Abstract
This article provides an insight on detailed current advances in molecular understandings of periodontal ligament cells and the influence of orthodontic force on them in the light of recent advances in molecular and genetic sciences. It sequentially unfolds the cellular events beginning from the mechanical force initiated events of cellular responses to bone remodeling. It also highlights the risks and limitations of orthodontic treatment in certain periodontal conditions, the important areas of team work, orthodontic expectations from periodontal treatment and the possibility of much more future combined research to improve the best possible periodontal health and esthetic outcome of the patient.
Keywords: Interdisciplinary approach, molecular biology, orthodontic tooth movement
INTRODUCTION
We live in an era where science and technology knows no boundaries. Our dental profession is no less; it is evolving into its best at present. The amalgamation of basic molecular science and engineering technology has had a huge impact on the world of medicine and dentistry alike. Advances in the studies of human genetics, stem cell biology, medicine, and proteomics have allowed for the application of tissue engineering to clinical problems in dentistry.
With this wide spread change in knowledge and awareness, the demand and responsibility of dentistry is increasingly huge. Today more adult patients seek orthodontic treatment as compared to earlier days. Invariably both the Orthodontist and the Periodontist need to be well equipped with thorough understanding of each other's fields, to deliver the best possible treatment to the patient. Research involving the biology of periodontal tissues, their reactions to mechanical, chemical or pathological stimuli is an exciting field as we are trying unravel several queries to meet demands of this ultramodern era, for example; “Can biology help us achieve better periodontal stable occlusion with good esthetics?,” “Can the chemical mediators of inflammation be better handled for our mutual benefit?” Can the ongoing projects with the aid of technology such as nanosciences help us reach the cellular or genetic material of our interest and affect the rate of orthodontic tooth movement (OTM)?
From a periodontist's point of view, the understanding of the changes occurring in the molecular and genetic levels of periodontal ligament (PDL) from a mechanical stimulus such as orthodontic force, will definitely open new pathways for common areas of research., as at cellular level both the orthodontist and the periodontist work on the PDL with similar host response to inflammation.
In the first part of the article we will comprehensively discuss the five types of cells identified in the bone with current molecular level research as needed by the craniofacial biologist common to both the Periodontist and the Orthodontist.
In later sections, we will discuss, in detail, the unfolding of PDL cellular phenomenon following mechanical loading during OTM. Here the inflammatory sequences can very well be correlated by a periodontist. In the third part the possible future molecular research that can be undertaken jointly to enhance the periodontal health will be discussed.
-
Osteoblasts, which are of mesenchymal origin, are primarily the bone forming cells. Osteoblasts synthesize and secrete the extra cellular organic matrix of bone including type I collagen, osteocalcin, osteopontin, osteonectin, alkaline phosphatase, proteoglycans and growth factors. Several factors are shown to influence the development of osteoblasts from mesenchymal pluripotent progenitors or mesenchymal stem cells (MSCs) in PDL and alveolar bone for e.g., certain growth factors like bone morphogenetic proteins (BMPs), transforming growth factor (TGF β-I and II), insulin-like growth factor (IGF-I and II), platelet derived growth factor (PDGF) and fibroblast growth factor (FGF) etc.[1] These growth factors promote osteoblast precursor proliferation, mineralization of new bone by mature osteoblasts and vasculogenesis. Extensive studies reveal that osteoblast differentiation and proliferation are separate processes controlled by different genes. Although many genes control the complex process of osteogenesis, the transcription factor Cbfa1 (Runx2/OSF2) is the earliest expressed and most specific marker of bone format[2]
Osterix is another gene playing a vital role in bone formation that functions downstream of Rxnx2.[3] In the absence of either Runx2 or osterix, no osteoblast formation takes place. Formation and proliferation of pre-oseoblast cells requires signaling through the Wnt-frizzled-low density lipoprotine 5 (LRP5 receptor-related protein)-β-catenin signaling pathway.[4] The deficiency of LRP5 lead to development of osteoporosis both in mice and humans.[5] The function of mature osteoblasts, including the ability to synthesize the extracellular matrix (ECM) proteins, also requires LRP5 as well as signaling protein ATF4.[6] Osteoblasts lining the bony socket are now believed to be directly responsive to strain such as orthodontic force through the proprioceptive receptor system.[7] One of the proteins in the membrane of osteoblasts is the integrin.[8] Integrins translate mechanical strain into a signal which in turn stimulates a gene to make the cell develop ligands. Ligands allow intracellular communication, which stimulates undermining resorption allowing OTM[9,10]
The field of influencing the development of osteoblasts is the present area of research in many of craniofacial research centers
The second type of cells, which are of interest are the osteocytes. They were histologically thought to be trapped osteoblasts in the matrix and whose function was considered to provide support and sustenance to the bone.[11] Osteocytes are now understood to be very proprioceptive and responsive cells of bone.[12] It has been demonstrated by Skerry et al., that an intermittent force within physiologic limits has an effect in increasing the expressions of glucose-6-phosphate dehydrogenase, 3H-urinidine, c-fos, and IGF-I in the osteocytes within 6 h after intermittent loading at physiological strain magnitude[13]
The third type of cells viz. osteoclasts which differentiate from monocyte-hemopoietic cells are multinucleated giant cells found in bay-like depressions of bone called Howship's lacunae. Active osteoclasts exhibit high content of a specific chemical marker, tartrate resistant acid phosphatase (TRAP), which participates in signaling active bone resorption
Chemical mediators of macrophage family are known to influence osteoclast differentiation. They are cytokines (tumor necrosis factor [TNF], interleukin-1 alpha, 6-alpha [I]), certain growth factors macrophage colony stimulating factor (M-CSF), granulocyte, macrophage colony stimulating factor (GM-CSF) and prostaglandin (PGE2). Osteoclast differentiation is also mediated by the interaction of two molecules produced by osteoblasts, namely osteoprotegerin (OPG) and RANK ligand (RANKL) Receptor activator of nuclear factor kappa B ligand[14,15]
Apart from the above three cells of bone; we also have osteoprogenitor cells and bone-lining cells. Osteoprogenitor cells are mesenchymal, fibroblast like cells, regarded to form a stem cell population to generate osteoblasts. They are situated in the vicinity of blood vessels of PDL.[16] Bone lining cells are the undifferentiated flattened cells lining the bone surface. They may represent active osteoblasts, but further confirmation is needed.[17]
In essence bone remodeling is orchestrated by cells of osteoblast linage and involves a complex network of cell-to-cell and cell-to-matrix interactions involving systemic hormones, locally produced cytokines, growth factors, many of which are sequestrated with in the bone matrix, as well as the mechanical environment of cells. An excess of resorption over formation leads to bone loss which may be associated with several factors including periodontal pathogens.
Cascade of events that follow after application of orthodontic force: The role of inflammation in orthodontic tissue remodeling
As we apply orthodontic force on the tooth, following events at the microscopic level occur, based on the current understanding [Figure 1].
Figure 1.
