Abstract
Duchenne muscular dystrophy (DMD) typically occurs as a result of truncating mutations in the DMD gene that result in a lack of expression of the dystrophin protein in muscle fibers. Various therapies under development are directed toward restoring dystrophin expression at the subsarcolemmal membrane, including gene transfer. In a trial of intramuscular adeno-associated virus (AAV)-mediated delivery of a therapeutic minidystrophin construct, we identified in two of six subjects the presence of a population of T cells that had been primed to recognize dystrophin epitopes before transgene delivery. As the presence of preexisting T cell immunity may have a significant effect on the success of therapeutic approaches for restoring dystrophin, we sought to determine the prevalence of such immunity within a DMD cohort from our Muscular Dystrophy Association clinic. Dystrophin-specific T cell immunity was evaluated in subjects with DMD who were either receiving the glucocorticoid steroid prednisone (n=24) or deflazacort (n=29), or who were not receiving steroids (n=17), as well as from normal age-matched control subjects (n=21). We demonstrate that increasing age correlates with an increased risk for the presence of anti-dystrophin T cell immunity, and that treatment with either corticosteroid decreases risk compared with no treatment, suggesting that steroid therapy in part may derive some of its benefit through modulation of T cell responses. The frequency of dystrophin-specific T cells detected by enzyme-linked immunospot assay was lower in subjects treated with deflazacort versus prednisone, despite similar overall corticosteroid exposure, suggesting that the effects of the two corticosteroids may not be identical in patients with DMD. T cells targeted epitopes upstream and downstream of the dystrophin gene mutation and involved the CD4+ helper and/or CD8+ cytotoxic subsets. Our data confirm the presence of preexisting circulating T cell immunity to dystrophin in a sizable proportion of patients with DMD, and emphasize the need to consider this in the design and interpretation of clinical gene therapy trials.
Flanigan and colleagues characterize the prevalence of preexisting dystrophin-specific T cells in Duchenne muscular dystrophy (DMD) patients. They identify CD4+ and CD8+ T cell populations targeting epitopes upstream and downstream of dystrophin mutations in a significant fraction of patients. They further demonstrate a lower frequency of dystrophin-specific T cells in patients receiving glucocorticoid therapy. These findings suggest important considerations for future DMD gene therapy trials and offer new insight into the mechanism of glucocorticoid therapy for DMD.
Introduction
Duchenne muscular dystrophy (DMD) is a severe and progressive X-linked degenerative muscle disorder in which truncating mutations in the DMD gene result in the absence of expression of the dystrophin protein in skeletal and cardiac muscle. As a result, myofiber degeneration occurs, with subsequent accelerated rounds of myofiber regeneration and degeneration leading to myofiber loss and replacement of contractile fibers with fibrosis and fat. Clinically, symptoms typically present as gait disturbance between ages 3 and 5 years, with loss of ambulation by age 12. Thereafter, cardiac and respiratory symptoms eventually predominate, and historically these have typically led to death by the end of the second decade. The only therapies that have been shown to alter this course are the use of the glucocorticoid corticosteroids prednisone and deflazacort. Although the specific mechanisms of actions of these drugs in DMD are unknown, their use results in improvement in motor function, and may increase ambulation up to 1–3 years (Manzur et al., 2008).
Clinical trials in DMD have brought to the fore the issue of circulating T cell responses to the dystrophin protein. In a trial of gentamicin as a nonsense suppression therapy (Malik et al., 2010), we identified a T cell response in one patient that was directed toward an epitope downstream of the nonsense mutation, suggesting that therapeutic expression may result in the acquisition of T cell immunity in patients without prior expression. Nevertheless, the idea of T cells being primed by an exogenous transgene was unexpected and not previously reported until a trial of intramuscular adeno-associated virus (AAV)-mediated delivery of a therapeutic minidystrophin. In that clinical gene therapy trial we identified a subject in whom T cell responses developed that were directed toward epitopes encoded by exons present in the transgene but absent in the patient's genomic DNA (patient 5 in Mendell et al., 2010). In fact, in two of six subjects we identified a population of T cells that had not only been primed to recognize dystrophin epitopes before transgene delivery, but were directed toward epitopes encoded by exons that were not deleted at the patients' DMD locus (Mendell et al., 2010). In one of these (patient 2 in that report) the response consisted of CD4+ cells, whereas in the other (patient 4) it was mediated by CD8+ cells. It had been postulated that such priming was unlikely, due to the presence of revertant fibers in many patients (estimated, using different degrees of sensitivity in the analysis, as occurring between 0.01 and 47% of patients with DMD [Kissel et al., 1991; Fanin et al., 1992, 1995; Klein et al., 1992; Thanh et al., 1995; Uchino et al., 1995; Winnard et al., 1995; Arechavala-Gomeza et al., 2010]). In these fibers, dystrophin protein is expressed because of secondary mutations within the individual muscle fibers—most commonly, altered splicing that restores the reading frame and allows translation of exons downstream of the primary mutation. Such expression had been generally expected to result in tolerization of the immune system to dystrophin gene replacement. In addition, the possibility that such T cell responses play a role in disease progression, perhaps by engendering inflammatory responses against revertant fibers, could not be excluded.
The prevalence of such preexisting anti-dystrophin responses is unknown. A high prevalence would have obvious implications for the design of clinical trials, and specifically could influence the success of therapies that result in reexpression of the dystrophin protein. We therefore set out to establish the prevalence of dystrophin-specific T cell responses in a survey of patients from a single muscular dystrophy clinic. Patients included those with DMD who had never been treated with steroids or were treated with deflazacort or prednisone, and normal age-matched control subjects. Our data demonstrate that circulating dystrophin-primed T cells are frequently seen in DMD, with a probability that increases with age, and that treatment with corticosteroids significantly decreases the likelihood of a positive response. These data demonstrate for the first time the prevalence of these responses; raise the intriguing possibility that part of the beneficial effect of corticosteroid treatment may be related to modulation of T cell immunity; and provide the first evidence of a differential effect of deflazacort and prednisone in modulating T cell responses in boys with DMD.
