Abstract
Background:
Pro-inflammatory markers are seen to increase in inflammatory diseases like periodontitis. Detecting an increase in these markers is one of the diagnostic modality. One such marker, which can be detected, is the ceruloplasmin. Ceruloplasmin induces hypoxia and generates oxygen radicals at the site of aggressive periodontitis. It also causes a state of hypoferremia leading to increase in the natural resistance of the body. The aim of this study was to evaluate the serum levels of cerruloplasmin in both aggressive and chronic periodontitis patients.
Materials and Methods:
Blood samples were collected from aggressive periodontitis patients (n = 20), chronic periodontitis patients (n = 20) and periodontally healthy patients (n = 20). The serum was extracted from all the blood samples and ceruloplasmin levels were spectroscopically evaluated through a new kinetic method, which used a norfloxacin based reagent.
Results:
Serum ceruloplasmin levels were found to be significantly higher in aggressive periodontitis patients (P > 0.05) than in chronic periodontitis patients (P > 0.05) even though increase in the level of ceruloplasmin was found in chronic periodontitis. Periodontally healthy patients did not show increase in the levels of serum ceruloplasmin. The levels of serum ceruloplasmin also increased with the disease severity whose manifestations were increased bleeding on probing, increased pocket depth and increased attachment loss.
Conclusion:
Serum ceruloplasmin levels increased in both aggressive and chronic periodontitis patients, but more in aggressive periodontitis patients making it a potential marker for diagnosis of periodontitis.
Keywords: Ceruloplasmin, hypoferremia, hypoxia, natural resistance
INTRODUCTION
Periodontitis comprise a group of diseases, which are inflammatory in origin and generally affect the connective tissue attachment and supporting bone present around the teeth. It generally results from interactions between periodontal microflora and the multifaceted response of the host.[1] The two common types of periodontal diseases are the chronic periodontitis and aggressive peridontitis. Aggressive periodontitis is a rapidly progressing disease that affects otherwise healthy individuals.[2] The main characteristic of this disease is that it is episodic and the destruction of periodontal tissues is very rapid, resulting in early tooth loss.[3,4] It is generally seen that aggressive periodontitis is more common in young individuals while chronic periodontitis is more frequent in adults. The main difference between these two diseases is the rate of progression of disease wherein aggressive periodontitis is seen to be faster in progression than the chronic periodontitis, even in the presence of minute amount of microbial deposits.[5]
The prevalence of severe form of periodontitis varies throughout the world with Brazil (50%)[6] leading followed by USA (32%)[7] and Germany (21%).[8] The diagnosis of these periodontal diseases and the identifying the patients who are at risk for these diseases always possess a challenge for clinicians. Early diagnosis of periodontal diseases will surely help in reducing the morbidity rate of the teeth. Periodontal disease risk can be identified and quantified by objective measures, like biomarkers.[9] It has been a feature of periodontal diseases that it creates an area of local inflammation as well as systemic inflammation, which are indicated by elevated serum levels of various pro-inflammatory markers such as alkaline phosphatase, tumor necrosis factor α, interleukin-1, C-reactive protein, vascular endothelial growth factor,[10,11,12,13,14] and reduced levels of various anti-inflammatory markers especially the interleulkin-10.[15] One such pro-inflammatory marker for periodontal disease recognized in recent times is the ceruloplasmin.
Ceruloplasmin a 122-kD multi-copper binding plasma protein containing ferroxidase activity[16] necessary for ferric ion saturation of transferrin. Ceruloplasmin helps in transferring of copper within our body and also influences the uptake of iron into the cells because of its property of conversion of ferrous form of iron to the ferric form, due to which alterations in serum iron are often accompanied by changes in serum ceruloplasmin.[16,17] It thus leads to a state of hypoferremia. Ceruloplasmin is also an acute phase reactant seen to increase in inflammatory conditions.[17,18,19] It has already been proved that ceruloplasmin levels are seen to increase in cases of localized aggressive periodontitis.[20]
Keeping the above mentioned facts in mind, the following cross-sectional study was conducted to evaluate the serum ceruloplasmin levels in patients diagnosed with generalized aggressive periodontitis and generalized chronic periodontitis.
