Abstract
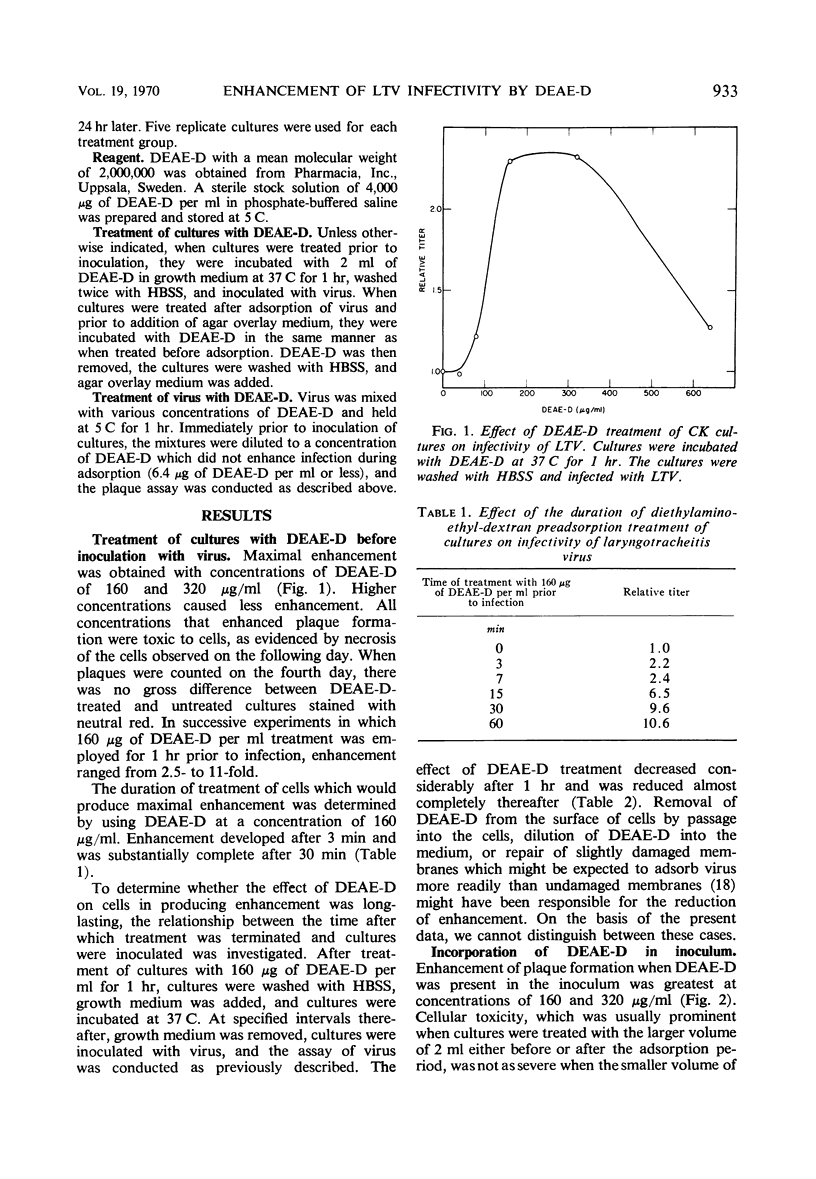
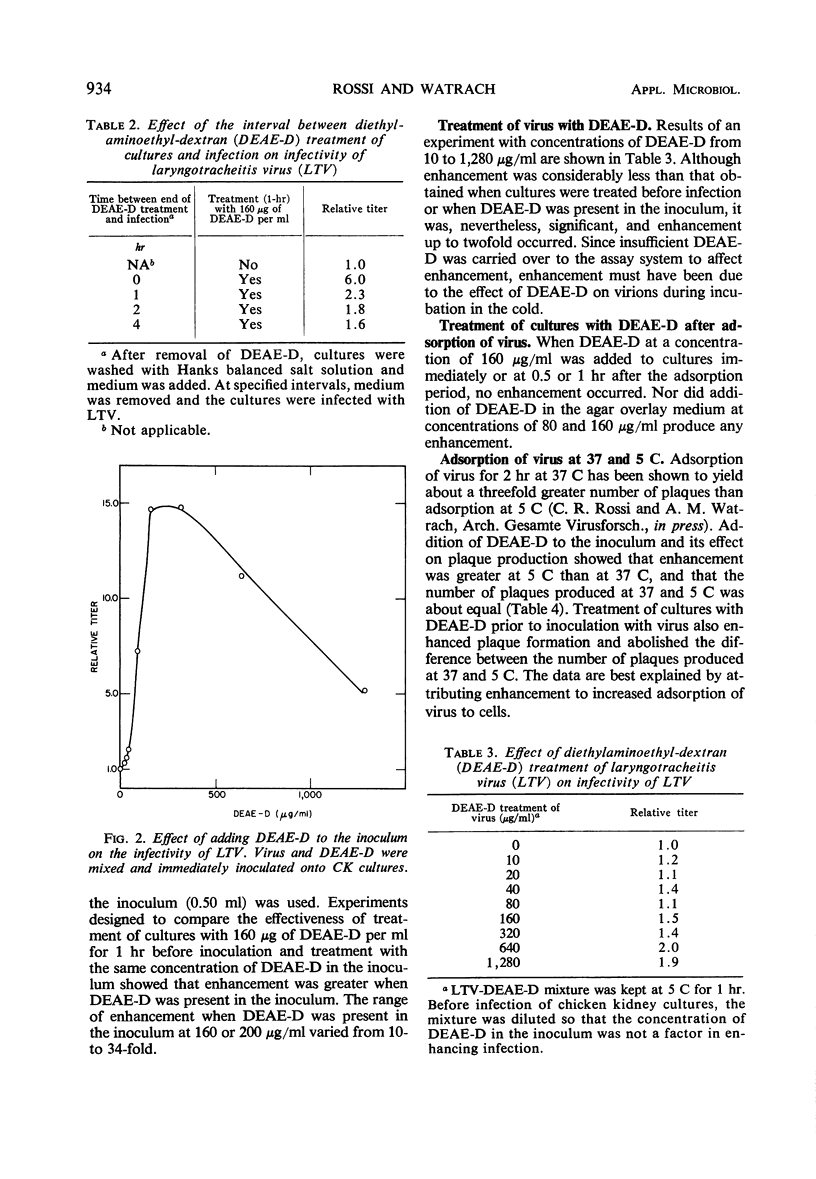
Diethylaminoethyl-dextran (DEAE-D) enhanced the infectivity of laryngotracheitis virus (LTV) for chicken kidney (CK) cells when cultures were treated before inoculation with virus and when DEAE-D was present in the inoculum. Infectivity was not increased when cultures were treated after virus had adsorbed to cells; since infection was not synchronized, most of the virus had probably already penetrated the plasma membrane by the time DEAE-D was added. Maximal enhancement occurred when DEAE-D was present in the inoculum. Enhancement of a lesser degree occurred when virus and DEAE-D were mixed, diluted, and inoculated onto cultures. Adsorption of LTV at 37 C as compared to that at 5 C usually yields about a threefold greater number of plaques after a 2-hr adsorption period. However, when DEAE-D was incorporated in the inoculum, greater enhancement occurred at 5 C than at 37 C, and the number of plaques produced at both adsorption temperatures was about equal. Results are compatible with the hypothesis that increased adsorption is a factor in enhancement of infectivity of LTV by DEAE-D.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach H. L. Ribonucleic acid of foot-and-mouth disease virus: an ultrasensitive plaque assay. Proc Soc Exp Biol Med. 1966 Dec;123(3):939–945. doi: 10.3181/00379727-123-31644. [DOI] [PubMed] [Google Scholar]
- Craighead J. E., Layne C. H. Contrasting effects of polycations on plaquing efficiency of encephalomyocarditis virus variants. J Virol. 1969 Jan;3(1):45–51. doi: 10.1128/jvi.3.1.45-51.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S. Pentration of animal viruses into cells. Prog Med Virol. 1965;7:1–43. [PubMed] [Google Scholar]
- Dunnebacke T. H., Levinthal J. D., Williams R. C. Entry and release of poliovirus as observed by electron microscopy of cultured cells. J Virol. 1969 Oct;4(4):505–513. doi: 10.1128/jvi.4.4.505-513.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Maes R. F., Campbell J. B., Koprowski H. Effect of polyions on the infectivity of rabies virus in tissue culture: construction of a single-cycle growth curve. J Virol. 