Abstract
Background
Previous studies have demonstrated alterations in the peripheral cholinergic system in Alzheimer’s disease (AD), though results have been inconsistent and not linked to in vivo biomarkers of pathology. We examined the relationship between amyloid-beta (Aβ) plaques and plasma cholinesterase activity in a heterogeneous dementia population.
Methods
29 participants with clinical AD and 35 with non-AD diagnoses underwent positron emission tomography (PET) with the amyloid ligand [11C] PIB and plasma measurements of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activity. Multi-linear regression was used to evaluate the relationship between AChE or BChE activity and PIB binding (adjusted for age, sex, apolipoprotein E4 and vascular risk), applying voxel-wise and region of interest (ROI) approaches. AChE activity was further adjusted for cholinesterase inhibitor (ChE-I) use. Global amyloid load was measured using a PIB Index, representing mean tracer binding in frontal, parietal, lateral temporal and cingulate cortex.
Results
AChE activity was correlated with PIB Index (β=0.39, p<0.001) and with regional PIB binding in frontal, temporal, parietal and occipital lobes, precuneus and posterior cingulate on both voxel-wise (p<0.001 uncorrected) and ROI (β=0.26-0.41, p<0.005) analysis. Correlations remained significant after covarying clinical diagnosis (β=0.42, p=0.001), and among participants naive to ChE-I (β=0.51, p=0.005). No correlation was found between BChE activity and PIB. Among AD participants, disease severity was not correlated with AChE, BChE or PIB Index.
Conclusion
AChE activity in plasma is correlated with brain Aβ load. Activation of the ‘anti-inflammatory cholinergic pathway’ may provide the link between Aβ plaques and peripheral cholinergic measures.
Introduction
Two of the main features of Alzheimer disease (AD) are amyloid-β (Aβ) plaques (1) and dysfunction of the cholinergic system (2, 3). Aβ plaque accumulation is thought to be a continuous process that takes place over 10-15 years and reaches a relative plateau before the evolution of cognitive impairment (4). In many (but probably not all) individuals, Aβ aggregation triggers a cascade of events that lead to network dysfunction,, neurodegeneration and ultimately to clinical dementia (5). Aβ aggregates, in particular soluble oligomers, are thought to have a direct neurotoxic effect (6). In addition, Aβ may induce neurodegeneration indirectly by initiating a pro-inflammatory cascade that results in the release of neurotoxic cytokines (7,8). The dysfunction and loss of basal forebrain cholinergic neurons and consequent decrease in acetylcholine (ACh) levels also contribute to cognitive impairment in AD (9). Cholinesterases, the enzymes that catalyze the hydrolysis of ACh, play a central role in the regulation of cholinergic neurotransmission. The predominant cholinesterases are acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Most studies report decreased AChE activity in the cerebrospinal fluid (CSF) of AD patients, compatible with the early loss of cholinergic neurons in AD. Reports regarding changes in BChE activity in AD are inconsistent (10-12). One study reported a negative correlation between BChE activity levels in the CSF and Aβ load in vivo (13). Increasing cholinergic activity in the central nervous system via cholinesterase inhibition is the current mainstay of the pharmacologic treatment of AD (14).
The inflammatory response triggered in the brain by Aβ deposition may affect peripheral cholinesterase (ChE) levels via the peripheral cholinergic system. Inflammatory stimuli induce the vagus nerve to increase ACh release, which in turn acts as an anti-inflammatory agent (15). Therefore, measuring peripheral ChE activity may inform us about the presence of Aβ plaques and could potentially serve as a serologic biomarker for AD pathology.
The objective of this study was to examine the relationship between serum ChE activity and brain Aβ load, as measured in vivo by positron emission tomography with the Aβ-specific tracer Pittsburgh Compound-B (PIB-PET) (16). We hypothesized that increased brain Aβ load would be associated with increased serum ChE activity, reflecting activation of the cholinergic anti-inflammatory pathway by the neuro-inflammation associated with Aβ deposition.
1. Methods
1.1. Participant selection and characterization
Sixty four participants were recruited from dementia research cohorts followed at the University of California San Francisco Memory and Aging Center. The clinical evaluation included a history and physical examination by a neurologist, a structured caregiver interview by a nurse, and a battery of neuropsychological tests (17). Global cognition was assessed using the Mini Mental State Examination (MMSE), and functional impairment was measured via the Clinical Dementia Rating (CDR). Clinical diagnosis was assigned by consensus at a multi-disciplinary conference. Participants with significant co-morbid medical, psychiatric illness or cerebrovascular disease were not eligible for the study. A heterogeneous dementia population was studied in order to include individuals with a broad continuum of brain Aβ load. Participants’ medical records were reviewed to assess for vascular risk factors that can impact systemic inflammation and thus impact peripheral ChE activity (15, 18). A ‘Vascular Index’ was calculated by adding 1 point each for hypertension, hypercholesterolemia, diabetes mellitus and recent (<10 years prior) tobacco use, and 2 points for active smoking and cardiovascular disease (modified from Framingham criteria (19) to integrate available data on participants’ past medical history).