MATERIALS AND METHODS
Data source
The study was conducted in the Division of Periodontology of Department of Dental Surgery, Armed Forces Medical College (AFMC), Pune between June 2010 and April 2011. The study population of 60 (Age 16-58 years, Male - 41 and Female - 19) subjects were selected and divided into three groups wherein Group A constituted 20 subjects with untreated generalized aggressive periodontitis (Age 16-31 years, Male - 12 and Female - 8), Group B constituted 20 subjects (Age 34-58 years, Male - 13 and Female - 7) with untreated generalized chronic periodontitis and Group C constituted 20 subjects (Age 24-54 years, Male - 16 and Female - 4) who were periodontal healthy. The research protocol was ethically approved by the Ethical Committee and Review Board of the AFMC, Pune. All subjects were verbally informed and written informed consent was obtained for their participation in the study.
Subject selection
Subjects selected had at least 20 teeth and were diagnosed as either generalized chronic or generalized aggressive periodontitis according to criteria based on the 1999 consensus classification of periodontal diseases.[21] Diagnosis of generalized aggressive periodontitis was based only on the clinical findings, which included periodontal breakdown without evidence of any disease, rapid attachment loss and bone destruction, history of familial aggregation and clinical and radiographic diagnosis. Diagnosis of generalized chronic periodontitis was based on periodontal destruction, which was consistent with amount of local factors and slow progression of the disease generally seen in adult patients. Periodontally healthy patients included patients who had no clinical signs of any form of periodontal disease. Clinical measurements were taken at 6 sites per tooth (mesiobuccal, buccal, distobuccal, mesiolingual/mesiopalatal, lingual/palatal and distolingual/distopalatal) of every tooth present, except third molars, with a University of North Carolina-15 (UNC-15) probe. The clinical parameters measured were percentage of sites that bleed on probing (BOP), probing depth (PD), clinical attachment level (CAL) and Ramfjord's periodontal disease index. Percentage of bleeding was evaluated by multiplying number of sites involved with hundred and dividing the result by number of sites present. PD was measured as the distance the UNC-15 probe penetrated the depth of the gingival sulcus/periodontal pocket. CAL was measured as distance of the marginal gingival from the cementoenamel junction. Full-mouth orthopantamograph were recorded to evaluate the bone condition.
Subjects were excluded if they had any systemic condition, which would influence the course of periodontal disease or treatment and medical conditions, which would require antibiotic prophylaxis for routine dental procedures. Individuals who had taken antibiotics in the previous 3 months or pregnant or lactating women were also excluded. Care was taken to exclude patients who were smokers or those who had other abnormal habits.
The cases diagnosed as generalized aggressive periodontitis and generalized chronic periodontitis were further divided into mild cases (BOP ≥ 40%, CAL 0-3 mm > 30% sites), moderate cases (BOP ≥ 40%, CAL 3-5 mm > 30% sites) and severe cases (BOP ≥ 40%, CAL > 5 mm > 30% sites). Examination of the patients was carried out twice, 1 day apart by two different examiners. Calibration was accepted if measurements at baseline and 1 day were similar to 1 mm at the 95% level.
Sample collection
Blood (5 ml from each patient) from all the 60 subjects was collected from the antecubital vein using a vacutainer and standard aseptic precautions. The blood samples were then taken to the Department of Biochemistry, AFMC for assessing the serum levels of ceruloplasmin.
Ceruloplasmin analysis
Serum was extracted from the subjects’ blood by centrifugation. Serum ceruloplasmin levels were assessed using a new kinetic technique wherein phenylenediamine has been replaced by norfloxacin based reagent which is more stable, cheaper and physiological.[22] The norfloxacin based reagents for the detection were discovered and manufactured in the Biochemistry Department of AFMC. The ceruloplasmin detection test is based on the detection of the ferroxidase activity of ceruloplasmin and the unit of measurement used here is IU/ml. Spectroscopic analysis of the serum samples for detection of ceruloplasmin levels were carried out. The levels of serum ceruloplsmin obtained from the selected subjects were tabulated then statistically analyzed. The results were analyzed keeping in mind the normal serum ceruloplasmin level, which is less than 1000 IU/ml in any healthy individual.[22]
Experimental results are presented as mean ± standard deviation. The analysis of variance (ANOVA) was used for the analysis and comparison of results between the groups. P < 0.05 if obtained would be considered as statistically significant.
