Abstract
We have developed a simple and inexpensive method that improves sensitivity of protein and antigen detection in standard PAGE procedures. Our technique uses a sample microloader device with a funnel-like structure, filled with a 4% stacking gel. When attach to the top of a polyacrylamide slab gel the proteins in a sample are concentrated by electrophoresis into a small volume as they emerge from the device's narrow outlet. Our microloader has several advantages over previous devices, including simple assembly, high versatility and absence of cross-contamination between lanes. Addition of this device to a slab gel results in a 5-fold increase in the sensitivity of antigen detection in a western blot. As a result less protein is required for loading and signal detection. Our protocol is a straightforward modification of a standard experimental technique, and is especially useful when only limited sample quantities are available.
Keywords: Microloader, phosphoproteins, polyacrylamide gels, western blot
Polyacrylamide gel electrophoresis (PAGE) is a broadly used laboratory technique that most commonly is used to resolve proteins or nucleic acids by size [1, 2]. PAGE may be followed by Western blot to immunodetect proteins, especially those that are of low abundance [3]. Western blotting is a powerful procedure developed in the late 1970s [4, 5] that makes it possible to analyze the presence, abundance, mass, modification, and interactions of individual proteins within an extract [3, 6], However, it has several drawbacks that include the amount of sample that must be loaded onto a gel in order to detect less abundant proteins. Such a limitation is a problem for rare, minute and difficult to obtain samples.
To overcome this challenge, many protocol modifications and technologies have emerged to increase protein concentration or western blot sensitivity [6], but they are often insufficient or require special equipment. In particular, a new method has been published in which a porous loader was used to concentrate protein samples in PAGE [7]. In this system, samples are first loaded onto filter paper strips mounted on a plastic backing sheet, and afterwards an empty loader is apposed to the sample loader, creating a sandwich that prevents lateral diffusion of samples. Finally, the loaded sandwich is placed on top of the stacking gel and electrophoresis is performed as usual. By using this loading method, the authors found a significant increase in sensitivity. However, hand construction of this device is complicated and time-consuming. In our hands, at least, the use of this sandwich resulted in significant cross-contamination between adjacent samples. Here, we present a protocol that minimizes cross-contamination and maintains high sensitivity through use of a novel, easy-to-use microloader device.
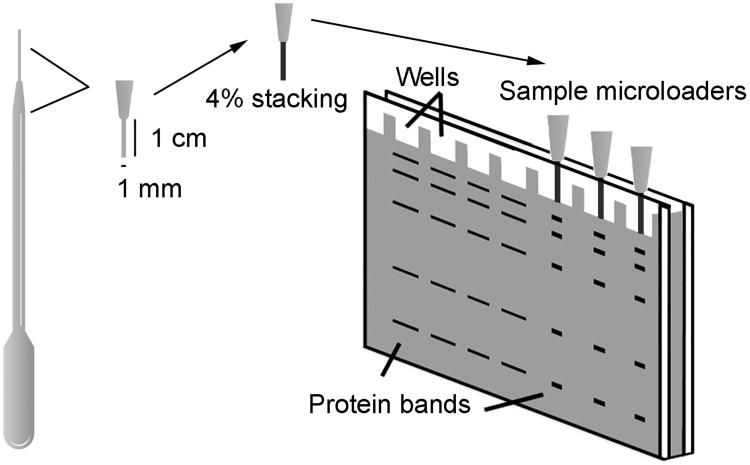
Our motivation for increasing western blot sensitivity was our need to quantify protein expression and phosphorylation using the minute quantities of tissue present in different regions of the heart in single embryonic day 12 (E12) mice [8]. Because the mutant mice that we were examining showed a variable phenotype, pooling of samples from multiple animals was undesirable. As a result, we had less than 10 μg of total protein in each sample that we needed to use to quantify phosphorylation status and total level of several different proteins by western blot. Using conventional 15-well blots, we were not able to detect most of these proteins and phospho-proteins in 2 μg of protein extracts even with the most sensitive ECL reagents (SuperSignal West Dura Chemiluminescent Substrate and Femto Trial Kit, Pierce). To increase sensitivity, we first tried the Swank protocol, but failed to obtain reproducible and high-quality western blots by using a sample loader based on their design. In our hands, protein loading was variable, mainly due to leakage and cross-contamination among adjacent wells. Next, we made several efforts to design our own sample loaders using different materials, such as pipette tips and capillary tubes. Our best results were obtained with transfer pipette tips (extended fine tip, #233, #235 Samco Scientific, USA) cut to fit the gel dimensions (Figure 1). These tips have a wide inlet that allows for easy loading of up to 15 μl and a 1 mm diameter outlet that allows them to fit into any polyacrylamide gel with at least 1 mm thickness. A 4% stacking gel was added to the outlet of the tips by using capillary action. Although not essential for sensitivity increase, we found that this gel addition improved band resolution and vertical protein separation, likely due to the different buffer composition between the stacking gel in the microloaders (125 mM Tris-HCl, pH 6.8) and the minigels (375 mM Tris-HCl, pH 8.8).