1.2. Plasma measurements
Plasma from participants was collected between March 2006 and November 2008. Plasma cholinesterase catalytic activity was measured by a spectrophotometric assay. Acetylthiocholine (ATCh, Sigma, 1 mM) hydrolysis rates were measured by placing 10 μL 1:20 diluted plasma in microtiter plate wells, after 20 min pre-incubation with or without 50uM iso-OMPA (Sigma), a specific BChE inhibitor. Readings at OD405 nm were repeated at 2-min intervals for 10 min. Non-enzymatic breakdown of substrate was subtracted from the total rate of hydrolysis. Enzyme activities were calculated using the OD405 for 5-thio-2-nitrobenzoate, 13,600 M/cm..
1.3. PIB-PET
1.3.1. PIB-PET acquisition
All participants underwent PIB-PET imaging at Lawrence Berkeley National Laboratory between April 2005 and February 2009. Average time between plasma collection and PIB was 0.69 ±0.70 years (range 0-2.87). [11C]PIB was synthesized at the Lawrence Berkeley National Laboratory’s Biomedical Isotope Facility. PET scans were performed with a Siemens ECAT EXACT HR PET scanner in 3D acquisition mode. 15 mCi of PIB were injected as a bolus into an antecubital vein and dynamic acquisition frames were obtained for 90 min, as previously described (20). PET data were reconstructed using an ordered subset expectation maximization algorithm with weighted attenuation. Images were smoothed with a 4 mm Gaussian kernel with scatter correction. All images were evaluated before analysis for participant motion and adequacy of statistical counts.
1.3.2. PIB-PET analysis
Image processing and analysis was performed using Statistical Parametric Mapping (SPM) software (http://www.fil.ion.ucl.ac.uk/spm). To allow for inter-subject comparisons, voxel-wise distribution volume ratios (DVR) were calculated using Logan graphical analysis, with the cerebellum time-activity curve used as a reference tissue input function (t = 35–90 min).
1.3.3. Voxel-wise analysis
Individual PIB volumes were spatially normalized to Montreal Neurological Institute (MNI) space using SPM5. Mean PIB images created during frame realignment were normalized to the SPM PET template, and the normalization parameters were then applied to the PIB DVR volumes. Spatially-normalized images were smoothed with a 12 mm kernel. Voxel-wise multi-linear regression was performed examining the relationship between PIB binding and AChE activity, adjusting for age, sex, apolipoprotein E4 (ApoE4) allele status (carrier or non- carrier), Vascular Index and cholinesterase inhibitor (ChE-I) treatment. A similar model was used to test the relationship between PIB and BChE activity with two exceptions: participants taking rivastigmine (a non-selective ChE-I that affects both AChE and BChE) were excluded, and ChE-I use was not included as a covariate since donepezil and galantamine do not have a biological effect on BChE). To allow broad visualization of the data, results were displayed on a template brain as T-maps threshold at p<0.001 uncorrected for multiple comparisons.
1.3.4. Region of interest (ROI) analysis
PIB DVR values were extracted in normalized space from regions of interest derived from the Automated Anatomic Labeling Atlas (21). The ROIs were: frontal, parietal, temporal and occipital cortex, hippocampus, precuneus and posterior cingulate cortex. In order to exclude white matter and cerebrospinal fluid, automated regions of interest were masked by the individual subject’s gray matter segmented images. A PIB index, representing the mean DVR throughout frontal, parietal, lateral temporal and cingulate cortices was used as a measure of global amyloid burden.
1.4. Statistical analysis
Multi linear regression was used to evaluate the relationship between PIB binding (independent variable) and AChE activity (dependent variable) across all subjects, and then separately among AD and non-AD participants. The following covariates were included in the model: age, sex, ApoE4 status (carrier or non- carrier), Vascular Index and ChE-I treatment (separately for donepezil, rivastigmine and galantamine, as each ChE-I may have a different effect on AChE activity level (22)). Due to the small sample size, we repeated the analysis with no covariates, using PIB Index as a single independent variable. For the evaluation of the relationship between PIB binding (independent variable) and BChE activity (dependent variable) the model included: age, sex, ApoE4 allele status and Vascular Index. As in the voxel-wise analysis, subjects using rivastigmine were excluded and ChE-I treatment was not included as a covariate. Again we repeated the analysis with no covariates, using PIB Index as a single independent variable. The model then evaluated separately the relationship among AD and non-AD participants. Among participants with clinical AD, the correlation between disease severity and cholinesterase (AChE and BChE) activity was tested using MMSE or CDR sum-of boxes (CDR-sb) as measures of disease severity. Additional covariates included in the model were: age, sex, ApoE4 allele status (carrier or non- carrier), Vascular Index and ChE-I treatment. The relationships between PIB Index (dependent variable) and MMSE/ CDR-sb (independent variables) were assessed using multi-linear regression adjusting for age, sex, education and ApoE4 allele status (carrier or non- carrier). All analyses were conducted in SPSS (version 18.0).