1967 Feb;1(1):145–151. doi: 10.1128/jvi.1.1.145-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Sharp D. G. The influence of DEAE-dextran on plain and synergistic plaque formation by two poxviruses. J Gen Virol. 1969 Jun;4(4):505–512. doi: 10.1099/0022-1317-4-4-505. [DOI] [PubMed] [Google Scholar]
- Koch G., Bishop J. M. The effect of polycations on the interaction of viral RNA with mammalian cells: studies on the infectivity of single- and double-stranded poliovirus RNA. Virology. 1968 May;35(1):9–17. doi: 10.1016/0042-6822(68)90300-0. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Howe C. Structure and development of viruses as observed in the electron microscope. IX. Entry of parainfluenza I (Sendai) virus. J Virol. 1968 Oct;2(10):1122–1132. doi: 10.1128/jvi.2.10.1122-1132.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M., Mednis B. Electron microscopy of herpes simplex virus. I. Entry. J Virol. 1968 May;2(5):507–516. doi: 10.1128/jvi.2.5.507-516.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M. Structure and development of viruses as observed in the electron microscope. 8. Entry of influenza virus. J Virol. 1968 Sep;2(9):925–936. doi: 10.1128/jvi.2.9.925-936.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Mednis B. Structure and development of viruses as observed in the electron microscope. V. Entry and uncoating of adenovirus. J Virol. 1969 Nov;4(5):777–796. doi: 10.1128/jvi.4.5.777-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S. Interaction of respiratory syncytial virus with polyions: enhancement of infectivity with diethylaminoethyl dextran. Proc Soc Exp Biol Med. 1968 May;128(1):163–166. doi: 10.3181/00379727-128-32969. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., McCutchan J. H., Vaheri A. Factors influencing the enhancement of the infectivity of poliovirus ribonucleic acid by diethylaminoethyl-dextran. J Virol. 1967 Oct;1(5):891–897. doi: 10.1128/jvi.1.5.891-897.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano J. S., Vaheri A. Enhancement of infectivity of poliovirus RNA with diethylaminoethyl-dextran (DEAE-D). Arch Gesamte Virusforsch. 1965;17(3):456–464. doi: 10.1007/BF01241201. [DOI] [PubMed] [Google Scholar]
