Abstract
Purpose
Challenges exist regarding antiretroviral quantitation in the female genital tract. Endocervical wicking using sterile tear flow test strips is an alternative to conventional methods due to the consistent sample volume obtained.
Methods
A novel method for measuring antiretrovirals in cervicovaginal secretions using Sno-strip® wicking was developed and tested by spiking Sno-strips® with known concentrations of tenofovir, nevirapine, atazanavir, lopinavir, and ritonavir in blank cervicovaginal lavage fluid. Drug concentrations were determined by high-performance liquid chromatography with ultraviolet or mass spectrometry detection.
Results
Mean extraction recoveries were 91% for tenofovir, 89% for nevirapine, 63% for atazanavir, 60% for lopinavir, and 61% for ritonavir relative to controls. Freezing spiked samples for 24 hours at –80°C had no effect on recovery.
Conclusions
Results suggest that the antiretrovirals tested can be efficiently extracted from Sno-strips®, although a greater percentage of tenofovir and nevirapine was recovered. Storage of Sno-strip® samples up to 24 hours before analysis showed no difference in the percentage of drug recovered compared with immediate analysis. Quantitating antiretroviral penetration into the female genital tract may assist in determining optimal therapeutic antiretroviral regimens to both decrease the risk of HIV transmission and prevent development of HIV drug resistance.
Keywords: antiretroviral, drug monitoring, women
Human immunodeficiency virus (HIV) can be detected in anatomical reservoirs outside of the blood and lymph nodes despite the use of highly active antiretroviral therapy. HIV-1 RNA has been detected and quantitated in semen and cervicovaginal secretions, commonly at lower concentrations compared with plasma.1–7 Virus in these compartments can be genetically distinct from that in the bloodstream due to local immune responses and varying penetration of drugs into the genital tract. Antiretroviral therapy has been shown to reduce the amount of HIV-1 RNA present in vaginal secretions.8–10 Although numerous data describe antiretroviral penetration into semen, data illustrating the degree of drug penetration into the female genital tract (FGT) are limited.
Antiretrovirals enter extravascular compartments from the bloodstream by passive or facilitated diffusion and/or active transport. Passive diffusion is regulated by the drug's physical and chemical characteristics, such as molecular weight, degree of ionization, lipid solubility, and protein binding.11 Physical properties of the specific blood-compartment barrier also help regulate the passive diffusion process. Antiretrovirals that utilize passive diffusion, such as protease inhibitors (PI), are likely to achieve low concentrations in biological fluids, because their high affinity for proteins does not allow passage across biological membranes. Drugs using facilitated diffusion, such as nucleoside reverse transcriptase inhibitors (NRTI), or active transport may penetrate biological fluids at concentrations greater than those found in plasma.
Once the drug enters extravascular compartments, such as biological fluids, influx is greater than efflux and the drug begins to accumulate. Drug sequestration aids the accumulation process by preventing drug efflux through diffusion and/or transport. This process occurs through drug ionization and/or protein binding. The degree of ionization depends upon the pKa of a drug and the pH of its environment. Because ionization increases hydrophilicity, only non-ionized drugs diffuse across membranes to reach biological fluids. When the pH in one compartment differs from another, the degree of drug ionization between the two compartments differs. This process is referred to as ion trapping. At equilibrium, non-ionized concentrations will be equal between the two compartments whereas total concentrations (ionized and un-ionized) will be unequal. This concept is important in regard to the FGT, because the pH of blood approximates 7.4 and the pH of the vagina approximates 4.0, which is more acidic. In this environment, weak bases, such as PIs, may accumulate. A drug bound to proteins in blood would have difficulties crossing into biological fluids, and a drug with affinity for proteins found in the FGT would have difficulties exiting the fluid.
