Abstract
Dynamic pupillary light reflex (PLR) is a simple neurological test that can be useful for assessment of autonomic disorders. In this study, we investigated the changes in PLR induced by mental arithmetic task and cold pressor trials which are often applied in research as model systems to elicit autonomic responses. PLR was recorded before, during and after mental arithmetic and cold pressor tasks in twenty healthy adults (ten males and ten females). Stress-induced sympathetic activation was evident as shown in the increased blood pressure during both tasks. Although the pupillary constriction amplitude did not show significant changes, both constriction time and redilation time changed during the tasks. A significant gender effect was observed in cold pressor that suggested more sympathetic activation in males and faster parasympathetic activation in females in response to light stimulation under cold pressor.
Keywords: pupillary light reflex, autonomic nervous system, stress
1. Introduction
Pupil size is controlled by the antagonistic dilator and sphincter muscles in the iris (Barbur 2004). The sphincter is innervated primarily by the parasympathetic nervous system and its contraction leads to pupil constriction. The dilator mediates pupillary dilation and is innervated primarily by the sympathetic nervous system. Pupil size undergoes a characteristic change under a sudden increase in retinal luminance: an initial rapid constriction followed with a slow redilation. Such pupillary response is referred to as pupillary light reflex (PLR). Autonomic nervous system (ANS) modulation is evident in PLR responses (Tavernor et al 2000) and the dynamic PLR parameters are considered useful for reliable ANS assessment (Bremner 2009). PLR parameters linked to the constriction phase such as the constriction amplitude and constriction time fall under parasympathetic control; whereas base pupil radius and PLR parameters measured in the redilation phase such as the redilation time are mainly governed by the sympathetic nervous system (Keivanidou et al 2010).
PLR has been found to be affected by stress and anxiety (Bakes et al 1990, Bitsios et al 2002). Bakes et al (1990) reported that the PLR constriction was smaller in patients with anxiety disorder. Bitsios et al (2002) found the threat-induced anxiety reduced the PLR constriction amplitude and a negative correlation existed between state anxiety level and PLR constriction. In addition, threat also increased the initial pupil diameter. Interestingly, two frequently applied laboratory stressors: mental stress (Yamanaka and Kawakami 2009) and cold pressor stress (Tavernor et al 2000) were also shown to cause autonomic nervous system-mediated pupil dilation. Mental arithmetic requires subjects to solve a series of arithmetic problems, which was shown to increases sympathetic activity (Freeman 2006, Liu et al 2011) and inhibit parasympathetic activity (Sloan et al 1991). Increases in blood pressure (Willemsen et al 2000) and marginal increases in heart rate (Tanida et al 2004) have been reported. The cold pressor test involves submerging the subject’s hand up to mid-forearm in a bath of ice water for 60–120s. Cold pressor induces pain and emotional distress which produce sympathetic activation with measurable increases in heart rate and blood pressure (Zygmun and Stanczyk 2010).
The effects of mental stress and cold pressor on resting pupil size have been documented previously (Yamanaka and Kawakami 2009, Tavernor et al 2000). In addition, Steinhauer et al (2000) found that an arithmetic task changed multiple dynamic PLR responses. However the effect of cold pressor on dynamic PLR responses is largely unknown. In this study, we compared changes in PLR induced by mental stress and cold pressor in healthy adult volunteers. We also examined the gender differences in dynamic pupil change, heart rate, and blood pressure during cold pressor and mental stress tests. With this study we hope to gain a better understanding of the different autonomic components of the PLR pathway, and how these components are affected by different types of stress.
2. Methods
2.1 Subjects
Twenty volunteers (age 18–21), 10 males (20.4±0.8 years old) and 10 females (19.9±0.9 years old) were recruited from the student population at the University of Missouri-Columbia. All were in good health and had no history of eye-related disorders. Before the experiment, each subject completed the State-Trait Anxiety Inventory for Adults (Spielberger et al 1970). Subject anxiety levels were examined based on their current state and enduring personality traits.
The study was approved by the university IRB board, and all subjects gave informed consent prior to participating in the experiment.
