Abstract
Background
Black women are less likely to be evaluated and treated for anginal symptoms, despite a higher premature cardiac mortality rate compared to white women. Our objective was to compare angina symptoms in black versus white women regarding (1) angina symptoms characterization; (2) relationship with obstructive coronary artery disease (CAD); and (3) relationship with subsequent mortality.
Methods
A cohort of 466 women (69 black and 397 white) undergoing coronary angiography for suspected ischemia and without prior history of CAD completed symptom checklists. Four symptom clusters (CHEST, UPPER, STOMACH, and TYPICAL TRIGGERS) were derived by factor analysis. All angiograms were analyzed by core lab. Mortality data over 10 years were obtained from National Death Index.
Results
(1) Black women had lower mean CHEST cluster scores (0.60±0.30 vs. 0.73±30, p=0.002), but higher STOMACH scores (0.41±0.25 vs. 0.30±0.25, p=0.011) than white women. (2) Prevalence and severity of CAD did not differ in black and white women and was not predicted by symptom cluster scores. (3) All-cause mortality rates were 24.9% in blacks versus 14.5% in whites, p=0.007; and cardiovascular mortality 22.5% vs.8.8%, p=0.001. Symptom clusters were not predictive of adverse events in white women. However, black women with a low TYPICAL score had significantly higher mortality compared to those with a high TYPICAL score (43% vs. 10%, p=0.006).
Conclusions
Among women undergoing coronary angiography, black women report fewer chest-related and more stomach-related symptoms, regardless of presence or severity of CAD, and these racial symptom presentation differences are linked with the more adverse prognosis observed in the black women. Atypical symptom presentation may be a barrier to appropriate and timely diagnosis and treatment and contribute to poorer outcomes for black women.
Introduction
Advances in cardiovascular (CV) therapies have led to significant declines in death rates for men. Similar declines have not been realized for women. Among middle-aged women, mortality has increased by 1.3% per year since 1997.1 Even less progress has been reported among black compared to white women.1–4 Studies comparing treatments and outcomes between black and white patients with acute coronary syndromes (ACS)2–4 or suspected coronary artery disease (CAD) support the presence of racial disparities in referrals to catheterization and revascularization procedures and preventive therapies.5–7 National health agencies have emphasized the goal of reducing racial minority disparities in health.8
The importance of addressing racial/ethnic disparities in health and health care is a national goal and clinical cultural competence is an important part of the greater framework to improve care for all Americans and eliminate disparities.9 Proposed explanatory factors for racial disparities include biological differences, cultural differences, differential access to health care, provider bias, and patient preferences and beliefs. One poorly understood factor is potential differences in the quality of chest pain. Chest pain is a major symptom driving triage decisions and treatments for CAD patients. Often women present with chest pain symptoms that differ in type, frequency and quality from “typical” anginal symptoms defined in primarily male populations.10–13 We hypothesized that black and white women undergoing coronary angiography would differ in the way they reported their symptoms.
We compared black and white women regarding: (1) characterization of anginal symptoms, (2) relationship between anginal symptoms and the presence of angiographically confirmed obstructive CAD; and (3) relationship between anginal symptoms and adverse outcomes, including all-cause and CV mortality and myocardial infarction (MI), heart failure (HF), or stroke.
Materials and Methods
Study population
Participants were enrolled in the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) study, designed to improve the understanding of the pathophysiology of myocardial ischemia in women with and without obstructive CAD and to improve diagnostic testing for CAD in a large sample of women undergoing clinically-indicated coronary angiography.14 Institutional review board approval was obtained from four participating sites (Birmingham, Alabama; Gainesville, Florida; and two hospitals in Pittsburgh, Pennsylvania). Prior to enrollment, participants provided written informed consent for baseline and follow-up testing. Women were eligible for inclusion if they were older than 18 years and were undergoing physician-referred coronary angiography for suspected CAD. Women were excluded if they were pregnant, had a history of cardiomyopathy or congenital heart disease, or a recent history of MI or revascularization procedures. In order to complete the study questionnaires women had to be able to read and speak English.14
Due to concerns that a prior CAD diagnosis could bias the symptom reports of participants, we excluded 291 women with a known history of CAD from the full WISE sample of 936. Seven women who were neither black nor white were excluded also. Of the remaining 645 women, 466 completed a symptom history questionnaire (added to the WISE baseline protocol several months after initiation of WISE baseline testing) and comprised the sample for these analyses.