The first investigation of antiretroviral penetration into the FGT collected cervicovaginal samples.12 However, the extent of sample dilution could not be determined and the lower limit of quantitation for many antiretrovirals approximated the mean concentrations reported for those drugs in cervicovaginal fluid; thus it is likely that the concentrations for several patients fell below the lower limit of quantitation. The most recent examination of antiretroviral penetration into the FGT collected cervicovaginal fluid from the posterior fornix of the vagina via direct aspiration with a volumetric vaginal aspirator (Rovumeter; Recipe Pharmaceuticals, Munich, Germany).13 Antiretroviral concentrations were determined based upon standard curves of these drugs in plasma instead of the more appropriate matrix, cervicovaginal fluid. To date, no data evaluating the percent recovery or stability of antiretrovirals in samples collected using direct aspirate have been reported. Thus, the degree of antiretroviral penetration into the FGT needs further exploration with a method that more accurately quantitates drug concentrations in this sanctuary site. The purpose of this study was to determine whether select antiretrovirals could be quantitated in vitro using spiked Sno-strips® and to assess short-term drug stability using frozen banked Sno-strips®.
METHODS
Antiretroviral Analysis
High-performance liquid chromatography (HPLC) analysis was performed using a Waters (Milford, Massachusetts, USA) Alliance chromatography system, including a Model 2695 Separations Module coupled to either a Model 2487 dual wavelength ultraviolet (UV) detector or ZQ2000 single quadrupole mass spectrometer (MS) for detection. For the nevirapine (NVP) assay, reverse phase chromatography was performed using a Microsorb MV C8 analytical column (250 × 4.6 mm, 5-μm particle size). A Supelguard™ Discovery® C8 (4 × 20 mm; 5-μm particle size) in-line guard column was used to extend the life of the analytical column. The UV detector was set to monitor the 284-nm wavelength. A binary isocratic mobile phase was used containing 0.3% triethylamine (TEA) in water and MeOH mixed at a ratio of 60:40 and run at a flow rate of 1 mL/min. This assay is a modification of a previously published method14 and was validated over the range 50–10,000 μg/L with inter- and intraday variability (coefficients of variation [CV]) of 9.8% and 7.8%, respectively. For the multi-PI assay, reverse phase liquid chromatography was performed using an YMC C8 analytical column (4.6 × 100 mm; 3-μm particle size). The column temperature was maintained at 30°C with a column heater to decrease variability between runs. A Supelguard™ Discovery® C8 (4 × 20 mm; 5-μm particle size) in-line guard column was also used. The UV detector was set to monitor the 212-nm wavelength. A binary isocratic mobile phase containing 55% 20 mM ammonium formate (pH 4.88) and 45% acetonitrile was used at a flow rate of 1.0 mL/min. This assay was validated over the range of 25–20,000 ng/mL with intra-assay CVs ranging from 2.3% to 6.6%, 2.2% to 7.5%, and 2.0% to 7.1% and an interassay variation of 4.7%, 6.1%, and 5.6% for lopinavir (LPV), ritonavir (RTV), and atazanavir (ATV), respectively. For the TDF assay, reverse phase chromatography was performed using a Waters Atlantis dC18 analytical column (2.1 × 100 mm; 3-μm particle size). A Supelguard™ Discovery® C8 (4 × 20 mm; 5-μm particle size) in-line guard column was used. Detection and quantitation of TDF and internal standard (IS) were achieved by selected ion monitoring, and the protonated molecular ion [M+H]+ was monitored at m/z 288.2 and 212.2 for TDF and IS, respectively. A binary isocratic mobile phase was used containing a 52.2 mM hydroxylamine/4.9 mM acetic acid buffer and methanol mixed at a ratio of 93:7 and run at a flow rate of 0.2 mL/min. This assay was validated over the range 1–750 μg/L with inter- and intraday variability (CV) of 5.2% and 1.3% to 8.8%, respectively. The Waters Millennium® software stored all data and performed the chromatographic integrations. Peak height ratios (PHR) or peak area ratios (PAR) were calculated as detector response of analyte versus detector response of the internal standard from baseline. The mean, standard deviation (SD), and %CVs of the PHR and PAR were calculated for each drug under control and test conditions and at all concentrations used. These values were used to determine antiretroviral extraction efficacy and short-term antiretroviral stability from Sno-strips®.
