Abstract
Human inhalation exposures to manufactured nanoparticles (NP) and airborne ultrafine particles (UFP) continues to increase in both occupational and environmental settings. UFP exposures have been associated with increased cardiovascular mortality and morbidity, while ongoing research supports adverse systemic and cardiovascular health effects after NP exposures. Adverse cardiovascular health effects include alterations in heart rate variability, hypertension, thrombosis, arrhythmias, increased myocardial infarction, and atherosclerosis. Exactly how UFP and NP cause these negative cardiovascular effects is poorly understood, however a variety of mediators and mechanisms have been proposed. UFP and NP, as well as their soluble components, are known to systemically translocate from the lung. Translocated particles could mediate cardiovascular toxicity through direct interactions with the vasculature, blood, and heart. Recent study suggests that sensory nerve stimulation within the lung may also contribute to UFP- and NP-induced acute cardiovascular alterations. Activation of sensory nerves, such as C-fibers, within the lung may result in altered cardiac rhythm and function. Lastly, release of pulmonary-derived mediators into systemic circulation has been proposed to facilitate cardiovascular effects. In general, these proposed pulmonary-derived mediators include pro-inflammatory cytokines, oxidatively-modified macromolecules, vasoactive proteins, and prothrombotic factors. These pulmonary-derived mediators have been postulated to contribute to the subsequent prothrombotic, atherogenic, and inflammatory effects after exposure. This review will evaluate the potential contribution of individual mediators and mechanisms in facilitating cardiopulmonary toxicity following inhalation of UFP and NP. Lastly we will appraise the literature and propose a hypothesis regarding the possible role of mast cells in contributing to these systemic effects.
Keywords: Mast cell, IL-33, LOX-1, RAGE, particulate matter
Introduction
Particulate matter (PM) is well-known to be associated with a variety of adverse health effects including diseases of the pulmonary, vascular, and cardiac systems (Pope 2000, Pope, Dockery 2006, Breitner et al. 2011, Hoek et al. 2001). PM is classified based upon its diameter into three groups PM10, PM2.5, and ultrafine particles (UFP). Of the different types of PM, UFP have been postulated to significantly contribute to the adverse health effects associated with PM exposure (Seaton et al. 1995). Due to the extremely small size (aerodynamic diameter < 100nm), UFP are able to deposit deep within the lung after inhalation and evade many mechanisms responsible for the clearance of larger particles (Sanchez-Crespo et al. 2011). Recently, there has been a dramatic increase in the development of engineered ultrafine particles or nanoparticles (NP) due to their high applicability in the biomedical field and various industries. Human exposures to NP are likely to increase as a result of this rapidly growing field and therefore warrant toxicological evaluation.
Epidemiology has identified correlations between exposure to UFP and increased pulmonary and cardiovascular morbidity and mortality (Sannolo et al. 2010, Strak et al. 2010, Ibald-Mulli et al. 2002). Furthermore, recent toxicological investigation has also demonstrated the ability of NP to cause negative cardiopulmonary health effects (Kang et al. 2011, Park et al. 2011, Wang et al. 2010). It has been postulated that upon inhalation, UFP and NP are increasingly toxic compared to larger particles because of their increased reactivity, surface area, particle number on a mass basis, deposition potential, and their ability to translocate to other organ systems such as the cardiovascular and/or neuronal system and illicit adverse effects (Frampton 2001).
The lung is the initial site of deposition after inhalation of UFP and NP and therefore is one of the primary targets of toxicity. Inhalation exposure of animals to nanoparticles such as CeO2, TiO2, SiO2, NiO, FeO, ZnO, single- and multi- walled carbon nanotubes have demonstrated a variety of pulmonary effects including cytotoxicity, inflammation, apoptosis, oxidative stress, damage to the epithelial air interface, fibrotic changes, and impaired pulmonary function in healthy animals (Wang et al. 2010, Horie et al. 2011, Yazdi et al. 2010, Ma et al. 2011, Cho et al. 2010, Li et al. 2010a, Bonner 2010, Wang et al. 2011). Despite the primary route of exposure to UFP and NP being inhalation, a principal site of toxicity appears to be the cardiovascular system. Specifically, epidemiological studies have demonstrated correlations between human UFP inhalation exposures and both increased cardiovascular and respiratory diseases compared to larger fractions of PM, however, the epidemiological or occupational evidence has yet to emerge for NP exposures (Franck et al. 2011, Belleudi et al. 2010). Individuals exposed to UFP are known to specifically suffer from alterations in heart rate variability, arrhythmias, and increased incidences of myocardial infarction and stroke (Delfino et al. 2005, Chuang et al. 2005, Lanki et al. 2006, Andersen et al. 2010). One could expect similar findings in humans exposed to NP, as animal exposures to NP have been shown to produce similar cardiovascular effects (Zhao et al. 2010, LeBlanc et al. 2010, Chen et al. 2008). As with UFP (Pope et al. 2006, Devlin et al. 2003), it is possible that those individuals with preexisting cardiovascular diseases are increasingly susceptible to adverse health effects of NP exposure. Both UFP and NP have been shown to cause vasoconstriction, initiate prothrombotic changes, and induce the development of atherosclerotic lesions in animal studies which likely contribute to adverse cardiovascular events (Elder et al. 2004).
Currently, little is known regarding the individual and/or combined contribution of specific mediators and mechanisms that are responsible for the cardiovascular effects associated with inhaled UFP and NP. Research however suggests a few possible mediators and mechanisms contributing to these cardiovascular effects as outlined in Figure 1. These mechanisms include: 1) UFP and NP may activate sensory nerve fibers within the lung resulting in modified cardiovascular function; 2) based on the size of UFP and NP they may be able to translocate from the lung and directly affect the cardiovascular system; 3) UFP and NP exposure may result in the release of a variety of pulmonary-derived mediators into circulation thereby initiating adverse cardiovascular outcomes. The focus of this review will be examining these mediators and mechanisms of cardiopulmonary interactions that lead to systemic and cardiovascular alterations following UFP and NP inhalation exposures. Although UFP and NP differ physicochemically, there are likely commonalities in biological responses after exposure based on their size and shared mechanisms of toxicity. Furthermore, due to the limited study of the most highly produced NP (Hendren et al. 2011), the toxicological examination of UFP can be utilized to understand potential mediators involved in NP-induced adverse health effects. Table 1 highlights our current knowledge and gaps in knowledge of NP cardiopulmonary interactions based upon the proposed mechanisms associated with UFP exposure. This review will critically analyze what is currently known regarding mediators of pulmonary and cardiovascular toxicity of inhaled ambient fine PM, ambient UFP and newly emerging work on NP to postulate hypotheses regarding cardiopulmonary interactions.
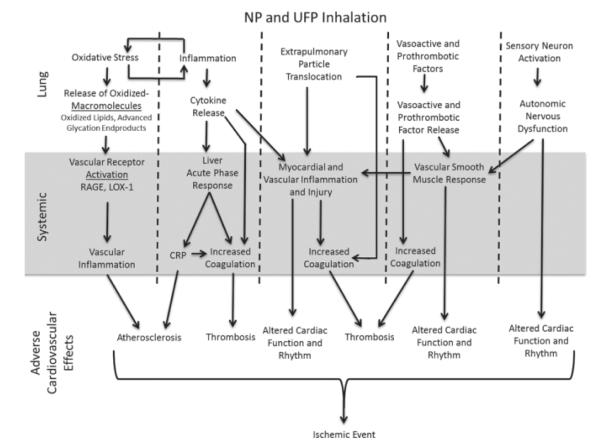
Figure 1.
Possible mediators and mechanisms of cardiovascular toxicity following NP and UFP inhalation exposure. After inhalation, NP and UFP may translocate and induce cardiovascular toxicity, while particles remaining within the lung may alter cardiac function via autonomic nervous system dysfunction. Pulmonary inflammation and oxidative stress may lead to increases in systemic levels of oxidized-macromolecules, cytokines, and vasoactive and prothrombotic factors which can systemically result in adverse cardiovascular health effects.
Table 1.
Assessment of current knowledge regarding possible mechanisms responsible for cardiovascular toxicity after pulmonary exposure to the five highest produced nanoparticles.
| Nanoparticle | Translocation | Autonomic Dysfunction |
Systemic Effects Following Pulmonary Exposure |
Adverse Mast Cell Responses |
|---|---|---|---|---|
| TiO2 | Altered alveolar-capillary barrier in mice (Li et al. 2010c) |
NA | Increased serum LDL and LDH in rats (Tang et al. 2011) Microvascular oxidative stress and dysfunction in Sprague Dawley rats (Nurkiewicz et al. 2009) |
Increased intracellular Ca2+ and histamine secretion after rat mast cell exposure (Chen et al. 2012) |
| Silver | NA | NA | NA | Increased degranulation of RBL-2H3 cells (Yang et al. 2010) |
| CeO2 | NA | NA | Liver damage and acute phase response in Sprague Dawley rats (Nalabutu et al. 2011) Impaired vascular relaxation and enhanced myocardial ischemia/reperfusion injury (Wingard et al. 2011) |
Increased production of PGD2, TNF-α, IL-6, and osteopontin and contribution to altered vascular reactivity and ischemia/reperfusion injury (Wingard et al. 2011) |
|
Carbon
Nanotubes |
Movement of MWCNT to lung- associated lymph nodes in F344 rats (Aiso et al. 2011) Movement of MWCNT to sub- pleural wall (Ryman-Rasmussen et al. 2009) |
Altered baroreflex function in Wistar Kyoto rats exposed to SWCNT (Legramante et al. 2009) |
Increased endothelin-1 and angiotensin 1- converting enzyme in plasma after SWCNT exposure in Spontaneously Hypertensive rats (Ge 2011) Increased serum oxidative stress after MWCNT exposure in Wistar rats (Reddy et al. 2011) Increased myocardial infarct size and decreased functional recovery after ex vivo ischemia / reperfusion in CD-1 mice exposed to acid functionalized SWCNT exposure (Tong et al. 2009) Accelerated plaque development in ApoE (-/-) mice exposed to SWCNT (Li et al. 2007) Increases in marker circulating markers of pro-thrombosis in Swiss mice exposed to MWCNT (Nemmar et al. 2007) Increase serum cytokines and acute phase response in mice exposed to CNT (Erdely et al. 2011) |
NA |
| Fullerenes | Alveolar epithelial and endothelial cell pinocytosis and movement to lung-associated lymph nodes in ICR mice after C60 exposure (Naota et al. 2009) |
NA | NA | NA |
The extensive study of combustion-source ultrafine particles has demonstrated mechanisms by which inhalation can result in cardiovascular toxicity. Possible mechanisms include autonomic dysfunction, the release of pulmonary-derived mediators into the circulation, direct systemic effects of particles after translocation, and the activation of mast cells. Based on similarities between ultrafine and nanoparticles, analogous mechanisms may be responsible for the cardiovascular toxicity following inhalation. The table above highlights what is currently known regarding these proposed mechanisms for nanoparticle exposures and also demonstrates the existing gaps in our knowledge.
Evidence of Autonomic Nervous System Impairment
Vagal sensory receptors such as C-fibers innervate the airways and the lung and are vital in regulating respiratory and cardiovascular function (Taylor-Clark and Undem 2011). The activation of C-fibers can initiate a protective chemoreflex response causing apnea, bronchospasm, vasodilation, and reduced cardiac output via bradycardia and decreased contractility after inhalation of various irritants (Coleridge, Coleridge 1994). Inhaled gas and PM have been shown to activate sensory receptors in the pulmonary system thereby stimulating the nervous system’s vagal response and ultimately mediating the acute cardiac effects following exposure. Specifically, exposure of rats to acrolein has been shown to stimulate airway C-fibers causing a decrease in breathing frequency and a drop in heart rate, while exposure to ozone was also found to activate C-fibers in the lung (Hazari et al. 2008, Ho, Lee 1998). It has been postulated that the ability of acrolein and ozone to induce C-fiber activation was directly related to their ability to deposit deep within the alveoli of the lung where C-fibers predominate (Widdicombe, Lee 2001). Furthermore C-fibers may become activated via localized inflammation and oxidative stress (Taylor-Clark and Undem 2011, Ruan et al. 2003, Undem et al. 1993). The activation of C-fibers could mediate the acute changes in cardiac function and rhythm associated with the inhalation of UFP and NP. UFP and NP may preferentially activate pulmonary C-fibers due to their deposition deep within the lung where C-fibers predominate compared to other sensory nerves. This activation could occur through direct interactions between C-fibers and particles or in response to particle-induced pulmonary inflammation and oxidative stress.
Few studies however have addressed the ability of UFP, NP, or other particles to induce the activation of pulmonary C-fibers. Nemmar et al. (1999) demonstrated that intratracheally instillation of UFP was able to activate release of the neuropeptide substance P from C-fibers within the lungs of rabbits. The release of substance P is known to cause vasodilation, increased mucus secretion, chemotaxis of inflammatory cells, and endothelial permeability. Furthermore, instillation of single walled carbon nanotubes was found to alter baroreflex function in rats (Legramante et al. 2009). It has been shown that C-fibers are distributed in the vasculature (Holzer 1991, Scotland et al. 2004) but their role in peripheral vascular resistance following exposure to UPM or NP is not known. The ability of UFP to stimulate the release of substance P and single walled carbon nanotubes to modify cardiac reflex function establishes the activation of sensory receptors as a possible mechanism by which UFP and NP inhalation could lead to cardiovascular effects via cardiopulmonary reflexes.
Autonomic contribution to cardiac function has been well studied after PM exposure. It is believed that acute cardiac contractility changes observed after PM exposure are mediated by imbalance or parasympathetic and sympathetic controls. Vagus stimulation as a result of PM inhalation can influence this balance (Godleski et al. 2000). Research has shown an increase in baroreceptor reflex sensitivity after PM exposure consistent with an up-regulation of vagal reflexes (Schwartz et al. 1988). The animal experimental studies of PM exposure and blood pressure changes are contradictory, i.e. some show decreased blood pressure (Watkinson et al. 2001, Wichers et al. 2004) while others show an increase (Nemmar et al. 2010, Huang et al. 2010). It is possible that differing experimental protocols and exposure to chemically different forms of PM may have variable effects on blood pressure and cardiac function depending on the resulting parasympathetic or sympathetic imbalance. Nevertheless it does appears that exposure to particles can affect the cardiovascular system via pulmonary nerve stimulation.
Due to this possible mechanism of inhalation-induced cardiovascular toxicity, current studies have begun to utilize rodent electrocardiograms to assess rapid changes in cardiac function during and following particle exposure. This method allows for assessment of cardiac function with the benefit of not having to anesthetize the animal and can be used for controlled human studies. Using a radiotelemetry approach, Wichers et al. (2004) showed a decrease in blood pressure in hypertensive rats for two days after intratracheal exposure to residual oil fly ash particles, a combustion source particulate matter rich in the transition metals Fe, V, and Ni. The authors speculated that these results were due to the influence of autonomic control on blood pressure. Our recent study involving healthy Wistar Kyoto rats and exposure to diesel exhaust by inhalation shows a decrease in blood pressure and increased QA interval reflecting decreased contractility but only during exposure periods (Gordon et al., 2012 submitted). (Farraj et al. 2009) demonstrated rapidly decreased heart rate variability and alterations in cardiac rhythm and function in spontaneously hypertensive rats exposed to residual oil fly ash. Furthermore, hypertensive rats exposed to combustion source particulate matter have shown sensitivity to ST depression compared to non-compromised rat models, which could increase their susceptibility to alterations in heart rate variability and cardiac arrhythmias (Farraj et al. 2009, Kodavanti et al. 2000). These studies assessing modifications in animal electrocardiograms provide further evidence for the stimulation of pulmonary sensory neurons and suggest the role of autonomic regulation in adverse cardiophysiological outcomes in healthy and susceptible subpopulations. Assessment of humans who had previously suffered a myocardial infarction demonstrated that exposure to PM0.25 caused decreased heart rate variability which was blocked in individuals treated with β-blockers (Scapellato 2010). These findings in humans are in agreement with animal studies suggesting autonomic nervous system impairment following exposure to particles leading to alterations in cardiac rhythm.
