Abstract
Objective/Background
Many patients with systemic lupus erythematosus (SLE) have working memory deficits. Few studies have evaluated working memory performance and neurometabolite profile using magnetic resonance spectroscopy (MRS) in SLE.
Methods
We gave the Paced Auditory Serial Addition Test (PASAT), a measure of working memory, to 73 patients with SLE. We calculated total score, dyads, chunking, and cognitive fatigue. Using MRS, we determined the ratio of choline to creatine (Ch/Cr) in normal-looking right and left frontal lobe white matter.
Results
Twenty-nine percent of patients showed impaired working memory on the PASAT. Total PASAT score inversely correlated with right and left frontal white matter Ch/Cr. Left frontal white matter Ch/Cr correlated with percent chunking and inversely correlated with total and percent of dyads. Right frontal white matter Ch/Cr correlated with percent chunking and inversely correlated with total and percent dyads. There was no relationship between cognitive fatigue and either left or right frontal white matter Ch/Cr. Longer disease duration was associated with higher left frontal white matter Ch/Cr. Correlations remained significant between left frontal white matter Ch/Cr and total PASAT score and total dyads when disease duration was considered.
Conclusions
Patients with SLE were impaired on the PASAT. Lower total PASAT score and fewer dyads correlated with higher left frontal microstructural white matter damage, while cognitive fatigue did not. This pattern suggests that early white matter damage interferes with working memory in SLE and provides further insight into the neurobiological basis of mild cognitive dysfunction related to microstructural white matter injury.
Keywords: systemic lupus erythematosus (SLE), working memory, Paced Auditory Serial Addition Test (PASAT), MRS, white matter
Many patients with systemic lupus erythematosus (SLE) develop neuropsychiatric disorders involving the central and peripheral nervous system. The American College of Rheumatology has identified 19 neuropsychiatric disorders in SLE, ranging from headache to mood and anxiety disorders to myasthenia gravis (“The American College of Rheumatology nomenclature,” 1999); one of these disorders, which can develop independently of the others, is cognitive impairment. Common manifestations of cognitive impairment in patients with SLE are deficits in attention, processing speed, and working memory (Kozora, 2008).
The Paced Auditory Serial Addition Test (PASAT) (Gronwall, 1977) assesses these cognitive processes by requiring examinees to pay attention to auditory input (a pair of numbers to be added), to inhibit the encoding of their response (the sum) while attending to the next pair in the series, and to perform the task at an externally determined pace (Spreen and Strauss, 1991). Although there are many versions of the PASAT, most of the commonly used forms include 4 trials of 50 to 61 numbers (Gronwall, 1977; Levin et al, 1982). During each trial, an audiorecorded voice says individual numbers between 1 and 9. Participants are instructed to add the number just heard, to the one heard immediately before it, and say the total. Each trial presents the numbers at a faster rate than the previous trial. The speeds of presentation depend on the version of the PASAT being given. For example, the first trial might give a new number every 2.4 seconds; the second trial, every 2.0 seconds; the third, every 1.6 seconds; and the fourth, every 1.2 seconds. PASAT performance is commonly impaired in people who have neurologic disorders involving alterations of white matter integrity, including traumatic brain injury, multiple sclerosis (MS), and SLE (Audoin et al, 2005; Kozora et al, 2004; Shucard et al, 2004; Tombaugh, 2006; Vanderploeg et al, 2005).
In 2004, we reported that global PASAT performance was significantly lower in patients with SLE, whether or not they had neuropsychiatric manifestations, than in controls (Kozora et al, 2004). Our patients with SLE and neuropsychiatric symptoms performed in the mildly to moderately impaired range; those without neuropsychiatric activity performed in the mildly impaired range. Covey et al (2012) found that patients with SLE or MS performed more poorly than controls on the PASAT total score, and the authors reported that the PASAT was the most sensitive measure of SLE-induced cognitive impairment in a battery of cognitive tests.
In a study of patients with SLE and controls, Shucard et al (2004) reported higher rates of impaired PASAT performance as the task sped up: 11.1% of the SLE group and 7.4% of the controls were impaired at the 2.4-second rate; 17.8% of the SLE group and 3.7% of controls at the 2.0-second rate. (People with intact information processing and working memory tend to perform better with faster presentation speeds.) Although the SLE group was more impaired than the controls at both presentation rates, the groups had no significant difference in total correct responses at either rate. This finding suggests that presentation speed could be an important factor in detecting impairment to information processing and working memory.
Interpreting the PASAT total score or impairment rates in isolation, however, has limitations. Many investigators have found that participants try to reduce the task-related cognitive load (ie, the number of discrete units of information that they must hold in working memory at once) to improve their performance on the PASAT (Snyder et al, 1993). For example, participants might skip every other item on the task to optimize their performance on the items that they do choose to address. This “alternate answer” strategy (Tombaugh 2006) allows for a “nonimpaired” score, but alters the test paradigm and reduces the sensitivity of the PASAT total score in detecting impaired cognitive processing. To address this problem, researchers have designed alternative scoring procedures that measure both working memory and speed of information processing. Snyder et al (1993) developed a “dyad” score that tracks the number of correct responses immediately preceded by another correct response. A higher dyad score thus implies that the participant is trying to tackle all the test items.
Fisk and Archibald (2001) later studied how patients with MS “chunked” items to reduce the PASAT’s cognitive load. By chunking, a participant forgoes any attempt to keep adding consecutive digits, and instead collapses multiple elements of information into more manageable sets, or “chunks.” For example, a person may add 2 numbers, skip 1 number, add the next 2 numbers, skip 1, etc. Another person may add 3 pairs of numbers, skip 1 number, add 3 pairs of numbers, etc. Because the PASAT is difficult, participants may chunk intentionally or may not even realize that they are doing it. They are also likely to chunk in several different ways during the task.
Beyond measuring chunking directly, Fisk and Archibald (2001) observed that this way of reducing cognitive load is reflected in other PASAT scores. Participants who chunk have a lower total number of dyads and a lower percentage of correct responses that are dyads. More specifically, the authors reported that the percentage of dyads fell as the PASAT presentation rate sped up over trials 1 through 4. Shucard et al (2004) reported that patients with SLE had fewer dyads than controls at faster PASAT rates, and more chunking at all rates. The authors concluded that chunked scores reflect performance differences more accurately than dyad scores. Notably, Shucard et al found no correlation between these findings and disease activity, steroid dosage, depression, or fatigue.
