Abstract
Background:
Platelet-rich fibrin (PRF), an intimate assembly of cytokines, glycan chains, and structural glycoproteins enmeshed within a slowly polymerized fibrin network, has the potential to accelerate soft and hard tissue healing. The purpose of the study was to clinically evaluate and compare the efficacy of autologous PRF combined with demineralized freeze-dried bone allograft (DFDBA) to DFDBA alone in the treatment of periodontal intrabony defects.
Materials and Methods:
In a split mouth study design, 10 patients having two almost identical intrabony defects with clinical probing depth of at least 6 mm were selected for the study. Selected sites were randomly divided into two groups. In Group I, mucoperiosteal flap elevation followed by the placement of DFDBA was done. In Group II, mucoperiosteal flap elevation followed by the placement of homogeneous mixture of PRF with DFDBA was done. Clinical and radiographic parameters were recorded at baseline and at 6 months post-operatively.
Results:
Both treatment groups showed a significant probing pocket depth reduction, clinical attachment gain, defect fill, and defect resolution 6 months after surgery compared to baseline. However, there was a significantly greater probing pocket depth reduction and clinical attachment gain when PRF was added to DFDBA.
Conclusion:
Within limits of the study it may be concluded that a combination of PRF with DFDBA demonstrated better results in probing pocket depth reduction and clinical attachment level gain as compared to DFDBA alone in the treatment of periodontal intrabony defects.
Keywords: Demineralized freeze dried bone allograft, growth factor, platelet rich fibrin, re-generation
INTRODUCTION
Regeneration of lost structures has become the primary therapeutic goal in periodontics. The objectives of periodontal regenerative therapy are to reconstitute the bone, cementum, and periodontal ligament on a previously diseased root surface. Numerous therapeutic modalities for restoring periodontal osseous defects have been investigated.[1] Many of these procedures include the use of bone grafts and bone replacement materials.[2] For the last few decades, demineralized freeze-dried bone allograft (DFDBA) has been used alone and in combination with other treatment modalities for periodontal regeneration. The presence of bone morphogenetic proteins contained within DFDBA aids in mesenchymal cell migration, attachment, and osteogenesis. DFDBA has both osteoinductive and osteoconductive activity and the ability to create and maintain the space.[3,4]
A different approach to periodontal regeneration is the use of polypeptide growth factors. These factors, abundant in alpha granules of platelets, may control the growth of cells and hence, the number of cells available to produce a tissue. Platelet rich plasma (PRP) is an autologous concentration of platelets in plasma.[5,6] PRP has been used to enhance the clinical outcome obtained by using bone grafts with and without guided tissue regeneration in the treatment of intrabony defects.[7] However, there are potential risks associated with the use of PRP.
Platelet-rich fibrin (PRF), a second generation platelet concentrate has been introduced by Choukroun et al. in 2001 that has several advantages over PRP.[8] Choukroun's PRF, a second generation platelet concentrate is an autologous leukocyte and PRF material. It requires neither anticoagulant nor bovine thrombin. This is produced in a totally natural manner, without using anticoagulant during blood harvest nor bovine thrombin and calcium chloride for platelet activation and fibrin polymerization. It has eliminated the redundant process of adding anticoagulant as well as the need to neutralize it. The absence of anticoagulant implies the activation in a few minutes of most platelets of the blood sample in contact with the tube walls and release of the coagulation cascades. The protocol is very simple and of low cost. PRF is a matrix of autologous fibrin, in which are embedded intrinsically a large quantity of platelet and leukocyte cytokines during centrifugation leading to their progressive release over time (7-11 days), as the network of fibrin disintegrates.[8,9]
Therefore, the aim of the present study was to clinically compare autologous PRF combined with DFDBA to DFDBA alone in the treatment of periodontal intrabony defects.