Baseline evaluation
Upon enrollment, demographic information, complete medical and reproductive histories, a physical exam, and core lab blood assays were collected. Functional status was assessed using the Duke Activity Status Index.15 Chronic environmental stress, previously shown to be associated with an increased risk for CAD16 was sampled at study entry by a single 5-point question that has been demonstrated to be predictive of future adverse cardiac events in patients with CAD. 17 Because atypical chest pain descriptors have been reported specifically in Southern blacks, 18 we included a regional (north versus south) variable. The complete WISE design and methodology have been described elsewhere.14
Symptom characteristics and anginal classification
At baseline and follow-up (6 weeks and annually), all women completed a brief symptom questionnaire to assess the presence of typical angina (defined as symptoms that were substernal, precipitated by emotional stress or exercise, and relieved within 10 minutes by rest or nitroglycerine).19 At baseline women completed a symptom checklist developed by WISE investigators to assess symptoms over the past 12 months. Questions included: 23 symptom type items (e.g., chest tightness, nausea, weakness/fatigue/faintness); 14 symptom locations (e.g., chest, throat, stomach); 9 symptom triggers (e.g., upper body exertion, strong emotions); 10 symptom relievers (e.g., rest, nitroglycerin); and 11 symptom descriptions (e.g. numbness, sharpness). Symptom frequency was rated from 1 to 9 times per day, week, month, or year. Symptom intensity was scored on a 5-point ordinal scale with 1 denoting tolerable, no relief needed to 5 denoting not tolerable, not relieved with usual measures. Symptom duration was rated as less than 1 minute, 1–5 minutes, 5–15 minutes, 15–30 minutes, 30–60 minutes, and more than 60 minutes.”
Forty percent of the women (32% black and 42% white) completed the symptom questionnaire prior to or on the same day as angiography. Those administered on the same day were usually scheduled before the patient entered the heart catheterization lab.
Quantitative angiographic assessment of CAD
All coronary angiograms performed at enrollment were analyzed quantitatively and qualitatively at the WISE angiographic core laboratory (Rhode Island Hospital) by investigators blinded to all other clinical data. The presence of obstructive CAD was defined as ≥50% stenosis in ≥1 major epicardial coronary artery. An angiographic CAD severity index was calculated based on stenosis severity weighted by proximal lesion.20 Interobserver variability for this lab was 0.196 mm with a 6.3% coefficient of variation.20
Follow-up procedures
Telephone or mail follow-up at six weeks and annually thereafter was conducted by experienced study clinicians using a scripted interview. It included queries regarding health status, hospitalizations, medications, diagnostic or revascularization procedures, and CV events since the last contact. In the event of death, a death certificate and/or physician narrative was obtained. Follow-up information was collected for 459 women for a median of 6.1 years.
We also conducted a National Death Index search for all women who were still alive at last contact. This increased the sample to 466 women and extended the follow up period for mortality (only) to a median of 9.3 years. All deaths were adjudicated as CV or non-CV by WISE investigators blinded to angiographic findings. In addition to individual events (MI, HF, stroke, death, and CV death), we created two composite adverse outcomes: (1) a major event was defined as a nonfatal MI, HF, stroke or death due to all causes; and (2) a CV event was defined as a nonfatal MI, HF, stroke, or death due to CV causes.
Statistical methods
Demographic and clinical data are reported as means±standard deviations for continuous variables or as percentages for dichotomous variables. All p-values comparing data in black versus white women were age adjusted using logistic or linear regression. For variables with skewed distributions, we present medians (interquartile ranges) and used their log transformations when calculating age-adjusted p-values.
To identify symptom types, 67 symptom descriptor variables from the WISE Symptom History checklist and baseline clinical history form (including diabetes, hypertension, age, etc.) were entered into an exploratory factor analysis, using orthogonal and oblique rotations. Exploratory factor analysis is a mathematical model that reduces a large set of observed variables (i.e., symptom descriptors) to a smaller set of “latent” variables (factors or clusters). Schwartz's criterion21 was used to determine the optimal number of clusters, which in this analysis was four. For each cluster, a symptom cluster score was derived for each woman by adding the standardized scoring coefficients for the symptom characteristics that loaded highly (>0.40) on that factor and that the woman had checked.
Multivariate linear regression analyses were performed to identify variables that were independently associated with each of the four symptom cluster scores. Previous information has solely been reported on men, which led us to explore a broad range of variables for women (Table 1).22,23,24 For each symptom cluster score, modeling was conducted in several steps: (1) forward stepwise regression of the symptom cluster score on all variables in Table 1 to obtain a preliminary model; (2) variables that did not enter the preliminary model were forced, one at a time, into the model and retained if p <0.05 or if they modulated the major effects in the model; and (3) if race did not enter significantly, it was forced into the model.
Table 1.