Shucard et al also discerned that not all PASAT responses are dyads or chunking. Dyads are correct responses following other correct responses, and chunks are correct responses following skipped items. A third possibility is a correct response following an attempted but incorrect response, which Shucard et al termed an “other” response. “Other” responses show errors made while trying to perform the PASAT according to the instructions. Interestingly, the controls had twice as many “other” responses as did patients with SLE. The authors wrote that although these errors could be interpreted as the controls making more mistakes than the patients when trying to do the PASAT as instructed, the controls’ high rate of “other” responses could also suggest their greater willingness to try to respond to the most possible items, thus maintaining more of a working memory burden.
If we compare PASAT subscores overall, a high proportion of dyad responses reflects the greatest working memory burden, a high rate of “other” responses (across trials and/or between groups) reflects the next greatest working memory burden, and a high rate of chunking responses (across trials and/or between groups) reflects the smallest working memory burden.
Taking a more holistic approach to interpreting the PASAT, Bryant et al (2004) found that patients with MS were susceptible to cognitive fatigue, defined as fewer correct responses generated during the second half of a PASAT trial than during the first half. These investigators developed a PASAT-based cognitive fatigue index that is “operationalized as the failure to sustain effort over the course of a continuous working memory task” (p 114).
Understanding the neurobiology of performance on information-processing tasks such as the PASAT has been the focus of many studies of SLE as well as other neurologic and medical disorders (Tombaugh, 2006). In SLE, we and other investigators have used magnetic resonance spectroscopy (MRS) to evaluate the ratio of choline to creatine (Ch/Cr) as an index of brain white matter integrity. Ch is essential to neuronal membranes and myelin; Cr is a storage form of high-energy phosphates used as a reference marker. An elevated Ch/Cr is regarded as a marker of increased membrane turnover related to inflammation, demyelination, ischemia, and/or gliosis.
We were the first to report an association between elevated frontal lobe white matter Ch/Cr and cognitive impairment in patients with SLE (Kozora et al, 2005). In a later study, we also found a higher frontal white matter Ch/Cr (with no other differences in white or gray matter structure) in patients with SLE and no overt neuropsychiatric disorders than in controls (Filley et al, 2009). A composite measure of attention, processing speed, and executive function correlated with total white matter volume and correlated inversely with left frontal white matter Ch/Cr (Filley, 2009). These findings suggest that early changes in white matter, particularly in myelin, may play a role in SLE-related impairment in attention, processing speed, and executive function (Kozora et al, 2012). We have proposed the pathogenesis of these white matter changes in SLE to be immune-mediated myelinopathy (Filley, 2009; Kozora et al, 2012).
Collectively, the prior studies of PASAT performance among persons with SLE suggest that the PASAT reveals subtle disturbances in attention, processing speed, and executive function, all of which can be subsumed under the unifying domain of working memory. Data also show an association between impaired PASAT performance and SLE-related frontal white matter abnormalities identified by MRS (Filley et al, 2009; Kozora et al, 2011). Questions remain about the processes that people with SLE use to perform the PASAT, including the extent to which their strategies to ease the cognitive load may mask performance deficits, and the manner in which SLE-associated white matter abnormalities might influence the use and benefits of those strategies. The primary objectives of our current study were to evaluate more closely the PASAT response pattern among persons with SLE and no overt neuropsychiatric disorders and to examine PASAT subscores in relation to white matter integrity as assessed by Ch/Cr on MRS.
METHODS
Participants
We studied 73 patients with SLE and no overt neuropsychiatric disorders. Our patients were enrolled in a larger, controlled study of cognitive function and neuroimaging in SLE at National Jewish Health in Denver, CO from 2004 to 2009 (Kozora et al, 2008, 2012). Our patients had been diagnosed with SLE based on the American College of Rheumatology Classification Criteria (Hochberg, 1997). We excluded candidates if they had another past or current neurologic disorder (stroke, seizures, etc), major psychiatric disorder (psychosis, major mood disorder, etc), learning disability, or substance use disorder. Our methods of screening, recruiting, confirming the SLE diagnosis, and completing disease activity indices are described in Kozora et al (2008). We recorded each patient’s duration of disease from the time of clinical diagnosis; recent SLE disease activity, quantified with the Systemic Lupus Disease Activity Index (Bombardier et al, 1992); and total score from the Beck Depression Inventory-Second Edition (Beck et al, 1996). Table 1 lists our sample’s demographic and health characteristics.
TABLE 1.
Demographics and Health Characteristics of 73 Patients With Systemic Lupus Erythematosus and No Overt Neuropsychiatric Disorders
| Mean | Standard Deviation | |
|---|---|---|
|
| ||
| Demographics | ||
|
| ||
| Age (years) | 36.79 | 8.52 |
|
| ||
| Education (years) | 14.62 | 2.53 |
|
| ||
| Women:men | 69:4 (94.52%:5.48%) | |
|
| ||
| Handedness: right:left | 68:5 (93.15%:6.85%) | |
|
| ||
| Ethnicity | ||
| Asian | 4.11% | |
| African American | 19.18% | |
| Caucasian | 63.01% | |
| Hispanic | 13.7% | |
|
| ||
| Heath characteristics | ||
|
| ||
| Months since diagnosis | 87.97 | 76.7 |
|
| ||
| Systemic Lupus Erythematosus Disease Activity Index total | 5.07 | 5.54 |
|
| ||
| % taking prednisone | 46.57 | |
|
| ||
| Prednisone dose (mg/day) | 4.15 | 6.6 |
|
| ||
| % taking hydroxychloroquine | 73.97 | |
|
| ||
| % taking mycophenolic acid | 13.7 | |
|
| ||
| % taking methotrexate | 5.48 | |
|
| ||
| % taking antidepressant | 21.91 | |
|
| ||
| % taking thyroid medication | 23.29 | |
|
| ||
| Behavioral characteristics | ||
|
| ||
| Beck Depression Inventory-II total score | 9.19 | 6.36 |
|
| ||
| Estimated Full-Scale Intelligence Quotient | 103.63 | 8.52 |
The study was approved by the Colorado Multiple Institutional Review Board at the University of Colorado Denver (also serving National Jewish Health. All participants were informed of the study’s procedures, risks, and benefits, and signed an informed consent before participating.
