Abstract
Background
The 3 year survival after pulmonary metastatectomy for osteosarcoma (OS) is approximately 30%. Resection of metastatic disease can prolong life in pediatric patients with OS. Our objective is to assess the outcome of pediatric patients with pulmonary metastases located centrally as compared to peripheral lesions.
Methods
A retrospective review of patients 0 to 21 years with a diagnosis of OS with pulmonary metastases on CT scan between 1985 and 2000 was completed. Demographics, metastasis location, survival, morbidity and mortality were evaluated.
Results
Of 115 patients who had pulmonary metastasis secondary to OS, there were 96 wedge resections and 13 lobectomy/pneumonectomies in 84 patients. The morbidity of wedge resection was 9% and lobectomy/pneumonectomy was 8%. There were no deaths from surgery. The median survival for patients undergoing lobectomy compared to wedge resection was 0.61 and 1.14 years respectively but did not reach statistical significance. The median overall survival for the entire cohort was 0.75 years. The median overall survival after initial detection of metastatic disease was 1.06 years among the patients with peripheral disease, compared to 0.38 years in patients with central disease (p=0.008).
Conclusion
Patients with central pulmonary metastases in osteosarcoma have a very poor prognosis, even after operative treatment, compared to those with peripheral disease. Patients with central lesions may benefit from other non-surgical treatment options.
Keywords: Osteosarcoma, pulmonary metastases, thoracotomy, lobectomy, wedge resection, surgery
Osteosarcoma is the most common bone tumor in children. The prognosis is based primarily on the development of metastasis, whether at presentation or at relapse. Patients with metastatic osteosarcoma to the lungs have a poor prognosis. Despite advances in chemotherapy, radiation therapy and surgery, the three year survival of patients with metastatic osteosarcoma is 20-45%1-4 Surgical resection of pulmonary metastasis has been shown in numerous studies to prolong survival and is part of the standard surgical treatment for osteosarcoma.2,5-7 It has also been shown that patients with pulmonary metastases do better than those with metastases in other sites.8 When planning a thoracic operation for metastatic osteosarcoma, the location of the lesion is important and preoperative imaging is essential to the planning process. While it has been shown that CT scans may underestimate the number of metastases, it remains the best diagnostic modality for evaluating metastases.9 Some pulmonary lesions are located in the peripheral aspect of the lungs and when few in number are amenable to wedge resection whereas others are located in the central portion of the lung close to the hilum and primary vascular and airway branches. Centrally located lesions may require anatomical resections such as a lobectomy instead of the more common wedge resections that are done for peripheral lesions. The prognostic significance of central versus peripheral pulmonary metastasis in osteosarcoma has not previously been evaluated and may support alternative non-surgical therapies.10 Our aim is to examine the outcome of osteosarcoma patients with CT identified central versus peripheral pulmonary metastasis who underwent surgical resection.
Materials and Methods
Institutional review board approval was obtained for this retrospective review. Using our database of patients with metastatic osteosarcoma, we evaluated a cohort of patients diagnosed between January 1, 1985 and December 31, 2000. Patients under the age of 21 were included if CT scans were available for review and clinical information was complete. Demographics, morbidity, mortality, survival and disease status were examined. Operative notes were reviewed to determine the extent of resection, and examine intra-operative morbidity.
Survival analysis was performed using the overall survival (OS), which was computed from the date of first operation to the date of death or the date of last follow up, whichever occurred first. The patients who were alive at the last follow-up date were censored. The Kaplan-Meier method was used to estimate the probability of the overall survival. The Log rank test and Cox proportional hazards regression analysis was used to determine the association of OS with the clinical factors and demographic parameters. The Wilcoxson rank sum test was used to compare the age between the central and peripheral procedure groups, and the Fisher's exact test was used to determine demographic parameters between the central and peripheral procedure groups. All the analysis were performed using S-plus 7 (Insightful corporate, Seattle, WA). Overall survival is defined by time from first operation until death, or first identification of a central lesion until death.
For our study we defined as central lesions those lesions that abut a first degree bronchus or blood vessel, such that a resection of that lesion would require resection of that structure in order to assure adequate margins. Central lesions were determined by CT scan. Essential lesions were determined by CT scan as assessed by the authors. Since operative therapy is planned based on CT imaging, we used CT instead of operative findings as the definition. The coding of central versus peripheral lesions was done before statistical analysis; therefore, the coder was blinded to the patient outcome.