Extraction of Antiretrovirals from Sno-strips®
To investigate the quantitation of antiretroviral agents using Sno-strips® (Chauvin Pharmaceuticals Ltd., Essex, England), we developed a simple extraction for this matrix that would produce acceptable recovery of drugs across different antiretroviral classes in a single method. We examined representative agents from three different classes including: the NRTI TDF, the non-nucleoside reverse transcriptase inhibitor (NNRTI) NVP, and the PIs LPV, RTV, and ATV. Drug concentrations were subsequently measured using current, validated HPLC with UV or LC/MS methodology. Each drug class was extracted and run separately on assays specific for the drug(s) of interest. Chromatographic examples are shown in Figure 1. Blank cervicovaginal fluid was obtained and spiked with drug standards at a low, mid, and high concentration. The concentrations were selected based on the calibration range of the specific assay used. The concentrations used for TDF were 50 ng/mL (LOW), 500 ng/mL (MID), and 5000 ng/mL (HIGH). For NVP and the PIs, the concentrations used were 1000 ng/mL (LOW), 5000 ng/mL (MID), and 10,000 ng/mL (HIGH). Drug-spiked cervicovaginal fluid was applied to the Sno-strips® by adding 7 μL to the end of the strip with a pipette. Strips were allowed to air dry, were cut at the shoulder, and then were placed in 1 mL of MeOH for extraction. IS was added (25 μL at 5 μg/mL for TDF; 50 μL at 10 μg/mL for NVP; and 10 μL at 20 μg/mL for the PI) to each sample to minimize intersample variability related to sample work-up and injection. Samples were then gently shaken for 2 hours at room temperature. This shaking time was chosen after time course experiments revealed increasing recovery when performed for up to 2 hours; longer time courses did not increase drug recovery. The Sno-strips® were then removed from the extraction solution and the samples were evaporated to dryness under a gentle stream of nitrogen. The resulting dried specimen residue was reconstituted with mobile phase, transferred to injector vials, and injected onto the HPLC system for analysis.
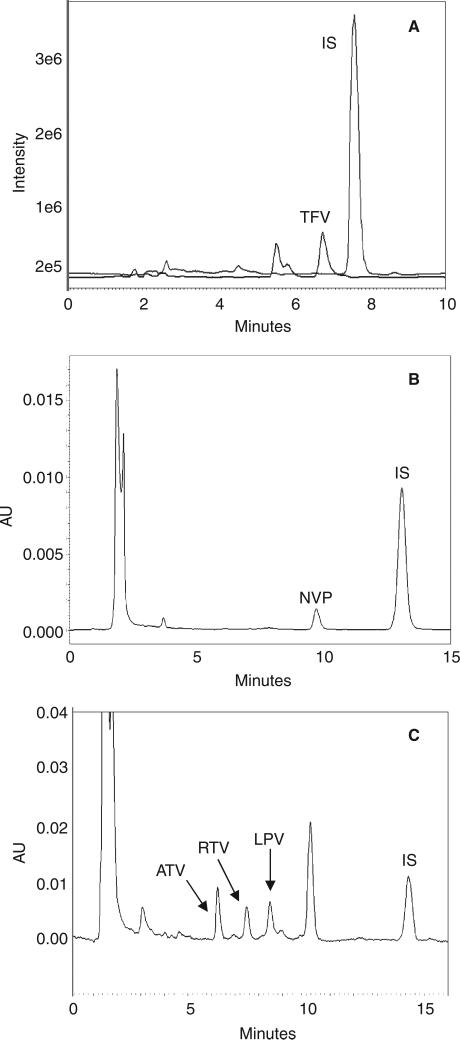
Figure 1.
Representative chromatograms of (A) tenofovir, (B) nevirapine, and (C) the three protease inhibitors atazanavir, ritonavir, and lopinavir freshly spiked and extracted from Sno-strips®.
Antiretroviral Recovery and Short-Term Stability
To determine the efficiency of this extraction method on the antiretrovirals of interest, extracted samples were compared to unextracted controls. For the control samples, Sno-strips® were spiked with blank cervicovaginal fluid and extracted as described earlier, but were reconstituted with mobile phase containing the drug(s) of interest at either the LOW, MID, or HIGH antiretroviral concentrations. Multiple aliquots (n = 3) at each of the three concentrations were assayed. This extraction method allowed recovery and quantitation of all tested drugs from the Sno-strips®. To determine the short-term stability of the selected antiretrovirals on this matrix, Sno-strips® were spiked as previously described and then stored at –80°C for 24 hours before processing. All test samples were analyzed simultaneously with freshly prepared samples of equivalent concentrations and the mean of triplicate PHRs or PARs were compared between the control, extracted, and stability test samples.