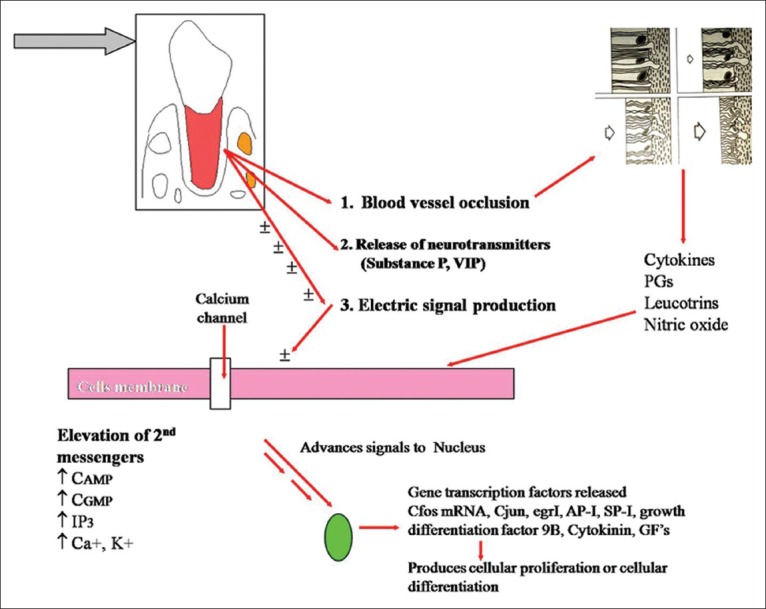
Cascade of histological events during orthodontic tooth movement
Primarily, alteration in the blood flow which results in reduced oxygen level at compressed area, and there might be an increased oxygen level at tension side.[18,19]
Secondly, generation of Piezo electric signal, which is now stated more appropriately as bioelectric potential in the form of small voltage of current, is released due to bending of bone and deformation of crystal structure.[20]
Thirdly, neurotransmitters (examples Substance P, vasointestinal polypeptide (VIP), calcitonin gene related peptide are possibly released on account of physical distortion imposed by peripheral forces on paradental tissues such as nerve fibers and terminals.[21]
Thus, the primary stimulus such as that of the orthodontic force may elicit its response to cells of PDL and bone in the form of release of -Bioelectric signals produced on account of bone bending, -inflammatory-chemical mediators such as PGE2s, cytokines nitric oxide (NO) etc., -release of neurotransmitters.
It has been proved that cells in PDL such as fibroblasts and bone cells such as osteoblasts possess receptors for these substances, and all these are highly interacting and interconnected, presenting number of possibilities of transducing mechanical force acting on cells and their adjacent matrices.[22] These interactions lead to transient increase in the intracellular levels of second messengers such as cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), inositol phospatase 3 (IP3) and calcium.[23,24,25,26] These second messengers advance signals to the nucleus through series of kinases. In the nucleus of each cell, different second messengers account for the differential patterning, protein synthesis and gene expression. Such recently identified immediate early gene expression proteins transcription factors include C-fos, C-jun AP-1 mRNA, Egr-1, SP-1 growth differentiation factor 9B and extracellular matrix gamma carboxyglutamic acid (GLA) protein. The transcription factors seem to increase when cells are exposed to mechanical stimulation, cytokines and growth factors.[27,28,29,30,31] These transcription factors can produce either cellular proliferation or cellular differentiation leading to osteoblastic bone formation or interactions to lead osteoclastic bone resorption.
In rat experiments, it was shown that within 3 h of applying orthodontic force there was an increase of 1.7 fold in C-fos mRNA expression, when compared to the controlled side where no orthodontic tooth movement was applied. At 24 h after initial orthodontic force application, significant induction of 1.9 fold was detected. Final 1.5 fold induction was seen after 7 days of force application. These results show a peak of C-fos induction in cyclic fashion, i.e., at 3 h and 24 h and at 7 days after appliance activation. The authors speculate that C-fos may play a role in both early and in the later phases of orthodontic tooth movement cycle.[32]
The sequence of events after the application of mechanical forces with the help of orthodontic appliances can thus be outlined as:[33]
Movement of PDL fluids from areas of compression into areas of tension
A gradual development of strain in cells and ECM in the paradental tissues involved
Release of phospholipase A2 and cleavage of phospholipids leading to release of PGE2 and leukotrienes
ECM remodelling and signal transduction through integrin trans-membrane channels
Cytoplasmic alterations and release of 2nd messengers of tooth movement - cAMP and cGMP, ionositol phosphates, calcium and tyrosine kinases
Release of kinases such as protein kinase A, kinase C and Mitigen activated protein MAP kinases
Direct transduction of mechanical forces to the nucleus of strained cells through the cytoskeleton, leading to activation of specific genes
Release of neuropeptides (nociceptive and vasoactive) from paradental afferent nerve endings
Interaction of vasoactive neuropeptides with endothelial cells in strained paradental tissue
Adhesion of circulated leukocytes to activated endothelial cells
Migration by diapedesis of leukocytes into the extravascular space
Synthesis and release of signalling molecules by leukocytes that have migrated into the strained paradental tissues
Interaction of various types of paradental cells with the signal molecules released by the migratory leukocytes
Activation of the cells to participate in the modelling and remodelling of the paradental tissues.
The above stated cascade of events, in fact, may be a brief summary of current understanding of whole lot of complex activities and interactions occurring in the PDL and alveolar bone after the application of primary stimulus such as mechanical force or action of hormones. In the next part, we will analyze certain important modes of actions of chemical mediators and their complex, internal interactions.
ROLE OF PROSTAGLANDINS IN MEDIATING OTM
Prostaglandins are synthesized from 20-carbon essential fatty acids by a microsomal enzyme complex (PG Prostangladin synthetase) found in all Mammalian tissues.[34] In humans, the most abundant precursor is arachidonic acid, which is present in membrane phospholipids of cells. Arachidonic acid can be released either by phospholipases activated by direct cellular damage or by any non-destructive perturbation of the membrane, be it physical, chemical, hormonal or neurohormonal. Prostaglandins can also be termed as local hormones functioning to co-ordinate effects of those other hormones which induce PGE2 synthesis. They function through G-protein linked receptors to elicit their cellular effects.[35]
Classically, Prostaglandins as one of the chief mediators of inflammation cause an increase in intracellular cAMP and calcium accumulation by monocytic cells, which then modulates and activates osteoclastic activity. (It is to be noted that elevation in cAMP is not only affected by PGE2s alone, but also influenced by substance P, VIP, calcitonin gene related peptide and many others).
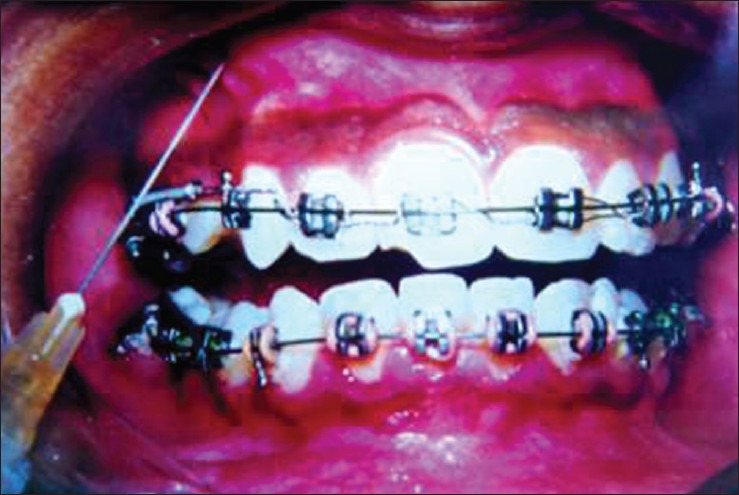
Klein and Riasz[36] reported for the first time the involvement of Prostaglandins in OTM. After that many in vivo and in vitro animal experiments have been conducted mainly by Davidovitch et al.; Davidovitch and Shanfeld; Davidovitch et al.,[37,38,39] Yamasaki et al. (first reported human study, 1982-83),[40] Lee et al.,[41] Liker,[42] Selinkale et al.[43] and many others. All these experiments indicate a very vital role of PGE2s in OTM. The human reports of Yamasaki et al.[40] and Anand et al.[44] demonstrated increased rate of orthodontic tooth movement with exogenous injections of PGE2 [Figures 2 and 3]. Studies indicate that Prostaglandins increase the number of osteoclasts as well as stimulate osteoblastic cell differentiation and new bone formation.
Figure 2.
Production and metabolism of prostaglandins
Figure 3.
