Abstract
The presence of donor human leukocyte antigen (HLA)-specific antibodies (DSA) increases engraftment failure risk in partially HLA-mismatched, or HLA-haploidentical, allogeneic marrow(alloBMT) transplantation. As preexisting sensitization to HLA antigens is not well characterized among candidates for HLA-haploidentical alloBMT, we retrospectively evaluated both the incidence and relative strength of DSA in this patient population. Based on correlations of solid-phase antibody assays on the Luminex (Luminex, Austin, TX) platform with actual crossmatch tests, DSA were characterized as weak for results that were consistent with negative flow cytometric crossmatch results or as moderate-to-strong for results consistent with positive flow cytometric or cytotoxicity crossmatches. We evaluated 296 alloBMT candidates; 111 (37.5%) were female. DSA were detected in 43 (14.5%) candidates, mostly among female candidates (42.9% female versus 12.5% male). Moderate-to-strong DSA strength was more frequently encountered when directed against haploidentical donors as compared with mismatched unrelated donors. DSAwere most commonly detected in female patients directed against their children. Because the presence of DSA has been considered prohibitive for HLA-mismatched alloBMT, we additionally report a desensitization methodology used to reduce DSA to negative or weak levels, ie, levels well below those detectable in a flow cytometric crossmatch. Nine patients without other available donors underwent desensitization. Eight who reduced their DSA to negative or weak levels proceeded to alloBMT and achieved full donor engraftment. These data support routine DSA evaluation in all patients considered for mismatched alloBMT; however, for patients with no other viable options, desensitization to weak or negative DSA levels may afford the opportunity for successful transplantation.
Keywords: Donor specific antibodies, Haploidentical allogeneic bone marrow transplantation, Desensitization
INTRODUCTION
Allogeneic blood or marrow transplantation (alloBMT) is a potentially curative treatment for many hematologic malignances [1]. Historically, graft-versus-host disease (GVHD) has constrained the applicability and availability of alloBMT. Despite large international unrelated donor registries, nearly half of searches fail to identify a suitable matched donor [2] and only a minority of registry donor searches are successful for some ethnic groups, such as African-Americans [3]. Even when a match is found, National Marrow Donor Program data indicate a median of 4 months is required to complete searches; thus, patients can succumb to disease during the process [2]. These limitations gave impetus for the identification of alternative allograft sources.
Translational studies show high-dose cyclophosphamide early after alloBMT effectively modulates alloreactivity, even in partially human leukocyte antigen (HLA)-mismatched donor-recipient pairs [4-6]. By utilizing high-dose posttransplantation cyclophosphamide, the normally prohibitive complication of severe GVHD associated with partially HLA-mismatched alloBMT is reduced to rates observed in fully HLA-matched transplants [5]; in fact, increasing HLA mismatch does not appear to be associated with increased GVHD [6]. Importantly, the safety and efficacy of haploidentical related donor bone marrow transplantation (BMT) utilizing high-dose post-BMT cyclophosphamide was confirmed by the BMT Clinical Trials Network and other studies [7].
Over 95% of patients, regardless of ethnicity, have readily available, potential related haploidentical donors among their parents, children, and siblings. In 2009, mismatched alloBMT represented 5% of the alloBMT procedures performed in the United States for acute myeloid leukemia [8], but represented 65% of all the alloBMT performed at the Sidney Kimmel Comprehensive Cancer Center (SKCCC) at Johns Hopkins. In 2011, that percentage increased to 76%.
However, the use of partially HLA-mismatched donors for alloBMT raises a barrier previously limited to solid organ transplantation recipients—antibody-meditated (graft) rejection. Patients sensitized through transfusion, pregnancy, or previous transplantations to the mismatched HLA antigens can produce donor HLA-specific antibodies (DSA). In solid organ transplantation, DSA are associated with increased rates of antibody-mediated rejection and are often considered a contraindication to transplantation [9]. Similarly, the presence of DSA before alloBMT, as detected by a positive crossmatch test (serum complement-fixing antidonor antibodies), increases the rate of engraftment failure, presumably due to antibody-mediated rejection [10,11]. Recent studies employing highly sensitive solid-phase immunoassays utilizing solubilized HLA antigens as targets—a more sensitive DSA assay than the crossmatch test—reveal a greater than 10-fold increased risk of engraftment failure in allogeneic BMT with DSA [12-16].
We report here the frequency and relative strength of DSA in patients considered for haploidentical alloBMT at the SKCCC at Johns Hopkins and describe an effective method of reducing DSA in patients being considered for alloBMT.