Although current research seems to support that UFP- and NP-induced impairment of the autonomic nervous system may be responsible for systemic effects (Folino et al. 2009, Elder et al. 2007), this area requires further extensive examination. Specifically, it is currently unknown if particles can directly interact with these sensory receptors within the lung or if they are activated primarily via inducible pulmonary inflammation and oxidative stress. Because of their predominance in the alveoli where UFP and NP are known to deposit, research on particle activation of pulmonary sensory receptors has focused primarily on C-fiber responses. However, other receptors are present in the upper airways including rapid adapting receptors which are thought to also respond to inhalable irritants, but have not been studied in the context of UFP and NP inhalation exposures. The activation of C-fibers has been postulated to account for acute changes in cardiac function and rhythm however it is unknown if these effects are associated with chronic diseases. Lastly, it is likely due to the almost immediate changes seen in cardiac function following exposure, that pulmonary nerve stimulation plays a vital role in the systemic effects following UFP and NP exposure, however, it is improbable that this mechanism mediates all effects.
Direct Effects of UFP and NP on the Cardiovascular System
Translocation of UFP, NP or chemical constituents across the pulmonary epithelium and into the circulation may mediate systemic cardiovascular effects after inhalation. The direct systemic effects of inhaled UFP and NP on the cardiovascular system after translocation have been evaluated and reviewed previously (Stone et al. 2007). After inhalation and deposition within the lung, the proportion of UFP and NP which could translocate and mediate cardiovascular effects is related to the particle size, charge, and surface chemistry.
Evidence of UFP and NP Translocation
Semmler-Behnke et al. 2008 demonstrated that translocation of NP was size dependent by measuring significantly more 1.4 nm gold NP at secondary organ sites than 18 nm gold NP after instillation in rats. Intratracheal instillation of hamsters with positively-charged 60nm polystyrene particles was found to induce systemic prothrombotic factors but no pulmonary inflammation while positively charged 400nm polystyrene particles resulted in pulmonary inflammation (Nemmar et al. 2003). In the same study, negatively-charged 60nm polystyrene particles were not found to cause pulmonary inflammation or changes in systemic prothrombotic factors. Similar effects of surface charge on translocation have also been reported for quantum dots (Geys et al. 2008). These studies together suggest that not only particle size but also charge contributes to systemic translocation. Exactly how these particles may translocate is a growing area of investigation. It is possible that particle-induced pulmonary injury and increased vascular permeability may contribute to translocation. Further, transport of nanoparticles through nanopores has been recently examined (Hall et al. 2011). Dispersion of single wall carbon nanotubes with single strand DNA has been shown to provide needed surface charge for single molecule carbon nanotubes to cross cell membrane, suggesting that surface charge of particles can govern the translocation through cell membranes. Overall these studies provide insight into the role of surface charge and shape in modulating intracellular transport and translocation to systemic circulation, however, because of the differences in the properties of particles, one may not be able to generalize quantitative similarities in translocation or the mechanisms by which this might occur with UFP and NP.
Additional animal studies have demonstrated the ability of UFP and NP to translocate from the lung to secondary organ systems such as the liver, kidney, spleen, blood, and heart but at varying rates (Oberdorster et al. 2002, Lipka et al. 2010, Liu et al. 2009). Nemmar et al. (2002a) provided evidence that UFP translocate in humans by exposing individuals via inhalation to 99m Technetium-labeled ultrafine carbon particles and examining their distribution. Nemmar et al. (2002a) concluded that the majority of 99m Technetium-labeled UFP remained in the lung, however, radioactivity was found in the blood and other organs, such as the liver, supporting extrapulmonary translocation as a possible mechanism of human cardiovascular toxicity. Studies such as Nemmar et al. (2002a) however are difficult to interpret as it is unclear if the extrapulmonary radioactive signal is due to solubilization of the label or if the label remains attached to a translocated particle.
UFP and NP may translocate from the lung directly into the circulation or their transport may be mediated via immune cell uptake and lymphatic circulation. Using near infrared fluorescent nanoparticles, it has been demonstrated that nanoparticles with a smaller hydrodynamic diameter of ~34 nm or less with a non-cationic surface charge translocate rapidly from the lung to mediastinal lymph nodes after intratracheal instillation in the rat lung (Choi et al. 2010). These authors further show that NP of < 6 nm diameter can be rapidly translocated from the lungs to lymph nodes and the bloodstream, and are subsequently cleared by the kidneys. Furuyama et al. (2009) demonstrated that the migration of gold UFP-laden alveolar macrophages to the circulation, liver, and heart after intratracheal instillation in mice. Carbon black UFP have also been found within macrophages in the mediastinal lymph nodes of mice that have undergone repeated intratracheal instillations (Shwe et al. 2005). These carbon black-laden macrophages were found to cause inflammation within the lymph nodes (Shwe et al. 2005). These findings suggest that the movement of particle-laden alveolar macrophages via the lymphatic circulation could facilitate particle translocation and contribute to cardiovascular inflammation and injury following UFP inhalation.
Toxicity Associated with Translocated UFP and NP
In order to determine the direct effects of UFP and NP due to translocation from the lung, studies have examined the effects of particles on blood and endothelial cells in vitro and after intravenous injection in vivo. These studies are useful in examining mechanisms of systemic toxicity which occur after translocation because the contribution of pulmonary-derived mediator release and autonomic nerve activation in the lung can be minimized.
Direct Effects on Endothelial Cells and Coagulation
Exposure of endothelial cells to ambient UFP was found to increase the expression of genes related to inflammation and coagulation (Karoly et al. 2007) while exposure to diesel exhaust UFP was determined to cause inflammation and oxidative stress (Li et al. 2009, Li et al. 2010). Single- and multi- walled carbon nanotubes induce aggregation of human platelets in vitro while causing vascular thrombosis in vivo in rats (Radomski et al. 2005). Intravenous injection of polystyrene UFP with positive charges and carbon UFP have been shown to increase coagulation in hamsters and rats through the activation of platelets (Khandoga et al. 2004, Nemmar et al. 2002b). Furthermore, supporting the role of translocation, intratracheal instillation of 60 nm UFP, but not 400 nm UFP, was also found to initiate coagulation in hamsters (Nemmar et al. 2003). The inability of 400 nm UFP to induce coagulation was postulated to be due to their inability to translocate into circulation due to their size. Intravenous injection of diesel exhaust UFP increased inflammatory cytokines and increased bleeding time suggesting that translocated UFP induce systemic effects such as inflammation (Nemmar et al. 2009). Exposure of human aortic endothelial cells to CeO2 NP resulted in a proinflammatory effect (Gojova et al. 2009). Interestingly, intravenous injection of CeO2 NP, which are also known to cause pulmonary inflammation after inhalation exposure, actually were found to have cardioprotective effects in monocyte chemoattractant protein-1-/- mice possibly through antioxidant properties (Ma et al. 2011, Niu et al. 2007). These findings may be due to differences in routes of exposure suggesting that cardiovascular effects from some UFP and NP may be due to the release of other mediators from the lung and not translocation of particles. These seemingly contrary results regarding CeO2 may also indicate differential cell-type specific responses to UFP and NP exposures. Lastly, since various forms of CeO2 are manufactured, it is important to recognize the contribution of the surface properties in differential responsiveness of CeO2. Overall these experiments demonstrate that UFP and NP can have direct effects systemically by inducing coagulation, and causing endothelial cell inflammation, possibly contributing to vasoconstriction and atherogenic lesion development.
Direct Effects on Vascular Reactivity
Translocated UFP and NP have also been postulated to stimulate direct changes in vascular reactivity. Exposure to 45 nm silver NP was shown to induce concentration-dependent endothelial cell cytotoxicity and eNOS activation resulting in NO-dependent proliferation in vitro, while also causing changes in the vasoreactivity of isolated rat aortic rings (Rosas-Hernandez et al. 2009). Furthermore, combustion-derived diesel exhaust NP were found to inhibit acetylcholine-induced relaxation when isolated rat aortic rings were directly exposed (Mills et al. 2011). This inhibitory effect was reversed in the presence of superoxide dismutase but unchanged with the addition of the hydroxyl radical scavenger mannitol (Mills et al. 2011). These findings suggest an inhibition of vascular nitric oxide production and/or activity and the possible role of oxidative stress leading to vasoconstriction after NP exposure. The relatively minor changes in agonist-induced vasoreactivity following the in vitro exposure of aortic rings to carbon NP and TiO2, (particles with comparatively minimal oxidative potential), further supports the role of oxidative stress in the vascular responses to NP (Mills et al. 2011, Knaapen et al. 2001).
Direct Effects on the Heart and Cardiac Myocytes
UFP and NP, if able to translocate to the circulation and cause direct vascular effects and changes in coagulation, could also translocate directly to the heart and cause effects. Cardiac myocytes and co-cultures of cardiac myocytes and fibroblasts exposed to carbon black UFP produced a proinflammatory response via increased release of IL-1 receptor-dependent IL-6 and IL-1β (Totlandsdal et al. 2008). In vitro cardiac myocycte exposure to diesel exhaust UFP and TiO2 NP were shown to generate dose-dependent oxidative stress, and changes in cell function, while only TiO2 NP caused alterations in myofibrillar structure (Helfenstein et al. 2008). Few changes, however, were observed in cardiomyoccyctes exposed to single walled carbon nanotubes (Helfenstein et al. 2008).
A study of different size fractions of ambient particulate matter determined that while coarse particles caused the most substantial pulmonary injury in mice after exposure, UFP were found to exacerbate cardiac reperfusion injury to the greatest degree (Tong et al. 2010). The limited pulmonary inflammation caused by UFP compared to coarse particles supports that this increased cardiovascular damage after UFP exposure may be directly related to the ability of UFP to translocate out of the lung. Overall, studies have demonstrated that direct exposure of cardiomyocytes to UFP and NP can induce cellular damage through eliciting an inflammatory response and generation of oxidative stress. However, the evidence for myocardial inflammation as a result of UFP and NP translocation in vivo after pulmonary exposure is weak and requires more studies. A recent study addressing this area has shown that diesel exhaust exposures, which primarily contain UFP, cause changes in myocardial gene expression profile which reflects impaired contractile function, oxidative stress and extracellular matrix alterations, but not inflammogenic effects in left ventricular tissues of healthy rats (Gottipolu et al. 2009).
Translocation of Transition Metals and Organic Components from UFP and NP
Many NP are produced utilizing reactive transition metals which could leach off from the surface of the particles and into the circulation thereby mediating systemic affects not attributable to particles. Recent studies in rats have demonstrated the translocation of various transition metals from the surface of particles which were later found in the liver, blood, and heart (Mani et al. 2007, Wallenborn et al. 2007). In order to determine the translocation of transition metals after leaching from inhaled particles we exposed rats through intratracheal instillation and gavage to (70) zinc, a stable and low abundance isotope (Wallenborn et al. 2009). We found the majority of (70) zinc remained in the lung 1h after instillation, however, after 24 h and 48 h significant increases were noted in the liver, kidney, spleen and heart. Interestingly, more (70) zinc translocated to secondary organs after intratracheal instillation than after gavage. In other experiments, we determined that animals exposed repeatedly to soluble zinc through intratracheal instillation and inhalation exhibited increased myocardial lesions, mitochondrial dysfunction, decreases in cardiac aconitase activity, and increased expression of cardiac genes related to oxidative stress, altered mitochondrial function, and cell-cycle control (Kodavanti et al. 2008, Wallenborn et al. 2008). Many NP are produced using reactive transition metals as catalysts which can lead to contamination of NP with these metals. These remaining metals could leach off of the surface of NP after exposure resulting in their translocation and possibly toxicity. It is also likely due to the large surface area and diverse surface modifications possible with NP, that they can accumulate a variety of metals in the environment which could contribute to translocation.
It is also possible that a variety of organic components could mediate the systemic effects of UFP after translocation. Due to the large surface area of UFP, they are able to absorb large amounts of organic molecules compared to larger particles. Ambient air particles collected in Los Angeles demonstrated that UFP particles have a higher percentage of both elemental and organic carbon as well as polyaromatic hydrocarbons (PAH) compared to coarse and fine particles (Li et al. 2003). This increased PAH content of the UFP was found to correlate with increased reactive oxygen species generation and oxidative stress in macrophages and epithelial cells (Li et al. 2003). It is likely that after translocation of UFP that both their organic and metal components can result in systemic oxidative stress.
Detection of Translocated UFP and NP in vivo
Although numerous studies have established extrapulmonary translocation of UFP and NP, their relative abundance in the circulation appears to be low. A major limitation to the study of translocated particles is our inability to precisely measure the low abundance of these particles that reach the circulation after inhalation exposure. This especially is true for carbon-based particles. While some translocated metal NPs can be detected using conventional mass spectrometry if the metal is not a natural biological component of the tissue, however, in many cases one may not be able to confirm if the translocated metal exists as a NP or reflects soluble fraction. This is especially true for iron- and zinc- based NPs. The ability to use TEM for carbon-based NPs and nanotubes measurements is limited since this technique cannot provide a quantitative estimate of translocation and the limited visualization due to the size and low refractory properties of the carbon based particles.
Because of the current limitations to accurately quantify translocated UFP and NP, it is difficult to ascertain their direct contribution to systemic, vascular, and cardiac effects. It is however likely that inhaled UFP and NP with the appropriate physiochemical properties can translocate systemically in sufficient quantities to induce inflammation in the cardiovascular system and modify vasoreactivity through oxidative stress and the inhibition of nitric oxide. Studies examining the direct effects of NP on the cardiovascular system using intravenous injection or utilizing in vitro methods however demonstrate variable toxicity. This supports that there may be a variety of other factors contributing to the cardiovascular effects of UFP and NP such as the activation and release of mediators from lung.
Pulmonary-Derived Mediator Release and Systemic Effects
The release of pulmonary-derived mediators into circulation may facilitate the negative cardiovascular effects of UFP and NP. Inhalation of UFP and NP has been shown to increase vascular permeability and membrane transport within the lung. This increased movement across air-blood barrier is associated with pulmonary edema and the possible release of pulmonary-derived mediators, such as pro-inflammatory cytokines, oxidative-damaged macromolecules (microparticles), and vasoactive and prothrombotic factors into circulation. Once distributed systemically, these mediators promote inflammation, directly modify vascular tone, and/or induce a prothrombotic state.
Inflammatory Cytokines and Thrombogenic Factors
Pulmonary exposure to larger forms of PM such as PM10 and PM2.5 which do not readily translocate compared to UFP, but induce significant pulmonary inflammation after exposure, can be utilized to examine the systemic effects of pulmonary-derived mediators. Mice exposed to ambient particles from Chapel Hill, NC demonstrated increased circulating PAI-1, fibrinogen, and soluble P-selectin with a reduction in bleeding times compared to controls (Cozzi et al. 2007). Additionally, mice exposed to PM collected in Dusseldorf, Germany demonstrated a reduced bleeding time which was dependent on IL-6 release from the lung (Mutlu et al. 2007). Amphibole asbestos fibers, which are extremely biopersistent within the lung, have demonstrated acute systemic effects including acute changes in platelet activity, and an acute phase response in rats possibly via the release of proinflammatory cytokines and/or oxidatively-modified proteins (Shannahan et al. 2012a in press, Shannahan et al. 2012b in press). Since the quantities of leachable and thus translocatable components in these studies are most likely biologically insignificant, these studies provide evidence that particles do not have to translocate to cause systemic effects.