The study was approved by the University of California Berkeley, University of California San Francisco and Lawrence Berkeley National Laboratory Institutional Review Boards for Human Research.
2. Results
2.1. Participant characteristics
Our final cohort consisted of 64 individuals (Table 1). 29 participants had a clinical diagnosis of AD (including 1 Lewy-body variant), and 35 had a non-AD diagnosis (27 frontotemporal lobar degeneration, 3 dementia of unknown cause, and 5 non-demented participants). The cohort was characterized by male preponderance and high education. 27 participants had either no cognitive impairment (CDR=0), or mild cognitive impairment (CDR=0.5) and 36 participants met criteria for dementia (CDR≥1). One non AD participant lacks a CDR score. 32/64 participants were taking ChE-I: donepezil (n=21) rivastigmine (n=5) or galantamine (n=6).
Table 1.
Participant characteristics
| AD | Non AD | |
|---|---|---|
| Number of Participant | 29 | 35 |
| Age at Sample | 66.8 ±8.6 | 63.7 ±7.4 |
| Sex | 20M,9F | 21M,14F |
| Education | 16.5 ±3.2 | 15.7 ±2.9 |
| MMSE | 19.6 ±8.2 | 23.2 ±7.7 |
| CDR (0,0.5,≥1) | 1,8,20 | 6,12,16 (1missing data) |
| ApoE4 carrier/non carrier | 18/29 | 8/35 |
| Vascular index | 1.3±1.4 | 1.1±1.1 |
| PIB index | 1.6±0.3 | 1.2±0.3 |
| PIB positive (PIB index>1.2) | 27/29 | 10/35 |
| Interval between plasma sample & PIB (years) |
0.6 ± 0.7 | 0.7 ± 0.7 |
| ChE-I use | 20/29 (69%) | 12/35 (34%) |
| Donepezil: Rivastigmine: Galantamine: none |
13:3:4:9 | 8:2:2:23 |
2.2. PIB: voxel-wise correlations
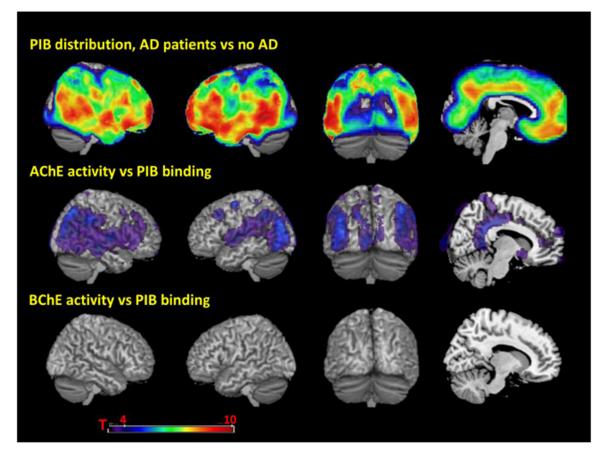
AChE activity correlated with PIB binding in diffuse cortical regions including bilateral middle frontal gyrus, precuneus, middle and posterior cingulate gyrus, inferior parietal lobule, superior greater than middle and inferior temporal gyrus, superior and middle occipital gyrus, calcarine cortex and lingual gyrus (p<0.001 uncorrected, Fig. 1). Additional correlations were found in right anterior cingulate, and right superior and inferior frontal gyrus. No correlation was found between BChE activity and PIB binding at p<0.001 uncorrected (Fig. 1).
Figure 1.
Top row, patterns of PEB binding in Alzheimer’s disease (AD) compared to non-AD participants. Middle row, voxel-wise multi-linear regression of PIB binding and AChE activity, adjusting for age. sex, apolipoprotem E4 (ApoE4) allele status (carrier or non- carrier). Vascular Index and cholinesterase inhibitor (C’hE-I) treatment. Bottom row, voxel-wise multi-linear regression of PIB binding and BC’hE activity, adjusting for age. sex. apolipoprotem (ApoE4) allele status (carrier or non- carrier), excluding participants taking rivastigmine (a non-selective ChE-I that affects both AChE and BChE). All results are presented at a threshold of P < 0.001. uncorrected for multiple comparisons.