Prostaglandin E1 with lignocaine as vehicle and injection method of administration of prostaglandin E1
Several cytokines and hormones induces PGE2 secretion in bone which in turn effects cytokine activity, stimulating osteoclast activation,[45] and loss of osteoblastic junctional complexes.[46] Prostaglandins stimulate osteoprogenitor cells to proliferate and differentiate so that osteogenesis is increased.[36]
Of the many investigations emerged the hypothesis that osteoblasts regulate the resorptive activities of osteoclasts.[47]
Osteoblasts carry receptors to all hormones involved in the maintenance of calcium homeostasis, such as (PTH) Parathyroid hormone, calcitonin and vitamin D3.[48] Osteoblasts also respond to locally produced agents such as growth factors, with elevations in cAMP contents and PG synthesis. Thus Prostaglandins could serve as a stimulatory link or a coupling factor between osteoblasts and osteoclasts.[49,50]
CYTOKINES AND GROWTH FACTORS IN OTM
The early phase of OTM involves an acute inflammatory response characterized by periodontal vasodilatation and migration of leukocytes out of PDL capillaries. The released inflammatory mediators such as Prostaglandins and IL-1 interact with bone cells. Cytokines secreted by leukocytes may interact directly with bone cells or indirectly, via neighboring cells, such as monocytes/macrophages, lymphocytes and fibroblasts.[6,31]
Cytokines released have multiple activities, which include bone remodeling, bone resorption and new bone deposition.
Cytokines family includes IL-1, Tumor necrosis factors, colony stimulating factors and Growth factors. Prominent cytokines that show demonstrated effects on bone remodeling are IL-1 Iβ, IL-6, TNF-alpha (TNF-α), GM-CSF and M-CSF.[51] These cytokines have been shown to stimulate bone resorption and induce osteoclast proliferation. IL-1 β attracts leukocytes, stimulates fibroblastic proliferation and enhances bone resorption; IL-2 is associated with active osteoclasts. IL-6 induces osteoclastogenesis and osteoclastic bone resorption. Elevation in IL-1 α, IL-1 β, and IL-6 following mechanical force application has been demonstrated by in-vivo studies[51,52,53] and in vitro studies.[54,55,56] TNF-α stimulates bone resorption and bone cell replication. M-CSF is the most potent in stimulating bone-marrow cells to produce osteoclasts.
Growth factors are also released during inflammation and repair by the cells of PDL and bone. Another theory states that the growth factors may be secreted by bone cells and stored (bound to bound matrix). They are likely to be released and activated during bone resorption.[57] They include FGF, IGF-I, IGF-II, TGF-β, PDGF and BMPs. FGF stimulates replication of both osteoblasts and progenitor population. PDGF plays a role in wound healing. IGF increases type 1-collagen and matrix synthesis by osteoblasts. TGF inhibits osteoclasts as well as promotes PGs production.
BMP's are now showing promising results in Periodontics in the field of PDL tissue reconstruction. BMP-2 has been shown to induce mesenchymal progenitors to differentiate into both osteoblasts and chondrocytes. BMP-2 also has been shown to stimulate committed osteo-progenitors (ROB – C 26(C26) cells) to differentiate into more mature osteoblasts.[58]
RANK-RANKL-OPG
The receptor activator of nuclear factor kappa B ligand (RANKL), its decoy receptor (RANK) and OPG were found to play important roles in regulation of bone metabolism.[59] Evidences suggest osteoblast itself regulates the differentiation of osteoclast.[60] The talk between an osteoblast and osteoclast is accomplished through an osteoblast membrane bond RANKL which can interact with osteoclast precursors to cause them to differentiate into osteoclasts. Another membrane bond molecule and its bonding ligand OPG can develop to block RANKL and prevent osteoclast formation.[61] Extensive studies done by Alhashimi et al.,[51] Aihara et al.,[62] Theoleyre et al.,[63] Kanzaki et al.,[64] and Yamaguchi et al.[65] have demonstrated that RANKL promotes osteoclastogenesis while OPG inhibits this effect.
Kanzaki and Chiba et al.[28] have demonstrated in rat experiments that local OPG gene transfer to periodontium inhibits OTM. It is also shown in the same experimental study that exogenous injection of PGE2 increases RANKL mRNA expression in PDL cells in rats. Researches of this kind may be an exciting thing in future to accelerate/block the tooth movement specifically at a particular site during the course of OTM.
Gingival crevicular fluid assessment
Various molecules are capable of passing through gingival sulcular epithelium and filter their way into GCF. The majority of these molecules are associated with remodeling of paradental tissues during situations such as normal maintenances, periodontal diseases and Orthodontic treatment. The collection of GCF is a non-invasive procedure, which provides periodontist and orthodontist useful diagnostic information on the nature and extent of the periodontal health or disease as well as patients response to mechanotheraphy.
Direct evidence for modified or enhanced cellular activities during orthodontic tooth movement can be found in GCF of treated teeth. Grieve et al.,[66] Ren et al.,[67] Sari et al.,[68] Dudic et al.,[69] Giannopoulou et al.[70] demonstrated elevated PGE2 levels in GCF 1 day after application of mechanical stimuli. Iwasaki et al.[71,72,73] measured concentration of IL-1β and its receptor antagonist IL-1RA Receptor antagonist periodically in GCF in 7 Orthodontic patients during the retraction of canines in extraction site. A correlation was found between the velocity of tooth movement and increase in concentrations of cytokine and its receptor antagonist.[4] Lee et al.[74] similarly measured 1 L-1β and PGE2 in GCF of young adult patients with orthodontic canine retraction. The retraction forces was continuous on one side and intermittent on the other side. The study showed significant increase in 1 L-1β and PGE2 in both the groups. Grieve et al.,[66] Uematsu et al.,[75] Tzannetou et al.,[76] Yamaguchi et al.,[77] Dudic et al.,[69] Giannopoulou et al.,[70] Ren et al.[67] also demonstrated elevated IL-1 β levels in GCF 1 h after application of mechanical stimulus. Uematsu et al.,[75] Ren et al.,[78] Basaran et al.[79] showed elevated IL-6, IL-8 levels in GCF after force application. Uematsu et al.,[76] Basaran et al.,[80] Ren et al.,[67] Lowney et al.,[81] Karacay et al.[82] demonstrated increased level of TNF-α in GCF after force application which peaked at day 1. Last et al.,[83] Samuels et al.,[84] Waddington,[85] Pender et al.[86] demonstrated elevated Hyaluronic acid in all GCF samples and chondroitin sulphate levels in GCF increased greatest in teeth that moved most. Saggese et al.[87] and Toia et al.[88] showed elevated IGF (bone remodeling marker) and its binding protein levels in GCF, 4 h after mechanical stimulation. Acid phosphatase[89] and Aspartate aminotransferase[90] levels after force application were higher on compressed side compared to tension side in GCF. Alkaline phosphatase levels were higher on tension side compared to compression side as shown by Perinetti et al.[91] and Batra et al.[92] Lactate dehydrogenase[93,94] levels were higher on compression side whereas Collagenase[95] levels were elevated on both mesial and distal sides after mechanical stimulus. Matrix metalloproteinases (MMPs) (MMP-1, MMP-2 and MMP-8) show elevated levels on compressed side than on tension side.[96,97,98] Elevated levels of Cathepsin B, an indicator of ECM degradation, were demonstrated in GCF by Sugiyama et al.[99] 1 day after force application. Wahab et al.[100] demonstrated elevated levels of TRAP in GCF on the compression side after force application. Griffiths et al.,[101] Isik et al.,[102] Kunimatsu et al.,[103] Nakashima et al.,[104] demonstrated significantly elevated levels of Osteocalcin, a bone turnover marker in GCF of patients with periodontal breakdown. Elevated levels of Osteonectin[105] and Osteopontin,[106,107]) detected in GCF with progressive increase in periodontal breakdown. Root resorption markers like dentin matrix protein-1, dentin phosphophoryn and dentin sialoprotein had significantly high concentrations in GCF of teeth showing severe root resorption.[108] Kereshanan et al.[109] demonstrated elevated levels of dentin sialoprotein in GCF samples of teeth at 12 weeks following commencement of fixed appliance therapy.
DETECTION OF MECHANICAL STRAIN BY BONE CELLS
OTM involves application of forces and moments from wires through brackets to teeth, with a goal of repositioning them in dental arches. The system of forces and moments is applied to the tooth which is a rigid body The PDL and alveolar bone houses the teeth. After the application of orthodontic force, the initial step is the detection of mechanical strain.
Researches indicate that the cells responsible for sensing mechanical strains in the bone are osteoblasts, Osteocytes or both.[110]
Three theories have been suggested on how these cells sense mechanical strain and how then the stimuli are passed into the cell and from one cell to another.