2.2 PLR Measurements
PLR was measured using a two-channel pupillographic system (Daluwatte et al 2012). During the test, participants looked at a screen 0.6 m away through a view port in the system. The screen was covered with a dark red film to avoid affecting pupil size. The 0.1 sec stimulation light was produced by green LEDs at 530 nm. The stimulation light had an intensity of 3.3×10−5 W/m2 and a field size of 5.3° measured at the position of the eye, leading to a luminous intensity of 0.74 cd/m2. All tests were conducted in the morning between 9:30 a.m. and 12:10 p.m in a light adapted condition with a room illumination of 255 Lux.
The light stimulus was applied to the right eye in odd numbered trials and to the left eye in even numbered trials during the mental arithmetic task. Due to the time limit in the cold pressor task, only right eyes were stimulated. The image sequences of both pupils were recorded on CCD cameras (119 fps) for the entirety of each measurement. The stimulus was presented 1 s after image acquisition started. Each measurement lasted 5 sec with a minimal of 30 sec interval in between consecutive measurements. The imaging system had a spatial resolution of 46 µm/pixel and approximately 120 pixels were subtended by an average pupil.
Custom image processing (Fan et al. 2009) was applied to automatically calculate the pupil size from each image frame recorded during the 5 sec acquisition. This method used a histogram-based thresholding (Fan et al. 2009) to segment pupils from eye images. The accuracy of pupil size calculation was on the scale of the pixel resolution of the imaging system (46 µm). To characterize pupillary response, the following six PLR parameters were calculated: (1) the resting pupil radius (R0); (2) the minimal pupil radius (Rm) during constriction; (3) the percentage constriction calculated as ; (4) the latency (TL) calculated as the time interval between stimulus onset and the beginning of pupil constriction; (5) the constriction time (TC) calculated as the time interval between the beginning of pupil constriction and when pupil reached minimal diameter Rm; (6) the redilation time (TR) calculated as the time interval between the minimal diameter Rm and when the pupil recovered to half of the constriction.
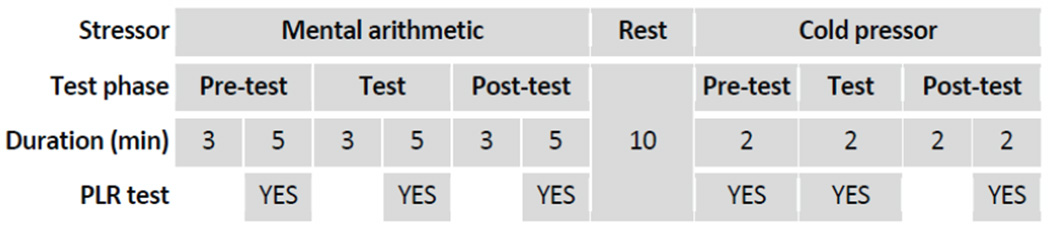
The PLR recording procedure in the mental arithmetic and cold pressor tests is illustrated in figure 1. As described before, each test was divided into three different test phases: pre-test, test, and post-test.
Figure 1.
An illustration of the PLR measurements during the mental arithmetic and cold pressor tests. Each test was divided into three phases: pre-test, test, and post-test.
2.3 Heart Rate and Blood Pressure Monitors
Each participant’s heart rate was measured by a heart rate monitor (RS800CX, Polar, Kempele, Finland) at 1 kHz acquisition rate during the entire test session. Participant’s blood pressure was also measured periodically throughout the test via blood pressure monitor (VSM 6000 series, Welch Allyn, Skaneateles Falls, New York, USA). Two blood pressures were taken in each segment of the test, for a total of twelve readings per subject. Blood pressures were taken from the left arm. Subjects were asked to minimize movement throughout the test to improve blood pressure acquisition.
All measurements obtained during each of the pre-test, test, and post-test phases were averaged to calculate the mean arterial blood pressure (MAP) and heart rate (HR) of each participant during each phase.
2.4 Procedure
Mental arithmetic
Before starting the mental arithmetic task (“pre-test” phase), subjects were first asked to read aloud numbers shown on the computer screen for three minutes in order to establish an attention baseline. The numbers were integer numbers from 5–8700. At the three minute mark, 8 measurements were taken while the subjects continued to read the numbers.