2.3. PIB: region of interest correlations
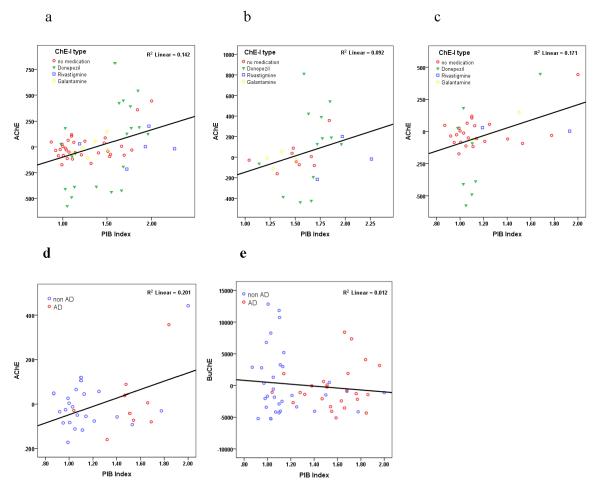
AChE activity was positively correlated with PIB DVR values throughout all ROIs (p<0.01) except hippocampus (Table 3). Global amyloid burden, measured by PIB index, was positively correlated with AChE activity (β=0.39, p<0.001) (Table 2, Fig. 2a), with similar results when using PIB Index as a single independent variable (β=0.395, p=0.001). A model in which clinical diagnosis (AD and non-AD) was added as an independent variable (to account for the potential confounding effect of clinical AD on both PIB and AChE activity) yielded similar results (β=0.42, p=0.001). On evaluation of non-AD participants the correlation remained positively significant (β=0.39, p=0.01) (Fig. 2c), while among AD participants the correlation was marginally significant (β=0.30, p=0.08) (Fig. 2b). The analysis was repeated including only individuals who were naïve to ChE-I treatment (n=32), to assure that the correlation between PIB Index and AChE activity was not confounded by ChE-I use among AD subjects, who are also likely to show high PIB binding. AChE remained positively correlated with PIB index (β=0.51, p=0.006) (Fig. 2d). BChE activity was not correlated with PIB DVR values in any of the ROIs (Table 3) or with PIB index (β=−0.123, p=0.383) (Table 2, Fig. 2e). Using the model with PIB Index as a single independent variable yielded similar results (β=−0.186, p=0.162). BChE correlated with sex (F>M) (β=0.29, p=0.03), compatible with previous observations (23), but not with any other variable in the model (Table 2).
Table 3.
Results of multi linear regression examining the relationship between PIB binding and cholinesterase activity in different ROIs
| ROI | AChE activity | BChE activity | ||||
|---|---|---|---|---|---|---|
| Standardized coefficient β |
T | Sig. | Standardized coefficient β |
t | Sig. | |
| Frontal cortex | 0.371 | 3.56 | 0.001 | −0.109 | −0.778 | 0.440 |
| Hippocampus | 0.170 | 1.662 | 0.102 | 0.038 | 0.284 | 0.778 |
| Lateral temporal cortex | 0.378 | 3.770 | <0.001 | −0.127 | −0.933 | 0.355 |
| Medial temporal cortex | 0.295 | 2.977 | 0.004 | −0.137 | −1.029 | 0.308 |
| Occipital cortex | 0.319 | 3.323 | 0.002 | −0.195 | −1.476 | 0.146 |
| Parietal cortex | 0.412 | 4.050 | <0.001 | −0.156 | −1.121 | 0.268 |
| Posterior cingulate cortex |
0.387 | 4.208 | <0.001 | −0.126 | −0.938 | 0.352 |
| Precuneus | 0.382 | 3.691 | 0.001 | −0.159 | −1.144 | 0.258 |
Table 2.
Results of multi linear regression, examining the relationship between PIB index and cholinesterase activity.
| AChE activity | BChE activity | |||||
|---|---|---|---|---|---|---|
| Standardized coefficient β |
T | Sig. | Standardized coefficient β |
t | Sig. | |
| PIB index | .389 | 3.784 | .000 | −.123 | −.880 | .383 |
| Sex | −.082 | −.939 | .352 | .286 | 2.211 | .031 |
| ApoE4 carrier | −.093 | −.991 | .326 | −.211 | −1.524 | .134 |
| Age at sample | .073 | .828 | .411 | −.109 | −.838 | .406 |
| Vascular index | .105 | 1.196 | .237 | .049 | .377 | .708 |
| Donepezil | .606 | 6.364 | .000 | - | - | - |
| Rivastigmine | −.199 | −1.994 | .051 | - | - | - |
| Galantamine | .071 | .783 | .437 | - | - | - |
Figure 2.
Partial regression plots show the relationships between PIB Index and Cholinesterase activity, (a) AChE vs PIB Index among all participants, with AChE-I symbols (b) AChE vs PIB Index among AD participants (c) AChE vs PIB Index among non AD participants (d) AChE vs PIB Index among participants naïve to AChE-I treatment (e) BChE vs PIB Index. Residuals are plotted for each participant to adjust for the effects of age, gender, Vascular Index, ApoE4 status and ChE-I treatment.
