski S, Armstrong L, Evans J, Murdoch A, Strachan T, Stojkovic M. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005;23:306–314. doi: 10.1634/stemcells.2004-0137. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Unger C, Skottman H, Blomberg P, Dilber MS, Hovatta O. Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum. Mol. Genet. 2008;17:R48–R53. doi: 10.1093/hmg/ddn079. [DOI] [PubMed] [Google Scholar]
- Xu C, Jiang J, Sottile V, McWhir J, Lebkowski J, Carpenter MK. Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells. 2004;22:972–980. doi: 10.1634/stemcells.22-6-972. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]