Strain released potentials
Activation of ion channels
ECM and cytoskeleton reorganization.
For the purpose of interdisciplinary understanding this article restricts explanation to ECM and cytoskeleton.
ECM AND CYTOSKELETON REORGANIZATION
The principle elements of ECM of either PDL or the bone may be considered as collagen fibrous network providing structural support embedded in and interacting with a non-collagenous matrix consisting of proteoglycans and various glycoproteins. The macromolecules, which make up the ECM, include collagen and glycosaminoglycans. These macro molecules are secreted at local levels by cells, particularly fibroblasts in the PDL and osteoblasts in the bone. The MMPs represent major class of enzymes responsible for ECM metabolism.
The growth and repair of connective tissue is a delicately balanced process of ECM removal and replacement, with significant control by MMPs and primary natural inhibitors or tissue inhibitors of metalloproteinases.
Cytoskeleton represents a framework attaching cell to cell or cell to ECM, thereby presenting a possibility of transducing mechanical forces acting on the cells or on their adjacent matrices.[7]
There are two types of cellular adhesions possible in the cytoskeleton framework. One is cell to cell adhesion and the other is cell to ECM adhesion. It is now clearly demonstrated histologically that any cytoskeleton framework has three main components, i.e., Microtubules, microfilaments and intermediate filaments. The major subunit protein of the microfilaments is actin. There are, however many associated protein such as myosin, tropomyosin, vinculin and talin.[7]
The cell membrane integral proteins are also identified as cell surface receptors termed Integrins which span the cell membrane from cytoplasm to extra cellular matrix. These Integrins mediate cell to cell attachment or cell attachment to ECM molecules such as fibronectin, laminin and talin. It has also been shown that actin and vinculin bind to this talin-integrin complex.
Integrins when analyzed at molecular levels, are a family of α/β heterodimeric cell surface receptors, composed of at least fourteen distinct α-sub units and eight or more β - subunits that can associate non-covalently in various combinations. Osteoblasts have been shown to express the Integrin subunits α1, α3 and β1. Osteoclasts express a different selection of Integrin subunits namely α2, αv, β1 and β3 which play an important role in adhesion of osteoclast to the bone surface.[60]
Integrins are found to regulate signaling pathways by changing intracellular calcium, regulating incitol lipid turnover, and phosphorylation of intracellular proteins. The individual binding of Integrins to osteoclasts and osteoblasts has been elucidated with the use of cell adhesion assays, monoclonal antibodies and affinity chromatography. The receptor binding site (RGD)(which is the one letter amino acid abbreviation for arginine-glycine-aspartic acid) was first defined. Similarly RGES (arginine- glycine- glutamic acid-serine) receptor binding site was also identified. In an engineered experimental study, it was found that both RGES and RGDs bind to osteoblasts.[9]
Both osteoblasts and osteoclasts express multiple Integrins or bind to many RGD containing proteins including osteopontin and cleaved type-I collagen, which are abundant in bone.
Periodontal tissue response to orthodontic forces
Light force i.e., force less than capillary blood pressure results in PDL ischemia with simultaneous bone resorption and formation resulting in more continuous tooth movement. Moderate force i.e., force exceeding capillary blood pressure leads to PDL strangulation resulting in delay in bone resorption. Strong/heavy force i.e., forces far exceeding capillary blood pressure results in ischemia and degeneration of PDL on pressure side resulting in hyalinization with more delay in tooth movement.
Possible combined future researches in the field of orthodontics and periodontics
Saliva biomarkers: Orthodontic tooth movement is a process of paradental remodeling mediated by inflammatory mediators like PGE2s, cytokines, neuropeptides, MMPs etc. These inflammatory mediators are also present in periodontitis and periodontal diseases. Hence detection of these inflammatory mediators is of paramount importance in detection and screening of periodontal diseases as well as demonstrating orthodontic tooth movement. GCF markers have several shortcomings like long collection times, easily prone to contamination, thick viscosity, questionable accuracy etc. Salivary biomarkers are rapidly gaining increasing popularity over GCF markers these days.
Advantages
Inexpensive, non-invasive and easy-to-use
Ease of collection, storing and shipping
Easier handling as it does not clot.
Disadvantages
Informative analytes generally present in lower amounts than in serum
Dilution of biomarkers common.
Pederson et al.[111] demonstrated that an increase in salivary levels of Cathepsin G, Elastase, Elastase inhibitors and C-reactive proteins correlated with increased periodontal breakdown. Craig Miller et al.[112] in his study revealed that salivary biomarkers IL-1 β and MMP-8 specific for three aspects of periodontitis i.e., inflammation, collagen degradation and bone turnover were significantly higher in subjects with periodontal breakdown. Todorovic et al.[113] showed in his study that the activity of creatine kinase, Lactate dehydrogenase, Aspartate aminotransferase, Alanine aminotransferase, Gamma glutamil transferase, acid phosphatase and alkaline phosphatase significantly increased in saliva of patients with periodontal breakdown. Frodge Ebersole et al.[114] demonstrated elevated mean salivary levels of TNF-α in subjects having periodontal diseases. Marracini et al.[115] in his study demonstrated that the mean myeloperoxidase activity in both GCF and saliva increased 2 h after orthodontic appliance activation. Gloria et al.[116] in her study aimed to determine whether variations in salivary concentrations of deoxypyridinoline (DPD) and bone-specific alkaline phosphatase (BAP) as detected with four consecutive visits may be linked with different phases of tooth movement. Results showed that although DPD values revealed an increasing nature after force application and BAP values showed a decreasing trend, only the former showed significant changes over time which implied that DPD dominates the earlier phases of tooth movement while BAP serves as an indicator of bone formation as soon as tooth movement stops. Thus qualitative changes in the composition of saliva biomarkers used in estimating OTM mediated by paradental remodeling could have significance in diagnosis and treatment of periodontal disease as well.
Periodontally accelerated osteogenic orthodontics
Wilcko et al.,[117,118] Nazarow et al.[119] demonstrated that adding periodontal regenerative surgery to the orthodontic protocol increased the quality of care in terms of clinical outcome and long term stability. Surgically accelerated modalities like selective alveolar decortication (SAD) and periodontally accelerated osteogenesis orthodontics can be used as an adjunct to conventional approaches to accelerate OTM with fewer adverse effects. SAD is a procedure where linear and punctuate decortications are made after reflecting the flap. The decortications should not impinge on root – PDL – cribriform plate complex and not extend to the alveolus crest. Accelerated OTM occurs due to inflammation and wound healing processes that are evoked by surgical trauma to alveolar bone. In addition alveolar bone surgery may also stimulate production of MSCs in marrow cavities which function synergistically with neighbouring PDL and alveolar bone cells resulting in accelerated OTM.
Murphy[120] demonstrated that addition of bone graft to a teeth moving through a surgical wound increases bone mass and enhances long term stability. Punctate and linear decortication in areas of alveolus where accelerated OTM is desired followed by addition of bone ad hoc where augmentation is needed provides stable results. Frost[121,122,123] investigated this phenomenon in depth and coined it as the “regional acceleratory phenomenon” and found that the normal metabolic rate of inflammation and wound healing process is accelerated.