Many patients in this study had multiple thoracotomies. Our criteria for re-operation on metastatic lesions included no disease or controlled disease outside of the lung or chest wall, the ability to achieve a complete resection of all metastasis without causing respiratory insufficiency or oxygen dependence based on pre-operative spirometry testing, and performance status.
Multiple different combinations of chemotherapy and rarely, radiation therapy were used but are not included in this report.
Results
The patient's ages ranged from 0-21 years. The median age was 14.1 years. The median follow-up was 9.72 years (95% CI: 6.54-NA years) and the longest follow-up time was 14.89 years. There were 36 males (65.5 %) and 19 females (34.5%) in the cohort. There were no significant differences in gender, (p=0.53 ) age (p=0.57 ) or ethnicity(p=0.63 ) between the group of patients with one or more central metastasis versus those with peripheral metastasis only . Table 1 shows other patient tumor characteristics.
Table 1. Analysis of outcome factors in osteosarcoma lung metastasis.
| Group | N | Death/(%) | Median survival time (Yrs) (95% CI) | Probability of 1 yr survival | Probability of 3 yr survival | p value |
|---|---|---|---|---|---|---|
| Lobectomy | 12 | 10/(83%) | 1.14 (0.46, NA) | 0.58 (0.36, 0.94) | 0.25 (0.09, 0.67) | 0.91 |
| Wedge | 33 | 25(75%) | 0.61 (0.40, 3.41) | 0.42 (0.29, 0.63) | 0.30 (0.18, 0.51) | |
| Central | 15 | 15/(100%) | 0.38 (0.25, 1.57) | 0.4 (0.22, 0.74) | 0.07 (0.01, 0.44) | 0.008 |
| Peripheral | 32 | 21(65%) | 1.06 (0.6, NA) | 0.53 (0.38, 0.74) | 0.41 (0.27, 0.62) | |
| Lobectomy | 12 | 10(83%) | 1.14 (0.46, NA) | 0.58 (0.36, 0.94) | 0.25 (0.09, 0.67) | 0.21 |
| Wedge number <5 | 11 | 7(63%) | 3.41 (0.61, NA) | 0.64 (0.41, 0.995) | 0.55 (0.32, 0.94) | |
| Wedge number >= 5 | 22 | 18(82%) | 0.45 (0.29, 1.1) | 0.32 (0.17, 0.59) | 0.18 (0.08, 0.44) |
Of the 115 patients who had pulmonary metastases secondary to osteosarcoma, we identified 84 that underwent surgical resection. Fifty-five of those patients had CT scans available for examination. This cohort underwent 118 total procedures: 96 wedge resections, and 12 lobectomies and one pneumonectomy. Ten patients had pulmonary metastasis at diagnosis. The remainder developed pulmonary metastasis after the initiation of therapy. (Patients who underwent biopsy alone were excluded.)
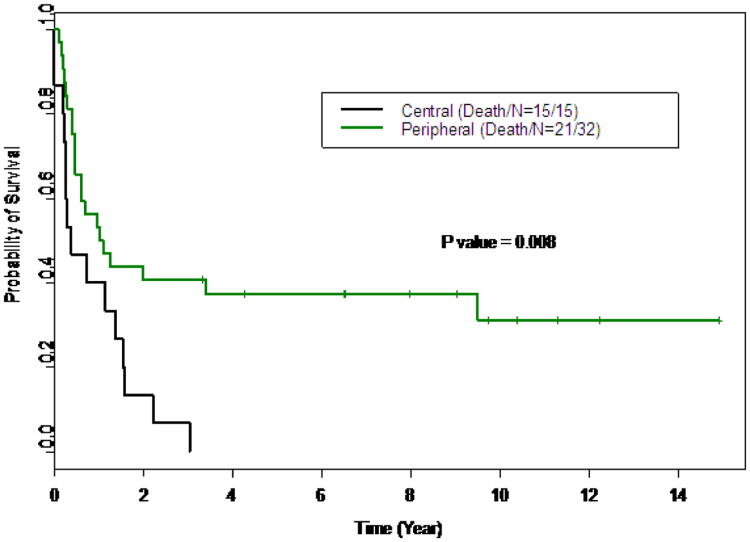
There were no surgical mortalities. The morbidity of wedge resection was 9% and lobectomy/pneumonectomy was 8%. Morbidity included fever of unknown origin, pneumothorax, hydropneumothorax, pneumonia, and prolonged air leak. One intended lobectomy had to be aborted secondary to intraoperative hemorrhage. There was no significant difference in surgical morbidity between the wedge resection and lobectomy group. The log rank test indicated that type of operation (wedge versus lobectomy), or the number of metastatic lesions was not a predictor of overall survival (Table 2). However, the central or peripheral locations of the lesions were significant predictors of survival. (p=0.008). The univariate Cox proportional hazards regression analysis indicates that the number of nodules (HR=1.1, p=0.003), but not age (HR=1.04, mp=0.35), was significantly associated with survival. (Table 2a) The multivariate analysis suggested that both location of the lesions and the number of nodules were significantly associated with the overall survival (Table 2b). Patients with peripheral lesions have increased survival compared to those to those with central lesions (HR=0.42, p=0.01, Figure 1), the risk of death increases as the number of nodules resected increases (HR=1.09, p=0.01).