As with larger PM fractions, the release of proinflammatory cytokines into the circulation may be relevant to UFP and NP toxicity. Specifically, carbon black UFP increase pulmonary inflammatory cytokine production when compared to fine carbon black particles (Donaldson et al. 2002). In the case of carbon UFP, inefficient phagocytosis has been shown to stimulate calcium signaling in macrophages thereby increasing production of inflammatory cytokines in the lung (Donaldson et al. 2002). Furthermore, this inefficient phagocytosis by macrophages results in increased contact time with epithelial cells possibly resulting in the release of additional mediators. Alveolar macrophages have been shown to uptake NP at differing rates due to variations in size, and surface functionality resulting in differential macrophage activation and cytotoxicity in vitro (Oh et al. 2010). Alveolar macrophage uptake of TiO2 UFP has been demonstrated to induce the activation of macrophages and the release of the proinflammatory cytokine TNF-α (Scherbart et al. 2011). In the same experiment alveolar macrophages did not readily internalize fine TiO2 particles or release TNF-α (Scherbart et al. 2011). These findings suggest an exacerbation of alveolar macrophage-mediated pulmonary inflammation by UFP compared to fine particles possibly due to differences in uptake. Specifically, these and other studies provide evidence that pulmonary mediator production may be exacerbated by UFP and NP compared to larger particles. This exacerbated response could influence systemic toxicity due to the direct vascular effects of circulating proinflammatory cytokines.
Inhalation exposures to UFP and NP have been shown to increase not only pulmonary but circulating levels of inflammatory cytokines such as IL-8, IL-6, TNF-α and others. These released pro-inflammatory cytokines contribute to myocardial and vascular inflammation, induce a prothrombotic state and an acute phase response from the liver. Budinger et al. (2011), determined that exposure to concentrated ambient PM2.5 caused an induction of PAI-1 that was dependent on pulmonary TNF-α increase and a prothrombotic effect that was dependent on alveolar macrophage release of IL-6 (Budinger et al. 2011). These systemic increases in PAI-1 were postulated to be due to the pulmonary prothrombotic effect of PM and the extrapulmonary release of TNF-α and IL-6 (Mutlu et al. 2007). Mice exposed to concentrated ambient PM2.5 have demonstrated elevated serum levels of many other markers of inflammation and coagulation (MIP-1α, MIP-1β, IL-6, IL-10, TNF-α, M-CSF, GM-CSF, PDGF-bb, and RANTES) (Wilson et al. 2010). The larger surface area and the reactivity of UFP and NP might result in greater pulmonary injury and thus enhanced oxidative stress and inflammation compared to larger particles. Further it is also likely that the deposition fraction of UFP and NP is greater than fine particles upon inhalation contributing to exacerbated response. These studies utilizing larger forms of PM support the role of pulmonary derived mediators in the systemic and cardiovascular response of UFP and NP.
The evidence of NP-induced systemic and cardiovascular toxicity has recently begun to emerge. Niwa et al. (2008), demonstrated by electron microscopy analysis that translocation of carbon black NP in rats after inhalation exposure does not occur, however, the authors did find significant increases in circulating IL-6, monocyte chemoattractant protein-1, and C-reactive protein. These carbon black NP were primarily found within alveolar macrophages and resulted in mild pulmonary inflammation which was postulated to lead to the release of these cytokines into circulation (Niwa et al. 2008). The release of pro-inflammatory cytokines into circulation could be responsible for inflammation in the heart and vasculature. Although many have attempted to measure systemic cytokine levels after PM, UFP and NP inhalation exposures, with rather robust pulmonary exposure and injury, they have failed to demonstrate increases in the levels of many of these cytokines. This may be due to a diverse set of responsible inflammatory cytokines, undetectable low levels of circulating cytokines, a temporality in circulating levels or a critical microenvironment that is undetectable in serum cytokine levels. While it is well established that cytokines can induce endothelial cell signaling and subsequent vascular inflammation and vasoconstrictive response, there is still a gap in our understanding regarding which types of PM and cytokines are responsible for vascular effects.
Cardiac inflammation has been shown to occur following exposure to TiO2 UFP without quantifiable increases in circulating pro-inflammatory cytokines raising further questions on the role of circulating mediators versus direct translocation (Kan et al. 2011). It is plausible that low levels of constant circulating inflammatory cytokines over an extended amount of time could be sufficient for the development of chronic cardiovascular diseases. Furthermore, at human relevant UFP and NP exposure levels it is possible that increases in systemic cytokines may not occur or that cytokines are not increased to high enough levels to illicit systemic effects. Some studies however have demonstrated undetectable pulmonary inflammation but with systemic alterations in cardiac function, vasoconstriction, and thrombosis (Upadhyay et al. 2008).
In order to determine the role of pulmonary inflammation versus translocated metals on cardiac impact, we conducted a study examining the effect of repeated pulmonary exposure of rats to Mount St. Helen’s ash with no leachable metals or leachable zinc sulfate and evaluated cardiac gene expression using expression arrays. In that study we showed that despite presence of pulmonary pathology, no changes occurred in cardiac gene expression in rats exposed to Mount St. Helen’s ash while rats exposed to soluble zinc demonstrated changes in cardiac gene expression (Kodavanti et al. 2008). It was postulated that these changes in cardiac gene expression resulting from zinc exposure were related to its ability to translocate. The lack of observable changes in cardiac gene expression following Mount St. Helen’s ash exposure despite extensive lung inflammation and pathology seems to suggest that the release of inflammatory mediators from the lung does not contribute to cardiovascular effects. Subsequently we have shown that rats exposed to ozone which does not translocate systemically produces cardiac and systemic vascular alterations (Kodavanti et al. 2011). Considering many other studies examining molecular cardiovascular effects of PM exposure, it is still not clear which mediators or mechanisms may be responsible for these cardiovascular effects following pulmonary exposure. The lack of temporality assessment in most published studies often provides inconsistent results. Extensive research needs to be undertaken to examine pulmonary-derived mediators responsible for the cardiovascular effects of inhaled UFP and NP. It is likely that there are other factors besides circulating cytokines that are likely to induce endothelial and vascular alterations. These factors may induce changes in peripheral vasculature which may influence cardiac function.
Oxidatively Modified Proteins and Lipids
Many UFP and NP have shown an ability to generate reactive oxygen species through redox cycling causing oxidative damage to proteins, lipids, and DNA. Oxidative damage to these macromolecules can lead to cytotoxicity and lung injury. It is possible that these oxidized proteins and lipids may get released from the lung into circulation where they can activate receptors in the vasculature resulting in vascular alterations and disease. Vascular receptors of interest include the receptor for advanced glycation endproducts (RAGE) and the leptin-like oxidized low-density lipoprotein receptor-1 (LOX-1).
RAGE is known to have a variety of ligands including advanced glycation endproducts which are produced via interaction of glucose with proteins in diabetic patients and also by oxidative damage to proteins. Activation of vascular RAGE is of importance because of its role in initiating leukocyte adhesion during pulmonary and vascular inflammation and its contribution to the development of atherosclerosis (Pollreisz et al. 2010, Barlovic et al. 2011). Specifically, RAGE has been shown to mediate the inflammatory response in airway epithelial cells in vitro after exposure to diesel PM while siRNA inhibition of RAGE reduced this response (Reynolds et al. 2011). These findings suggest the presence of oxidatively-damaged macromolecules within the lung and their role in stimulating an inflammatory response via RAGE activation. RAGE activation has also been shown to be of importance in the injury produced in the heart after an ischemic event. Specifically, RAGE-/- mice have demonstrated decreased pulmonary and cardiac ischemic reperfusion injury after acutely-induced hyperglycemia compared to wild type mice (Lapar et al. 2011). Furthermore inhalation of a combination of ozone and diesel exhaust PM has demonstrated increased aortic RAGE mRNA expression in rats (Kodavanti et al. 2011). These findings support that following an inhalation exposure to particles vascular RAGE is up-regulated suggesting the presence of oxidatively-damaged macromolecules systemically.
Another vascular receptor of interest, LOX-1, is expressed primarily by endothelial cells. LOX-1 is responsible for binding oxidized low-density lipoprotein (oxidized-LDL) and removing it from circulation. Binding and internalization of oxidized-LDL via LOX-1 causes activation of endothelial cells leading to increased expression of LOX-1, the expression of adhesion molecules, and the recruitment of monocytes to the subendothelial space (Mitra et al. 2011). LOX-1 receptor activation is known to signal through MAPK and NFkB and induce downstream changes such as CD40/CD80 ligand expression which is involved in monocytes recruitment, foam cell production, expression of adhesion molecules on endothelium, and inflammatory gene expression (Kita et al. 2001, Mehta 2004). This recruitment of monocytes promotes vascular inflammation, foam cell accumulation and eventually the development of atherosclerosis. Endothelial cell LOX-1 levels can be used as a biomarker of oxidized-LDL levels and may be useful as a possible biomarker related to the progression of atherosclerosis. We have recently shown increases in aortic LOX-1 mRNA and protein in rats exposed to ozone and diesel exhaust particles (Kodavanti et al. 2011). Apolipoprotein E-/- mice, which have increased LOX-1 as compared to wild-type mice, when exposed to combustion source PM have demonstrated increased levels of oxidized-LDL, endothelial expression of LOX-1 mRNA, vascular oxidative stress, and subendothelial macrophage accumulation compared to controls (Lund et al. 2011). A one-time single walled carbon nanotube exposure in the same mouse model was found to cause increased oxidative stress in the lung, aorta, and heart, while multiple exposures were found to increase aortic and brachiocephalic artery atherosclerosis lesion development (Li et al. 2007). Furthermore, exposure of apolipoprotien E-/- mice to concentrated ambient UFP has been shown to be more proatherogenic compared to concentrated ambient fine particles (Araujo et al. 2008). These findings suggest that inhalation of PM and specifically the UFP portion or single-walled carbon nanotubes can contribute to vascular inflammation and the development of atherosclerosis possibly through increasing circulating oxidized-LDL and activation of vascular receptors such as LOX-1.
Prothrombotic and Vasoactive Mediator Release
Many studies have assessed the impact of procoagulative and prothrombotic changes after exposure to PM and UFP in order to determine their contribution to possible adverse cardiovascular effects. Coagulation in general is an activation of platelets resulting in the formation of blood clots. Coagulation is a complex process which includes a variety of clotting factors, many of which are often assessed following inhalation exposure including tissue factor, thrombin, fibrinogen, thrombomodulin, Von Willibrand Factor, and Factor V, VII, VIII, X, and XIII (Bonzini et al. 2010, Ghio et al. 2003). Changes causing an increase in coagulation are termed as a prothrombotic and can lead to the occlusion of blood vessels. Thrombi typically form at areas of cell injury in microvasculature and those of atherosclerosis in larger vessels. Breakage of thrombi within an atherosclerotic plaque can produce an infarct in the vessel where it lodges.
It is not well understood how injury within the lung can induce coagulation within microvessels and how this may activate platelets systemically to cause increased thrombus formation in peripheral and major vessels. Tissue factor is thought to be the primary protein which is involved in initiating extrinsic pathways of coagulation and has been shown to be markedly increased within lung, especially in macrophages after an exposure to PM or its components (Budinger et al. 2011, Li et al. 2010b). The release of tissue factor microvesicles after multi-walled carbon nanotube-induced pulmonary inflammation acutely activates platelets systemically and P-selectin mediated thrombosis (Nemmar et al. 2007). Several studies using fine and UFP with various characteristics have examined thrombus formation by using rose bengal dye accumulation at the sight of injury in peripheral vessels after exposure of rats, mice and hamsters (Nemmar et al. 2002b, Nemmar et al. 2007, Nemmar et al. 2003, Silva et al. 2005). Obstruction of the coronary artery by a thrombus can result in a myocardial infarct. Inhalation of particles has been demonstrated to cause a prothrombotic effect which could mediate the increased risk of myocardial infarction and ischemia as has been noted following PM exposure.
It is also probable that inhalation exposure to particles can release vasoactive factors from the lung causing altered vascular tone and blood flow through vessels thereby mediating cardiopulmonary toxicity. Interestingly the lung is a primary site of production and clearance for endothelin in the body (Dupuis et al. 1996). Furthermore it has been suggested that release of endothelin from the lung is responsible for the increased vascular resistance in patients with chronic heart disease (Tsutamoto et al. 1994). Endothelin and nitric oxide (NO) synthase are primary modifiers of vascular tone and their expression is altered in blood vessels after particle exposure (Kodavanti et al. 2011). Endothelin can stimulate an increase in blood pressure through binding endothelin receptor-A in smooth muscle causing calcium influx and vasoconstriction (Pollock et al. 1995). Endothelin also mediates a decrease in blood pressure through binding the endothelin receptor-B of endothelial cells causing the release of NO and the relaxation of smooth muscle resulting in vasodilation (Rodriguez-Pascual et al. 2011). The contribution of the potent vasoconstrictor endothelin and the vasorelaxant NO in inhaled PM-induced systemic vascular contractility has been examined in a number of studies. It has been shown that PM and ozone regulate lung endothelin by increasing expression of MMP-2 (Thomson et al. 2005). Plasma endothelin levels have also been shown to increase after PM exposure (Calderon-Garciduenas et al. 2007).
Typically there is baseline expression of endogenous nitric oxide synthase (eNOS) which produces NO in vessels therefore keeping vessels dilated to allow adequate blood flow to tissues. The vasorelexant NO is produced in the lung upon PM or UFP-induced injury by induction of inducible nitric oxide synthase (iNOS) where it could have a bronchodilatory effect (Yokohira et al. 2007, Becher et al. 2007); however, because the half-life of NO is short, it is not likely to be released systemically and have a vasorelaxant effect on the vasculature. In the systemic vasculature, NO is primarily produced by the action of eNOS within endothelium and in the smooth muscle cells. Some studies have shown that exposure of rats and mice to diesel exhaust particles inhibits NO-dependent vasorelaxation by possibly removing it through oxidation and uncoupling of NOS (Cherng et al. 2009, Knuckles et al. 2008). However, the literature on how the mediators which influence the production of NO within vessels after exposure to PM, UFP or NP is still not sufficient to draw any mechanistic insights and the role of specific characteristics. Particle-induced vasoconstriction via induction of endothelin-dependent constriction or inhibition of NO relaxation can lead to additional stress on the heart by means of increasing blood pressure and impairing cardiac function as well as stimulating the possible rupture of a thrombus.