Materials and Methods
Clinical subjects
All subjects were recruited from the Muscular Dystrophy Association clinic at Nationwide Children's Hospital (Columbus, OH). Diagnoses of DMD were made or confirmed by a neuromuscular clinician expert in the diagnosis of muscle disease (K.M.F. and J.R.M.), and all subjects had molecular diagnostic confirmation of a DMD gene mutation consistent with DMD (out-of-frame deletions, nonsense, or splice-site mutations). Under a protocol approved by the Nationwide Children's Hospital Institutional Review Board and after informed consent was given, blood samples were obtained for immune studies.
Enzyme-linked immunospot assay
Peripheral blood T cell responses to dystrophin were quantified by interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay. Briefly, peripheral blood mononuclear cells (PBMCs) isolated on Ficoll Hypaque gradients were cultured with synthetic peptides (20 amino acids in length, overlapping by 10 residues) that spanned the entire dystrophin protein. Peptides were organized into nine pools designated DP1 to DP9, representing exons of the entire dystrophin protein as follows: DP1, exons 1–9; DP2, exons 10–17; DP3, exons 18–26; DP4, exons 27–34; DP5, exons 35–41; DP6, exons 42–50; DP7, exons 51–58; DP8, exons 59–69; and DP9, exons 70–79. Peptide mapping pools were used to further localize the immune response in a subset of some positive patients. After incubation at 37°C for 36 hr, IFN-γ spot-forming cells (SFCs) were counted. A response to a dystrophin peptide pool was considered positive when it exceeded an average of 15 SFCs/106 PBMCs in duplicate wells and was at least three times greater than the number of SFCs (5 SFCs/106 PBMCs) in duplicate wells containing pooled peptides from an irrelevant antigen (enhanced green fluorescent protein).
MHC restriction
Frozen PBMCs from patients were sent to University of Oklahoma Health Sciences Center (Oklahoma City, OK) for complete HLA typing. To restrict the dystrophin T cell response to a particular allele, semi-HLA-matched cell lines expressing at least one of the possible alleles were pulsed with the peptide of interest, washed, and incubated with T cells in the standard IFN-γ ELISPOT assay described previously.
Flow cytometry
Direct intracellular cytokine staining (ICS) was performed to determine the phenotype of IFN-γ-producing cells. Briefly, PBMCs were incubated with peptides for 1 hr at 37°C before the addition of GolgiPlug (diluted 1:1000; BD Biosciences, San Jose, CA) and GolgiStop (diluted 1:1500; BD Biosciences) to each well. Cells were incubated for an additional 15 hr, and then washed and stained for the surface markers CD4, CD8, and CD3 (4°C, 30 min) before staining with a live cell discriminator (Live/Dead Fixable Blue Dead Cell Stain; Invitrogen, Carlsbad, CA). Next, cells were fixed, permeabilized (Cytofix/Cytoperm kit; BD Biosciences), and incubated with antibodies to the intracellular cytokines (4°C, 30 min). Cells were analyzed on a BD LSR II flow cytometer (BD Biosciences).
Data analysis
Because age did not closely follow a normal distribution, the nonparametric Wilcoxon two-sample test was used to compare age between two groups, and the Kruskal–Wallis test was used to compare age among three or more groups. Logistic regression was used to test the effects of age and group on the probability of a positive immune response. All tests were conducted in SAS 9.2 (SAS, Cary, NC).
Results
Ninety-one subjects were enrolled, including 70 patients with DMD and 21 age-matched normal control subjects. Among the patients with DMD, 29 were treated with deflazacort (DMD-Deflaz), 24 were treated with prednisone (DMD-Pred), and 17 were untreated (DMD-Steroid Naive). Anti-dystrophin T cell responses were detected in none of the normal control patients, and responses were found in 9 of 17 (52.9%) untreated patients with DMD. In contrast, they were found in only 11 of 53 (20.8%) treated patients with DMD (Fig. 1), including 5 of 29 DMD-Deflaz patients (17.2%) and 6 of 24 DMD-Pred patients (25.0%).
FIG. 1.
Statistical probability of a T cell response in corticosteroid-treated versus treatment-naive patients. *Odds ratio, 0.011; 95% CI, (<0.001, 0.440).
Taking age into account, there is no significant difference in age among the three DMD groups (p=0.77), or among all groups (p=0.16) (Fig. 2). Logistic regression analysis demonstrates that older subjects have a higher probability of having a positive immune response regardless of clinical phenotype (as modeled in Fig. 3) (p=0.0036; odds ratio, 1.223; 95% CI of odds ratio, [1.068, 1.401]). Because of this apparent overall age effect, we used several models of logistic regression to test the effects of diagnostic class on the probability of a positive immune response, controlled by age. Compared with DMD-Steroid Naive subjects as the reference group, subjects receiving any steroid (either DMD-Pred or DMD-Deflaz) were less likely to have a positive T cell response (p=0.0425; odds ratio, 0.248; 95% CI, [0.065, 0.954]). We performed logistic regression of the same data with DMD-Deflaz and DMD-Pred analyzed as separate groups, maintaining DMD-Steroid Naive as the reference group. In this model, DMD-Deflaz subjects again demonstrated less likelihood of having a positive immune response (p=0.0278; odds ratio, 0.173; 95% CI, [0.036, 0.8260]), although DMD-Pred subjects did not (p=0.20; odds ratio, 0.358; 95% CI, [0.074, 1.740]). However, direct comparison of the incidence of T cell responses between the DMD-Deflaz and DMD-Pred cohorts reveals that the distribution is not significantly different (p=0.5175 by Fisher exact test).
FIG. 2.
Distribution by age of subjects in each cohort, with mean age marked by a horizontal line.
FIG. 3.
Estimated probability of a positive ELISPOT response by age, using a logistic regression model (p=0.0036; odds ratio, 1.223; 95% CI of odds ratio, [1.068, 1.401]). Patients with actual positive responses are marked with an arrow.