Baseline Characteristics by Race
| Combined (n=466) | Black (n=69) | White (n=397) | Age-adjusted p* | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 57±11 | 54±11 | 57±11 | 0.029 |
| High school education or more (%) | 83 | 74 | 85 | 0.013 |
| Body size | ||||
| Waist circumference (inches) | 36.3±6.6 | 38.2±6.1 | 35.9±6.6 | 0.009 |
| BMI (kg/M2) | 29.6±6.5 | 31.3±5.6 | 29.3±6.6 | 0.036 |
| Risk factors | ||||
| HX diabetes (%) | 18 | 36 | 15 | <0.0001 |
| HX hypertension (%) | 53 | 78 | 49 | <0.0001 |
| Presence of ≥1 comorbidities (%) | 34 | 49 | 32 | 0.003 |
| No. of comorbidities (mean±SD) | 0.39±0.62 | 0.61±0.73 | 0.35±0.59 | 0.009 |
| Blood pressure measures | ||||
| Systolic BP (mmHg) | 136±20 | 145±22 | 135±20 | <.0001 |
| Diastolic BP (mmHg) | 77±10 | 80±11 | 77±10 | 0.009 |
| Lab values | ||||
| Total cholesterol (mg/dL) | 197±44 | 185±39 | 200±45 | 0.019 |
| Triglycerides (mg/dL) (median[interquartile range])† | 119 (79, 180) | 83 (53, 124) | 100 (86, 147) | <0.0001 |
| Triglycerides/HDL ratio (medians) | 2.2 (1.4, 3.6) | 1.5 (0.9, 2.5) | 2.4 (1.5, 3.8) | <0.0001 |
| Non-HDL cholesterol (mg/dL) | 143±44 | 129±38 | 146±44 | 0.004 |
| Fasting blood glucose (mg/dL) (medians) | 95 (84, 116) | 100 (86, 147) | 95 (84, 112) | 0.003 |
| Insulin (μIU/mL), (medians) | 7.6 (4.3, 12.1) | 11.2 (6.2, 17.1) | 7.1 (4.1, 11.5) | 0.006 |
| eGFR (mL/minute) | 98±35 | 97±38 | 99±34 | 0.08 |
| Hemoglobin (g/dL) | 13.1±1.3 | 12.3±1.1 | 13.2±1.2 | <0.0001 |
| HOMA (medians) | 2.1 (1.0, 3.2) | 2.9 (1.9, 5.5) | 1.9 (1.0, 3.0) | 0.001 |
| Recent medications | ||||
| Anti-HTN meds (%) | 42 | 65 | 38 | <0.0001 |
| Lipid lowering meds (%) | 21 | 28 | 20 | 0.059 |
| Other risk factors | ||||
| No. of live births | 2.8±1.8 | 3.3±2.6 | 2.7±1.6 | 0.003 |
| Functional capacity (DASI) | 22.7±15.4 | 17.9±14.3 | 23.5±15.4 | 0.004 |
| Angiographic findings | ||||
| CAD (50% stenosis†) (%) | 22 | 26 | 21 | 0.18 |
| CAD severity score | 10.6±10.8 | 10.2±11.6 | 10.6±10.6 | 0.87 |
| Residential location | ||||
| South (Alabama, Florida) (%) | 51 | 87 | 45 | <0.0001 |
| Symptom history | ||||
| Typical angina (%) | 31 | 27 | 31 | 0.46 |
| Symptom frequency almost 1/day or more (%) | 37 | 30 | 38 | 0.14 |
| Symptom severity at worst >3 (%) | 63 | 64 | 63 | 0.93 |
| Number of symptom types checked (mean) | 10.2±4.6 | 11.3±4.9 | 10.2±4.6 | 0.070 |
| Number of symptom types checked ≥12 (%) | 36 | 48 | 34 | 0.03 |
Nonsignificant risk factors and their age-adjusted p-values: post-menopausal status, waist to hip ratio (0.27); low-density lipoprotein cholesterol (LDL-C) (0.15); high-density lipoprotein cholesterol (HDL-C) (0.08); family history of coronary artery disease (CAD) (0.31); history of dyslipidemia (0.45); ever smoking (0.52), current smoker (0.19); self-reported stress level (0.10), ever hormone replacement therapy (HRT) user (0.52); current HRT user (0.20); other medication user.
Means±standard deviations (SD), percentages (%), or medians and interquartile ranges for highly skewed distributions.
Comorbidities included diabetes, chronic obstructive pulmonary disease, chronic renal dysfunction, congestive heart failure (HF), autoimmune disease, anorexia nervosa, alcoholism (≥25 drinks/week), other (included HIV, ulcerative colitis, myasthenia gravis, Parkinson's disease, Sheehan's syndrome, multiple sclerosis, Hashimoto disease, and Wilson's disease).
Except for age, all p-values were age-adjusted using linear regression for continuous variables (e.g., BMI) and logistic regression for dichotomous variables (e.g., history of diabetes).
BMI, body mass index; BP, blood pressure; DASI, Duke activity status index; eGFR, estimated glomerular filtration rate; HOMA, homeostatic model assessment: HOMA-IR=[(Glucose×Insulin)/22.5]; glucose in molar units mmol/L; HTN, hypertension; HX, history of.
A two-way analysis of covariance was conducted to estimate joint effect of race (black versus white) and obstructive CAD (presence versus absence) on each of the four symptom cluster scores while adjusting for covariates identified in the regression modeling. A race by CAD interaction term was evaluated.
Adverse event rates were estimated using Kaplan-Meier methods. Following the same modeling procedure, we used stepwise Cox proportional hazard regression analyses to select independent covariates of adverse events. All analyses were performed using the SAS 9.2 software. Statistical significance was set at p<0.05.
Results
Baseline demographics and CAD risk factors
The mean age of the 466 women was 57 (21–85) years and 13% were <45 years old; 22% had obstructive CAD. Sixty-nine women (15%) were black. Although black women were significantly younger (p=0.03), they had more CAD risk factors than white women (Table 1), including more obesity; higher rates of diabetes; hypertension; higher levels of blood glucose, insulin, and creatinine; and lower levels of hemoglobin and functional capacity. Blood pressure was higher in black women despite an almost double use of hypertension medications. White women had more dyslipidemia, particularly elevated triglycerides, than did black women. Despite these differences, there were no significant racial differences in the prevalence or severity of obstructive CAD (Table1). As described in the Methods, women with prior history of CAD were excluded from the analyses. The women not included in this analysis were older, had more prevalence and severity of CAD, and had higher rates and severity of CAD risk factors including diabetes, dyslipidemia, and less functional capacity.