Figure 1. Microloader device preparation.
Fine tip transfer pipettes (#233, #235, Samco Scientific) were used for the assembly of sample microloaders. Pipette tips were cut to obtain an outlet passage length of 1 cm and a reservoir length less than 1 cm and a 4% polyacrylamide stacking gel was introduced through the outlet passage by capillary action. After stacking gel polymerization, sample microloaders were firmly inserted into a 1 mm-thick acrylamide gel and the reservoir was loaded with running buffer. Special care was taken to avoid bubbles and to ensure close contact between microloaders and gel. A total of 1-15 μl of sample was loaded into each microloader, and electrophoresis was performed at a maximum of 100 V until samples entered into the gel, after which voltage could be increased up to 200 V.
Using this device, by loading 1.5 μg of protein sample per lane, we were able to analyze the relative abundance of a battery of proteins and phospho-proteins in each sample, including FAK and phospho-FAK, Erk1/2 and phospho-Erk1/2, CrkL and phospho-CrkL, phospho-Smad2/3 and GAPDH [8]. In comparison, conventional SDS-PAGE followed by western blotting required more than 5 μg of total protein per 15-well lane for detecting many of these proteins and phospho-proteins. Without this device, we could not have carried out these studies without pooling of tissues from multiple embryos.
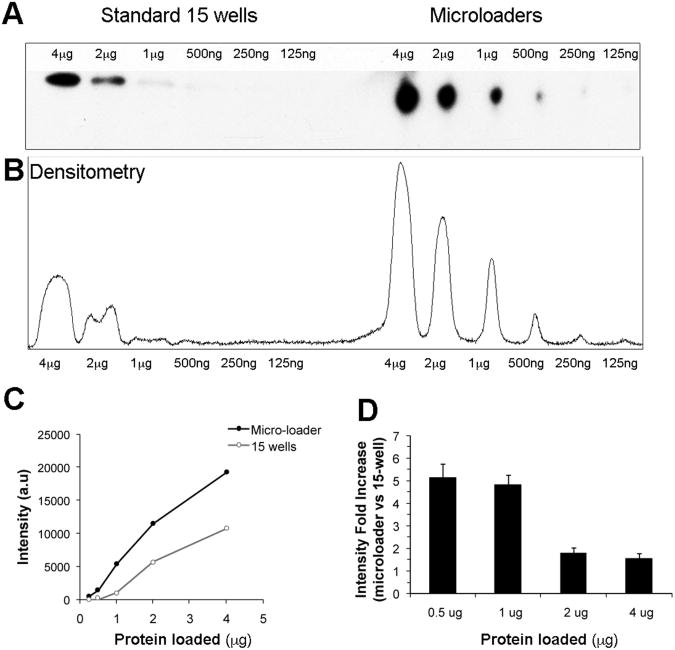
Since the linear range of the ratio of chemiluminescence signal intensity to protein concentration is quite limited [9, 10], it is important to generate a calibration curve for each protein of interest in order to accurately compare relative signal intensities across numerous samples. We have found that our method yields increased sensitivity but similar dynamic range compared to conventional western blotting (Figure 2). For this analysis, electrophoresis in a tris-glycine discontinuous buffer system was performed using the RIPA buffer extract of E12 mouse embryos outflow tract, as previously described [8]. Two-fold protein dilutions (4 μg to 125 ng) were loaded onto 15-well 4-15% SDS-PAGE gradient gels (Bio-Rad). In order to compare sensitivity on the same gel, microloaders were attached on top of the last 6 wells. As in Figure 1, the microloader device was filled partially with 4% polyacrylamide stacking gel. FAK primary antibody (A17, Santa Cruz Biotechnology, Inc) was detected with anti-rabbit HRP-conjugated secondary antibodies (10 ng/ml, Pierce), and signal was visualized using SuperSignal West Dura Chemiluminescent Substrate (Pierce). Quantification of protein bands densitometry was carried out using ImageJ 1.34s software (Wayne Rasband, NIH). Figure 2 shows a representative experiment where we obtained a 5-fold increase in FAK detection sensitivity using our microloader device when 1 μg of total protein was loaded. In our samples FAK signal was difficult to detect of reproducibly quantify when less than 2 μg of protein was loaded into a regular well. However, signal in the sub-microgram range was consistently detected and could be quantified when the same sample was loaded into a microloader attached on top of the wells. Also, linearity was maintained from 0.5 μg to 4 μg of protein loads when using microloader devices (r2 = 0.98). Interestingly, the increase in sensitivity is related with the total protein loaded, and is highest when minimum amounts of protein are loaded onto the microloader. We found that FAK signal sensitivity was increased up to 5-fold when 0.5 or 1 μg of protein extract was loaded (Figure 2D, n=3). This result could vary depending on the relative amount of the protein of interest in the protein extract.