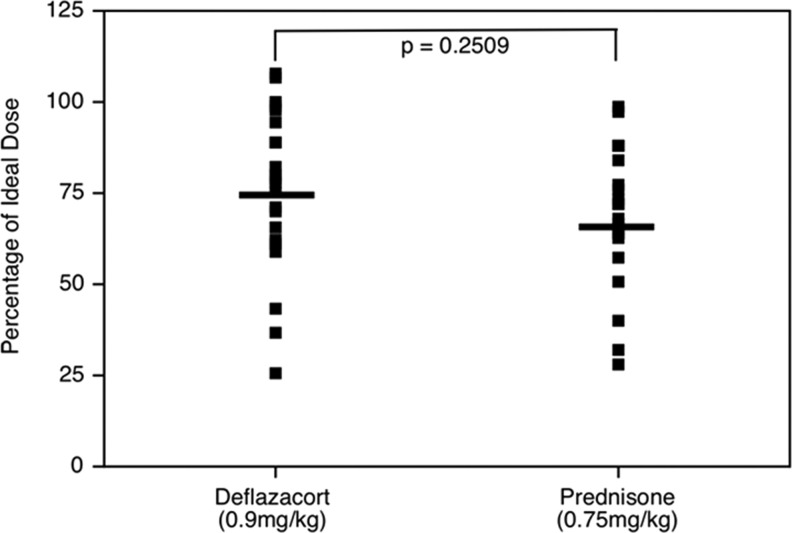
One possible reason for different results between the DMD-Deflaz and DMD-Pred groups would be a differential effect of the two steroids. A second reason, however, would involve differences in steroid exposure, either by duration or by dose. To test these possibilities, we first looked at the length of time during which patients were treated with steroids. In comparing the two drugs, there was no difference in time on treatment between the prednisone group (31.85±19.75 months) and the deflazacort group (25.7±19.3 months) (p=0.27; Mann–Whitney U test). Within each group, there was similarly no difference in duration on steroids between those patients with or without immune responses. In the prednisone-treated group, the mean duration for patients with a response was 25.67±18.32 months, and for those without a response it was 34.50±20.40 months (p=0.510). In the deflazacort-treated group, the mean for the positive response group was 23.08±13.22 months versus 26.04±20.05 months for the group without a response (p=1.0). To assess dose, we used the percentage of the ideal treatment by body weight that the patient was receiving on the day blood was drawn. To estimate the relative exposure of each patient, we calculated their dose in milligrams per kilogram at the time of the blood draw, and expressed it as a fraction of the recommended dose (prednisone, 0.75 mg/kg; deflazacort, 0.9 mg/kg). There was no significant difference in the mean corrected dosage of the prednisone (75.4±20.7%) versus the deflazacort (68.8±19.0%) cohorts (p=0.2509; Fig. 4). Logistic regression analysis of the two treated DMD cohorts, controlling for dose, demonstrates no significant difference observed for age (p=0.7273) or for type of steroid (p=0.8376).
FIG. 4.
Distribution of corticosteroid doses at the time of blood drawing, expressed as a percentage of the ideal recommended dose.
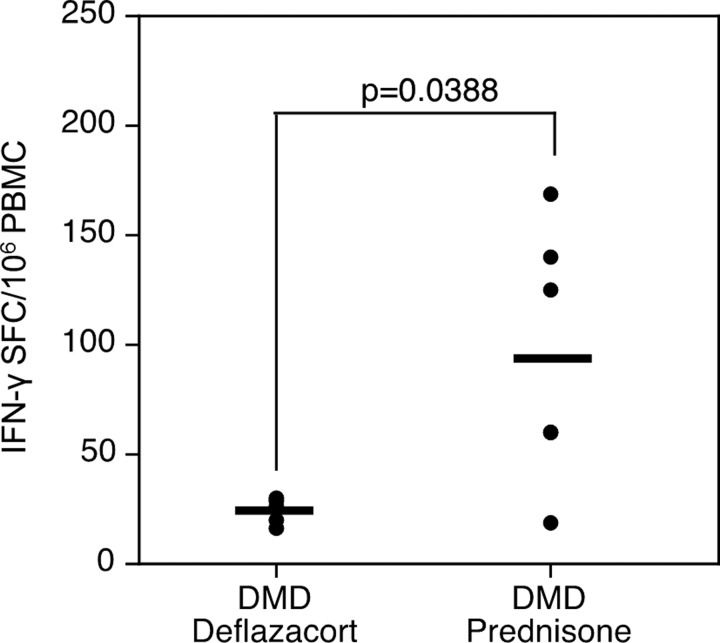
We looked further for evidence of a differential steroid effect, using an alternative analysis. In contrast to scoring a response as simply positive or negative, we can describe the overall frequency of dystrophin-specific T cells by quantifying on the basis of the number of positive spot-forming cells per million PBMCs. There was a significant difference in frequency of circulating antigen-specific T cells between the deflazacort and prednisone groups (p=0.0388) (Fig. 5), but no correlation between ideal dose percentage and response intensity (with a correlation coefficient of −0.26, which is not significantly different from zero [p=0.46]).
FIG. 5.
Frequency of antigen-specific T cells in the blood (expressed as SFCs/106 PBMCs, ordinate axis) of deflazacort- versus prednisone-treated groups.
We previously described dystrophin-specific CD4+ helper T cell activity in the blood of one patient before treatment with a gene therapy vector (Mendell et al., 2010). The class II-restricted epitope targeted by this response was downstream of a frame-shifting deletion but within a domain of revertant dystrophin expressed in a small percentage of his myofibers. This observation fit a model of T cell priming against epitopes that cannot be expressed from the defective gene in the absence of a second spontaneous mutation that promotes in-frame production of an antigenic revertant fiber. We reasoned that epitopes would not be located upstream of the mutation because of the potential for partial in-frame expression from the defective gene that should promote immunological tolerance to dystrophin. Identification of the dystrophin peptide pools targeted by the T cells revealed that this was not always the case. In six of nine subjects, the epitope(s) were encoded by exons upstream of deletions or nonsense mutations that disrupted the dystrophin open reading frame (Table 1). Further characterization revealed that the response was narrowly focused on one or two epitopes, despite the large size of the dystrophin protein, and composed of circulating CD4+ helper and/or CD8+ cytotoxic T cells that could be measured by intracellular staining for IFN-γ immediately after antigen stimulation (Table 1). There was no relationship between glucocorticoid treatment history and detection of circulating dystrophin-specific CD4+ versus CD8+ T cells.