Procedures
We assessed our patients’ cognitive function with the 200-item Levin version of the PASAT (Levin et al, 1982; Levin et al, 1987) and the SLE test battery recommended by the American College of Rheumatology (“The American College of Rheumatology nomenclature,” 1999; Kozora et al, 2004). The Levin version of the PASAT has 4 trials, each with 50 items. As explained earlier, the stimuli are digits ranging from 1 to 9, presented by an audio recording. We gave the test according to the standard instructions and procedures described by Diehr et al (1998). Patients were told to add each consecutive pair of numbers, making sure to add each number to the one immediately preceding it, and to say the sum. Each trial presented the numbers at a faster rate than the previous trial: 3.0, 2.4, 2.0, and 1.6 seconds. Trials were separated by 15 seconds of recorded silence. The entire test was presented in a single session lasting just under 10 minutes. We gave patients a practice list first, to help them understand the task instructions. When they showed that they could perform the task correctly, we switched to the real test.
We recorded all responses, correct and incorrect. Then we transformed the PASAT total score into a T-score based on normative data, correcting for age, education, and sex (Heaton et al, 1991). We considered T-scores below 40 to show impaired working memory.
In addition to calculating the total number of correct responses, we scored the PASAT for total dyads, total chunking, total “other” responses, percentage of total responses accounted for by dyads, and percentage of total responses accounted for by chunking (Table 2). We also determined cognitive fatigue for each trial and derived a composite cognitive fatigue index for all 4 trials by comparing the total number of dyads during the first half of each trial vs the total dyads during the second half of each trial (Table 2). We considered patients to have cognitive fatigue if they made fewer correct responses during the second half of a trial than during the first half; we considered them not to have cognitive fatigue if they made as many or more correct responses during the second half of the trial than the first (Bryant et al, 2004; Fisk and Archibald, 2001; Shucard et al, 2004). Table 2 also shows how we interpreted the score for each measure.
TABLE 2.
Paced Auditory Serial Addition Test (PASAT): Methods and Interpretation of Scoring for Select Measures in This Study
| Measure | Method of Scoring | Interpretation of Score | |
|---|---|---|---|
| Total correct | Number of correct responses | Traditional measure | |
| Total dyads | Correct responses after ≥1 other correct response | Extent to which participants continue to hold PASAT digits in working memory while calculating sums, ie, performing the test according to instructions. A higher score indicates better working memory. | |
| Total chunking | Correct responses after a skipped response | A cognitive strategy for reducing required working memory and attentional workload. A higher score indicates poorer working memory. | |
| Total “other” responses | Correct responses after an incorrect response (ie, total correct responses not considered dyads or chunking) | This score reflects an attempt to perform the task correctly, meaning that the task is more taxing on working memory and attentional processes than with chunking. | |
| Dyads as % of total correct responses |
|
Proportion of total correct responses accounted for by dyads. | |
| Chunking as % of total correct responses |
|
Proportion of total correct responses accounted for by chunking. | |
| Cognitive fatigue | Yes, if participants made fewer correct responses in 2nd half of a trial than in 1st half. No, if correct responses in 2nd half equal or exceed those in 1st half |
Cognitive fatigue suggests inability to sustain attention while engaging working memory over course of a trial. |
Within 2 weeks of the cognitive testing session, which included the PASAT, we performed magnetic resonance imaging (MRI) and MRS using a research-dedicated 3.0T GE MR SIGNA whole-body (long-bore) scanner (GE Healthcare, Waukesha, WI) at the University of Colorado Brain Imaging Center. Specific details of the MRS procedure and techniques are described in Filley et al (2009) and Kozora et al (2010). Briefly, we acquired single-voxel spectra from 2 × 2 × 2 cm2 voxels centered in normal-appearing left and right frontal white matter using the GE Probe-P protocol (pulse sequence = Point Resolved Spectroscopy echo time/repetition time [TE/TR] = 136/2000 ms, 192 averages, with chemical-shift-selective water suppression and outer-volume suppression spatial saturation bands, time = 7:12). We used LCModel software (Provencher, 1993) to analyze spectra and obtained left and right frontal white matter Ch/Cr values.
Data Analysis
We used the SAS statistical analysis package (Version 9.2; SAS Institute Inc, Cary, NC) for descriptive and correlation analyses. We calculated correlations between clinical measures and cognitive scores using Spearman correlation coefficients. We compared high and low frontal white matter Ch/Cr groups in terms of demographics (age, education, sex, and ethnicity), health characteristics (duration of disease, disease activity and severity, and medication use), and behavioral characteristics (using the Beck Depression Inventory-Second Edition). We used a nonparametric Wilcoxon rank sum test to compare all continuous variables, and a Fisher’s exact test to compare all categorical variables. For all of these analyses, we used 2-tailed tests with statistical significance set at P < 0.05.
We used Statistica 8.0 (StatSoft, Inc, Tulsa, OK) for backward stepwise regression modeling to estimate the proportion of variance in left frontal white matter Ch/Cr and right frontal white matter Ch/Cr accounted for by PASAT variables and disease duration. We defined the relevant PASAT variables for each of these models as those that had a significant correlation with either left or right frontal white matter Ch/Cr. We entered each relevant PASAT variable into a regression model with disease duration to determine whether the model was significant and, if so, whether the PASAT variable and/or disease duration were significant independent predictors of left or right frontal white matter Ch/Cr. We assessed the normality of the residual error in each model to evaluate its integrity, and interpreted only those models in which residual error was normally distributed.
RESULTS
PASAT Scores
Table 3 shows our patients’ PASAT performance. We found that 29% (21/73) had total PASAT T-scores below 40, indicating impaired working memory. For all patients across the 4 trials of the PASAT, the mean number of dyads was 71.96 (standard deviation [SD] = 43.11), and the mean total percent dyads was 59.84% (SD = 22.59). For trial 1 (the 3.0-second presentation rate), the mean total percent dyads was 73.6%; for trial 2 (the 2.4-second rate), it was 61.4%; for trial 3 (the 2.0-second rate), 49.7%; and for trial 4 (the 1.6-second rate), 40.3%. Mean total chunking was 31.33 (SD = 13.32), with mean total percent chunking of 34.95% (SD = 21.85). The mean total “other” response was 4.89 (SD = 3.53), with a mean total percentage of 4.86% (SD = 3.75). Finally, 61.64% of patients showed cognitive fatigue on the PASAT as a whole.
TABLE 3.