MATERIALS AND METHODS
In this 6 month follow-up interventional study, a total of 10 systemically healthy patients undergoing periodontal therapy at the Department of Periodontology, Govt. Dental College and Hospital, Patiala (Punjab) were selected for the study. Intraoral periapical radiographs (IOPAs) were taken to confirm the presence of suitable bony defects for the selection of subjects.
The inclusion criteria were the presence of two almost identical interproximal intrabony defects, one on either side of arch based on radiographic observations with clinical probing depth of at least 6 mm in teeth without furcation involvement.
Patients with any systemic disease that contraindicate periodontal surgery, patients having insufficient platelet count for PRF preparation, patients with coagulation defect or anticoagulation treatment, pregnant or lactating mothers, a smoker or an alcoholic patient, those with unacceptable oral hygiene after the re-evaluation of phase I therapy were excluded from the study. In addition, teeth with endodontic involvement, or Miller grade II or greater mobility were also excluded.
It was made clear to the potential subjects that participation was voluntary. Written informed consent was obtained from those who agreed to participate.
A general assessment of selected subjects was made through their history, clinical examination and routine investigations. All subjects were treated with the initial phase I therapy involving oral hygiene instructions, scaling and root planning. Following phase I therapy the subjects were re-evaluated after 6 weeks, and those who still satisfied the selection criteria were finally taken up for the study.
Selected sites were randomly divided into two groups. In Group I, mucoperiosteal flap elevation followed by the placement of DFDBA was done. In Group II, mucoperiosteal flap elevation followed by the placement of homogeneous mixture of PRF with DFDBA was done. Probing pocket depth, gingival marginal position and clinical attachment level of the selected sites were recorded using customized acrylic occlusal stents at baseline before surgery and again recorded at 3 months and at 6 months post-operatively. Site-specific plaque index and sulcus bleeding index were recorded at baseline and again at 3 months, at 6 months post-operatively. For measuring radiographic parameters, IOPAs of each defect was exposed at baseline before surgery and again recorded at 6 months post-operatively using extension cone paralleling instrument (paralleling technique) using 1 × 1 mm calibrated grid. Radiographic measurements were taken using a vernier caliper to achieve accuracy of 1/10 mm in measurement. The measurements included the distance from cemento enamel junction to the base of defect, distance from cemento enamel junction to the alveolar crest and the distance from the alveolar crest to the base of the defect [Figures 1 and 2].
Figure 1.
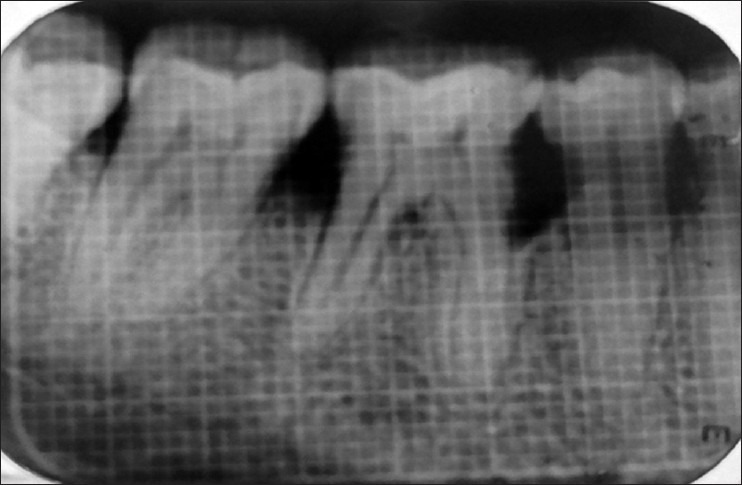
Intraoral peri-apical radiograph at baseline (Group I)
Figure 2.