METHODS
Cohort Selection
After IRB approval, we retrospectively reviewed HLA antibody results for individuals who were screened for haploidentical alloBMT at the SKCCC between January 2006 and March 2011. For the first 214 consecutive haploidentical alloBMT candidates screened, eligibility required the absence of readily available HLA-matched related or unrelated donor. Subsequently, a potential matched unrelated donor was not a contraindication to related haploidentical transplantation screening. Thereafter, our institutional standard for alloBMT donor screening changed to evaluate 5 donors at initial screening. If fewer than 5 siblings were evaluable, partially HLA-matched related donors (parents, children were or half-siblings) were also evaluated. Thus, the subsequent 82 alloBMT candidates had simultaneous sibling, unrelated, and haploidentical donor searches. The presence of DSA was considered as an exclusion criterion for potential donors, except for 9 patients with no other donor options who underwent desensitization.
HLA Typing
Patients and donors were HLA typed at the HLA-A, B, C, DRB1, DRB3-5, DQB1, and DPB1 loci. Typing was performed using a combination of reverse sequence specific oligonucleotide probe hybridization assays (One Lambda, Inc., Canoga Park, CA) on the Luminex 100 instrument (Luminex, Austin, TX) and high resolution using Sanger Sequencing BigDye V1.1 Terminator reagents (Applied Biosystems, Foster City, CA) with local and commercial (Abbott Molecular, Abbott Park, IL) assays on the 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Crossmatch Tests
Patient sera were crossmatched against lymphocytes from potential donors at the time of their initial evaluation and within 1 week before alloBMT. The crossmatch techniques used were standard complement dependent cytotoxicity assays and/or flow cytometric crossmatch tests. The cytotoxicity assays were an antiglobulin enhanced test against donor T lymphocytes and a 1-wash, basic crossmatch against donor B cells. The flow cytometric crossmatch, which is substantially more sensitive than a cytotoxicity test, was used to detect reactivity in patients with lower antibody levels. The flowcytometric assaywas performed by 3-color fluorescence after gating on the lymphocyte population. Details of the crossmatch tests have been previously reported [17]. Interpretation of crossmatch resultswas made in conjunction with analysis of the presence of any donor HLA-specific antibodies measured with solid-phase immunoassays, described below.
Analysis of HLA-Specific Antibodies
HLA-specific antibodies were assessed by solid-phase immunoassays on the Luminex platform. The target antigens included pooled HLA antigens, single HLA class I or class II phenotypes, and single HLA antigens (GenProbe Lifecodes, Inc., San Diego, CA; One Lambda, Inc., Canoga Park, CA). To reduce interference in the HLA single-antigen assays, euglobulins were depleted from test sera by hypotonic dialysis before testing [18].
Estimates of relative antibody strength measured by degrees of reactivity in solid-phase immunoassays were based on prior correlations with crossmatch test results [17,19]. For the retrospective analysis of antibody frequencies, reactions with test beads yielding mean fluorescent intensities (MFI) of ≥1000 were considered positive. Although the sensitivity of detection varies for different HLA antibodies, in general, positive flow cytometric crossmatch tests correlated with the following MFI values from the solid-phase immunoassays: ≥5000 on phenotype panels, and ≥10,000 to 15,000 on single-antigen panels. Positive complement-dependent cytotoxicity crossmatch (CDC) results were associated with ≥10,000 MFI on phenotype panels. For comparisons of DSA, results from assays on phenotype panels were used as the primary criterion, as these assays have been shown to give better correlations with crossmatch tests than do results from the single-antigen panels [17]. Antibodies that would likely yield negative flowcytometric crossmatch tests were categorized as weak when MFI values were from 1000 to 3000 on HLA phenotype panels. Antibodies were considered moderate-to-strong when MFI values were >3000 on HLA phenotype panels, as these values were predicatively consistent with positive crossmatch results. Because the immunoassays have been enhanced for detection of antibodies to HLA-C, -DQ, and-DP antigens, higher cutoff values for these antibody specificities were used [17]. Antibodies to HLA-DP antigens were assessed on single-antigen panels, as these antigens are not included on the phenotype panel assays.
In addition to antibody strength, the breadth of HLA sensitization or the number of antibodies to different HLA antigenswas assessed to determine the extent to which HLA antibodies might limit a candidate’s HLA-mismatched donor options. The breadth of sensitization was measured by a calculated panel reactive antibody (CPRA), which was developed as a measure of sensitization among solid organ transplant candidates and is derived from the frequencies of HLA antigens among potential donors that correspond to the HLA specific antibodies identified for each candidate [20]. In this analysis, CPRA values were based only on antibodies sufficient in strength to be crossmatch positive. Statistical evaluation of differences in the incidence of HLA antibodies among different patient subgroups was by chi-square tests.