Paced Auditory Serial Addition Test Raw Data
| Total (n = 73) | Ratio of Choline/Creatine in Frontal White Matter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High Left | Low Left | High Right | Low Right | |||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | |
| Trial 1 total | 35.47 | 11.6 | 33.46 | 11.77 | 37.53 | 11.21 | 32.19 | 11.45 | 38.83 | 10.9 |
| Trial 2 total | 29.32 | 10.82 | 26.22 | 10.21 | 32.5 | 10.63 | 26.11 | 10.09 | 32.61 | 10.68 |
| Trial 3 total | 24.42 | 10.04 | 22.05 | 9.95 | 26.86 | 9.67 | 21.68 | 9.64 | 27.25 | 9.77 |
| Trial 4 total | 19.34 | 8.04 | 17.92 | 8.79 | 20.81 | 7.01 | 17.89 | 8.5 | 20.83 | 7.36 |
| Total | 108.55 | 37.22 | 99.65 | 36.45 | 117.69 | 36.25 | 97.86 | 36.23 | 119.53 | 35.43 |
| T-score | 45.32 | 10.42 | 42.86 | 10.06 | 47.83 | 10.32 | 42.57 | 10.3 | 48.14 | 9.91 |
| % impaired | 28.77 | 37.84 | 19.44 | 40.54 | 16.67 | |||||
| Total % fatigued | 61.64 | 64.87 | 58.33 | 70.27 | 52.78 | |||||
| Total dyads | 71.96 | 43.11 | 62.43 | 40.39 | 81.75 | 44.18 | 58.76 | 40.76 | 85.83 | 41.73 |
| Total % dyads | 59.84 | 22.59 | 56.45 | 22.49 | 63.34 | 22.47 | 53.72 | 23.23 | 66.14 | 20.36 |
| Total % dyads fatigued | 80.82 | 81.02 | 80.56 | 70.27 | 91.67 | |||||
| Total chunking | 31.33 | 13.32 | 32.05 | 13.61 | 30.58 | 13.17 | 34 | 14.56 | 28.58 | 11.48 |
| Total % chunking | 34.95 | 21.85 | 37.73 | 20.41 | 32.1 | 23.18 | 40.47 | 21.48 | 29.27 | 21.03 |
| Total “other” | 4.89 | 3.53 | 4.54 | 3.54 | 5.25 | 3.52 | 4.59 | 3.09 | 5.19 | 3.95 |
| Total % “other” | 4.86 | 3.75 | 5.16 | 4.22 | 4.55 | 3.22 | 5.27 | 3.77 | 4.44 | 3.72 |
M = mean. SD = standard deviation.
Left and Right Frontal White Matter Ch/Cr
The mean left frontal white matter Ch/Cr was 0.376 (SD = 0.06) and the median value was 0.374. The mean right frontal white matter Ch/Cr value was 0.413 (SD = 0.57) and the median value was 0.403. Using a median split, we placed 37 patients into “high” (above the median) right and left frontal white matter Ch/Cr groups, and 36 into “low” right and left frontal white matter Ch/Cr groups.
Associations Between PASAT and Left and Right Frontal Ch/Cr
As shown in Table 4, the PASAT total T-score correlated negatively with left frontal Ch/Cr (Spearman correlation coefficient [r] = −0.338, P = 0.004) and right frontal Ch/Cr (r = −0.248, P = 0.034). Left frontal Ch/Cr correlated negatively with total dyads (r = −0.311, P = 0.007) and total percent dyads (r = −0.271, P = 0.02), and correlated positively with total percent chunking (r = 0.289, P = 0.01). Right frontal Ch/Cr correlated negatively with total dyads (r = −0.272, P = 0.02) and total percent dyads (r = −0.251, P = 0.03), and correlated positively with total percent chunking (r = 0.240, P = 0.04). Using group comparisons (P values > 0.55), we found no significant difference between patients with and without total PASAT cognitive fatigue on left or right frontal Ch/Cr.
TABLE 4.
Correlations Between Paced Auditory Serial Addition Test Scores and Left and Right Ch/Cr
| Ch/Cr in Frontal White Matter | ||||
|---|---|---|---|---|
| Left | Right | |||
| r | P | r | P | |
| Total T-score | −0.34 | 0.004 | −0.25 | 0.034 |
| Total dyads | −0.31 | 0.007 | −0.27 | 0.020 |
| Total % dyads | −0.27 | 0.021 | −0.25 | 0.033 |
| Total chunking | 0.10 | 0.404 | 0.13 | 0.274 |
| Total % chunking | 0.29 | 0.013 | 0.24 | 0.041 |
| Total “other” | −0.20 | 0.098 | −0.07 | 0.58 |
Significant correlations are in bold type. P < 0.05.
Ch/Cr = ratio of choline/creatine. r = Spearman correlation coefficient.
As a secondary analysis, we separated the groups into high and low right and left frontal white matter Ch/Cr using the median split mentioned in “Left and Right Frontal White Matter Ch/Cr” above. Patients with high Ch/Cr in both the left and right frontal white matter showed much higher levels of overall impairment on the PASAT (Table 3). Total PASAT scores were impaired in 37.8% of patients in the high left frontal white matter Ch/Cr group and in 19.4% of those in the low left frontal white matter Ch/Cr group (Figure 1). Total PASAT scores were impaired in 40.5% of those in the high right frontal white matter Ch/Cr group and in 16.7% of those in the low right frontal white matter Ch/Cr group (Figure 1). Patients with a high Ch/Cr in both the left and right frontal white matter had a higher percentage of dyads and a lower percentage of chunking than patients with a low Ch/Cr in left and right frontal white matter (Figures 2 and 3).
FIGURE 1.
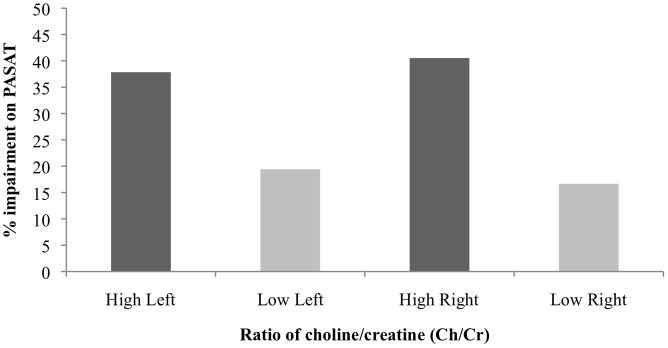
Percent impairment on the Paced Auditory Serial Addition Test (PASAT) in 73 patients with systemic lupus erythematosus and no overt neuropsychiatric manifestations. Scores are shown by group: high vs low ratio of choline/creatine (Ch/Cr) in left vs right frontal white matter.
FIGURE 2.