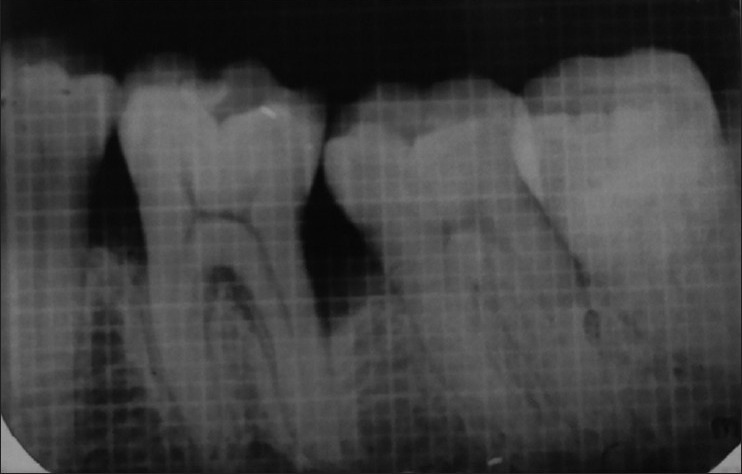
Intraoral peri-apical radiograph at baseline (Group II)
Using above measurements, following parameters were calculated as:
Defect fill: It was calculated as the difference between the values of the distance from the cemento enamel junction to the base of defect at 6 months and baseline.
Alveolar crest resorption: It was calculated as the difference between the values of the distance from the cemento enamel junction to the alveolar crest at 6 months and baseline.
Defect resolution: It was calculated as the difference between the values of the distance from the alveolar crest to the base of the defect at 6 months and baseline.
After taking informed consent of the patient, a 10 ml blood sample of the patient without anticoagulant was taken in a test tube and centrifuged immediately at 3000 rpm for 10 min.
The resultant product consisted of the following three layers
Top most layer of acellular platelet poor plasma
PRF clot in the middle
Red blood cells at the bottom.
The platelet rich fibrin clot was recovered and was cut in few millimeter fragments and mixed with DFDBA to get a homogeneous mass.
All patients were operated under local anesthesia with a solution of 2% lignocaine with 1:200,000 adrenaline. Anesthetic solution was administered by either nerve block or local infiltration to adequately anesthetize the surgical site. The patients were then subjected to periodontal flap surgery.
Periodontal flap surgery was done by giving sulcular incision and a full thickness mucoperiosteal flap was reflected both on facial and lingual side. Thorough debridement and root planning of the exposed root surface was performed by hand instrumentation. In Group I, the rehydrated DFDBA was then carried to the defect and carefully packed into the defect. In Group II, after proper debridement [Figure 3], autologous PRF was prepared. PRF clots were recovered and were cut in few millimeter fragments and mixed with DFDBA to get a homogeneous mass. The grafted mixture was properly condensed in the defect to the level of surroundings bony walls [Figure 4]. The flap was adapted back to its original position and suturing was done using non-resorbable silk thread (3-0) [Figure 5]. The surgical site was dried using gauze and Coe-pak was then applied.
Figure 3.
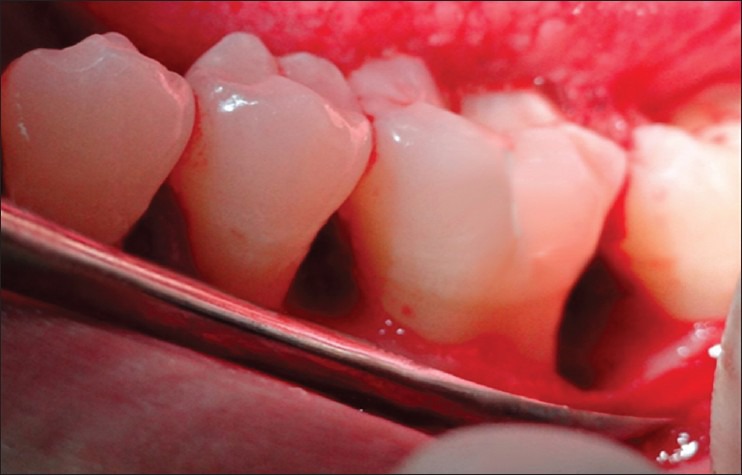
Intrabony defect after flap reflection
Figure 4.