Factor structure of symptoms
Table 2 presents four symptom cluster scores generated by factor analysis. Together, they explained 62% of the total symptom variance. The following symptom clusters were identified and named: (1) UPPER, explaining 34% of the total variance and ranging from 0 to 1.3, loaded highly on upper body symptoms, including arm, hand, shoulder, neck, back, and jaw; (2) CHEST, explaining 12% of the total variance and ranging from 0 to 1.04, loaded highly on chest symptoms and general malaise, including chest discomfort, pressure, tightness, fatigue, and shortness of breath; (3) STOMACH, explaining 9% of the total variance and ranging from 0 to 1.09, loaded highly on abdominal symptoms, including indigestion, esophagus, throat, abdomen; and (4) TYPICAL, explaining 7% of the total variance and ranging from 1 to 1.24, loaded highly on angina triggers and relievers, including triggers of exertion and emotion, and relief by resting or stopping the activity. Chronbach's alphas for the UPPER, CHEST, STOMACH, and TYPICAL symptom clusters were 0.82, 0.76, 0.73, and 0.76, respectively.
Table 2.
Symptom Domains Derived by Varimax Rotation and Corresponding Symptom Variables
| Symptom description | Factor loading | Standardized scoring coefficients |
|---|---|---|
| Factor 1: upper body, no chest | ||
| Arm location | 0.65 | 0.18 |
| Hand location | 0.61 | 0.17 |
| Arm or shoulder pain | 0.60 | 0.18 |
| Shoulder location | 0.58 | 0.13 |
| Numbness, tingling in arm or hand | 0.57 | 0.14 |
| Neck location | 0.51 | 0.12 |
| Neck pain | 0.48 | 0.10 |
| Numbness | 0.48 | 0.10 |
| Back location | 0.42 | 0.08 |
| Jaw location | 0.42 | 0.10 |
| Factor 2: chest, general malaise | ||
| Chest pressure | 0.62 | 0.22 |
| Chest tightness | 0.57 | 0.17 |
| Pressure | 0.51 | 0.13 |
| Weakness, fatigue, faintness | 0.50 | 0.14 |
| Shortness of breath | 0.47 | 0.10 |
| Tightness | 0.45 | 0.10 |
| Feel lousy, generally blah | 0.43 | 0.11 |
| Chest discomfort | 0.40 | 0.07 |
| Factor 3: abdominal discomfort | ||
| Heart burn, indigestion | 0.52 | 0.15 |
| Esophagus location | 0.52 | 0.15 |
| During or after meals | 0.51 | 0.14 |
| Description: indigestion | 0.51 | 0.15 |
| Throat location | 0.50 | 0.14 |
| Stomach location | 0.50 | 0.13 |
| Description: burning | 0.49 | 0.12 |
| Abdominal pain | 0.44 | 0.11 |
| Factor 4: typical triggers/relievers | ||
| Lower body exertion trigger | 0.63 | 0.22 |
| Stops when stopping activity | 0.59 | 0.20 |
| Whole body exertion trigger | 0.57 | 0.19 |
| Relieved by rest | 0.50 | 0.14 |
| Exertion or emotional stress trigger | 0.49 | 0.15 |
| Pain goes away with rest | 0.49 | 0.15 |
| Strong emotions, stress trigger | 0.41 | 0.10 |
| Upper body exertion trigger | 0.40 | 0.09 |
Symptom cluster scores in black versus white women
Black women had lower mean CHEST cluster scores (p=0.002), but higher STOMACH scores (p=0.011) than white women (Table 3). UPPER and TYPICAL cluster scores did not differ between black and white women and remained consistent in multivariate modeling. White race remained strongly predictive of higher CHEST cluster scores (beta=0.14, standard error [SE] 0.04, p=0.0006) in a model that included younger age (p<0.0001), low functional capacity (p<0.0001), high self-reported stress (p=0.009), higher number of live births (p=0.002), ever hormone therapy use (p=0.015), and residing in the South (Alabama or Florida vs. Pittsburgh) (p=0.002). Similarly, black race remained predictive of higher STOMACH scores (beta=–0.10, SE 0.03, p=0.003) in a model that included high self-reported stress (p=0.008) and body mass index (p=0.032) (models not shown). The other variables (diabetes, hypertension, age, etc) from Table 1 were not independent predictors of symptom clusters and did not act as effect modifiers on the relationships between race and symptom clusters. Race continued not to be predictive of the UPPER and TYPICAL symptom clusters in multivariate modeling.
Table 3.