Table 1.
Location and Nature of Dystrophin Mutation and Epitopes Targeted by Circulating CD4+ and/or CD8+ T Cells
| Patient ID | Treatment regimen | Mutationa | Truncating mutation (prediction) | Location of immune response | Location of response relative to mutation location | T Cell phenotype |
|---|---|---|---|---|---|---|
| 12 | Naive | Splice exon 12 | + | Exons 42–50 | Downstream | CD4 |
| 21 | Prednisone | Del ex 45 | + | Exons 1–9 | Upstream | CD4 |
| 74 | Naive | Del ex 46–50 | + | Exons 42–50 | Upstream | CD8 |
| 39 | Naive | Del ex 48–50 | + | Exons 42–50 | Upstream | CD4 |
| 19 | Naive | Del ex 48–50 | + | Exons 17–26 | Upstream | CD4/CD8 |
| 35 | Prednisone | Del ex 50 | + | Exons 50–59 | Downstream | CD4 |
| 11 | Naive | Del ex 49–54 | + | Exons 17–26 | Upstream | CD8 |
| 14 | Naive | Nonsense ex 59 | + | Exons 70–79 | Downstream | CD4 |
| 59 | Prednisone | Nonsense ex 69 | + | Exons 59–69 | Upstream | CD4 |
Del, deletion; ex, exon. All are truncating mutations that are predicted to result in an interrupted mRNA reading frame.
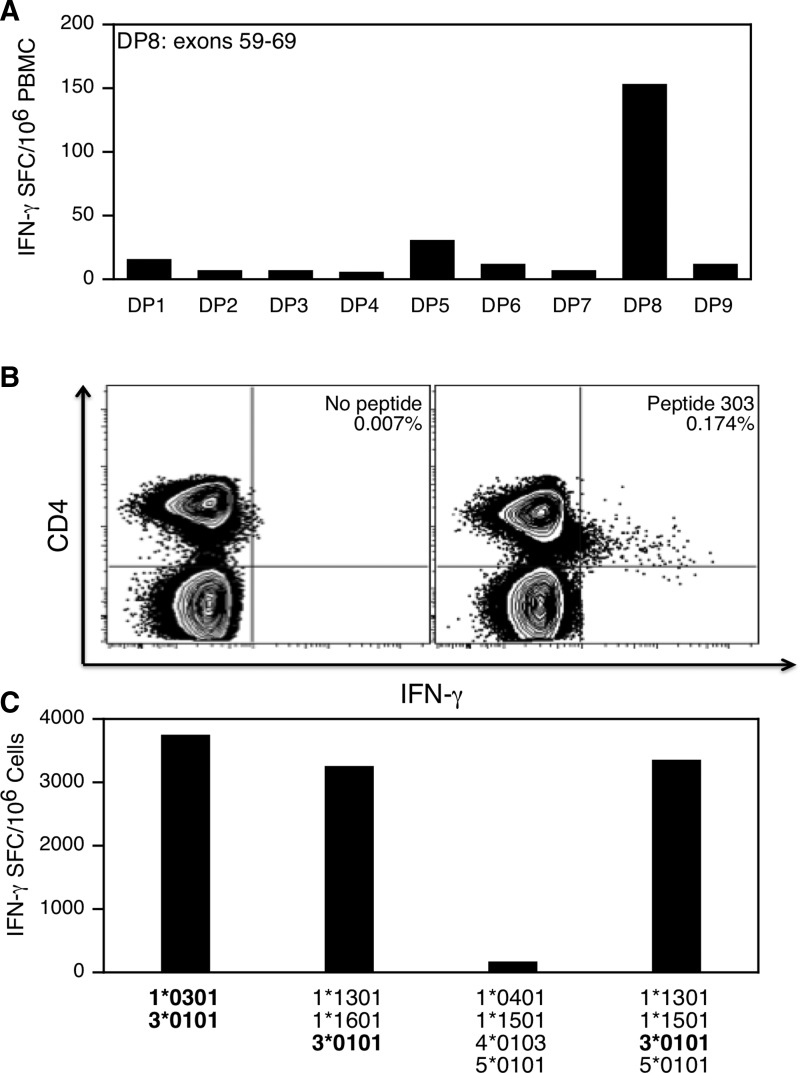
Detailed immunological characterization of the response was performed for two subjects to better understand the functional characteristics of dystrophin-specific T cells. T cell immunity was first characterized in patient 59, who had a nonsense mutation in exon 69 and was receiving prednisone at a dosage of 0.24 mg/kg/day at the time of study. A T cell response was detected directly out of blood to dystrophin peptide pool 8 (DP8), which spanned exons 59–69 of the protein (Fig. 6A). The T cell response was mapped to a single peptide (peptide 303; NTRWKLLQVAVEDRVRQLHE) spanning amino acids 3021–3040 of the central rod subdomain repeat 24 encoded by exons 60–61 (data not shown). The epitope is therefore located upstream of this patient's truncating mutation. The response was mediated by CD4+ T cells (Fig. 6B) and restricted by the HLA class II allele DRB3*0101 (Fig. 6C).
FIG. 6.
Detailed immunological characterization of dystrophin-specific T cells in patient 59. Responses were characterized after stimulation of PBMCs with the indicated peptide pool or the class II epitope (QVAVEDRVRQLH). (A) IFN-γ ELISPOT response to nine peptide pools spanning dystrophin, including peptide pool DP8, which represented exons 59–69. Data are presented as the number of IFN-γ spot-forming cells (SFCs) per million PBMCs. (B) Intracellular staining for cytokine (IFN-γ) and expression of CD4 by PBMCs after stimulation with the peptide epitope. (C) IFN-γ production by PBMCs after stimulation with autologous and semiallogeneic B-lymphoblastoid cell line production. The HLA class II molecules displayed by each cell line are indicated. The common HLA- DRB3*0101 class II molecule expressed by B-LCL that stimulated a response is boldfaced.