Participants were then given instructions to solve either math problems or visual patterns that appeared on the screen. The problems consisted of simple addition, subtraction, multiplication, division, and algebra. The visual pattern questions depicted four segments of a pattern and asked the participant to identify the fifth segment. Participants were informed that their answers would be recorded. They were given a maximum of 8 seconds to solve each problem. They were asked to say the solutions out loud and to solve the problems as quickly as possible. At the three minute mark, 8 measurements were taken while the subjects continued to solve problems.
Immediately after the problem solving section, subjects were asked to rate their feelings about the problems on a four level scale (1=easy, 2=ok, 3=difficult, 4=frustrated). Subjects were then shown scenic pictures selected to be easily visible through the red film. At the three minute mark, 8 measurements were taken while the subjects continued to look at the pictures. Subjects rested for 10 minutes between the mental stress and cold pressor sections of the test.
Cold pressor
For the cold pressor portion of the test, four baseline measurements were taken while participants were shown scenic pictures on the screen. Participants were then asked to place their right hand and forearm in cold water (5°C) for two minutes. Four more measurements were taken during submersion. At the end of 2 minutes, the subjects were instructed to remove their hand and dry it. Immediately after the test, participants were asked to subjectively rate their pain level during the test on a four degree scale from 1=none to 4=severe. Subjects were allowed to rest for 2 minutes. At the end of the rest period, four measurements were taken.
2.5 Data processing and statistical analysis
The Kolmogorov-Smirnov test (Massey 1951) was used to verify normal distributions of all measured parameters. A repeated measures ANOVA model (PROC ANOVA procedure in SAS) was used to test the effects of test phase, gender, as well as their interaction. Test phase was treated as the within-subject effect and gender was treated as a between-subject effect. Post hoc paired t-test was used to confirm effects of tasks and one way ANOVA model was used to confirm gender effect. A p value < 0.05 was considered significant.
3. Results
The total score for state anxiety ranged from 20–46 (30.9±6.4), and scores for trait anxiety ranged from 20–52 (34.4±8.3). Neither male subjects (State=31.1±7.9, Trait=32.3±8.4) nor female subjects (State=30.6±4.9, Trait=36.8±7.4) differed significantly from the sample of college students reported by Spielberg (1970). There was no significant difference between male and female subjects on state or trait anxiety (Student t-test t18 = −0.17 p=0.9 and t18 = 1.27, p = 0.2 for state anxiety and trait anxiety, respectively). The Kolmogorov-Smirnov test indicated that all measured PLR parameters, MAP and HR values followed a normal distribution at every test condition.
3.1. Mental arithmetic task
Subjects reported an average score of (3.4±0.9) on the subjective difficulty scale administered at the conclusion of the mental arithmetic section. Females reported a significantly higher difficulty rating (4.0±0.0) than male subjects (2.8±0.9) (Student t-test t9 = −4.12, p < 0.01).
3.1.1 Blood pressure and heart rate
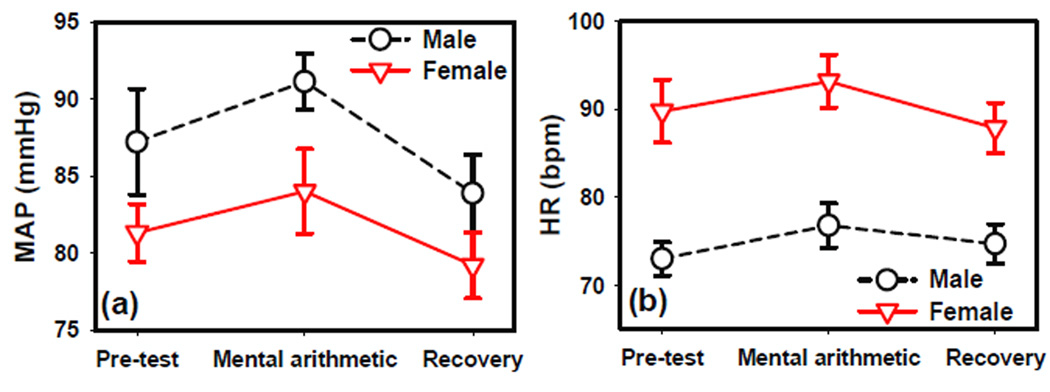
The repeated measures ANOVA reported significant test phase effect (F2,28=6.19 p=0.006) and significant gender effect (F1,14=4.85 p=0.045) on MAP, while the interaction between gender and test phase was not significant. Males showed higher mean baseline MAP than females (figure 2a). During the mental arithmetic task, MAP increased significantly in both genders. In females, the MAP decreased during the recovery phase to a level lower than that of the pre-test (Paired t-test between recovery and pre-test: t7 = 3.25, p = 0.014) (figure 2b). A similar trend was observed in males, but did not reach statistical significance.