2.4. AChE activity and ChE-I use
Use of donepezil, a rapidly-reversible ChE-I, correlated positively with AChE activity in the serum (β=0.61, p<0.001). There was a trend for a negative correlation between AChE activity levels and use of rivastigmine, a pseudo-irreversible ChE-I (β=−0.20, p<0.06). Use of galantamine, a rapidly-reversible ChE-I that also acts as a weak nicotinic receptor agonist (24), showed no correlation with AChE activity levels (Table 2).
2.5. Disease severity and cholinesterase activity among participants with clinical AD
Among participants with clinical AD, neither AChE nor BChE activity were correlated with disease severity, as measured by MMSE (β=0.13, p=0.40 and β=0.12, p=0.36) or CDR-sb (β=−0.27, p=0.05 and β=−0.02, p=0.86). In addition, no correlation was found between PIB Index and disease severity as measured by MMSE (β=0.02, p=0.94) and CDR-sb (β=−0.14, p=0.51).
3. Discussion
This is, to our knowledge, the first study to examine the relationship between plasma cholinesterase activity and brain Aβ plaques measured in vivo by PIB-PET. As hypothesized, we found a positive correlation between AChE activity and PIB binding. As PIB is a specific marker of Aβ fibrillar deposits which can be found both in AD and non-AD subjects, we correlated PIB binding and ChE activity across all subjects. This correlation remained significant after adjusting for the potential confounding effects of clinical diagnosis (by separately examining AD vs. non-AD groups, or by adding diagnostic group as a covariate) and ChE treatment. These findings suggest that deposition of Aβ in the brain is reflected in peripheral cholinergic activity. However, whether increased cholinergic activity in the periphery drives or is caused by Aβ deposition is unclear.
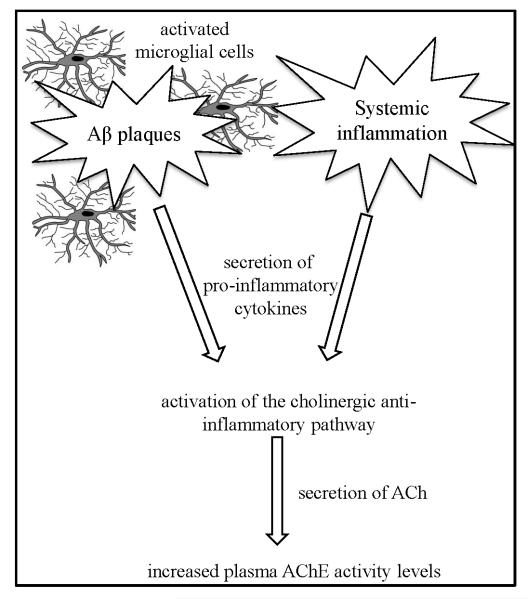
The link between brain Aβ and the peripheral cholinergic activity may be mediated by the ‘cholinergic anti-inflammatory pathway’ (Fig. 3). Minor signs of neuroinflammation can be found in the normal aging brain and are exacerbated by AD (7, 25). Aβ aggregates play a pivotal role as inducers of neuroinflammation via activation of microglial cells, which in turn release neurotoxic cytokines (8, 26). While pro-inflammatory cytokines exacerbate inflammation, other cytokines act to restrain it by activating anti-inflammatory responses. One of the rapid anti-inflammatory responses is the ‘cholinergic anti-inflammatory pathway’, which alleviates inflammation by increased release of ACh to the periphery via the vagus nerve (27, 28). ACh then acts as an anti-inflammatory agent by deactivating tissue macrophages (29, 30) and glial cells in the brain (31). Hyper-activation of the ‘cholinergic anti-inflammatory pathway’ increases ACh release and therefore leads to an increase in AChE activity, which then acts to dampen the excessive cholinergic signal (32, 33). Activation of the cholinergic anti-inflammatory pathway by Aβ-mediated processes thus provides a plausible mechanism for the observed positive correlation between PIB binding and peripheral ChE activity.
Figure 3.
Activation of the cholinergic anti-inflammatory pathway - Microglial cells activated by Aβ plaques/ongoing systemic inflammation increase the secretion of proinflammatory cytokines. The cytokines activate the ‘cholinergic anti-inflammatory pathway’ which increases ACh release. As a response to increased ACh levels, AChE activity levels increase to dampen the effect.
Since cytokines cross the blood brain barrier (34), it is also possible that activation of the ‘cholinergic anti-inflammatory pathway’ is mediated by peripheral pro-inflammatory cytokines. Studies suggest that systemic inflammation might be associated with increased risk of developing AD (35-37). Pro-inflammatory cytokines have been shown to enhance Aβ deposition through increased expression of BACE1, which mediates the initial step in the cleavage of amyloid precursor protein to Aβ, and suppression of Aβ clearance (38). Therefore, exacerbation of Aβ deposition due to increased peripheral pro-inflammatory cytokines might provide another plausible explanation of our findings.