Many studies examining the inhalation effects of NP and UFP demonstrate increased levels of prothrombotic and vasoactive factors in circulation. These factors may be released directly from the lung or may be related to the release of proinflammatory cytokines and their induction systemically. As discussed previously, pulmonary-derived IL-6 and TNF-α have been found necessary for systemic increases in prothrombotic factors and coagulation after UFP exposure (Budinger et al. 2011). These elevations in circulating inflammatory cytokines were also linked to decreased clotting times and increased fibrinogen and Factors II, VIII, and X (Mutlu et al. 2007). Furthermore, inhalation exposure of mice to TiO2 NP was found to have significantly increased expression of genes involved in pathways related to the complement system and coagulation (Halappanavar et al. 2011). Supporting the role of inflammation, Gilmour et al. 2004 demonstrated that rats exposed to carbon black UFP exhibited a modest inflammatory response in the lung and did not modify circulating prothrombotic factors fibrinogen, vWF, or Factor VII. These findings suggest that a robust pulmonary inflammatory response is needed to modify circulating levels of prothrombotic factors. Not all studies however demonstrate the need for pulmonary inflammation in order to induce thrombosis. Nemmar et al. 2004b demonstrated that after inhalation of 60nm polystyrene particles there was no correlation between pulmonary inflammation and thrombosis. Furthermore it was shown in the same study that 400nm particles which induced the most pulmonary inflammation had no effect on acute thrombotic markers, suggesting that prothrombotic changes may not be exclusively related to inflammation (Nemmar et al. 2004a). Based on these findings by Nemmar et al. 2004 other mediators may be required to induce a prothrombotic state or the thrombotic effects resulting from pulmonary inflammation occur at later time points.
Acute Phase Response
The release of pro-inflammatory cytokines as well as oxidatively-modified proteins into the circulation from the lung could to lead to a systemic innate immune response known as the acute phase response (Cray et al. 2009). Specifically, the release of pro-inflammatory mediators known to be produced in the lung and released into circulation following UFP and NP exposures including IL-1, IL-6, and TNF-α as well as the release of oxidatively-modified macromolecules are known to stimulate the liver to synthesize and release specific acute phase response proteins (Cray et al. 2009). The acute phase response involves increased circulating levels of C-reactive protein, serum amyloid A, ceruloplasmin, haptoglobulin, complement C3, fibrinogen, Factor VII, α1-acid glycoprotein, α-2-macroglobulin and others, while proteins such as transferrin, apolipoprotein A, albumin, are decreased (Ceron et al. 2005, Lakota et al. 2011). Various air pollutants have demonstrated the ability to induce an acute phase response including ozone, diesel exhaust particles, amphibole asbestos, and single walled carbon nanotubes (Laskin et al. 1998, Lewis et al. 2007, Erdely et al. 2011, Shannahan et al. 2012b). Increases in acute phase response proteins have been associated with metabolic syndrome and the eventual development of diabetes and cardiovascular disease (Saiki et al. 2011, Wellen, Hotamisligil 2005, Venteclef et al. 2011). The release of acute phase response proteins such as C-reactive protein, fibrinogen, and Factor VII from the liver into circulation also promote a prothrombotic state and are recognized as risk factors for myocardial infarction (Donaldson et al. 2001, Upadhyay et al. 2010, Wilhelmsen et al. 1984, Moyssakis et al. 2010). Specifically C-reactive protein is increased following particulate exposure and has been shown to correlate with the development of atherosclerosis and to induce coagulation (Donaldson et al. 2001). The mechanism by which C-reactive protein contributes to UFP or NP -induced cardiovascular disease is not clear. It is also not known if there is a role for other acute phase proteins in modulating systemic vascular alterations following PM exposure. It is possible that this acute phase response after inhalation assists in the removal of circulating cytokines and oxidatively-damaged macromolecules further diminishing our ability to detect these pulmonary-derived mediators. The acute phase response however may be a reliable general marker of systemic effects following UFP and NP exposure.
One could hypothesize that an individual previously exposed to an air pollutant such as ozone or suffering from disease-related pulmonary edema could exist in a state of increased vascular permeability which would not only increase the direct translocation of UFP and NP but also enhance the release of pulmonary mediators into the circulation. No studies to date have specifically examined the direct role of these mediators by intravenous treatment of animals to inflammatory cytokines, oxidatively-damaged macromolecules, prothrombotic factors, or vasoreactive proteins. If the leakage of mediators from the lung is responsible for these cardiovascular effects, more sensitive methods for detecting circulating cytokines and oxidatively-damaged macromolecules need to be established. Currently, increases in circulating cytokines and other mediators, well below the limit of detection for the available assays, have been reported causing many to question the validity of their results. It may be necessary due to the transient nature and our current limitations in measurement of these pulmonary-derived mediators to examine downstream systemic responses of their presence such as changes in acute phase response proteins, LOX-1, and RAGE vascular expression, and changes in coagulation. A major disadvantage to these markers, however, is lack of specificity as they are increased in general as a response to diseases and non-pulmonary injuries. Thus, our understanding of cardiopulmonary interactions is deficient in regards to how pulmonary exposure to UFP and NP might result in systemic and cardiovascular alterations. In addition, it has not been examined if the primary target of UFP or NP exposure is the peripheral vasculature and alterations in vascular function are responsible for cardiac physiological compensation by adjusting blood flow.
The Role of Mast Cells in Mediating Cardiopulmonary Effects
Mast cells are a major focus of our laboratory and may well be a critical cell in the initiation of both pulmonary and cardiovascular events following UFP and NP exposure. Mast cells are present in most tissues including the skin, gut, lung, heart, and blood vessels. Mast cells are an essential cell of the innate immune system, mediating allergic reactions and inflammation through degranulation and the release of chemical mediators typically following crosslinking of the high affinity IgE receptor (FcεRI) with an allergen (Brown et al. 2008). Degranulation of mast cells causes allergic reactions through the release of histamine and other preformed mediators during the immediate phase response and contributes to late phase inflammatory responses through the production of a variety of cytokines (Table 2). Once released these mast cell-derived mediators could result in a variety of physiological effects which may well contribute to cardiovascular disease development. Many of the previously described mechanisms may result in the activation and degranulation of mast cells contributing to the systemic effects of inhaled UFP and NP.
Table 2.
Mast Cell Mediators
| Class | Mediator | Physiological Effects |
|---|---|---|
| Preformed | Histamine, serotonin, heparin, neutral proteases, acid hydrolases, peroxidase, phospholipases |
Vasodilation Vasoconstriction Angiogenesis Mitogenesis Pain Protein processing/degradation Lipid/proteoglycan hydrolysis Arachidonic acid generation Tissue damage Inflammation |
| Lipid | LTB4, LTC4, PGE2, PAF | Leukocyte chemotaxis Vasoconstriction Bronchoconstriction Platelet Activation Vasodilation |
| Cytokines | TNF-α, TGF-β, IFN-α, IFN-γ, IL-1α, IL-1β, IL-3, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-11, IL-12, IL-13, IL-15, IL-16, IL- 17, IL-18, IL-25, IL-33 SCF, TSLP, MIF |
Inflammation Leukocyte migration/proliferation |
| Chemokines | CXCL8, CCL2, CCL3, CCL5, CCL7, CCL13, CCL11, CCL19 |
Chemoattraction and tissue infiltration of leukocytes |
| Growth Factors | CSF, GM-CSF, bFGF, VEGF, NGF, LIF |
Cell growth Vasodilation Neovascularization Angiogenesis |
PAF, platelet activating factor; SCF, stem cell factor; TSLP, thymic stromal lymphopoietin; MIF, macrophage migration inhibitory factor; GM-CSF, granulocyte macrophage-colony stimulating factor; VEGF, vascular endothelial growth factor, bFGF, basic fibroblast growth factor: NGF, nerve growth factor; LIF, leukemia inhibitory factor
There is evidence suggesting the role of mast cells in the pathogenesis of a variety of diseases including autoimmune, cancer, asthma, and cardiovascular (Weller et al. 2011, Levick et al. 2011, Bot, Biessen 2011). In respect to cardiovascular disease, the release of mediators from mast cells has been found to promote atherosclerotic plaque growth and destabilization (Bot et al. 2007). Specifically, histamine has been shown to increase macrophage uptake of lipids (Kokkonen, Kovanen 1987), while cytokines such as IL-6, IL-8, and TNF-α have been shown to contribute to the inflammatory state (Bot et al. 2007, Zhang et al. 2011). Furthermore inhibition of chymase, a protease released by mast cells, has been found to inhibit plaque progression and destabilization (Bot et al. 2011). Inhibition of chymase has also been shown to reduce inflammation and infarct size following ischemia in pigs (Oyamada et al. 2011). It is possible that mast cell activation contributes to the adverse cardiovascular effects occurring after UFP and NP exposures.
Due to the location of mast cells at interfaces of the lung and the external environment, it is also plausible that UFP or NP could directly activate mast cells. In this regards, mast cells may well be an initial source of inflammatory mediators within the lung after UFP and NP exposure and contribute to circulating factors thereby facilitating systemic effects. In addition to preformed mediators such as histamine and chymase, mast cells also store preformed TNF-α which can be released within minutes of activation. Further, the preformed TNF-α in mast cells can be released in heparin-based microparticles and translocated to draining lymph nodes facilitating communication between peripheral sites of inflammation and remote lymphoid tissue (Kunder CA, JEM, 2009). Several studies have examined the direct activation of mast cell by UFP or NP. Specifically silver NP have been shown to directly stimulate mast cell degranulation in vitro (Yang et al. 2010). Exposure of cultured mast cells to CeO2 NP has been found to stimulate the release of a variety of inflammatory cytokines including TNF-α, IL-6, and osteopontin in vitro (Wingard et al. 2010). These findings suggest that mast cells contribute to the inflammatory response induced by UFP and NP. Furthermore in a study assessing the role of mast cells in NP-induced cardiovascular toxicity, mice deficient in mast cells did not exhibit the same inflammatory response as wild-type mice. Interestingly, mast cell deficient mice did not have an impairment in vascular relaxation nor was there an exacerbation of cardiac ischemia reperfusion injury following NP exposure as seen in wild-type mice (Wingard et al. 2010). In general these findings suggest a role of mast cells in mediating the cardiovascular toxicity induced following NP exposure possibly through vasoconstriction or induction of inflammation following an ischemic event. It is likely that pulmonary mast cells release mediators, such as heparin-based microparticles containing TNF-α, into the circulation which are responsible for these effects.
The mechanism by which NP directly affect mast cells is currently poorly understood. It has been demonstrated that some forms of PM, UFP, and NP can stimulate mast cells to degranulate (Yang et al. 2010) while others have been shown to inhibit degranulation and thus reduce allergic inflammation (Maurer-Jones et al. 2010). Mast cells have been shown to internalize NP including SiO2, and TiO2, as well as the UFP ZnO, resulting in a reduced ability to degranulate (Maurer-Jones et al. 2010, Yamaki, Yoshino 2009). The internalization of fullerene NP by mast cells through nonspecific endocytosis has been shown to inhibit the allergic response (Dellinger et al. 2010, Love, Haynes 2010, Ryan et al. 2007). Once internalized by mast cells, NP may interfere with cell signaling thereby inhibiting degranulation. Specifically, the UFP ZnO once internalized by mast cells has demonstrated an ability to inhibit the phosphorylation of Akt and tyrosine kinase activity responsible for mast cell degranulation (Yamaki, Yoshino 2009). Endocytosis may be responsible for the internalization and inhibitory effects of NP on mast cells, however others have provided evidence that NP are able to interact with cell surface receptors resulting in variable effects on mast cell degranulation (Huang et al. 2009). Specifically (Huang et al. 2009) determined that the ability of gold NP to either stimulate or inhibit degranulation was related to both its size and the FcεRI receptor density of the cell. The release of serotonin by mast cells exposed to gold NP was found to be concentration and time dependent (Marquis et al. 2009). Gold NP were found to be internalized by mast cells over time, as the exposure concentration of gold NP increased and at later time points the serotonin release of mast cells was inhibited, while at acute time points and at lower concentrations serotonin release was increased. One could postulate that at earlier time points and lower concentrations NP interact with cell surface receptors stimulating mast cell degranulation while at higher concentrations and at later time points more gold NP are internalized which inhibit serotonin release. It is also likely that low concentrations of inhaled and translocated UFP and NP could directly activate mast cell surface receptors leading to degranulation before being internalized over time and causing an inhibitory effect. Some research has postulated that inhibition of mast cell degranulation by internalization of NP could be utilized as a therapeutic treatment of asthma and other allergic conditions (Ryan et al. 2007, Norton et al. 2010). The mechanisms by which UFP and NP directly interact with mast cells and influence degranulation requires further examination, but may contribute to systemic toxicity.
The degranulation and/or activation of systemic mast cells may occur through other mechanisms not involving direct interactions with particles. It is possible that many of the mediators previously described could activate mast cells in the vasculature and heart thereby resulting in the adverse cardiovascular effects of UFP and NP. Perivascular mast cells have been found to localize with nerve fibers (Huet al. 2008, Laine et al. 2000) and express receptors for neuropeptides (Krishnaswamyet al. 2006). Experimentation has demonstrated that substance P, a neuropeptide released by C-fibers, can activate mast cells resulting in the recruitment of mast cells to atherosclerotic lesions and plaque destabilization in apolipoprotein-/- mice (Bot et al. 2010). Substance P release from nerve fibers has been suggested to activate cardiac mast cells in rats resulting in mast cell recruitment and myocardial inflammation (Melendez et al. 2011). Also, mast cells are well known to release histamine which can activate C fibers and is thought to enhance neuropeptide release (Harvima et al. 2010). This possible interaction between neuronal cells and mast cells may result in systemic inflammation and influence cardiovascular function following UFP and NP inhalation.
The release of pulmonary-derived mediators into the circulation could contribute to the activation of systemic mast cells. Specifically, mast cell activation via the ST2 receptor and the inflammatory cytokine IL-33 has recently been described as a major mechanism leading to mast cell activation independent of FcεRI. It is postulated that UFP and NP-induced lung injury is associated with release of IL-33 from lung into the systemic circulation which can activate mast cells and induce an inflammatory response through the release of cytokines and other mediators (Figure 2). Inhaled UFP and NP can stimulate alveolar epithelial cells and macrophages to produce IL-33 (Wang et al. 2011, and unpublished data). This IL-33 can activate mast cells within the lung via the ST2 receptor or possibly leave the lung and activate systemic mast cells. This activation causes the release of a variety of mast cell-derived cytokines, chemokines, and other mediators and may result in altered vascular reactivity and inflammation systemically. Soluble ST2, a decoy receptor for IL-33, has been found to be elevated in cardiac myocytes subjected to mechanical strain and is also found in mice and humans following myocardial infarction (Weinberg et al. 2002). Since the only known ligand for the ST2 receptor is IL-33 (Schmitz et al. 2005), these findings suggest that soluble ST2 is elevated after myocardial infarct inhibiting mast cell-induced inflammation. Work in our laboratory has demonstrated that IL-33 mRNA is elevated in lung as well as protein levels in the bronchoalveolar lavage fluid after mice were exposed to multi-walled carbon nanotubes thereby supporting IL-33-mediated mast cell activation within the lung (Wang et al. 2011). IL-33 has also been shown to cause increased mRNA expression of leukocyte adhesion molecules and pro-inflammatory cytokine in human endothelial cells which may promote atherosclerosis (Demyanets et al. 2011). In summary the release of inflammatory cytokines such as IL-33 from the lung could induce the degranulation of systemic mast cells and contribute to systemic toxicity of UFP and NP.
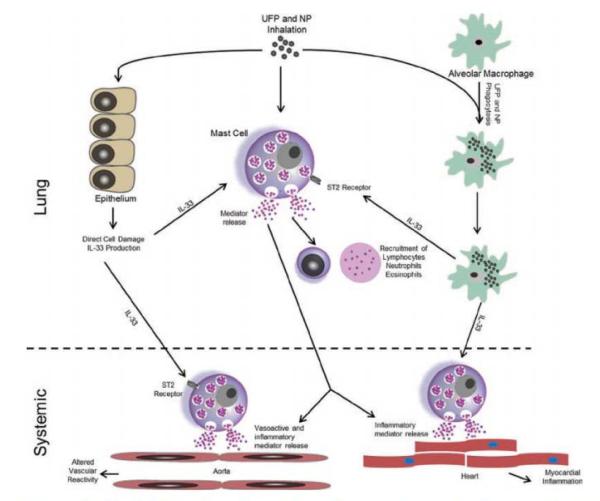
Figure 2.