Figure 2.
The effects of mental arithmetic on (a) mean arterial blood pressure (MAP) and (b) heart rate (HR). Error bars shown indicate standard error.
The repeated measures ANOVA also reported significant test phase effect (F2,36=8.58 p=0.0009) and gender effect (F1,18=17.49 p=0.0006) on average heart rate, while the interaction between gender and test phase was not significant. Males had lower HR than females (figure 2b). During the mental arithmetic task, HR increased significantly and during the recovery phase, recovered to pre-test level in both genders.
3.1.2 PLR parameters
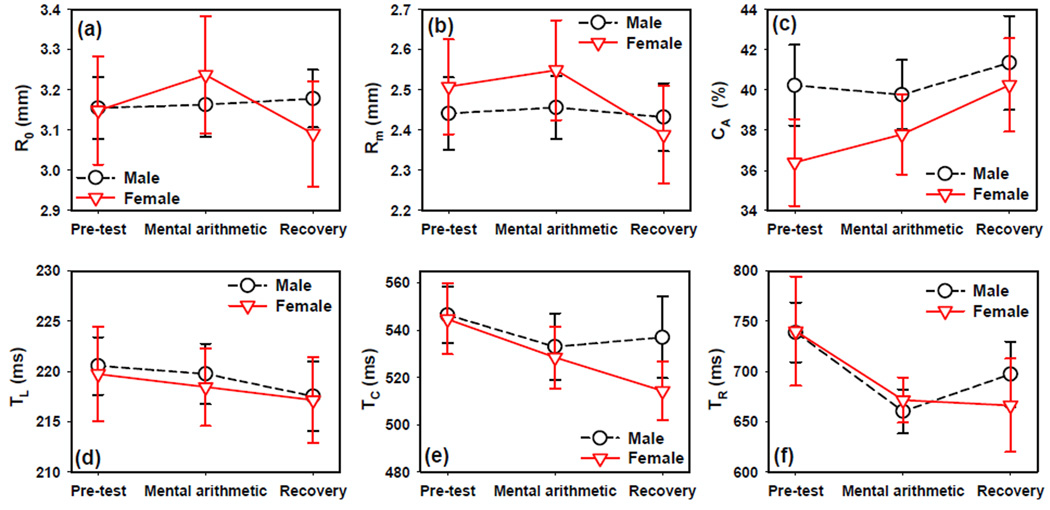
During the pre-test phase, females and males had similar PLR parameters (figure 3). A trend for higher PLR constriction CA in males than females was observed (figure 3c), though the difference was not statistically significant (Student t-test t18 = −1.30, p = 0.21).
Figure 3.
The effects of mental arithmetic task on PLR parameters including (a) resting pupil radius R0, (b) minimal pupil radius Rm, (c) relative constriction CA, (d) latency TL, (e) constriction time TC, and (f) recovery time TR. Error bars indicate standard error.
The repeated measures ANOVA reported significant test phase effect on minimal pupil radius Rm (F2,36=3.38 p=0.045), constriction CA (F2,36=6.35 p=0.005), latency TL (F2,36=3.39 p=0.045), constriction time TC (F2,36=5.79 p=0.007) and recovery time TR (F2,36=8.35 p=0.001). Neither gender nor the interaction between gender and test phase had a significant effect on any PLR parameters.
The average recovery time TR and constriction time TC decreased during the mental arithmetic task in both males and females (figure 3). However, these decreasing trends reached statistically significance only in males on TR (Paired t-test t9 = 3.85, p=0.004). The resting pupil size R0, minimal pupil radius Rm, and constriction CA had an increasing trend during the mental arithmetic task in females only, but these increases did not reach statistical significance in paired t-test.