RESULTS
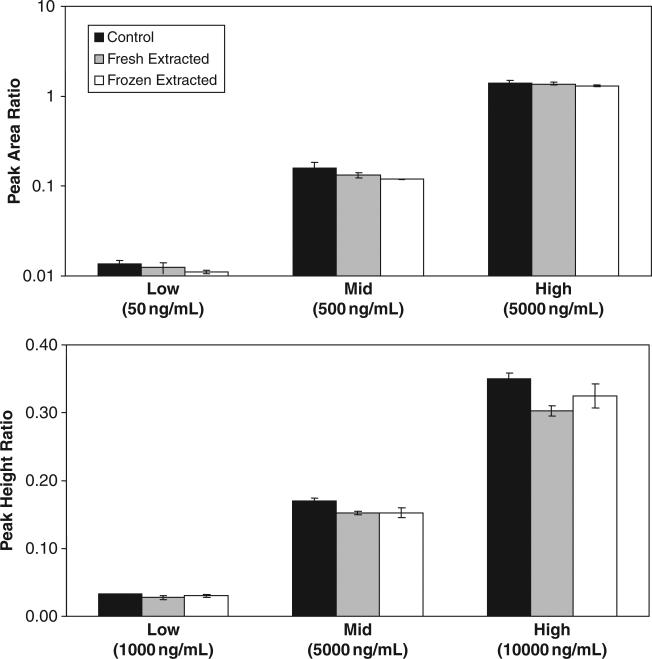
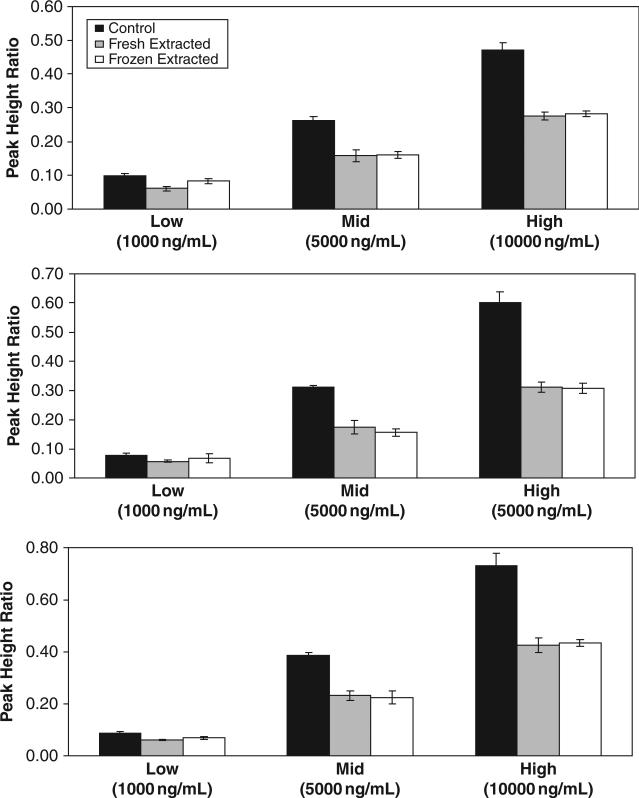
The extraction efficiency of various antiretrovirals from Sno-strips® are shown in Figure 2 (TDF and NVP) and Figure 3 (PIs). Mean extraction recoveries for fresh extracted test samples were 91% for TDF, 89% for NVP, 63% for ATV, 60% for LPV, and 61% for RTV. Although extraction efficiency of the PIs was somewhat lower, other extraction solvents tested either did not substantially increase PI recovery or negatively affected recovery of antiretrovirals from other drug classes. Short-term stability of selected antiretrovirals in cervicovaginal fluid is also presented in Figures 2 and 3. Results reveal that specimens banked under stability test conditions are not significantly different from nontest samples (.02 < p < .86), using Student t test (α = 0.01), indicating all tested antiretrovirals are stable in cervicovaginal fluid on Sno-strips® at –80°C for at least 24 hours.
Figure 2.
Tenofovir (top panel) and nevirapine (bottom panel) concentrations in unextracted control samples (black bars), fresh extracted Sno-strip® test samples (gray bars), and previously frozen extracted Sno-strip® test samples (white bars).