The association between the cholinergic and inflammatory pathways is quite complex. Recent studies have shown that the brain’s cholinergic signaling activates a peripheral blockade over inflammation (39). This in turn limits the penetration of pro-inflammatory cytokines into the brain and reduces the production of AChE in cholinergic neurons. Within the brain, as well as in nucleated blood cells, AChE is by far the major cholinesterase, and changes in one of these enzymes do not necessarily predict similar changes in the other. An example is post-stroke conditions, where plasma AChE levels decline whereas BChE levels increase (40)
Clinical decline in AD is correlated with decreased AChE activity in the cerebral cortex (41, 42), but not with amyloid burden, which has either reached a plateau in early stages of AD or continues to progress very slowly (43). Consistent with previous studies, we found no correlation between PIB load and disease severity (44, 45). The lack of correlation between disease severity and plasma cholinesterase activity in AD patients in our cohort strengthens our hypothesis that acetylcholinesterase activity in the plasma is a reflection of amyloid burden rather than central cholinergic function.
We also found a positive relationship between ChE-I treatment with donepezil and plasma AChE activity. This relationship is perhaps counter-intuitive, given that the goal of ChE-I treatment is to dampen AChE activity and thus enhance cholinergic transmission. However, our results are consistent with previous studies which reported increased AChE activity in plasma and CSF among AD patients who were treated with donepezil (22, 46-48). It is possible that suppression of ChE activity in AD patients is transient, and that treatment may lead to a “rebound” of activity via compensatory mechanisms (49), perhaps explaining the apparently transient benefit of ChE-I treatment (50). Rivastigmine has a pseudo-irreversible inhibitory effect on acetyl- and butyrylcholinesterase and also was shown to have a potential neuroprotective effect, by altering APP (Aβ precursor) and Aβ secretions (51). The negative trend between rivastigmine use and AChE is compatible with previous studies (46, 52). The lack of relationship between galantamine and AChE activity can be attributed to its weak AChE inhibitory activity (45). However, due to the small sample size of ChE-I treated individuals in this study (particularly with galantamine and rivastigmine), these observations should be considered preliminary. Further studies are needed to examine the biological effects of ChE-I treatment over time in AD.
Our study has limitations. No direct inflammatory markers were measured and thus we could not directly test the hypothesis that Aβ deposition and peripheral ChE activity are linked by activation of the cholinergic anti-inflammatory pathway. Our cohort is primarily composed of patients with dementia, and most have some degree of neurodegeneration (related to either AD or non-AD pathology). Thus, it is conceivable that inflammation is driven by brain pathology other than Aβ plaques. We do not have biomarkers of non-Aβ pathology in this cohort (e.g. CSF tau levels). Structural MRIs were performed on a variety of different MRI scanners and field strengths (1.5T, 3T and 4T), precluding a primary analysis of the relationship between atrophy/neurodegeneration and ChE activity. We measured total AChE activity and thus cannot distinguish the relative contributions of AChE subtypes, which are known to have different effects on Aβ accumulation. Finally, though our heterogeneous dementia population included a spectrum of Aβ pathology, patients with clinical AD were highly likely to have high PIB (93%) and be treated with ChE-I (69%), potentially confounding our analysis. Reassuringly, the correlation between PIB and ChE activity remained significant when adjusting for ChE-I use and clinical AD and in the subset of patients naïve to ChE-I treatment, suggesting it was not spuriously driven by ChE-I treatment in AD patients.
Future studies investigating the in vivo relationship between Aβ and cholinergic activity should incorporate measurements of inflammatory markers in the serum and in the CSF, to establish the connection between Aβ and the ‘cholinergic anti-inflammatory pathway’. As CSF Aβ1-42 and PIB-PET was shown in previous studies are highly correlated (53), studying the correlation between Aβ levels in the CSF and plasma AChE would reinforce the correlation we found between brain Aβ plaques and plasma AChE, and its potential role as a biomarker for AD. It would also be informative to study the relationship between peripheral ChE activity and the integrity of cholinergic nuclei in the basal forebrain, as well as the relationship between ChE activity and more direct measures of neurodegeneration in AD, such as temporoparietal hypometabolism and cortical and hippocampal atrophy.
Acknowledgements
This study was supported by the Rosetrees Foundation and a Senior Miller fellowship (to H.S for work at UCB); an ICNC pre-doctoral fellowship (to G.Z); National Institute on Aging (grant numbers K23-AG031861 to G.D.R, R01-AG034570 to W.J.J, P01-AG1972403 and P50-AG023501 to B.L.M.); Alzheimer’s Association (grant numbers NIRG-07-59422 to G.D.R. and ZEN-08-87090 to W.J.J); John Douglas French Alzheimer’s Foundation (to G.D.R.) and State of California Department of Health Services Alzheimer’s Disease Research Center of California (grant number 04-33516 to B.L.M).