RESULTS
The periodontal parameters recorded showed that the Group A patients had BOP in 46% of sites, PD of 6.4 mm (±0.8) and CAL of 3.4 mm (±0.1). Group B patients had BOP in 68% of sites, PD of 5.6 mm (±0.7) and CAL of 2.7 mm (±0.3). Group C patients had BOP in 7% sites, PD of 2.4 mm (±0.4) and no CAL [Table 1].
Table 1.
The clinical parameters recorded of all the subjects enrolled in the study

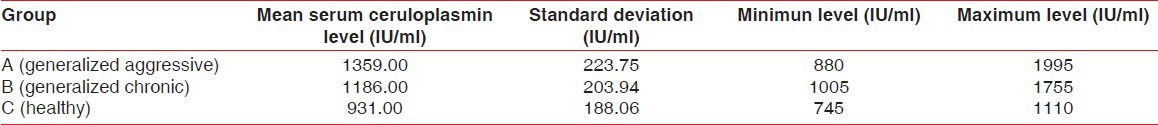
The results from the analysis of the data examining the serum levels of ceruloplasmin reveals that the patients of Group A having generalized aggressive periodontitis had a higher mean level of serum ceruloplasmin (1359 ± 223.75 IU/ml, min level-889 IU/ml, max level 1995 IU/ml) when compared to patients of Group B having chronic generalized periodontits (1186 ± 233.94 IU/ml, min level - 1005 IU/ml, max level 1755 IU/ml) and patients of Group C not having any form of periodontitis (931 ± 188.06 IU/ml, min level - 745 IU/ml, max level 1110 IU/ml) [Table 2].
Table 2.
Serum ceruloplasmin levels in all the three groups
On correlating the serum ceruloplasmin levels with the disease severity (Increase in BOP and CAL), it revealed that the levels of ceruloplasmin increased as the disease severity increased. In generalized aggressive periodontitis cases, it was seen that that the ceruloplasmin levels in mild, moderate and severe cases were 1290 ± 180.75 IU/ml, 1340.00 ± 125.00 IU/ml and 1475.00 ± 170.50 IU/ml respectively. In generalized chronic periodontitis cases, it was seen that that the ceruloplasmin levels in mild, moderate and severe cases were 1065.00 ± 75.00 IU/ml, 1190.00 ± 115.5 IU/ml and 1275.00 ± 170.00 IU/ml respectively [Table 3].
Table 3.
Correlation between serum ceruloplasmin levels and disease severity

The statistical analysis in the form of ANOVA revealed that the level of significance in between groups (927126.6 – mean square), within groups (42341.9) and the sum total were all not statistically significant (>0.05). A degree of freedom of 2 was for in between groups, 57 for within groups and 59 for the sum total [Table 4].
Table 4.
Statistical analysis using ANOVA test

DISCUSSION
The aim of the current study evaluated the serum levels of ceruloplasmin in patients with generalized aggressive as well in patients with generalized chronic peridontitis. This study is the first of its kind and there are no previous studies available comparing the level of ceruloplasmin in generalized chronic and aggressive periodontitis patients. Hence, comparing the results with any previous study is not possible.
The identification of susceptible individuals or sites at risk from disease and the diagnosis of active phases of periodontal disease, represents a challenge for both clinicians and oral health researchers.[23] It has long been established that simple and non-invasive diagnostic tools that allows rapid screening, provides accurate predictive information and enables reliable evaluation of periodontal disease status would be of great value to both dentists and patients.[24] Traditional diagnostic measures, such as periodontal pocket depth, attachment level, plaque index, bleeding on probing and radiographic assessment of alveolar bone loss,[25] are informative to evaluate disease severity, but provide few useful determinants of disease activity. Potential diagnostic biomarkers for diagnosis of periodontal disease is the latest modality and mainly includes locally produced proteins of host and bacterial origin such as enzymes, immunoglobulins, cytokines and also includes genetic/genomic biomarkers such as deoxyribonucleic acid and messenger ribonucleic acid of host origin, bacteria and bacterial products, ions, steroid hormones and volatile compounds.[26] One such potential marker recognized is the ceruloplasmin.