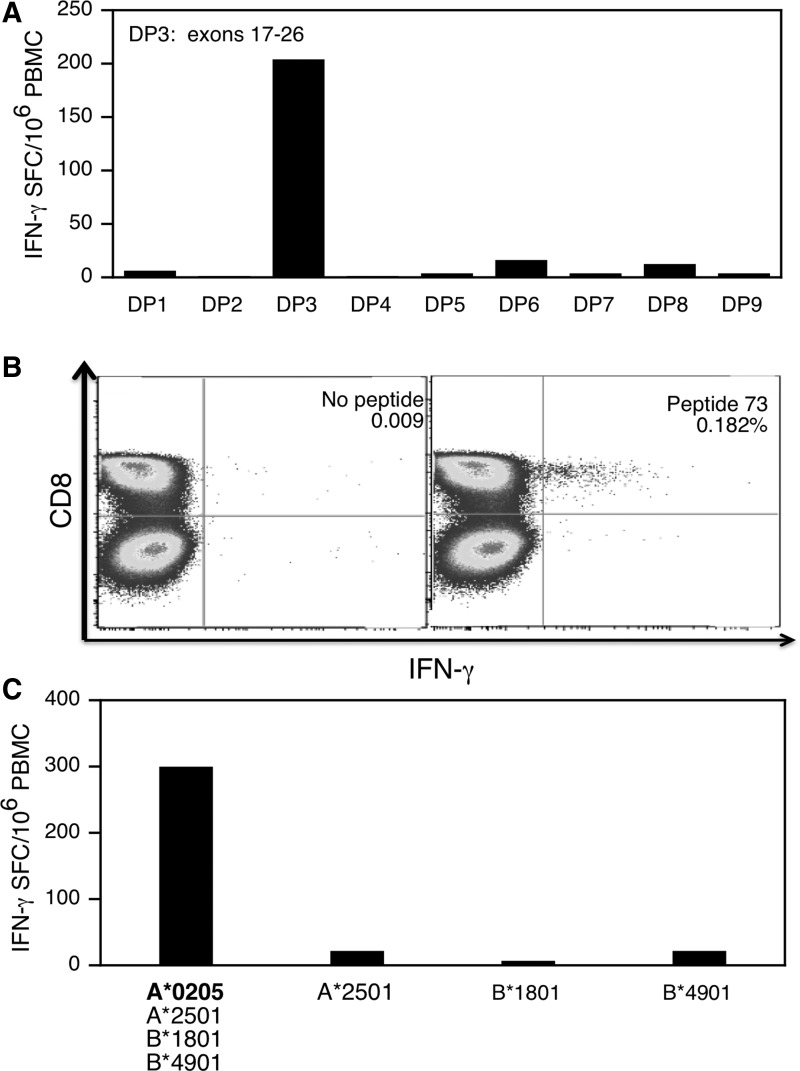
Patient 11 carried an out-of-frame deletion of exons 49–54. T cell immunity was further characterized in this patient because he fit a different clinical and immunological profile from subject 59. Specifically, he was steroid naive at the time of study and preliminary analysis indicated that the dystrophin-specific T cell response was mediated by CD8+ T cells rather CD4+ T cells as in subject 59. We detected a strong T cell response to dystrophin peptide pool 3 (DP3) directly out of blood (i.e., without expansion in cell culture) (Fig. 7A). DP3 represented exons 17–26 and is thus located upstream of this boy's truncating DMD mutation. The epitope was further mapped to a single peptide (peptide 73) contained in DP3 that spanned dystrophin amino acids 721–740 incorporated in repeat 4 of the central rod domain (data not shown). Intracellular staining for IFN-γ production revealed that the T cells belonged to the CD8+ cytotoxic subset (Fig. 7B) and the epitope was presented by the HLA-A*0205 class I allele (Fig. 7C).
FIG. 7.
Characterization of dystrophin-specific T cells in patient 11. Responses obtained directly from blood samples (not an expanded PBMC population) are shown. (A) IFN-γ ELISPOT response to nine peptide pools spanning dystrophin, including peptide pool DP3, which represented exons 18–26 that were upstream of the patient's mutation (deletion of exons 49–54). (B) Intracellular staining for cytokine (IFN-γ) and expression of CD8 by PBMCs after stimulation with peptide 73. (C) IFN-γ production by PBMCs after stimulation with autologous and semiallogeneic B-lymphoblastoid cell lines pulsed with the class I epitope. The four HLA class I molecules matched between the semiallogeneic and autologous B-LCL lines are indicated.