Symptom Cluster Scores in Black and White Women
| Symptom cluster | Black (n=69) | White (n=397) | p |
|---|---|---|---|
| UPPER | 0.61±0.38 | 0.54±0.38 | 0.16 |
| CHEST | 0.60±0.30 | 0.73±0.30 | 0.002 |
| STOMACH | 0.41±0.25 | 0.30±0.25 | 0.011 |
| TYPICAL | 0.64±0.35 | 0.64±0.36 | 0.95 |
The association of the four symptom clusters with obstructive CAD in black versus white women
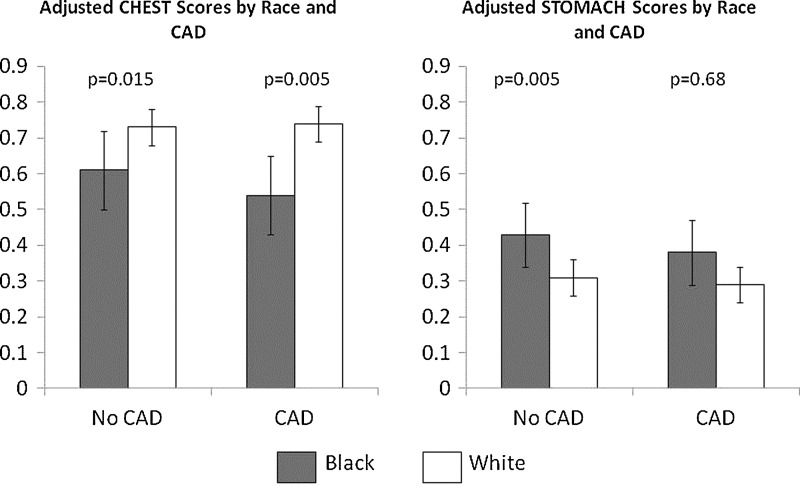
When forced into the multivariate regression models, neither presence nor severity of obstructive CAD was significantly correlated with any of the symptom cluster scores. Similarly, race by CAD interaction terms were not significant (p-values from 0.14 to 0.69). Figure 1 gives the adjusted CHEST and STOMACH scores stratified by race and presence vs. absence of obstructive CAD and adjusted for the independent predictors identified in the multivariate modeling. The higher STOMACH scores and lower CHEST scores remained evident in black women regardless of CAD status, while presence versus absence of CAD did not differ across the races.
FIG. 1.
Mean adjusted CHEST and STOMACH scores stratified by race (black vs. white) and presence vs. absence of obstructive CAD. The “error bars” represent standard deviations. Means were adjusted by significant correlates of CHEST and STOMACH scores. Means for CHEST scores were adjusted by age, functional capacity, self-reported stress, number of live births, ever hormone therapy use and location (south vs. north). Means for STOMACH scores were adjusted by self-reported stress and BMI. CAD, coronory artery disease; BMI, body mass index.
Noting that women tended to check a large number of symptom types and locations on the symptom history checklist, we counted the number of symptom types that were checked by each woman (data not shown). Out of a possible 23 symptom types, we evaluated the percent of women who checked ≥12 (>50% of available) symptom types. Overall, 48% of black women and 34% of white women checked ≥12 symptom types (p=0.030). Although there was no difference between blacks and whites without CAD (p=0.15), black women with CAD were twice as likely as whites to check a large number and variety of symptoms (50% vs. 26%, p=0.043).
Symptom cluster scores as predictors of adverse events
Kaplan-Meier estimated event rates are given in Table 4. Among 459 women with a median of 6.1 years follow-up for nonfatal events, 2.5% experienced an MI, 6.1% HF, and 5.6% stroke. Black women had double the number of nonfatal events compared to whites; this difference was significant for HF (p=0.045) and stroke (p=0.028). Similarly, among women with a median of 9.3 years of follow-up for death, blacks had double the mortality rates of whites for all-cause (p=0.007) and CV (p=0.001) mortality. Over 1 out of 4 black women had a major adverse event (including mortality), compared to 1 out of 7 white women. In the black women, 87% of the deaths versus 68% in white women were due to CV-related causes. Because major non-fatal events were tracked for a median of only 6.1, the rates of the composite major and CV events are an under-estimate.
Table 4.
Adverse Events in Black and White Women: Kaplan-Meier Estimated Rates
| Event | Observation time (years) | n (%) with event overall | n (%) with event black | n (%) with event white | p (black vs. white) |
|---|---|---|---|---|---|
| Nonfatal events (median=6.1 years of follow-up)* | n=459 | n=69 | n=390 | ||
| MI | 6.1 | 2.5% | 3.6% | 2.8% | 0.66 |
| HF | 6.1 | 6.1% | 10.8% | 5.4% | 0.045 |
| Stroke | 6.1 | 5.6% | 11.1% | 4.6% | 0.028 |
| Fatal and nonfatal events (median=9.3 years of follow-up) | n=466 | n=69 | n=397 | ||
| All-cause mortality | 9.3 | 11.7% | 19.9% | 10.2% | 0.007 |
| CV mortality | 9.3 | 8.4% | 17.4% | 6.9% | 0.001 |
| Major event† | 9.3 | 18.7% | 29.0% | 16.9% | 0.007 |
| CV event‡ | 9.3 | 16.1% | 27.8% | 14.1% | 0.002 |
These are not mutually exclusive.