Potential mechanism by which IL-33 release could lead to the activation of mast cells and mediate the systemic effects of inhaled NP and UFP. Inhalation of NP and UFP can stimulate the production of IL-33 from alveolar epithelial cells and macrophages resulting in activation of pulmonary mast cells. This activation causes the released causes the released of variety of mast cell-derived mediators resulting in pulmonary inflammation and injury. These mast cell-derived mediators could also leak into the circulation and mediate the cardiovascular effects of NP and UFP. The IL-33 produced within the lung could also reach the circulation and activate systemic mast cells, thereby mediating the cardiovascular effects of NP and UFP.
Other factors besides inflammatory cytokines could be released from the lung and result in mast cell degranulation and cardiovascular toxicity. For instance, oxidized-LDL is known to promote atherosclerosis through the activation of mast cells (Liao et al.1997) and has been shown to be increased following PM exposure (Lund et al. 2011). It is probable that oxidized-LDL released from the lung could activate mast cells and contribute to systemic inflammation. Many studies of UFP have demonstrated activation of coagulation pathways following inhalation while comparatively few have evaluated the ability of UFP to activate the complement system. One study assessing the adjuvant effects of ambient UFP demonstrated increases in bronchoalveolar lavage fluid levels of complement C3 after mice were challenged with ovalbumin following ambient UFP exposure compared to those exposed to filter air (Kang et al. 2010). Complement C3 is an acute phase reactant increased during inflammation and central to activation of the complement system. Complement C3 as wells as other proteins involved in the complement system have been shown to activate mast cells (Guo et al. 2011). These findings suggest that not only can UFP exposures potentiate allergic reactions but that UFP can induce the complement system possibly releasing complement components into the circulation which may mediate systemic inflammation and platelet activation. This ability of UFP and NP to induce the complement system may be important in understanding cardiovascular toxicity and is an area that requires further study.
Overall, the studies assessing the role of mast cells in UFP and NP-induced systemic and cardiovascular effects are just emerging. Currently however it remains unclear which population of mast cells contribute to these systemic effects. Specifically, the activation of pulmonary mast cells may release mast cell-derived mediators into the circulation contributing to cardiovascular toxicity or the leakage of inflammatory cytokines into the circulation may activate systemic mast cells. Furthermore, the individual mast cell-derived mediators which are responsible for each specific cardiovascular effect also remain unidentified. Future studies will undoubtedly provide additional insights into the role of mast cells in mediating systemic alterations after UFP and NP exposure and may influence therapeutic interventions.
Conclusions
In conclusion, epidemiology has established that inhalation exposure to particulate matter increases the risk for cardiovascular mortality and morbidity. UFP are thought to contribute to cardiovascular effects more readily than larger forms of inhaled PM. Furthermore, data from toxicological studies in rodents support similar adverse cardiovascular health effects after exposure to NP. It is not likely that one mediator or mechanism is solely responsible for these systemic cardiovascular effects, rather, that these effects are due to multiple contributing factors which are dependent on the physicochemical characteristics of inhaled UFP and NP. These cardiopulmonary mediators of toxicity possibly include impairment of the autonomic nervous system, direct systemic effects by translocated particles, and the release of pulmonary-derived mediators into circulation. Impairment of the autonomic nervous system through activation of sensory neurons in the lung may be responsible for the acute changes in cardiac function and rhythm following exposure via directly modulating cardiac function or through altering the vasculature. The direct effects of particles via translocation and the release of pulmonary-derived mediators however remains obscure since at ambient levels of particle inhalation it is not known if there are sufficient quantities of particle components or mediators released into the circulation to facilitate adverse outcomes. Pulmonary inflammation appears to be a key step in progression from inhalation exposure to cardiovascular events, however the role of inflammatory cytokines is also questionable based on the available literature showing lack of sufficient release of cytokines from lung. Specifically, the activation of pulmonary and/or systemic mast cells either directly or indirectly by the inhalation of UFP and NP does constitute a likely mechanism influencing cardiovascular toxicity. The role of mast cells has been supported by recent studies however further evaluation is needed.
The interactions between the pulmonary and cardiovascular system resulting in adverse health outcomes are complex, dynamic, and exposure-specific. Many more focused temporal inhalation studies will be needed with relatively high levels of exposures to various chemically and physically characterized materials addressing specific mechanisms of cardiopulmonary interaction in a systematic manner. Furthermore studies are needed at low concentrations to determine the validity of each mechanism at relevant human exposure levels as well as the risk of health effects.
Acknowledgements
This work was funded by NIH RO1 ES019311 (JMB).
Footnotes
Disclaimer: This article has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and the policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Competing interests: The authors declare that they have no competing interests.
References
- Aiso S, Kubota H, Umeda Y, Kasai T, Takaya M, Yamazaki K, Nagano K, Sakai T, Koda S, Fukushima S. Translocation of intratracheally instilled multiwall carbon nanotubes to lung-associated lymph nodes in rats. Industrial health. 2011;vol. 49(no. 2):215–220. doi: 10.2486/indhealth.ms1213. [DOI] [PubMed] [Google Scholar]
- Andersen ZJ, Olsen TS, Andersen KK, Loft S, Ketzel M, Raaschou-Nielsen O. Association between short-term exposure to ultrafine particles and hospital admissions for stroke in Copenhagen, Denmark. European heart journal. 2010;vol. 31(no. 16):2034–2040. doi: 10.1093/eurheartj/ehq188. [DOI] [PubMed] [Google Scholar]
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circulation research. 2008;vol. 102(no. 5):589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlovic DP, Soro-Paavonen A, Jandeleit-Dahm KA. RAGE biology, atherosclerosis and diabetes. Clinical science (London, England : 1979) 2011;vol. 121(no. 2):43–55. doi: 10.1042/CS20100501. [DOI] [PubMed] [Google Scholar]
- Becher R, Bucht A, Ovrevik J, Hongslo JK, Dahlman HJ, Samuelsen JT, Schwarze PE. Involvement of NADPH oxidase and iNOS in rodent pulmonary cytokine responses to urban air and mineral particles. Inhalation toxicology. 2007;vol. 19(no. 8):645–655. doi: 10.1080/08958370701353528. [DOI] [PubMed] [Google Scholar]
- Belleudi V, Faustini A, Stafoggia M, Cattani G, Marconi A, Perucci CA, Forastiere F. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology (Cambridge, Mass.) 2010;vol. 21(no. 3):414–423. doi: 10.1097/EDE.0b013e3181d5c021. [DOI] [PubMed] [Google Scholar]
- Bonner JC. Nanoparticles as a potential cause of pleural and interstitial lung disease. Proceedings of the American Thoracic Society. 2010;vol. 7(no. 2):138–141. doi: 10.1513/pats.200907-061RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzini M, Tripodi A, Artoni A, Tarantini L, Marinelli B, Bertazzi PA, Apostoli P, Baccarelli A. Effects of inhalable particulate matter on blood coagulation. Journal of thrombosis and haemostasis : JTH. 2010;vol. 8(no. 4):662–668. doi: 10.1111/j.1538-7836.2009.03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot I, Biessen EA. Mast cells in atherosclerosis. Thrombosis and haemostasis. 2011;vol. 106(no. 5):820–826. doi: 10.1160/TH11-05-0291. [DOI] [PubMed] [Google Scholar]
- Bot I, Bot M, van Heiningen SH, van Santbrink PJ, Lankhuizen IM, Hartman P, Gruener S, Hilpert H, van Berkel TJ, Fingerle J, Biessen EA. Mast cell chymase inhibition reduces atherosclerotic plaque progression and improves plaque stability in ApoE-/- mice. Cardiovascular research. 2011;vol. 89(no. 1):244–252. doi: 10.1093/cvr/cvq260. [DOI] [PubMed] [Google Scholar]
- Bot I, de Jager SC, Bot M, van Heiningen SH, de Groot P, Veldhuizen RW, van Berkel TJ, von der Thusen JH, Biessen EA. The neuropeptide substance P mediates adventitial mast cell activation and induces intraplaque hemorrhage in advanced atherosclerosis. Circulation research. 2010;vol. 106(no. 1):89–92. doi: 10.1161/CIRCRESAHA.109.204875. [DOI] [PubMed] [Google Scholar]
- Bot I, de Jager SC, Zernecke A, Lindstedt KA, van Berkel TJ, Weber C, Biessen EA. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;vol. 115(no. 19):2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- Breitner S, Liu L, Cyrys J, Bruske I, Franck U, Schlink U, Leitte AM, Herbarth O, Wiedensohler A, Wehner B, Hu M, Pan XC, Wichmann HE, Peters A. Sub-micrometer particulate air pollution and cardiovascular mortality in Beijing, China. The Science of the total environment. 2011;vol. 409(no. 24):5196–5204. doi: 10.1016/j.scitotenv.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Brown JM, Wilson TM, Metcalfe DD. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2008;vol. 38(no. 1):4–18. doi: 10.1111/j.1365-2222.2007.02886.x. [DOI] [PubMed] [Google Scholar]
- Budinger GR, McKell JL, Urich D, Foiles N, Weiss I, Chiarella SE, Gonzalez A, Soberanes S, Ghio AJ, Nigdelioglu R, Mutlu EA, Radigan KA, Green D, Kwaan HC, Mutlu GM. Particulate matter-induced lung inflammation increases systemic levels of PAI-1 and activates coagulation through distinct mechanisms. PloS one. 2011;vol. 6(no. 4):e18525. doi: 10.1371/journal.pone.0018525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Vincent R, Mora-Tiscareno A, Franco-Lira M, Henriquez-Roldan C, Barragan-Mejia G, Garrido-Garcia L, Camacho-Reyes L, Valencia-Salazar G, Paredes R, Romero L, Osnaya H, Villarreal-Calderon R, Torres-Jardon R, Hazucha MJ, Reed W. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environmental health perspectives. 2007;vol. 115(no. 8):1248–1253. doi: 10.1289/ehp.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceron JJ, Eckersall PD, Martynez-Subiela S. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Veterinary clinical pathology / American Society for Veterinary Clinical Pathology. 2005;vol. 34(no. 2):85–99. doi: 10.1111/j.1939-165x.2005.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Chen EY, Garnica M, Wang YC, Mintz AJ, Chen CS, Chin WC. A mixture of anatase and rutile TiO2 nanoparticles induces histamine secretion in mast cells. Particle and fibre toxicology. 2012;vol. 9(no. 1):2. doi: 10.1186/1743-8977-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Meng H, Xing G, Yuan H, Zhao F, Liu R, Chang X, Gao X, Wang T, Jia G, Ye C, Chai Z, Zhao Y. Age-related differences in pulmonary and cardiovascular responses to SiO2 nanoparticle inhalation: nanotoxicity has susceptible population. Environmental science and technology. 2008;vol. 42(no. 23):8985–8992. doi: 10.1021/es800975u. [DOI] [PubMed] [Google Scholar]
- Cherng TW, Campen MJ, Knuckles TL, Gonzalez Bosc L, Kanagy NL. Impairment of coronary endothelial cell ET(B) receptor function after short-term inhalation exposure to whole diesel emissions. American journal of physiology.Regulatory, integrative and comparative physiology. 2009;vol. 297(no. 3):R640–7. doi: 10.1152/ajpregu.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WS, Duffin R, Poland CA, Howie SE, MacNee W, Bradley M, Megson IL, Donaldson K. Metal oxide nanoparticles induce unique inflammatory footprints in the lung: important implications for nanoparticle testing. Environmental health perspectives. 2010;vol. 118(no. 12):1699–1706. doi: 10.1289/ehp.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A, Insin N, Bawendi MG, Semmler-Behnke M, Frangioni JV, Tsuda A. Rapid translocation of nanoparticles from the lung airspaces to the body. Nature biotechnology. 2010;vol. 28(no. 12):1300–1303. doi: 10.1038/nbt.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KJ, Chan CC, Chen NT, Su TC, Lin LY. Effects of particle size fractions on reducing heart rate variability in cardiac and hypertensive patients. Environmental health perspectives. 2005;vol. 113(no. 12):1693–1697. doi: 10.1289/ehp.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC. Neural regulation of bronchial blood flow. Respiration physiology. 1994;vol. 98(no. 1):1–13. doi: 10.1016/0034-5687(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Cozzi E, Wingard CJ, Cascio WE, Devlin RB, Miles JJ, Bofferding AR, Lust RM, Van Scott MR, Henriksen RA. Effect of ambient particulate matter exposure on hemostasis. Translational research : the journal of laboratory and clinical medicine. 2007;vol. 149(no. 6):324–332. doi: 10.1016/j.trsl.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Cray C, Zaias J, Altman NH. Acute phase response in animals: a review. Comparative medicine. 2009;vol. 59(no. 6):517–526. [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environmental health perspectives. 2005;vol. 113(no. 8):934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger A, Zhou Z, Norton SK, Lenk R, Conrad D, Kepley CL. Uptake and distribution of fullerenes in human mast cells. Nanomedicine : nanotechnology, biology, and medicine. 2010;vol. 6(no. 4):575–582. doi: 10.1016/j.nano.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanets S, Konya V, Kastl SP, Kaun C, Rauscher S, Niessner A, Pentz R, Pfaffenberger S, Rychli K, Lemberger CE, de Martin R, Heinemann A, Huk I, Groger M, Maurer G, Huber K, Wojta J. Interleukin-33 induces expression of adhesion molecules and inflammatory activation in human endothelial cells and in human atherosclerotic plaques. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;vol. 31(no. 9):2080–2089. doi: 10.1161/ATVBAHA.111.231431. [DOI] [PubMed] [Google Scholar]
- Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. The European respiratory journal.Supplement. 2003;vol. 40:76s–80s. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Brown D, Clouter A, Duffin R, MacNee W, Renwick L, Tran L, Stone V. The pulmonary toxicology of ultrafine particles. Journal of aerosol medicine : the official journal of the International Society for Aerosols in Medicine. 2002;vol. 15(no. 2):213–220. doi: 10.1089/089426802320282338. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Stone V, Seaton A, MacNee W. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environmental health perspectives. 2001;vol. 109(Suppl 4):523–527. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J, Stewart DJ, Cernacek P, Gosselin G. Human pulmonary circulation is an important site for both clearance and production of endothelin-1. Circulation. 1996;vol. 94(no. 7):1578–1584. doi: 10.1161/01.cir.94.7.1578. [DOI] [PubMed] [Google Scholar]