The authors would like to thank Tony Wyss-Coray and Sartoris Inc. for sharing samples.
Footnotes
Conflict of Interest: The authors confirm that this article content has no conflicts of interest.
References
- 1.The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 2.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982 Jul 30;217(4558):408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 3.Coyle JT, Price DL, DeLong MR. Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science. 1983 Mar 11;219(4589):1184–90. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010 Jan;9(1):119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011 May;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. Epub 2011 Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlgren KN, Manelli AM, Stine WB, Jr., Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002 Aug 30;277(35):32046–53. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 7.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and alzheimer’s disease. Neurobiol Aging. 2000 May-Jun;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heneka MT, O’Banion MK. Inflammatory processes in alzheimer’s disease. J Neuroimmunol. 2007 Mar;184(1-2):69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res Dec. :9. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 10.Arendt T, Bruckner MK, Lange M, Bigl V. Changes in acetylcholinesterase and butyrylcholinesterase in alzheimer’s disease resemble embryonic development--a study of molecular forms. Neurochem Int. 1992 Oct;21(3):381–96. doi: 10.1016/0197-0186(92)90189-x. [DOI] [PubMed] [Google Scholar]
- 11.Kuhl DE, Koeppe RA, Snyder SE, Minoshima S, Frey KA, Kilbourn MR. In vivo butyrylcholinesterase activity is not increased in alzheimer’s disease synapses. Ann Neurol. 2006 Jan;59(1):13–20. doi: 10.1002/ana.20672. [DOI] [PubMed] [Google Scholar]
- 12.Perry EK, Perry RH, Blessed G, Tomlinson BE. Changes in brain cholinesterases in senile dementia of alzheimer type. Neuropathol Appl Neurobiol. 1978 Jul-Aug;4(4):273–7. doi: 10.1111/j.1365-2990.1978.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 13.Darreh-Shori T, Forsberg A, Modiri N, Andreasen N, Blennow K, Kamil C, et al. Differential levels of apolipoprotein E and butyrylcholinesterase show strong association with pathological signs of alzheimer’s disease in the brain in vivo. Neurobiol Aging. 2010 Jun 8; doi: 10.1016/j.neurobiolaging.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: Evidence review for a clinical practice guideline. Ann Intern Med. 2008 Mar 4;148(5):379–97. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 15.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000 May 25;405(6785):458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 16.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in alzheimer’s disease with pittsburgh compound-B. Ann Neurol. 2004 Mar;55(3):306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 17.Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and alzheimer disease. Cogn Behav Neurol. 2003 Dec;16(4):211–8. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard RE, O’Mahony MS, Calver BL, Woodhouse KW. Plasma esterases and inflammation in ageing and frailty. Eur J Clin Pharmacol. 2008 Sep;64(9):895–900. doi: 10.1007/s00228-008-0499-1. [DOI] [PubMed] [Google Scholar]
- 19.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991 Jan;121(1 Pt 2):293–8. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 20.Rabinovici GD, Furst AJ, O’Neil JP, Racine CA, Mormino EC, Baker SL, et al. 11C-PIB PET imaging in alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007 Apr 10;68(15):1205–12. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- 21.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002 Jan;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 22.Nordberg A, Darreh-Shori T, Peskind E, Soininen H, Mousavi M, Eagle G, et al. Different cholinesterase inhibitor effects on CSF cholinesterases in alzheimer patients. Curr Alzheimer Res. 2009 Feb;6(1):4–14. doi: 10.2174/156720509787313961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sklan EH, Lowenthal A, Korner M, Ritov Y, Landers DM, Rankinen T, et al. Acetylcholinesterase/paraoxonase genotype and expression predict anxiety scores in health, risk factors, exercise training, and genetics study. Proc Natl Acad Sci U S A. 2004 Apr 13;101(15):5512–7. doi: 10.1073/pnas.0307659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samochocki M, Zerlin M, Jostock R, Groot Kormelink PJ, Luyten WH, Albuquerque EX, et al. Galantamine is an allosterically potentiating ligand of the human alpha4/beta2 nAChR. Acta Neurol Scand, Suppl. 2000;176:68–73. doi: 10.1034/j.1600-0404.2000.00310.x. [DOI] [PubMed] [Google Scholar]