Ceruloplasmin is a 132-kDa pro-inflammatory marker protein with multiple copper-binding domains[27] seen to increase in systemic infections. Ceruloplasmin has the ability to create a state of hypoferraemia, which increases the natural resistance of the body to fight the disease.[28] So, it is postulated that there is an increase in the level of ceruloplasmin level during these infections. Ceruloplasmin also acts as a downstream target for hypoxia inducible factor (HIF-1α) which is created in an area of local inflammation during the infections. It is also seen to play a central role in excessive superoxide generation in phenotypically hyperactive and primed peripheral blood polymorphonuclear neutrophils (PMNs).[20] The functions of ceruloplasmin are slightly confusing. Ceruloplasmin functions an anti-inflammatory agent and it can also work as a proinflammatory molecule.[29,30]
Study by Iwata et al. has already proved that ceruloplasmin causes priming of the neutrophils in localized aggressive periodontitis.[20] Oxygen tension is generally lower in inflammed tissues. Local hypoxia as a result of this severe inflammation results in increased activation of HIF-1α. The outcome of this is that ceruloplasmin mediates iron ion conversion from ferrous to ferric form and hence down regulates this HIF-1α. Ceruloplasmin mediated conversion of ferrous ion to ferric ion increases the intracellular ferric ion content, leading to increased binding of gp91phox.[31] Thus, as previously proved PMNs can produce a faster and more response to secondary challenges and present a “primed” phenotype. In the presence of these primed PMNs, we expect a faster and severe form of tissue destruction. As proven in our study, it is this reason why the ceruloplasmin content is drastically increased in aggressive periodontitis.
The potential pathogens acquire the iron necessary for growth through mechanisms, which are extremely complex.[28] It is significant that in spite of bacterial iron acquiring mechanisms, the natural resistance to infection operates effectively in the normal low iron environment.[28,32]
Only when iron is freely available these protective mechanisms are reduced. The factors contributing for the natural resistance to infection are ample, but it is now clear that these protective systems can only function successfully in an environment where the normal concentration of free ionic iron is about 10−18 M,[33,34] which can be regarded as virtually zero. This low iron environment is due to the iron binding protein transferrin, which is normally only 30-40% saturated with iron and its presence is regulated by ceruloplasmin.[28] The ability of freely available iron to diminish or destroy normal resistance and increase bacterial virulence has been demonstrated repeatedly in experimental infections involving many different bacterial species.[35] So, it can be postulated and also proved by our study that even the periopathogenic bacteria of chronic perodontitis can also lead to an increase in the activity of ceruloplasmin in absence of any other disease leading to systemic infection.
One of the important features of this study was the use of new kinetic technique for detection of ceruloplasmin. This cheaper version of previous method for ceruloplasmin detection uses a norfloxacin based reagent and is based in on basic principle of ceruloplasmin function that of its ferroxidase activity. The norfloxacin based reagent used here is very different from the previous phenylenediamine based reagents which are more stable, cheaper and much more physiological.
As the disease severity increases, it is natural for any proinflammatory marker to increase.[26] This is the other salient feature this study has noted wherein the level of ceruloplasmin was more in periodontal disease with higher CAL. We observed that as the CAL increased corresponding to the percentage of bleeding sites, the serum level of ceruloplasmin showed higher values. The statistical results were noteworthy. Even though the results were clinically significant, they were not statistically significant. The possible reason attributable to this is the fact that the sample size selected for this study was very small. Further studies with an increase in the sample size will surely help in making it a statistically significant result.