During the recovery phase, the recovery time TR returned to the pre-test value in males. However TR did not change in females and was still significantly smaller than the pre-test value (Paired t-test between pre-test and recovery: t9 = 3.47, p = 0.007). TC continued to decrease into the recovery phase in females (Paired t-test between pre-test and recovery: t9 = 2.97, p = 0.016), but not in males. CA increased in both gender groups during the recovery period, but was significant only in females (Paired t-test t9 = −3.54, p=0.0063 in females). The decreasing trend in TL continued into the recovery phase but only males had a significantly smaller TL than the pre-test phase (Paired t-test t9 = 2.41, p = 0.039 in males). During the recovery phase, Rm remained the same in males but decreased in females and became smaller than the pre-test values (Paired t-test between pre-test and recovery: t9 = 3.22 p = 0.011).
3.2. Cold pressor task
Participants reported an average score of (2.8±1.1) on the subjective pain scale administered after CP. There was no significant difference in subjective pain reported by males (2.4±1.0) and females (3.1±1.1).
3.2.1 Blood pressure and heart rate
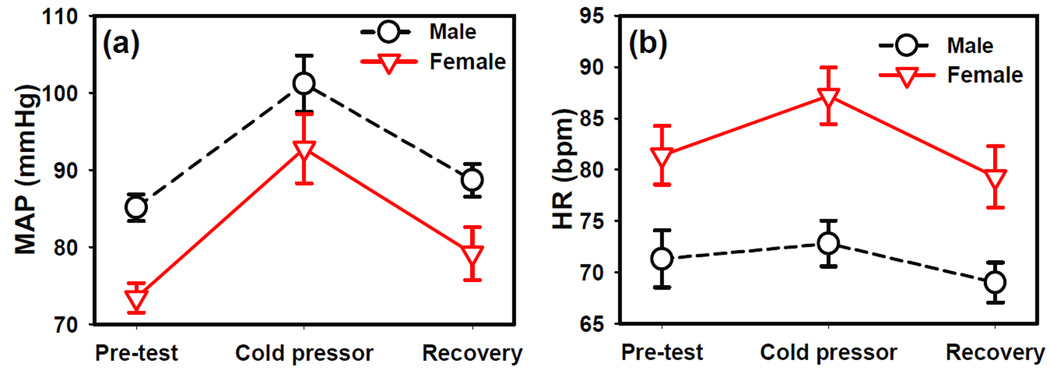
The repeated measures ANOVA reported significant test phase effect (F2,26=34.69 p<0.0001) and gender effect (F1,13=7.29 p=0.0182) on MAP. The interaction between gender and test phase was not significant. The baseline MAP was higher in males than females (figure 4a). MAP increased significantly during cold pressor in both genders (figure 4a) and dropped back to baseline during the recovery period.
Figure 4.
The effects of cold pressor task on (a) mean arterial blood pressure (MAP) and (b) heart rate (HR). Error bars shown indicate standard error.
The repeated measures ANOVA also revealed significant test phase effect (F2,36=5.65 p=0.007) and gender effect (F1,18=13.96 p=0.002) on average heart rate, while the interaction between gender and test phase was not significant. The baseline HR was higher in females than males (figure 4b). The HR increased during cold pressor and recovered during the recovery period.