Desensitization Methodology
For sensitized patients with moderate-to-strong DSA levels, and for whom no other donors were identifiable, desensitization was initiated. The desensitization treatment was based on the protocol developed for renal transplant candidates at the Johns Hopkins Comprehensive Transplant Center [21-23] and included alternate-day, single-volume plasmapheresis (PP) with anti-CMV hyper-intravenous immune immunoglobulin (IVIg) (100 mg/kg), tacrolimus (1 mg, IV per day) and mycophenolate mofetil (1 g, twice daily) starting 1 to 2 weeks before the beginning of BMT conditioning, depending upon each patient’s starting DSA levels. The number of PP/IVIg treatments planned for each patient was based on experience with desensitization of renal transplant candidates with modifications to accommodate the BMT conditioning [22]. The number of PP/IVIg treatments was estimated based on the starting donor specific antibody levels correlated with flow cytometric or CDC crossmatch tests. As PP could not be given during the pretransplantation conditioning, nor with the post-transplantation cyclophosphamide on days +3 and +4, treatments were given before conditioning with 1 additional treatment on the day before infusion. A total of 4 or 5 treatments were planned for patients with antibodies at flow cytometric crossmatch positive levels: 3 or 4 treatments performed before conditioning with an additional treatment on day −1. A total of 7 treatments were planned for 1 patient who had a CDC-crossmatch positive titer of 8. The number of treatments was increased for 1 patient because of a delay in the availability of an unrelated donor. Additional risk factors, such as child-to-mother transplantations or repeat mismatches, were also considered in planning the number of treatments. For patients exhibiting a rebound of their DSA on day −1, 1 or 2 additional PP/IVIG treatments were performed on days +1 and +2. Therapeutic plasma exchange was performed using the COBE Spectra (TerumoBCT, Lakewood, CO), exchanging 1 plasma volume and replacing at 100% volume with 5% albumin. The tacrolimus and mycophenolate mofetil were discontinued on the day before transplantation (day −1). Patient 4 additionally received 4 infusions of bortezomib 15½ weeks before initiation of desensitization.
Conditioning and Primary GVHD Prophylaxis Regimen
Nonmyeloablative conditioning consisted of cyclophosphamide (14.5 mg/kg/day) on days −6 and −5, fludarabine (30 mg/m/day) on days −5 through −2, total body irradiation (200 cGy) on day −1, infusion of an unmanipulated bone marrow graft (target 4.0 × 108 nucleated cells/kg recipient ideal body weight) on day 0, cyclophosphamide (50 mg/kg) on days +3 and +4, mycophenolate mofetil on days +5 through +35, tacrolimus on days +5 through day +180, and filgrastim (5 mcg/kg/day) on day +5 through neutrophil recovery (>1000/μL) [5,7].
Desensitization Monitoring
Reactions on solid-phase antibody assays of pre- and post-PP serum samples were used to evaluate antibody levels. To control for interassay variation, the reactivity of DSA and third-party, nondonor beads were expressed as ratios to the positive controls in each assay. The percentage of antibody reductionwas then determined fromthesenormalized values. In the absence of established risk levels for DSA, to minimize the risk of engraftment failure, the goal was to reduce DSA levels to weak or negative reactivity, (ie, levels that would be well below those consistent with positive flow cytometric crossmatch tests). The target levels varied for different DSA specificities as the correlation with positive crossmatch tests has been shown to vary with different antigens [17]. In general, desensitizationwas monitored to determine if the DSA were reduced to MFI reactivity <3000 on HLA phenotype panels and ≤5000 on a single-antigen panel. For antibodies to HLA-A, B, C, and DR antigens, based on previous correlations with crossmatch tests, these levels would be well below that detectable in a flow cytometric crossmatch assay. As noted above, a higher cutoff was applied for 1 patient with antibody to an HLA-DPantigen measuredon single-antigenpanel assays.
Engraftment Monitoring
Engraftment was evaluated at days +30, +60, and +180 via micro-satellite PCR detection of peripheral blood chimerism for both total leukocyte and sorted T lymphocytes. Mixed-donor chimerism was defined as ≥5%, but <95%, donor. Full-donor chimerism was defined as ≥95% donor.
RESULTS
The study group consisted of 296 patients: 111 (37.5%) females and 185 (62.5%) males. Patient characteristics are provided in Table 1. A total of 957 donors were evaluated, including 853 related (741 [86.9%] mismatched and 112 [13.1%] matched) and 104 unrelated donors. The overall incidence of any anti-HLA antibodies, whether directed against donor-mismatched antigens or against third-party antigens, was 23% (Table 2a). The incidence of anti-HLA antibodies was higher among females than males, (43.2 versus 10.8%, P < .0001), and substantially higher among parous compared with nulliparous females (52.4% versus 31.3%, P = .026).
Table 1.