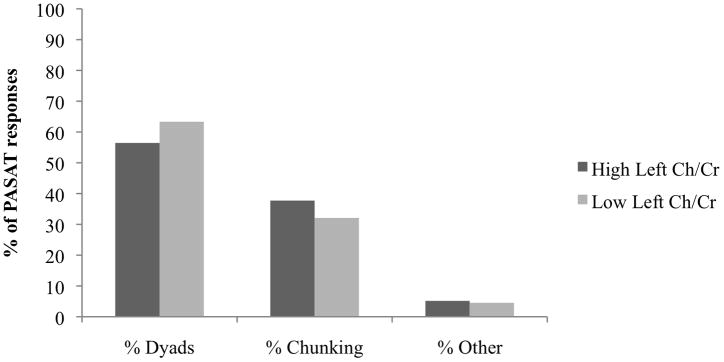
Total percentage of dyads, chunking, and “other” responses on the Paced Auditory Serial Addition Test (PASAT), by high vs low ratio of choline/creatine (Cr/Ch) in the left frontal white matter. Differences between the high and low Ch/Cr groups were not statistically significant (P < 0.05), but had trends similar to those for the right frontal white matter (see Figure 3).
FIGURE 3.
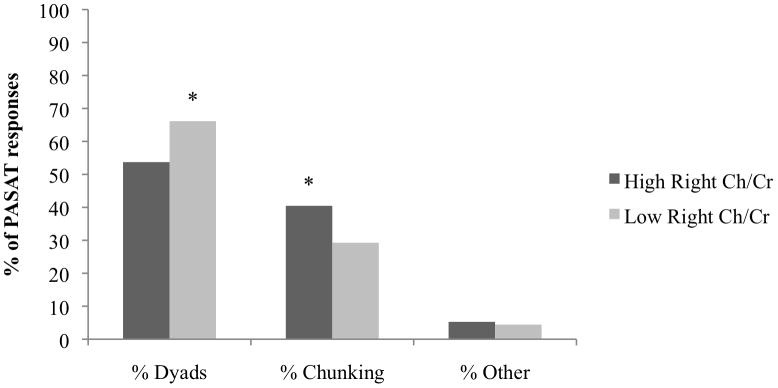
Total percentage of dyads, chunking, and “other” responses on the Paced Auditory Serial Addition Test (PASAT), by high vs low ratio of choline/creatine (Ch/Cr) in the right frontal white matter. The asterisks indicate a significant difference between the high and low Ch/Cr groups (P < 0.05).
Associations between Disease Characteristics and Left and Right Frontal White Matter Ch/Cr
Disease duration correlated with left frontal white matter Ch/Cr (r = 0.029, P = 0.01) but not right frontal white matter Ch/Cr (r = 0.15; P = 0.22). Neither the left nor the right frontal white matter Ch/Cr group showed any correlation with disease activity according to the Systemic Lupus Erythematosus Disease Activity Index total, or with prednisone dose or total Beck Depression Inventory-Second Edition score. Use of antidepressant or thyroid medication was not associated with right or left white matter Ch/Cr.
Backwards Stepwise Regression Models of Left and Right Frontal White Matter Ch/Cr
Based on frontal white matter Ch/Cr associations with not only PASAT variables but also disease duration, we examined backward stepwise regression models of left or right frontal white matter Ch/Cr using a specific PASAT variable (total T-score, total dyads, total percent dyads, and total percent chunking) and disease duration as predictor variables. We evaluated models for white matter Ch/Cr in both frontal lobes, predicating each model on the hypothesis that the relevant PASAT variable and disease duration would predict frontal white matter Ch/Cr.
Table 5 presents detailed results of these analyses. The combination of PASAT total T-score and disease duration accounted for 14% of the variance in left frontal white matter Ch/Cr, and both variables independently contributed to this model. The combination of PASAT total dyads and disease duration accounted for 13% of the variance in left frontal white matter Ch/Cr, and both of these variables independently contributed to this model. The combination of PASAT total percent dyads or PASAT total percent chunking with disease duration accounted for 7% of the variance in left frontal white matter Ch/Cr; however, only disease duration contributed significantly to these models.
TABLE 5.
Regression Models of the Relationships Among Left and Right Frontal Ch/Cr, Disease Duration, and Paced Auditory Serial Addition Test (PASAT) Scores
| Whole Model | Predictor Variables | Standardized Regression Coefficient | ||||
|---|---|---|---|---|---|---|
| Adjusted R2 | F | P | β | P | ||
| Left frontal white matter Ch/Cr | 0.14 | 6.93 | <0.002 | PASAT Total T-score | −0.29 | 0.01 |
| Disease duration | 0.23 | 0.04 | ||||
| 0.13 | 6.69 | <0.003 | PASAT Total dyads | −0.28 | 0.01 | |
| Disease duration | 0.24 | 0.03 | ||||
| 0.07 | 6.46 | <0.013 | PASAT Total % dyad score | n.s. | n.s. | |
| Disease duration | 0.29 | 0.01 | ||||
| 0.07 | 6.46 | <0.013 | PASAT Total % chunking score | n.s. | n.s. | |
| Disease duration | 0.29 | 0.01 | ||||
| Right frontal white matter Ch/Cr | n.s. | n.s. | n.s. | PASAT Total T-score | n.s. | n.s. |
| Disease duration | n.s. | n.s. | ||||
| 0.06 | 5.88 | <0.02 | PASAT Total dyads | −0.28 | 0.02 | |
| Disease duration | n.s. | n.s. | ||||
| n.s. | n.s. | n.s. | PASAT Total % dyad score | n.s. | n.s. | |
| Disease duration | n.s. | n.s. | ||||
| n.s. | n.s. | n.s. | PASAT Total % chunking score | n.s. | n.s. | |
| Disease duration | n.s. | n.s. | ||||
Ch/Cr = ratio of choline/creatine. n.s. = not significant.
For right frontal white matter Ch/Cr, the combination of PASAT total dyads and disease duration yielded the only significant model, accounting for 6% of the variance in this white matter measure. Only PASAT total dyads contributed significantly. There were no other significant predictive models of right frontal white matter Ch/Cr.
