Abstract
Modern hand and wrist prostheses afford a high level of mechanical sophistication, but the ability to control them in an intuitive and repeatable manner lags. Commercially available systems using surface electromyographic (EMG) or myoelectric control can supply at best two degrees of freedom (DOF), most often sequentially controlled. This limitation is partially due to the nature of surface-recorded EMG, for which the signal contains components from multiple muscle sources. We report here on the development of an implantable myoelectric sensor using EMG sensors that can be chronically implanted into an amputee’s residual muscles. Because sensing occurs at the source of muscle contraction, a single principal component of EMG is detected by each sensor, corresponding to intent to move a particular effector. This system can potentially provide independent signal sources for control of individual effectors within a limb prosthesis. The use of implanted devices supports inter-day signal repeatability. We report on efforts in preparation for human clinical trials, including animal testing, and a first-in-human proof of principle demonstration where the subject was able to intuitively and simultaneously control two DOF in a hand and wrist prosthesis.
Keywords: Myoelectric sensor, Prosthesis control, Electromyographic control
Out of approximately 100 000 people in the USA with upper-limb loss (1), 57% are transradial (below elbow) amputees (2,3). About 80% use a prosthesis (4), of which 50% are myoelectric-controlled (5) and 50% are body-powered and controlled. In myoelectric control, electrodes on the skin of the residual limb detect electric potentials from underlying muscles (electromyograms, or EMGs). These EMG signals contain components from several active muscles in the residual limb (6). Algorithmic processing has been attempted for differentiation of distinct signal sources, but this technique is not optimized. A maximum of four independent surface EMG sites can be located on a residual limb (7), and typically, only two sites are used in an agonist–antagonist (flexor/extensor muscles) arrangement.
In a three-state, two-site EMG controller, rapid co-contraction of extensors and flexors switches between the modes of hand opening/closing and wrist supination/pronation. Sequential control is slow and unintuitive; thus, many amputees abandon their prostheses because it is easier to perform tasks with their intact hand.
Mechanical prostheses have been developed with high numbers of degrees of freedom (DOF), including the DARPA RP2007 DEKA arm with 18 DOF and the DARPA RP2009 Applied Physics Laboratory arm with over 25 DOF. Unfortunately, the lack of independent control sources, lack of simultaneous multi-DOF control ability, and lack of repeatable control sources limit commercially available transradial prostheses to, at best, two DOF with sequential control.
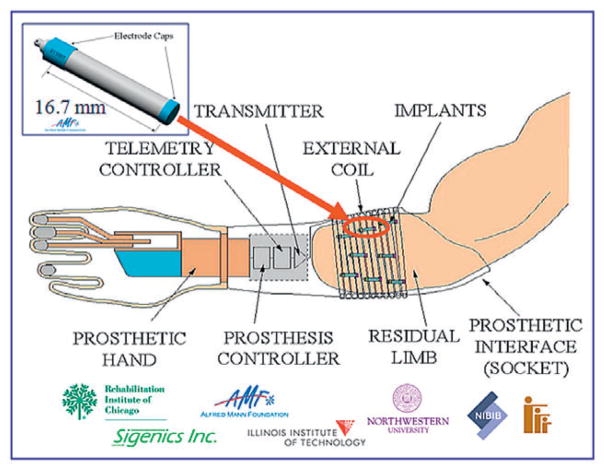
We have previously reported on the development of the implantable myoelectric sensor (IMES) system (8,9), which uses devices chronically implanted into the residual muscles of an amputee’s arm using minimally invasive surgical techniques (Fig. 1). By using a stable EMG sensor implanted within the source muscle, a single principal component of EMG is detected. These signals, corresponding to the intent to move a particular part of the anatomy, can be decoded and used to drive the appropriate effectors in a prosthesis. By using an implanted device, we have mitigated the problem of multiple-component EMGs inherent in surface recording. By using a leadless telemetered device, we have mitigated the infection risk present with any transcutaneous recording system, as well as issues with inter-day donning and doffing electrode placement.
FIG. 1.