CONCLUSION
Biomarkers have come a long way as indicators of periodontal disease. These indicators should not only depict the presence or absence of a disease but should also show some light on the severity of the disease. The findings of this study shows can guide us in using the ceruloplasmin as one such biomarker. Increased tissue damage in the locally inflammed tissues of the periodontium in peridontitis patients and increased host resistance in periodontitis are very much attributable to ceruloplasmin. Further studies with a much bigger sample size will without doubt prove our point statistically. A newer epitope can be used just to detect even a milder increase in the ceruloplasmin level just to diagnose periodontitis in its early stages. The day is not far off that this marker can be used a chair side detection kit for the diagnosis of periodontal disease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.de Queiroz AC, Taba M, Jr, O’Connell PA, da Nóbrega PB, Costa PP, Kawata VK, et al. Inflammation markers in healthy and periodontitis patients: A preliminary data screening. Braz Dent J. 2008;19:3–8. doi: 10.1590/s0103-64402008000100001. [DOI] [PubMed] [Google Scholar]
- 2.Niklaus Lang, Bartold M, Cullinan M, Jeffcoat M, Mombelli A. Consensus report. Aggressive periodontitis. Ann Periodontol. 1999;4:53. [Google Scholar]
- 3.Califano JV. Position paper: Periodontal diseases of children and adolescents. J Periodontol. 2003;74:1696–704. doi: 10.1902/jop.2003.74.11.1696. [DOI] [PubMed] [Google Scholar]
- 4.Albandar JM, Tinoco EM. Global epidemiology of periodontal diseases in children and young persons. Periodontol 2000. 2002;29:153–76. doi: 10.1034/j.1600-0757.2002.290108.x. [DOI] [PubMed] [Google Scholar]
- 5.Armitage GC, Cullinan MP. Comparison of the clinical features of chronic and aggressive periodontitis. Periodontol 2000. 2010;53:12–27. doi: 10.1111/j.1600-0757.2010.00353.x. [DOI] [PubMed] [Google Scholar]
- 6.Susin C, Oppermann RV, Haugejorden O, Albandar JM. Periodontal attachment loss attributable to cigarette smoking in an urban Brazilian population. J Clin Periodontol. 2004;31:951–8. doi: 10.1111/j.1600-051x.2004.00588.x. [DOI] [PubMed] [Google Scholar]
- 7.Genco RJ, Falkner KL, Grossi S, Dunford R, Trevisan M. Validity of self-reported measures for surveillance of periodontal disease in two western New York population-based studies. J Periodontol. 2007;78:1439–54. doi: 10.1902/jop.2007.060435. [DOI] [PubMed] [Google Scholar]
- 8.Holtfreter B, Schwahn C, Biffar R, Kocher T. Epidemiology of periodontal diseases in the study of health in pomerania. J Clin Periodontol. 2009;36:114–23. doi: 10.1111/j.1600-051X.2008.01361.x. [DOI] [PubMed] [Google Scholar]
- 9.O’Connel PA. Effects of periodontal therapy on glycemic control and inflammatory markers. J Periodontol. 2008;79:774–83. doi: 10.1902/jop.2008.070250. [DOI] [PubMed] [Google Scholar]
- 10.Perinetti G, Paolantonio M, Femminella B, Serra E, Spoto G. Gingival crevicular fluid alkaline phosphatase activity reflects periodontal healing/recurrent inflammation phases in chronic periodontitis patients. J Periodontol. 2008;79:1200–7. doi: 10.1902/jop.2008.070519. [DOI] [PubMed] [Google Scholar]
- 11.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391–40. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 12.Tsai CC, Ho YP, Chen CC. Levels of interleukin-1 beta and interleukin-8 in gingival crevicular fluids in adult periodontitis. J Periodontol. 1995;66:852–9. doi: 10.1902/jop.1995.66.10.852. [DOI] [PubMed] [Google Scholar]
- 13.Peng Li L, Ataullah K. Periodontitis and C-reactive protein as a cardiovascular risk factor a causal relationship? Hong Kong Dent J. 2008;5:100–1009. [Google Scholar]
- 14.Prapulla DV, Sujatha PB, Pradeep AR. Gingival crevicular fluid VEGF levels in periodontal health and disease. J Periodontol. 2007;78:1783–7. doi: 10.1902/jop.2007.070009. [DOI] [PubMed] [Google Scholar]