- Elder A, Couderc JP, Gelein R, Eberly S, Cox C, Xia X, Zareba W, Hopke P, Watts W, Kittelson D, Frampton M, Utell M, Oberdorster G. Effects of on-road highway aerosol exposures on autonomic responses in aged, spontaneously hypertensive rats. Inhalation toxicology. 2007;vol. 19(no. 1):1–12. doi: 10.1080/08958370600985735. [DOI] [PubMed] [Google Scholar]
- Elder AC, Gelein R, Azadniv M, Frampton M, Finkelstein J, Oberdorster G. Systemic effects of inhaled ultrafine particles in two compromised, aged rat strains. Inhalation toxicology. 2004;vol. 16(no. 6-7):461–471. doi: 10.1080/08958370490439669. [DOI] [PubMed] [Google Scholar]
- Erdely A, Liston A, Salmen-Muniz R, Hulderman T, Young SH, Zeidler-Erdely PC, Castranova V, Simeonova PP. Identification of systemic markers from a pulmonary carbon nanotube exposure. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2011;vol. 53(no. 6 Suppl):S80–6. doi: 10.1097/JOM.0b013e31821ad724. [DOI] [PubMed] [Google Scholar]
- Farraj AK, Haykal-Coates N, Winsett DW, Hazari MS, Carll AP, Rowan WH, Ledbetter AD, Cascio WE, Costa DL. Increased non-conducted P-wave arrhythmias after a single oil fly ash inhalation exposure in hypertensive rats. Environmental health perspectives. 2009;vol. 117(no. 5):709–715. doi: 10.1289/ehp.0800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folino AF, Scapellato ML, Canova C, Maestrelli P, Bertorelli G, Simonato L, Iliceto S, Lotti M. Individual exposure to particulate matter and the short-term arrhythmic and autonomic profiles in patients with myocardial infarction. European heart journal. 2009;vol. 30(no. 13):1614–1620. doi: 10.1093/eurheartj/ehp136. [DOI] [PubMed] [Google Scholar]
- Frampton MW. Systemic and cardiovascular effects of airway injury and inflammation: ultrafine particle exposure in humans. Environmental health perspectives. 2001;vol. 109(Suppl 4):529–532. doi: 10.1289/ehp.01109s4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck U, Odeh S, Wiedensohler A, Wehner B, Herbarth O. The effect of particle size on cardiovascular disorders--the smaller the worse. The Science of the total environment. 2011;vol. 409(no. 20):4217–4221. doi: 10.1016/j.scitotenv.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Furuyama A, Kanno S, Kobayashi T, Hirano S. Extrapulmonary translocation of intratracheally instilled fine and ultrafine particles via direct and alveolar macrophage-associated routes. Archives of Toxicology. 2009;vol. 83(no. 5):429–437. doi: 10.1007/s00204-008-0371-1. [DOI] [PubMed] [Google Scholar]
- Ge C, Meng L, Xu L, Bai R, Du J, Zhang L, Li Y, Chang Y, Zhao Y, Chen C. Acute pulmonary and moderate cardiovascular responses of spontaneously hypertensive rats after exposure to single-wall carbon nanotubes. Nanotoxicology. 2011 doi: 10.3109/17435390.2011.587905. [DOI] [PubMed] [Google Scholar]
- Geys J, Nemmar A, Verbeken E, Smolders E, Ratoi M, Hoylaerts MF, Nemery B, Hoet PH. Acute toxicity and prothrombotic effects of quantum dots: impact of surface charge. Environmental health perspectives. 2008;vol. 116(no. 12):1607–1613. doi: 10.1289/ehp.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Hall A, Bassett MA, Cascio WE, Devlin RB. Exposure to concentrated ambient air particles alters hematologic indices in humans. Inhalation toxicology. 2003;vol. 15(no. 14):1465–1478. doi: 10.1080/08958370390249111. [DOI] [PubMed] [Google Scholar]
- Gilmour PS, Ziesenis A, Morrison ER, Vickers MA, Drost EM, Ford I, Karg E, Mossa C, Schroeppel A, Ferron GA, Heyder J, Greaves M, MacNee W, Donaldson K. Pulmonary and systemic effects of short-term inhalation exposure to ultrafine carbon black particles. Toxicology and applied pharmacology. 2004;vol. 195(no. 1):35–44. doi: 10.1016/j.taap.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Godleski JJ, Verrier RL, Koutrakis P, Caralano P, Coull B, Reinisch U, Lovett EG, Lawrence J, Murthy GG, Wolfson JM, Clarke RW, Nearing BD, Killingsworth C. Mechanisms of morbidity and mortality from exposure to ambient air particles’. vol. 91. Research report/health effects institute; 2000. pp. 5–88. discussion 89-103. [PubMed] [Google Scholar]
- Gojova A, Lee JT, Jung HS, Guo B, Barakat AI, Kennedy IM. Effect of cerium oxide nanoparticles on inflammation in vascular endothelial cells. Inhalation toxicology. 2009;vol. 21(Suppl 1):123–130. doi: 10.1080/08958370902942582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ, Schladweiler MC, Krantz C, Kodavanti UP. Cardiovascular and thermoregulatory responses of unrestrained rats exposed to filtered or unfiltered diesel exhaust. Inhalation toxicology. 2012 doi: 10.3109/08958378.2012.670811. Submitted. [DOI] [PubMed] [Google Scholar]
- Gottipolu RR, Wallenborn JG, Karoly ED, Schladweiler MC, Ledbetter AD, Krantz T, Linak WP, Nyska A, Johnson JA, Thomas R, Richards JE, Jaskot RH, Kodavanti UP. One-month diesel exhaust inhalation produces hypertensive gene expression pattern in healthy rats. Environmental health perspectives. 2009;vol. 117(no. 1):38–46. doi: 10.1289/ehp.11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Subramanian H, Gupta K, Ali H. Regulation of C3a receptor signaling in human mast cells by G protein coupled receptor kinases. PloS one. 2011;vol. 6(no. 7):e22559. doi: 10.1371/journal.pone.0022559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halappanavar S, Jackson P, Williams A, Jensen KA, Hougaard KS, Vogel U, Yauk CL, Wallin H. Pulmonary response to surface-coated nanotitanium dioxide particles includes induction of acute phase response genes, inflammatory cascades, and changes in microRNAs: a toxicogenomic study. Environmental and molecular mutagenesis. 2011;vol. 52(no. 6):425–439. doi: 10.1002/em.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AR, Keegstra JM, Duch MC, Hersam MC, Dekker C. Translocation of single-wall carbon nanotubes through solid-state nanopores. Nano letters. 2011;vol. 11(no. 6):2446–2450. doi: 10.1021/nl200873w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvima IT, Nilsson G, Naukkarinen A. Role of mast cells and sensory nerves in skin inflammation. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia. 2010;vol. 145(no. 2):195–204. [PubMed] [Google Scholar]
- Hazari MS, Rowan WH, Winsett DW, Ledbetter AD, Haykal-Coates N, Watkinson WP, Costa DL. Potentiation of pulmonary reflex response to capsaicin 24h following whole-body acrolein exposure is mediated by TRPV1. Respiratory physiology and neurobiology. 2008;vol. 160(no. 2):160–171. doi: 10.1016/j.resp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Helfenstein M, Miragoli M, Rohr S, Muller L, Wick P, Mohr M, Gehr P, Rothen-Rutishauser B. Effects of combustion-derived ultrafine particles and manufactured nanoparticles on heart cells in vitro. Toxicology. 2008;vol. 253(no. 1-3):70–78. doi: 10.1016/j.tox.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Hendren CO, Mesnard X, Droge J, Wiesner MR. Estimating production data for five engineered nanomaterials as a basis for exposure assessment. Envionmental science and technology. 2011;vol. 45(no. 7):2562–2569. doi: 10.1021/es103300g. [DOI] [PubMed] [Google Scholar]
- Ho CY, Lee LY. Ozone enhances excitabilities of pulmonary C fibers to chemical and mechanical stimuli in anesthetized rats. Journal of applied physiology (Bethesda, Md.: 1985) 1998;vol. 85(no. 4):1509–1515. doi: 10.1152/jappl.1998.85.4.1509. [DOI] [PubMed] [Google Scholar]
- Hoek G, Brunekreef B, Fischer P, van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology (Cambridge, Mass.) 2001;vol. 12(no. 3):355–357. doi: 10.1097/00001648-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacological reviews. 1991;vol. 43(no. 2):143–201. [PubMed] [Google Scholar]
- Horie M, Fukui H, Nishio K, Endoh S, Kato H, Fujita K, Miyauchi A, Nakamura A, Shichiri M, Ishida N, Kinugasa S, Morimoto Y, Niki E, Yoshida Y, Iwahashi H. Evaluation of acute oxidative stress induced by NiO nanoparticles in vivo and in vitro. Journal of occupational health. 2011;vol. 53(no. 2):64–74. doi: 10.1539/joh.l10121. [DOI] [PubMed] [Google Scholar]
- Hu CL, Xiang JZ, Hu FF. Vanilloid receptor TRPV1, sensory C-fibers, and activation of adventitial mast cells. A novel mechanism involved in adventitial inflammation. Medical hypotheses. 2008;vol. 71(no. 1):102–103. doi: 10.1016/j.mehy.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Huang CH, Lin LY, Tsai MS, Hsu CY, Chen HW, Wang TD, Chang WT, Cheng TJ, Chen WJ. Acute cardiac dysfunction after short-term diesel exhaust particles exposure. Toxicology letters. 2010;vol. 192(no. 3):349–355. doi: 10.1016/j.toxlet.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Huang YF, Liu H, Xiong X, Chen Y, Tan W. Nanoparticle-mediated IgE-receptor aggregation and signaling in RBL mast cells. Journal of the American Chemical Society. 2009;vol. 131(no. 47):17328–17334. doi: 10.1021/ja907125t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibald-Mulli A, Wichmann HE, Kreyling W, Peters A. Epidemiological evidence on health effects of ultrafine particles. Journal of aerosol medicine : the official journal of the International Society for Aerosols in Medicine. 2002;vol. 15(no. 2):189–201. doi: 10.1089/089426802320282310. [DOI] [PubMed] [Google Scholar]
- Kan H, Wu Z, Young SH, Chen TH, Cumpston JL, Chen F, Kashon ML, Castranova V. Pulmonary exposure of rats to ultrafine titanium dioxide enhances cardiac protein phosphorylation and substance P synthesis in nodose ganglia. Nanotoxicology. 2011 doi: 10.3109/17435390.2011.611915. [DOI] [PubMed] [Google Scholar]
- Kang GS, Gillespie PA, Gunnison A, Moreira AL, Tchou-Wong KM, Chen LC. Long-term inhalation exposure to nickel nanoparticles exacerbated atherosclerosis in a susceptible mouse model. Environmental health perspectives. 2011;vol. 119(no. 2):176–181. doi: 10.1289/ehp.1002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X, Li N, Wang M, Boontheung P, Sioutas C, Harkema JR, Bramble LA, Nel AE, Loo JA. Adjuvant effects of ambient particulate matter monitored by proteomics of bronchoalveolar lavage fluid. Proteomics. 2010;vol. 10(no. 3):520–531. doi: 10.1002/pmic.200900573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly ED, Li Z, Dailey LA, Hyseni X, Huang YC. Up-regulation of tissue factor in human pulmonary artery endothelial cells after ultrafine particle exposure. Environmental health perspectives. 2007;vol. 115(no. 4):535–540. doi: 10.1289/ehp.9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandoga A, Stampfl A, Takenaka S, Schulz H, Radykewicz R, Kreyling W, Krombach F. Ultrafine particles exert prothrombotic but not inflammatory effects on the hepatic microcirculation in healthy mice in vivo. Circulation. 2004;vol. 109(no. 10):1320–1325. doi: 10.1161/01.CIR.0000118524.62298.E8. [DOI] [PubMed] [Google Scholar]
- Kita T, Kume N, Minami M, Hayashida K, Murayama T, Sano H, Moriwaki H, Kataoka H, Nishi E, Horiuchi H, Arai H, Yokode M. Role of oxidized LDL in atherosclerosis. Annals of the New York Academy of Sciences. 2001;vol. 947:199–205. doi: 10.1111/j.1749-6632.2001.tb03941.x. discussion 205-6. [DOI] [PubMed] [Google Scholar]
- Knaapen AM, den Hartog GJ, Bast A, Borm PJ. Ambient particulate matter induces relaxation of rat aortic rings in vitro. Human and experimental toxicology. 2001;vol. 20(no. 5):259–265. doi: 10.1191/096032701678227677. [DOI] [PubMed] [Google Scholar]
- Knuckles TL, Lund AK, Lucas SN, Campen MJ. Diesel exhaust exposure enhances venoconstriction via uncoupling of eNOS. Toxicology and applied pharmacology. 2008;vol. 230(no. 3):346–351. doi: 10.1016/j.taap.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Schladweiler MC, Gilmour PS, Wallenborn JG, Mandavilli BS, Ledbetter AD, Christiani DC, Runge MS, Karoly ED, Costa DL, Peddada S, Jaskot R, Richards JH, Thomas R, Madamanchi NR, Nyska A. The role of particulate matter-associated zinc in cardiac injury in rats. Environmental health perspectives. 2008;vol. 116(no. 1):13–20. doi: 10.1289/ehp.10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti UP, Schladweiler MC, Ledbetter AD, Watkinson WP, Campen MJ, Winsett DW, Richards JR, Crissman KM, Hatch GE, Costa DL. The spontaneously hypertensive rat as a model of human cardiovascular disease: evidence of exacerbated cardiopulmonary injury and oxidative stress from inhaled emission particulate matter. Toxicology and applied pharmacology. 2000;vol. 164(no. 3):250–263. doi: 10.1006/taap.2000.8899. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Thomas R, Ledbetter AD, Schladweiler MC, Shannahan JH, Wallenborn JG, Lund AK, Campen MJ, Butler EO, Gottipolu RR, Nyska A, Richards JE, Andrews D, Jaskot RH, McKee J, Kotha SR, Patel RB, Parinandi NL. Vascular and cardiac impairments in rats inhaling ozone and diesel exhaust particles. Environmental health perspectives. 2011;vol. 119(no. 3):312–318. doi: 10.1289/ehp.1002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkonen JO, Kovanen PT. Stimulation of mast cells leads to cholesterol accumulation in macrophages in vitro by a mast cell granule-mediated uptake of low density lipoprotein. Proceedings of the National Academy of Sciences of the United States of America. 1987;vol. 84(no. 8):2287–2291. doi: 10.1073/pnas.84.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy G, Ajitawi O, Chi DS. The human mast cell: an overview. Methods in molecular biology (Clifton, N.J.) 2006;vol. 315:13–34. doi: 10.1385/1-59259-967-2:013. [DOI] [PubMed] [Google Scholar]
- Kunder C, St John A, Li G, Leong K, Berwin B, Staats H, Abraham S. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. Journal of Experimental Medicine. 2009;vol. 206(no. 11):2455–2467. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine P, Naukkarinen A, Heikkila L, Penttila A, Kovanen PT. Adventitial mast cells connect with sensory nerve fibers in atherosclerotic coronary arteries. Circulation. 2000;vol. 101(no. 14):1665–1669. doi: 10.1161/01.cir.101.14.1665. [DOI] [PubMed] [Google Scholar]