- 25.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002 Aug 1;35(3):419–32. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 26.Heneka MT, O’Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in alzheimer’s disease. J Neural Transm. Aug;117(8):919–47. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- 27.Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007 Apr;35(4):1139–44. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 28.van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF, et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005 Jun 15;191(12):2138–48. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 29.Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, et al. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity. 2009 Dec 18;31(6):965–73. doi: 10.1016/j.immuni.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Tracey KJ. The inflammatory reflex. Nature. 2002 Dec 19-26;420(6917):853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 31.Pollak Y, Gilboa A, Ben-Menachem O, Ben-Hur T, Soreq H, Yirmiya R. Acetylcholinesterase inhibitors reduce brain and blood interleukin-1beta production. Ann Neurol. 2005 May;57(5):741–5. doi: 10.1002/ana.20454. [DOI] [PubMed] [Google Scholar]
- 32.Kaufer D, Friedman A, Seidman S, Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature. 1998 May 28;393(6683):373–7. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- 33.Soreq H, Seidman S. Acetylcholinesterase--new roles for an old actor. Nat Rev Neurosci. 2001 Apr;2(4):294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 34.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995 Jul-Aug;2(4):241–8. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 35.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, et al. Inflammatory proteins in plasma and the risk of dementia: The rotterdam study. Arch Neurol. 2004 May;61(5):668–72. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 36.Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, et al. Systemic inflammation and disease progression in alzheimer disease. Neurology. 2009 Sep 8;73(10):768–74. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, et al. Inflammatory markers and the risk of alzheimer disease: The framingham study. Neurology. 2007 May 29;68(22):1902–8. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, et al. Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in swedish mutant APP transgenic mice. Am J Pathol. 2007 Feb;170(2):680–92. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011 Oct 7;334(6052):98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben Assayag E, Shenhar-Tsarfaty S, Ofek K, Soreq L, Bova I, Shopin L, et al. Serum cholinesterase activities distinguish between stroke patients and controls and predict 12-month mortality. Mol Med. 2010 Jul-Aug;16(7-8):278–86. doi: 10.2119/molmed.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haense C, Kalbe E, Herholz K, Hohmann C, Neumaier B, Krais R, et al. Cholinergic system function and cognition in mild cognitive impairment. Neurobiol Aging. Oct 18; doi: 10.1016/j.neurobiolaging.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Shinotoh H, Namba H, Fukushi K, Nagatsuka S, Tanaka N, Aotsuka A, et al. Progressive loss of cortical acetylcholinesterase activity in association with cognitive decline in alzheimer’s disease: A positron emission tomography study. Ann Neurol. 2000 Aug;48(2):194–200. [PubMed] [Google Scholar]
- 43.Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: Testing the amyloid hypothesis in vivo. Behav Neurol. 2009;21(1):117–28. doi: 10.3233/BEN-2009-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furst AJ, Rabinovici GD, Rostomian AH, Steed T, Alkalay A, Racine C, et al. Cognition, glucose metabolism and amyloid burden in alzheimer’s disease. Neurobiol Aging. Apr 22; doi: 10.1016/j.neurobiolaging.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009 Oct 13;73(15):1193–9. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amici S, Lanari A, Romani R, Antognelli C, Gallai V, Parnetti L. Cerebrospinal fluid acetylcholinesterase activity after long-term treatment with donepezil and rivastigmina. Mech Ageing Dev. 2001 Nov;122(16):2057–62. doi: 10.1016/s0047-6374(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 47.Davidsson P, Blennow K, Andreasen N, Eriksson B, Minthon L, Hesse C. Differential increase in cerebrospinal fluid-acetylcholinesterase after treatment with acetylcholinesterase inhibitors in patients with alzheimer’s disease. Neurosci Lett. 2001 Mar 16;300(3):157–60. doi: 10.1016/s0304-3940(01)01586-5. [DOI] [PubMed] [Google Scholar]
- 48.Parnetti L, Chiasserini D, Andreasson U, Ohlson M, Huls C, Zetterberg H, et al. Changes in CSF acetyl- and butyrylcholinesterase activity after long-term treatment with AChE inhibitors in alzheimer’s disease. Acta Neurol Scand. Sep 29; doi: 10.1111/j.1600-0404.2010.01435.x. [DOI] [PubMed] [Google Scholar]
- 49.Kaufer D, Friedman A, Seidman S, Soreq H. Anticholinesterases induce multigenic transcriptional feedback response suppressing cholinergic neurotransmission. Chem Biol Interact. 1999 May 14;119-120:349–60. doi: 10.1016/s0009-2797(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 50.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005 Jun 9;352(23):2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 51.Bailey JA, Ray B, Greig NH, Lahiri DK. Rivastigmine Lowers Aβ and Increases APPα Levels, Which Parallel Elevated Synaptic Markers and Metabolic Activity in Degenerating Primary Rat Neurons. PLoS One. 2011;6(7):e21954. doi: 10.1371/journal.pone.0021954. Epub 2011 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darreh-Shori T, Almkvist O, Guan ZZ, Garlind A, Strandberg B, Svensson AL, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology. 2002 Aug 27;59(4):563–72. doi: 10.1212/wnl.59.4.563. [DOI] [PubMed] [Google Scholar]