Patient Characteristics
| Variable | Result |
|---|---|
| Number evaluable | 296 |
| Patient age, years | |
| Median (range) | 55 (1-74) |
| ≤30 | 20 (6.75%) |
| 31-40 | 31 (10.5%) |
| 41-50 | 56 (18.9%) |
| 51-60 | 90 (30.4%) |
| 61-70 | 79 (26.7%) |
| >70 | 20 (6.75%) |
| Male | 185 (62.5%) |
| Female | 111 (37.5%) |
| Parous | 63 (56.8%) |
| Null parous | 48 (43.2%) |
| Illness | |
| Myeloid leukemias | 97 |
| Lymphoid leukemias | 41 |
| High-grade lymphomas | 80 |
| Low-grade lymphomas | 53 |
| Multiple myeloma | 6 |
| Aplastic anemia | 2 |
| In-born errors of metabolism | 15 |
| Other | 2 |
Table 2.
a The Overall Incidence of Any HLA-Specific Abs and DSA by Gender and Pregnancy History
| Patient Group (N) | Any HLA-Specific Abs | Donor HLA-Specific Abs |
|---|---|---|
| All candidates (296) | 68 (23.0%) | 43 (14.5%) |
| Males (185) | 20 (10.8%) | 9 (4.9%) |
| Females (111) | 48 (43.2%) | 34 (30.6%) |
| Parous females (63) | 33 (52.4%) | 27 (42.9%) |
| Nulliparous females (48) | 15 (31.3%) | 6 (12.5%) |
|
| ||
|
b Characterization of the DSA for the 42 Candidates with DSA
| ||
| DSA to Donors Evaluated (N) | Weak DSA* | Moderate-to-Strong DSA† |
|
| ||
| Haploidentical (117) | 37 (31.6%) | 80 (68.4%) |
| Mismatched unrelated (15) | 8 (53.3%) | 7 (46.7%) |
HLA indicates human leukocyte antibodies; Abs, antibodies; DSA, donorspecific antibodies.
Data are presented as n (%).
DSA indicates donor-specific antibodies.
Number and percentage of donors to whom there was only weak DSA detected (median fluorescence values (MFI) values between 1000 and 3000 on solid-phase immunoassay).
Number and percentage of the donors to whom the DSA level was moderate-to-strong (MFI values 3000 to >15,000 on phenotype assays, except for antibodies to HLA-DP antigens).
DSA
DSA to 1 or more potential donors were found in 43 (14.5%) BMT candidates. DSA were detected against 117 potential haploidentical donors and 15 unrelated donors mismatched for 1 or more HLA alleles. DSA were most commonly detected in female patients (30.6% versus 4.9%, P < .00001), with prior pregnancy increasing the risk of DSA (42.9% versus 12.5%, P = .0005, Table 2a), The DSA, based on the relative strength in antibody assays, had moderate to strong reactivity against 80 (68.4%) of the haploidentical donors and 7 (46.7%) of the unrelated donors (Table 2b).
Of the 32 females with DSA, antibody formationwas most frequently detected against their children. Similarly, DSA of moderate-to-strong strength was more commonly encountered against their children than their siblings or parents (data not shown). Only 9 male candidates exhibited DSA and, not surprisingly, were found equally against children, siblings, and parents. Only 2 males had moderate-to-strong DSA.
Desensitization
Nine patients without other viable donor options, but who remained otherwise excellent BMT candidates, underwent desensitization (Table 3). Desensitization was performed against 7 haploidentical related donors and against 2 mismatched, unrelated donors. One of the unrelated donors was mismatched for 3 HLA alleles; the other was mismatched only for a single HLA-DPB1 allele. Six of the 9 patients were broadly sensitized with antibodies reactive with 92% to 99% of potential donors as estimated by their CPRA values. Three patients were more moderately sensitized with CPRAs of 44%, 47%, and 51% (patients 1, 3, and 9, respectively).
Table 3.
Extent of Sensitization among Nine Desensitized Patients
| Patient No. | Pre-PP CPRA*
|
DSA HLA Specificity | Crossmatch Tests† | Additional Risk Factors | Donors Evaluated
|
||
|---|---|---|---|---|---|---|---|
| Class I | Class II | Related | Unrelated | ||||
| 1 | 44 | 0 | B7 | CDC− | child to mother | 2 children | 4 |
| vFCXM+ | 2 siblings | ||||||
| 2 | 98 | 94 | DP1 | CDC− | 1 sibling | 1 | |
| vFCXM± | |||||||
| 3 | 47 | 0 | A2 | CDC− | child to mother | 2 children | 0 |
| vFCXM+ | |||||||
| 4 | 0 | 96 | DR13, DR52, DQ6 | FCXM+ | multiple DSA | 2 children | 5 |
| 2 siblings | |||||||
| 5 | 63 | 99 | DR16 | FCXM+ | 1 child | 5 | |
| 2 parents | |||||||
| 3 half-siblings | |||||||
| 2 cousins | |||||||
| 6 | 0 | 99 | DQ4 | FCXM+ | 2 children | 6 | |
| 3 siblings | |||||||
| 7 | 55 | 92 | A2 | CDC+ | DSA strength | 2 siblings | 0 |
| Titer 8 | 2 parents | ||||||
| 8 | 98 | 0 | A3, B27 | FCXM+ | child to mother | 3 children | 1 |
| 3 half-siblings | |||||||
| 9 | 0 | 51 | DR4, DR53 | FCXM+ | repeat DR4, DR53 mismatches | 2 siblings | 0 |
| 2 parents | |||||||
| Mean | 45.0 | 59.0 | |||||
| SD | 38.9 | 46.6 | |||||
PP indicates plasmapheresis; CPRA, calculated panel reactive antibody; CDC, complement-dependent cytotoxicity crossmatch; FCXM, flow cytometric crossmatch; vFCXM, virtual FCXM when actual crossmatch was not performed, but antibody levels were consistent with positive results.