Schematic of the implantable myoelectric sensor system. Implant devices are 2.4 mm diameter × 16.7 mm length, and act as differential amplifiers. One or more devices can be implanted into gross muscle, and telemeter EMG data to an external coil connected to a telemetry controller (TC). The TC passes the EMG to the prosthesis controller to drive a prosthesis.
We report here on our further development of the system in preparation for deployment into clinical trials for prosthesis control.
MATERIALS AND METHODS
IMES technical description
The implanted sensors receive power and commands from an external telemetry controller (TC) driving a coil that will ultimately be built into an amputee’s prosthetic socket. Each implant acts as an independent differential amplifier connected to two electrodes (on the ends of the implant) to detect the EMG generated during muscle contraction. The implants transmit the EMG signals as digital data over a transcutaneous magnetic link to the TC, which then reforms the analog EMG signals for presentation to a prosthesis controller. Each implant is housed in a hermetic, biocompatible ceramic package originally developed by the Alfred Mann Foundation for the Radio Frequency Microstimulator. These packages have a qualified benign lifetime in vivo of 80 years.
The system is designed to telemeter EMG data on one of two bands. Integrated EMG (band 1) is the format used in commercial myoelectric systems, thus allowing for direct replacement of surface recordings to drive current myoelectric prostheses. Raw EMG capability (band 2) has been designed to support future advanced control algorithms requiring higher sampling rates. The frame-based transmission scheme uses 32 time slots per frame, which can be assigned to individual IMES to optimize sample rates for a particular set of implants. In the human demonstration reported here, four IMES were used. Band 1 was set for 120 samples/s/IMES (480 samples/s aggregate). Band 2 was not used in the demonstration, but is capable of transmitting raw EMG signals at 444 frames/s, which, for four IMES, yields 3552 samples/s/IMES (14 208 samples/s aggregate).
Animal experiments
Three cats were each implanted with two IMES sensors into the tibialis anterior and lateral gastrocnemius muscles (9). Biweekly recordings were taken for 12 months. Chronic stability was evaluated using statistical markers in the power spectral density. X-rays were taken immediately postimplant and at 6 and 11 months postimplant.
Nine IMES sensors were implanted into the forearm of a rhesus monkey. Simultaneous recordings have been taken for over 2 years while the monkey performed individuated and combined finger flexions on a manipulandum (10). Off-line pattern recognition was performed using a parallel linear discriminant analysis to decode finger activity. A second monkey has subsequently been implanted with IMES. This experiment is ongoing.
First-in-human demonstration
To demonstrate proof of principle, a human volunteer was acutely implanted with four pairs of fine-wire bipolar electrodes into the supinator, pronator teres, extensor digitorum, and flexor digitorum muscles (Fig. 2). The leads exited the forearm percutaneously and were connected to four IMES implants, contained within the TC external coil. On the same volunteer, four pairs of surface EMG electrodes were placed over these same muscles. A two-DOF hand and wrist prosthesis (Motion Control, Salt Lake City, UT, USA) was driven by either the four pairs of intramuscular (IMES) recordings or the four surface recordings, allowing a head-to-head performance comparison. The comparison was video recorded.
FIG. 2.
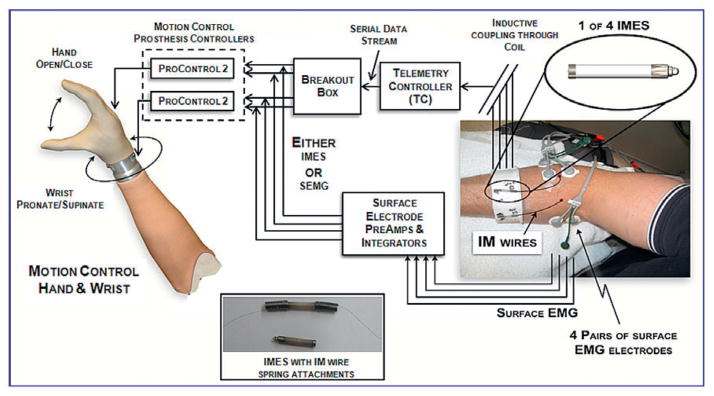
System used in 2 DOF prosthesis control demonstration. Fine-wire intramuscular electrodes were attached to the IMES electrodes via springs. The coil is not shown so the IMES cuff can be seen.