3.2.2 PLR parameters
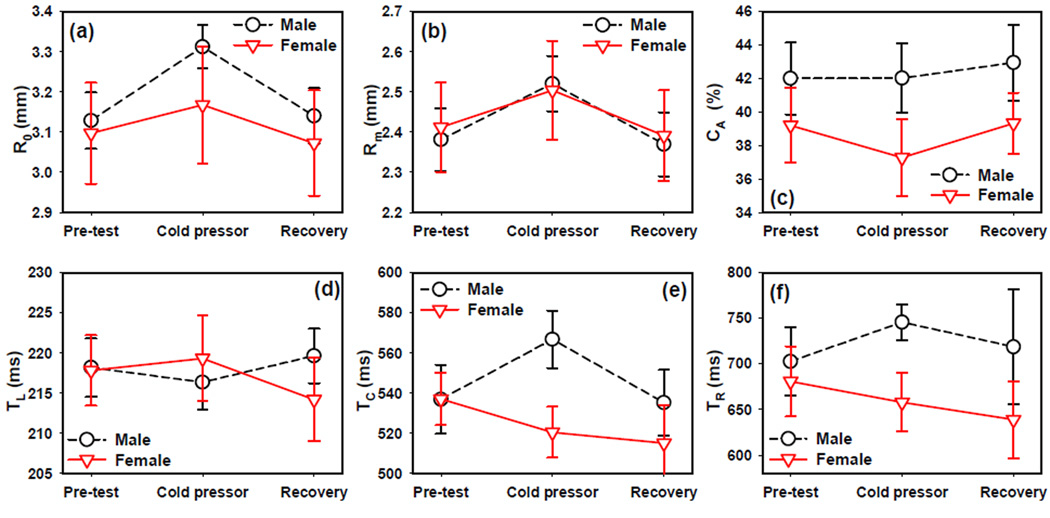
The repeated measures ANOVA indicated that test phase had significant effects on base pupil radius R0 (F2,36=20.12 p<0.0001) and minimal pupil radius Rm (F2,36=19.49 p<0.0001). The interaction between gender and test phase was significant for PLR latency TL (F2,36=4.13 p=0.032) and constriction time TC (F2,36=3.61 p=0.049). No other effects or interactions were found significant.
The base pupil radius R0 and minimal pupil radius Rm increased in both genders (figure 5a and figure 5b respectively) during the cold pressor submersion. However this increase was significant only in males for R0 (Paired t-test t9 = −6.86, p<0.0001) while the increase in Rm was significant for both genders (Paired t-test t9 = −5.48, p=0.0004 in males and t9 = −2.50, p = 0.034 in females). In males, the constriction time TC increased during cold pressor (Paired t-test t9 = −3.72, p=0.005) but showed a decreasing trend in females which was not significant (figure 5e). PLR latency TL did not change significantly during cold pressor submersion in either gender (figure 5d). The PLR constriction CA decreased slightly in females during cold pressor submersion, but this change was not statistically significant (figure 5c). The redilation time TR showed an increasing trend in males but a decreasing trend in females although neither reached statistical significance in paired t-test.
Figure 5.
The effects of cold pressor task on PLR parameters including (a) resting pupil radius R0, (b) minimal pupil radius Rm, (c) relative constriction CA, (d) latency TL, (e) constriction time TC, and (f) recovery time TR. Error bars indicate standard error.
During the recovery phase, R0, Rm, and TC all recovered to the pre-test level in both genders. TR in males reversed the increase trend in cold pressor and recovered back to the pre-test level; whereas it continued to decrease in females although its value was still not significantly different from the pre-test value. The latency TL decreased during recovery period in females and became smaller with respect to pre-test (Paired t-test t9 = 2.38, p = 0.041).
4. Discussion
Consistent with previous reports (Hellstrom and Lundberg 2000), females had a lower resting blood pressure which suggested a lower sympathetic activity and was attributed to greater baroreflex inhibitive control of sympathetic activity in females (Hogarth et al 2007). In addition, females had a higher resting heart rate due to vagal withdrawal (Hogarth et al 2007).
The MAP and HR increased in both genders during both the mental arithmetic (MA) and cold pressor (CP) tasks. This observation has been frequently documented in literature (Sloan et al 1991, Willemsen et al 2000, Tanida et al 2004, Freeman 2006, Zygmunt and Stanczyk 2010, Liu et al 2011). These changes suggest an elevated sympathetic activation during these two tasks. Notably, the MAP increased more significantly in CP than MA (26.4% vs. 3.3% increase in females and 18.9% vs. 4.5% increase in males during CP task), suggesting that CP has a stronger effect on sympathetic activation than MA.
The resting pupil size is controlled by the balance between the sympathetic tone and parasympathetic tone. The sympathetic activation during MA and CP shifts the original balance and increases the resting pupil size as reported in previous studies (Tavernor et al 2000, Yamanaka and Kawakami 2009). The increase in pupil size is related to the strength of stimulus or task demand (Hess and Polt 1964, Beatty 1982, Steinhauer et al 2000). In this study, the resting pupil size only showed an increasing trend in females during MA, most likely because females considered MA more challenging in this study. In CP, both genders reported a similar pain level. However males had much larger increases in resting pupil size than females during CP (5.8% increase in males vs. 2.3% in females). This seems to be consistent with the prior observation that males show more sympathetic activity than females under stress (Dart et al 2002, Sato and Miyake 2004).