DISCUSSION
Consistent with prior studies, nearly one third of our patients with SLE and no overt neuropsychiatric disorders showed impairment on the PASAT, a measure of auditory information processing (Kozora et al, 2004; Shucard et al, 2004). Detailed subanalysis revealed that across the 4 trials for all patients, 59.8% of responses were categorized as dyads (correct response after another correct response), 34.9% as chunking (correct response after a skipped response), and only 4.89% as “other” (correct response after an incorrect response). These data indicate that our patients used chunking more than ”other” to help them through the task, and that chunking accounted for a significant proportion of total responses. The patients’ lower proportion of dyads and “other” responses and their higher proportion of chunking further demonstrate their prevalent use of less demanding working memory strategies to complete the task. These findings are consistent with the observation of Shucard et al (2004) that people with SLE are more likely to use chunking than ”other,” while controls are more likely to use ”other” than chunking.
Few prior studies list PASAT total percent dyad and chunking scores, thus making it difficult to compare their findings directly with ours. However, we can compare our results with 2 other reports, Shucard et al (2004) and Bryant et al (2004).
Our patients scored 61.4% dyads at the 2.4-second presentation rate and 49.7% dyads at the 2.0-second rate. Very similarly, Shucard et al’s (2004) patients with SLE scored 61.4% dyads at the 2.4-second rate and 49.1% dyads at the 2.0-second rate. The slight differences at the 2.0-second rate likely relate to the participants’ demographic and health characteristics: The participants in the Shucard et al study were older than our patients and had greater disease activity and higher depression scores. Overall, however, patients with SLE seem to have a consistent percentage of dyad responses on the PASAT between groups and over time.
We can also compare our results for percentage of dyads from the 2.4, 2.0, and 1.6-second rate trials (61.4%, 49.7%, and 40.3%, respectively) with those of Bryant et al (2004), who performed a controlled study of the PASAT in cognitively impaired and intact patients with MS. Our patients with SLE performed better than Bryant et al’s cognitively impaired patients with MS, whose percentage of dyads was 51.2% for the 2.4-second rate, 38.4% for the 2.0-second rate, and 28.7% for the 1.6-second rate. Our patients performed worse than Bryant’s cognitively intact patients with MS, whose percentage of dyads was 75.2% for the 2.4-second rate, 68.2% for the 2.0-second rate, and 58.9% for the 1.6-second rate. Our patients also performed worse than Bryant’s healthy controls, who scored 84.4% for the 2.4-second rate, 70.5% for the 2.0-second rate, and 65.1% for the 1.6-second rate. This comparison suggests that patients with SLE have less working memory impairment than cognitively impaired patients with MS but more working memory impairment than cognitively intact patients with MS and healthy controls. It also suggests that patients with SLE commonly reduce cognitive demand when performing the PASAT, a finding that implies impaired working memory.
Thus, our key neuropsychological finding is that persons who have SLE and no overt psychiatric disorders try to make the PASAT easier to do, as reflected by a relative decrease in their total number and percentage of dyads, and a relative increase in their total and percentage of chunking. Moreover, patients who simplify the task have an elevated Ch/Cr. In both right and left frontal white matter, Ch/Cr values correlated negatively with lower PASAT total scores, and were associated with fewer dyads and more chunking. Detailed analysis including disease duration revealed that total PASAT score and fewer dyads correlated only with left frontal white matter Ch/Cr. As the neuropathology of SLE is unlikely to display a predilection for 1 cerebral hemisphere, we presume that correlations with right frontal white matter Ch/Cr would also become apparent if we could analyze a larger sample with adequate power.
In earlier analyses of the large population from which our current study sample was drawn, Ch/Cr was higher in normal-looking white matter of the frontal lobes in patients with SLE than in controls, and was associated with a lower score on a composite measure of processing speed, attention, and executive function (Filley et al, 2009; Kozora and Filley, 2011). Our current study, therefore, suggests that microstructural white matter abnormalities in patients with SLE correlate with specific PASAT deficits that can be summarized as “impaired working memory.”
Prior studies evaluating the neuroanatomic structures and physiologic processes associated with the PASAT suggested that multiple regions in both gray and white matter may contribute to performance on the PASAT (Tombaugh, 2006). For example, a study using a blood flow neuroimaging technique showed that the PASAT significantly activated clusters containing voxels (in approximately descending Z-score order) in the cerebellum, superior temporal gyri, anterior cingulate, precuneus/angular gyrus, and medial and inferior aspects of the frontal lobes (Lockwood et al, 2004). Our earlier studies of our large population did not find any difference in structural (total brain volume) or metabolic (N-acetylaspartate/Cr levels) measures of gray matter between patients with SLE and controls; neither was there any difference between the groups in overall number and volume of white matter lesions. We suspect, therefore, that elevated Ch/Cr in SLE may identify early white matter changes that precede metabolic or structural neuronal damage and any white matter abnormalities observable on MRI.
Our current study was the first to use MRS in relation to PASAT performance in SLE. Our regions of interest were the normal-looking white matter in both frontal lobes. It is possible that SLE also affects white matter regions elsewhere in the brain similarly to those that we studied, but neither our study nor others address this question directly.
Studies of other white matter disorders such as MS suggest that multiple white matter tracts and regions may be involved in the development of working memory impairment, as indexed by the PASAT. For example, Van Hecke et al (2010) correlated diffusion tensor imaging abnormalities (reduced fractional anisotropy) in several frontal, parietal, and temporal white matter regions with impaired PASAT performance in 20 patients with MS. Lin et al (2008) reported that PASAT performance correlated with diffusion tensor imaging abnormalities in the corpus callosum of 36 patients with MS. Because white matter lesions on MRI may correlate better with PASAT dyads, chunking, and cognitive fatigue than with PASAT total scores (Snyder and Cappelleri, 2001), continued investigation using subanalysis of PASAT scores and advanced MR metrics is warranted to further our understanding of white matter-behavior relationships.
Our results show that 61.64% of our patients demonstrated objective cognitive fatigue while performing the PASAT. This figure is consistent with another study that found higher levels of cognitive fatigue in persons with MS than in controls, although the authors did not report the actual percentage of cognitive impairment for their groups (Bryant et al, 2004). In our SLE sample, however, cognitive fatigue was not associated with Ch/Cr. Self-reported measures of general fatigue have been related to white matter hyperintensities in SLE and other disorders (Harboe et al, 2008; Schwid et al, 2003), but the relationship of general fatigue to cognitive fatigue is unclear. In our earlier work with SLE, self-reported fatigue was related to cognitive dysfunction only in patients who had overt neuropsychiatric manifestations (Kozora et al, 2006). Although our current study did not find a correlation between cognitive fatigue and Ch/Cr in SLE, cognitive fatigue seems to be a more prominent problem than PASAT inefficiency, and we speculate that cognitive fatigue might be expected to correlate with more florid white matter lesions. In other words, subtle white matter lesions (on MRS) produce subtle cognitive deficits (PASAT inefficiency), while more obvious white matter lesions (on MRI) may produce more obvious problems (measurable cognitive fatigue).