Table 2.
Univariate and multivariate analysis a)Statistical association of age and number of nodules with overall survival in univariate analysis. Number of nodules but not age is a significant predictor of overall survival when analyzed as a continuous variable b)In multivariate analysis, two variables, location of lung metastasis by CT scan and number of nodules continued to be significant on multivariate analysis.
| a. | ||
|---|---|---|
| variable | HR (95% CI) | p value |
| age | 1.04 (0.96, 1.13) | 0.35 |
| Num of Nodules | 1.1 (1.03, 1.17) | 0.003 |
| b. | ||
| variable | HR (95% CI) | p value |
| Peripheral vs. central | 0.42 (0.21, 0.84) | 0.01 |
| Num of Nodules | 1.09 (1.02, 1.17) | 0.01 |
Figure 1.
Kaplan Meier curve comparing survival of patients with centrally located versus peripheral lesions.
Although data is not complete on all patients, at operation central lesions identified on preoperative imaging were indeed very close to airway or vascular structures and required either segementectomy or lobectomy. At the time of operation few ‘central lesions’ were in hilar lymph nodes. Hilar lymph node dissection was not routine in these metastatic patients. Operative reports were reviewed and central lesions were not found to be peripheral at operation and vice-versa. Pathology revealed metastatic osteosarcoma in resected lung lesions. Pathologic correlation with imaging revealed central lesions were not mistaken lymph nodes but were parenchymal metastatic disease.
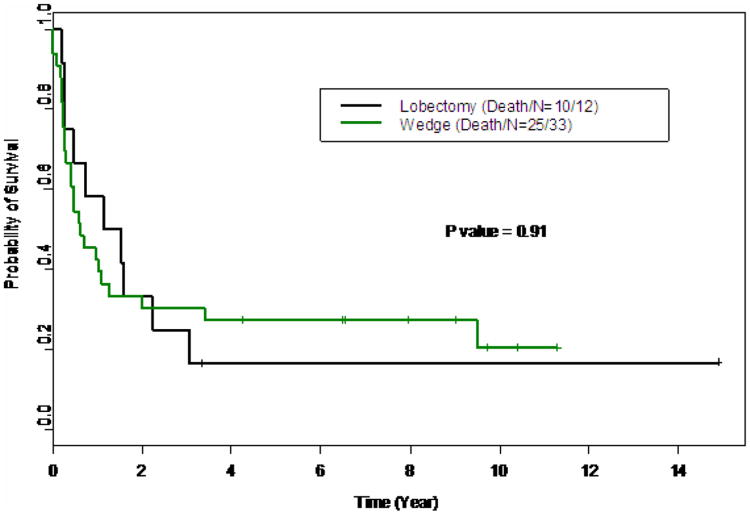
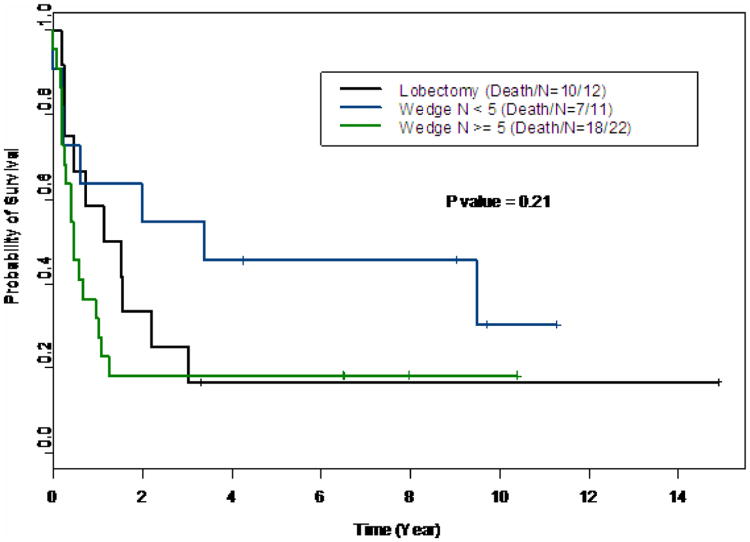
There was no significant difference in 3 year overall survival when comparing lobectomy to wedge resection (18% vs 30% p=0.91, Figure 3). Thirty-two of these patients, 58%, underwent more than one thoracotomy for relapsed disease. No significant difference in survival existed among patients with lobectomy, wedge resections of at least 5 lesions, and wedge resections less than 5 lesions (Figure 2, p=0.21). The most significant difference was found in patients with a central versus a peripheral location of their pulmonary metastasis. Patients with peripheral lesions had an estimated 3 year survival probability of 41% (95% CI: 0.27-0.62) compared to 7% (95% CI: 0.01, 0.44) for those with central lesions (table 1, p=0.008). The estimated median survival time for the entire cohort was 0.73 years (95% CI 0.47-1.57).
Figure 3.
Kaplan Mefercurve comparing over survival of patients who underwent lobectomy versus those who underwent wedge resection.
Figure 2.
Kaplan Meier curve comparing patients who underwent lobectomy to those who underwent wedge resections of <5 lesions or ≥5.
Interestingly, there was no difference in outcome between those patients who presented with pulmonary metastasis and those who developed pulmonary metastasis after the completion of chemotherapy. In the 10 patients who had metastasis at presentation, the estimated median1 and 3 year survival was 50% and 30% and for the remaining 38 patients who developed pulmonary metastasis after diagnosis estimated 1 and 3 year median survival was 47% and 29% respectively. (p=0.58)
Discussion