The CPRA values were based on the frequencies for HLA antigens to which patients had antibodies at strengths consistent with positive complement dependent cytotoxicity and/or flow cytometric crossmatches. Accessed from: http://optn.transplant.hrsa.gov/resources/professionalResources.asp?index=78, CPRA Calculator.
Crossmatch results correlate with the following median fluorescence values (MFI) from the solid-phase immunoassays: FCXM+ on phenotype panels ≥5000; FCXM+ on single-antigen panels ≥10-15,000 MFI; positive complement-dependent cytotoxicity crossmatch (CDC+) ≥10,000 on a phenotype panel.
All of the patients who underwent desensitization had starting DSA levels consistent with positive crossmatches. In 5 cases, the DSA levels detected in the solid-phase immunoassays were confirmed flow cytometric crossmatch positive in actual tests. The strongest antibody levels were observed in patient 7, whose donor crossmatch was positive in a complement-dependent cytotoxicity test with a 2-fold dilution titer of 8.
Desensitization substantially reduced the DSA levels in all 9 patients after an average of 5.3 treatments with a mean reduction in DSA of 68.1% at the end of treatment (Table 4). Only expected toxicities occurred during the PP. After desensitization, 8 patients’ DSA were well below levels associated with positive flow cytometric crossmatches with an average of 1742 ± 1560 MFI on solid-phase immunoassay phenotype panels (data not shown). Of note, further DSA reductions occurred after alloBMT and the last PP/IVIG, resulting in an average DSA reduction of 94.9% at last followup. Based on MFI values <500 in HLA single-antigen assays, patients 2, 3, 5, 8, and 9 could be considered to have completely cleared their DSA. Interestingly, the reduction of nondonor or third party HLA antibodies was less pronounced. Among 5 patients who had third-party antibodies of comparable strength to their DSA, the average antibody reductionwas only 37% at the end of treatment and 36% at last follow-up.
Table 4.
Alloantibody Reduction after Desensitization
| Patient No. | Number of PP/IVIG Treatments | % Antibody Reduction*
|
|||
|---|---|---|---|---|---|
| DSA
|
Third-Party Antibodies†
|
||||
| EOT‡ | F/u§ | EOT | F/u | ||
| 1 | 7 | 93.9 | |||
| 2∥ | 5 | 59.5 | 93.8 | 46.9 | −16.4 |
| 3 | 3 | 33.1 | 96 | ||
| 4 - DSA 1 | 3 | 86 | 99.1 | 83.4 | 83.8 |
| 4 - DSA 2 | 82 | 98.6 | |||
| 4 - DSA 3 | 81.6 | 98.7 | |||
| 5 | 5 | 52.1 | 96.9 | 11.4 | 29.5 |
| 6 | 4 | 44.3 | 73 | 4.3 | 6.4 |
| 7 | 7 | 64.7 | |||
| 8 | 3 | 74 | 99.9 | 39.1 | 76.8 |
| 9 | 11 | 77.4 | 98.4 | ||
| Mean | 5.3 | 68.1 | 94.9 | 37.0 | 36.0 |
| SD | 2.6 | 19.0 | 8.4 | 31.5 | 43.6 |
PP indicates plasmapheresis; IVIG, intravenous immunoglobulin; DSA, donor-specific antibody; EOT, end of desensitization treatment; F/u, follow-up.
To control for inter-assay variation, test median fluorescence values (MFI) were expressed as a ratio to the positive control value in each assay. The percentage of antibody reduction from the pretreatment level was determined using these normalized values.
Five patients had nondonor, third-party antibodies that were comparable in strength to the donor-specific antibodies. The percent reduction is given for the highest reactive antibodies.
Desensitization treatment for patients 5, 6, and 9 included 1 plasmapheresis/IVIG treatment day +1.
The percent reduction at last follow-up calculated from normalized MFI values. Based on MFI values < 500, patients 2, 3, 5, 8 and 9 could be considered to have cleared their DSA completely.
Patient 2 received additional PP and intravenous IVIG treatments because of a delay in availability of the unrelated donor. The number listed corresponds to the treatments immediately before transplant. No substantial reduction was achieved in DSA for patient 9 until the third PP/IVIG, and additional treatments, including 1 on day +1, were scheduled to achieve sufficient reduction and accommodate the delay in the pretransplantation conditioning.