Our current findings may have been impacted by confounds related to MRS and PASAT performance. For example, SLE disease duration was associated with frontal white matter Ch/Cr values, reaching significance in the left hemisphere. Our regression analyses suggest that disease duration is an independent predictor of left Ch/Cr abnormality. This confound did not eliminate the PASAT total T-score as an independent predictor of left frontal white matter Ch/Cr. However, the standardized regression coefficient (β = −0.29) of this association is less than the observed Spearman correlation coefficient (r = −0.338), suggesting that there is shared variance between PASAT total T-score and disease duration, and that both must be considered concurrently to gauge accurately the strength of the association between each of them and left frontal white matter Ch/Cr.
A similar issue applies to the relationship among PASAT total dyads, disease duration, and left frontal white matter Ch/Cr. Moreover, our regression analyses suggest that the observed relationship between left frontal white matter Ch/Cr and either PASAT total percent dyad score or PASAT total percent chunking score is accounted for fully by disease duration alone.
Earlier studies have also shown that SLE disease activity and duration may exert important effects on MRS-measured levels of neurometabolites (Appenzeller et al, 2005; Chinn et al, 1997; Sabet et al, 1998; Sibbitt et al, 1997; Sibbitt and Sibbitt, 1993) and the levels’ relationship with cognitive performance. Our current study echoes this finding, but reveals that SLE disease duration alone does not account for the relationship between impaired PASAT performance and SLE-induced frontal white matter Ch/Cr abnormalities. In fact, specifically with respect to PASAT total T-score and total dyads, disease duration only modestly attenuates the significance of the independent relationship between PASAT performance and frontal white matter Ch/Cr. Nonetheless, accurately understanding the clinical relevance of MRI-based biomarkers of SLE-induced neuropathology requires analyses more sophisticated than the simple correlation often reported in this rapidly developing field. Indeed, methods must be able to account for multiple concurrent and potentially interactive effects of demographics, disease characteristics, and neuropsychological test performance.
The PASAT may also be affected by such factors as increasing age, decreasing IQ, and low mathematical ability (Tombaugh, 2006). We tried to correct for these factors by using a PASAT total score adjusted for age and education, but evaluation should continue of factors impacting PASAT performance by medical patients such as those with SLE.
In conclusion, about 30% of our SLE sample without overt neuropsychiatric disorders had deficits in working memory, and examinee strategies associated with impaired PASAT performance – fewer dyads and more chunking – were related to MRS-identified neurometabolic abnormalities in normal-looking frontal white matter. These findings suggest that working memory may be impaired by functionally relevant but relatively subtle SLE-induced disturbances of frontal myelin, and that SLE-induced dysfunction of frontal white matter may precede the appearance of abnormalities on conventional MRI. Our data also support the intriguing notion that impaired working memory may appear earlier in the course of a white matter disorder than cognitive fatigue.
Continued study is warranted of how white matter abnormalities and immune-mediated myelinopathy relate to cognition in patients with SLE. Investigation should also continue into both microstructural and macrostructural involvement throughout the white matter. We suspect that the mechanisms of white matter abnormalities and immune-mediated myelinopathy in SLE will be most usefully approached by analysis of inflammatory mediators in the cerebrospinal fluid. Other areas meriting further study center on the role of working memory impairment in the proposed syndrome of mild cognitive dysfunction in SLE (Lockwood et al, 2004) and the clinical utility of the PASAT as a sensitive instrument for evaluating patients.
Acknowledgments
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases RO1 AR049152.
Glossary
- Ch/Cr
ratio of choline to creatine
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- MS
multiple sclerosis
- PASAT
Paced Auditory Serial Addition Test
- SD
standard deviation
- SLE
systemic lupus erythematosus
Footnotes
The authors declare no conflicts of interest.
Statement of reader benefit: Systemic lupus erythematosus may impair working memory before white matter damage can be detected; the Paced Auditory Serial Addition Test and analysis of neurometabolites in the cerebrospinal fluid may allow earlier diagnosis.
References
- Appenzeller S, Rondina JM, Li LM, et al. Cerebral and corpus callosum atrophy in systemic lupus erythematosus. Arthritis Rheum. 2005;52:2783–2789. doi: 10.1002/art.21271. [DOI] [PubMed] [Google Scholar]
- Audoin B, Au Duong MV, Ranjeva JP, et al. Magnetic resonance study of the influence of tissue damage and cortical reorganization on PASAT performance at the earliest stage of multiple sclerosis. Hum Brain Mapp. 2005;24:216–228. doi: 10.1002/hbm.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, et al. Comparison of Beck Depression Inventories -IA and -II inpsychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- Bryant D, Chiaravalloti ND, DeLuca J. Objective measurement of cognitive fatigue in multiple sclerosis. Rehabilitation Psychology. 2004;49:114–122. [Google Scholar]
- Chinn RJ, Wilkinson ID, Hall-Craggs MA, et al. Magnetic resonance imaging of the brain and cerebral proton spectroscopy in patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:36–46. doi: 10.1002/art.1780400107. [DOI] [PubMed] [Google Scholar]
- Covey TJ, Shucard JL, Shucard DW, et al. Comparison of neuropsychological impairment and vocational outcomes in systemic lupus erythematosus and multiple sclerosis patients. J Int Neuropsychol Soc. 2012;18:530–540. doi: 10.1017/S1355617712000057. [DOI] [PubMed] [Google Scholar]
- Diehr MC, Heaton RK, Miller W, et al. The Paced Auditory Serial Addition Task (PASAT): norms for age, education, and ethnicity. Assessment. 1998;5:375–387. doi: 10.1177/107319119800500407. [DOI] [PubMed] [Google Scholar]