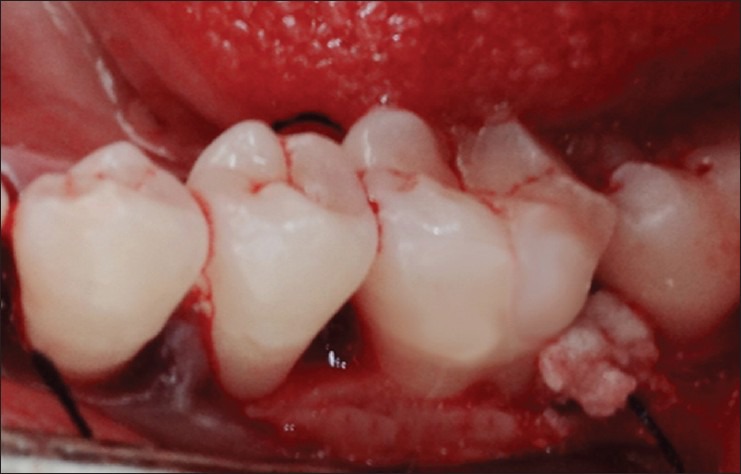
Mixture of platelet-rich fibrin and demineralized freeze-dried bone allograft placed in the intrabony defect
Figure 5.
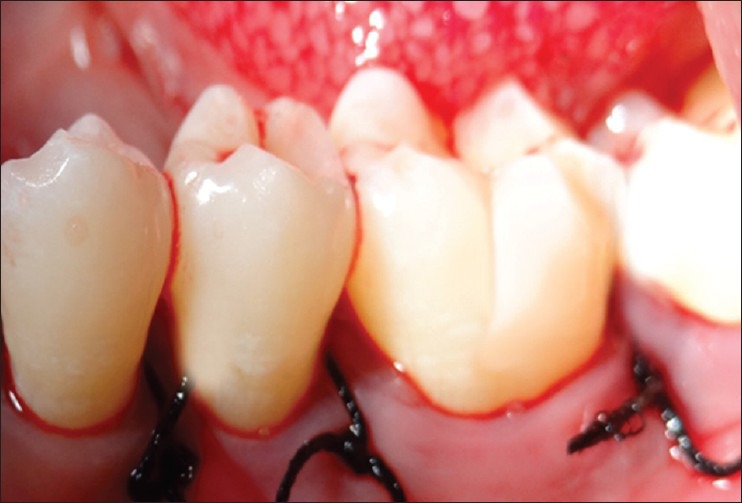
Sutures placed
Amoxycillin 500 mg thrice a day was prescribed for 5 days. Ibuprofen 400 mg thrice daily and vitamin-B complex, 1 capsule daily was also prescribed for 5 days. The subjects were instructed not to brush the operated area for 1 week and to rinse the oral cavity twice a day with Chlorhexidine (0.12%) mouthwash daily. Periodontal dressing and sutures were removed after 10 days post-operatively.
Clinical parameters were again recorded at 3 months and at 6 months. Radiographic parameters were again recorded at 6 months [Figures 6 and 7]. Clinical attachment level was the primary outcome of the study. Other parameters have been taken as the secondary outcomes.
Figure 6.