Besides the resting pupil size, other PLR parameters provide assessment of the dynamic ANS activation induced by the flash light stimulation. The constriction time and redilation time showed noticeable changes during the two tasks. Females had decreased constriction time in both MA and CP. Because the constriction amplitude was relatively stable, a faster pupil constriction process indicated that MA and CP induced stress accelerated parasympathetic activation in females. This seems to be in agreement with previous reports of increased parasympathetic response in females under stress (Sato and Miyake 2004, Nugent et al 2011). However, it is interesting to note that in males the constriction time increased in CP. As shown in previous studies, stress may induce more sympathetic increase in males (Dart et al 2002, Sato and Miyake 2004). Therefore, an elevated sympathetic activity may have suppressed the parasympathetic activation in males during CP and slowed the pupillary constriction.
Under the traditional model of autonomic pupillary control (Barbur 2004), pupil constriction is under the control of the parasympathetic nervous system, and pupil dilation is under the control of the sympathetic nervous system. Due to the stress-induced imbalance in the parasympathetic and sympathetic system, one would think a different trend would have been observed in the constriction and redilation times. However, the same trend (decrease or increase) was observed in both constriction time and redilation time across both test conditions and genders. An explanation for this correlation may lie in the timeline of constriction and dilation; parasympathetic withdrawal is believed to influence the first stage of redilation (Bremner 2009). In other words, the redilation time measured in this study may still be influenced by the parasympathetic activity.
5. Conclusion
Both MA and CP tests were able to elicit changes in HR, MAP, as well as in PLR parameters. Our results showed that static and dynamic parameters of the pupil are modulated differently by the changes in autonomic nervous system. The resting pupil size is more strongly affected by the sympathetic nervous system. In addition, males and females have differing levels of sympathetic and parasympathetic tone, which predisposes them to react to the same stimulus in different ways. Males generally have a higher sympathetic tone than females at rest, and they appear more likely to produce a sympathetic nervous response to a stressor. Females generally have a higher parasympathetic tone than males at rest, and thus are more likely to mount a parasympathetic response to stressful stimuli. These results provide useful information to further validate and understand the effects of autonomic nervous system modulation on the pupil light reflex.
Acknowledgement
The authors thank Randima Dinalankara for his technical assistance on the pupillogram recording system. This study was partially supported by National Institute of Neurological Disorders and Stroke (1R21NS070299-01) and U. S. Army Medical Research Materiel Command (DoD W81XWH-10-1-0474). However neither funding source was involved in the study design, data collection, analysis, interpretation, and manuscript writing.
References
- Bakes A, Bradshaw CM, Szabadi E. Attention of the pupillary light reflex in anxious patients. Br. J. Clin. Pharmacol. 1990;30:377–381. doi: 10.1111/j.1365-2125.1990.tb03787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbur JL. The Visual Neurosciences. Cambridge, MA: MIT Press; 2004. Learning from the pupil. [Google Scholar]
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin. 1982;91:276–292. [PubMed] [Google Scholar]
- Bitsios P, Szabadi E, Bradshaw CM. Relationship of the 'fear-inhibited light reflex' to the level of state/trait anxiety in healthy subjects. Int. J. Psychophysiol. 2002;43:177–184. doi: 10.1016/s0167-8760(01)00173-8. [DOI] [PubMed] [Google Scholar]
- Bremner F. Pupil evaluation as a test for autonomic disorders. Clin. Auton. Res. 2009;19:88–101. doi: 10.1007/s10286-009-0515-2. [DOI] [PubMed] [Google Scholar]