Figure 3.
Protease inhibitor recoveries for lopinavir (top panel), ritonavir (middle panel), and atazanavir (bottom panel) in unextracted control samples (black bars), fresh extracted Sno-strip® test samples (gray bars), and previously frozen extracted Sno-strip® test samples (white bars).
DISCUSSION
Methods to quantitate antiretroviral concentrations in the FGT originated from techniques used to sample HIV-1 RNA from this compartment, including cervicovaginal lavage with sterile saline or swabs of the cervix.15,16 Collection of samples by cervicovaginal lavage involves dilution by large fluid volumes that are difficult to estimate. Even when the volume of fluid used to flush the cervix is known, factors such as volume losses at collection and an exponentially lower volume of endocervical secretion make it difficult to calculate an accurate dilution factor for precise determination of endocervical antiretroviral concentrations. Swabs provide an imprecise amount of analyte in the sample, and the sample may be contaminated with blood due to mucosal disruption. Newer sampling methods include direct aspirates of the cervical mucus, cytobrush, and Sno-strip® wicking. Direct aspirates allow precise localization of sample; however, it presents problems similar to those for cervicovaginal fluid with volume standardization and has not been validated against other collection methods. Although the cytobrush collects more cells than wicking or swabbing, its disadvantages are similar to the swab, and of all the techniques it may be the most disruptive to the mucosal surface.
The Sno-strip® and TearFlo® strips (Contacare Opthalmic Pvt. Ltd., Baroda, India) are ophthalmic fluid collection devices adapted for collection of cervical secretions. They allow genital fluid to be collected from a specific anatomical location such as the vaginal fornix or endocervix with little or no disruption of the epithelial surface. One limitation of the ophthalmic test strips is the inability to collect large volumes of genital secretions (~8 μL per Sno-strip® and ~25 μL per TearFlo® strip). However, this technique allows accurate calculation of the dilution factor and has recently been shown to be more accurate than cervicovaginal fluid for quantifying HIV-1 RNA.17 Results from our study suggest that TDF, NVP, LPV, ATV, and RTV can be efficiently extracted from Sno-strips®, although quantitating low levels of TDF and NVP may be easier, because a greater percentage of these drugs were recovered compared with the PIs. Mass spectrometry will likely be needed for most drugs in order to quantitate low concentrations from small sample volumes. Also, collection and storage of Sno-strip® samples up to 24 hours before analysis showed no difference in the percentage of drug recovered compared with immediate analysis.
CONCLUSION
Genital secretions constitute the primary vehicle for HIV transmission, and antiretroviral therapy has been shown to reduce HIV-1 RNA concentrations in this sanctuary site. Over time, increased viral replication and the emergence of drug-resistant variants may occur due to differences in antiretroviral penetration and local antiviral selective pressure. Current knowledge on the degree of antiretroviral penetration into the FGT and the role it plays in the evolution of drug resistance virus needs expansion. Development of a precise pharmacologic method that quantifies antiretroviral concentrations on Sno-strips® or other wicking materials, as presented here, provides an initial tool to examine these relationships. Full validation of this methodology including quantitation using specially developed analytical assay(s) and stability of patient samples stored over longer durations of time will ensue. Ultimately, this methodology has the potential to expand our knowledge on drug penetration into the FGT, subsequently leading to the design of antiretroviral regimens that will sufficiently penetrate the FGT to decrease cervicovaginal HIV-1 RNA concentrations and positively impact the rate of viral transmission.
ACKNOWLEDGMENTS
This work was supported by Dr. Victoria A. Johnson's VA Merit Review entitled “Drug Resistance in Genital Tract Viruses Derived from HIV-Infected Women.” The pharmacology laboratory was supported, in part, by the Adult AIDS Clinical Trials Group Central Grant U01AI38858 of the National Institute of Allergy and Infectious Diseases. Dr. Johnson also acknowledges P30 AI27767 and the Birmingham VA Medical Center and UAB CFAR core clinic and laboratory facilities.