- Filley CM. Exploring white matter microstructure: new insights from diffusion tensor imaging. Neurology. 2009;73:1718–1719. doi: 10.1212/WNL.0b013e3181c2936b. [DOI] [PubMed] [Google Scholar]
- Filley CM, Kozora E, Brown MS, et al. White matter microstructure and cognition in non-neuropsychiatric systemic lupus erytematosus. Cogn Behav Neurol. 2009;22:38–44. doi: 10.1097/WNN.0b013e318190d174. [DOI] [PubMed] [Google Scholar]
- Fisk JD, Archibald CJ. Limitations of the Paced Auditory Serial Addition Test as a measure of working memory in patients with multiple sclerosis. J Int Neuropsychol Soc. 2001;7:363–372. doi: 10.1017/s1355617701733103. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, et al. Revised comprehensive norms for an expanded Halstead-Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources, Inc; 1991. [Google Scholar]
- Harboe E, Greve OJ, Beyer M, et al. Fatigue is associated with cerebral white matter hyperintensities in patients with systemic lupus erythematosus. J Neurol Neurosurg Psychiatry. 2008;79:199–201. doi: 10.1136/jnnp.2007.120626. [DOI] [PubMed] [Google Scholar]
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- Kozora E. Neuropsychological functioning in systemic lupus erythematosus. In: Morgan JE, Ricker JH, editors. Textbook of Clinical Neuropsychology. New York: Taylor and Francis; 2008. pp. 636–649. [Google Scholar]
- Kozora E, Arciniegas DB, Filley CM, et al. Cognition, MRS neurometabolites, and MRI volumetrics in non-neuropsychiatric systemic lupus erythematosus: preliminary data. Cogn Behav Neurol. 2005;18:159–162. doi: 10.1097/01.wnn.0000181543.05064.4b. [DOI] [PubMed] [Google Scholar]
- Kozora E, Ellison MC, West S. Reliability and validity of the proposed American College of Rheumatology neuropsychological battery for systemic lupus erythematosus. Arthritis Rheum. 2004;51:810–818. doi: 10.1002/art.20692. [DOI] [PubMed] [Google Scholar]
- Kozora E, Ellison MC, West S. Depression, fatigue, and pain in systemic lupus erythematosus (SLE): relationship to the American College of Rheumatology SLE neuropsychological battery. Arthritis Rheum. 2006;55:628–635. doi: 10.1002/art.22101. [DOI] [PubMed] [Google Scholar]
- Kozora E, Filley CM. Cognitive dysfunction and white matter abnormalities in systemic lupus erythematosus. J Int Neuropsychol Soc. 2011;17: 385–392. doi: 10.1017/S1355617711000191. [DOI] [PubMed] [Google Scholar]
- Kozora E, Filley CM, Zhang L, et al. Immune function and brain abnormalities in patients with systemic lupus erythematosus without overt neuropsychiatric manifestations. Lupus. 2012;21:402–411. doi: 10.1177/0961203311429116. [DOI] [PubMed] [Google Scholar]
- Kozora E, Hanly JG, Lapteva L, et al. Cognitive dysfunction in systemic lupus erythematosus: past, present, and future. Arthritis Rheum. 2008;58:3286–3298. doi: 10.1002/art.23991. [DOI] [PubMed] [Google Scholar]
- Kozora E, West SG, Maier SF, et al. Antibodies against N-methyl-D-aspartate receptors in patients with systemic lupus erythematosus without major neuropsychiatric syndromes. J Neurol Sci. 2010;295:87–91. doi: 10.1016/j.jns.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Benton AL, Grossman RG. Neurobehavioral consequences of closed head injury. New York: Oxford University Press; 1982. [Google Scholar]
- Levin HS, Mattis S, Ruff RM, et al. Neurobehavioral outcome following minor head injury: a three-center study. J Neurosurg. 1987;66:234–243. doi: 10.3171/jns.1987.66.2.0234. [DOI] [PubMed] [Google Scholar]
- Lin X, Tench CR, Morgan PS, et al. Use of combined conventional and quantitative MRI to quantify pathology related to cognitive impairment in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79:437–441. doi: 10.1136/jnnp.2006.112177. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Linn RT, Szymanski H, et al. Mapping the neural systems that mediate the Paced Auditory Serial Addition Task (PASAT) J Int Neuropsychol Soc. 2004;10:26–34. doi: 10.1017/S1355617704101045. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Sabet A, Sibbitt WL, Stidley CA, et al. Neurometabolite markers of cerebral injury in the antiphospholipid antibody syndrome of systemic lupus erythematosus. Stroke. 1998;29:2254–2260. doi: 10.1161/01.str.29.11.2254. [DOI] [PubMed] [Google Scholar]
- Schwid SR, Petrie MD, Murray R, et al. A randomized controlled study of the acute and chronic effects of cooling therapy for MS. Neurology. 2003;60:1955–1960. doi: 10.1212/01.wnl.0000070183.30517.2f. [DOI] [PubMed] [Google Scholar]
- Shucard JL, Parrish J, Shucard DW, et al. Working memory and processing speed deficits in systemic lupus erythematosus as measured by the Paced Auditory Serial Addition Test. J Int Neuropsychol Soc. 2004;10:35–45. doi: 10.1017/S1355617704101057. [DOI] [PubMed] [Google Scholar]
- Sibbitt WL, Jr, Haseler LJ, Griffey RR, et al. Neurometabolism of active neuropsychiatric lupus determined with proton MR spectroscopy. AJNR Am J Neuroradiol. 1997;18:1271–1277. [PMC free article] [PubMed] [Google Scholar]
- Sibbitt WL, Jr, Sibbitt RR. Magnetic resonance spectroscopy and positron emission tomography scanning in neuropsychiatric systemic lupus erythematosus. Rheum Dis Clin North Am. 1993;19:851–868. [PubMed] [Google Scholar]
- Snyder PJ, Aniskiewicz AS, Snyder AM. Quantitative MRI correlates and diagnostic utility of multi-modal measures of executive control in multiple sclerosis. J Clin Exp Neuropsychology. 1993;15:18. [Google Scholar]
- Snyder PJ, Cappelleri JC. Information processing speed deficits may be better correlated with the extent of white matter sclerotic lesions in multiple sclerosis than previously suspected. Brain Cogn. 2001;46:279–284. doi: 10.1016/s0278-2626(01)80084-1. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1991. [Google Scholar]
- The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 42:599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT) Arch Clin Neuropsychol. 2006;21:53–76. doi: 10.1016/j.acn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Curtiss G, Belanger HG. Long-term neuropsychological outcomes following mild traumatic brain injury. J Int Neuropsychol Soc. 2005;11:228–236. doi: 10.1017/S1355617705050289. [DOI] [PubMed] [Google Scholar]
- Van Hecke W, Nagels G, Leemans A, et al. Correlation of cognitive dysfunction and diffusion tensor MRI measures in patients with mild and moderate multiple sclerosis. J Magn Reson Imaging. 2010;31:1492–1498. doi: 10.1002/jmri.22198. [DOI] [PubMed] [Google Scholar]