After a reduction of DSA to negative or weak levels, eight of the 9 patients proceeded to alloBMT. Despite an initial 65% decrease in DSA level for patient 7, no further reduction was achieved after the fourth treatment. The DSA rebounded between the final treatments to a positive flow cytometric crossmatch level andwas deemed too high-risk to proceed to transplantation. Full donor engraftment occurred for all 8 transplanted patients. Two patients developed grade 1, cutaneous GVHD, and 1 patient developed chronic dermal GVHD. Four patients suffered AML recurrence ranging from 3 to 16 months, despite retaining donor hematopoiesis (Table 5). No DSA increase was observed posttransplantation. The course of antibody reduction is illustrated in Figure 1 for a patient with DSA and third-party antibodies; by day +31, this patient’s DSA was <500 MFI, but therewas only a 6.4% reduction in third-party antibodies.
Table 5.
Post-transplantation Clinical Status
| Patient No. | Donor Chimerism* | GVHD | Disease Relapse | Follow-Up Time (Months)† |
|---|---|---|---|---|
| 1 | 100% | no | no | 35 |
| 2 | 94% | no | 3 months | died: 17 months |
| 3 | >95% | no | 5 months | died: 7 months |
| 4 | 100% | chronic skin | no | 21 |
| 5 | 100% | grade 1 skin | 12 months | died: 18 months |
| 6 | 100% | no | 16 months | 18 |
| 8 | 100% | grade 1 skin | no | 7 |
| 9 | 100% | no | no | 3 |
Degree of donor chimerism determined at transplant day +60.
Continued donor hematopoiesis was documented for all patients at last follow-up.
Figure 1.
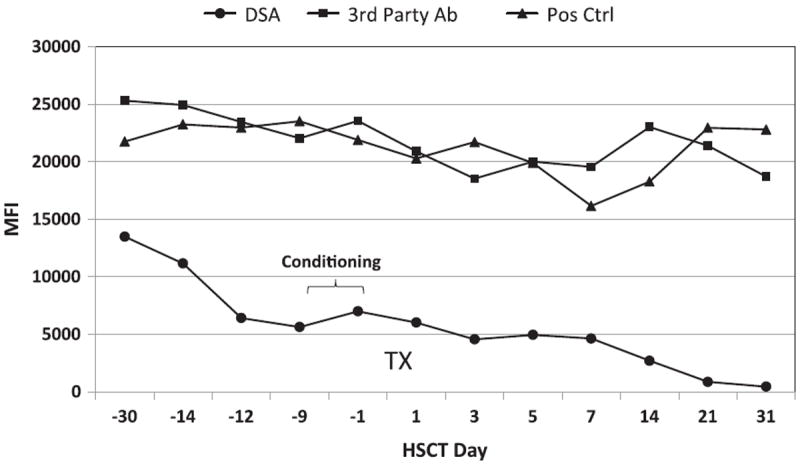
The antibody course during desensitization and posthematopoietic stem cell transplantation is illustrated for patient 5. The donor-specific antibody (DSA) is shown by the line with closed circles. Third-party antibody course is illustrated by the line with squares and the positive control values for each assay are indicated by the triangles. The relative antibody strength is indicated by the mean fluorescence intensity values (MFI) determined with single-antigen immunoassays. Before desensitization, the DSA resulted in a positive flow cytometric crossmatch (FCXM+).
DISCUSSION
The use of haploidentical donors eliminates one of the remaining barriers to alloBMT donor availability. However, DSA, a phenomenon not encountered with fully HLAmatched donors, increases the risk of graft failure [10]. Additionally, DSA only detectable by highly sensitive immunoassays also significantly increases the risk of graft failure [12-16,24]. Although the adverse impact of DSA on alloBMT outcomes is well recognized, their overall incidence is unknown. Ciurea et al. [12] reported a 21% incidence of DSA in 28 haploidentical alloBMT patients with 14% having clinically significant DSA to 1 or more potential mismatched donors. In our series of 294 screened alloBMT candidates, we report an overall DSA incidence of 14% and a 42% incidence in parous females.
The criteria for what constitutes prohibitive DSA levels are unknown. Importantly, solid-phase assays are variable and subject to interference from elevated IgMantibodies, immune complexes, and extrinsic factors, including therapeutic monoclonal antibodies [17,18]. Moreover, there is no established positive reference range, with reported positive cutoff values ranging from 500 to 2000 MFI [12,15]. We based our evaluation of DSA among alloBMT candidates on our institutional experience that used these assays for screening and monitoring solid organ allograft recipients [17,18,21-23]. Because the best correlations were obtained using single HLA class I or class II antigen phenotype panels as targets, these assays were used for assessing relative antibody strength. For some antibodies, particularly for antibodies to HLA-DP antigens that cannot be identified on phenotype panels, relative strength was assessed on HLA single-antigen panels. As the single-antigen assays are more sensitive than the phenotype assays, patients positive on a phenotype assay were substantially more reactive on the single-antigen assays.