Even where the bucco-lingual dimension of the alveolus is great, the decortication depth should not exceed the thickness of the cortex by more than 1-2 mm. The pattern of punctate or linear decortication does not seem to be as critical to the therapeutic effect as the degree of surgical manipulation per se Kole[124] summarized decortication of dento alveolar process with some refinements. The Kole surgery was limited to cortex of dental alveolus but subapical decortication was embelished by extending buccal and lingual cortical plate incisions until they communicated through subapical spongiosa. However, it had periodontal risks like attachment loss, periodontal pocket formation, alvelolar necrosis etc. Hence inspite of accelerated OTM, Koles method did not gain popularity. 32 years later Suya[125] revived interest in “corticotomy facilitated orthodontics”. He made a significant change in that he did not connect the buccal and lingual incisions through the alveolus simply relying on the effects of linear interproximal decortication. This procedure found universal acceptance as OTM was accelerated without any attachment loss, pocket formation or alveolar necrosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–55. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 2.Komori T, Kishimoto T. Cbfa1 in bone development. Curr Opin Genet Dev. 1998;8:494–9. doi: 10.1016/s0959-437x(98)80123-8. [DOI] [PubMed] [Google Scholar]
- 3.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 4.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: Arrows point the way. Development. 2004;131:1663–77. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 5.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–23. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 6.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–14. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandy JR, Farndale RW, Meikle MC. Recent advances in understanding mechanically induced bone remodeling and their relevance to orthodontic theory and practice. Am J Orthod Dentofacial Orthop. 1993;103:212–22. doi: 10.1016/0889-5406(93)70002-6. [DOI] [PubMed] [Google Scholar]
- 8.Graber TM, Vanardsdall RL, Vig KW. 4th ed. St. Louis: Elsevier-Mosby; 2005. Bone physiology, metabolism and biomechanics in orthodontic practice. Orthodontics: Current Principles and Techniques; pp. 266–80. [Google Scholar]
- 9.Covell D. Can biology help us move teeth faster? Presented by Dr. David Covell, Annual Session, Palm Springs. [Last accessed 2004 Sept 20]. Available from: http//www.pcsortho.org/bulletin 05 .
- 10.Wang Y, McNamara LM, Schaffler MB, Weinbaum S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci U S A. 2007;104:15941–6. doi: 10.1073/pnas.0707246104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. J Cell Biochem. 1994;55:287–99. doi: 10.1002/jcb.240550304. [DOI] [PubMed] [Google Scholar]
- 12.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–60. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 13.Skerry TM, Bitensky L, Chayen J, Lanyon LE. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. J Bone Miner Res. 1989;4:783–8. doi: 10.1002/jbmr.5650040519. [DOI] [PubMed] [Google Scholar]
- 14.Holtrop ME, King GJ. The ultrastructure of the osteoclast and its functional implications. Clin Orthop Relat Res. 1977;123:177–96. [PubMed] [Google Scholar]
- 15.Schoppet M, Preissner KT, Hofbauer LC. RANK ligand and osteoprotegerin: Paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol. 2002;22:549–53. doi: 10.1161/01.atv.0000012303.37971.da. [DOI] [PubMed] [Google Scholar]
- 16.Agata H, Asahina I, Yamazaki Y, Uchida M, Shinohara Y, Honda MJ, et al. Effective bone engineering with periosteum-derived cells. J Dent Res. 2007;86:79–83. doi: 10.1177/154405910708600113. [DOI] [PubMed] [Google Scholar]
- 17.Miller SC, Jee WS. The bone lining cell: A distinct phenotype? Calcif Tissue Int. 1987;41:1–5. doi: 10.1007/BF02555122. [DOI] [PubMed] [Google Scholar]
- 18.Baumrind S. A reconsideration of the propriety of the “pressure-tension” hypothesis. Am J Orthod. 1969;55:12–22. doi: 10.1016/s0002-9416(69)90170-5. [DOI] [PubMed] [Google Scholar]
- 19.Gianelly AA. Force-induced changes in the vascularity of the periodontal ligament. Am J Orthod. 1969;55:5–11. doi: 10.1016/s0002-9416(69)90169-9. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro E, Roeber FW, Klempner LS. Orthodontic movement using pulsating force-induced piezoelectricity. Am J Orthod. 1979;76:59–66. doi: 10.1016/0002-9416(79)90299-9. [DOI] [PubMed] [Google Scholar]
- 21.Kato J, Wakisaka S, Kurisu K. Immunohistochemical changes in the distribution of nerve fibers in the periodontal ligament during an experimental tooth movement of the rat molar. Acta Anat (Basel) 1996;157:53–62. doi: 10.1159/000147866. [DOI] [PubMed] [Google Scholar]
- 22.Andersen KL, Pedersen EH, Melsen B. Material parameters and stress profiles within the periodontal ligament. Am J Orthod Dentofacial Orthop. 1991;99:427–40. doi: 10.1016/S0889-5406(05)81576-8. [DOI] [PubMed] [Google Scholar]
- 23.Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958;232:1077–91. [PubMed] [Google Scholar]
- 24.Rodan GA, Bourret LA, Harvey A, Mensi T. Cyclic AMP and cyclic GMP: Mediators of the mechanical effects on bone remodeling. Science. 1975;189:467–9. doi: 10.1126/science.168639. [DOI] [PubMed] [Google Scholar]
- 25.Berridge MJ. Inositol trisphosphate and diacylglycerol: Two interacting second messengers. Annu Rev Biochem. 1987;56:159–93. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- 26.Sandy JR, Farndale RW. Second messengers: Regulators of mechanically-induced tissue remodelling. Eur J Orthod. 1991;13:271–8. doi: 10.1093/ejo/13.4.271. [DOI] [PubMed] [Google Scholar]
- 27.Dolce C, Malone JS, Wheeler TT. Current concepts in Biology of orthodontic tooth movement. Semin Orthod. 2002;8:6–12. [Google Scholar]
- 28.Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res. 2002;17:210–20. doi: 10.1359/jbmr.2002.17.2.210. [DOI] [PubMed] [Google Scholar]
- 29.Robert A.W. Furhmann. Three dimensional evaluation of periodontal remodeling during orthodontic tooth movement. Semin Orthod. 2002;8:23–8. [Google Scholar]
- 30.Dolce C, Kinniburgh AJ, Dziak R. Immediate early-gene induction in rat osteoblastic cells after mechanical deformation. Arch Oral Biol. 1996;41:1101–8. doi: 10.1016/s0003-9969(96)00098-2. [DOI] [PubMed] [Google Scholar]
- 31.Reyna J, Moon HB, Muang V, Panahpour M. Gene expression induced by orthodontic tooth movement and/or root resorption. In: Davidovitch Z, Mah J, Suthanarak S, editors. Biological Mechanisms of Tooth Eruption, Resorption and Movement. Boston: Harvard Society for the Advancement of Orthodontics; 2006. pp. 45–75. [Google Scholar]
- 32.King GJ, Keeling SD, McCoy EA, Ward TH. Measuring dental drift and orthodontic tooth movement in response to various initial forces in adult rats. Am J Orthod Dentofacial Orthop. 1991;99:456–65. doi: 10.1016/S0889-5406(05)81579-3. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129:469.e1–32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Robbins . 3rd Ed. Philadelphia: W.B. Saunders International Edition; 1984. Pathologic basis of disease. Textbook of General Pathology, Inflammation and Repair; pp. 40–84. [Google Scholar]
- 35.Samuelsson B, Granström E, Green K, Hamberg M, Hammarström S. Prostaglandins. Annu Rev Biochem. 1975;44:669–95. doi: 10.1146/annurev.bi.44.070175.003321. [DOI] [PubMed] [Google Scholar]