A major event was defined as non-fatal myocardial infarction (MI), HF, stroke, or death due to all causes.
A cardiovascular (CV) event was defined as nonfatal MI, HF, stroke, or death due to CV causes.
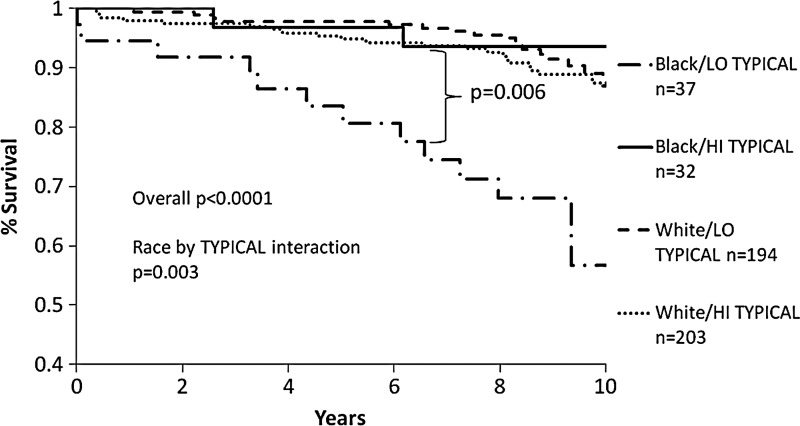
To determine whether symptom clusters predicted adverse outcomes differentially by race, we performed separate multivariate Cox proportional hazards regressions, one for each symptom cluster, and fit race-by-symptom cluster interaction terms. After adjusting for predictors of adverse events (diabetes, total cholesterol, history of hypertension, and CAD severity score), only the TYPICAL-by-race interaction term reached statistical significance for predicting all-cause mortality (p=0.003). When adjusting for location (South vs. North), southern location was an independent predictor of worse major outcomes (p=0.0006). Even with that term in the model, the TYPICAL-by-race interaction term remained a statistically significant predictor of major outcomes (p=0.008). The differential effect of the TYPICAL cluster score on all-cause mortality stratified by race and high versus low TYPICAL symptoms is shown in Fig. 2. High versus low TYPICAL cluster scores were based on a median split cut point of 0.71. Compared to white women with low TYPICAL scores, black women with low TYPICAL scores had significantly higher mortality (57% survival rate over 9.3 years). In contrast, all white women and black women with high TYPICAL trigger scores had about 90% survival over 9.3 years.
FIG. 2.
Kaplan-Meier curves of % survival from major events among 4 subgroups stratified by race (black vs. white) and low vs. high TYPICAL symptom presentation scores (median split of 0. 71). Major events are defined as all-cause mortality, CV mortality, MI, HF and stroke.
Discussion
Using factor analysis to identify symptom clusters in a well-characterized population of women with suspected CAD, we found important differences in symptom presentation among black versus white women. Black women were more likely to report stomach symptoms and less likely to report chest symptoms than white women. These differences persisted regardless of the presence of obstructive CAD, which occurred about equally, and its severity, which was similar, in black and white women. Black women had double the rates of both mortality and non-fatal adverse events over 6 and 9 years of follow-up. Although, chest, upper body and stomach symptoms were not predictive of adverse events, a low score on the TYPICAL symptom cluster predicted downstream mortality in black but not white women. This association was independent of major risk factors, suggesting a novel pathway contributing to the adverse prognosis experienced in black women.
Our results are consistent with and expand observations made by Klinger et al.5 who reported that black post MI patients were more likely to attribute their symptoms to the stomach (p=0.05), and white patients were more likely to attribute symptoms to the heart (p=0.05)5 Our results extend this observation to actual symptom reporting and its relationship to obstructive CAD and adverse outcomes.
We also found differences in the quantity of symptoms. Our finding that black women with CAD checked a greater number and variety of symptoms than white women with CAD are consistent with other studies.25,26 These differences may be explained in part by cultural differences in symptom expression, as the majority of the black women in this cohort were from the south. Studies have documented that regional differences may affect the description and experience of pain, which may result in misleading portrayals of CAD in southern U.S. populations.8 Whether these are semantic or physiological distinctions is less important than the need for cultural sensitivity on the part of treating clinicians.
Atypical symptom presentation in black women may be a barrier to appropriate clinical diagnostic and treatment regimens. Consistent with our findings, Brieger et al.9 compared treatments and outcomes of patients without chest pain to those with chest pain and found that patients lacking chest pain received less effective care and had worse outcomes. Prior data indicate that black women do not receive care in accordance with best practice guidelines as often as white women.8,21,27 Their atypical presenting symptomology may be key to this problem. Our findings may help clinicians to either raise or lower their index of suspicion based on the symptom clusters their patients endorse. Knowing which symptom clusters are less likely to be associated with CAD is as important as knowing which symptoms are. Our findings inform clinicians that atypical symptoms of CAD are common in women, and even more so in black women. In addition, there is compelling evidence that signs and symptoms of ischemia in the absence of obstructive disease can be due to abnormal microvascular coronary flow reserve and macrovascular endothelial dysfunction. Prior WISE work demonstrates that women with persistent chest pain but no obstructive disease have relatively high rates of adverse CVD outcomes.28 These results suggest that practitioners should carefully evaluate women presenting with symptoms, especially black women who have a heavier burden of risk factors and warrant aggressive risk factor modification. Raising awareness among black women and health care providers may increase early diagnosis/treatment and decrease delay-related consequences in black women.