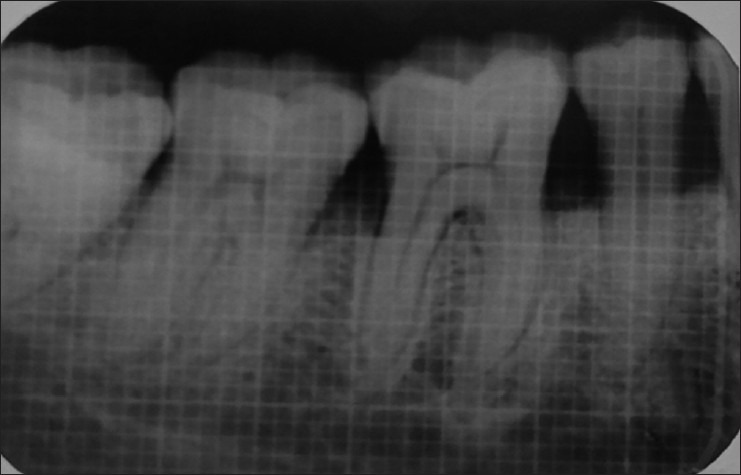
Intraoral peri-apical radiograph at 6 months (Group I)
Figure 7.
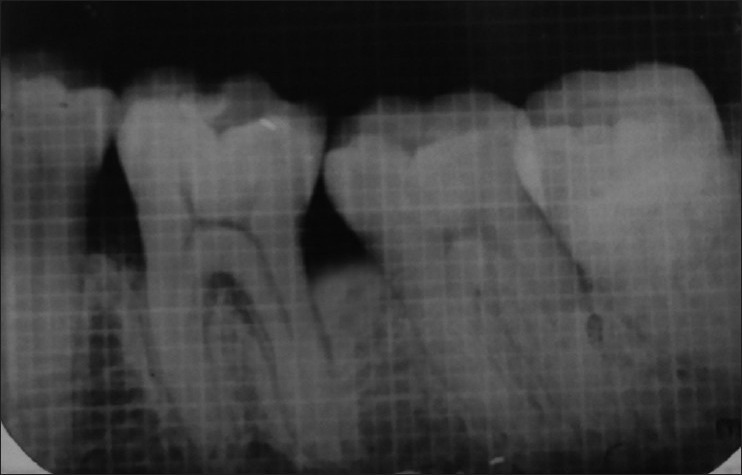
Intraoral peri-apical radiograph at 6 months (Group II)
The data thus collected was compiled, tabulated and statistically analysed to arrive at the results.
Statistical analysis
The results were averaged (mean ± SD) for each clinical and radiographic parameter at each time interval. The difference between each pair of measurements was calculated (baseline-6 months). The paired t-test was applied to assess the statistical significance between time intervals within each group for the clinical and radiographic parameters.
RESULTS
Power of the study was calculated based on comparing means of our two study groups; with a mean difference of clinical attachment level of 0.7 and SD of 0.57. Power of the study came out to be 80% at a confidence interval of 95% with sample size of 10 in each group.
There was statistically significant probing pocket depth reduction and clinical attachment level gain found in both groups. On comparison, Group II showed statistically significant more probing pocket depth reduction and more clinical attachment level gain than Group I at all-time intervals [Tables 1 and 2]. Statistically non-significant change in gingival marginal position at all-time intervals was found on both intragroup and intergroup comparison [Table 3]. Reductions in the plaque index score and sulcus bleeding index score were observed in both groups, but they were statistically non-significant. On comparison, the difference between the two groups was also statistically non-significant at all-time intervals [Tables 4 and 5]. There was statistically significant mean defect fill and mean defect resolution observed in both groups from baseline to 6 months. On comparison, the difference in mean defect fill and mean defect resolution between the two groups was statistically non-significant [Tables 6 and 7]. Slight alveolar crest resorption was observed in both groups from baseline to 6 months, but it was statistically non-significant. On comparison, the difference in alveolar crest resorption between the two groups was statistically non-significant [Table 8].
Table 1.
Comparison of mean probing pocket depth reduction (in millimeters) between Group I and II at different time intervals
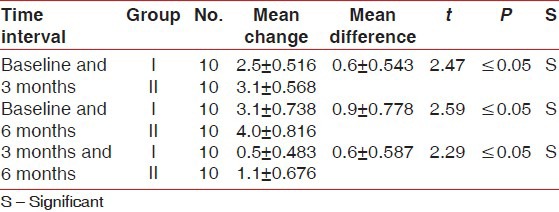
Table 2.