Recent studies have shown that aggressive surgical approaches to pulmonary metastases in osteosarcoma improve survival.2,6-7 However, it has been previously unknown if outcome is dependent on the location of pulmonary metastasis. Our results suggest that there is a survival advantage to peripherally located metastases and that this difference was not due to surgical approach.
A previous study of osteosarcoma demonstrated that in patients with pulmonary metastasis, tumor necrosis after neoadjuvant chemotherapy of greater than 98%, (p<0.05) and initial disease free interval of greater than one year, (p<0.001) were the strongest predictors of survival, but that age, number of pulmonary surgeries and greater than or less than 10 metastasis, was not predictive of survival.7 Since relatively few patients had greater than 10 lesions, we stratified by less than or greater than 5 lesions. A study from the Cooperative Osteosarcoma Study Group of Germany also found a negative correlation between tumor burden, as measured by number of nodules, and prognosis, but did not specify location of the lesion. They found that patients with 2 or fewer pulmonary metastasis had a survival advantage compared to those with over 2 metastasis.(p<0.001) They also demonstrate a survival advantage with surgical resection. P<0.0001)3
Several recent studies have demonstrated the utility of an aggressive approach to resection of pulmonary metastases in osteosarcoma patients.2,6,7,11-13 Many studies have shown a survival advantage to repeated thoracotomies for metastatic osteosarcoma.2,6,7 Briocolli and colleagues in Italy have shown in 267 patients, with every month of disease free interval, there is a 3% improvement in survival. Also, patients that undergo a second metastatectomy, have the same probability of survival as those that were operated the first time within 5 years of their first operation. Their calculated overall 3 year survival was 37%, similar to our 25% for lobectomy and 30% for those undergoing multiple wedge resections only. The Briocolli study did not evaluate survival based on surgical approach.
All patients who underwent lobectomy had at least one central lesion. However, because surgical approach is only a surrogate variable for the location of the lesion, and may be subject to surgeon preference, we evaluated the location of the lesion as identified by CT scan and found a significant difference in survival between central (hilar) and peripherally located lesions. No other previous study to our knowledge has evaluated this as a variable. There were no survivors at 3 years after identification of a central lesion with a median survival of 4 months, compared to 41% survival at 3 years with a median survival of 12 months in patients with peripheral lesions. The reason for a survival advantage in patients who have peripheral pulmonary metastatic disease is unknown. This difference may be because patients with central lesions had more extrapulmonary metastasis diagnosed after their central lesion and those with peripheral lesions mostly had lung only metastatic disease although not statistically significant.(data not shown) In addition, the study population is from a tertiary referral center and suffers from relatively small sample size.