From the experience with desensitization for renal transplantation, the most significant factors associated with successful desensitization are initial DSA strength and the antibody specificity [26,27]. Based on crossmatch test correlations, we classified antibodies as weak (MFI values from 1000 to 3000) or as moderate-to-strong (MFI values ranging from 3000 to >15,000). By these criteria, weak antibodies are predictably negative in a flow cytometric crossmatch test. Given no established level of DSA that increases the risk of engraftment failure in alloBMT, for monitoring the patients who underwent desensitization, the goal was set to reduce DSA to weak or negative levels of reactivity (ie, at levels that would be well below those associated with a positive flow cytometric crossmatch). DSA levels consistent with either a positive cytotoxicity or flow cytometric crossmatch were considered as absolute contraindications to alloBMT. Because the flow cytometric crossmatch is recognized to be significantly more sensitive than the complement-dependent cytotoxicity test [25], correlated weak or negative DSA levels in solid-phase assays were considered acceptable for alloBMT. The successful engraftment of the 8 patients whose DSA levels were reduced to such low levels is suggestive that this approach is valid. The failure to adequately reduce the DSA levels in patient 7 is likely attributable to a high starting DSA level, as this patient had the highest DSA titer. Moreover, the patient had experienced a substantial increase in antibody titer a few weeks before initiation of desensitization after an emergent appendectomy. Increasing antibody titers associated with ongoing immune responses have been noted to reduce the likelihood of successful desensitization [19].
Although most centers consider strong DSA levels a contraindication for alloBMT, our data suggest this should not be an absolute barrier. After desensitization, 8 of 8 transplanted patients engrafted. Although we cannot establish that primary nonengraftment would have definitely occurred without desensitization, available data suggest a 75% primaryengraftment failure rate in such patients [12]. In fact, since many centers nowexclude sensitized patients from BMT, a randomized trial is probably unethical. To our knowledge, 2 other desensitization BMT reports have been published: Ciurea et al. reported successful engraftment in 3 of 4 patients after PP and rituximab reduced their DSA to low/negative levels and the 1 patient with persistent DSA experienced graft failure [12]; Yoshihara et al. reported that PP and rituximab produced only a temporary reduction in DSA in 2 patients [28]. The higher rate of successful DSA reduction in our series may reflect our desensitization regimen. It should be noted that it is very important to monitorDSAlevels during desensitization, as illustrated by the course of patient 9 (Table 4) whose treatment plan needed modificationwhen no DSA reduction was noted until the third PP/IVIG.
DSA reduction continued after hematopoietic stem cell transplantation in the 8 patients successfully transplanted. A similar, continued post-transplantation decline in DSA has been observed in renal transplant patients. Interestingly, among desensitized renal transplant patients, antibody reduction was found to be significantly higher for DSA than for third-party antibodies, with 89% of patients losing DSA versus 19% demonstrating reductions in third-party antibodies [27]. Greater reduction of DSA was also noted among 5 of our desensitized HCT patients than for third-party antibodies of comparable strength, with an average 94.9% reduction of DSA at last follow-up compared with 36% of third-party antibodies. Two possibilities may account for this divergence: (1) the post-transplantation cyclophosphamide, which is thought to reduce the incidence of GVHD through elimination of alloreactive T lymphocytes and may have a similar effect against alloreactive B lymphocytes, resulting in the continued DSA decline [29]; or (2) engrafting donor cells may have absorbed circulating antidonor HLA antibodies resulting in the DSA decline.
Although our experience has shown that DSA can be reduced sufficiently by desensitization to permit successful alloBMT, it should be reserved for those patients with no or limited other treatment options. Such candidates would include those with very broad HLA sensitization, which would rule out most, if not all, HLA-mismatched donors or those whose disease status would not permit the time required for an unrelated donor search. Thorough analysis of DSA specificity and strength before initiating desensitization is critical to determine both the extent of treatment that may be required and the likelihood of success. If possible, alternative transplant or treatment options should be considered for patients with very strong DSA levels. Despite these caveats, our results have established that the presence of DSA need not be an absolute barrier to HLA-mismatched alloBMT.
Acknowledgments
Financial disclosure: This work was supported by National Institutes of Health Grant PO1 CA15396.
Footnotes
Authorship Statement: E.J.F., L.L., Y.L.K., and R.J.J. designed the research; K.E.K. performed research; Y.L.K. collected the data; and A.A.Z., R.A.B., and R.J.J. analyzed and interpreted the data. D.E.G is the primary author of all sections of the manuscript; M.S.L. is the senior author of all sections of the manuscript; and all authors participated in writing the manuscript.
Conflict of Interest Statement: There are no conflicts of interest to report.