These findings suggest that black women themselves may not recognize atypical symptoms as cardiac-related, leading to treatment-seeking delays and lack of optimal treatment. 13,26,29,30 Public campaigns to date have focused on typical chest pain presentations8,10 and significantly lower rates of awareness of heart disease risks have been documented in black women than white women.31–33
Limitations
This study included women undergoing coronary angiography for suspected CAD; our results are limited to this population and may not be more broadly generalizable. Although we over-sampled black women and have a higher proportion compared to the U.S. Census, our subsample of black women was relatively small compared with white women. Nonetheless, our findings of racial differences suggest that further study should target this area with the goal of reducing racial disparities, particularly among black women who face the most adverse CAD risk.
Conclusions
Among women undergoing coronary angiography, black women report fewer chest-related and more stomach-related symptoms, regardless of presence or severity of CAD. These racial symptom presentation differences are linked with more adverse prognoses in black women. These results suggest that atypical symptom presentation may be a barrier to appropriate and timely diagnosis and treatment and contribute to poorer outcomes for black women. However although typical symptoms and mortality rates were not high in white women, providers still need to have a high index of suspicion for CAD presentations in ALL women.
Future research should evaluate whether symptom clusters by race are related to differential utilization of diagnostic and treatment strategies and quality of life. Additional race and symptom research is needed to determine the influence of culture on symptom presentation and to develop and test interventions aimed at raising awareness of symptom presentation, especially in black women, among healthcare providers and the general population.
Acknowledgments
This work was supported by contracts from the National Heart, Lung, and Blood Institutes, Nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, and N01-HV-68164; grants U0164829, U01HL649141, U01HL649241, T32HL69751, and 1R03AG032631 from the National Institute on Aging; General Clinical Research Center (GCRC) grant MO1-RR00425 from the National Center for Research Resources; and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, New Jersey; The Women's Guild of Cedars-Sinai Medical Center, Los Angeles, California; The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania; and QMED, Inc., Laurence Harbor, New Jersey, as well as the Edythe L. Broad Women's Heart Research Fellowship, Cedars-Sinai Medical Center; the University of California Los Angeles School of Nursing; and the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars-Sinai Medical Center.
All authors have contributed to the development of this manuscript in important ways, including study design, data collection, participating in multiple internal drafts for editorial purposes, group discussion of findings, and data analysis. The paper has received approval for publication by the WISE P&P committee and the participating authors.
Disclosure Statement
Dr. C. Noel Bairey Merz has the following potential conflicts of interest: Honorarium and consulting paid to Cedars-Sinai Medical Center; Amarin; Allegheny Hospital; Brigham and Women's Hospital; Bristol-Myers Squibb; Cardiometabolic Congress; CV Institute of San Diego; Christian Health System; El Camino Hospital; Expert Exchange; Gilead; Mayo Foundation; Montefiore Medical Center; Medscape; National Institutes of Health; National Heart, Lung and Blood Institute; Pozen; SCS Healthcare; Society for Women's Health Research; Slocum-Dickson; and Women's Health Congress. Honorarium and consulting paid to Dr. C. Noel Bairey Merz: Abbott Vascular, Los Robles Medical Center, and University of Pennsylvania.
The other listed authors have no financial disclosures or conflicts of interest with the findings in this paper.
The first author takes full responsibility for the accuracy of the statistical results and all authors had access to the WISE data.