- 36.Klein DC, Raisz LG. Prostaglandins: Stimulation of bone resorption in tissue culture. Endocrinology. 1970;86:1436–40. doi: 10.1210/endo-86-6-1436. [DOI] [PubMed] [Google Scholar]
- 37.Davidovitch Z, Shanfeld JL, Batastini PJ. Increased production of cyclic AMP in mechanically stressed alveolar bone in cats. Am J Orthod Dentofacial Orthop. 1974;65:320–1. doi: 10.1016/s0002-9416(74)90340-6. [DOI] [PubMed] [Google Scholar]
- 38.Davidovitch Z, Shanfeld JL. Prostaglandin E2 levels in alveolar bone. Int Assoc Dent Res. 1980;362:972. [Google Scholar]
- 39.Davidovitch Z, Finkelson MD, Steigman S, Shanfeld JL, Montgomery PC, Korostoff E. Electric currents, bone remodeling, and orthodontic tooth movement. II. Increase in rate of tooth movement and periodontal cyclic nucleotide levels by combined force and electric current. Am J Orthod Dentofacial Orthop. 1980;77:33–47. doi: 10.1016/0002-9416(80)90222-5. [DOI] [PubMed] [Google Scholar]
- 40.Yamasaki K, Shibata Y, Imai S, Tani Y, Shibasaki Y, Fukuhara T. Clinical application of prostaglandin E1 (PGE1) upon orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1984;85:508–18. doi: 10.1016/0002-9416(84)90091-5. [DOI] [PubMed] [Google Scholar]
- 41.Lee WC. Experimental study of the effect of prostaglandin administration on tooth movement – With particular emphasis on the relationship to the method of PGE1 administration. Am J Orthod Dentofacial Orthop. 1990;98:231–41. doi: 10.1016/s0889-5406(05)81600-2. [DOI] [PubMed] [Google Scholar]
- 42.Leiker BJ, Nanda RS, Currier GF, Howes RI, Sinha PK. The effects of exogenous prostaglandins on orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 1995;108:380–8. doi: 10.1016/s0889-5406(95)70035-8. [DOI] [PubMed] [Google Scholar]
- 43.Kale S, Kocadereli I, Atilla P, Aşan E. Comparison of the effects of 1,25 dihydroxycholecalciferol and prostaglandin E2 on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2004;125:607–14. doi: 10.1016/j.ajodo.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Patil AK, Keluskar KM, Gaitonde SD. The clinical application of PGE1 in orthodontic tooth movement. J Indian Orthod Soc. 2005;38:91–8. [Google Scholar]
- 45.Schelling SH, Wolfe HJ, Tashjian AH., Jr Role of the osteoclast in prostaglandin E2-stimulated bone resorption: A correlative morphometric and biochemical analysis. Lab Invest. 1980;42:290–5. [PubMed] [Google Scholar]
- 46.Shen V, Rifas L, Kohler G, Peck WA. Prostaglandins change cell shape and increase intercellular gap junctions in osteoblasts cultured from rat fetal calvaria. J Bone Miner Res. 1986;1:243–9. doi: 10.1002/jbmr.5650010302. [DOI] [PubMed] [Google Scholar]
- 47.Rodan GA, Martin TJ. Role of osteoblasts in hormonal control of bone resorption – A hypothesis. Calcif Tissue Int. 1981;33:349–51. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- 48.Bringhurst FR, Marie BD, Stephen MK, Henry MK. Bone and mineral metabolism in health and disease. In: Anthony SF, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al., editors. Harrison's Principles of Internal Medicine. 17th ed. Ch. 346. New York: McGraw Hill Companies Inc; 2008. pp. 2365–76. [Google Scholar]
- 49.Marks SC, Jr, Miller S. Local infusion of prostaglandin E1 stimulates mandibular bone formation in vivo. J Oral Pathol. 1988;17:500–5. doi: 10.1111/j.1600-0714.1988.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 50.Meghji S. Bone remodelling. Br Dent J. 1992;172:235–42. doi: 10.1038/sj.bdj.4807835. [DOI] [PubMed] [Google Scholar]
- 51.Alhashimi N, Frithiof L, Brudvik P, Bakhiet M. Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. Am J Orthod Dentofacial Orthop. 2001;119:307–12. doi: 10.1067/mod.2001.110809. [DOI] [PubMed] [Google Scholar]
- 52.Davidovitch Z, Nicolay OF, Ngan PW, Shanfeld JL. Neurotransmitters, cytokines, and the control of alveolar bone remodeling in orthodontics. Dent Clin North Am. 1988;32:411–35. [PubMed] [Google Scholar]
- 53.Bletsa A, Berggreen E, Brudvik P. Interleukin-1alpha and tumor necrosis factor-alpha expression during the early phases of orthodontic tooth movement in rats. Eur J Oral Sci. 2006;114:423–9. doi: 10.1111/j.1600-0722.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 54.Saito S, Ngan P, Saito M, Kim K, Lanese R, Shanfeld J, et al. Effects of cytokines on prostaglandin E and cAMP levels in human periodontal ligament fibroblasts in vitro. Arch Oral Biol. 1990;35:387–95. doi: 10.1016/0003-9969(90)90186-e. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu N, Yamaguchi M, Goseki T, Ozawa Y, Saito K, Takiguchi H, et al. Cyclic-tension force stimulates interleukin-1 beta production by human periodontal ligament cells. J Periodontal Res. 1994;29:328–33. doi: 10.1111/j.1600-0765.1994.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto T, Kita M, Kimura I, Oseko F, Terauchi R, Takahashi K, et al. Mechanical stress induces expression of cytokines in human periodontal ligament cells. Oral Dis. 2006;12:171–5. doi: 10.1111/j.1601-0825.2005.01179.x. [DOI] [PubMed] [Google Scholar]
- 57.Daviddovitch Z, Norton LA. Biological Mechanisms of Tooth movement and Craniofacial Adaptation. Vol. 1. Alabama: Harvard Society; 1996. Signal Pathways activated by growth factors in bone cells; pp. 195–205. [Google Scholar]
- 58.Lee MB. Bone morphogenetic proteins: Background and implications for oral reconstruction. A review. J Clin Periodontol. 1997;24:355–65. doi: 10.1111/j.1600-051x.1997.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 59.Ogasawara T, Yoshimine Y, Kiyoshima T, Kobayashi I, Matsuo K, Akamine A, et al. In situ expression of RANKL, RANK, osteoprotegerin and cytokines in osteoclasts of rat periodontal tissue. J Periodontal Res. 2004;39:42–9. doi: 10.1111/j.1600-0765.2004.00699.x. [DOI] [PubMed] [Google Scholar]
- 60.Raisz LG. Physiology and pathophysiology of bone remodeling. Clin Chem. 1999;45:1353–8. [PubMed] [Google Scholar]
- 61.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–49. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 62.Aihara N, Yamaguchi M, Kasai K. Low-energy irradiation stimulates formation of osteoclast-like cells via RANK expression in vitro. Lasers Med Sci. 2006;21:24–33. doi: 10.1007/s10103-005-0368-4. [DOI] [PubMed] [Google Scholar]
- 63.Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15:457–75. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Kanzaki H, Chiba M, Takahashi I, Haruyama N, Nishimura M, Mitani H. Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J Dent Res. 2004;83:920–5. doi: 10.1177/154405910408301206. [DOI] [PubMed] [Google Scholar]
- 65.Yamaguchi M, Aihara N, Kojima T, Kasai K. RANKL increase in compressed periodontal ligament cells from root resorption. J Dent Res. 2006;85:751–6. doi: 10.1177/154405910608500812. [DOI] [PubMed] [Google Scholar]
- 66.Grieve WG, 3rd, Johnson GK, Moore RN, Reinhardt RA, DuBois LM. Prostaglandin E (PGE) and interleukin-1 beta (IL-1 beta) levels in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1994;105:369–74. doi: 10.1016/s0889-5406(94)70131-8. [DOI] [PubMed] [Google Scholar]
- 67.Ren Y, Maltha JC, Van’t Hof MA, Von Den Hoff JW, Kuijpers-Jagtman AM, Zhang D. Cytokine levels in crevicular fluid are less responsive to orthodontic force in adults than in juveniles. J Clin Periodontol. 2002;29:757–62. doi: 10.1034/j.1600-051x.2002.290813.x. [DOI] [PubMed] [Google Scholar]