REFERENCES
- 1.Coombs RW, Speck CE, Hughes JP, et al. Association between cultureable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Bujalance S, Ruiz G, Landron de Guevara C, et al. Quantitation of human immunodeficiency virus type 1 RNA loads in cervicovaginal secretions in pregnant women and relationship between viral loads in the genital tract and blood. Eur J Clin Microbiol Infect Dis. 2004;23:111–115. doi: 10.1007/s10096-003-1058-4. [DOI] [PubMed] [Google Scholar]
- 3.Hart CE, Lennox JL, Pratt-Palmore M, et al. Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J Infect Dis. 1999;179:871–882. doi: 10.1086/314656. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs A, Chan LS, Chen Z, et al. HIV-1 RNA in plasma and genital tract secretions in women infected with HIV-1. J Acquir Immune Defic Syndr. 1999;22:124–131. doi: 10.1097/00126334-199910010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Pilcher CD, Shugars DC, Fiscus SA, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS. 2001;15:837–845. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- 6.Shaheen F, Sison AV, McIntosh L, Mukhtar M, Pomerantz RJ. Analysis of HIV-1 in the cervicovaginal secretions and blood of pregnant and nonpregnant women. J Hum Virol. 1999;2:154–166. [PubMed] [Google Scholar]
- 7.Tachet A, Dulioust E, Salmon D, et al. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS. 1999;13:823–831. doi: 10.1097/00002030-199905070-00012. [DOI] [PubMed] [Google Scholar]
- 8.Cu-Uvin S, Caliendo AM, Reinert S, et al. Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS. 2000;14:415–421. doi: 10.1097/00002030-200003100-00015. [DOI] [PubMed] [Google Scholar]
- 9.Debiaggi M, Zara F, Spinillo A, et al. Viral excretion in cervicovaginal secretions of HIV-1-infected women receiving antiretroviral therapy. Eur J Clin Microbiol Infect Dis. 2001;20:91–96. doi: 10.1007/s100960000442. [DOI] [PubMed] [Google Scholar]
- 10.Fiore JR, Suligoi B, Saracino A, et al. Correlates of HIV-1 shedding in cervicovaginal secretions and effects of antiretroviral therapies. AIDS. 2003;17:2169–2176. doi: 10.1097/00002030-200310170-00004. [DOI] [PubMed] [Google Scholar]
- 11.Taylor S, Pereira AS. Antiretroviral drug concentrations in the semen of HIV-1 infected men. Sex Transm Inf. 2001;77:4–11. doi: 10.1136/sti.77.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Si-Mohamed A, Kazatchkine MD, Heard I, et al. Selection of drug-resistant variants in the female genital tract of human immunodefi ciency virus type-1-infected women receiving antiretroviral therapy. J Infect Dis. 2000;182:112–122. doi: 10.1086/315679. [DOI] [PubMed] [Google Scholar]
- 13.Min SS, Corbett AH, Rezk N, et al. Protease inhibitor and nonnucleoside reverse transcriptase inhibitor concentrations in the genital tract of HIV-1-infected women. J Acquir Immune Defic Syndr. 2004;37:1577–1580. doi: 10.1097/00126334-200412150-00008. [DOI] [PubMed] [Google Scholar]
- 14.Tribut O, Arvieux C, Michelet C, Chapplain JM, Allain H, Bentue-Ferrer D. Simultaneous quantitative assay of six HIV protease inhibitors, one metabolite, and two nonnucleoside reverse transcriptase inhibitors in human plasma by isocratic reversed-phase liquid chromatography. Ther Drug Monit. 2002;24:554–562. doi: 10.1097/00007691-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Baron P, Bremer J, Wasserman SS, et al. Detection and quantitation of human immunodefi ciency virus type 1 in the female genital tract. The Division of AIDS Treatment Research Initiative 009 Study Group. J Clin Microbiol. 2000;38:3822–3824. doi: 10.1128/jcm.38.10.3822-3824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajjar AM, Lewis PF, Endeshaw Y, et al. Efficient isolation of human immunodeficiency virus type 1 RNA from cervical swabs. J Clin Microbiol. 1998;36:2349–2352. doi: 10.1128/jcm.36.8.2349-2352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John GC, Sheppard H, Mbori-Ngacha D, et al. Comparison of techniques for HIV-1 RNA detection and quantitation in cervicovaginal secretions. J Acquir Immune Defic Syndr. 2001;26:170–175. doi: 10.1097/00042560-200102010-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]