- Lakota K, Zigon P, Mrak-Poljsak K, Rozman B, Shoenfeld Y, Sodin-Semrl S. Antibodies against acute phase proteins and their functions in the pathogenesis of disease: a collective profile of 25 different antibodies. Autoimmunity reviews. 2011;vol. 10(no. 12):779–789. doi: 10.1016/j.autrev.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Lanki T, Pekkanen J, Aalto P, Elosua R, Berglind N, D’Ippoliti D, Kulmala M, Nyberg F, Peters A, Picciotto S, Salomaa V, Sunyer J, Tiittanen P, von Klot S, Forastiere F. Associations of traffic related air pollutants with hospitalisation for first acute myocardial infarction: the HEAPSS study. Occupational and environmental medicine. 2006;vol. 63(no. 12):844–851. doi: 10.1136/oem.2005.023911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapar DJ, Sharma AK, Hajzus VA, Zhao Y, Lau CL, French BA, Kron IL, Laubach VE. Acute Hyperglycemic Exacerbation of Lung Ischemia-Reperfusion Injury is Mediated by RAGE Signaling. American journal of respiratory cell and molecular biology. 2011 doi: 10.1165/rcmb.2011-0247OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin DL, Heck DE, Laskin JD. Role of inflammatory cytokines and nitric oxide in hepatic and pulmonary toxicity. Toxicology letters. 1998;vol. 102-103:289–293. doi: 10.1016/s0378-4274(98)00316-6. [DOI] [PubMed] [Google Scholar]
- LeBlanc AJ, Moseley AM, Chen BT, Frazer D, Castranova V, Nurkiewicz TR. Nanoparticle inhalation impairs coronary microvascular reactivity via a local reactive oxygen species-dependent mechanism. Cardiovascular toxicology. 2010;vol. 10(no. 1):27–36. doi: 10.1007/s12012-009-9060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legramante JM, Valentini F, Magrini A, Palleschi G, Sacco S, Iavicoli I, Pallante M, Moscone D, Galante A, Bergamaschi E, Bergamaschi A, Pietroiusti A. Cardiac autonomic regulation after lung exposure to carbon nanotubes. Human and experimental toxicology. 2009;vol. 28(no. 6-7):369–375. doi: 10.1177/0960327109105150. [DOI] [PubMed] [Google Scholar]
- Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovascular research. 2011;vol. 89(no. 1):12–19. doi: 10.1093/cvr/cvq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JA, Rao KM, Castranova V, Vallyathan V, Dennis WE, Knechtges PL. Proteomic analysis of bronchoalveolar lavage fluid: effect of acute exposure to diesel exhaust particles in rats. Environmental health perspectives. 2007;vol. 115(no. 5):756–763. doi: 10.1289/ehp.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Muralikrishnan S, Ng CT, Yung LY, Bay BH. Nanoparticle-induced pulmonary toxicity. Experimental biology and medicine (Maywood, N.J.) 2010a;vol. 235(no. 9):1025–1033. doi: 10.1258/ebm.2010.010021. [DOI] [PubMed] [Google Scholar]
- Li M, Yu D, Williams KJ, Liu ML. Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010b;vol. 30(no. 9):1818–1824. doi: 10.1161/ATVBAHA.110.209577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li J, Yin J, Li W, Kang C, Huang Q, Li Q. Systematic influence induced by 3 nm titanium dioxide following intratracheal instillation of mice. Journal of nanoscience and nanotechnology. 2010c;vol. 10(no. 12):8544–8549. doi: 10.1166/jnn.2010.2690. [DOI] [PubMed] [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environmental health perspectives. 2003;vol. 111(no. 4):455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Ning Z, Cui J, Khalsa B, Ai L, Takabe W, Beebe T, Majumdar R, Sioutas C, Hsiai T. Ultrafine particles from diesel engines induce vascular oxidative stress via JNK activation. Free radical biology and medicine. 2009;vol. 46(no. 6):775–782. doi: 10.1016/j.freeradbiomed.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Ning Z, Majumdar R, Cui J, Takabe W, Jen N, Sioutas C, Hsiai T. Ultrafine particles from diesel vehicle emissions at different driving cycles induce differential vascular pro-inflammatory responses: implication of chemical components and NF-kappaB signaling. Particle and fibre toxicology. 2010;vol. 7:6. doi: 10.1186/1743-8977-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hulderman T, Salmen R, Chapman R, Leonard SS, Young SH, Shvedova A, Luster MI, Simeonova PP. Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes. Environmental health perspectives. 2007;vol. 115(no. 3):377–382. doi: 10.1289/ehp.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L, Starzyk RM, Granger DN. Molecular determinants of oxidized low-density lipoprotein-induced leukocyte adhesion and microvascular dysfunction. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;vol. 17(no. 3):437–444. doi: 10.1161/01.atv.17.3.437. [DOI] [PubMed] [Google Scholar]
- Lipka J, Semmler-Behnke M, Sperling RA, Wenk A, Takenaka S, Schleh C, Kissel T, Parak WJ, Kreyling WG. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials. 2010;vol. 31(no. 25):6574–6581. doi: 10.1016/j.biomaterials.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gao Y, Zhang L, Wang T, Wang J, Jiao F, Li W, Liu Y, Li Y, Li B, Chai Z, Wu G, Chen C. Potential health impact on mice after nasal instillation of nano-sized copper particles and their translocation in mice. Journal of nanoscience and nanotechnology. 2009;vol. 9(no. 11):6335–6343. doi: 10.1166/jnn.2009.1320. [DOI] [PubMed] [Google Scholar]
- Love SA, Haynes CL. Assessment of functional changes in nanoparticle-exposed neuroendocrine cells with amperometry: exploring the generalizability of nanoparticle-vesicle matrix interactions. Analytical and bioanalytical chemistry. 2010;vol. 398(no. 2):677–688. doi: 10.1007/s00216-010-3735-3. [DOI] [PubMed] [Google Scholar]
- Lund AK, Lucero J, Harman M, Madden MC, McDonald JD, Seagrave JC, Campen MJ. The oxidized low-density lipoprotein receptor mediates vascular effects of inhaled vehicle emissions. American journal of respiratory and critical care medicine. 2011;vol. 184(no. 1):82–91. doi: 10.1164/rccm.201012-1967OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JY, Zhao H, Mercer RR, Barger M, Rao M, Meighan T, Schwegler-Berry D, Castranova V, Ma JK. Cerium oxide nanoparticle-induced pulmonary inflammation and alveolar macrophage functional change in rats. Nanotoxicology. 2011;vol. 5:312–325. doi: 10.3109/17435390.2010.519835. [DOI] [PubMed] [Google Scholar]
- Mani U, Prasad AK, Suresh Kumar V, Lal K, Kanojia RK, Chaudhari BP, Murthy RC. Effect of fly ash inhalation on biochemical and histomorphological changes in rat liver. Ecotoxicology and environmental safety. 2007;vol. 68(no. 1):126–133. doi: 10.1016/j.ecoenv.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Marquis BJ, Maurer-Jones MA, Braun KL, Haynes CL. Amperometric assessment of functional changes in nanoparticle-exposed immune cells: varying Au nanoparticle exposure time and concentration. The Analyst. 2009;vol. 134(no. 11):2293–2300. doi: 10.1039/b913967b. [DOI] [PubMed] [Google Scholar]
- Maurer-Jones MA, Lin YS, Haynes CL. Functional assessment of metal oxide nanoparticle toxicity in immune cells. ACS nano. 2010;vol. 4(no. 6):3363–3373. doi: 10.1021/nn9018834. [DOI] [PubMed] [Google Scholar]
- Mehta JL. The role of LOX-1, a novel lectin-like receptor for oxidized low density lipoprotein, in atherosclerosis. The Canadian journal of cardiology. 2004;vol. 20(Suppl B):32B–36B. [PubMed] [Google Scholar]
- Melendez GC, Li J, Law BA, Janicki JS, Supowit SC, Levick SP. Substance P induces adverse myocardial remodelling via a mechanism involving cardiac mast cells. Cardiovascular research. 2011;vol. 92(no. 3):420–429. doi: 10.1093/cvr/cvr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NL, Miller MR, Lucking AJ, Beveridge J, Flint L, Boere AJ, Fokkens PH, Boon NA, Sandstrom T, Blomberg A, Duffin R, Donaldson K, Hadoke PW, Cassee FR, Newby DE. Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. European heart journal. 2011 doi: 10.1093/eurheartj/ehr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Goyal T, Mehta JL. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2011;vol. 25(no. 5):419–429. doi: 10.1007/s10557-011-6341-5. [DOI] [PubMed] [Google Scholar]
- Moyssakis I, Vlahodimitris IE, Kanakis MA, Kapsimali V, Tsoucala C, Vaiopoulos GA. Behavior of coagulation factors and normal inhibitors of coagulation during the acute phase of myocardial infarction. Blood coagulation and fibrinolysis : an international journal in haemostasis and thrombosis. 2010;vol. 21(no. 7):670–673. doi: 10.1097/MBC.0b013e32833e479a. [DOI] [PubMed] [Google Scholar]
- Mutlu GM, Green D, Bellmeyer A, Baker CM, Burgess Z, Rajamannan N, Christman JW, Foiles N, Kamp DW, Ghio AJ, Chandel NS, Dean DA, Sznajder JI, Budinger GR. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. The Journal of clinical investigation. 2007;vol. 117(no. 10):2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalabotu SK, Kolli MB, Triest WE, Ma JY, Manne ND, Katta A, Addagarla HS, Rice KM, Blough ER. Intratracheal instillation of cerium oxide nanoparticles induces hepatic toxicity in male Sprague-Dawley rats. International journal of nanomedicine. 2011;vol. 6:2327–2335. doi: 10.2147/IJN.S25119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naota M, Shimada A, Morita T, Inoue K, Takano H. Translocation pathway of the intratracheally instilled C60 fullerene from the lung into the blood circulation in the mouse: possible association of diffusion and caveolae-mediated pinocytosis. Toxicologic pathology. 2009;vol. 37(no. 4):456–462. doi: 10.1177/0192623309335059. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Al-Salam S, Zia S, Dhanasekaran S, Shudadevi M, Ali BH. Time-course effects of systemically administered diesel exhaust particles in rats. Toxicology letters. 2010;vol. 194(no. 3):58–65. doi: 10.1016/j.toxlet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Delaunois A, Nemery B, Dessy-Doize C, Beckers JF, Sulon J, Gustin P. Inflammatory effect of intratracheal instillation of ultrafine particles in the rabbit: role of C-fiber and mast cells. Toxicology and applied pharmacology. 1999;vol. 160(no. 3):250–261. doi: 10.1006/taap.1999.8762. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Dhanasekaran S, Yasin J, Ba-Omar H, Fahim MA, Kazzam EE, Ali BH. Evaluation of the direct systemic and cardiopulmonary effects of diesel particles in spontaneously hypertensive rats. Toxicology. 2009;vol. 262(no. 1):50–56. doi: 10.1016/j.tox.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoet PH, Dinsdale D, Vermylen J, Hoylaerts MF, Nemery B. Diesel exhaust particles in lung acutely enhance experimental peripheral thrombosis. Circulation. 2003;vol. 107(no. 8):1202–1208. doi: 10.1161/01.cir.0000053568.13058.67. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoet PH, Vandervoort P, Dinsdale D, Nemery B, Hoylaerts MF. Enhanced peripheral thrombogenicity after lung inflammation is mediated by platelet-leukocyte activation: role of P-selectin. Journal of thrombosis and haemostasis : JTH. 2007;vol. 5(no. 6):1217–1226. doi: 10.1111/j.1538-7836.2007.02557.x. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002a;vol. 105(no. 4):411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Hoet PH, Dinsdale D, Smith T, Xu H, Vermylen J, Nemery B. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. American journal of respiratory and critical care medicine. 2002b;vol. 166(no. 7):998–1004. doi: 10.1164/rccm.200110-026OC. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Hoet PH, Nemery B. Possible mechanisms of the cardiovascular effects of inhaled particles: systemic translocation and prothrombotic effects. Toxicology letters. 2004a;vol. 149(no. 1-3):243–253. doi: 10.1016/j.toxlet.2003.12.061. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Hoet PH, Nemery B. Possible mechanisms of the cardiovascular effects of inhaled particles: systemic translocation and prothrombotic effects. Toxicology letters. 2004b;vol. 149(no. 1-3):243–253. doi: 10.1016/j.toxlet.2003.12.061. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Hoet PH, Vermylen J, Nemery B. Size effect of intratracheally instilled particles on pulmonary inflammation and vascular thrombosis. Toxicology and applied pharmacology. 2003;vol. 186(no. 1):38–45. doi: 10.1016/s0041-008x(02)00024-8. [DOI] [PubMed] [Google Scholar]
- Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovascular research. 2007;vol. 73(no. 3):549–559. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y, Hiura Y, Sawamura H, Iwai N. Inhalation exposure to carbon black induces inflammatory response in rats. Circulation journal : official journal of the Japanese Circulation Society. 2008;vol. 72(no. 1):144–149. doi: 10.1253/circj.72.144. [DOI] [PubMed] [Google Scholar]
- Norton S, Dellinger A, Zhou Z, Lenk R, Macfarland D, Vonakis B, Conrad D, Kepley C. A new class of human mast cell and peripheral blood stavilizers that differentially control allergic mediator release. Clinical Translation Science. 2010;vol. 3(no. 4):158–169. doi: 10.1111/j.1752-8062.2010.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Stone S, Chen BT, Frazer DG, Boegehold MA, Castranova V. Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicological sciences : an official journal of the Society of Toxicology. 2009;vol. 110(no. 1):191–203. doi: 10.1093/toxsci/kfp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. Journal of toxicology and environmental health.Part A. 2002;vol. 65(no. 20):1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Oh WK, Kim S, Choi M, Kim C, Jeong YS, Cho BR, Hahn JS, Jang J. Cellular uptake, cytotoxicity, and innate immune response of silica-titania hollow nanoparticles base on size and surface functionality. American chemical society nano. 2010;vol. 4(no. 9):5301–5313. doi: 10.1021/nn100561e. [DOI] [PubMed] [Google Scholar]
- Oyamada S, Bianchi C, Takai S, Chu LM, Sellke FW. Chymase inhibition reduces infarction and matrix metalloproteinase-9 activation and attenuates inflammation and fibrosis after acute myocardial ischemia/reperfusion. The Journal of pharmacology and experimental therapeutics. 2011;vol. 339(no. 1):143–151. doi: 10.1124/jpet.111.179697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Choi K, Park K. Induction of inflammatory responses and gene expression by intratracheal instillation of silver nanoparticles in mice. Archives of Pharmacal Research. 2011;vol. 34(no. 2):299–307. doi: 10.1007/s12272-011-0216-y. [DOI] [PubMed] [Google Scholar]
- Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1995;vol. 9(no. 12):1196–1204. doi: 10.1096/fasebj.9.12.7672512. [DOI] [PubMed] [Google Scholar]