References
- 1.Ford ES. Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: Concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 2.Barnhart J. Bernstein SJ. Is coronary angiography underused in an inner-city population? Ethn Dis. 2006;16:659–665. [PubMed] [Google Scholar]
- 3.Hravnak M. Whittle J. Kelley ME, et al. Symptom expression in coronary heart disease and revascularization recommendations for black and white patients. Am J Public Health. 2007;97:1701–1708. doi: 10.2105/AJPH.2005.084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendricks AS. Goodman B. Stein JH. Carnes M. Gender differences in acute myocardial infarction: the University of Wisconsin experience. WMJ. 1999;98:30–33. , 36. [PubMed] [Google Scholar]
- 5.Klingler D. Green-Weir R. Nerenz D, et al. Perceptions of chest pain differ by race. Am Heart J. 2002;144:51–59. doi: 10.1067/mhj.2002.122169. [DOI] [PubMed] [Google Scholar]
- 6.Vaccarino V. Rathore SS. Wenger NK, et al. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005;353:671–682. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin MH. Walters AE. Cook SC. Huang ES. Interventions to reduce racial and ethnic disparities in health care. Med Care Res Rev. 2007;64(5 Suppl):7S–28S. doi: 10.1177/1077558707305413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. JAMA. 2002;94:666–668. [PMC free article] [PubMed] [Google Scholar]
- 9.Canto JG. Kiefe CI. Rogers WJ, et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011;306:2120–2127. doi: 10.1001/jama.2011.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canto JG. Goldberg RJ. Hand MM, et al. Symptom presentation of women with acute coronary syndromes: Myth vs. reality. Arch Intern Med. 2007;167:2405–2413. doi: 10.1001/archinte.167.22.2405. [DOI] [PubMed] [Google Scholar]
- 11.DeVon HA. Zerwic JJ. Symptoms of acute coronary syndromes: Are there gender differences? A review of the literature. Heart Lung. 2002;31:235–245. doi: 10.1067/mhl.2002.126105. [DOI] [PubMed] [Google Scholar]
- 12.Fiebach NH. Viscoli CM. Horwitz RI. Differences between women and men in survival after myocardial infarction. Biology or methodology? JAMA. 1990;263:1092–1096. [PubMed] [Google Scholar]
- 13.Canto AJ. Kiefe CI. Goldberg RJ. a. Differences in symptom presentation and hospital mortality according to type of acute myocardial infarction. Am Heart J. 2012;163:572–579. doi: 10.1016/j.ahj.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Merz CN. Kelsey SF. Pepine CJ, et al. The Women's Ischemia Syndrome Evaluation (WISE) study: Protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–1461. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 15.Hlatky MA. Boineau RE. Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 16.Strike PC. Steptoe A. Psychosocial factors in the development of coronary artery disease. Prog Cardiovasc Dis. 2004;46:337–347. doi: 10.1016/j.pcad.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Rosengren A. Tibblin G. Wilhelmsen L. Self-perceived psychological stress and incidence of coronary artery disease in middle-aged men. Ame J Cardiol. 1991;68:1171–1175. doi: 10.1016/0002-9149(91)90189-r. [DOI] [PubMed] [Google Scholar]
- 18.Summers RL. Cooper GJ. Carlton FB. Andrews ME. Kolb JC. Prevalence of atypical chest pain descriptions in a population from the southern United States. Am J Med Sci. 1999;318:142–145. doi: 10.1097/00000441-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Diamond GA. Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–1358. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 20.Sharaf BL. Pepine CJ. Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory) Am J Cardiol. 2001;87:937–941. doi: 10.1016/s0002-9149(01)01424-2. , A933. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz GE. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 22.Lo MY. Bonthala N. Holper EM. Banks K. Murphy SA. McGuire DK. de Lemos JA. Khera A. A risk score for predicting coronary artery disease in women with angina pectoris and abnormal stress test finding. Am J Cardiol. 2013;111:781–785. doi: 10.1016/j.amjcard.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 23.Olson MB. Kelsey SF. Matthews K. Shaw L. Sharaf BL. Pohost G. Cornell CE. McGorray S. Vido D. Bairey Merz C. Symptoms, myocardial ischaemia and quality of life in women: results from the NHLBI-sponsored WISE Study. Eur Heart J. 2003;24:1506–1514. doi: 10.1016/s0195-668x(03)00279-3. [DOI] [PubMed] [Google Scholar]
- 24.Brezinka V. Kittel F. Psychosocial factors of coronary heart disease in women: a review. Soc Sci Med. 1996;42:1351–1365. doi: 10.1016/0277-9536(95)00284-7. [DOI] [PubMed] [Google Scholar]
- 25.Maynard C. Beshansky JR. Griffith JL. Selker HP. Causes of chest pain and symptoms suggestive of acute cardiac ischemia in African-American patients presenting to the emergency department: a multicenter study. J Natl Med Assoc. 1997;89:665–671. [PMC free article] [PubMed] [Google Scholar]
- 26.McSweeney JC. O'Sullivan P. Cleves MA, et al. Racial differences in women's prodromal and acute symptoms of myocardial infarction. Am J Crit Care. 2010;19:63–73. doi: 10.4037/ajcc2010372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gopalakrishnan P. Ragland MM. Tak T. Gender differences in coronary artery disease: review of diagnostic challenges and current treatment. Postgrad Med. 2009;121:60–68. doi: 10.3810/pgm.2009.03.1977. [DOI] [PubMed] [Google Scholar]
- 28.Johnson BD. Shaw LJ. Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women's Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27:1408–1415. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 29.Canto JG. Rogers WJ. Goldberg RJ, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307:813–822. doi: 10.1001/jama.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canto JG. Kiefe CI. Rogers WJ, et al. Atherosclerotic risk factors and their association with hospital mortality among patients with first myocardial infarction (from the National Registry of Myocardial Infarction) Am J Cardiol. 2012;110:1256–1261. doi: 10.1016/j.amjcard.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosca L. Mochari-Greenberger H. Dolor RJ. Newby LK. Robb KJ. Twelve-year follow-up of American women's awareness of cardiovascular disease risk and barriers to heart health. Circ Cardiovasc Qual Outcomes. 2010;3:120–127. doi: 10.1161/CIRCOUTCOMES.109.915538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roger VL. Go AS. Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferris A. Robertson RM. Fabunmi R. Mosca L. American Heart Association and American Stroke Association national survey of stroke risk awareness among women. Circulation. 2005;111:1321–1326. doi: 10.1161/01.CIR.0000157745.46344.A1. [DOI] [PubMed] [Google Scholar]