- Daluwatte C, Miles JH, Yao G. Simultaneously measured pupillary light reflex and heart rate variability in healthy children. Physiol. Meas. 2012;33:1043–1052. doi: 10.1088/0967-3334/33/6/1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart AM, Du XJ, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc. Res. 2002;53:678–687. doi: 10.1016/s0008-6363(01)00508-9. [DOI] [PubMed] [Google Scholar]
- Fan X, Miles JH, Takahashi N, Yao G. Sex-specific lateralization of contraction anisocoria in transient pupillary light reflex. Invest. Ophthalmol. Vis. Sci. 2009;50:1137–1144. doi: 10.1167/iovs.08-2329. [DOI] [PubMed] [Google Scholar]
- Freeman R. Assessment of cardiovascular autonomic function. Clin. Neurophysiol. 2006;117:716–730. doi: 10.1016/j.clinph.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Hellstrom B, Lundberg U. Pain Perception to the Cold Pressor Test during the Menstrual Cycle in Relation to Estrogen Levels and a Comparison with Men. Integr. Psychol. Behav. Sci. 2000;35:132–141. doi: 10.1007/BF02688772. [DOI] [PubMed] [Google Scholar]
- Hess EH, Polt JM. Pupil size in relation to mental activity during simple problem-solving. Science. 1964;143:1190–1192. doi: 10.1126/science.143.3611.1190. [DOI] [PubMed] [Google Scholar]
- Hogarth AJ, Mackintosh AF, Mary DASG. Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin. Sci. 2007;112:353–361. doi: 10.1042/CS20060288. [DOI] [PubMed] [Google Scholar]
- Keivanidou A, Fotiou D, Arnaoutoglou C, Arnaoutoglou M, Fotiou F, Karlovasitou A. Evaluation of autonomic imbalance in patients with heart failure: A preliminary study of pupillomotor function. Cardiol. J. 2010;17:65–72. [PubMed] [Google Scholar]
- Liu X, Iwanaga K, Koda S. Circulatory and central nervous system responses to different types of mental stress. Ind. Health. 2011;49:265–273. doi: 10.2486/indhealth.ms1272. [DOI] [PubMed] [Google Scholar]
- Massey FJ. The Kolmogorov-Smirnov Test for Goodness of Fit. J. Am. Statist. Assoc. 1951;46:68–78. [Google Scholar]
- Nugent AC, Bain EE, Thayer JF, Sollers JJ, Drevets WC. Sex differences in the neural correlates of autonomic arousal: A pilot PET study. Int. J. Psychophysiol. 2011;80:182–191. doi: 10.1016/j.ijpsycho.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Miyake S. Cardiovascular reactivity to mental stress: Relationship with menstrual cycle and gender. J. Physiol. Anthropol. Appl. Human Sci. 2004;23:215–223. doi: 10.2114/jpa.23.215. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Korten JB, Myers MM. Components of heart rate reactivity during mental arithmetic with and without speaking. Physiol. Behav. 1991;50(1039) doi: 10.1016/0031-9384(91)90434-p. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch A, Lushene R. The State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Steinhauer SR, Condray R, Kasparek A. Cognitive modulation of midbrain function: task-induced reduction of the pupillary light reflex. Int. J. Psychophysiol. 2000;39:21–30. doi: 10.1016/s0167-8760(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Siegle GJ, Condray R, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. Int. J. Psychophysiol. 2004;52:77–86. doi: 10.1016/j.ijpsycho.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Tanida M, Sakatani K, Takano R, Tagai K. Relation between asymmetry of prefrontal cortex activities and the autonomic nervous system during a mental arithmetic task: near infrared spectroscopy study. Neurosci. Lett. 2004;369:69–74. doi: 10.1016/j.neulet.2004.07.076. [DOI] [PubMed] [Google Scholar]
- Tavernor SJ, Abduljawad KAJ, Langley RW, Bradshaw CM, Szabadi E. Effects of pentagastrin and the cold pressor test on the acoustic startle response and pupillary function in man. J. Psychopharmacol. 2000;14:387–394. doi: 10.1177/026988110001400407. [DOI] [PubMed] [Google Scholar]
- Willemsen G, Ring C, Mckeever S, Carroll D. Secretory immunoglobulin A and cardiovascular activity during mental arithmetic: effects of task difficulty and task order. Biol. Psychol. 2000;52:127–141. doi: 10.1016/s0301-0511(99)00028-9. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Kawakami M. Convenient evaluation of mental stress with pupil diameter. Int. J. Occup. Saf. Ergon. 2009;15:447–450. doi: 10.1080/10803548.2009.11076824. [DOI] [PubMed] [Google Scholar]
- Zygmunt A, Stanczyk J. Methods of evaluation of autonomic nervous system function. Arch. Med. Sci. 2010;6:11–18. doi: 10.5114/aoms.2010.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]