- 68.Sari E, Olmez H, Gürton AU. Comparison of some effects of acetylsalicylic acid and rofecoxib during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2004;125:310–5. doi: 10.1016/S0889540603009144. [DOI] [PubMed] [Google Scholar]
- 69.Dudic A, Kiliaridis S, Mombelli A, Giannopoulou C. Composition changes in gingival crevicular fluid during orthodontic tooth movement: Comparisons between tension and compression sides. Eur J Oral Sci. 2006;114:416–22. doi: 10.1111/j.1600-0722.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 70.Giannopoulou C, Dudic A, Kiliaridis S. Pain discomfort and crevicular fluid changes induced by orthodontic elastic separators in children. J Pain. 2006;7:367–76. doi: 10.1016/j.jpain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Iwasaki LR, Haack JE, Nickel JC, Reinhardt RA, Petro TM. Human interleukin-1 beta and interleukin-1 receptor antagonist secretion and velocity of tooth movement. Arch Oral Biol. 2001;46:185–9. doi: 10.1016/s0003-9969(00)00088-1. [DOI] [PubMed] [Google Scholar]
- 72.Iwasaki LR, Crouch LD, Tutor A, Gibson S, Hukmani N, Marx DB, et al. Tooth movement and cytokines in gingival crevicular fluid and whole blood in growing and adult subjects. Am J Orthod Dentofacial Orthop. 2005;128:483–91. doi: 10.1016/j.ajodo.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 73.Iwasaki LR, Gibson CS, Crouch LD, Marx DB, Pandey JP, Nickel JC. Speed of tooth movement is related to stress and IL-1 gene polymorphisms. Am J Orthod Dentofacial Orthop. 2006;130:698.e1–9. doi: 10.1016/j.ajodo.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 74.Lee KJ, Park YC, Yu HS, Choi SH, Yoo YJ. Effects of continuous and interrupted orthodontic force on interleukin-1beta and prostaglandin E2 production in gingival crevicular fluid. Am J Orthod Dentofacial Orthop. 2004;125:168–77. doi: 10.1016/j.ajodo.2003.03.006. [DOI] [PubMed] [Google Scholar]
- 75.Uematsu S, Mogi M, Deguchi T. Interleukin (IL)-1 beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta 2-microglobulin levels are elevated in gingival crevicular fluid during human orthodontic tooth movement. J Dent Res. 1996;75:562–7. doi: 10.1177/00220345960750010801. [DOI] [PubMed] [Google Scholar]
- 76.Tzannetou S, Efstratiadis S, Nicolay O, Grbic J, Lamster I. Interleukin-1beta and beta-glucuronidase in gingival crevicular fluid from molars during rapid palatal expansion. Am J Orthod Dentofacial Orthop. 1999;115:686–96. doi: 10.1016/s0889-5406(99)70295-7. [DOI] [PubMed] [Google Scholar]
- 77.Yamaguchi M, Yoshii M, Kasai K. Relationship between substance P and interleukin-1beta in gingival crevicular fluid during orthodontic tooth movement in adults. Eur J Orthod. 2006;28:241–6. doi: 10.1093/ejo/cji100. [DOI] [PubMed] [Google Scholar]
- 78.Ren Y, Hazemeijer H, de Haan B, Qu N, de Vos P. Cytokine profiles in crevicular fluid during orthodontic tooth movement of short and long durations. J Periodontol. 2007;78:453–8. doi: 10.1902/jop.2007.060261. [DOI] [PubMed] [Google Scholar]
- 79.Baºaran G, Ozer T, Kaya FA, Hamamci O. Interleukins 2, 6, and 8 levels in human gingival sulcus during orthodontic treatment. Am J Orthod Dentofacial Orthop. 2006;130:7.e1–6. doi: 10.1016/j.ajodo.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 80.Başaran G, Ozer T, Kaya FA, Kaplan A, Hamamci O. Interleukine-1 beta and tumor necrosis factor-alpha levels in the human gingival sulcus during orthodontic treatment. Angle Orthod. 2006;76:830–6. doi: 10.1043/0003-3219(2006)076[0830:IATNFL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 81.Lowney JJ, Norton LA, Shafer DM, Rossomando EF. Orthodontic forces increase tumor necrosis factor alpha in the human gingival sulcus. Am J Orthod Dentofacial Orthop. 1995;108:519–24. doi: 10.1016/s0889-5406(95)70052-8. [DOI] [PubMed] [Google Scholar]
- 82.Karacay S, Saygun I, Bengi AO, Serdar M. Tumor necrosis factor-alpha levels during two different canine distalization techniques. Angle Orthod. 2007;77:142–7. doi: 10.2319/120905-430R.1. [DOI] [PubMed] [Google Scholar]
- 83.Last KS, Donkin C, Embery G. Glycosaminoglycans in human gingival crevicular fluid during orthodontic movement. Arch Oral Biol. 1988;33:907–12. doi: 10.1016/0003-9969(88)90021-0. [DOI] [PubMed] [Google Scholar]
- 84.Samuels RH, Pender N, Last KS. The effects of orthodontic tooth movement on the glycosaminoglycan components of gingival crevicular fluid. J Clin Periodontol. 1993;20:371–7. doi: 10.1111/j.1600-051x.1993.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 85.Waddington RJ, Embery G, Samuels RH. Characterization of proteoglycan metabolites in human gingival crevicular fluid during orthodontic tooth movement. Arch Oral Biol. 1994;39:361–8. doi: 10.1016/0003-9969(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 86.Pender N, Samuels RH, Last KS. The monitoring of orthodontic tooth movement over a 2-year period by analysis of gingival crevicular fluid. Eur J Orthod. 1994;16:511–20. doi: 10.1093/ejo/16.6.511. [DOI] [PubMed] [Google Scholar]
- 87.Saggese R, Federico G, Gandini P. The IGF-1 – IGFBPs system in the crevicular fluid: Its changes during orthodontic movement. Prog Orthod. 2005;6:114–8. [PubMed] [Google Scholar]
- 88.Toia M, Galazzo R, Maioli C, Granata R, Scarlatti F. The IGF-I/IGFBP-3 system in gingival crevicular fluid and dependence on application of fixed force. J Endocrinol Invest. 2005;28:1009–14. doi: 10.1007/BF03345340. [DOI] [PubMed] [Google Scholar]
- 89.Insoft M, King GJ, Keeling SD. The measurement of acid and alkaline phosphatase in gingival crevicular fluid during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1996;109:287–96. doi: 10.1016/s0889-5406(96)70152-x. [DOI] [PubMed] [Google Scholar]
- 90.Perinetti G, Paolantonio M, D’Attilio M, D’Archivio D, Dolci M, Femminella B, et al. Aspartate aminotransferase activity in gingival crevicular fluid during orthodontic treatment. A controlled short-term longitudinal study. J Periodontol. 2003;74:145–52. doi: 10.1902/jop.2003.74.2.145. [DOI] [PubMed] [Google Scholar]
- 91.Perinetti G, Paolantonio M, D’Attilio M, D’Archivio D, Tripodi D, Femminella B, et al. Alkaline phosphatase activity in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2002;122:548–56. doi: 10.1067/mod.2002.126154. [DOI] [PubMed] [Google Scholar]
- 92.Batra P, Kharbanda O, Duggal R, Singh N, Parkash H. Alkaline phosphatase activity in gingival crevicular fluid during canine retraction. Orthod Craniofac Res. 2006;9:44–51. doi: 10.1111/j.1601-6343.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 93.Serra E, Perinetti G, D’Attilio M, Cordella C, Paolantonio M, Festa F, et al. Lactate dehydrogenase activity in gingival crevicular fluid during orthodontic treatment. Am J Orthod Dentofacial Orthop. 2003;124:206–11. doi: 10.1016/s0889-5406(03)00407-4. [DOI] [PubMed] [Google Scholar]
- 94.Perinetti G, Serra E, Paolantonio M, Bruè C, Meo SD, Filippi MR, et al. Lactate dehydrogenase activity in human gingival crevicular fluid during orthodontic treatment: A controlled, short-term longitudinal study. J Periodontol. 2005;76:411–7. doi: 10.1902/jop.2005.76.3.411. [DOI] [PubMed] [Google Scholar]
- 95.Sorsa T, Ingman T, Mikkonen T, Suomalainen K, et al. Characterization of interstitial collagenase in gingival crevicular fluid during orthodontic tooth movement in man. In: Davidovitch Z, editor. The Biological mechanisms of Tooth Movement and Craniofacial Adaptation. Columbus, Ohio State: University College of Dentistry; 1992. pp. 47–51. [Google Scholar]