Comparison of mean clinical attachment level gain (in millimeters) between Group I and II at different time intervals
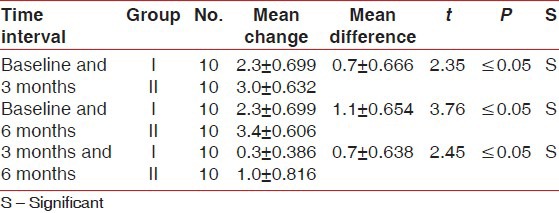
Table 3.
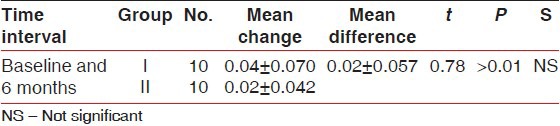
Comparison of mean change in gingival marginal position from fixed reference point of stent (in millimeters) between Group I and II at different time intervals
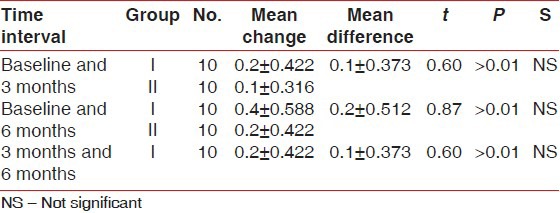
Table 4.
Comparison of mean change in plaque index score between Group I and II at different time intervals
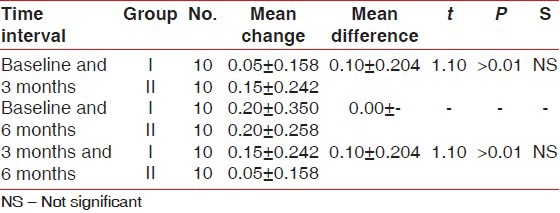
Table 5.
Comparison of mean change in sulcus bleeding index score between Group I and II at different time intervals
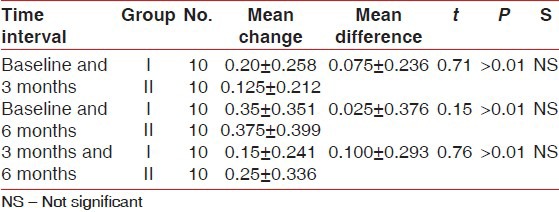
Table 6.
Comparison of mean defect fill (in millimeters) between Group I and II

Table 7.
Comparison of mean defect resolution (in millimeters) between Group I and II

Table 8.
Comparison of mean alveolar crest resorption (in millimeters) between Group I and II
DISCUSSION
Periodontal disease is among the most prevalent diseases worldwide and is characterized by the presence of gingival inflammation, periodontal pocket formation, loss of periodontal attachment and loss of alveolar bone around the affected teeth.[10,11] The goal of periodontal therapy includes not only the arrest of periodontal disease progression, but also the regeneration of structures lost due to disease.[12] Bone grafting is one of the most common forms of regenerative therapy and is usually essential for restoring periodontal supporting tissue.[11] A wide range of bone grafting materials, including bone grafts and bone graft substitutes, have been applied and evaluated clinically, including autografts, allografts, xenografts, and alloplasts (synthetic/semisynthetic materials).[13] DFDBA is widely used in periodontal therapy and has been demonstrated to be safe and capable of inducing new bone formation. DFDBA is shown to be both osteoconductive and osteoinductive. Urist et al. showed through numerous animal experiments that DFDBA could stimulate the formation of new bone by osteoinduction.[14] That is, the graft material induces host undifferentiated mesenchymal cells to differentiate into osteoblasts with subsequent formation of new bone.[11] Moreover, DFDBA also provides a scaffold for osteoconduction.[3] DFDBAs have repeatedly demonstrated significant improvements in soft and hard tissue clinical parameters for the treatment of intraosseous periodontal defects.[15]
Recently, the use of growth factors in periodontal regeneration has shown promising results.[12] Growth factors are a class of natural biologic mediators that regulate key cellular events in tissue regeneration including cell proliferation, chemotaxis, differentiation, and matrix synthesis via binding to specific cell surface receptors.[7] Platelet alpha (α) granules form an intracellular storage pool of growth factors including platelet-derived growth factor, transforming growth factor β (including β-1 and β-2-isomers), vascular endothelial growth factor, and epidermal growth factor and insulin-like growth factor-1.[8]