Figure 2. Sensitivity and linear range of western blot with microloaders.
A) Representative western blot showing FAK protein detection in a single 15-well 4-15% minigel with and without use of a microloader. This figure illustrates comparison within the same gel between samples loaded directly onto wells versus samples loaded onto microloaders prefilled with a 4% stacking gel that were attached to adjacent wells. Sequential 2-fold dilutions of protein extracts from a E12 embryonic mouse were loaded and FAK signal was visualized using SuperSignal West Dura Chemiluminescent Substrate. B) The films were scanned, and ImageJ software was used to obtain densitometry plots and to quantify signal intensity. C) Densitometry results are plotted into a graph to better show sensitivity and linear range comparison. D) Comparison of FAK signal detection sensitivity in 15-wells and microloader systems in the same gels. Data are obtained from 3 independent experiments and expressed as mean ± SEM.
Our results appear to be very similar to the ones described in a previous study that used porous loaders [7]. Indeed, the final gel volume occupied by the concentrated protein is similar in both procedures. However, our modified protocol shows several advantages over previous methods. First, construction of microloaders is simple and may be done in any laboratory without special equipment. Also, attaching a microloader to the PAGE setup is straightforward it is even easier to load a sample than into a regular well, since a special pipette tip is not needed. As additional advantages, these microloaders are compatible with all formats of custom-made and precast polyacrylamide gels and can be used for other applications, such as nucleic acid polyacrylamide electrophoresis or elution-concentration of proteins cut from 2D polyacrylamide gels [11].
In summary, we have developed a simple new method for loading samples on SDS-PAGE gels that results in a significant increase in sensitivity for western blotting and therefore, requires far less total sample. The benefits to the end user are substantial compared with other techniques that require especial equipment. This procedure is rapid, inexpensive and only requires knowledge of a standard experimental technique. It is also compatible with in-gel digestion, microsequencing and other methods used for protein characterization. These features make this an appealing new tool for investigators who use SDS-PAGE and Western blotting in their laboratories. Further improvements may be possible by modification of the loading device, changes in buffer to increase the efficiency of protein concentration during stacking phase of electrophoresis, and reductions in temperature to minimize diffusion during the fractionation phase of electrophoresis and transfer of proteins onto filters [12, 13].
Acknowledgments
This work was supported by the National Institute of Health Grant 1 R01 NS19090 (L.F.R) and a Marie Curie International Reintegration Grant (A.V).
Abbreviations
- E12
Embryonic day 12
- HRP
Horseradish peroxidase
- RIPA
Radioimmunoprecipitation assay buffer
Footnotes
The authors declare that no conflict of interest exists.
References
- 1.Chrambach A, Rodbard D. Science. 1971;172:440–451. doi: 10.1126/science.172.3982.440. [DOI] [PubMed] [Google Scholar]
- 2.Weber K, Osborns M. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 3.Kurien BT, Scofield RH. In: Methods in molecular biology, protein blotting and detection. Kurien BT, Scofield RH, editors. Vol. 536. Humana Press; Totowa: 2009. pp. 9–22. [DOI] [PubMed] [Google Scholar]
- 4.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnette WN. Analytical Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 6.Kurien BT, Scofield RH. In: Methods in molecular biology, protein blotting and detection. Kurien BT, Scofield RH, editors. Vol. 536. Humana Press; Totowa: 2009. pp. 557–571. [Google Scholar]
- 7.Swank MW, Kumar V, Zhao J, Wu GY. J Neurosci Methods. 2006;158:224–233. doi: 10.1016/j.jneumeth.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Vallejo-Illarramendi A, Zang K, Reichardt LF. J Clin Invest. 2009;119:2218–2230. doi: 10.1172/JCI38194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson J, Fowler SJ. In: The Protein Protocols Handbook. Walker JM, editor. Humana Press; Totowa: 2002. pp. 429–437. [Google Scholar]
- 10.Mathrubutham M, Vattem K. Pierce Protein Research Application Note #12. Rockford: Thermo Scientific; 2005. [Google Scholar]
- 11.Kristensen DB, Inamatsu M, Yoshizato K. Electrophoresis. 1997;18:2078–2084. doi: 10.1002/elps.1150181134. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler D, Chrambach A, Ashburn P, Jovin TM. Electrophoresis. 2004;25:973–974. doi: 10.1002/elps.200305799. [DOI] [PubMed] [Google Scholar]
- 13.Harlow E, Lane D. Using Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring: 1999. pp. 267–309. [Google Scholar]