References
- 1.Blume KG, editor. Thomas’ Hematopoietic Cell Transplantation. 3. Malden, Mass: Blackwell; 2004. [Google Scholar]
- 2.Grewal SS, Barker JN, Davies SM, Wagner JE. Unrelated donor hematopoietic cell transplantation: marrow or umbilical cord blood? Blood. 2003;101:4233–4244. doi: 10.1182/blood-2002-08-2510. [DOI] [PubMed] [Google Scholar]
- 3.Dew A, Collins D, Artz A, et al. Paucity of HLA-identical unrelated donors for African-Americans with hematologic malignancies: the need for new donor options. Biol Blood Marrow Transplant. 2008;14:938–941. doi: 10.1016/j.bbmt.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luznik L, Jalla S, Engstrom LW, et al. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–3464. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 5.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose post-transplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16:482–489. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Marrow Donor Program, Health Resources and Services Administration, Healthcare Systems Bureau. Donor Registry Transplant Data. 2011 Feb 5; http://bloodcell.transplant.hrsa.gov/RESEARCH/Transplant_Data/Registry_Tx_Data/index.html.
- 9.Zachary AA, Leffell MS. Barriers to successful transplantation of the sensitized patient. Expert Rev Clin Immunol. 2010;6:449–460. doi: 10.1586/eci.10.14. [DOI] [PubMed] [Google Scholar]
- 10.Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320:197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 11.Taylor PA, Ehrhardt MJ, Roforth MM, et al. Preformed antibody, not primed T cells, is the initial and major barrier to bone marrow engraftment in allosensitized recipients. Blood. 2007;109:1307–1315. doi: 10.1182/blood-2006-05-022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciurea SO, de Lima M, Cano P, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88:1019–1024. doi: 10.1097/TP.0b013e3181b9d710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciurea SO, Thall PF, Wang X, et al. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011;118:5957–5964. doi: 10.1182/blood-2011-06-362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutler C, Kim HT, Sun L, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118:6691–6697. doi: 10.1182/blood-2011-05-355263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spellman S, Bray R, Rosen-Bronson S, et al. The detection of donordirected, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115:2704–2708. doi: 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takanashi M, Atsuta Y, Fujiwara K, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116:2839–2846. doi: 10.1182/blood-2009-10-249219. [DOI] [PubMed] [Google Scholar]
- 17.Zachary AA, Sholander JT, Houp JA, Leffell MS. Using real data for a virtual crossmatch. Hum Immunol. 2009;70:574–579. doi: 10.1016/j.humimm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Zachary AA, Lucas DP, Detrick B, Leffell MS. Naturally occurring interference in Luminex assays for HLA-specific antibodies: characteristics and resolution. Hum Immunol. 2009;70:496–501. doi: 10.1016/j.humimm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Zachary AA, Leffell MS. Detecting and monitoring human leukocyte antigen-specific antibodies. Hum Immunol. 2008;69:591–604. doi: 10.1016/j.humimm.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Leffell MS. The calculated panel reactive antibody policy: an advancement improving organ allocation. Curr Opin Organ Transplant. 2011;16:404–409. doi: 10.1097/MOT.0b013e3283489910. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLAincompatible kidney recipients and survival. New Engl J Med. 2011;365:318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery RA, Zachary AA. Transplanting patients with a positive donor-specific crossmatch: a single center’s perspective. Pediatr Transplant. 2004;8:535–542. doi: 10.1111/j.1399-3046.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery RA, Zachary AA, Racusen LC, et al. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation. 2000;70:887–895. doi: 10.1097/00007890-200009270-00006. [DOI] [PubMed] [Google Scholar]
- 24.Focosi D, Zucca A, Scatena F. The role of anti-HLA antibodies in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1585–1588. doi: 10.1016/j.bbmt.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Gebel HM, Bray RA. Sensitization and sensitivity: defining the unsensitized patient. Transplantation. 2000;69:1370–1374. doi: 10.1097/00007890-200004150-00027. [DOI] [PubMed] [Google Scholar]
- 26.Zachary AA, Montgomery RA, Leffell MS. Factors associated with and predictive of persistence of donor-specific antibody after treatment with plasmapheresis and intravenous immunoglobulin. Hum Immunol. 2005;66:364–370. doi: 10.1016/j.humimm.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 27.Zachary AA, Montgomery RA, Ratner LE, et al. Specific and durable elimination of antibody to donor HLA antigens in renal-transplant patients. Transplantation. 2003;76:1519–1525. doi: 10.1097/01.TP.0000090868.88895.E0. [DOI] [PubMed] [Google Scholar]
- 28.Yoshihara S, Maruya E, Taniguchi K, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012;47:508–515. doi: 10.1038/bmt.2011.131. [DOI] [PubMed] [Google Scholar]
- 29.Brodsky RA, Fuller AK, Ratner LE, et al. Elimination of alloantibodies by immunoablative high-dose cyclophosphamide. Transplantation. 2001;71:482–484. doi: 10.1097/00007890-200102150-00025. [DOI] [PubMed] [Google Scholar]