A combination of PRF with DFDBA demonstrated better results in probing pocket depth reduction and clinical attachment level gain as compared to DFDBA alone in the treatment of periodontal intrabony defects. This result may be attributed to beneficial effects of PRF. PRF has been introduced by Choukroun et al. in 2001. PRF consists of a fibrin matrix polymerised in a tetramolecular structure; the incorporation of platelets, leukocytes, and cytokines; and circulating stem cells. Slow fibrin polymerization during PRF processing leads to the intrinsic incorporation of platelet cytokines and glycan chains in the fibrin meshes. This result implies that PRF, unlike the other platelet concentrates, is able to progressively release cytokines during fibrin matrix remodeling.[16] It is also found that PRF organizes as a dense fibrin scaffold with a high number of leukocytes concentrated in one part of the clot. Leukocytes seem to have a strong influence on growth factor release, immune regulation, anti-infectious activity, and matrix remodeling during healing. It is an optimal matrix for migration of endothelial cells and fibroblasts. It permits a rapid angiogenesis and an easier remodeling of fibrin in a more resistant connective tissue. Such a mechanism might explain the clinically observed soft tissue healing properties of PRF.[17] However, histological studies are needed to establish the exact nature of this clinical attachment gain.
In our study, PRF does not seem to have any additional favorable effect on defect fill and defect resolution in the treatment of periodontal intrabony defects. However, various in vitro studies have shown a beneficial effect of PRF on bone healing like its effect on proliferation and differentiation on osteoblasts (Ehrenfest et al. 2009, He et al. 2009).[18,19] When mixed with the graft, PRF fragments serve as a biological connector between bone particles. Moreover, the gradual release of cytokines plays a significant role in the self-regulation of inflammatory and infectious phenomena within the grafted material.[20]
Thus, in the present study PRF with DFDBA demonstrated better results in probing pocket depth reduction and clinical attachment level gain as compared to DFDBA alone in the treatment of periodontal intrabony defects. This result may be attributed to beneficial effects of PRF. However, histological studies are needed to establish the exact nature of this clinical attachment gain. PRF does not seem to have any additional favorable effect on defect fill and defect resolution. However, various in vitro studies have shown a beneficial effect of PRF on bone healing.