- Reynolds H. A., Wetrach A. M., Hanson L. E. Development of the nuclear inclusion bodies of infectious laryngotracheitis. Avian Dis. 1968 May;12(2):332–347. [PubMed] [Google Scholar]
- Rossi C. R., Reynolds H. A., Watrach A. M. Studies of laryngotracheitis virus in avian tissue cultures. I. Plaque assay in chicken embryo kidney tissue cultures. Arch Gesamte Virusforsch. 1969;28(2):219–228. doi: 10.1007/BF01249387. [DOI] [PubMed] [Google Scholar]
- Ryser H. J. Uptake of protein by mammalian cells: an underdeveloped area. The penetration of foreign proteins into mammalian cells can be measured and their functions explored. Science. 1968 Jan 26;159(3813):390–396. doi: 10.1126/science.159.3813.390. [DOI] [PubMed] [Google Scholar]
- Somers K. D., Kirsten W. H. Focus formation by murine sarcoma virus: enhancement by DEAE-dextran. Virology. 1968 Sep;36(1):155–157. doi: 10.1016/0042-6822(68)90129-3. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Sedwick W. D., Plotkin S. A. Growth of rubella virus in BHK21 cells. II. Enhancing effect of DEAE-dextran, semicarbazide and low doses of metabolic inhibitors. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1092–1098. doi: 10.3181/00379727-125-32284. [DOI] [PubMed] [Google Scholar]
- WATRACH A. M., HANSON L. E. Cytopathic effect of infectious laryngotracheitis virus in cultures of chicken embryo kidney cells. Proc Soc Exp Biol Med. 1963 Jan;112:230–232. doi: 10.3181/00379727-112-28002. [DOI] [PubMed] [Google Scholar]
- WATRACH A. M., HANSON L. E., WATRACH M. A. THE STRUCTURE OF INFECTIOUS LARYNGOTRACHEITIS VIRUS. Virology. 1963 Dec;21:601–608. doi: 10.1016/0042-6822(63)90233-2. [DOI] [PubMed] [Google Scholar]
- Warden D., Thorne H. V. Influence of diethylaminoethyl-dextran on uptake and degradation of polyoma virus deoxyribonucleic acid by mouse embryo cells. J Virol. 1969 Oct;4(4):380–387. doi: 10.1128/jvi.4.4.380-387.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