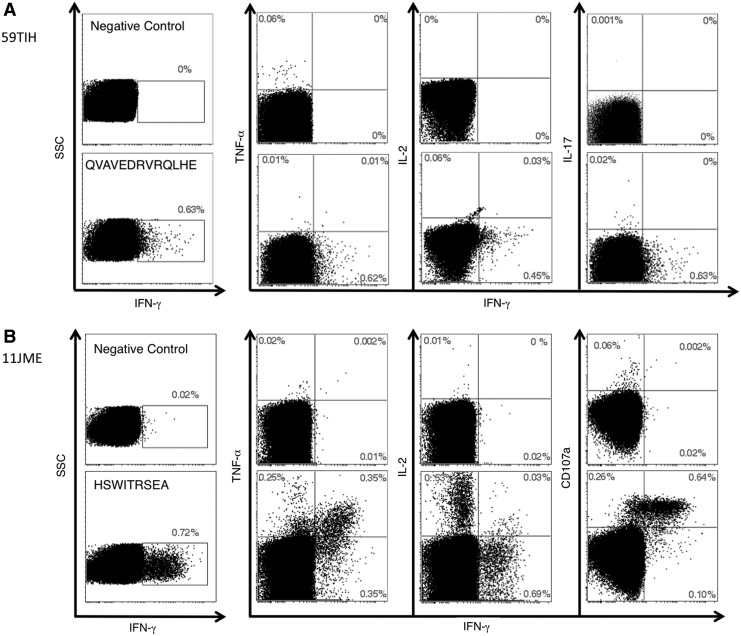
We next examined patterns of cytokine production by circulating dystrophin-specific T cells from patients 59 and 11. After dystrophin peptide stimulation, CD4+ T cells were detected in the blood of patient 59 at a frequency of 0.63% by staining for intracellular production of IFN-γ (Fig. 8A). These CD4+ T cells lacked the polyfunctional profile expected of effector populations because they failed to coproduce tumor necrosis factor (TNF)-α, interleukin (IL)-2, or IL-17 (Fig. 8A). Next, we examined cytokine production and the capacity to degranulate for dystrophin-specific CD8+ T cells from subject 11 (Fig. 8B). The response to the A*0205-restricted HSWITRSEA epitope was strong because the frequency of cognate CD8+ T cells was about 1.2% when populations that produced IFN-γ, TNF-α, or IL-2 were considered. CD8+ T cell that produce multiple cytokines and have cytotoxic capacity are considered the most highly differentiated of effector cells (Seder et al., 2008). From this perspective the response was highly unusual because it was composed of two functionally distinct CD8+ T cell populations. The first population defined by production of IFN-γ was present at a frequency of about 0.7%. They resembled effector CD8+ T cells because antigen stimulation caused coproduction of TNF-α and expression of CD107a/LAMP-1 (Fig. 8B), a marker of cytotoxic potential via secretion of lytic granules containing such molecules as perforin and granzyme. The second population of CD8+ T cells specific for the same dystrophin peptide was also present at a relatively high frequency (0.53%) in blood, produced IL-2 but not effector molecules such as IFN-γ, TNF-α, or CD107A (Fig. 8B). Thus the response in this patient was composed of CD8+ T cells with the ability to (1) produce effector cytokines (IFN-γ and TNF-α) and degranulate on engagement of antigen-positive myocytes and (2) produce IL-2 that may be necessary to generate or sustain the effector population.
FIG. 8.
Multicytokine analysis of the dystrophin-specific T cell response in patient 59 (A) and patient 11 (B). PBMCs from each patient were stimulated with the relevant dystrophin class I or II peptides. Production of the indicated cytokines was measured by polychromatic flow cytometry after intracellular staining. Cell surface expression of CD107A was also determined after antigen stimulation. Gates were set on live CD3+CD4+ (A) or CD3+CD8+ (B) lymphocytes.
Discussion
We previously reported dystrophin-specific cellular immune responses in two of six patients with DMD before treatment with a gene therapy vector designed to restore muscle function (Mendell et al., 2010). This study was undertaken to better define the scope of T cell immunity in subjects with DMD because of its potential to interfere with therapies that increase expression of dystrophin. Here we report that 20 of 70 (28.6%) patients with DMD in an unselected sample from a single clinic observation had T cell immunity against dystrophin. This immune response developed spontaneously during the course of disease because none of the subjects had received treatment with gene therapy vectors or small molecules designed to induce expression of dystrophin in muscle. Our results also indicate for the first time that the risk of developing dystrophin-specific T cell response increases with age and is reduced in those who receive corticosteroid therapy.
In our earlier study, dystrophin-specific T cell immunity was associated with expression of revertant dystrophin that might have served as an antigen for induction of the response (Mendell et al., 2010). Revertant fibers arise spontaneously in a small number of myofibers after development of second site mutation(s) that restore the open reading frame (Klein et al., 1992; Winnard et al., 1995). In that study, we found that T cells can target dystrophin epitopes downstream of a deletion or missense nucleotide substitution. Here we document that epitopes were also located upstream of DMD mutations in six of nine subjects. A predominance of epitopes in upstream regions of the gene was unexpected because of the potential for in-frame expression of protein that might impose immunological tolerance. It is possible that tolerance did not develop because nonsense-mediated decay of dystrophin RNA prevented expression of even a truncated version of the protein. We interpret our results to suggest that spontaneous production of revertant fibers is necessary to prime T cells against epitopes, regardless of their location relative to the mutation. These results suggest a complex relationship between expression of truncated dystrophin proteins and induction of cellular immunity in a disease that spans a spectrum from relatively mild to severe. Whether immunity to dystrophin is generated may depend on how early in the course of disease revertant fibers first appear, and whether the pattern of expression in myofibers is sporadic and rare or more sustained and widespread.
Dystrophin immunity in most subjects was dominated by either CD4+ or CD8+ T cells that targeted a single epitope. Detailed characterization of the CD4+ versus CD8+ T cell response in two subjects also revealed substantial differences in effector function(s) and thus the potential for destruction of myocytes. Dystrophin-specific CD4+ T cells detected in the blood of subject 59 are unlikely, on their own, to pose a substantial threat for tissue injury because they produced only IFN-γ and not other effector cytokines with the potential for cytotoxicity. On the other hand, subject 11 had a functionally complex CD8+ T cell response, and at least half of the primed effector cells produced TNF and released cytotoxic granules capable of killing antigen-positive myocytes. It is not clear why CD8+ T cells segregated into two functionally distinct populations. CD8+ T cells that respond to virus infections are able to produce IL-2 or exert cytotoxic activity, but not both, perhaps because of differential regulation by the transcriptional activators such as T-bet (Makedonas et al., 2010). CD8+ T cells that produce perforin are usually dominant during the acute phase of infection and transition to IL-2 production as memory develops (Makedonas et al., 2010). Cocirculation of both dystrophin-specific CD8+ T cell populations in this patient may indicate interdependence, with the IL-2 producers providing help for the generation or survival of effectors that express IFN-γ, TNF-α, and CD107A (Zhang and Bevan, 2011). Alternatively, IL-2 production may define a self-renewing memory population that continuously replenishes a terminally differentiated effector population. Production of IL-2 but not TNF-α or IFN-γ is also somewhat unusual for CD8+ T cells, even after differentiation to memory is complete. Further studies are required to determine whether this mixed functional profile is common for dystrophin-specific CD8+ T cells in patients with DMD and whether the pattern of cytokine production is associated with other effector or memory phenotypic markers.