Within limits of the study, it can be concluded that future long-term studies with a large sample size and utilization of advanced radiological techniques should be carried out to further explore the role of PRF in the management of periodontal intrabony defects and to verify the results of in vitro studies in a clinical study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bender SA, Rogalski JB, Mills MP, Arnold RM, Cochran DL, Mellonig JT. Evaluation of demineralized bone matrix paste and putty in periodontal intraosseous defects. J Periodontol. 2005;76:768–77. doi: 10.1902/jop.2005.76.5.768. [DOI] [PubMed] [Google Scholar]
- 2.Froum S, Cho SC, Rosenberg E, Rohrer M, Tarnow D. Histological comparison of healing extraction sockets implanted with bioactive glass or demineralized freeze-dried bone allograft: A pilot study. J Periodontol. 2002;73:94–102. doi: 10.1902/jop.2002.73.1.94. [DOI] [PubMed] [Google Scholar]
- 3.Gajiwala AL, Kumar BD, Chokhani P. Evaluation of demineralised, freeze-dried, irradiated bone allografts in the treatment of osseous defects in the oral cavity. Cell Tissue Bank. 2007;8:23–30. doi: 10.1007/s10561-006-9014-z. [DOI] [PubMed] [Google Scholar]
- 4.Kimble KM, Eber RM, Soehren S, Shyr Y, Wang HL. Treatment of gingival recession using a collagen membrane with or without the use of demineralized freeze-dried bone allograft for space maintenance. J Periodontol. 2004;75:210–20. doi: 10.1902/jop.2004.75.2.210. [DOI] [PubMed] [Google Scholar]
- 5.Sunitha J, Manjunath K. A combination of platelet rich plasma and hydroxyapatite (osteogen) bone graft in the treatment of intrabony defects-A case report: A preliminary study. Clin Diagnc Res. 2010;4:2984–8. [Google Scholar]
- 6.Ouyang XY, Qiao J. Effect of platelet-rich plasma in the treatment of periodontal intrabony defects in humans. Chin Med J (Engl) 2006;119:1511–21. [PubMed] [Google Scholar]
- 7.de Obarrio JJ, Araúz-Dutari JI, Chamberlain TM, Croston A. The use of autologous growth factors in periodontal surgical therapy: Platelet gel biotechnology: Case reports. Int J Periodontics Restorative Dent. 2000;20:486–97. [PubMed] [Google Scholar]
- 8.Toffler M, Toscano N, Holtzcaw D, Corso MD, Ehrenfest DM. Introducing Choukroun's PRF to the reconstructive surgery millieu. The Journal of Implant and Advanced Clinical Dentistry. 2009;1:21–32. [Google Scholar]
- 9.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Newman MG, Takei H, Klokkveold PR, Carranza FA, Klokkevold PR, Takei HH, Newman MG. 10th ed. St Louis: Saunders, Elsevier; 2006. Carranza's Clinical Periodontology; p. 494. [Google Scholar]
- 11.Rosenberg E, Rose LF. Biologic and clinical considerations for autografts and allografts in periodontal regeneration therapy. Dent Clin North Am. 1998;42:467–90. [PubMed] [Google Scholar]
- 12.Kanakamedala A, Ari G, Sudhakar U, Vijaylakhsmi R, Ramakrishnan T, Emmadi P. Treatment of a furcation defect with combination of PRF and bone grafts-a case report. ENDO (Lond Engl) 2009;3:127–35. [Google Scholar]
- 13.Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL. Regeneration of periodontal tissue: Bone replacement grafts. Dent Clin North Am. 2010;54:55–71. doi: 10.1016/j.cden.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Urist MR. Bone formation by auto induction. Science. 1965;150:893. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 15.Piemontese M, Aspriello SD, Rubini C, Ferrante L, Procaccini M. Treatment of periodontal intrabony defects with demineralized freeze-dried bone allograft in combination with platelet-rich plasma: A comparative clinical trial. J Periodontol. 2008;79:802–10. doi: 10.1902/jop.2008.070436. [DOI] [PubMed] [Google Scholar]
- 16.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Sharma A, Pradeep AR. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: A randomized clinical trial. J Periodontol. 2011;82:1396–403. doi: 10.1902/jop.2011.100731. [DOI] [PubMed] [Google Scholar]
- 18.Ehrenfest DMD, Diss A, Odin G, Doglioli P, Hippolyte MP, Charrier JB. In vitro effects of Choukroun's PRF on human gingival fibroblasts, dental prekeratinocytes, preadipocytes, and maxillofacial osteobalasts in primay cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:341–352. doi: 10.1016/j.tripleo.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 19.He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of PRF and PRP on effect of proliferation and differentiation at rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:707–13. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 20.Simonpieri A, Del Corso M, Sammartino G, Dohan Ehrenfest DM. The relevance of Choukroun's platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. Part I: A new grafting protocol. Implant Dent. 2009;18:102–11. doi: 10.1097/ID.0b013e318198cf00. [DOI] [PubMed] [Google Scholar]