We chose to include patients beginning at 1985 since this was the onset of modern CT scanning. However, CT scans have improved significantly over this time period and older CT scans may have underestimated the number of lung metastasis, and small central lesions may have been missed. This is a potential selection bias. Chemotherapeutic approaches have also changed over this time period but, unfortunately, survival in patients with metastatic disease has not changed, and pulmonary metastases continue to be chemoresistant.
Patients with centrally located pulmonary metastatic disease in osteosarcoma have a very poor prognosis. We use the CT definition, and not intra-operative findings, of central versus peripheral so that it could be used in the future to perhaps avoid thoracotomy in patients with central disease. Because of very short post-surgical survival, other non-surgical approaches may be beneficial for patients with centrally located metastasis and surgical exploration may not be warranted. If unexpected central disease is found at the time of surgery, surgical removal should be completed if possible. However, postoperative discussion with the family should reflect the poor prognosis in this instance. More extensive study is necessary to identify any genetic or other differences in tumors of patients who develop central pulmonary metastatic disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsuchiya H, Kanazawa Y, Abdel-Wanis ME, et al. Effect of timing of pulmonary metastases identification on prognosis of patients with osteosarcoma: the Japanese Musculoskeletal Oncology Group Study. J Clin Oncol. 2002;20:3470–3477. doi: 10.1200/JCO.2002.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Briocolli A, Rocca M, Salone M, et al. Resection of recurrent pulmonary metastases in patients with osteosarcoma. Cancer. 2005;104:1721–1725. doi: 10.1002/cncr.21369. [DOI] [PubMed] [Google Scholar]
- 3.Kempf-Bielack B, Bielack S, Jurgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2005;23:559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 4.Carter SR, Grimer RJ, Sneath RS, et al. Results of thoracotomy in osteogenic sarcoma with pulmonary metastases. Thorax. 1991;46:727–731. doi: 10.1136/thx.46.10.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacci G, Mercuri M, Bricolli A, et al. Osteogenic sarcoma of the extremity with detectable lung metastases at presentation. Cancer. 1997;79:245–254. [PubMed] [Google Scholar]
- 6.Snyder CL, Saltzman DA, Ferrell KL, et al. A new approach to the resection of pulmonary osteosarcoma metastases: results of aggressive metastectomy. Clinical Orthopedics and Related Research. 1991:247–253. [PubMed] [Google Scholar]
- 7.Harting MT, Blakely ML, Jaffe N, et al. Long-Term Survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg. 2006;41:194–199. doi: 10.1016/j.jpedsurg.2005.10.089. [DOI] [PubMed] [Google Scholar]
- 8.Blielack S, Kempf-Blielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 9.Kayton ML, Huvos AG, Casher J, et al. Computed tomographic scan of the chest underestimates the number of metastatic lesions in osteosarcoma. J Pediatr Surg. 2006;41:200–206. doi: 10.1016/j.jpedsurg.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Graat H, van Beusechem VW, Schagen F, et al. Intravenous administration of the conditionally replicative adenovirus Ad5-Δ24RGD induces regression of osteosarcoma lung metastases. Molecular Cancer. 2008;7:9. doi: 10.1186/1476-4598-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacker FM, Scweinitz Dv, Gambazzi F. The relevance of surgical therapy for bilateral and/or multiple pulmonary metastases in children. Eur J Pediatr Surg. 2007;17:84–89. doi: 10.1055/s-2007-964873. [DOI] [PubMed] [Google Scholar]
- 12.Karnak I, Senocak ME, Kutlak T. Pulmonary Metastases in children: an analysis of surgical spectrum. Eur J Pediatr Surg. 2002;12:151–158. doi: 10.1055/s-2002-32728. [DOI] [PubMed] [Google Scholar]
- 13.Kandioler D, Kromer E, Tuchler H, et al. Long-Term Results after repeated surgical removal of pulmonary metastases. Ann Thorac Surg. 1998;65:909–912. doi: 10.1016/s0003-4975(98)00019-8. [DOI] [PubMed] [Google Scholar]