- Pollreisz A, Hudson BI, Chang JS, Qu W, Cheng B, Papapanou PN, Schmidt AM, Lalla E. Receptor for advanced glycation endproducts mediates pro-atherogenic responses to periodontal infection in vascular endothelial cells. Atherosclerosis. 2010;vol. 212(no. 2):451–456. doi: 10.1016/j.atherosclerosis.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA., 3rd Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who’s at risk? Environmental health perspectives. 2000;vol. 108(Suppl 4):713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. Journal of the Air and Waste Management Association (1995) 2006;vol. 56(no. 6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;vol. 114(no. 23):2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, Radomski MW. Nanoparticle-induced platelet aggregation and vascular thrombosis. British journal of pharmacology. 2005;vol. 146(no. 6):882–893. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AR, Rao MV, Krishna DR, Himabindu V, Reddy YN. Evaluation of oxidative stress and anti-oxidant status in rat serum following exposure of carbon nanotubes. Regulatory toxicology and pharmacology : RTP. 2011;vol. 59(no. 2):251–257. doi: 10.1016/j.yrtph.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Reynolds PR, Wasley KM, Allison CH. Diesel particulate matter induces receptor for advanced glycation end-products (RAGE) expression in pulmonary epithelial cells, and RAGE signaling influences NF-kappaB-mediated inflammation. Environmental health perspectives. 2011;vol. 119(no. 3):332–336. doi: 10.1289/ehp.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pascual F, Busnadiego O, Lagares D, Lamas S. Role of endothelin in the cardiovascular system. Pharmacological research : the official journal of the Italian Pharmacological Society. 2011;vol. 63(no. 6):463–472. doi: 10.1016/j.phrs.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Rosas-Hernandez H, Jimenez-Badillo S, Martinez-Cuevas PP, Gracia-Espino E, Terrones H, Terrones M, Hussain SM, Ali SF, Gonzalez C. Effects of 45-nm silver nanoparticles on coronary endothelial cells and isolated rat aortic rings. Toxicology letters. 2009;vol. 191(no. 2-3):305–313. doi: 10.1016/j.toxlet.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Ruan T, Ho CY, Kou YR. Afferent vagal pathways mediating respiratory reflexes evoked by ROS in the lungs of anesthetized rats. Journal of applied physiology (Bethesda, Md.: 1985) 2003;vol. 94(no. 5):1987–1998. doi: 10.1152/japplphysiol.01047.2002. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Bateman HR, Stover A, Gomez G, Norton SK, Zhao W, Schwartz LB, Lenk R, Kepley CL. Fullerene nanomaterials inhibit the allergic response. Journal of immunology (Baltimore, Md.: 1950) 2007;vol. 179(no. 1):665–672. doi: 10.4049/jimmunol.179.1.665. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, Bonner JC. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nature nanotechnology. 2009;vol. 4(no. 11):747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki O, Kuhara M, Kikuchi N, Shiraishi S, Uda H. Evaluation of Lasting High Levels of CRP among the Patients with Metabolic Syndrome. Inflammation. 2011 doi: 10.1007/s10753-011-9368-7. [DOI] [PubMed] [Google Scholar]
- Sanchez-Crespo A, Klepczynska-Nystrom A, Lundin A, Larsson BM, Svartengren M. (1)(1)(1)Indium-labeled ultrafine carbon particles; a novel aerosol for pulmonary deposition and retention studies. Inhalation toxicology. 2011;vol. 23(no. 3):121–128. doi: 10.3109/08958378.2010.549856. [DOI] [PubMed] [Google Scholar]
- Sannolo N, Lamberti M, Pedata P. Human health effects of ultrafine particles. Giornale italiano di medicina del lavoro ed ergonomia. 2010;vol. 32(no. 4 Suppl):348–351. [PubMed] [Google Scholar]
- Scapellato ML. Individual exposure to particulate matter and autonomic activity in patients with myocardial infarction. Giornale italiano di medicina del lavoro ed ergonomia. 2010;vol. 32(no. 4 Suppl):344–347. [PubMed] [Google Scholar]
- Scherbart AM, Langer J, Bushmelev A, van Berlo D, Haverzettl P, van Schooten FJ, Schmidt AM, Rose CR, Schins RP, Albrecht C. Contrasting macrophage activation by fine and ultrafine titanium dioxide particles is associated with different uptake mechanisms. Particle and fiber toxicology. 2011;vol. 8(no. 31) doi: 10.1186/1743-8977-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;vol. 23(no. 5):479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation. 1988;vol. 78(no. 4):969–979. doi: 10.1161/01.cir.78.4.969. [DOI] [PubMed] [Google Scholar]
- Scotland RS, Chauhan S, Davis C, De Felipe C, Hunt S, Kabir J, Kotsonis P, Oh U, Ahluwalia A. Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation: a novel mechanism involved in myogenic constriction. Circulation research. 2004;vol. 95(no. 10):1027–1034. doi: 10.1161/01.RES.0000148633.93110.24. [DOI] [PubMed] [Google Scholar]
- Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet. 1995;vol. 345(no. 8943):176–178. doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Semmler-Behnke M, Kreyling WG, Lipka J, Fertsch S, Wenk A, Takenaka S, Schmid G, Brandau W. Biodistribution of 1.4- and 18-nm gold particles in rats. Small (Weinheim an der Bergstrasse, Germany) 2008;vol. 4(no. 12):2108–2111. doi: 10.1002/smll.200800922. [DOI] [PubMed] [Google Scholar]
- Shannahan JH, Schladweiler MC, Thomas RF, Ward WO, Ghio AJ, Gavett SH, Kodavanti UP. Vascular and thrombogenic effects of pulmonary exposure to Libby amphibole. Journal of toxicology and environmental health perspectives part A. 2012a doi: 10.1080/15287394.2012.652055. (in press) [DOI] [PubMed] [Google Scholar]
- Shannahan JH, Alzate O, Winnik WM, Andrews D, Schladweiler CJ, Ghio AJ, Gavett SH, Kodavanti UP. Acute phase response, inflammation, and metabolic syndrome biomarkers of Libby asbestos exposure. Toxicology and applied pharmacology. 2012b doi: 10.1016/j.taap.2012.02.006. (in press) [DOI] [PubMed] [Google Scholar]
- Shwe TT, Yamamoto S, Kakeyama M, Kobayashi T, Fujimaki H. Effect of intratracheal instillation of ultrafine carbon black on proinflammatory cytokine and chemokine release and mRNA expression in lung and lymph nodes of mice. Toxicology and applied pharmacology. 2005;vol. 209(no. 1):51–61. doi: 10.1016/j.taap.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Silva VM, Corson N, Elder A, Oberdorster G. The rat ear vein model for investigating in vivo thrombogenicity of ultrafine particles (UFP) Toxicological sciences : an official journal of the Society of Toxicology. 2005;vol. 85(no. 2):983–989. doi: 10.1093/toxsci/kfi142. [DOI] [PubMed] [Google Scholar]
- Stone V, Johnston H, Clift MJ. Air pollution, ultrafine and nanoparticle toxicology: cellular and molecular interactions. IEEE transactions on nanobioscience. 2007;vol. 6(no. 4):331–340. doi: 10.1109/tnb.2007.909005. [DOI] [PubMed] [Google Scholar]
- Strak M, Boogaard H, Meliefste K, Oldenwening M, Zuurbier M, Brunekreef B, Hoek G. Respiratory health effects of ultrafine and fine particle exposure in cyclists. Occupational and environmental medicine. 2010;vol. 67(no. 2):118–124. doi: 10.1136/oem.2009.046847. [DOI] [PubMed] [Google Scholar]
- Tang M, Zhang T, Xue Y, Wang S, Huang M, Yang Y, Lu M, Lei H, Kong L, Wang Y, Pu Y. Metabonomic studies of biochemical changes in the serum of rats by intratracheally instilled TiO2 nanoparticles. Journal of nanoscience and nanotechnology. 2011;vol. 11(no. 4):3065–3074. doi: 10.1166/jnn.2011.3604. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ. Sensing pulmonary oxidative stress by lung vagal afferents. Respiratory physiology and neurobiology. 2011;vol. 178(no. 3):406–413. doi: 10.1016/j.resp.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson E, Kumarathasan P, Goegan P, Aubin RA, Vincent R. Differential regulation of the lung endothelin system by urban particulate matter and ozone. Toxicological sciences : an official journal of the Society of Toxicology. 2005;vol. 88(no. 1):103–113. doi: 10.1093/toxsci/kfi272. [DOI] [PubMed] [Google Scholar]
- Tong H, McGee JK, Saxena RK, Kodavanti UP, Devlin RB, Gilmour MI. Influence of acid functionalization on the cardiopulmonary toxicity of carbon nanotubes and carbon black particles in mice. Toxicology and applied pharmacology. 2009;vol. 239(no. 3):224–232. doi: 10.1016/j.taap.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Tong H, Cheng WY, Samet JM, Gilmour MI, Devlin RB. Differential cardiopulmonary effects of size-fractionated ambient particulate matter in mice. Cardiovascular toxicology. 2010;vol. 10(no. 4):259–267. doi: 10.1007/s12012-010-9082-y. [DOI] [PubMed] [Google Scholar]
- Totlandsdal AI, Skomedal T, Lag M, Osnes JB, Refsnes M. Pro-inflammatory potential of ultrafine particles in mono- and co-cultures of primary cardiac cells. Toxicology. 2008;vol. 247(no. 1):23–32. doi: 10.1016/j.tox.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Tsutamoto T, Wada A, Maeda Y, Adachi T, Kinoshita M. Relation between endothelin-1 spillover in the lungs and pulmonary vascular resistance in patients with chronic heart failure. Journal of the American College of Cardiology. 1994;vol. 23(no. 6):1427–1433. doi: 10.1016/0735-1097(94)90387-5. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Hubbard W, Weinreich D. Immunologically induced neuromodulation of guinea pig nodose ganglion neurons. Journal of the Autonomic Nervous System. 1993;vol. 44(no. 1):35–44. doi: 10.1016/0165-1838(93)90376-6. [DOI] [PubMed] [Google Scholar]
- Upadhyay S, Ganguly K, Stoeger T, Semmler-Bhenke M, Takenaka S, Kreyling WG, Pitz M, Reitmeir P, Peters A, Eickelberg O, Wichmann HE, Schulz H. Cardiovascular and inflammatory effects of intratracheally instilled ambient dust from Augsburg, Germany, in spontaneously hypertensive rats (SHRs) Particle and fibre toxicology. 2010;vol. 7:27. doi: 10.1186/1743-8977-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay S, Stoeger T, Harder V, Thomas RF, Schladweiler MC, Semmler-Behnke M, Takenaka S, Karg E, Reitmeir P, Bader M, Stampfl A, Kodavanti UP, Schulz H. Exposure to ultrafine carbon particles at levels below detectable pulmonary inflammation affects cardiovascular performance in spontaneously hypertensive rats. Particle and fibre toxicology. 2008;vol. 5:19. doi: 10.1186/1743-8977-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venteclef N, Jakobsson T, Steffensen KR, Treuter E. Metabolic nuclear receptor signaling and the inflammatory acute phase response. Trends in endocrinology and metabolism: TEM. 2011;vol. 22(no. 8):333–343. doi: 10.1016/j.tem.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Wallenborn JG, Evansky P, Shannahan JH, Vallanat B, Ledbetter AD, Schladweiler MC, Richards JH, Gottipolu RR, Nyska A, Kodavanti UP. Subchronic inhalation of zinc sulfate induces cardiac changes in healthy rats. Toxicology and applied pharmacology. 2008;vol. 232(no. 1):69–77. doi: 10.1016/j.taap.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Wallenborn JG, Kovalcik KD, McGee JK, Landis MS, Kodavanti UP. Systemic translocation of (70)zinc: kinetics following intratracheal instillation in rats. Toxicology and applied pharmacology. 2009;vol. 234(no. 1):25–32. doi: 10.1016/j.taap.2008.09.024. [DOI] [PubMed] [Google Scholar]
- Wallenborn JG, McGee JK, Schladweiler MC, Ledbetter AD, Kodavanti UP. Systemic translocation of particulate matter-associated metals following a single intratracheal instillation in rats. Toxicological sciences : an official journal of the Society of Toxicology. 2007;vol. 98(no. 1):231–239. doi: 10.1093/toxsci/kfm088. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang L, Ding W, Zhang F. Acute toxicity of ferric oxide and zinc oxide nanoparticles in rats. Journal of nanoscience and nanotechnology. 2010;vol. 10(no. 12):8617–8624. doi: 10.1166/jnn.2010.2483. [DOI] [PubMed] [Google Scholar]
- Wang X, Katwa P, Podila R, Chen P, Ke PC, Rao AM, Walters DM, Wingard CJ, Brown JM. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Particle and fibre toxicology. 2011;vol. 8:24. doi: 10.1186/1743-8977-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson WP, Campen MJ, Nolan JP, Costa DL. Cardiovascular and systemic responses to inhaled pollutants in rodents: effects of ozone and particulate matter. Environmental health perspectives. 2001;vol. 109(Suppl 4):539–546. doi: 10.1289/ehp.01109s4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;vol. 106(no. 23):2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. The Journal of clinical investigation. 2005;vol. 115(no. 5):1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller CL, Collington SJ, Williams T, Lamb JR. Mast cells in health and disease. Clinical science (London, England : 1979) 2011;vol. 120(no. 11):473–484. doi: 10.1042/CS20100459. [DOI] [PubMed] [Google Scholar]
- Wichers LB, Nolan JP, Winsett DW, Ledbetter AD, Kodavanti UP, Schladweiler MC, Costa DL, Watkinson WP. Effects of instilled combustion-derived particles in spontaneously hypertensive rats. Part I: Cardiovascular responses. Inhalation toxicology. 2004;vol. 16(no. 6-7):391–405. doi: 10.1080/08958370490439696. [DOI] [PubMed] [Google Scholar]
- Widdicombe J, Lee LY. Airway reflexes, autonomic function, and cardiovascular responses. Environmental health perspectives. 2001;vol. 109(Suppl 4):579–584. doi: 10.1289/ehp.01109s4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen L, Svardsudd K, Korsan-Bengtsen K, Larsson B, Welin L, Tibblin G. Fibrinogen as a risk factor for stroke and myocardial infarction. The New England journal of medicine. 1984;vol. 311(no. 8):501–505. doi: 10.1056/NEJM198408233110804. [DOI] [PubMed] [Google Scholar]
- Wilson DW, Aung HH, Lame MW, Plummer L, Pinkerton KE, Ham W, Kleeman M, Norris JW, Tablin F. Exposure of mice to concentrated ambient particulate matter results in platelet and systemic cytokine activation. Inhalation toxicology. 2010;vol. 22(no. 4):267–276. doi: 10.3109/08958370903278069. [DOI] [PubMed] [Google Scholar]
- Wingard CJ, Walters DM, Cathey BL, Hilderbrand SC, Katwa P, Lin S, Ke PC, Podila R, Rao A, Lust RM, Brown JM. Mast cells contribute to altered vascular reactivity and ischemia-reperfusion injury following cerium oxide nanoparticle instillation. Nanotoxicology. 2010 doi: 10.3109/17435390.2010.530004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaki K, Yoshino S. Comparison of inhibitory activities of zinc oxide ultrafine and fine particulates on IgE-induced mast cell activation. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2009;vol. 22(no. 6):1031–1040. doi: 10.1007/s10534-009-9254-z. [DOI] [PubMed] [Google Scholar]
- Yang W, Lee S, Lee J, Bae Y, Kim D. Silver nanoparticle-induced degranulation observed with quantitative phase microscopy. Journal of Biomedical Optics. 2010;vol. 15(no. 4):045005. doi: 10.1117/1.3470104. [DOI] [PubMed] [Google Scholar]
- Yazdi AS, Guarda G, Riteau N, Drexler SK, Tardivel A, Couillin I, Tschopp J. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1alpha and IL-1beta. Proceedings of the National Academy of Sciences of the United States of America. 2010;vol. 107(no. 45):19449–19454. doi: 10.1073/pnas.1008155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokohira M, Takeuchi H, Yamakawa K, Saoo K, Matsuda Y, Zeng Y, Hosokawa K, Imaida K. Bioassay by intratracheal instillation for detection of lung toxicity due to fine particles in F344 male rats. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie. 2007;vol. 58(no. 4):211–221. doi: 10.1016/j.etp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Zhang J, Alcaide P, Liu L, Sun J, He A, Luscinskas FW, Shi GP. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PloS one. 2011;vol. 6(no. 1):e14525. doi: 10.1371/journal.pone.0014525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Xie Y, Qian X, Jiang R, Song W. Acute effects of fine particles on cardiovascular system: differences between the spontaneously hypertensive rats and wistar kyoto rats. Toxicology letters. 2010;vol. 193(no. 1):50–60. doi: 10.1016/j.toxlet.2009.12.002. [DOI] [PubMed] [Google Scholar]