RESULTS
Animal experiments
The signal content recorded from chronic cats was stable over time, and X-rays indicated no migration of devices. All devices operated without problems throughout the study.
In the chronic monkey experiment, EMG from different IMES demonstrated very little cross-correlation. Training was stable across data sets that were collected months apart.
The animal experiments suggest that IMES implants do not migrate over time and yield stable EMG signals.
First-in-human demonstration
The IMES control system provided excellent tracking of the prosthesis to the movement of the natural hand in an intuitive manner, demonstratively better than the control obtained with the surface electrodes. The combined time for IMES parameter setup and training of the volunteer was about 1 h. Using the IMES devices, the volunteer was able to simultaneously and intuitively control two DOF, in a manner which could not be matched with the surface control system.
DISCUSSION
An IMES system has been designed and tested which we believe will offer superior control, relative to surface EMG, for upper-limb prostheses. We have demonstrated the stability of control signals in chronic animals and simultaneous two-DOF control in a human volunteer.
We are now continuing with design verification, which will be followed by miniaturization of the TC for incorporation into a prosthesis shell in preparation for human clinical trials.
CONCLUSIONS
The IMES offers promise as a stable control signal sensor which will allow acquisition of independent principal components of EMG from distinct muscles, thus mitigating the problem of multiple-component EMGs inherent in surface recording, and facilitating true intuitive and simultaneous multiple-DOF prosthesis control.
Acknowledgments
The authors would like to acknowledge the help of Alex Birdwell of the Biomechatronics Development Laboratory, Rehabilitation Institute of Chicago in acquiring the intramuscular signals.
Footnotes
Presented in part at the 10th Vienna International Workshop on Functional Electrical Stimulation and 15th IFESS Annual Conference held September 8–12, 2010 in Vienna, Austria.
References
- 1.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–9. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Kay H, Newman J. Relative incidence of new amputations. Orth Prosth. 1975;29:3–16. [Google Scholar]
- 3.Glatty HW. A statistical study of 12 000 new amputees. South Med J. 1964;57:1373–8. doi: 10.1097/00007611-196411000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Biddiss EA, Chau TT. Upper limb prosthesis use and abandonment: a survey of the last 25 years. Prosthet Orthot Int. 2007;31:236–57. doi: 10.1080/03093640600994581. [DOI] [PubMed] [Google Scholar]
- 5.Whiteside SR, Alaimo J, Barringer WJ, et al. Practice Analysis Task Force. Alexandria, VA: American Board for Certification in Orthotics and Prosthetics, Inc; 2000. [Google Scholar]
- 6.Basmajian J, De Luca C. Muscles Alive: Their Functions Revealed by Electromyography. 5. Baltimore, MD: Williams and Williams; 1985. [Google Scholar]
- 7.Ajiboye AB, Weir RF. A heuristic fuzzy logic approach to EMG pattern recognition for multifunctional prosthesis control. IEEE Trans Neural Syst Rehabil Eng. 2005;13:280–91. doi: 10.1109/TNSRE.2005.847357. [DOI] [PubMed] [Google Scholar]
- 8.Weir RF, Troyk PR, DeMichele G, Kerns D. Technical details of the implantable myoelectric sensor (IMES) for multifunction prosthesis control. Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference; Shanghai, China. 2005. pp. 7337–40. [DOI] [PubMed] [Google Scholar]
- 9.Weir RF, Troyk PR, DeMichele GA, et al. Implantable myoelectric sensors (IMESs) for intramuscular electromyogram recording. IEEE Trans Biomed Eng. 2009;56:159–71. doi: 10.1109/TBME.2008.2005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker JJ, Scheme E, Englehart K, Hutchinson DT, Greger B. Continuous detection and decoding of dexterous finger flexions with implantable myoelectric sensors. IEEE Trans Neural Syst Rehabil Eng. 2010;18(4):424–32. doi: 10.1109/TNSRE.2010.2047590. [DOI] [PubMed] [Google Scholar]