- 96.Apajalahti S, Sorsa T, Railavo S, Ingman T. The in vivo levels of matrix metalloproteinase-1 and-8 in gingival crevicular fluid during initial orthodontic tooth movement. J Dent Res. 2003;82:1018–22. doi: 10.1177/154405910308201216. [DOI] [PubMed] [Google Scholar]
- 97.Ingman T, Apajalahti S, Mäntylä P, Savolainen P, Sorsa T. Matrix metalloproteinase-1 and-8 in gingival crevicular fluid during orthodontic tooth movement: A pilot study during 1 month of follow-up after fixed appliance activation. Eur J Orthod. 2005;27:202–7. doi: 10.1093/ejo/cjh097. [DOI] [PubMed] [Google Scholar]
- 98.Cantarella G, Cantarella R, Caltabiano M, Risuglia N, Bernardini R, Leonardi R. Levels of matrix metalloproteinases 1 and 2 in human gingival crevicular fluid during initial tooth movement. Am J Orthod Dentofacial Orthop. 2006;130:568.e11–6. doi: 10.1016/j.ajodo.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 99.Sugiyama Y, Yamaguchi M, Kanekawa M, Yoshii M, Nozoe T, Nogimura A, et al. The level of cathepsin B in gingival crevicular fluid during human orthodontic tooth movement. Eur J Orthod. 2003;25:71–6. doi: 10.1093/ejo/25.1.71. [DOI] [PubMed] [Google Scholar]
- 100.Wahab RM, Dasor MM, Senafi S, Abdullah AA, Jemain AA, Kasim NA, et al. Crevicular tartrate resistant acid phosphatase activity and rate of tooth movement under different continuous force applications. Afr J Pharm Pharmacol. 2011;5:2213–9. [Google Scholar]
- 101.Griffiths GS, Moulson AM, Petrie A, James IT. Evaluation of osteocalcin and pyridinium crosslinks of bone collagen as markers of bone turnover in gingival crevicular fluid during different stages of orthodontic treatment. J Clin Periodontol. 1998;25:492–8. doi: 10.1111/j.1600-051x.1998.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 102.Isik F, Sayinsu K, Arun T, Unlüçerçi Y. Bone marker levels in gingival crevicular fluid during orthodontic intrusive tooth movement: A preliminary study. J Contemp Dent Pract. 2005;6:27–35. [PubMed] [Google Scholar]
- 103.Kunimatsu K, Mataki S, Tanaka H, Mine N, Kiyoki M, Hosoda K, et al. A cross-sectional study on osteocalcin levels in gingival crevicular fluid from periodontal patients. J Periodontol. 1993;64:865–9. doi: 10.1902/jop.1993.64.9.865. [DOI] [PubMed] [Google Scholar]
- 104.Nakashima K, Demeurisse C, Cimasoni G. The recovery efficiency of various materials for sampling enzymes and polymorphonuclear leukocytes from gingival crevices. J Clin Periodontol. 1994;21:479–83. doi: 10.1111/j.1600-051x.1994.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 105.Bowers MR, Fisher LW, Termine JD, Somerman MJ. Connective tissue-associated proteins in crevicular fluid: Potential markers for periodontal diseases. J Periodontol. 1989;60:448–51. doi: 10.1902/jop.1989.60.8.448. [DOI] [PubMed] [Google Scholar]
- 106.Kido J, Nakamura T, Asahara Y, Sawa T, Kohri K, Nagata T. Osteopontin in gingival crevicular fluid. J Periodontal Res. 2001;36:328–33. doi: 10.1034/j.1600-0765.2001.360509.x. [DOI] [PubMed] [Google Scholar]
- 107.Sharma P, Kumar S, Kundu GC. Transcriptional regulation of human osteopontin promoter by histone deacetylase inhibitor, trichostatin A in cervical cancer cells. Mol Cancer. 2010;9:178. doi: 10.1186/1476-4598-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Balducci L, Ramachandran A, Hao J, Narayanan K, Evans C, George A. Biological markers for evaluation of root resorption. Arch Oral Biol. 2007;52:203–8. doi: 10.1016/j.archoralbio.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kereshanan S, Stephenson P, Waddington R. Identification of dentine sialoprotein in gingival crevicular fluid during physiological root resorption and orthodontic tooth movement. Eur J Orthod. 2008;30:307–14. doi: 10.1093/ejo/cjn024. [DOI] [PubMed] [Google Scholar]
- 110.Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function: A review. Osteoporos Int. 2002;13:688–700. doi: 10.1007/s001980200095. [DOI] [PubMed] [Google Scholar]
- 111.Pederson ED, Stanke SR, Whitener SJ, Sebastiani PT, Lamberts BL, Turner DW. Salivary levels of alpha 2-macroglobulin, alpha 1-antitrypsin, C-reactive protein, cathepsin G and elastase in humans with or without destructive periodontal disease. Arch Oral Biol. 1995;40:1151–5. doi: 10.1016/0003-9969(95)00089-5. [DOI] [PubMed] [Google Scholar]
- 112.Miller CS, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: A cross-sectional study. J Am Dent Assoc. 2006;137:322–9. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- 113.Todorovic T, Dozic I, Vicente-Barrero M, Ljuskovic B, Pejovic J, Marjanovic M, et al. Salivary enzymes and periodontal disease. Med Oral Patol Oral Cir Bucal. 2006;11:E115–9. [PubMed] [Google Scholar]
- 114.Frodge BD, Ebersole JL, Kryscio RJ, Thomas MV, Miller CS. Bone remodeling biomarkers of periodontal disease in saliva. J Periodontol. 2008;79:1913–9. doi: 10.1902/jop.2008.080070. [DOI] [PubMed] [Google Scholar]
- 115.Marcaccini AM, Amato PA, Leão FV, Gerlach RF, Ferreira JT. Myeloperoxidase activity is increased in gingival crevicular fluid and whole saliva after fixed orthodontic appliance activation. Am J Orthod Dentofacial Orthop. 2010;138:613–6. doi: 10.1016/j.ajodo.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 116.Flórez-Moreno GA, Marín-Restrepo LM, Isaza-Guzmán DM, Tobón-Arroyave SI. Screening for salivary levels of deoxypyridinoline and bone-specific alkaline phosphatase during orthodontic tooth movement: A pilot study. Eur J Orthod Adv Acess. 2012;34:1–8. doi: 10.1093/ejo/cjr138. [DOI] [PubMed] [Google Scholar]
- 117.Wilcko WM, Wilcko MT, Bouquot JE, Ferguson DJ. Accelerated orthodontics with alveolar reshaping. J Orthod Pract. 2000;10:63–70. [Google Scholar]
- 118.Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: Two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21:9–19. [PubMed] [Google Scholar]
- 119.Nazarow AD, Ferguson DJ, Wilkcko WM, Wilcko MT. Improved orthodontics retention following corticotomy using ABO oblective grading system. J Dent Res. 2004;83 Abs 2644. [Google Scholar]
- 120.Murphy NC. In vivo tissue engineering for orthodontists: A modest first step. In: Davidovitch Z, Mah J, Suthanarak S, editors. Biological Mechanisms for Tooth Eruption, Resorption and Movement. Boston: Harvard Society for the Advancement of Orthodontics; 2006. pp. 385–410. [Google Scholar]
- 121.Frost HM. The regional acceleratory phenomenon: A review. Henry Ford Hosp Med J. 1983;31:3–9. [PubMed] [Google Scholar]
- 122.Frost HM. Perspectives: A proposed general model of the “mechanostat” (suggestions from a new skeletal-biologic paradigm) Anat Rec. 1996;244:139–47. doi: 10.1002/(SICI)1097-0185(199602)244:2<139::AID-AR1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 123.Frost HM. The Utah paradigm of skeletal physiology: An overview of its insights for bone, cartilage and collagenous tissue organs. J Bone Miner Metab. 2000;18:305–16. doi: 10.1007/s007740070001. [DOI] [PubMed] [Google Scholar]
- 124.Kole H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol. 1959;12:515–29. doi: 10.1016/0030-4220(59)90153-7. [DOI] [PubMed] [Google Scholar]
- 125.Hosl E, Baldauf A, editors. Mechanical and Biological Basics in Orthodontic Therapy. Heidelberg: Huthig Buch Verlag; 1991. Corticotomy in orthodontics; pp. 207–26. [Google Scholar]