Factors that determine whether the response was composed of CD4+ or CD8+ T cells, and their functional characteristics, are not known. There was no common pattern of class I or class II epitope recognition by the nine subjects in this study; CD4+ and CD8+ T cells targeted many different domains of dystrophin that were both upstream and downstream of the deletion. Priming of a CD4+ versus CD8+ T cell response may simply depend on whether a class I or II epitope is available for presentation. Indeed, in any individual patient the response in blood appeared to be highly restricted to a single epitope despite the large size of the dystrophin protein. It is possible that the potential for a broader response by CD4+ and CD8+ T cells is constrained by the repeating nature of some dystrophin domains that can also share amino acid homology with other self-proteins. The possibility that only a small subset of primed T cells circulates in blood, and that the response is much broader in muscle, also cannot be excluded. Nevertheless, direct measurement of dystrophin-specific T cells in blood, without prolonged culture with antigen and cytokines that promote growth and differentiation, excludes the possibility of artifactual expansion and detection of antigen-specific responses. Consistent with this view, T cell responses were not detected in age-matched control subjects who did not have DMD.
In the DMD population, age was clearly defined as an independent risk factor for the development of dystrophin-specific T cell immunity. Our model derived from logistic regression (see Appendix) predicts the probability of a response at age 20 years (as an example) as 82% in the DMD-Steroid Naive group and 55% in the DMD-Steroid group (deflazacort or prednisone). The mechanism responsible for this age-related risk remains unclear. It may be related to an increased frequency of revertant fibers with advancing age, as reported in studies of DMD cohorts (Fanin et al., 1992, 1995), but an age-related increase in revertant fiber frequency was not seen in a series of patients rebiopsied over a mean interval of 8.2 years (Arechavala-Gomeza et al., 2010). Alternatively, the nonspecific inflammation that accompanies progressive muscle deterioration in many older subjects with DMD could create an environment that facilitates priming of T cells, although whether this priming is a cause or consequence of muscle deterioration is not explained by our results. Future studies in a variety of other degenerative muscle disorders should shed light on this issue.
Perhaps the most intriguing finding is the difference in immune responses between patients who are treated with corticosteroids and those who are not. There are multiple potential effects of corticosteroids in DMD including membrane stabilization and decreased myofiber degeneration (Jacobs et al., 1996), utrophin upregulation (Courdier-Fruh et al., 2002), and decreased fibrosis, but studies in both patients with DMD (Engel and Biesecker, 1982; Arahata and Engel, 1984) and mdx mice (Spencer et al., 2001) suggest that inflammation may contribute early and significantly to myofiber deterioration. The dose of prednisone used to treat patients with DMD for prolonged periods of time is relatively low and does not increase the risk of opportunistic infections or reactivation of latent viruses when compared with untreated patients. It is possible that doses typically used to treat DMD inhibit the relatively weak dystrophin-specific T cell responses measured in our patients without causing more generalized immune suppression. By comparison, the frequency and breadth of T cell immunity to a mix of dominant cytomegalovirus, Epstein–Barr virus, and influenza virus (CEF) class I epitopes was much stronger in all groups of subjects, and no difference was observed between prednisone-treated and untreated patients with DMD (data not shown). Together, our data provide the first evidence that therapy with corticosteroids may diminish the amount of dystrophin-primed T cells, which may contribute to their therapeutic effect.
These data also raise the compelling possibility that deflazacort and prednisone may affect T cells in quantitatively different ways. The difference in the mean number of spot-forming colonies per 106 PBMCs between the two groups is both striking (Fig. 5) and statistically significant, raising the possibility that deflazacort is more efficacious in modulating T cell pathways. This is an observational study rather than a prospective one, limiting the certainty with which conclusions can be drawn; nonetheless, such a differential effect would be consistent with several previous reports of increased immunosuppressive activity with deflazacort in comparison with prednisolone in a variety of models, including ex vivo thymus explants (Luzzani et al., 1983), and in suppressing experimental models of anaphylaxis and delayed hypersensitivity (Omote et al., 1994). Compared with prednisone in patients with rheumatoid arthritis, deflazacort has been shown to result in more profound T cell suppression; to result in more prolonged suppression after a single dose; and to alter the CD4+/CD8+ ratio by diminishing CD4+ cells and increasing CD8+ cells (Scudeletti et al., 1987).
These results raise several issues that require future experimental exploration. The first is that the specific function of these T cells is not clear. The presence of both CD4+ and CD8+ cells as mediators of the response suggests that both regulatory and cytotoxic functions may be primed by dystrophin, and suggests the possibility that revertant fibers may in different cases initiate or sustain any immune response. A second and related issue is whether the presence of these cells is related causally to disease progression, or whether they accumulate with age as a by-product of muscle degeneration and antigen presentation. A third, requiring longitudinal studies, is whether steroid therapy decreases existing T cell primed populations, or prevents effective priming. Finally, it remains unclear whether circulating dystrophin-primed T cells reflect the T cell repertoire and burden within muscle, the tissue of interest; this relationship between circulating and tissue-resident T cells in DMD remains to be explored, and is the object of ongoing muscle biopsy studies in our group. In the meantime, our results have immediate implications for the design and interpretation of clinical trials intended to result in dystrophin gene expression, and argue that preexisting T cell immunity status should be considered for stratification in the design of gene therapy trials. A notable difference was encountered in our ongoing eteplirsen exon-skipping trial (Sarepta Therapeutics, Cambridge, MA). In 12 patients with proven dystrophin expression after 6 months of treatment we did not encounter T cell immunity (Mendell et al., 2013). One possibility to consider is that viral gene therapy induces a proinflammatory state that may have an adjuvant effect.
Appendix
Probability of a positive response by age uses the following formula, where the term DMD-Defla/Pred'=1 for patients on steroids and = 0 for DMD-Naïve patients:
 |
Acknowledgments
This work has been supported by a Sen. Paul Wellstone Muscular Dystrophy Cooperative Research Center award (NICHD U54HD066409). The authors acknowledge the study coordinator assistance of Ms. Chelsea Rankin, and thank the participants and their families for taking part in the study.