- 15.Lappin DF, MacLeod CP, Kerr A, Mitchell T, Kinane DF. Anti-inflammatory cytokine IL-10 and T cell cytokine profile in periodontitis granulation tissue. Clin Exp Immunol. 2001;123:294–300. doi: 10.1046/j.1365-2249.2001.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segelmark M, Persson B, Hellmark T, Wieslander J. Binding and inhibition of meyleoperoxidase (MPO). A major function of ceruloplasmin? Clin Expo Immunol. 1997;108:167–74. doi: 10.1046/j.1365-2249.1997.d01-992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris ZL, Takahashi Y, Miyajima H, Serizawa M, MacGillivray RT, Gitlin JD. Aceruloplasminemia: Molecular characterization of this disorder of iron metabolism. Proc Natl Acad Sci U S A. 1995;92:2539–43. doi: 10.1073/pnas.92.7.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein IM, Kaplan HB, Edelson HS, Weissmann G. Ceruloplasmin: An acute phase reactant that scavenges oxygen-derived free radicals. Ann N Y Acad Sci. 1982;389:368–79. doi: 10.1111/j.1749-6632.1982.tb22150.x. [DOI] [PubMed] [Google Scholar]
- 19.Martin F, Linden T, Katschinski DM, Oehme F, Flamme I, Mukhopadhyay CK, et al. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: Implications for ceruloplasmin regulation. Blood. 2005;105:4613–9. doi: 10.1182/blood-2004-10-3980. [DOI] [PubMed] [Google Scholar]
- 20.Iwata T, Kantarci A, Yagi M, Jackson T, Hasturk H, Kurihara H, et al. Ceruloplasmin induces polymorphonuclear leukocyte priming in localized aggressive periodontitis. J Periodontol. 2009;80:1300–6. doi: 10.1902/jop.2009.090092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Somani BL, Ambade V. A kinetic method amenable to automation for ceruloplasmin estimation with inexpensive and stable reagents. Clin Biochem. 2007;40:571–4. doi: 10.1016/j.clinbiochem.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Kamma JJ, Giannopoulou C, Vasdekis VG, Mombelli A. Cytokine profile in gingival crevicular fluid of aggressive periodontitis: Influence of smoking and stress. J Clin Periodontol. 2004;31:894–902. doi: 10.1111/j.1600-051X.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- 24.Xiang X, Sowa MG, Iacopino AM, Maev RG, Hewko MD, Man A, et al. An update on novel non-invasive approaches for periodontal diagnosis. J Periodontol. 2010;81:186–98. doi: 10.1902/jop.2009.090419. [DOI] [PubMed] [Google Scholar]
- 25.Armitage GC. The complete periodontal examination. Periodontol 2000. 2004;34:22–33. doi: 10.1046/j.0906-6713.2002.003422.x. [DOI] [PubMed] [Google Scholar]
- 26.Taba M, Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am. 2005;49:551–71. doi: 10.1016/j.cden.2005.03.009. vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox PL, Mukhopadhyay C, Ehrenwald E. Structure, oxidant activity, and cardiovascular mechanisms of human ceruloplasmin. Life Sci. 1995;56:1749–58. doi: 10.1016/0024-3205(95)00146-w. [DOI] [PubMed] [Google Scholar]
- 28.Bullen JJ, Rogers HJ, Spalding PB, Ward CG. Iron and infection: The heart of the matter. FEMS Immunol Med Microbiol. 2005;43:325–30. doi: 10.1016/j.femsim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Broadley C, Hoover RL. Ceruloplasmin reduces the adhesion and scavenges superoxide during the interaction of activated polymorphonuclear leukocytes with endothelial cells. Am J Pathol. 1989;135:647–55. [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KH, Yun SJ, Nam KN, Gho YS, Lee EH. Activation of microglial cells by ceruloplasmin. Brain Res. 2007;1171:1–8. doi: 10.1016/j.brainres.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 31.Roman DG, Dancis A, Anderson GJ, Klausner RD. The fission yeast ferric reductase gene frp1 + is required for ferric iron uptake and encodes a protein that is homologous to the gp91-phox subunit of the human NADPH phagocyte oxidoreductase. Mol Cell Biol. 1993;13:4342–50. doi: 10.1128/mcb.13.7.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bullen JJ, Rogers HJ, Spalding PB, Ward CG. Natural resistance, iron and infection: A challenge for clinical medicine. J Med Microbiol. 2006;55:251–8. doi: 10.1099/jmm.0.46386-0. [DOI] [PubMed] [Google Scholar]
- 33.Bullen JJ, Rogers HJ, Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- 34.Elin RJ, Wolff SM. The role of iron in nonspecific resistance to infection induced by endotoxin. J Immunol. 1974;112:737–45. [PubMed] [Google Scholar]
- 35.Griffiths E. Iron and infection: Molecular, physiological and clinical aspects. Chichester: Wiley; 1999. Iron in biological systems; pp. 1–26. [Google Scholar]