Author Contributions
Kevin M. Flanigan: literature search, study design, data analysis, data interpretation, figures, writing of manuscript; Katie Campbell: data collection, figures, data analysis, writing; Laurence Viollet: study design, data collection; Wei Wang: biostatistical analysis; Ana Maria Gomez: data collection; Christopher M. Walker: literature search, study design, data analysis, data interpretation, figures, writing of manuscript; Jerry R. Mendell: literature search, study design, data analysis, data interpretation, figures, writing of manuscript.
Author Disclosure Statement
None of the authors has any conflict of interest related to this work.
References
- Arahata K. Engel A.G. Monoclonal antibody analysis of mononuclear cells in myopathies. I. Quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann. Neurol. 1984;16:193–208. doi: 10.1002/ana.410160206. [DOI] [PubMed] [Google Scholar]
- Arechavala-Gomeza V. Kinali M. Feng L., et al. Revertant fibres and dystrophin traces in Duchenne muscular dystrophy: Implication for clinical trials. Neuromuscul. Disord. 2010;20:295–301. doi: 10.1016/j.nmd.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Courdier-Fruh I. Barman L. Briguet A. Meier T. Glucocorticoid-mediated regulation of utrophin levels in human muscle fibers. Neuromuscul. Disord. 2002;12(Suppl. 1):S95–S104. doi: 10.1016/s0960-8966(02)00089-5. [DOI] [PubMed] [Google Scholar]
- Engel A.G. Biesecker G. Complement activation in muscle fiber necrosis: Demonstration of the membrane attack complex of complement in necrotic fibers. Ann. Neurol. 1982;12:289–296. doi: 10.1002/ana.410120314. [DOI] [PubMed] [Google Scholar]
- Fanin M. Danieli G.A. Vitiello L., et al. Prevalence of dystrophin-positive fibers in 85 Duchenne muscular dystrophy patients. Neuromuscul. Disord. 1992;2:41–45. doi: 10.1016/0960-8966(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Fanin M. Danieli G.A. Cadaldini M., et al. Dystrophin-positive fibers in Duchenne dystrophy: Origin and correlation to clinical course. Muscle Nerve. 1995;18:1115–1120. doi: 10.1002/mus.880181007. [DOI] [PubMed] [Google Scholar]
- Jacobs S.C. Bootsma A.L. Willems P.W., et al. Prednisone can protect against exercise-induced muscle damage. J. Neurol. 1996;243:410–416. doi: 10.1007/BF00869001. [DOI] [PubMed] [Google Scholar]
- Kissel J.T. Burrow K.L. Rammohan K.W. Mendell J.R. CIDD Study Group. Mononuclear cell analysis of muscle biopsies in prednisone-treated and untreated Duchenne muscular dystrophy. Neurology. 1991;41:667–672. doi: 10.1212/wnl.41.5.667. [DOI] [PubMed] [Google Scholar]
- Klein C.J. Coovert D.D. Bulman D.E., et al. Somatic reversion/suppression in Duchenne muscular dystrophy (DMD): Evidence supporting a frame-restoring mechanism in rare dystrophin-positive fibers. Am. J. Hum. Genet. 1992;50:950–959. [PMC free article] [PubMed] [Google Scholar]
- Luzzani F. Barone D. Galliani G. Glasser A. Ex vivo binding to glucocorticoid receptors in the thymus of the adrenalectomized rat. Eur. J. Pharmacol. 1983;87:61–66. doi: 10.1016/0014-2999(83)90050-x. [DOI] [PubMed] [Google Scholar]
- Makedonas G. Hutnick N. Haney D., et al. Perforin and IL-2 upregulation define qualitative differences among highly functional virus-specific human CD8 T cells. PLoS Pathog. 2010;6:e1000798. doi: 10.1371/journal.ppat.1000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik V. Rodino-Klapac L.R. Viollet L., et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann. Neurol. 2010;67:771–780. doi: 10.1002/ana.22024. [DOI] [PubMed] [Google Scholar]
- Manzur A.Y. Kuntzer T. Pike M. Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2008;1:CD003725. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- Mendell J.R. Campbell K. Rodino-Klapac L., et al. Dystrophin immunity in Duchenne's muscular dystrophy. N. Engl. J. Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J.R. Rodino-Klapac L.R. Sahenk Z. Roush K., et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 2013 (in press) [Google Scholar]
- Omote M. Sakai K. Mizusawa H. Acute effects of deflazacort and its metabolite 21-desacetyl-deflazacort on allergic reactions. Arzneimittelforschung. 1994;44:149–153. [PubMed] [Google Scholar]
- Scudeletti M. Piccardo C. Piovano P., et al. Effects of a new heterocyclic glucocorticoid, deflazacort (DFC), on the functions of lymphocytes from patients with rheumatoid arthritis (RA) Int. J. Immunopharmacol. 1987;9:133–139. doi: 10.1016/0192-0561(87)90087-7. [DOI] [PubMed] [Google Scholar]
- Seder R.A. Darrah P.A. Roederer M. T-cell quality in memory and protection: Implications for vaccine design. Nat. Rev. Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- Spencer M.J. Montecino-Rodriguez E. Dorshkind K. Tidball J.G. Helper (CD4+) and cytotoxic (CD8+) T cells promote the pathology of dystrophin-deficient muscle. Clin. Immunol. 2001;98:235–243. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- Thanh L.T. Nguyen T.M. Helliwell T.R. Morris G.E. Characterization of revertant muscle fibers in Duchenne muscular dystrophy, using exon-specific monoclonal antibodies against dystrophin. Am. J. Hum. Genet. 1995;56:725–731. [PMC free article] [PubMed] [Google Scholar]
- Uchino M. Tokunaga M. Mita S., et al. PCR and immunocytochemical analyses of dystrophin-positive fibers in Duchenne muscular dystrophy. J. Neurol. Sci. 1995;129:44–50. doi: 10.1016/0022-510x(94)00245-j. [DOI] [PubMed] [Google Scholar]
- Winnard A.V. Mendell J.R. Prior T.W., et al. Frameshift deletions of exons 3–7 and revertant fibers in Duchenne muscular dystrophy: Mechanisms of dystrophin production. Am. J. Hum. Genet. 1995;56:158–166. [PMC free article] [PubMed] [Google Scholar]
- Zhang N. Bevan M.J. CD8+ T cells: Foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]